Abstract
The wound-activated biosynthesis of phytoalexin hydroxycinnamic acid amides of tyramine was compared in untransformed and transgenic tobacco (Nicotiana tabacum) lines that express tryptophan decarboxylase (TDC), tyrosine decarboxylase (TYDC), or both activities. Transgenic in vitro-grown tobacco lines expressing TDC activity accumulated high levels of tryptamine but not hydroxycinnamic amides of tryptamine. In contrast, transgenic tobacco lines expressing TYDC accumulated tyramine as well as p-coumaroyltyramine and feruloyltyramine. The MeOH-soluble and cell wall fractions showed higher concentrations of wound-inducible p-coumaroyltyramine and feruloyltyramine, especially at and around wound sites, in TYDC and TDC ×TYDC tobacco lines compared to wild-type or TDC lines. All the enzymes involved in the biosynthesis of hydroxycinnamic acid amides of tyramine were found to be similarly wound inducible in all tobacco genotypes investigated. These results provide experimental evidence that, under some circumstances, TYDC activity can exert a rate-limiting control over the carbon flux allocated to the biosynthesis of hydroxycinnamic acid amides of tyramine.
The importance of tyramine during suberization of wounded potato (Solanum tuberosum) tubers is well established. Borg-Olivier and Monties (1993) used alkaline hydrolysis to fractionate and characterize the MeOH-extractable components of wound periderm in healing potato tuber discs. Fourteen days postwounding, their results established that tyramine accounts for 23% of total phenylpropanoids and Tyr-derived metabolites occurring in the MeOH-extractable free residue, whereas tyramine was undetected in the control nonwounded potato tuber discs. The presence of tyramine in suberizing potato periderm has also been corroborated by in situ noninvasive solid-state 13C-NMR spectroscopy that allows the characterization of the polyaromatic domain of suberin in its native state within the plant cell wall matrix (Bernards et al., 1995; Bernards and Lewis, 1998).
Phytoalexin hydroxycinnamic acid amides of tyramine (PCAAT), which are formed by the conjugation of tyramine with cinnamoyl-CoA thioesters (Negrel and Javelle, 1997), represent a major class of tyramine-derived metabolites. The biosynthesis of PCAAT is elicited by wounding (Pearce et al., 1998; Ishihara et al., 2000) or pathogen inoculation (Keller et al., 1996; Muhlenbeck et al., 1996; Schmidt et al., 1998; Newman et al., 2001) in many species (Martin-Tanguy et al., 1996), especially in solanaceous plants (Clarke, 1982; Negrel and Javelle, 1995; Keller et al., 1996; Muhlenbeck et al., 1996). PCAAT can be further integrated into cell walls via a peroxidase-mediated process (Negrel and Lherminier, 1987; Keller et al., 1996) that yields mono- or dicovalent ether bonds between cinnamoyl or tyramine moieties of PCAAT and the plant phenolic cell wall matrix (Lapierre et al., 1996). Although some PCAAT possess cytotoxic properties (Yamamoto et al., 1991; Park and Schoene, 2002), it is generally speculated that their major function in plants is to reinforce cell walls to prevent the invasion of pathogens within suberizing tissues.
Some studies have suggested that the availability of tyramine may be limiting for the biosynthesis of PCAAT. Transgenic canola lines transformed with a heterologous opium poppy (Papaver somniferum) Tyr decarboxylase (TYDC) gene (Facchini and De Luca, 1995) had lower protoplast release efficiency than wild type when their leaf tissue was incubated with hydrolytic enzymes (Facchini et al., 1999) as a result of increased accumulation of tyramine, hydroxycinnamic acid amides of tyramine, and increased oxidative cross-linking of hydroxycinnamic acid amides of tyramine in the cell wall. On the other hand, lower metabolite flux through the PCAAT pathway by overexpressing an alternative pathway (Yao et al., 1995) results in decreased lignification. The immunolocalization of acyltransferase in the vascular tissues of opium poppy (Yu and Facchini, 1999) further corroborates the importance of PCAAT for cell wall cross-linking. On the other hand, an external supply of tyramine (0.5 mm) provided through watering solution or leaf spraying can significantly increase the levels of p-coumaroyltyramine (p-CT) or feruloyltyramine (FT) in leaf tissue of wild-type tobacco (Nicotiana tabacum; Martin-Tanguy et al., 1996) and rice plants transformed with a heterologous pepper hydroxycinnamoyltransferase (Jang et al., 2004). Altogether, these results indicate that modifications of aromatic amino acid pools or modifications of later steps may affect the biosynthesis and integration of phenolic compounds and/or PCAAT within the cell wall.
TYDC may constitute a limiting step for the biosynthesis of tyramine-derived secondary metabolites like PCAAT since the availability of tyramine is tightly associated with transcript levels and enzyme activities of TYDC (Facchini and De Luca, 1994; Facchini et al., 1998; Schmidt et al., 1999; Newman et al., 2001). This hypothesis is assessed in this study by comparing the wound-inducible accumulation of PCAAT in a TYDC-expressing transgenic tobacco line accumulating high constitutive levels of tyramine and an untransformed control line. A comparative study was also performed with a Trp decarboxylase (TDC; EC 4.1.1.28) transgenic tobacco line and a TYDC × TDC cross to assess whether a high level of tryptamine, the corresponding amine generated by the decarboxylation of Trp, could lead to the formation of tryptamine-derived phytoalexin amides (Fig. 1).
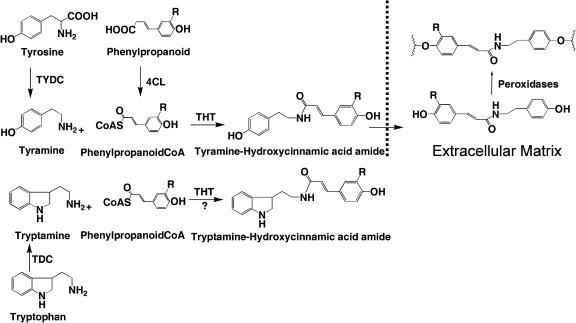
Figure 1.
Biosynthesis of tobacco PCAAT and their cell wall-bound forms. Tyr is converted to tyramine by tobacco TYDC and caffeic or ferulic acid are converted to their respective CoA esters by 4CL in the presence of coenzyme A. THT catalyzes amide bond formation between tyramine and one of these CoA esters to yield caffeoyltyramine and FT, respectively. Amides are released from the cell into the cell wall matrix where they become bound by the action of peroxidases (Negrel and Lherminier, 1987). The area beyond the broken lines represents phenolic amides associated with the cell wall matrix, where R is H, OH, or OCH3 depending if the phytoalexin amide is p = coumaroyltryamine, caffeoyltyramine, or feruloyltyramine. Transgenic tobaccos expressing TDC and/or TYDC make high levels of tryptamine and/or tyramine (Guillet et al., 2000). This hypothetical diagram also displays the possibility that transgenic plants expressing the TDC gene could also accumulate novel phenolic amides of tryptamine that are not known to occur in tobacco.
RESULTS
TYDC-Expressing Tobacco Plants Accumulate Higher Levels of PCAAT in Response to Wounding
Physical wounding induced a time-dependent increase of MeOH-soluble PCAAT in all 4 genotypes tested, although the levels were about 250% higher for the TYDC-expressing lines (TYDC and TDC × TYDC) compared to the untransformed control or TDC lines (Fig. 2). These results also show that the high constitutive levels of tyramine found in transgenic TYDC plants, but not the high tryptamine levels found in TDC plants, are converted to PCAAT.
Figure 2.
Time course of accumulation of tyramine-based PCAAT in wounded leaves of untransformed control (wild-type) tobacco, transformed TDC-expressing tobacco, transformed TYDC-expressing tobacco, and crossed transformed TDC × TYDC tobacco. Three replicates were performed for each experiment and the bars represent sd.
In Vivo Conversion of l-[U-14C]Tyr and l-[5-3H]Trp into Respective Amines
Wounded leaves from untransformed, TDC-, TYDC-, and TDC × TYDC-expressing tobacco were harvested and fed with l-[5-3H]Trp (20–30 Ci/mmol) by petiolar absorption. This experiment revealed that Trp was converted to tryptamine in transformed tobacco plants that expressed TDC (Fig. 3A, TDC and TDC × TYDC), but not in untransformed controls (Fig. 3A, wild type) or in plants expressing only TYDC (Fig. 3A, TYDC). The lack of other radioactive MeOH-soluble products suggests that tryptamine was not further converted into other tryptamine-derived metabolites in transgenic tobacco. In separate experiments, leaves from untransformed, TDC-, TYDC-, and TDC × TYDC-expressing tobacco were harvested and fed with l-[U-14C]Tyr through their petioles. The labeled Tyr was converted into tyramine in transformed tobacco plants that expressed TYDC (Fig. 3B, TYDC and TDC × TYDC), but not in untransformed (Fig. 3B, wildl type) or in plants expressing only TDC (Fig. 3B, TDC). The labeling with l-[U-14C]Tyr in these TYDC-expressing plants produced three additional, more rapidly migrating radioactive spots (Fig. 3B, TYDC and TDC × TYDC), two of which comigrated with p-CT and FT, respectively. This remarkable difference in conversion rates of l-[U-14C]Tyr into tyramine and into p-CT or FT in TYDC-expressing tobacco compared to the untransformed control or TDC-expressing lines was further investigated.
Figure 3.
Autoradiography of TLCs obtained from the MeOH-soluble fraction of wounded leaves from untransformed control (WT) tobacco, transformed TDC-expressing tobacco, transformed TYDC-expressing tobacco, and crossed transformed TDC × TYDC tobacco labeled by petiolar uptake with l-[U-14C]Tyr and l-[5-3H]Trp. Samples were run on a silica TLC plate (silica gel 60 F254) using CHCl3:MeOH:25% ammonia solution (6:3:1). These results are representative of typical experiments that were repeated three times. WT, Wild type.
Effect of Wounding on Mobilization of l-[U-14C]Tyr in Leaves of Different Tobacco Backgrounds
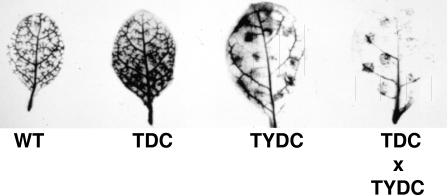
Healthy leaves from untransformed, TDC-, TYDC-, and TDC × TYDC-expressing tobacco were harvested, wounded, and incubated with l-[U-14C]Tyr as described in “Materials and Methods.” After labeling, leaves were washed extensively with MeOH to extract soluble radioactive products and the leaves were then submitted to autoradiography to localize the accumulation sites of cell wall-bound radiolabeled metabolites. The autoradiogram revealed a distinct labeling pattern around wound sites in the two TYDC-expressing lines (Fig. 4, TYDC and TDC × TYDC) compared with the untransformed control (Fig. 4, wild type) or TDC-expressing lines (Fig. 4, TDC). A similar l-[5-3H]Trp-labeling experiment failed to reveal any differences in cell wall-bound radiolabeled metabolites between any of the tobacco lines (data not shown).
Figure 4.
Autoradiography of wounded leaves after extensive washing out of soluble radioactive metabolites with MeOH from untransformed control (WT) tobacco, transformed TDC-expressing tobacco, transformed TYDC-expressing tobacco, and crossed transformed TDC × TYDC tobacco labeled through petiolar uptake with l-[U-14C]Tyr.
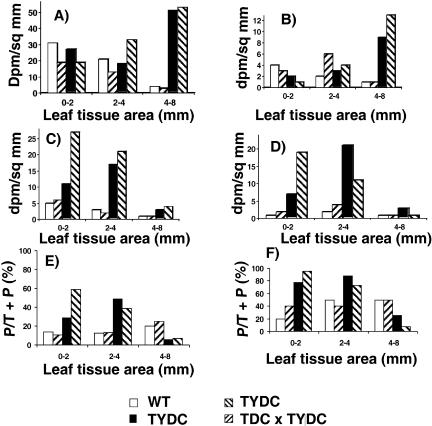
This distinct labeling pattern around wound sites in TYDC lines fed with l-[U-14C]Tyr was further investigated by extraction of the wound sites as described in “Materials and Methods” (Fig. 5). The MeOH-soluble fraction was analyzed for radioactive tyramine and PCAAT content, respectively. The TYDC-expressing lines accumulated up to 15.9 times and 4.5 times more labeled soluble and wall-bound PCAAT, respectively, than the control untransformed or the TDC line at the 0- to 2- and 2- to 4-mm-diameter concentric zones around the wound site (Fig. 5, C and D). These results suggest that wound sites of TYDC-expressing leaves are integrating larger amounts of PCAAT into cell walls compared to the other lines as a result of enhanced TYDC expression. In addition, the TYDC-expressing tobacco lines accumulated lower levels of MeOH-soluble and -insoluble p-CT and FT in more distant leaf tissues (4- to 8-mm-diameter zone) compared to those closer to the wound site (0- to 2-mm-diameter zone or the 2- to 4-mm-diameter zone; Fig. 5, C and D). In contrast, the free and wall-bound tyramine content was nearly 10 times higher in leaf tissues more distant from the wound site (4- to 8-mm-diameter zone) in TYDC-expressing lines than in the wild-type or the TDC line (Fig. 5, A and B). It is also noticeable that the conversion rate of tyramine into PCAAT is approximately 2 to 3 times higher for TYDC-expressing lines compared to wild-type or TDC lines at or near wound sites, although the reverse trend is observed for the 4- to 8-mm zone (Fig. 5, E and F). These results indicate that wounding triggered the induction of the PCAAT pathway in all leaves and that the expression of TYDC in tobacco leads to greater and more efficient localized accumulation of these soluble and wall-bound metabolites.
Figure 5.
Determination of soluble and cell wall-associated radioactive tyramine and PCAAT at different distances from the wound site in untransformed (WT) control tobacco, transformed TDC-expressing tobacco, transformed TYDC-expressing tobacco, and crossed transformed TDC × TYDC tobacco labeled through petiolar uptake with l-[U-14C]Tyr. The data in Figure 5, A to D, are expressed in disintegrations/mm of tissue. The total radioactivity (in disintegrations per minute) observed for soluble tyramine, cell wall-bound tyramine, soluble PCAAT, and cell wall-bound PCAAT for 0 to 2 mm, 2 to 4 mm, and 4 to 8 mm from the wound site were as follows. A (soluble tyramine): (WT 390, 793, 603); (T201 246, 494, 451); (T2U9 351, 694, 7704); (T201 × T2u9 233, 1219, 8102). B (cell wall-bound tyramine): (WT 48, 73, 13); (T201 39, 233, 40); (T2U9 19, 119, 1368); (T201 × T2u9 none detected, 147, 2019). C (soluble PCAAT): (WT 66, 107, 47); (T201 72, 78, 57); (T2U9 141, 661, 427); (T201 × T2u9 343, 759, 611). D (cell wall-bound PCAAT): (WT 15, 68, 33); (T201 23, 154, 17); (T2U9 91, 804, 439); (T201 × T2u9 239, 381, 64). E and F, Percent of tyramine that is incorporated into PCAAT out of the combined total of tyramine plus PCAAT ([P/T+P] × 100) in the soluble (E) and wall-bound (F) fractions.
Enzyme Activity of the Phytoalexin Amide Pathway
Mechanical wounding of leaves induced a nearly 10-fold increase of 4-coumarate:CoA ligase (4CL) and tyramine hydroxycinnamoyltransferase (THT) activities in all tobacco lines (Fig. 6, B and C), whereas peroxidase activity increased 20-fold over the same time period (Fig. 6D). TYDC activity was, as expected, 15- to 26-fold higher in the TYDC transgenic lines, compared to wild-type or TDC lines. Interestingly, an 8- to 15-fold induction of TYDC activity was still observed in response to physical wounding for both wild-type and TDC-expressing transgenic lines (Fig. 6A) compared to unwounded control cells, reflecting the endogenous activity of native tobacco TYDC. It is remarkable that coordinate increases of all enzymes encoding the PCAAT pathway were observed for all genotypes investigated, except the two TYDC transgenic lines that now had constitutive levels of TYDC activity.
Figure 6.
Relative wound-inducible activity of TYDC (A), 4CL (B), THT (C), and peroxidases (D) that may be involved in the biosynthesis of PCAAT of tyramine in untransformed control (WT) tobacco, transformed TDC-expressing tobacco, transformed TYDC-expressing tobacco, and crossed transformed TDC × TYDC tobacco. Bars are for sds and treatments were performed three times. Note that, for peroxidase activity, 1 unit is the amount of enzyme that decreases absorbance of 0.1 A units mg protein−1 5 min−1 incubation at 330 nm/min.
DISCUSSION
Tryptamine Is Not an Acceptable Substrate for THT
The wounding of leaves from control untransformed or TDC-expressing tobacco lines activated the accumulation of similar levels of p-CT and FT, unlike the higher rates of accumulation of PCAAT observed in wounded TYDC-expressing lines (Fig. 2). In addition, the l-[5-3H]Trp feeding experiment corroborated a previous study (Guillet et al., 2000) that only the two TDC-expressing lines accumulated severalfold higher levels of labeled tryptamine, compared to the wild-type and TYDC-expressing tobacco lines (Fig. 3A). The l-[5-3H]Trp-labeling experiment showed that wounded leaves from TDC-expressing tobacco plants will decarboxylate Trp to accumulate tryptamine (Fig. 3A) without further metabolizing it into tryptamine-derived phytoalexin amides. These results are consistent with the substrate specificity of TDC (De Luca et al., 1989) and TYDC (Facchini and De Luca, 1994, 1995) for their respective substrates (Tyr and Trp) and the fact that tryptamine is not an acceptable substrate for the tobacco THT (Negrel and Martin, 1984).
TYDC as a Rate-Limiting Step for the Biosynthesis of PCAAT at Wound Sites
It is remarkable that the TYDC and the TYDC × TDC lines producing high levels of tyramine (Guillet et al., 2000) also had higher wound-inducible levels of soluble and insoluble PCAAT compared to the untransformed control or TDC tobacco lines (Fig. 2). Feeding studies with l-[U-14C]Tyr showed that wounded leaves from TYDC-expressing tobacco plants will decarboxylate Tyr to accumulate tyramine that will be further transformed into tryamine-derived PCAAT (Fig. 3B). Autoradiograms of MeOH-washed wounded leaves fed with l-[U-14C]Tyr further showed that, unlike the even distribution of radioactivity observed in untransformed control or TDC-expressing lines (Fig. 4), cell wall-bound 14C-labeled compounds were localized to the wound sites of TYDC-expressing tobacco lines. Direct extraction of wound sites for soluble and cell wall-bound radioactive compounds clearly showed that TYDC-expressing tobacco lines incorporate much higher levels of l-[U-14C]Tyr into soluble and cell wall-bound tyramine and tyramine-based PCAAT (Fig. 5). In addition, leaf tissues more distant from the wound site (4- to 8-mm-diameter zone) had the lowest concentrations of soluble and bound PCAAT compared with the levels found directly at (0- to 2-mm-diameter zone) or around (2- to 4-mm-diameter zone) the wound site (Fig. 5, C and D).
Together, these results indicate a localized activation of the PCAAT pathway in and around the wound sites in tobacco leaves and that increased availability of tyramine leads to the higher levels of wound site production of PCAAT in TYDC-expressing lines. Wound site-localized biosynthesis and accumulation of FT has also been observed in wounded tissue of tomato leaves (Pearce et al., 1998). Localized biosynthesis of PCAAT has been associated with the induction of THT activity that was shown to be typically restricted to the outermost cell layers of wound-healing potato tuber discs (Negrel et al., 1995). Accordingly, TYDC-expressing lines, TYDC, and TDC × TYDC had high levels of tyramine in all leaf parts except at wound sites (Fig. 5, A and B; compare wild type and TDC to TYDC and TDC × TYDC for the different leaf tissue areas) where the conversion into p-CT and FT was mediated by an increase of THT activity (Fig. 6A). These results also mean that the larger tyramine pools in TYDC-expressing lines can be used to feed the PCAAT pathway. A coordinate local induction of the PCAAT pathway observed at wound sites in wild-type and TDC lines could be explained by the presence of the endogenous pathway for producing the localized increase of tyramine, p-CT, and FT (Figs. 5 and 6). Overall, the results obtained strongly suggest that TYDC encodes a rate-limiting step under these conditions for the biosynthesis of PCAAT in young tobacco leaves. However, the data do not preclude other explanations for the results obtained, and it is not possible to eliminate the possibility that other components of PCAAT biosynthesis may be rate limiting.
The wound-inducible activation of the PCAAT pathway was shown to be coordinately activated by pectinase or pronase treatment of cell suspension cultures of tobacco (Negrel and Javelle, 1995). Wounding of tobacco leaves in this report showed an almost identical pattern of wound-inducible activity of several enzymes of the PCAAT pathway in all tobacco lines, in spite of the high levels of TYDC activity found in TYDC-expressing transgenic lines (Fig. 6).
Conversion Yield of Tyramine into PCAAT in TYDC-Expressing Tobacco Lines
The tyramine levels in leaves of 1-month-old tobacco plants were 800 μg/g dry weight in the TYDC-expressing tobacco, whereas those of untransformed and TDC lines were only 10 μg/g dry weight (data not shown). That data combined with those in Figure 2, which were obtained by extensive extraction of amides by MeOH washing of whole tobacco leaves, reveal that the conversion rate of tyramine into PCAAT at 8 h postwounding is nearly 0.1% and 1.5% in TYDC-expressing tobacco lines and TDC or wild-type lines, respectively. Those conversion estimates for whole tobacco leaves are 1 to 2 orders of magnitude lower than the ones directly measured by labeling with [U-14C]tyramine at and around wound sites (Fig. 6, E and F). It is noticeable that both TYDC-expressing lines have 2 to 3 times higher conversion rates of tyramine into PCAAT than the wild-type and TDC lines within the 0- to 2-mm and 2- to 4-mm leaf tissue areas and for both the MeOH-soluble and cell wall-bound fractions.
The relatively low conversion rates of tyramine into PCAAT that were observed at longer distances from wound sites in TYDC-expressing lines (Fig. 6, E and F, 4- to 8-mm tissue area) imply that the synthesis of tyramine is not the only limiting step for the biosynthesis of PCAAT. The cosubstrate cinnamoyl-CoA ester, which is required for the biosynthesis of PCAAT and other phenylpropanoids, such as flavonoids and lignin, may become a limiting factor for the biosynthesis of p-CT and FT, especially when two tobacco 4CL genes are wound inducible (Lee and Douglas, 1996). A temporal and spatial consistency between the THT activity and the immobilization of PCAAT into cell walls (Negrel et al., 1993; Facchini, 1998) implies that THT is another important regulatory step for the biosynthesis and incorporation of PCAAT into cell walls. It is concluded that the conversion of tyramine into PCAAT might be optimized by the coexpression in tobacco plants of TYDC, 4CL, and THT.
MATERIALS AND METHODS
Growth of Tobacco Plants
Seeds of untransformed (wild-type) and transgenic lines of tobacco (Nicotiana tabacum) cv Xanthi overexpressing TDC (T-201-1), TYDC (T-2u9), or both enzyme activities (T-201-1 × T-2u9; Guillet et al., 2000) were sterilized with 70% ethanol, washed with sterile water, and germinated in darkness for 4 d. Sterile seedlings were transferred to magenta vessels containing 1% agar in Murashige and Skoog medium and were grown under a 16-h photoperiod (10 μmol photons m−2 s−1) at 25°C for 1 month.
Wounding of Tobacco Leaves
Sterile in vitro-grown plants were used for all experiments. The 2 largest leaves (12–17 mm in length) of 1-month-old plants were wounded by placing a finger under the leaf and by slightly compressing the upper surface using the circular section (2.2-mm diameter) of a metal rod. Ten wounds were made per leaf in a way that each wound site was overlapping a secondary vein without affecting the larger primary veins. Whole tobacco leaves were harvested at different postwounding times for analysis of p-CT and FT content. The harvested material was also used to measure enzyme activities.
Measurement of p-CT and FT after Mechanical Wounding
Four wounded leaves were collected from two distinct tobacco plants for each postwounding time (0, 4, and 8 h). After grinding in liquid nitrogen using a mortar and pestle, the leaves were extracted with MeOH (Pearce et al., 1998) to determine the levels of PCAAT. The 2 most abundant PCAAT found in tobacco, p-CT and FT, were quantified by HPLC using a 3.9 × 300-mm C-18 reverse-phase column (Waters, Mississauga, Canada) coupled to a Waters chromatograph system equipped with a 600E system controller, a 717 plus autosampler, a 991 photodiode array detector, and a 470 fluorescence detector. The identity of FT and p-CT was confirmed by comparing HPLC retention times and UV absorption and emission spectra to the ones obtained for authentic standards. For the purpose of this study, the FT to p-CT ratio, which varied from 9.5 to 12.7:1 depending on the experimental treatment of tissues, was considered as constant and only the combined levels of both p-CT and FT were provided in the results.
Enzyme Activity Measurements
Protein Extraction
Four wounded leaves were collected from two distinct tobacco plants for each postwounding time (0, 1, 3, 6, 12, and 24 h). After grinding the leaves in liquid nitrogen using a mortar and pestle, proteins were extracted at 4°C in 4 mL of 100 mm Tris-HCl, pH 7.8, containing 14 mm β-mercaptoethanol (buffer A). The crude extract was filtered through cheesecloth and the supernatant was centrifuged at 10,000 rpm for 10 min at 4°C. The supernatant was desalted on PD-10 columns (Amersham Biosciences, Baie d'Urfé, Canada) preequilibrated with buffer A.
TYDC (EC 4.1.1.25)
TYDC activity was determined by measuring 14CO2 released from l-[carboxyl-14C]Tyr (Amersham Biosciences) using filter discs saturated with quaternary ammonium as a trapping system (Facchini and De Luca, 1994). The assay mixture contained 50 mm BisTris, pH 7.2, 1 mm EDTA, 25 μm pyridoxal-1-phosphate, 0.1 μCi (specific activity 55 mCi/mmol) [carboxyl-14C]Tyr, and 50 to 100 μL of protein extract in a total volume of 1 mL. The reactions were incubated under constant agitation (200 rpm) on a rotary shaker for 60 min at 35°C. The reactions were stopped by addition of 50 μL of 0.2 n HCl and the decarboxylated 14CO2, which was released by shaking (200 rpm) the reaction tubes for1 h at 35°C, and trapped on the filter impregnated with quaternary ammonium.
4CL (EC 6.2.1.12)
4CL activity was measured spectrophotometrically (Knobloch and Hahlbrock, 1977) in the presence of 5 mm ATP, 5 mm MgCl2, 0.33 mm CoA, and 0.2 mm 4-coumarate. Formation rate of p-coumaroyl:CoA was determined by measuring the increase in absorption at 333 nm.
THT (EC 2.3.1.110)
To determine THT activity, a radiolabeled assay was used to measure formation of 14C-labeled p-CT in the presence of p-coumaroyl-CoA and [U-14C]tyramine. A tobacco recombinant 4CL gene expressed in Escherichia coli (pQE19; Lee and Douglas, 1996) was used to synthesize p-coumaroyl-CoA as previously described (Yu and Facchini, 1999), and the product obtained after purification with Sep-Pak C18 column (Waters) had an identical UV spectrum with that of the published standard. Another clone encoding for opium poppy (Papaver somniferum) TYDC protein was expressed in E. coli (Facchini and Deluca, 1995) to convert l-[U-14C]Tyr (Amersham Biosciences; 450 mCi/mmol) into -[U-14C]tyramine. A crude desalted protein extract from the TYDC-expressing E. coli cells was incubated for 4 h under the same conditions as described for the TYDC assay. The [U-14C]tyramine product, which cochromatographed with an authentic tyramine standard (Sigma, St. Louis), was purified by thin-layer chromatography (TLC; silica gel 60 F254) using CHCl3:MeOH:25% ammonia solution (6:3:1). The [U-14C]tyramine band was scraped off from the silica plate and extracted with MeOH. After centrifugation of the suspension for 5 min at 10,000g, the MeOH phase was recuperated and evaporated to dryness before being dissolved in 50 μL of 50% ethanol. For THT assay, 1 mL of the desalted crude protein extract was incubated with 50 nCi of [U-14C]tyramine and 100 μm p-coumaroyl-CoA for 30 min at 37°C (Facchini, 1998). The reactions were stopped by adding 0.2 mL of 1 m HCl and an aliquot of 20 μL of the reaction mixture was chromatographed on a TLC plate (silica gel 60 F254; EM Science, Mülheim, Germany) with CHCl3:MeOH (1:1) as a solvent system. A nonlabeled p-CT standard (Rf = 0.9) was cochromatographed and the corresponding band in samples was scraped off and the radioactivity determined with a scintillation counter.
Peroxidase Activity (EC 1.11.1.7)
Tobacco leaves were ground in liquid nitrogen, resuspended in 100 mm phosphate buffer (1 mL g−1 fresh weight), pH 6.0, and desalted on preequilibrated PD-10 columns. Peroxidase activity was assessed in the presence of 20 mm guaiacol, 100 mm sodium phosphate buffer, pH 6.0, and 30 mm of H2O2 in a total volume of 1 mL to which 2.0 μL of a desalted protein extract were added. Peroxidase activity was detected at 420 nm for 5 min and the mean slope was determined. For all enzyme assays, total proteins were determined using the Bradford (1976) method.
Labeling Studies Using l-[U-14C]Tyr and -[5-3H]Trp
Production of Radiolabeled Amines and By-Products in TDC, TYDC, TDC × TYDC, and Control Untransformed Tobacco
Tobacco leaves were wounded (as described above) and immediately harvested by cutting the base of the petiole with a razor blade. Leaves were individually transferred to 0.5-mL Eppendorf tubes and fed by petiolar absorption with 50 nCi of l-[U-14C]Tyr or 100 nCi of l-[5-3H]Trp (Amersham Biosciences) in 50 μL of water. Leaves were maintained at 25°C and 80% to 90% relative humidity until complete absorption of the radiolabeled solution (2–4 h). Sterile water was then added to the tubes that were kept for 48 h under a 16-h photoperiod (10 μmol photons m−2 s−1) at 25°C and 95% relative humidity. At the end of this incubation, each tobacco leaf was transferred into a 100-mL Erlenmeyer flask containing 40 mL of MeOH and was incubated for 48 h on a rotary shaker (75 rpm) at room temperature. Each tobacco leaf was then removed, wiped gently, and exposed to x-ray film (X-Omat; Eastman-Kodak, Rochester, NY), whereas each MeOH fraction was evaporated and dissolved in 50 μL of MeOH and a 20-μL aliquot was chromatographed onto a TLC silica plate (silica gel 60 F254) using CHCl3:MeOH:25% ammonia solution (6:3:1). TLC plates were dried and exposed to x-ray film for 24 to 72 h before they were developed.
Spatial Localization of Radiolabeled Amines and By-Products in TDC, TYDC, TDC × TYDC, and Control Untransformed Tobacco
A separate experiment was performed to assess the spatial localization of PCAAT around wound sites. A single wound per leaf, rather than the 10 described above, was made before feeding the leaves with 100 nCi [U-14C]Tyr through their petioles. Leaves were maintained under a 16-h photoperiod (10 μmol photons m−2 s−1) at 25°C to allow for the wound-inducible biosynthesis of p-CT and FT. Concentric leaf tissue discs were cut around wound sites with increasing size cork borers (2.0-, 4.0-, and 8.0-mm diameter) while avoiding to take samples in the highly lignified main vein. Ten leaf discs from each concentric size were pooled and extracted with MeOH, as described above, to determine the incorporation of labeled Tyr derivatives into soluble PCAAT around the wound site. In each case, the remaining MeOH-insoluble residue was submitted to alkaline hydrolysis to release bound forms of radioactive tyramine from cell walls. The insoluble residues were homogenized in 10 mL of 1 m NaOH and incubated with agitation for 5 h at 55°C before adding 10 mL of MeOH and adjusting the pH to 3 ± 0.5 with 5 m HCl. After each sample was extracted twice with 10 mL ethyl acetate, the ethyl acetate fractions were pooled, evaporated to dryness, and dissolved in 50 μL of MeOH. The two 50-μL aliquots corresponding to the MeOH-soluble and the alkaline hydrolysis-solubilized fractions were separately applied on a TLC plate, and the bands corresponding to tyramine and p-CT and FT were removed and counted for radioactivity as described above.
This work was funded by the Natural Sciences and Engineering Research Council of Canada (research grant to V.D.L. and postdoctoral fellowship to G.G.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.050294.
References
- Bernards MA, Lewis NG (1998) The macromolecular aromatic domain in suberized tissue: a changing paradigm. Phytochemistry 47: 915–933 [DOI] [PubMed] [Google Scholar]
- Bernards MA, Lopez ML, Zajicek J, Lewis NG (1995) Hydroxycinnamic acid-derived polymers constitute the polyaromatic domain of suberin. J Biol Chem 270: 7382–7386 [DOI] [PubMed] [Google Scholar]
- Borg-Olivier O, Monties B (1993) Lignin, suberin, phenolic acids and tyramine in the suberized, wound-induced potato periderm. Phytochemistry 32: 601–606 [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Clarke DD (1982) The accumulation of cinnamic acid amides in the cell walls of potato tissue as an early response to fungal attack. In RKS Wood, ed, Active Defense Mechanisms in Plants. Plenum Press, New York, pp 321–322
- De Luca V, Marineau C, Brisson N (1989) Molecular cloning and analysis of cDNA encoding a plant tryptophan decarboxylase: comparison with animal DOPA decarboxylases. Proc Natl Acad Sci USA 86: 2582–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini PJ (1998) Temporal correlation of tyramine metabolism with alkaloid and amide biosynthesis in elicited opium poppy cell cultures. Phytochemistry 49: 481–490 [Google Scholar]
- Facchini PJ, De Luca V (1994) Differential and tissue-specific expression of a gene family for tyrosine/dopa decarboxylase in opium poppy. J Biol Chem 269: 26684–26690 [PubMed] [Google Scholar]
- Facchini PJ, De Luca V (1995) Expression in Escherichia coli and partial characterization of two tyrosine/dopa decarboxylases from opium poppy. Phytochemistry 38: 1119–1126 [DOI] [PubMed] [Google Scholar]
- Facchini PJ, Penzes-Yost C, Samanani N, Kowalchuk B (1998) Expression patterns conferred by tyrosine/dihydroxyphenylalanine decarboxylase promoters from opium poppy are conserved in transgenic tobacco. Plant Physiol 118: 69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini PJ, Yu M, Penzes-Yost C (1999) Decreased cell wall digestibility in canola transformed with chimeric tyrosine decarboxylase genes from opium poppy. Plant Physiol 120: 653–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet G, Poupart J, Basurco J, De Luca V (2000) Expression of tryptophan decarboxylase and tyrosine decarboxylase genes in tobacco results in altered biochemical and physiological phenotypes. Plant Physiol 122: 933–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara A, Kawata N, Matsukawa T, Iwamura H (2000) Induction of N-hydroxycinnamoyltyramine synthesis and tyramine N-hydroxycinnamoyltransferase (THT) activity by wounding in maize leaves. Biosci Biotechnol Biochem 64: 1025–1031 [DOI] [PubMed] [Google Scholar]
- Jang S-M, Ishihara A, Back K (2004) Production of coumaroylserotonin and feruloylserotonin in transgenic rice expressing pepper hydroxycinnamoyl-coenzyme A:serotonin N-(hydroxycinnamoyl)transferase. Plant Physiol 135: 346–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller H, Hohlfeld H, Wray V, Hahlbrock K, Scheel D, Strack D (1996) Changes in the accumulation of soluble and cell wall-bound phenolics in elicitor-treated cell suspension cultures and fungus-infected leaves of Solanum tuberosum. Phytochemistry 42: 389–396 [Google Scholar]
- Knobloch K-H, Hahlbrock K (1977) 4-Coumarate:CoA ligase from cell suspencion cultures of Petroselinum hortense Hoffm. Partial purification, substrate specificity and further properties. Arch Biochem Biophys 184: 237–248 [DOI] [PubMed] [Google Scholar]
- Lapierre C, Pollet B, Négrel J (1996) The phenolic domain of potato suberin: structural comparison with lignins. Phytochemistry 42: 949–953 [Google Scholar]
- Lee D, Douglas CJ (1996) Two divergent members of a tobacco 4-coumarate:coenzyme A ligase (4CL) gene family. cDNA structure, gene inheritance and expression, and properties of recombinant proteins. Plant Physiol 112: 193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Tanguy J, Sun LY, Burtin D, Vernoy R, Rossin N, Tepfer D (1996) Attenuation of the phenotype caused by the root-inducing, left-hand, transferred DNA and its ROLA gene: correlations with changes in polyamine metabolism and DNA methylation. Plant Physiol 111: 259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlenbeck U, Kortenbusch A, Barz W (1996) Formation of hydroxycinnamoyl amides and alpha-hydroxyacetovanillone in cell cultures of Solanum khasianum. Phytochemistry 42: 1573–1579 [Google Scholar]
- Negrel J, Javelle F (1995) Induction of phenylpropanoid and tyramine metabolism in pectinase- or pronase-elicited cell suspension cultures of tobacco (Nicotiana tabacum). Physiol Plant 95: 569–574 [Google Scholar]
- Negrel J, Javelle F (1997) Purification, characterization and partial amino acid sequencing of hydroxycinnamoyl-CoA:tyramine N-(hydroxycinnamoyl)transferase from tobacco cell-suspension cultures. Eur J Biochem 247: 1127–1135 [DOI] [PubMed] [Google Scholar]
- Negrel J, Javelle F, Paynot M (1993) Wound-induced tyramine hydroxycinnamoyl transferase in potato (Solanum tuberosum) tuber disks. J Plant Physiol 142: 518–524 [Google Scholar]
- Negrel J, Lherminier J (1987) Peroxidase-mediated integration of tyramine into xylem cell walls of tobacco leaves. Planta 172: 494–501 [DOI] [PubMed] [Google Scholar]
- Negrel J, Lotfy S, Javelle F (1995) Modulation of the activity of two hydroxycinnamoyltransferasees in wound-healing potato tuber discs in response to pectinase and abscisic acid. J Plant Physiol 146: 318–322 [Google Scholar]
- Negrel J, Martin C (1984) The biosynthesis of feruloyltyramine in Nicotiana tabacum. Phytochemistry 23: 2797–2801 [Google Scholar]
- Newman MA, von Roepenack-Lahaye E, Parr A, Daniels MJ, Dow JM (2001) Induction of hydroxycinnamoyl-tyramine conjugates in pepper by Xanthomonas campestris, a plant defense response activated by hrp gene-dependent and hrp gene-independent mechanisms. Mol Plant Microbe Interact 14: 785–792 [DOI] [PubMed] [Google Scholar]
- Park JB, Schoene N (2002) Synthesis and characterization of N-coumaroyltyramine as a potent phytochemical which arrests human transformed cells via inhibiting protein tyrosine kinases. Biochem Biophys Res Commun 292: 1104–1110 [DOI] [PubMed] [Google Scholar]
- Pearce G, Marchand PA, Griswold J, Lewis NG, Ryan CA (1998) Accumulation of feruloyltyramine and p-coumaroyltyramine in tomato leaves in response to wounding. Phytochemistry 47: 659–664 [Google Scholar]
- Schmidt A, Grimm R, Schmidt J, Scheel D, Strack D, Rosahl S (1999) Cloning and expression of a potato cDNA encoding hydroxycinnamoylCoA:tyramine N-(hydroxycinnamoyl)transferase. J Biol Chem 274: 4273–4280 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Scheel D, Strack D (1998) Elicitor-stimulated biosynthesis of hydroxycinnamoyltyramines in cell suspension cultures of Solanum tuberosum. Planta 205: 51–55 [Google Scholar]
- Yamamoto I, Matsunaga T, Kobayashi H, Watanabe K (1991) Analysis and pharmacotoxicity of feruloytyramine as a new constituent and p-coumaroyltyramine in Cannabis sativa L. Pharmacol Biochem Behav 40: 465–469 [DOI] [PubMed] [Google Scholar]
- Yao K, De Luca V, Brisson N (1995) Creation of a metabolic sink for tryptophan alters the phenylpropanoid pathway and the susceptibility of potato to Phytophthora infestans. Plant Cell 7: 1787–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Facchini PJ (1999) Purification, characterization, and immunolocalization of hydroxycinnamoyl-CoA:tyramine N-(hydroxycinnamoyl)transferase from opium poppy. Planta 209: 33–44 [DOI] [PubMed] [Google Scholar]