Abstract
Arabidopsis (Arabidopsis thaliana) plants display a number of root developmental responses to low phosphate availability, including primary root growth inhibition, greater formation of lateral roots, and increased root hair elongation. To gain insight into the regulatory mechanisms by which phosphorus (P) availability alters postembryonic root development, we performed a mutant screen to identify genetic determinants involved in the response to P deprivation. Three low phosphate-resistant root lines (lpr1-1 to lpr1-3) were isolated because of their reduced lateral root formation in low P conditions. Genetic and molecular analyses revealed that all lpr1 mutants were allelic to BIG, which is required for normal auxin transport in Arabidopsis. Detailed characterization of lateral root primordia (LRP) development in wild-type and lpr1 mutants revealed that BIG is required for pericycle cell activation to form LRP in both high (1 mm) and low (1 μm) P conditions, but not for the low P-induced alterations in primary root growth, lateral root emergence, and root hair elongation. Exogenously supplied auxin restored normal lateral root formation in lpr1 mutants in the two P treatments. Treatment of wild-type Arabidopsis seedlings with brefeldin A, a fungal metabolite that blocks auxin transport, phenocopies the root developmental alterations observed in lpr1 mutants in both high and low P conditions, suggesting that BIG participates in vesicular targeting of auxin transporters. Taken together, our results show that auxin transport and BIG function have fundamental roles in pericycle cell activation to form LRP and promote root hair elongation. The mechanism that activates root system architectural alterations in response to P deprivation, however, seems to be independent of auxin transport and BIG.
Phosphorus (P) is one of the most limiting nutrients for plant growth in many natural and agricultural ecosystems. In response to low P availability in the soil, plants have developed several adaptive strategies to increase P acquisition, including enhanced expression of P transport genes, exudation of P-solubilizing substances, such as organic acids and phosphatases, and alterations in postembryonic root development (for review, see Raghothama, 1999; López-Bucio et al., 2000; Abel et al., 2002; López-Bucio et al., 2003; Vance et al., 2003).
Plants typically respond to P deficiency by allocating more carbon to roots, thus increasing their root-to-shoot ratio. In addition, low P availability can dramatically alter the spatial configuration of the root system by increasing root hairs and promoting lateral root formation. Such plastic root alterations are believed to play a crucial role in exploring increased soil volumes in search of nutrient-rich patches (Neumann and Martinoia, 2002; López-Bucio et al., 2003). In Lupinus albus, P deficiency promotes the formation of cluster roots, also termed proteoid roots (Johnson et al., 1996). Increased root branching in response to P limitation has also been reported for Arabidopsis (Arabidopsis thaliana). Growth of Arabidopsis in a moderate-to-limiting supply of P results in a redistribution of root growth from the primary to lateral roots (Williamson et al., 2001). Reduced primary root elongation in low P conditions was accompanied by increased root branching, perhaps concentrating root biomass near the soil surface for more efficient nutrient foraging (López-Bucio et al., 2002, 2003).
Despite several detailed anatomical studies on root architectural alterations in response to P limitation, little is known about the physiological and molecular mechanisms that coordinate these developmental changes. Phytohormones control most root system characteristics in angiosperms, including primary root growth and the formation of lateral roots. For example, auxin, ethylene, and cytokinin are thought to play important roles in P deficiency-induced alterations in lateral root development and cluster root development (López-Bucio et al., 2003; Vance et al., 2003). In Lupin and Arabidopsis, an important role for auxins in the formation of lateral roots in response to P limitation has been suggested. In low P conditions, the auxin transport inhibitors 2,3,5-triiodobenzoic acid (TIBA) and N-(1-napthyl) phtalamic acid (NPA) inhibit the formation of proteoid or lateral roots in Lupin and Arabidopsis, respectively (Gilbert et al., 2000; Skene and James, 2000; López-Bucio et al., 2002). In addition, auxin treatments of P-starved plants and gene expression analysis support the conclusion that auxin signaling is involved in the adaptive response of the root system architecture to P limitation (López-Bucio et al., 2002; Al-Ghazi et al., 2003).
Although these data collectively support the hypothesis that auxin is involved in the responses of the root system architecture to P limitation, several mutants defective in either auxin transport or signaling display normal changes in root system architecture in response to P availability. For example, the auxin transport mutants aux1 and eir1 and the auxin response mutants axr1 and axr4, which have reduced lateral root formation in P-sufficient conditions, show typical primary root growth inhibition and enhanced production of lateral roots on low P media (Williamson et al., 2001; López-Bucio et al., 2002). To date, it is not clear whether changes in local concentration, transport, or sensitivity to auxin play important roles in the root architectural changes that occur during P deprivation.
To elucidate the low P-signaling pathway responsible for alterations in root development, we conducted a visual screen for Arabidopsis mutants with reduced lateral root formation when grown in P-limiting conditions. Here, we report the isolation of mutants that have reduced lateral root formation in low P conditions. Recombination mapping identified the gene defective in the mutants as BIG, a gene required for normal auxin transport (Ruegger et al., 1997; Gil et al., 2001) and lateral root development (Ruegger et al., 1997). Analysis of root system architecture and DR5:uidA expression in wild-type and BIG plants in high and low P conditions indicates that low P-induced alterations in postembryonic root development are likely independent of auxin transport and BIG function. Moreover, our results point to a key role of BIG in pericycle cell activation to form lateral root primordia (LRP), a process that is not modified by P availability but is required for increased LRP emergence in low P conditions.
RESULTS
Isolation of Arabidopsis Mutants with Altered Lateral Root Formation in Response to Low P Conditions
To identify mutants that are defective in lateral root formation in low P conditions, we screened ethyl methane sulfonate (EMS) and T-DNA insertion mutant collections (Krysan et al., 1999) by observing the root architecture of plants growing over the surface of agar plates with low (1 μm) P content. Three mutant lines showing a reduced number and density of lateral roots during P deprivation were identified (Fig. 1, A and B). Other aspects of root architecture induced by low P, such as primary root growth inhibition and root hair growth stimulation, were apparently unaffected in the mutants. The mutants were back-crossed to either wild-type Columbia (Col-0) or Wassilewskija (Ws) four times prior to detailed phenotypic analyses. In F2 progeny from these crosses, the 3 lines segregated the mutant phenotype in a 1:3 ratio, indicating that each resulted from a single recessive nuclear mutation. All three mutants appeared to belong to the same phenotypic class, showing reduced primary root growth, absence of lateral roots, and very short root hairs in high (1 mm) P conditions (Fig. 1C). Complementation tests revealed that the three lines were allelic (data not shown). We named this locus low phosphate-resistant root 1 (lpr1), and the three alleles were designated lpr1-1 (Col-0), lpr1-2 (Ws), and lpr1-3 (Col-0). Adult lpr1 mutants had shoot developmental alterations (Fig. 1, D–F). Mutant plants have a compact rosette caused by a reduction in leaf petiole length and smaller, round leaves (Fig. 1E) compared to wild type (Fig. 1D). At later stages of development, lpr1 plants showed reduced apical dominance (Fig. 1F). Aside from these morphological alterations, lpr1 mutants were fertile and produced fully viable seed (data not shown). Since the three lpr1 alleles appeared to be phenotypically indistinguishable, we performed most of our detailed studies on lpr1-1 (Col-0) and lpr1-2 (Ws).
Figure 1.
lpr1-1 mutant characterization. Twelve-day-old wild-type (Col-0; A) and lpr1-1 (B) seedlings growing on media containing low P. C, Eighteen-day-old Col-0 (left) and lpr1-1 (right) seedlings growing in high P. Note that no lateral roots are visible in the mutant at this advanced stage of seedling development. Col-0 (D) and lpr1-1 (E) grown under 16-h photoperiod for 3 weeks at 22°C/18°C. F, Mature Col-0 and lpr1-1 plants.
Recombinant Mapping of lpr1 Locus: lpr1 IsAllelic to doc1/tir3/big
The chromosomal location of the lpr1 locus was mapped genetically. We out-crossed an lpr1 mutant plant (Col-0 background) to Ws. DNA from F2 progeny with the lpr1 mutant phenotype was analyzed for linkage to published markers (Konieczny and Ausubel, 1993; Bell and Ecker, 1994). The lpr1 mutation mapped to the top of chromosome 3, north of nga172 (Fig. 2). The doc1/tir3 locus that encodes BIG, a calossin-like protein required for polar auxin transport in Arabidopsis, also maps to this region (Gil et al., 2001). To test the possibility that lpr1 mutants were allelic to doc1/tir3, we crossed lpr1-1 with doc1 (Li et al., 1994). F1 plants from this cross had lpr1 mutant phenotypes (data not shown), indicating that lpr1 mutants represent new mutant alleles of BIG.
Figure 2.
Recombinant mapping of lpr1-1. The lpr1-1 mutation was localized to the top arm of chromosome 3 using published markers (Konieczny and Ausubel, 1993; Bell and Ecker, 1994). The lpr1 mutation was mapped to north of nga172, a region that includes the BIG locus (Ruegger et al., 1997; Gil et al., 2001). Fractions represent the number of recombinant chromosomes over the total number of chromosomes scored.
Effects of Phosphate Availability on Root System Architecture in Wild Type and BIG Mutants
To more clearly define the alterations in the root architectural response to low P caused by mutations in BIG, we performed temporal and single-point measurements of primary root growth, lateral root density, and root hair length. To test whether mutations in BIG could alter primary root growth in response to varying P availability, a kinetic analysis of primary root growth in wild-type and lpr1-2 seedlings was carried out in high and low P conditions. As previously reported for tir3 (Ruegger et al., 1997), primary roots in the lpr1-2 mutants were 30% to 40% shorter than wild type at 12 d in high P conditions (Fig. 3A). In low P conditions, the primary roots of both wild-type and mutant plants were similar, growing for 6 to 9 d before arresting at lengths of only 1.5 to 1.8 cm (Fig. 3A). In addition, root hair length on primary roots of wild-type and lpr1-2 plants were measured following 10 d of growth on high and low P media. In high P conditions, in lpr1-2, root hairs were 50% shorter than wild type (Fig. 3B). In contrast, low P conditions restored root hair elongation to wild-type levels (Fig. 3B). Similar results were obtained for doc1 (data not shown).
Figure 3.
Effects of P availability on the root system architecture of wild-type (Ws) and lpr1-2 plants. A, Primary root growth kinetic assay. Seedlings were grown for the indicated times over the surface of agar plates containing high or low P and primary root lengths were measured. B, Mean root hair length at 10 d in the above experiment. C, Mean lateral root density expressed as the number of lateral roots per centimeter, recorded at 15 d in the experiment. In B and C, black bars represent growth in high P and white bars represent growth in low P. All values shown represent the mean of 20 seedlings ± sd. Letters represent means statistically different (P < 0.05).
To further analyze the effects of low P availability on lateral root development and the effects of mutations in BIG on this process, the density of emerged lateral roots in wild-type and lpr1-2 seedlings was determined. After 15 d of growth in low P conditions, wild-type seedlings showed a highly branched root system harboring second- and third-order lateral roots (data not shown), with a 5- to 6-fold increased density of lateral roots (Fig. 3C). At this stage, in high P conditions, first-order lateral roots had emerged from the primary root. As previously reported for tir3 (Ruegger et al., 1997), lpr1-2 mutant seedlings produced less than 10% of the lateral roots observed in wild type in high P conditions. In low P, the density of lateral roots was 25% of wild type (Fig. 3C). Interestingly, although lpr1-2 produced fewer lateral roots compared to wild type in both low and high P conditions, exposure to low P conditions induced a 3- to 5-fold increase in lateral root density in the mutant, similar to that observed for wild type. Most lateral roots that emerged in low P in the mutants elongated normally and were located close to the root tip (Fig. 1B). Similar results were obtained with doc1 (data not shown). Taken together, these results show that the primary root and root hair responses to low P were unaltered in lpr1-2 mutants and that mutations in BIG may alter more specific developmental traits related to lateral root formation.
Effect of P Availability on LRP Initiation
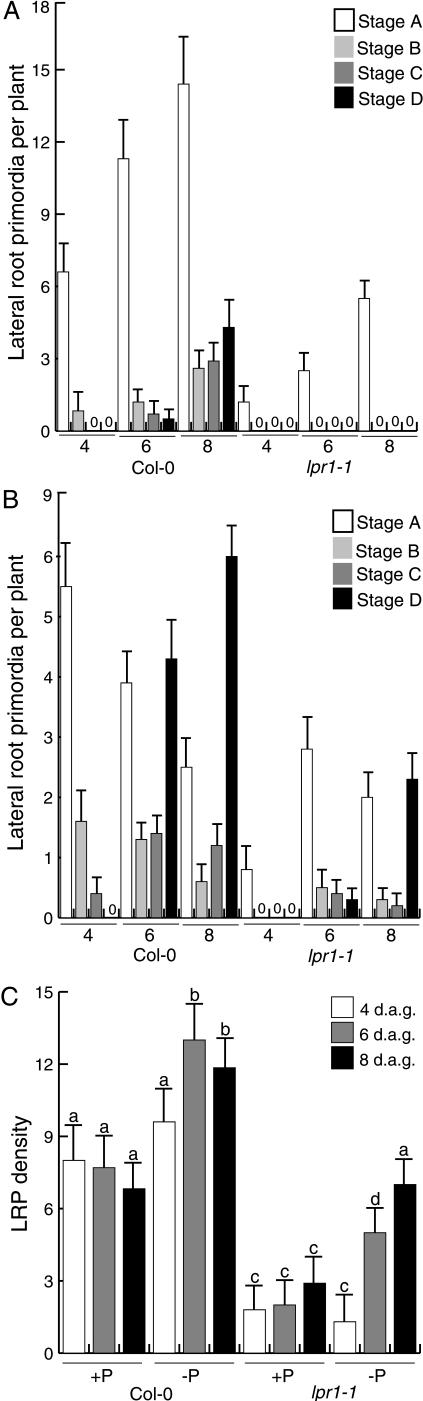
To more closely analyze the effects of P availability on lateral root development, and to investigate whether lpr1-1 is defective in LRP formation or in the subsequent elongation of these primordia, LRP originating in the primary root were counted at 4, 6, and 8 d after germination (see “Materials and Methods”). The developmental stage of each LRP was classified according to Zhang et al. (1999): stage A, up to 3 cell layers; stage B, unemerged, of more than 3 cell layers; stage C, emerged lateral roots of less than 0.5 mm in length; stage D, lateral root longer than 0.5 mm. In wild-type plants grown in high P conditions, the number of LRP at the four developmental stages increased with time. At 8 d, however, most LRP remained at an early developmental stage (Fig. 4A). Interestingly, low P conditions stimulated LRP transition from stage A to stage D. This effect was clearly observed at 4 d and increased at 8 d after germination. At day 8, most wild-type LRP were classified as stage D (Fig. 4B). In high P conditions, lpr1-1 mutants showed a reduction in total LRP formation when compared to wild type; no LRPs at stages B, C, and D were observed at 4, 6, or 8 d (Fig. 4A). At 6 and 8 d of growth in low P conditions, we observed an increase in LRP transition from early-to-late stages of development in lpr1-1 roots (Fig. 4B).
Figure 4.
Effects of P availability on wild-type (Col-0) and lpr1-1 lateral root development. A, Lateral root stage distribution in 4-, 6-, and 8-d-old primary roots grown on media containing high P. B, Lateral root stage distribution in 4-, 6-, and 8-d-old primary roots grown on media containing low P. C, LRP density at 4, 6, and 8 d after germination in high or low P conditions. Wild-type (Col-0) and lpr1-1 seedlings were cleared and the number and stage of LRP recorded according to Zhang et al. (1999). This analysis was repeated twice with similar results.
The LRP density was calculated by dividing the number of LRP by the length of the primary root to normalize for the effects of P availability on root length. LRP density significantly increased in plants grown at low P when compared with high P-grown plants. Although low P conditions increased the density of LRP in lpr1-1 mutants when compared to high P conditions, lpr1 always showed lower LRP density than wild type at the two P treatments (Fig. 4C). These results indicate that low P conditions modify the Arabidopsis root system architecture, most likely accelerating the emergence of LRP from the primary root and mutations in BIG interfere with the process of LRP initiation.
BIG Mutants Display Reduced Auxin Maximum in Primary and Lateral Roots
To evaluate the contribution of auxin responsiveness in the altered lateral root formation in response to P availability in BIG mutants, we crossed lpr1-2 with a transgenic plant expressing the DR5:uidA construct, which is useful in studying auxin-regulated gene expression in Arabidopsis (Ulmasov et al., 1997). As previously reported (Sabatini et al., 1999), we observed β-glucuronidase (GUS) activity in the columella and quiescent center of primary roots of 10-d-old transgenic DR5:uidA seedlings grown in high P (Fig. 5A). In these conditions, GUS staining in primary root tips of lpr1-2 seedlings was dramatically reduced (Fig. 5M). In low P conditions, both wild type and lpr1-2 showed little or no GUS activity (Fig. 5, G and Q). This reduction of the DR5:uidA maximum in primary root tips was accompanied by differentiation processes in this region. For instance, long root hairs were formed very close to the root meristem (Fig. 5, G and Q). We further examined GUS expression in LRP at different developmental stages in wild-type and lpr1-2 seedlings grown in high and low P conditions. For transgenic DR5:uidA seedlings, cells of LRP stained for GUS activity when plants were cultivated on high P medium (Fig. 5, B–F). Interestingly, LRP of wild-type plants grown in low P showed increased GUS activity at most developmental stages analyzed (Fig. 5, H–L). In contrast, GUS staining in LRP of lpr1-2 seedlings grown in high P was undetectable (Fig. 5, N–P). When grown in low P conditions, GUS activity was detected in the LRP of lpr1-2 seedlings, albeit at a lower level than in wild type (Fig. 5, R–T). Although the number of LRP at advanced developmental stages in lpr1-2 was partially recovered by low P treatment, only a limited number progressed to emerge from the primary root (Fig. 4B). These primordia clearly showed reduced GUS expression when compared to wild-type primordia at similar developmental stages (Fig. 5, K and T, L and U). These data suggest that low P enhances auxin responsiveness in LRP.
Figure 5.
Effect of P availability on auxin-regulated gene expression. Ten-hour GUS staining of DR5:uidA primary roots and LRP at various stages of development in wild-type seedlings grown for 10 d in medium with high (A–F) or low (G–L) P content. Ten-hour GUS staining of DR5:uidA primary roots and LRP at various stages of development in lpr1-2 seedlings grown for 10 d in medium with high (M–P) or low (Q–U) P content. Photographs are representative individuals of at least 20 plants stained. Scale bar = 100 μm.
Effect of Exogenous Auxin on Root Development in Wild Type and BIG Mutants
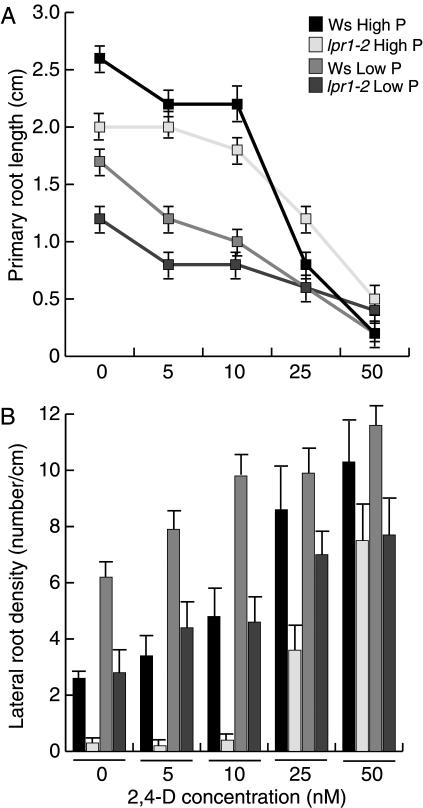
Exogenous auxin inhibits primary root elongation and stimulates lateral root formation (Casimiro et al., 2001). The observation that the expression of the auxin response marker DR5:uidA (Fig. 5G) was not increased in low P conditions suggested that primary root inhibition by low P treatment was not caused by auxin accumulation at the primary root tip region. Instead, it shows that P deprivation negatively affects the auxin response maximum normally present at the root tip under high P conditions. To investigate whether exogenously supplied auxins can restore the auxin maximum and in this way restore primary root growth in low P-grown plants, the effect of the synthetic auxin 2,4,-dichlorophenoxyacetic acid (2,4-D) on the growth and development of wild-type and lpr1-2 root systems was determined at low and high P conditions. Seedlings were grown for 5 d in media with low or high P content and then transferred to media containing the same P level supplemented with varying 2,4-D concentrations. The increase in primary root length was measured after 5 d. As shown in Figure 6A, primary roots of wild-type and lpr1-2 plants transferred from medium containing low P or high P to similar medium supplemented with 2,4-D all decreased primary root growth with increasing auxin concentrations, although plants grown on high P were more sensitive to the inhibitory effects of 2,4-D on primary root elongation than those grown on low P. These results suggest that, although high concentrations of exogenously supplied auxins and low P conditions both inhibit primary root growth, they likely operate through independent signaling mechanisms. To further determine whether exogenous auxin could stimulate lateral root formation in lpr1-2 mutants, the number of lateral roots was determined 9 d after transfer to medium containing 2,4-D. Interestingly, low P-grown plants significantly increased their lateral root density in the presence of 10 nm 2,4-D when compared to high P-grown plants (Fig. 6B). When exposed to 25 or 50 nm 2,4-D, lateral root density of wild-type plants grown in high P was similar to that of plants grown in low P. Treatment of lpr1-2 roots grown in low P conditions with 2,4-D gradually increased lateral root density in a dose-dependent manner (Fig. 6B). In contrast, lpr1-2 seedlings grown in high P and transferred to 5 or 10 nm 2,4-D did not have a significant effect on lateral root density when compared to the untreated control. Fifty nanomolar 2,4-D allowed normal lateral root formation in the mutant in high P conditions (Fig. 6B). Since auxin rescued the lpr1-2 lateral root phenotype both in high and low P, our results are consistent with the hypothesis that BIG has an important role in auxin transport. Moreover, as both wild-type and lpr1-2 plants displayed an amplification of the effect of 2,4-D on lateral root density at low P, it is possible that P limitation increases auxin sensitivity in the Arabidopsis root.
Figure 6.
Effects of exogenous auxin on wild-type (Ws) and lpr1-2 root development in low and high P. A, Mean primary root length of Ws and lpr1-2 seedlings grown on 2,4-D. Seedlings were transferred at 5 d after germination from auxin-free media to media containing varying concentrations of 2,4-D. Primary root length was recorded 5 d after transfer. B, Mean lateral root density of Ws and lpr1-2 seedlings grown on 2,4-D. Plants were transferred at 5 d after germination from auxin-free media to media containing varying concentrations of 2,4-D. Lateral root density was recorded 9 d after transfer (n = 15).
Effects of Brefeldin A on Lateral Root Developmentin High and Low P Conditions
Treatments of Arabidopsis roots with inhibitors that reduce polar auxin transport, such as TIBA and NPA, inhibit lateral root formation in high and low P conditions (López-Bucio et al., 2002). Recently, it was found that several auxin transport inhibitors are not specific, but rather generally affect the transport of membrane proteins (Geldner et al., 2001). Interestingly, the vesicle-trafficking inhibitor brefeldin A (BFA) was found to mimic most physiological effects of auxin transport inhibitors (Geldner et al., 2001). We therefore tested whether BFA-treated wild type could mimic the lateral root defects of BIG mutants by growing wild-type seedlings on vertical agar plates with high or low P content, supplemented with low concentrations of BFA. In high P-grown plants, treatment with 10 μm BFA dramatically altered lateral root formation, whereas 20 μm BFA inhibited primary root growth and altered root gravitropism (Fig. 7, A–E). Similar results were previously reported (Geldner et al., 2001). In P limitation, the most important effect of 20 μm BFA treatment on root system architecture was the inhibition of lateral root formation (Fig. 7, B–F). Further LRP analysis in low P conditions showed that, similarly to mutations in BIG, BFA inhibition of lateral root development was mainly caused by a reduction in LRP formation (data not shown).
Figure 7.
Effect of BFA on wild-type seedlings grown in high and low P availability. Seedlings were grown on agar plates containing different concentrations of BFA. Twelve-day-old wild-type seedlings grown in media containing high P (A) or low P (B) without BFA. High P-grown wild type treated with 10 μm (C) or 20 μm (E) BFA. Low P-grown wild type treated with 10 μm (D) or 20 μm (F) BFA. Photographs are representative individuals of at least 30 plants analyzed. Scale bar = 1 cm.
DISCUSSION
The Role of Auxin Transport in Root Architectural Response to Low P Conditions in Arabidopsis
When Arabidopsis plants are grown in limiting P conditions, primary root growth is inhibited and the lateral root density is increased, resulting in the formation of a shallower and wider root system (Williamson et al., 2001; López-Bucio et al., 2002; Ticconi et al., 2004). Little is known about the physiological and molecular mechanisms by which plants alter their growth and development when exposed to limiting P supply. Auxins are a class of phytohormones required for myriad plant developmental processes. Many plant species respond to the exogenous application of auxins by inhibiting primary root growth and producing large numbers of lateral roots. A regulated, differential distribution of auxin underlies several adaptive processes, including organogenesis, meristem patterning, and postembryonic root development (Bhalerao et al., 2002; Friml, 2003). We previously provided pharmacological data pointing to an important role for auxins in the root architectural changes induced by P limitation (López-Bucio et al., 2002). From these studies, the auxin transport inhibitor TIBA was found to inhibit the formation of lateral roots in P-deprived Arabidopsis plants. In this article, we show that mutations in BIG impair lateral root induction in low P conditions and provide genetic support for the requirement of auxin transport in lateral root proliferation during low P stress. Since low P conditions similarly inhibited primary root growth and stimulated root hair growth in lpr1 mutants and wild type, we conclude that an auxin transport independent pathway is involved in low P stress-induced root architectural alterations in Arabidopsis.
To date, several mutants in BIG have been isolated, including doc1 (Li et al., 1994), tir3 (Ruegger et al., 1997), and umb1 (Kanyuka et al., 2003). Although these mutants were isolated in very different screens, complementation tests demonstrated that they are allelic (Gil et al., 2001; Kanyuka et al., 2003). Furthermore, tir3-1 and doc1-1 stem segments transport reduced amounts of radioactively labeled indole-3-acetic acid (IAA) compared with wild type. Therefore, it was proposed that BIG is required for polar auxin transport in Arabidopsis (Gil et al., 2001). As previously reported for tir3 (Ruegger et al., 1997), lpr1 mutants showed reduced primary root growth when compared to wild type grown in high P conditions. This result suggests that auxin transport is required to provide sufficient auxin to promote root growth. However, treatment with 2,4-D showed that excess auxin still results in root growth inhibition (Fig. 5A), suggesting that a precise balance between auxin transport and inactivation is required to sustain root growth.
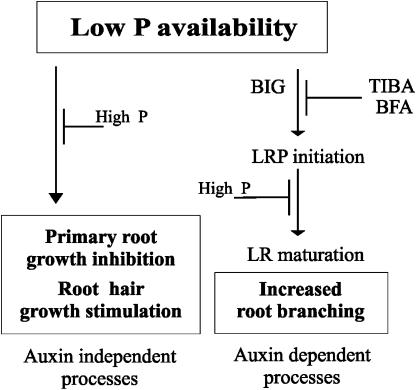
We also showed that growth of the primary root is dramatically reduced in P-limiting conditions in wild-type and lpr1 plants, thus suggesting that low P-induced primary root growth inhibition likely operates through a different mechanism than that regulating primary root growth in high P conditions. Therefore, lpr1 mutants separate lateral root proliferation from primary root growth arrest during P limitation and reveal a key role for BIG in lateral root initiation both in high and low P conditions. No other Arabidopsis mutants with defects in auxin transport/signaling have been found to show clear root developmental alterations to P stress (Williamson et al., 2001; López-Bucio et al., 2002). Since auxin fails to rescue the short-root phenotype of wild-type and lpr1-2 plants in low P, and DR5:uidA expression in primary roots of lpr1-2 plants is similarly reduced when compared to wild type in low P, we suggest that the inhibition of primary root growth and the stimulation of lateral root emergence triggered by low P operate through at least two independent mechanisms: one triggering primary root growth arrest that is independent of auxin, and one stimulating LRP emergence, a process that requires auxin transport-induced lateral root initiation and normal BIG function (Fig. 8).
Figure 8.
Root architectural responses to P limitation and their regulation. Low P availability alters root architecture by at least two mechanisms, one that is independent of auxin transport and BIG function leads to primary root growth inhibition and root hair growth stimulation, and one where low P promotes root branching interacting with auxin. In low P, lateral root development is a two-step process involving LRP initiation from the pericycle and the further maturation of these LRP into fast-growing lateral roots. In our model, LRP initiation requires auxin transport and normal BIG function, whereas LRP maturation is a BIG independent process. High P availability represses low P-induced root developmental responses.
Potential Role of BIG in Regulating Acropetal Auxin Transport and Lateral Root Emergence in P-Limiting Conditions
Two distinct auxin transport streams have been described in roots, acropetal from the shoot system to the root and basipetal from the root tip to the root base (Rashotte et al., 2000; Bhalerao et al., 2002). As both of these are inhibited by phytotropins, it is difficult to pharmacologically delineate the roles of the two transport streams in regulating lateral root formation in different P supplies.
The removal of primary root tips or cell ablation of root cap cells by expression of a diphtheria toxin stimulate lateral root formation (Torrey, 1950; Tsugeki and Fedoroff, 1999). Therefore, we propose that inhibition of primary root growth by low P can be considered a physiological decapitation that triggers lateral root development. Since lateral root growth requires auxin, it is possible that elongating LRP during the low P root architectural response become potent new sinks for auxin, reducing the supply of auxin to older root meristems. This premise may explain the drastic reduction of DR5:uidA expression in primary root tips of low P-grown Arabidopsis plants and the further increase in GUS expression in their LRP (Fig. 5). Our detailed analyses of LRP (Fig. 4) and DR5:uidA expression in wild-type and lpr1 root systems (Fig. 5) provide compelling evidence for the hypothesis that P deprivation accelerates the emergence of LRP and that BIG is required mainly for LRP initiation. Since lateral root formation and further lateral root growth that occurs in BIG mutants in low P takes place close to the primary root tip and root gravitropism is little or not affected in the mutants in high P conditions (Fig. 1, B and C), we speculate that basipetal transport of auxin is unaltered in BIG mutants and that BIG may play an essential role in acropetal transport of root auxin. Furthermore, since doc1 and tir3 mutants have a 70% reduction in the transport of auxin in inflorescence stems (Gil et al., 2001), we suggest that the lpr1 alterations in root developmental responses to P availability likely result from reduced acropetal auxin transport.
We also found that roots of lpr1 mutants may have suboptimal auxin levels or responsiveness, because 2,4-D application restored lpr1 lateral root formation in high and low P conditions to wild-type levels. This result suggests that, although BIG may be an integrator of several hormonal and environmental signals (Kanyuka et al., 2003), the primary root developmental alterations reported for big mutants are likely dependent on auxin action.
Increased Lateral Root Emergence in Low P Conditions Involves a BFA-Sensitive Auxin Transport Pathway
BIG is thought to be required for the correct expression, localization, or stability of the NPA-binding protein, which may affect auxin transport by influencing trafficking of various proteins to the plasma membrane, including the auxin efflux carrier PIN1 (Gil et al., 2001; Muday et al., 2003). Monensin and BFA, inhibitors of Golgi-mediated protein secretion, rapidly perturb the transport activity of plasma membrane-associated efflux carriers for IAA and inhibit polar transport of IAA (Wilkinson and Morris, 1994; Morris and Robinson, 1998). In our experiments, low concentrations of BFA dramatically inhibited lateral root formation in both high and low P conditions. This result suggests that the low P pathway for lateral root induction requires normal cycling of auxin transport proteins and that BIG may participate in vesicle transport. Currently, it is unknown how BIG could regulate vesicle transport. Gil et al. (2001) reported that BIG encodes a protein extraordinary in size (560 kD) that contains several zinc finger domains. BIG is similar to the Drosophila calossin/pushover (CalO/PUSH) gene, a member of a gene family also present in Caenorhabditis elegans and human genomes. In Drosophila, CalO affects synaptic transmission at the neuromuscular junctions, with specific defects in neurotransmitter release evoked by nerve stimulation (Richards et al., 1996). This process is dependent on the synaptic vesicle cycle that involves a cascade of protein-protein interactions (Sudhof, 1995). ADP ribosilation factors-GTP exchange factors are thought to determine the destination of membrane-trafficking vesicles by specific recruitment of protein coats for vesicles that determine their cellular location (Muday et al., 2003). ADP ribosilation factors-GTP exchange factors are also a target of BFA, which blocks vesicle delivery of proteins to the membrane surface. Whatever the target of BIG, the signaling cascade by which it controls lateral root development deserves further attention. In this regard, Grebe et al. (2002), while investigating the components of the auxin machinery that regulates root hair polarity, found that the presumptive auxin influx carrier AUX1 contributes to apical-basal cell polarity. The authors showed that AUX1 function is required for polarity changes induced by exogenous application of auxin. Interestingly, similar to aux1 mutants, treatment with BFA interferes with root hair initiation by inhibiting membrane trafficking of aux1. Although our observation that wild-type plants treated with BFA resemble lpr1 mutants, it is unlikely that the reported lateral root alterations involve AUX1 function. Both aux1-7 and eir1 auxin transport mutants respond normally to low P conditions producing large numbers of lateral roots (López-Bucio et al., 2002).
Our data provide evidence that increased lateral root development in low P conditions involves a BFA-sensitive auxin transport pathway. However, we cannot exclude that local auxin synthesis in LRP or increased sensitivity to auxins in LRP of low P-grown plants also accounts for earlier maturation of lateral roots. Further research is needed to provide unequivocal insight into how P limitation regulates the different traits that comprise the final configuration of the Arabidopsis root system and which of these developmental alterations involve auxin action.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotypes Col-0 and Ws were used for all experiments. Seeds were surface sterilized with 95% (v/v) ethanol for 5 min and 20% (v/v) bleach for 7 min. After 5 washes in distilled water, seeds were germinated and grown on agar plates containing 0.1× Murashige and Skoog medium, pH 5.7, 0.5% (w/v) Suc, and 1% (w/v) agar (López-Bucio et al., 2002). The basic medium contained 2.0 mm NH4NO3, 1.9 mm KNO3, 0.3 mm CaCl2 2H2O, 0.15 mm MgSO4 7H2O, 5 μm KI, 25 μm H3BO3, 0.1 mm MnSO4 H2O, 0.3 mm ZnSO4 7H2O, 1 μm Na2Mo04 2H2O, 0.1 μm CuSO4 5H2O, 0.1 μm CoCl2 6H2O, 0.1 mm FeSO4 7H2O, 0.1 mm Na2EDTA 2H2O, inositol (10 mg L−1), and Gly (0.2 mg L−1).
Plates were placed at an angle of 65° to allow root growth along the agar surface and to allow unimpeded hypocotyl growth into the air. Plants were grown at 22°C to 24°C in a plant growth cabinet (Percival Scientific, Perry, IA), with a photoperiod of 16 h of light, 8 h of darkness, and a light intensity of 300 μmol m−2 s−1. Seeds of transgenic DR5:uidA Arabidopsis plants (Ulmasov et al., 1997) were provided by Dr. Tom Guilfoyle, and homozygous F3 progeny of crosses with lpr1-2 were used for analysis of GUS expression.
To test the effects of 2,4-D and BFA, low (1 μm NaH2PO4) and high (1 mm NaH2PO4) P nutrient medium was supplemented with ethanol-dissolved compounds. The compounds were added to cooled (50°C) molten medium and poured into plates. Chemicals were purchased from Sigma (St. Louis).
Mutant Isolation Procedure
EMS-mutagenized seeds (Col-0) were purchased from Lehle Seeds (Round Rock, TX). T-DNA lines (Ws; Krysan et al., 1999) were provided by the Ohio Arabidopsis Seed Stock Center. Seeds were surface sterilized and plated on low P (1 μm NaH2PO4) 0.1× Murashige and Skoog medium. A total of approximately 25,000 M2 seedlings descended from EMS-mutagenized seed and 38,000 T-DNA lines were screened for reduced lateral root formation by placing seeds on 100-cm2 nutrient agar plates (20 seeds/plate). The seeds were distributed in 2 rows on the agar surface at a density of 1 seed/centimeter, stratified at 4°C for 48 h, and then incubated at 22°C. Fourteen days after germination, low P-grown plants have a short primary root and a large number of lateral roots formed close to the root apex. Putative mutants with short primary roots and reduced number of lateral roots were selected, transferred to soil, and allowed to self-fertilize. Homozygous M3 seeds were rescreened for altered lateral root formation in low P and back-crossed four times to wild type (either Col-0 or Ws) to remove unlinked mutations.
Genetic Analysis of lpr1 Mutants
The lpr1-1 allele was isolated in a Col-0 background from an EMS mutant population. The lpr1-2 allele was isolated in a Ws background from the Sussman and Amasino T-DNA mutant collection. The lpr1 mutants were back-crossed 4 times with the corresponding wild-type parent. To determine the segregation pattern of the lpr1 phenotype, 500 F2 seedlings derived from each cross between lpr1-1 × Col-0 and lpr1-2 × Ws were analyzed in solid low P medium. A typical 3:1 recessive segregation was observed for the wild-type/lpr1 phenotype. For allelism tests, homozygous lpr1-1, lpr1-2, and doc1 were crossed to each other and plants from the F1 population were analyzed for the lpr1 phenotype. Chromosomal localization of mutations corresponding to lpr1-1 was determined using a combination of cleaved amplified polymorphic sequences (Konieczny and Ausubel, 1993) and simple sequence length polymorphism (Bell and Ecker, 1994) molecular markers. Homozygous lpr1-1 plants in the Col-0 background were out-crossed to Ws. Genomic DNA for PCR was prepared from F2 plants with lpr1 mutant phenotypes (Celenza et al., 1995).
Histochemical Analysis
For histochemical analysis of GUS activity, Arabidopsis seedlings were incubated overnight at 37°C in a GUS reaction buffer (0.5 mg/mL of 5-bromo-4-chloro-3-indolyl-β-d-glucuronide in 100 mm sodium phosphate, pH 7), and the stained seedlings were cleared (Malamy and Benfey, 1997). For each marker line and for each treatment, at least 10 transgenic plants were analyzed. A representative plant was chosen for each P or auxin treatment and photographed using the Nomarski optics on a Leica (Wetzlar, Germany) DMR microscope.
Data Analysis
Arabidopsis root systems were viewed with an AFX-II-A stereomicroscope (Nikon, Tokyo). All lateral roots emerged from the primary one and observed at the 3× objective were included in the lateral root number data. Primary root length was determined for each root using a ruler. The stages of LRP were analyzed on cleared roots using a Nikon UW microscope in the 40× objective.
For all experiments, the overall data were statistically analyzed in the SPSS 10 program (SPSS, Chicago). Univariate and multivariate analyses with a Tukey's post hoc test were used for testing differences in primary root length, lateral root number, and lateral root density in P treatments in wild-type and mutant plants. Different letters are used to indicate means that differ significantly (P < 0.05).
Acknowledgments
We are thankful to Drs. Joanne Chory and Tom Guilfoyle for kindly providing us with seeds of mutant and transgenic lines. J.L.-B. gratefully acknowledges the Mexican Academy of Sciences and the Mexico-USA Foundation for Science for financial support to visit the Department of Biochemistry and Cell Biology at Rice University, Houston. We thank Dr. Bethany K. Zolman for her support and technical improvement for lpr1-1 mapping.
This work was supported in part by the Howard Hughes Medical Institute (grant no. Nbr55003677), by the European Commission (grant no. ICA–4–CT2000–30017), and by the Robert A. Welch Foundation (C–1309).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.049577.
References
- Abel S, Ticconi CA, Delatorre CA (2002) Phosphate sensing in higher plants. Physiol Plant 115: 1–8 [DOI] [PubMed] [Google Scholar]
- Al-Ghazi Y, Muller B, Pinloche S, Tranbarger TJ, Nacry P, Rossignol M, Tardieu F, Doumas P (2003) Temporal responses of Arabidopsis root architecture to phosphate starvation: evidence for the involvement of auxin signaling. Plant Cell Environ 26: 1053–1066 [Google Scholar]
- Bell CJ, Ecker JR (1994) Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19: 137–144 [DOI] [PubMed] [Google Scholar]
- Bhalerao RP, Eklof J, Ljung K, Marchant A, Bennet M, Sandberg G (2002) Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29: 325–332 [DOI] [PubMed] [Google Scholar]
- Casimiro I, Merchant A, Bhalerao RP, Beekman T, Dhoog S, Swarup R, Graham N, Inze D, Sandberg G, Casero PJ, et al (2001) Auxin transport promotes lateral root initiation. Plant Cell 13: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza JL, Grisafi PL, Fink GR (1995) A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev 9: 2131–2142 [DOI] [PubMed] [Google Scholar]
- Friml J (2003) Auxin transport: shaping the plant. Curr Opin Plant Biol 6: 7–12 [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Dieter Stierhof Y, Jurgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putterill J, Palme K, Estelle M, Chory J (2001) BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev 15: 1985–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert GA, Knight JD, Vance CP, Allan DL (2000) Proteoid root development of phosphorus deficient lupin is mimicked by auxin and phosphonate. Ann Bot (Lond) 85: 921–928 [Google Scholar]
- Grebe M, Friml J, Swarup R, Ljung K, Sandberg G, Terlou M, Palme K, Bennet M, Scheres B (2002) Cell polarity signaling in Arabidopsis involves a BFA-sensitive auxin influx pathway. Curr Biol 12: 329–334 [DOI] [PubMed] [Google Scholar]
- Johnson JF, Vance CP, Allan DL (1996) Phosphorus deficiency in Lupinus albus. Altered lateral root development and enhanced expression of phosphoenolpyruvate carboxylase. Plant Physiol 112: 31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanyuka K, Praekelt U, Franklin KA, Billingham OE, Hooley R, Whitelam GC, Halliday KJ (2003) Mutations in the huge Arabidopsis gene BIG affect a range of hormonal and light responses. Plant J 35: 57–70 [DOI] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J 4: 403–410 [DOI] [PubMed] [Google Scholar]
- Krysan PJ, Young JC, Sussman MR (1999) T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 12: 2283–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HM, Altschmied L, Chory J (1994) Arabidopsis mutants define downstream branches in the phototransduction pathway. Genes Dev 8: 339–349 [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6: 280–287 [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L (2002) Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol 129: 244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Nieto-Jacobo MF, Ramírez-Rodríguez V, Herrera-Estrella L (2000) Organic acid metabolism in plants: from adaptive physiology to transgenic verieties for cultivation in extreme soils. Plant Sci 160: 1–13 [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Morris DA, Robinson JS (1998) Targeting of auxin carriers to the plasma membrane: differential effects of brefeldin A on the traffic of auxin uptake and efflux carriers. Planta 205: 606–612 [Google Scholar]
- Muday GK, Peer WA, Murphy AS (2003) Vesicular cycling mechanisms that control auxin transport polarity. Trends Plant Sci 8: 301–304 [DOI] [PubMed] [Google Scholar]
- Neumann G, Martinoia E (2002) Cluster roots: an underground adaptation for survival in extreme environments. Trends Plant Sci 7: 162–167 [DOI] [PubMed] [Google Scholar]
- Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday G (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122: 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Hillman T, Stern M (1996) Mutations in the Drosophila pushover gene confer increased neuronal excitability and spontaneous synaptic vesicle fusion. Genetics 142: 1215–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M (1997) Reduced napthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 9: 745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, et al (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472 [DOI] [PubMed] [Google Scholar]
- Skene KR, James WM (2000) A comparison of the effects of auxin on cluster root initiation and development in Grevillea robusta Cunn. Ex R. Br. (Proteaceae) and the genus Lupinus (Leguminosae). Plant Soil 219: 221–229 [Google Scholar]
- Sudhof TC (1995) The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature 375: 645–673 [DOI] [PubMed] [Google Scholar]
- Ticconi CA, Delatorre CA, Lahner B, Salt DE, Abel S (2004) Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. Plant J 37: 801–814 [DOI] [PubMed] [Google Scholar]
- Torrey JG (1950) Induction of lateral roots by indoleacetic acid and decapitation. Am J Bot 37: 257–264 [Google Scholar]
- Tsugeki R, Fedoroff NV (1999) Genetic ablation of root cap cells in Arabidopsis. Proc Natl Acad Sci USA 96: 12941–12946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle T (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable source. New Phytol 157: 423–447 [DOI] [PubMed] [Google Scholar]
- Wilkinson S, Morris DA (1994) Targeting of auxin carriers to the plasma membrane: effects of monensin on transmembrane auxin transport in Cucurbita pepo L. tissue. Planta 193: 194–202 [Google Scholar]
- Williamson LC, Ribrioux S, Fitter AH, Leyser O (2001) Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol 126: 875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Jennings A, Barlow PW, Forde BG (1999) Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA 96: 6529–6534 [DOI] [PMC free article] [PubMed] [Google Scholar]