Abstract
Tocopherol belongs to the Vitamin E class of lipid soluble antioxidants that are essential for human nutrition. In plants, tocopherol is synthesized in plastids where it protects membranes from oxidative degradation by reactive oxygen species. Tocopherol cyclase (VTE1) catalyzes the penultimate step of tocopherol synthesis, and an Arabidopsis (Arabidopsis thaliana) mutant deficient in VTE1 (vte1) is totally devoid of tocopherol. Overexpression of VTE1 resulted in an increase in total tocopherol of at least 7-fold in leaves, and a dramatic shift from α-tocopherol to γ-tocopherol. Expression studies demonstrated that indeed VTE1 is a major limiting factor of tocopherol synthesis in leaves. Tocopherol deficiency in vte1 resulted in the increase in ascorbate and glutathione, whereas accumulation of tocopherol in VTE1 overexpressing plants led to a decrease in ascorbate and glutathione. Deficiency in one antioxidant in vte1, vtc1 (ascorbate deficient), or cad2 (glutathione deficient) led to increased oxidative stress and to the concomitant increase in alternative antioxidants. Double mutants of vte1 were generated with vtc1 and cad2. Whereas growth, chlorophyll content, and photosynthetic quantum yield were very similar to wild type in vte1, vtc1, cad2, or vte1vtc1, they were reduced in vte1cad2, indicating that the simultaneous loss of tocopherol and glutathione results in moderate oxidative stress that affects the stability and the efficiency of the photosynthetic apparatus.
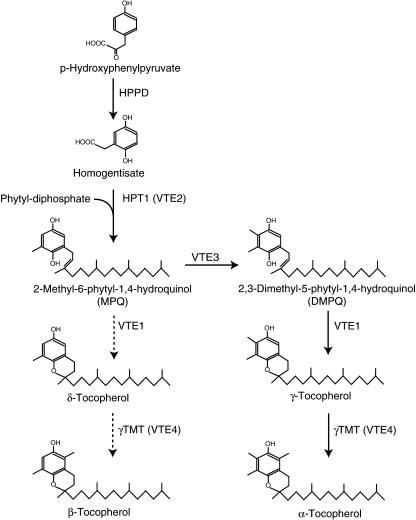
Vitamin E encompasses a class of lipid antioxidants consisting of four forms each of tocopherol and tocotrienol. Because of its high economical value and importance for human nutrition, much effort has been invested to elucidate the tocopherol biosynthetic pathway in plants and Cyanobacteria and to identify limiting steps by overexpression of candidate genes in transgenic plants. The hydroquinone ring of tocopherol is derived from the shikimate pathway of aromatic amino acid synthesis. Homogentisate, the precursor for the synthesis of tocopherol, tocotrienol, and plastoquinone, is synthesized by p-hydroxyphenylpyruvate dioxygenase (HPPD; Fig. 1; Norris et al., 1998). After attachment of the hydrophobic side chain by homogentisate phytyltransferase (HPT1/VTE2; Collakova et al., 2001; Savidge et al., 2002) and methylation (VTE3; Cheng et al., 2003; van Eenennaam et al., 2003), 2,3-dimethyl-5-phytyl-1,4-hydroquinol (DMPQ) is formed that is converted to γ-tocopherol by tocopherol cyclase (VTE1; Porfirova et al., 2002; Sattler et al., 2003). An increase in the activity of HPPD or HPT1 in transgenic plants resulted in an elevated tocopherol content in seeds and leaves of Arabidopsis (Arabidopsis thaliana; Tsegaye et al., 2002; Collakova et al., 2003a). It was concluded that flux into tocopherol is predominantly controlled by HPPD and HPT1, but that biosynthetic steps further downstream in the pathway, e.g. VTE1, are not limiting (Collakova et al., 2003a). Final methylation by γ-tocopherol methyltransferase (γ-TMT, VTE4) results in the production of α-tocopherol. α-Tocopherol is the predominant form in leaves, whereas γ-tocopherol is most abundant in seeds of Arabidopsis (Shintani and DellaPenna, 1998). Side reactions of VTE1, VTE3, and VTE4 result in the production of minor tocopherol forms in Arabidopsis seeds or leaves (δ-tocopherol, β-tocopherol; Fig. 1).
Figure 1.
Tocopherol synthesis in Arabidopsis. p-Hydroxyphenylpyruvate derived from the shikimate pathway is converted to homogentisate by action of HPPD. Attachment of a phytyl group by HPT1 (VTE2) results in the synthesis of MPQ. After methylation (VTE3), the second ring is formed by cyclization of DMPQ via the VTE1 reaction. The product of this reaction, γ-tocopherol, is converted to α-tocopherol by γ-TMT (VTE4). Side activities of VTE1 and γ-TMT in leaves resulting in the production of δ-tocopherol and β-tocopherol are indicated by dashed arrows.
Under oxidative stress, the amounts of different antioxidants strongly increase in plants, and it is believed that this results in an increased capacity to scavenge reactive oxygen species (Noctor and Foyer, 1998; Collakova et al., 2003b). The accumulation of antioxidants under stress is at least in part mediated by induction of gene expression. For example, HPPD and HPT1, two steps of tocopherol synthesis, were shown to be induced under light stress (Collakova et al., 2003b). However, expression of VTE1 was not strongly altered during stress (Collakova et al., 2003b). Tocopherol is a strong antioxidant, and it is crucial for scavenging reactive oxygen species released during oxidative stress (Fryer, 1992; Munné-Bosch and Alegre, 2002). Tocopherol is synthesized in the inner envelope of chloroplasts and accumulates in all chloroplast membranes (Lichtenthaler et al., 1981; Soll et al., 1985). Therefore, it has been suggested that tocopherol is involved in the protection of pigments and proteins of the photosynthetic apparatus and of thylakoids lipids against oxidative degradation. As a consequence, loss of tocopherol in plants would be expected to severely affect growth and photosynthesis. However, isolation and characterization of the Arabidopsis vte1 mutant showed that total loss of tocopherol has only a minor impact on growth, chlorophyll content, photosynthetic capacity, and fatty acid composition under normal and high light stress conditions (Porfirova et al., 2002; Bergmüller et al., 2003; Sattler et al., 2003). The SXD1 (Suc export deficient) gene that is orthologous to Arabidopsis VTE1 has previously been isolated from maize (Zea mays; Provencher et al., 2001; Sattler et al., 2003). Interestingly, the corresponding maize mutant, sxd1, shows a much more severe phenotype than the Arabidopsis vte1 plant, because anthocyanins, sugars, and starch strongly accumulate in sxd1 leaves (Russin et al., 1996; Provencher et al., 2001). Similarly, down-regulation of VTE1 expression in transgenic potato resulted in reduced growth and accumulation of carbohydrates (Hofius et al., 2004).
Ascorbate and glutathione are the most abundant low Mr compounds with antioxidant activity in higher plants (Noctor and Foyer, 1998; Smirnoff, 2000a, 2000b). Thus, these two antioxidants are crucial to maintain the intracellular redox status, and in addition, they are key substrates and cofactors of many enzymatic reactions. Similar to tocopherol, ascorbate and glutathione accumulate during oxidative stress (Noctor and Foyer, 1998). The pools of ascorbate and glutathione are linked via the ascorbate-glutathione cycle, because after oxidation of ascorbate (e.g. by hydrogen peroxide), dehydroascorbate can be reduced by a glutathione-dependent dehydroascorbate reductase (Smirnoff, 2000b). Subsequently, the oxidized form of glutathione (GSSG) is reduced by glutathione reductase in an NADPH dependent manner. Therefore, the ascorbate-glutathione cycle is thought to be critical for the removal of hydrogen peroxide from metabolism. Previous experiments already suggested that the oxidized form of tocopherol (the tocopheroxyl radical) can be regenerated by interaction with ascorbate and glutathione at the surface of the lipid bilayer (Leung et al., 1981; Niki et al., 1984; Liebler et al., 1986; Wefers and Sies, 1988; Kago and Terao, 1995; Kamal-Eldin and Appelqvist, 1996).
Different Arabidopsis mutants with deficiencies in the synthesis of ascorbate or glutathione have been isolated. The vtc1 (synonymous with soz1) mutant carries a mutation in the gene encoding GDP-Man pyrophosphorylase involved in ascorbate synthesis, and therefore this plant contains only 30% of wild-type amounts of ascorbate (Conklin et al., 1996, 1999). The vtc1 plant has been used to study the function of ascorbate in growth, photosynthesis, and oxidative stress (Conklin et al., 1996; Veljovic-Jovanovic et al., 2001). A glutathione deficient plant (cad2) was isolated based on an altered sensitivity to cadmium (Howden et al., 1995). In cad2, the gene encoding γ-glutamylCys synthetase, the first step of glutathione synthesis, carries a short deletion resulting in a reduction of glutathione to about 20% of wild type (Cobbett et al., 1998; Vernoux et al., 2000; Xiang et al., 2001).
To further our understanding of the regulation of tocopherol biosynthesis and of the role of tocopherol in the antioxidant network of Arabidopsis, transgenic plants overexpressing VTE1 were generated and subjected to tocopherol analysis, and double mutants of vte1 and lines deficient in the synthesis of ascorbate (vtc1) or glutathione (cad2) were produced. From these studies, it became clear that tocopherol cyclase is limiting tocopherol synthesis in leaves, and that the simultaneous loss of tocopherol and glutathione affects photosynthesis and growth in a way different from what was observed for the parental lines.
RESULTS
Overexpression of VTE1 Results in Accumulation of Tocopherol and in a Shift in Tocopherol Composition in Leaves
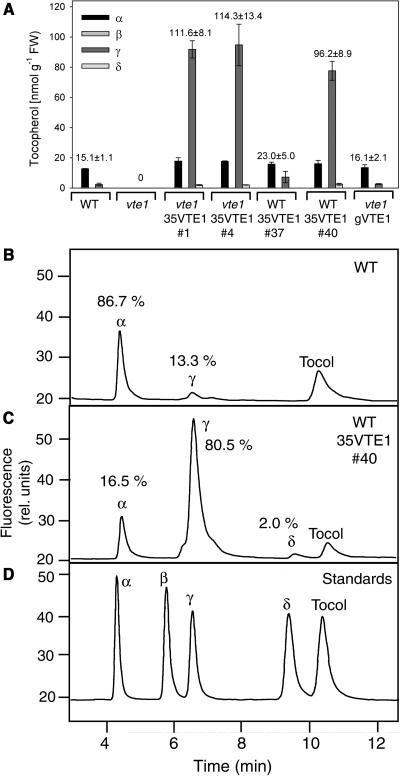
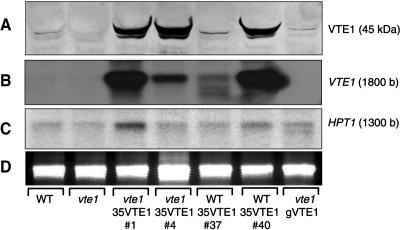
VTE1 catalyzes the cyclization of DMPQ resulting in the formation of γ-tocopherol that is subsequently converted to α-tocopherol in leaves (Fig. 1; Porfirova et al., 2002). To test whether VTE1 is limiting for tocopherol production in leaves, VTE1 was overexpressed in Arabidopsis under the control of two different promoters. Transformation of the vte1 mutant with a genomic VTE1 construct under the control of the endogenous VTE1 promoter (line vte1-gVTE1) resulted in the accumulation of tocopherol in transgenic plants in amounts very similar to wild type (Fig. 2A). Furthermore, the composition of tocopherol in the leaves of the transformants (86.5% α-tocopherol, 13.4% γ-tocopherol) was similar to wild type (Fig. 2, A and B). Polyclonal antiserum was raised to heterologously expressed VTE1 protein and its specificity tested by western analysis of wild-type and vte1 mutant leaf protein (Fig. 3A). The vte1 mutant carries a splicing site mutation and is devoid of VTE1 mRNA (Porfirova et al., 2002). The anti-VTE1 antibodies immunoreacted with the VTE1 band at 45 kD in wild type but not in the vte1 mutant, suggesting that they are highly specific. Complementation of tocopherol deficiency in vte1 carrying a genomic VTE1 construct (vte1-gVTE1) was accompanied by the accumulation of a 45-kD protein corresponding to the size of VTE1 that reacted with anti-VTE1 antibodies (Fig. 3A).
Figure 2.
Overexpression of VTE1 results in accumulation of γ-tocopherol in leaves. The cDNA (under control of the cauliflower mosaic virus 35S promoter, 35VTE1) or genomic DNA of VTE1 (gVTE1) was introduced into Col-2 wild-type or vte1 mutant plants as indicated. A, The amounts of different forms of tocopherol (α, β, γ, δ) were determined by fluorescence HPLC. The numbers above the bars indicate total amount of tocopherol (nmol g−1 fresh weight). Data show mean ± se of three experiments. B, HPLC chromatogram of tocopherols extracted from wild-type leaves. The numbers indicate tocopherol composition in percent. C, HPLC chromatogram showing tocopherol composition of line WT-35VTE1 number 40 with overexpression of the VTE1 cDNA under control of the 35S promoter. D, HPLC chromatogram of tocopherol and tocol standards.
Figure 3.
Expression of HPT1 and VTE1 in VTE1 overexpression lines. A, Expression of VTE1 was measured by western analysis with anti-VTE1 antiserum. B, Accumulation of mRNA in transgenic lines was measured by northern hybridization using VTE1 cDNA as a probe. C, HPT1 mRNA was detected after hybridization with a HPT1 probe amplified from genomic DNA by PCR. D, 25S rRNA bands of the northern gel after ethidium bromide staining.
A second construct that was introduced into Arabidopsis wild type and vte1 mutant contained the VTE1 cDNA under the control of the strong constitutive 35S promoter (lines WT-35VTE1 and vte1-35VTE1). Overexpression of VTE1 with 35S promoter resulted in an up to 7-fold increase in tocopherol in several lines, including transgenic plants with wild-type or vte1 mutant background (Fig. 2C). Furthermore, the dramatic increase in tocopherol led to a shift in tocopherol composition, because leaves of transgenic plants accumulated large amounts of γ-tocopherol (80.5%) instead of α-tocopherol (16.5%) and low amounts of δ-tocopherol (2%; Fig. 2C). The accumulation of γ-tocopherol can be explained by a low activity of γ-TMT, which might become limiting for α-tocopherol synthesis in the transgenic lines (Fig. 1). The presence of low amounts of δ-tocopherol is supposedly derived from conversion of 2-methyl-6-phytyl-1,4-hydroquinol (MPQ) to δ-tocopherol in VTE1 overexpressing lines. The high accumulation of total tocopherol in the 35S promoter plants correlated with a dramatic increase in VTE1 expression as shown by western analysis (Fig. 3A) and northern analysis (Fig. 3B). It is interesting to note that transgenic VTE1 plants of wild-type background (WT-35VTE1 no. 37) with low expression level had wild-type amounts of tocopherol. Therefore, accumulation of tocopherol was correlated with high expression of VTE1. It was previously reported that HPT1 limits tocopherol synthesis and that HPT1 overexpression leads to elevated tocopherol content in transgenic plants (Collakova and DellaPenna, 2003b). Therefore, expression of HPT1 was analyzed in VTE1 overexpression plants to study whether in these transgenic plants induction of HPT1 contributes to the increased tocopherol content (Fig. 3C). However, expression of HPT1 in the strongVTE1 overexpression lines number 1 and number 40 was only slightly increased (2- and 1.5-fold, respectively) as compared to wild type or vte1 carrying a genomic fragment of VTE1. Therefore, we concluded that the high tocopherol content observed for the VTE1 overexpression plants results from increased expression of VTE1 rather than HPT1.
Tocopherol Content and VTE1 Expression Are Increased under Oxidative Stress and in Antioxidant Mutants
It is well established that the amount of tocopherol increases during oxidative stress in leaves (e.g. Collakova and DellaPenna, 2003b). The increase in tocopherol under oxidative stress was accompanied by a shift in tocopherol composition from 5% γ-tocopherol in nonstressed wild type to about 15% after high light stress (Collakova and DellaPenna, 2003b). These results were explained by the strong induction of HPPD and HPT1, accompanied by a limitation in γ-TMT (VTE4) activity. Expression of VTE1 as measured by quantitative reverse transcription (RT)-PCR was found to be up-regulated at day 3 of high light stress but indistinguishable from wild type at later time points (Collakova and DellaPenna, 2003b).
To unravel the contribution of VTE1 for tocopherol synthesis during stress, gene expression was measured in wild-type leaves exposed to high light conditions using northern hybridization. Expression of VTE1 was strongly induced, particularly during the early time points (days 1 to 4) of high light stress (Fig. 4B).
Figure 4.
Accumulation of tocopherol and induction of VTE1 under high light stress and in oxidative stress mutants. A, Total tocopherol was determined by fluorescence HPLC. The black and the gray parts of the stacked bars indicate γ-tocopherol and α-tocopherol, respectively. The amounts of β-tocopherol and δ-tocopherol were below detection limit. Data represent the means and se of at least five measurements each. Asterisk, Total amount of tocopherol is significantly different to wild type after t test analysis (P < 0.05). B, Induction of VTE1 expression in Arabidopsis plants under high light conditions and in the antioxidant mutants vtc1 and cad2 as determined by northern analysis. C, 25S rRNA (photo of northern gel) as loading control.
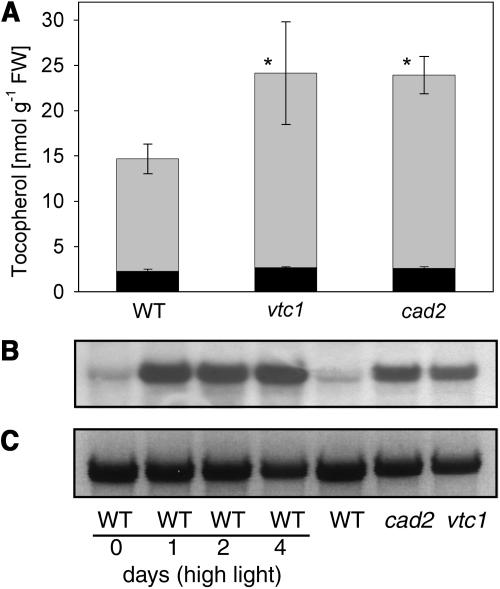
Mutants deficient in antioxidant synthesis offer an alternative approach to study the effects of oxidative stress on plant physiology. We selected two lines, vtc1 and cad2, which are deficient in ascorbate and glutathione synthesis, respectively (Howden et al., 1995; Conklin et al., 1996). In agreement with the hypothesis that oxidative stress is increased in these lines, the total amount of tocopherol was increased by approximately 50% in vtc1 and cad2 (Fig. 4A). Interestingly, we observed a slight shift in tocopherol composition toward an increased content of α-tocopherol and a decrease in γ-tocopherol in vtc1 (90.0% α-tocopherol; 9.9 γ-tocopherol) and cad2 (90.2% α; 9.7% γ) as compared to wild type (86.6% α; 13.3 γ; Fig. 4A). Thus, in contrast to the change in tocopherol composition observed under oxidative stress, the increase in tocopherol in vtc1 and cad2 was accompanied by a relative increase in α-tocopherol, suggesting that γ-TMT was not limiting in antioxidant mutants. To address the question of whether the stimulation of tocopherol synthesis observed in leaves of vtc1 and cad2 originates from induction of gene expression, northern analysis was done. Indeed, we observed a strong up-regulation of VTE1 expression (Fig. 4B) in these two mutants. As judged by northern hybridization, expression levels for HPT1 and HPPD in vtc1 and cad2 were in the range of wild type (data not shown). Therefore, the increase in tocopherol synthesis observed under oxidative stress and in antioxidant mutants (vtc1 and cad2) clearly correlates with induction of VTE1 expression.
Deficiency in Tocopherol, Ascorbate, or Glutathione in Mutant Plants Results in Compensatory Increases in Alternative Antioxidants
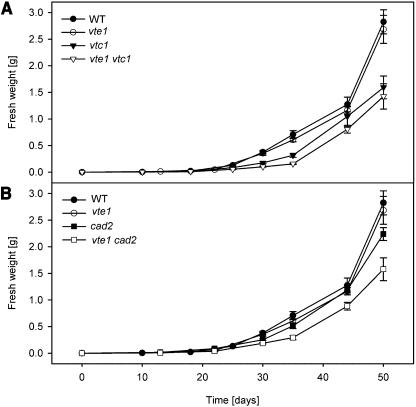
Growth and photosynthetic efficiency of the vte1 mutant of Arabidopsis were very similar to wild type under standard growth conditions (Porfirova et al., 2002; Bergmüller et al., 2003; Sattler et al., 2003). To test the hypothesis whether other antioxidants compensate for the loss of tocopherol in vte1, double mutants were created of vte1, vtc1, and cad2. The two double mutant lines vte1vtc1 and vte1cad2 were fertile and easily grown on soil. Growth of vte1vtc1 was very similar to vtc1, but these two lines were always smaller than wild type (Fig. 5A). As previously demonstrated, vtc1 mutant plants flower about 1 week later than wild type (Veljovic-Jovanovic et al., 2001). Flowering time of the vte1vtc1 was similar to that of vtc1 (data not shown). Apparently, the combination of the two mutations affecting tocopherol and ascorbate synthesis had no further effect on growth and physiology. However, growth of vte1cad2 was more severely retarded as compared with vte1 or cad2 (Fig. 5B), suggesting that the simultaneous deficiency in tocopherol and glutathione in vte1cad2 affects plant antioxidant status and might have an impact on photosynthesis.
Figure 5.
The growth phenotype of antioxidant mutants. A, Growth curves were recorded by measuring fresh weight of the aerial part of the plants wild type (WT), vte1, vtc1, and vte1vtc1 (mean ± se of five experiments). B, Growth curves for WT, vte1, cad2, and vte1cad2.
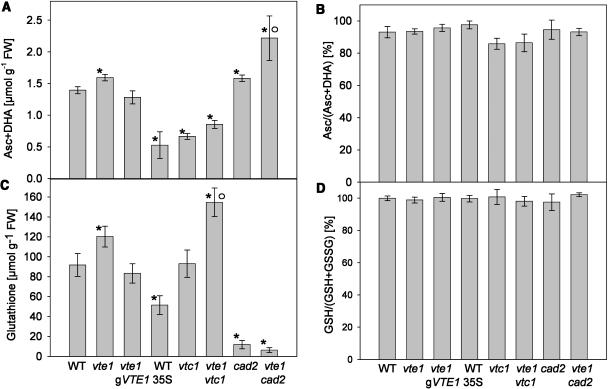
Ascorbate and glutathione were quantified in single and double mutant lines deficient in antioxidants (Fig. 6). Furthermore, vte1 carrying a genomic VTE1 construct and a VTE1 overexpression line were included in these analyses. Deficiency in tocopherol (in vte1) or glutathione (cad2) led to a moderate but significant increase in ascorbate (Fig. 6A). The accumulation of ascorbate was also visible when comparing vte1vtc1 with vtc1 and resulted in an even stronger ascorbate accumulation in vte1cad2 as compared to vte1 or cad2. Interestingly, the VTE1 overexpression line accumulated significantly reduced amounts of ascorbate as compared with wild type. The redox state of ascorbate was not affected by the vte1 or cad2 mutations (Fig. 6B). Total glutathione was increased in lines deficient for tocopherol (vte1) and increased even further in the vte1vtc1 double mutant (Fig. 6C). Glutathione was reduced to less than 10% of wild-type amounts in lines homozygous for cad2. The VTE1 overexpression lines contained reduced amounts of glutathione as compared to wild type. No change in the redox state of glutathione was observed (Fig. 6D). Taken together (Figs. 4A and 6), the reduction in one of the three antioxidants, tocopherol, ascorbate, or glutathione, resulted in an increase in the remaining antioxidants in single and double mutant plants, whereas high tocopherol contents resulted in a reduction of ascorbate and glutathione in VTE1 overexpression lines.
Figure 6.
Glutathione and ascorbate content in antioxidant mutants. A, Dehydroascorbate and ascorbate and (C) total glutathione were measured in leaves of 5-week-old single and double mutant plants in a VTE1 overexpression line (WT 35VTE1) and in vte1 carrying a genomic DNA construct (vte1 gVTE1). B, The redox status of ascorbate is presented as the ratio of ascorbate to the sum of ascorbate and dehydroascorbate. D, The redox status of glutathione is indicated by the ratio of (total glutathione − 2 × GSSG) to total glutathione. Data represent the means and se of at least five measurements each. Asterisk or white circle indicates significantly different to wild type or vte1, respectively, after t test analysis (P < 0.05).
Single and double mutant lines were exposed to high light stress conditions (800 μE light for 4 d) and antioxidant contents determined. Under high light, all antioxidants (tocopherol, glutathione, and ascorbate) increased severalfold (data not shown). A large variation of antioxidant contents was observed under stress conditions. Furthermore, the differences in antioxidant levels as observed under control conditions (Figs. 4A and 6) were smaller under high light stress, probably because under oxidative stress, antioxidants increased to a maximal level in all mutant lines regardless of the deficiency in alternative antioxidants.
NPQ of vte1 Is Increased under High Light
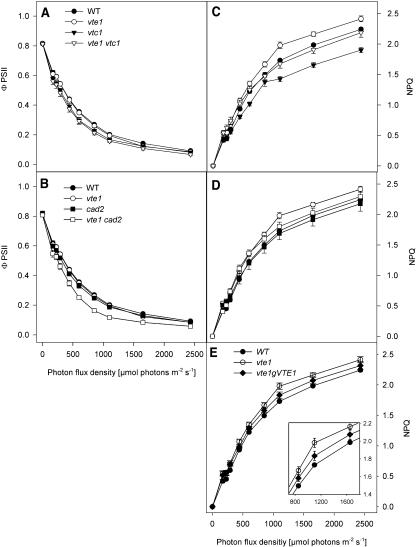
The susceptibility of the photosynthetic apparatus to oxidative stress prompted us to analyze the efficiency of photosynthesis in antioxidant mutants in more detail. Therefore, light response curves of chlorophyll fluorescence were recorded for wild-type and mutant plants. The maximum photochemical efficiency of PSII in the dark-adapted state (Fv/Fm) was very similar for all lines (0.816, 0.815, 0.816, 0.816, 0.813, 0.821, and 0.808 for wild type, vte1, vtc1, vte1vtc1, cad2, and vte1cad2, respectively). In addition, nonphotochemical quenching (NPQ) was calculated to reveal the fraction of light energy that is dissipated by means other than photosynthesis, e.g. heat. In accordance with previous studies (Smirnoff, 2000; Müller-Moulé et al., 2002), a decrease in NPQ was observed for vtc1 at high photon flux densities (PPFD; Fig. 7C). Interestingly, all lines homozygous for the vte1 mutation showed a slight increase (about 10%) in NPQ at high PPFD (Fig. 7, C and D). Transformation of vte1 with a genomic fragment of VTE1 resulted in reduction of NPQ, almost reaching wild-type values (Fig. 7E).
Figure 7.
Light response curves of fluorescent ΦPSII and NPQ ΦPSII (A and B) and NPQ (C, D, and E) were determined by chlorophyll fluorescence analysis. Data represent mean ± se derived from fluorescence measurements of at least five leaves each. The following lines were analyzed: Wild-type Col-2, vte1 single mutant (Col-2 background), vtc1 single mutant (Col-0), cad2 single mutant (in Col-0), vte1vtc1 double mutant, vte1cad2 double mutant, and vte1 transformed with a genomic fragment containing the entire VTE1 gene.
Deficiency in Tocopherol and Glutathione in vte1cad2 Affects Photosynthetic Efficiency and Pigment Content
The quantum yield of PSII (ΦPSII) was determined as a measure of the efficiency of PSII light reactions. ΦPSII was comparable at all light intensities for wild type, vte1, vtc1, cad2, and the vte1vtc1 double mutant (Fig. 7, A and B). However, ΦPSII in vte1cad2 was reduced at elevated light intensities by approximately 37% as compared to wild-type and the parental mutant lines (Fig. 7B), indicating that the simultaneous loss of tocopherol and glutathione affects photosynthetic efficiency in a way not observed in the parental lines.
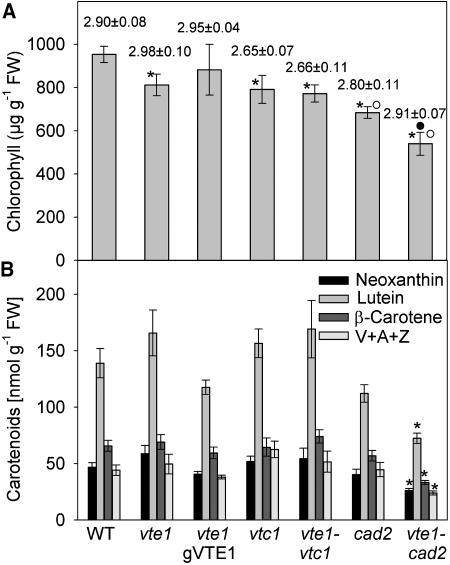
Because antioxidants, in particular tocopherol, were implicated in protecting the photosynthetic apparatus from oxidative damage, chlorophyll and carotenoids were quantified in the mutant lines. Total chlorophyll content was only slightly reduced in vte1, vtc1, and the respective double mutant as compared to wild type (Fig. 8A). The chlorophyll a to chlorophyll b ratio was close to 2.90, except for lines homozygous for vtc1, where it was reduced to about 2.65. Chlorophyll content was lower in cad2, and the vte1cad2 double mutant showed the lowest chlorophyll content of all lines analyzed. To unravel whether the reduction in chlorophyll observed in vte1cad2 was caused by a general decline in photosynthetic units, carotenoids (neoxanthin, lutein, β-carotene, and xanthophyll cycle components) were quantified by HPLC in wild-type and mutant lines (see Fig. 8B). The contents of the carotenoids were very similar to wild type for the vte1 and vtc1 mutants, except for the vte1cad2 double mutant. In vte1cad2, the amounts of neoxanthin, lutein, β-carotene, and xanthophyll cycle components (violaxanthin, antheraxanthin, and zeaxanthin) were reduced to about 60% of wild type, indicating that the simultaneous reduction of tocopherol and glutathione in this plant led to a concerted decrease of all photosynthetic pigments.
Figure 8.
Photosynthetic pigment content in antioxidant mutants. A, Total chlorophyll in leaves was measured spectrophotometrically. The numbers indicate the chlorophyll a to chlorophyll b ratio. B, The content of neoxanthin, lutein, β-carotene, and xanthophyll cycle components (violaxanthin, antheraxanthin, zeaxanthin; V+A+Z) in leaves was determined by HPLC analysis. Data represent the mean and se of at least five experiments. Asterisk, white circle, or black circle indicates significantly different as compared to wild type, vte1 or cad2, respectively, after t test analysis (P < 0.05).
DISCUSSION
Overexpression of VTE1 under the strong constitutive 35S promoter resulted in the accumulation of large amounts of tocopherol in leaves of transgenic plants. Tocopherol in transgenic VTE1 leaves was increased by a factor of 7 (Fig. 2A), representing the largest increase in total tocopherol content obtained in any transgenic plant to date. Previous studies reported an increase in leaf tocopherol by a factor of 1.4 and 4.4 by overexpression of HPPD or HPT1, respectively (Tsegaye et al., 2002; Collakova and DellaPenna, 2003a). Overexpression of homogentisate geranylgeranyl transferase resulted in a strong increase in tocotrienols of 10- to 15-fold as compared to the tocopherol content of Arabidopsis wild-type leaves (Cahoon et al., 2003). Interestingly, expression of HPPD and a yeast prephenate dehydrogenase in tobacco leaves resulted in a 10-fold increase in total tocol content, predominately tocotrienols (Rippert et al., 2004). The use of geranylgeranyl-pyrophosphate instead of phytyl-pyrophosphate for tocol synthesis is unusual in leaves. However, no tocotrienols were detected in VTE1 overexpressing lines (Fig. 2C).
Overexpression of HPPD or HPT1 resulted in a strong increase in tocopherol synthesis (Tsegaye et al., 2002; Collakova and DellaPenna, 2003a). Because the two substrates of tocopherol cyclase, MPQ and DMPQ do not accumulate in HPT1 overexpressing plants or in wild-type plants exposed to high light stress, it was concluded that VTE1 is not limiting for tocopherol synthesis (Collakova and DellaPenna, 2003a, 2003b). In addition, quantitative RT-PCR analysis revealed that expression of HPPD and HPT1 is induced during high light stress, but VTE1 expression remains more or less unchanged (Collakova and DellaPenna, 2003b). VTE1 expression as measured by quantitative RT-PCR was only slightly increased after 3 d at high light and was very similar to wild type at later time points (up to 12 d at high light; Collakova and DellaPenna, 2003b). In contrast to these studies, we observed a clear induction of VTE1 expression after 1 to 4 d of high light stress. The abundance of VTE1 mRNA as measured by northern analysis was increased by a factor of about 10-fold (Fig. 4B). Furthermore, we showed that VTE1 expression was induced in vtc1 and cad2, but expression of HPT1 or HPPD was not induced in these two mutants. The discrepancy of the results obtained by RT-PCR or by northern analysis might be caused by the specificity of the different techniques employed or by differences in the physiological stress conditions (e.g. light, plant age, and temperature). However, taken together, our results clearly demonstrate that VTE1 is strongly induced during oxidative stress and that it is a major factor limiting tocopherol synthesis in leaves.
In leaves of plants overexpressing VTE1, tocopherol was increased by a factor of 7, and this increase could be almost entirely attributed to an increase in γ-tocopherol, whereas the amount of α-tocopherol remained very similar to wild type. As a consequence, γ-tocopherol accounted for about 80% of total leaf tocopherol. The relative increase in γ-tocopherol in VTE1 overexpressing plants might be caused by a limitation in γ-TMT activity. Similarly, overexpression of HPT1 resulted in a 4.4-fold accumulation of total tocopherol accompanied by a slight shift toward γ-tocopherol (10%–30% of total tocopherol; Collakova and DellaPenna, 2003a). This change in tocopherol composition was explained by limitation in γ-TMT activity. Indeed coexpression of HPT1 with γ-TMT in leaves resulted in the conversion of a large fraction of γ-tocopherol into α-tocopherol (Collakova and DellaPenna, 2003a).
Expression of HPT1 and HPPD in VTE1 overexpression plants was slightly induced (2- and 1.5-fold as estimated from scanning northern blots). In contrast, overexpression of HPT1 or HPPD in transgenic plants using 35S promoter resulted in a much stronger accumulation of transgenes (e.g. 20–100-fold for HPT1; Tsegaye et al., 2002; Collakova and DellaPenna, 2003a), but tocopherol accumulation was lower than in transgenic VTE1 plants. Therefore, the high increase in tocopherol in VTE1 plants can only be explained by the strong expression of VTE1 rather than by coinduction of HPT1 or HPPD in the transgenic plants (Fig. 2C). In addition, the VTE1 overexpressing lines show a totally different tocopherol composition than wild type or plants overexpressing HPT1 or HPPD, and this is further evidence supporting the view that the primary cause of tocopherol increase in VTE1 plants is overexpression of VTE1 and not of another gene involved in tocopherol synthesis. These results demonstrate that VTE1 is limiting for tocopherol biosynthesis, and that the upstream enzymes are capable of providing sufficient precursor, DMPQ, as substrate for the VTE1 reaction in VTE1 overexpressing plants.
Interestingly, the pools of ascorbate and glutathione in tocopherol deficient lines were increased (Fig. 6). On the other hand, the pools of ascorbate and glutathione decreased in plants with high amounts of tocopherol. Ascorbate and glutathione are the two major soluble antioxidants in plant cells, and it is known that they are linked via the ascorbate-glutathione cycle (for review, see Noctor and Foyer, 1998; Smirnoff, 2000a, 2000b; Mittler, 2002). The ascorbate-glutathione cycle was implicated in the reduction of the tocopheroxyl radical to tocopherol (Fryer, 1992). In vitro experiments showed that the tocopherol mediated protection against lipid peroxidation is strongly enhanced by the presence of ascorbate and glutathione (Leung et al., 1981; Niki et al., 1984; Liebler et al., 1986; Wefers and Sies, 1988). Furthermore, ascorbate and glutathione were found to act in concert to keep tocopherol in the reduced, active state in membranes isolated from human platelets and erythrocytes (Chan et al., 1991; Constantinescu et al., 1993). Presumably, the absence of one or more of these three antioxidants in the single and double mutants leads to an increase in oxidative stress in the plant cell, and as a consequence, the amounts of the remaining antioxidants increase. On the other hand, the decrease in ascorbate and glutathione observed in VTE1 overexpression plants implies that a sensing mechanism for the different antioxidants and for the status of oxidative stress exists, resulting in the production of alternative antioxidants in mutant or transgenic plants.
The vtc1 and cad2 mutants used in this study still contain residual amounts of ascorbate and glutathione, respectively (Conklin et al., 1996; Cobbett et al., 1998). Furthermore, although vte1 represents a null allele and is totally devoid of tocopherol, DMPQ, the precursor of the tocopherol cyclase reaction, accumulates in vte1 (Porfirova et al., 2002), and recent studies indicated that this hydroquinol lipid might partially compensate for tocopherol deficiency in germinating seedlings (Sattler et al., 2004). However, no data on the activity of DMPQ are available for leaves, and the fact that all higher plants produce tocopherol instead of DMPQ suggests that conversion of DMPQ to tocopherol by VTE1 has physiological significance. Furthermore, the electron carrier of photosynthesis, plastoquinone, which is abundant in chloroplasts of leaves, contains the same head group as DMPQ. Therefore, plastoquinone could also serve as antioxdant and replace tocopherol or DMPQ in tocopherol mutants.
Chlorophyll content was slightly decreased in all mutant lines (Fig. 8A), supposedly as a result of accumulation of reactive oxygen species. Nevertheless, photosynthetic efficiency of PSII was not affected in vte1, vtc1, vte1vtc1, and cad2, but was affected in the vte1cad2 double mutant (Fig. 7, A and B). Furthermore, the pool sizes of carotenoids (neoxanthin, lutein, β-carotene, and xanthophyll cycle components) were decreased in vte1cad2 (Fig. 8B). This result implies a concerted reduction of chlorophyll and all carotenoids, i.e. reduction of entire photosynthetic units. The fact that growth, chlorophyll, and carotenoid content as well as photosynthetic ΦPSII are reduced in vte1cad2 as compared to the parental lines suggests that the presence of at least one of the two antioxidants, tocopherol or glutathione, is required to sustain normal photosynthetic efficiency. Previously, it was suggested that oxidized tocopherol is reduced via the ascorbate-glutathione cycle (Leung et al., 1981; Niki et al., 1984; Liebler et al., 1986; Wefers and Sies, 1988). Although the exact mechanism is unclear, the simultaneous deficiency in tocopherol and glutathione might result in an accumulation of reactive oxygen species, finally leading to a degradation of chlorophyll and a decrease in photosynthetic quantum yield. However, additional experiments are required, including the analysis of additional mutants affected in oxidative stress, to address these questions.
MATERIALS AND METHODS
Origin of Mutant Lines and Plant Growth Conditions
Plants were grown on soil at long day conditions (16 h light, 8 h dark) under a light intensity of 120 μmol m−2 s−1, 60% relative humidity, and temperatures of 20°C (day) and 18°C (night). Isolation of the tocopherol deficient vte1 mutant (Columbia [Col]-2 background) was described previously (Porfirova et al., 2002). The ascorbate mutant vtc1 (Col-0 background; Conklin et al., 1996, 1999) was obtained from the Nottingham Arabidopsis Stock Center. The glutathione deficient plant cad2-1 (Col-0; Howden et al., 1995; Cobbett et al., 1998) was provided by Dr. C. Cobbett (University of Melbourne, Australia).
Overexpression of Tocopherol Cyclase in Arabidopsis
The entire open reading frame of VTE1 was amplified from first strand cDNA (RT of leaf mRNA; Superscript II, Invitrogen, Karlsruhe, Germany) by PCR using the oligonucleotides PD211 (AGCTGGTACCTATGGAGATACGGAGCTTGATTGTTT) and PD212 (GACTTCTAGAGTTACAGACCCGGTGGCTTGAAGAAA), introducing an Asp718 and XbaI site at the 5′ and 3′ end of the PCR product, respectively. The VTE1 cDNA was first cloned into the pGEM-Teasy vector (Promega, Mannheim, Germany) and then ligated into the Asp718, XbaI sites of the binary vector pBINAR containing the strong constitutive cauliflower mosaic virus 35S promoter (Höfgen and Willmitzer, 1990).
A genomic fragment encompassing the VTE1 locus from chromosome 4 was isolated by colony hybridization of a cosmid library using the VTE1 PCR fragment as a probe. This library contains genomic DNA fragments (about 20,000 bp) from wild-type Arabidopsis (Arabidopsis thaliana) cloned into the HindIII site that is located inside a T-DNA cassette (Meyer et al., 1996). The positive clone was purified by cell plating/hybridization and characterized by HindIII restriction and Southern blotting using VTE1 as a probe.
Binary vectors containing the VTE1 gene or cDNA were transferred into Arabidopsis plants by Agrobacterium mediated infiltration (Bent et al., 1994). Transgenic plants were selected by kanamycin resistance and screened by tocopherol measurements.
Western Analysis
The open reading frame of VTE1 lacking the apparent transit peptide was ligated into the Escherichia coli expression plasmid pQE31 (Qiagen, Hilden, Germany) and used for expression of a protein with N-terminal His6 fusion as described in Porfirova et al. (2002). VTE1 was purified by nickel affinity chromatography (Qiagen, Hilden, Germany) and used to generate polyclonal anti-VTE1 antiserum in rabbits. Total protein was isolated from Arabidopsis leaves with phenol according to Cahoon et al. (1992), separated in SDS polyacrylamide gels, and blotted onto nitrocellulose membranes following standard protocols (Sambrook et al., 1989). Immunoblots were incubated with anti-VTE1 antiserum and goat anti-rabbit antibodies coupled to alkaline phosphatase. VTE1 bands were visualized with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate p-toluidine salt (Roche, Mannheim, Germany).
Northern Analysis
Total RNA was isolated from leaves and used for northern hybridization according to standard protocols (Sambrook et al., 1989). The VTE1 probe was obtained after PCR amplification of the entire VTE1 cDNA in pGEM-Teasy using the oligonucleotides PD211 and PD212 (see above). The HPT1 probe represented an approximately 3,000-bp fragment obtained after PCR amplification of genomic DNA using the primers PD255 (ATGCCGATTCCTCCCTTGTCTAAA) and PD256 (AAATTGGAGCGCATAAAAAGGCAGTA).
Isolation of Double Mutants
A double homozygous mutant vte1vtc1 was isolated from an F2 population of a cross between vte1 and vtc1 by first selecting F2 plants homozygous for vte1 using HPLC analysis of tocopherol. Subsequently, the VTC1 locus was amplified from genomic DNA of candidate plants by PCR using the primers PD323 (GATTTGATGACATAATGTCCCAGCCTT) and PD324 (TCCTTCAAGAAGTTCAGCATCACCTGT) and the PCR products sequenced. The vtc1 mutation results in a C-to-T base exchange in exon 1 of the structural gene encoding GDP-Man pyrophosphorylase (Conklin et al., 1999). One double homozygous vte1vtc1 line was identified by sequencing DNA of the progeny of two tocopherol deficient F2 plants.
The vte1 mutant was crossed to cad2-1 and tocopherol deficient lines were selected in the F2 population by HPLC analysis. A cleaved amplified polymorphic sequences marker (Konieczny and Ausubel, 1993) was developed for cad2-1 based on PCR with the primers PD290 (CCAAATGGCGTCGGGAGGATA) and PD291 (TGTAAAGCAAGACCAGCACGAAACT) and BslI restriction of the PCR product. Because of a 6-bp deletion in exon 6 of the cad2-1 locus (Cobbett et al., 1998), the mutant PCR product is lacking one BslI site. Screening of 20 F2 lines homozygous for vte1 resulted in the isolation of only one line heterozygous for cad2-1. This line was used to obtain double homozygous vte1cad2-1 plants in the F3 progeny. The non-Mendelian segregation in the F2 generation can be explained by the fact that the VTE1 and CAD2 loci are genetically linked, because they are located within a distance of only 14 cM on chromosome 4 of Arabidopsis.
Quantification of Tocopherol, Ascorbate, and Glutathione
Tocopherol was extracted from leaves in 300 μL of 1 m KCl/0.2 m H3PO4 and 1 mL diethylether. The organic phase was removed and the solvent evaporated under a stream of nitrogen gas. Tocopherols were dissolved in 100 μL hexane and quantified by fluorescence HPLC using tocol as internal standard (Thompson and Hatina, 1979).
Ascorbate and dehydroascorbate were extracted from frozen leaf material with 6% (w/v) TCA. Ascorbate was quantified by reduction of Fe3+ to Fe2+ and detection of a Fe2+ complex with 2,2′-dipyridyl as described by Kampfenkel et al. (1995). The Fe3+ to Fe2+ assay does not cross-react with free thiol groups (e.g. glutathione; data not shown) and has been successfully employed to detect ascorbate in plant material (Gatzek et al., 2002). The sum of ascorbate and dehydroascorbate was determined after reduction of dehydroascorbate with dithiothreitol and the redox state calculated as Asc/(Asc + DHA) (Kampfenkel et al., 1995).
Glutathione was extracted from frozen Arabidopsis leaves with 6% (v/v) TCA. The extract was neutralized with 1 m K2CO3. Total glutathione was determined using a cycling assay based on the reaction with 2-nitrobenzoic acid and the reduction by glutathione reductase as described by Griffith (1980). The amount of GSSG was determined after masking reduced glutathione (GSH) with 2-vinylpyridine. The redox state was calculated as the ratio of reduced (total glutathione − 2 × GSSG) to total glutathione.
Quantification of Carotenoids and Chlorophyll
Pigments were isolated from frozen leaf material with 80% acetone and analyzed by HPLC using a C18 reverse phase column according to Thayer and Björkman (1990). Peaks were identified by comparing their retention times with commercially available standards and by their UV spectra. Chlorophyll was extracted from leaves with 1 mL 80% (v/v) acetone and quantified photometrically as described by Lichtenthaler (1987).
Chlorophyll Fluorescence
Chlorophyll fluorescence of leaves was measured with a pulse amplitude modulation fluorimeter (PAM-2000, Heinz Walz, Effeltrich, Germany). A saturating light pulse was applied to dark-adapted plants and subsequently the PPFD stepwise increased to record light-response curves. The photosynthetic quantum yield of PSII was calculated from (Fm′−Ft)/Fm′ (Schreiber et al., 1986; Krause and Weis, 1991). The NPQ was obtained according to the fluorescence ratio (Fm−Fm′)/Fm′.
Acknowledgments
We thank Dr. C. Cobbett (University of Melbourne, Australia) for seeds of the Arabidopsis cad2-1 mutant. We are grateful to Dr. D. Büssis and Dr. Joachim Fisahn (MPI Golm, Germany) for help with chlorophyll fluorescence measurements. We thank Regina Wendenburg (Max Planck Institute Golm) for isolating cosmid clones of VTE1 and for northern analysis.
This work was supported in part by the Deutsche Forschungsgemeinschaft (research fellowship Por757/1–1 to S.P. and grant Do520/7–1 to P.D.).
Present address: Laboratory of Plant Biotechnology, ETH Zürich, LFW E57.1, 8092 Zürich, Switzerland.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.054908.
References
- Bent AF, Kunkel BN, Dahlbeck D, Brown KL, Schmidt R, Giraudat J, Leung J, Staskawicz BJ (1994) RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance gene. Science 265: 1856–1860 [DOI] [PubMed] [Google Scholar]
- Bergmüller E, Porfirova S, Dörmann P (2003) Characterization of an Arabidopsis mutant deficient in γ-tocopherol methyltransferase. Plant Mol Biol 52: 1181–1190 [DOI] [PubMed] [Google Scholar]
- Cahoon E, Shanklin J, Ohlrogge JB (1992) Expression of a coriander desaturase results in petroselinic acid production in transgenic tobacco. Proc Natl Acad Sci USA 89: 11184–11188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoon EB, Hall SE, Ripp KG, Ganzke TS, Hitz WD, Coughlan SJ (2003) Metabolic redesign of vitamin E biosynthesis in plants for tocotrienol production and increased antioxidant content. Nat Biotechnol 21: 1082–1087 [DOI] [PubMed] [Google Scholar]
- Chan AC, Khai T, Raynor T, Ganz PR, Chow CK (1991) Regeneration of vitamin E in human platelets. J Biol Chem 266: 17290–17295 [PubMed] [Google Scholar]
- Cheng Z, Sattler S, Maeda H, Sakuragi Y, Bryant DA, DellaPenna D (2003) Highly divergent methyltransferases catalyze a conserved reaction in tocopherol and plastoquinone synthesis in Cyanobacteria and photosynthetic eukaryotes. Plant Cell 15: 2343–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B (1998) The glutathione-deficient, cadmium-sensitive mutant, cad2-1 of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. Plant J 16: 73–78 [DOI] [PubMed] [Google Scholar]
- Collakova E, DellaPenna D (2001) Isolation and functional analysis of homogentisate phytyltransferase from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol 127: 1113–1124 [PMC free article] [PubMed] [Google Scholar]
- Collakova E, DellaPenna D (2003. a) Homogentisate phytyltransferase activity is limiting for tocopherol biosynthesis in Arabidopsis. Plant Physiol 131: 632–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collakova E, DellaPenna D (2003. b) The role of homogentisate phytyltransferase and other tocopherol pathway enzymes in the regulation of tocopherol synthesis during abiotic stress. Plant Physiol 133: 930–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL (1999) Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc Natl Acad Sci USA 96: 4198–4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Williams EH, Last RL (1996) Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA 93: 9970–9974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu A, Han D, Packer L (1993) Vitamin E recycling in human erythrocyte membranes. J Biol Chem 268: 10906–10913 [PubMed] [Google Scholar]
- Fryer MJ (1992) The antioxidant effects of thylakoid vitamin E (α-tocopherol). Plant Cell Environ 15: 381–392 [Google Scholar]
- Gatzek S, Wheeler GL, Smirnoff N (2002) Antisense suppression of L-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated L-galactose synthesis. Plant J 30: 541–553 [DOI] [PubMed] [Google Scholar]
- Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106: 207–212 [DOI] [PubMed] [Google Scholar]
- Höfgen R, Willmitzer L (1990) Biochemical and genetic analysis of different patatin isoforms expressed in various organs of potato (Solanum tuberosum). Plant Sci 66: 221–230 [Google Scholar]
- Hofius D, Hajirezaei M-R, Geiger M, Tschiersch H, Melzer M, Sonnewald U (2004) RNAi-mediated tocopherol deficiency impairs photoassimilate export in transgenic potato plants. Plant Physiol 135: 1256–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Andersen CR, Coldsbrough PB, Cobbett CS (1995) A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol 107: 1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kago T, Terao J (1995) Phospholipids increase radical scavenging activity of vitamin E in a bulk oil model system. J Agric Food Chem 43: 1450–1454 [Google Scholar]
- Kamal-Eldin A, Appelqvist LA (1996) The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31: 671–701 [DOI] [PubMed] [Google Scholar]
- Kampfenkel K, van Montagu M, Inzé D (1995) Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 225: 165–167 [DOI] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype specific PCR-based markers. Plant J 4: 403–410 [DOI] [PubMed] [Google Scholar]
- Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42: 313–349 [Google Scholar]
- Leung H, Vang MJ, Mavis RD (1981) The co-operative interaction between vitamin E and vitamin C in suppression of peroxidation of membrane phospholipids. Biochim Biophys Acta 664: 266–272 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler H, Prenzel U, Douce R, Joyard J (1981) Localization of prenylquinones in the envelope of spinach chloroplasts. Biochim Biophys Acta 641: 99–105 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148: 350–382 [Google Scholar]
- Liebler DC, Kling DS, Reed DJ (1986) Antioxidant protection of phospholipid bilayers by α-tocopherol. Control of α-tocopherol status and lipid peroxidation by ascorbic acid and glutathione. J Biol Chem 261: 12114–12119 [PubMed] [Google Scholar]
- Meyer K, Benning G, Grill E (1996) Cloning of plant genes based on the genetic map position. In AH Patterns, ed, Genome Mapping in Plants. Academic Press, New York, pp 137–154
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Müller-Moulé P, Conklin PL, Niyogi KK (2002) Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol 128: 970–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L (2002) The function of tocopherols and tocotrienols in plants. CRC Crit Rev Plant Sci 21: 31–57 [Google Scholar]
- Niki E, Saito T, Kawakami A, Kamiya Y (1984) Inhibition of oxidation of methyl linoleate in solution by vitamin E and vitamin C. J Biol Chem 259: 4177–4182 [PubMed] [Google Scholar]
- Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49: 249–279 [DOI] [PubMed] [Google Scholar]
- Norris SR, Shen X, DellaPenna D (1998) Complementation of the Arabidopsis pds1 mutation with the gene encoding p-hydroxyphenylpyruvate dioxygenase. Plant Physiol 117: 1317–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porfirova S, Bergmüller E, Tropf S, Lemke R, Dörmann P (2002) Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc Natl Acad Sci USA 99: 12495–12500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher LM, Miao L, Sinha N, Lucas WJ (2001) Sucrose export defective1 encodes a novel protein implicated in chloroplast-to-nucleus signaling. Plant Cell 13: 1127–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippert P, Scimemi C, Dubald M, Matringe M (2004) Engineering plant shikimate pathway for production of tocotrienol and improving herbicide resistance. Plant Physiol 134: 92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russin WA, Evert RF, Vanderveer PJ, Sharkey TD, Briggs SP (1996) Modification of a specific class of plasmodesmata and loss of sucrose export ability in the sucrose export defective1 maize mutant. Plant Cell 8: 645–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sattler SE, Cahoon EB, Coughlan SJ, DellaPenna D (2003) Characterization of tocopherol cyclases from higher plants and Cyanobacteria. Evolutionary implications for tocopherol synthesis and function. Plant Physiol 132: 2184–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D (2004) Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16: 1419–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidge B, Weiss JD, Wong Y-HH, Lassner MW, Mitsky TA, Shewmaker CK, Post-Beittenmiller D, Valentin HE (2002) Isolation and characterization of homogentisate phytyltransferase genes from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol 129: 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and nonphotochemical quenching with a new type of modulation fluorometer. Photosynth Res 10: 51–62 [DOI] [PubMed] [Google Scholar]
- Shintani D, DellaPenna D (1998) Elevating the vitamin E content of plants through metabolic engineering. Science 282: 2098–2100 [DOI] [PubMed] [Google Scholar]
- Smirnoff N (2000. a) Ascorbic acid: metabolism and functions of a multi-facetted molecule. Curr Opin Plant Biol 3: 229–235 [PubMed] [Google Scholar]
- Smirnoff N (2000. b) Ascorbate biosynthesis and function in photoprotection. Philos Trans R Soc Lond B Biol Sci 355: 970–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J, Schultz G, Joyard J, Douce R, Block MA (1985) Localization and synthesis of prenylquinones in isolated outer and inner envelope membranes from spinach chloroplasts. Arch Biochem Biophys 238: 290–299 [DOI] [PubMed] [Google Scholar]
- Thayer SS, Björkman O (1990) Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res 23: 331–343 [DOI] [PubMed] [Google Scholar]
- Thompson JN, Hatina G (1979) Determination of tocopherols and tocotrienols in foods and tissues by high performance lipid chromatography. J Liq Chromatogr 2: 327–344 [Google Scholar]
- Tsegaye Y, Shintani DK, DellaPenna D (2002) Overexpression of the enzyme p-hydroxyphenolpyruvate dioxygenase in Arabidopsis and its relation to tocopherol biosynthesis. Plant Physiol Biochem 40: 913–920 [Google Scholar]
- van Eenennaam AL, Lincoln K, Durrett TP, Valentin HE, Shewmaker CK, Thorne GM, Jiang J, Baszis SR, Levering CK, Aasen ED, et al (2003) Engineering vitamin E content: from Arabidopsis mutant to soy oil. Plant Cell 15: 3007–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH (2001) Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol 127: 426–435 [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, Reichheld J-P, Muroy S, Brown S, Maughan SC, Cobbett CS, van Montagu M, Inzé D, et al (2000) The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefers H, Sies H (1988) The protection of ascorbate and glutathione against microsomal lipid peroxidation is dependent on vitamin E. Eur J Biochem 174: 353–357 [DOI] [PubMed] [Google Scholar]
- Xiang C, Werner BL, Christensen EM, Liver DJ (2001) The biological functions of glutathione revisited in Arabidopsis transgenic plants with altered glutathione levels. Plant Physiol 126: 564–574 [DOI] [PMC free article] [PubMed] [Google Scholar]