Abstract
Context
Activity-based therapy initiated within days of the accident could prevent complications and improve neurofunctional outcomes in patients with traumatic spinal cord injury (TSCI). However, it has never been attempted in humans with TSCI because of practical obstacles and potential safety concerns. The PROMPT-SCI trial is the first attempt at implementing ABT within the first days following a TSCI (i.e. very early ABT; VE-ABT). The objective is to determine if VE-ABT can be initiated safely in the intensive care unit (ICU) within 48 h of early decompressive surgery.
Design
As part of the PROMPT-SCI trial, 15 adult patients with severe TSCI were enrolled between April and November of 2021. The intervention consisted of 30-minute sessions of motor-assisted in-bed leg cycling starting within 48 h of early spinal surgery. Safety was assessed through continuous monitoring of vital signs and recording of adverse events during and after sessions. The main outcome measure was the achievement (yes or no) of a full and safe session within 48 h of early surgery.
Findings
Out of the 15 participants, 10 (66.6%) achieved this outcome. Out of the remaining 5, 2 were not cleared to engage in cycling within 48 h of surgery and 3 initiated cycling within 48 h but stopped prematurely. All 5 eventually completed a full and safe session within the next 1–2 days. In all 15 participants, there were no neurological deteriorations after the first completed session.
Conclusion
Our results suggest that it is safe and feasible to perform a first session of VE-ABT within days of a severe TSCI with no serious adverse events and excellent completion rates.
Keywords: Spinal cord injury, Traumatology, Early mobilization, Clinical trial, In-bed exercise
Introduction
In-bed exercise can prevent immobility-associated complications and promote faster and improved recovery in critically ill patients.1,2 Specifically for patients with traumatic spinal cord injury (TSCI), pre-clinical evidence strongly suggests that early activity-based therapy (E-ABT) could also enhance neurological recovery.3 In particular, studies indicate that E-ABT could be particularly effective when initiated within days of the initial insult to the spinal cord.3 Unfortunately, despite these potentially great benefits, “very early” activity-based therapy (VE-ABT) – i.e. ABT started within days of the injury – has never been attempted in humans before, with the earliest attempt reported only around 3 weeks following the TSCI by Everaert et al.4,5
There are two main plausible explanations behind this including: (a) the existence of numerous practical obstacles to performing ABT in the intensive care unit after major trauma and surgery and (b) possible doubts regarding the neurological safety of early mobilization after a TSCI. Indeed, one particular animal study by Smith et al. reported neurological deteriorations in rats that were exposed to swimming 3 days after a TSCI. However, it has since been suggested that these deteriorations were caused by the excessive physiological demands incurred by swimming (as opposed to lower intensity exercises like passive cycling).6,7
In this context, our team has designed the first trial of VE-ABT in acute TSCI individuals. This preliminary analysis aims to determine if ABT can be initiated safely within 48 h of early decompressive surgery with a particular focus on hemodynamic and neurological aspects.
Methods
This research was performed within the framework of the Protocol for Rapid Onset of Mobilization in Patients with Traumatic SCI (PROMPT-SCI) study, an ongoing single-arm pilot trial aimed primarily at determining the safety of performing VE-ABT during the acute phase of hospitalization in patients with TSCI (NCT04699474). This study was approved by the Comité d’Éthique de la recherche du CIUSSS du Nord-de-L’Île de Montréal (“Mobilisation précoce suite à une lésion médullaire”, study #2020-1901, approved on March 12, 2020) and was conducted according to the principles of the Declaration of Helsinki. The full protocol is published elsewhere, but the inclusion process and the intervention are briefly summarized below.8
As part of the PROMPT-SCI trial, all patients who presented with a severe TSCI (i.e. American Spinal Injury Association Impairment Scale (AIS) grade A, B or C) at our Level-1 trauma center in Montreal, Canada, between April and November of 2021 were screened for eligibility. Patients were only included if they sustained a blunt trauma injury and if they were operated within 48 h of the injury (Table 1A). Potential participants were then excluded mainly if they presented a condition that could limit their ability to safely engage in cycling (e.g. lower limb injury, active cardiac event). Other minor exclusion criteria are described in detail in Table 1B and in the full study protocol.8 All participants provided informed written consent.
Table 1.
Inclusion, exclusion, daily exemption and stopping criteria.
| A. Inclusion criteria | B. Exclusion criteria | ||
|---|---|---|---|
| Adult aged 18 years or older | Severe SCI with AIS grade A, B or C | Condition limiting patient’s ability to engage into cycling (e.g. pelvis or lower extremity injury or deformity, body mass index 35 kg/m2) | Inability to walk independently prior to SCI |
| Blunt (non-penetrating) traumatic SCI | Medical condition that might endanger patients if submitted to cycling | Pre-existing neurological disorder | |
| Neurological level of injury from C0 to L2 | Moderate or severe brain injury | Complete spinal cord transection confirmed by MRI and/or surgery | |
| Spine surgery performed within 48 h of SCI | Unwilling or unable to comply with scheduled follow-up visits | ||
| C. Daily exemption criteria | D. Stopping criteria | ||
| Caring team determines that patient is haemodynamically or medically unstable | Pressure ulcer at sacrum, buttock or heel area grade 2* | Sustained or symptomatic heart rate <40 or >140 bpm | Sustained SpO2 < 90% |
| Resting heart rate <40 or >140 bpm | Severe agitation† | New arrhythmia | Clinical signs of cardiorespiratory distress |
| Unstable or uncontrolled arrhythmia | Uncontrolled pain | Concern for coronary ischaemia (eg, chest pain, changes on ECG) | Severe agitation† |
| Active coronary ischaemia | Caring team considers that in-bed cycling is not appropriate for a condition other than above criteria (eg, active bleeding, incision or wound precluding cycling, risk of compartment syndrome, etc) | Sustained or symptomatic mean arterial pressure <60 or >110 mm Hg | Termination of in-bed cycling session requested by patient or caring team |
| Mean arterial pressure <60 or >110 mm Hg | Patient refuses in-bed cycling | Unplanned extubation or endotracheal tube dislodgment | |
| SpO2 < 90% | Sustained or symptomatic heart rate <40 or >140 bpm | ||
The intervention consisted of daily 30-min sessions of continuous in-bed leg cycling for 14 consecutive days, starting within 48 h of spinal surgery, as soon as patients were deemed medically/hemodynamically stable. Prior to every session, participants were screened to assess their fitness to engage in cycling and ensure that there was no medical contraindication to cycling (Table 1C).
During sessions, patients engaged either in passive, active-assisted or active cycling depending on their motor ability and tolerance. The level of motor assistance from the ergometer (APT-5, Tzora Active Systems, Ohio, USA) was progressively increased to achieve a target cadence of 40 RPM. During cycling, mean arterial pressure (MAP), heart rate (HR), respiratory rate (RR) and blood oxygen saturation (SpO2) were closely monitored. Adverse events were systematically noted and reported to the principal investigator. Sessions were stopped if patients or their caring team requested termination, if vital signs fluctuated outside of the following ranges in a sustained fashion: MAP: 60–110 mmHg; HR: 40–140 bpm; SpO2: ≥ 90%, or if there were signs of medical instability (Table 1D). After each session, the neurological status of patients was assessed to ensure neurological safety. All neurological exams were conducted in accordance with the International Standards for the Neurological Classification of Spinal Cord Injuries (ISNCSCI).9 Post-session neurological deteriorations were ruled out based on patients’ motor score, neurological level of injury and AIS grade.
In addition to the intervention, patients received standard care, which includes maintenance of MAP ≥ 85 mmHg for 1 week with vasopressors if needed, physiotherapy 6 times/week (focusing on passive range of motion exercises, resistance training and balance training), occupational therapy 2–3 times/week and other paramedical interventions as needed.10
Results
Between April and November of 2021, 90 patients were admitted to our institution for a TSCI. Of these, 50 were AIS grade D of the remaining 40 patients, 25 did not meet the criteria for enrollment. The most frequent reasons for exclusion were: surgery ≥ 48 h of the injury (n = 7), medical contraindication to cycling according to the intensive care physician and/or surgeon (n = 8) and fracture/injury to the lower limbs (n = 4). In total, 15 participants remained eligible to the project. After being presented with the details of the research project, all 15 provided their consent (100% consent rate). Demographics and injury characteristics are described in Table 2.
Table 2.
Participant characteristics and cycling outcomes.
| Participant # | Sex | Age | AISa grade | NLIb | Timing of surgeryc(h) | 1st session attempted within … h of surgery | 1st completed session within … h of surgery | Comments |
|---|---|---|---|---|---|---|---|---|
| P1 | M | 42 | A | T11 | 5.8 | 48 | 48 | |
| P2 | M | 48 | B | C4 | 10.6 | 24 | 24 | |
| P3 | M | 77 | B | C5 | 11.3 | 24 | 48 | S24d reached stopping criterion: prolonged hypertension |
| P4 | M | 29 | B | C4 | 12.4 | 48 | 48 | |
| P5 | M | 74 | A | C5 | 10.5 | 48 | 48 | |
| P6 | M | 67 | A | L1 | 12.6 | 24 | 24 | |
| P7 | M | 70 | C | C6 | 22.8 | 48 | 72 | S48 not attempted due to medical contraindication |
| P8 | M | 30 | A | T10 | 9.0 | 48 | 48 | |
| P9 | M | 19 | C | C7 | 15.9 | 48 | 72 | S48 reached stopping criterion: SpO2 decrease |
| P10 | F | 42 | A | C4 | 10.2 | 48 | 72 | S48 not attempted due to medical contraindication |
| P11 | F | 24 | A | T2 | 15.4 | 48 | 96 | S48 & 72 reached stopping criterion: persisting abdominal pain |
| P12 | M | 69 | A | T6 | 11.1 | 48 | 48 | |
| P13 | M | 50 | C | C4 | 26.0 | 48 | 48 | |
| P14 | M | 73 | A | T9 | 12.2 | 24 | 72 | S24 & 48 reached stopping criterion: persisting abdominal pain |
| P15 | M | 71 | A | C4 | 35.2 | 24 | 24 |
a: American Spinal Injury Association Impairment Scale (Kirshblum et al.); b: Neurological Level of Injury (as defined by the ISNCSCI) (Kirshblum et al.); c: Number of hours from initial accident to incision; d: S24, “Session within 24 h of surgery”, etc.
The first full and safe sessions were all conducted in passive cycling, except for P6 (with conus medullaris syndrome and functional antigravity motor function in lower extremities) who engaged in active cycling. In all 15 participants, there were no neurological deteriorations after the first full session of cycling. Ten participants (66.6%) completed their first full 30-min session of cycling within 48 h of surgery with no serious adverse events. Out of the 5 remaining participants, 2 were not cleared to initiate cycling within 48 h of surgery because of medical contraindications (P7 – investigated for acute coronary syndrome; P10 – investigated for lung-associated injury). Both were eventually cleared and performed full sessions within 72 h of surgery without any adverse event. The 3 remaining participants initiated a session within 48 h of surgery, but it was interrupted prematurely due to stopping criteria (see Table 1). More specifically, P9’s session was terminated due to a progressive SpO2 decrease (from 98% prior to session to 83% at 10 min of cycling despite appropriate oxygen supplementation), while P11 and P14 requested premature termination due to pre-existing abdominal pain that limited their ability to sustain cycling. All three performed full and safe sessions shortly after, respectively within 72, 96 and 72 h of surgery. It is also important to point out that P3 performed a first attempt at cycling within 24 h of surgery and a stopping criterion was reached (sustained MAP > 110 mmHg), but a safe and full session was completed within the first 48 h after surgery. These central findings are also summarized in Table 2.
In these 15 patients, 4 (P1, P3, P6 and P13) had a single event of transient increase in MAP that slightly met or exceeded our threshold of 110 mmHg (111; 111; 110; 112; mm Hg), but none of these increases were sustained and MAP quickly normalized in all 4 patients with no specific medical intervention.
Finally, we report no major variation in average HR, or RR during sessions that reached the stopping criteria.
Discussion and conclusion
This study is the first ever to report on the safety of initiating VE-ABT within days following a TSCI. Prior to the PROMPT-SCI trial, acute care clinicians were reluctant to introduce VE-ABT in the management of TSCI patients mainly because of doubts regarding its neurological safety. With the absence of neurological deterioration and/or serious adverse event with the initiation of VE-ABT, our results suggest that passive cycling can be performed safely as soon as 24 h after early decompressive surgery.
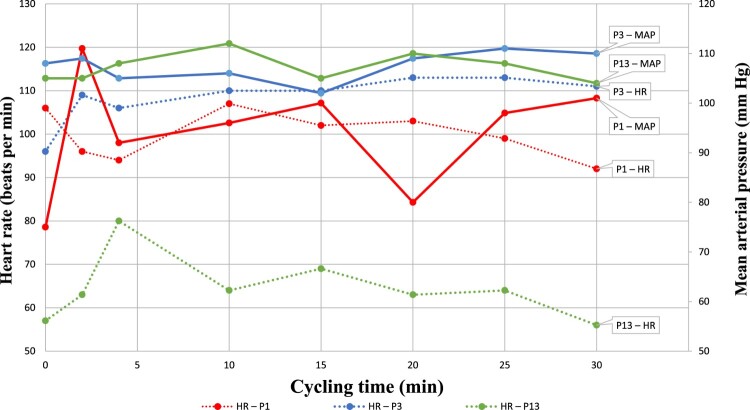
First of all, our results suggest that starting low-intensity, passive VE-ABT within 48 h of early surgery is safe and feasible, as long as close hemodynamic and respiratory monitoring is ensured during sessions, and stopping criteria described in the protocol are strictly enforced.8 In particular, the occurrence of transient – and self-resolved – increases in MAP > 110 mmHg in 3 patients who engaged in passive cycling (P1, P3 and P13) highlights the need to carefully monitor blood pressure during sessions even if it is a low-intensity exercise. Indeed, ensuring MAP remains controlled is important to minimize the risks of end-organ damage and spinal cord hyperperfusion, which could theoretically exacerbate the secondary injury to the spinal cord by causing extravasation of macromolecules and altering molecular cascades.2,7 Indeed, passive cycling may result in increased sympathetic drive (as the trend lines from P1 and P3 may suggest, with strong elevations in HR accompanying rising MAPs – Fig. 1), or increased venous return due to the elevation/mobilization of the lower limbs, which are a known site of fluid pooling in TSCI individuals (as was most probably the case in P13, where a marked increase in MAP was recorded, without a concomitant rise in HR – Fig. 1).11 Finally, it is important to remain aware that for patients with severe SCI above T7, large MAP variations can be due to severe dysautonomia or early autonomic dysreflexia.12
Figure 1.
HR and MAP variations in patients reaching MAP ≥ 110 mmHg during cycling sessions. The pattern in variation of HR (dotted lines) and MAP (solid lines) during cycling sessions suggest 2 potential mechanisms of increased blood pressure. The trends for P1 and P3 show reciprocal increases in HR and MAP, which points towards increased sympathetic drive as the main cause for MAP elevation. This contrasts with the trends for P13 which only show an increase in MAP (and not HR), which is more characteristic of increased venous return.
Since one patient required premature termination of their first session due to prolonged desaturation (P9) our results also stress the importance of close respiratory monitoring during sessions even if no serious aetiologies (such as pulmonary embolism) were found to be causative upon subsequent chart review. In this case, since the patient had received opiates, it was determined by the medical care team of the ICU that this desaturation was probably caused by central hypoventilation, perhaps also exacerbated by peripheral hypoventilation due to upper airway atelectasis in a context of drowsiness (patient was −1 on Richmond Agitation and Sedation Scale) and the semi-recumbent position adopted to engage in cycling.
Importantly, 13/15 included participants met all the daily criteria initiate to a session within 48 h of surgery and the 2 individuals that were kept from starting cycling within this timeframe due to medical precautions were delayed for only 24 h. This result is crucially important as it strongly supports the feasibility of VE-ABT in TSCI individuals, even in patients with severe injuries and major polytrauma.
There are several limitations to this study. First, the authors acknowledge that the presented results only support the feasibility of initiating early in-bed leg cycling within days of the TSCI and cannot be extrapolated to the complete 14-day cycling protocol planned in the PROMPT-SCI trial. Further results on the complete 14-day protocol will eventually be published in subsequent papers. Second, although none of our patients reported to feel pain as a result of cycling (only 2 out of 15 participants complained of pre-existing abdominal pain that persisted during sessions) it must be acknowledged that the intervention could theoretically have generated nociceptive stimuli under the level of the injury, which would not have been perceived by our participants. However, the cycling setup was designed to prevent any movement outside of comfortable and physiological ranges with adapted crank length, bed-head angulation at 30°, immobilization of the trunk, adequate leg supports and relatively low cycling cadence (40 RPM). In addition, any patient presenting injuries to the lower limbs (starting at the pelvis) was excluded in order to minimize the risk of pain under the level of injury. Finally, it is important to highlight that although the objective of the intervention was to serve as a form of ABT – i.e. a form of rhythmic mobilization achieving neuromuscular activation below the level of the injury – the effect of cycling on the neuromuscular system was not examined in this preliminary study.13 As a result, we cannot certify without a doubt that the intervention performed was, in fact, true ABT. However, at this stage of the PROMPT-SCI trial, we can reveal that preliminary analyses of electromyography and plantar pressure recordings obtained during passive cycling sessions showed patterns of cyclical stimulation of the plantar soles and rhythmic muscle contractions in the thigh and leg muscles in several participants. These results are currently being prepared for submission in another manuscript. However, irrespective of whether the intervention did represent true ABT or not, the fact that it was possible to perform cycling sessions safely this early after the accident – which can be viewed as a form of preparatory activity to later ABT or as a way to prevent immobilization-associated complication – remains a major result in and of itself.
In conclusion, these preliminary results from the PROMPT-SCI trial suggest that VE-ABT is as safe in severe TSCI individuals as it is in other populations of ICU patients. However, we recommend close monitoring during sessions and active titration of vasoactive agents to ensure hemodynamic stability. In supporting the feasibility and safety of VE-ABT after TSCI, these findings may stimulate more research aiming to facilitate the clinical translation of pre-clinical evidence while also paving the way for a more generalized use of VE-ABT principles in the standard of care.
Disclaimer statements
Contributors None.
Funding This research was funded by the Craig H. Neilsen Foundation [grant #649997].
Conflicts of interest Authors have no conflict of interests to declare.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- 1.Zhang L, Hu W, Cai Z, Liusd J, Wu J, Deng Y, et al. Early mobilization of critically ill patients in the intensive care unit: a systematic review and meta-analysis. PLoS One 2019;14(10):e0223185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medrinal C, Combret Y, Prieur G, Robledo Quesada A, Bonnevie T, Gravier FE, et al. Comparison of exercise intensity during four early rehabilitation techniques in sedated and ventilated patients in ICU: a randomised cross-over trial. Crit Care 2018;22(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown AK, Woller SA, Moreno G, Grau JW, Hook MA.. Exercise therapy and recovery after SCI: evidence that shows early intervention improves recovery of function. Spinal Cord 2011;49(5):623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everaert DG, Okuma Y, Abdollah V, Ho C.. Timing and dosage of FES cycling early after acute spinal cord injury: a case series report. J Spinal Cord Med 2021;44(sup1):S250–S2S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galea MP, Panisset MG, El-Ansary D, Dunlop SA, Marshall R, Clark JM, et al. SCIPA switch-on: a randomized controlled trial investigating the efficacy and safety of functional electrical stimulation-assisted cycling and passive cycling initiated early after traumatic spinal cord injury. Neurorehabil Neural Repair 2017;31(6):540–551. [DOI] [PubMed] [Google Scholar]
- 6.DeVeau KM, Harman KA, Squair JW, Krassioukov AV, Magnuson DSK, West CR.. A comparison of passive hindlimb cycling and active upper-limb exercise provides new insights into systolic dysfunction after spinal cord injury. Am J Physiol Heart Circ Physiol 2017;313(5):H861–HH70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith RR, Brown EH, Shum-Siu A, Whelan A, Burke DA, Benton RL, et al. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J Neurotrauma 2009;26(7):1017–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mac-Thiong JMR-DA, Petit Y, Bernard F, Barthélémy D, Dionne A, Magnuson D.. Protocol for rapid onset of mobilization in patients with traumatic spinal cord injury (PROMPT-SCI) study: a single-arm proof-of-concept trial of early in-bed leg cycling following acute traumatic spinal cord injury. Brit Med J Open 2021;11(11):e049884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirshblum S, Snider B, Rupp R, Read MS.. International standards committee of A, IscoS. updates of the international standards for neurologic classification of spinal cord injury: 2015 and 2019. Phys Med Rehabil Clin N Am 2020;31(3):319–330. [DOI] [PubMed] [Google Scholar]
- 10.Hawryluk G, Whetstone W, Saigal R, Ferguson A, Talbott J, Bresnahan J, et al. Mean arterial blood pressure correlates with neurological recovery after human spinal cord injury: analysis of high frequency physiologic data. J Neurotrauma 2015;32(24):1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Partida E, Mironets E, Hou S, Tom VJ.. Cardiovascular dysfunction following spinal cord injury. Neural Regen Res 2016;11(2):189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver JR. Early autonomic dysreflexia. Spinal Cord 2000;38(4):229–233. [DOI] [PubMed] [Google Scholar]
- 13.Behrman AL, Ardolino EM, Harkema SJ.. Activity-Based therapy: from basic science to clinical application for recovery after spinal cord injury. J Neurol Phys Ther 2017;41(Suppl 3 IV STEP Spec Iss):S39–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]