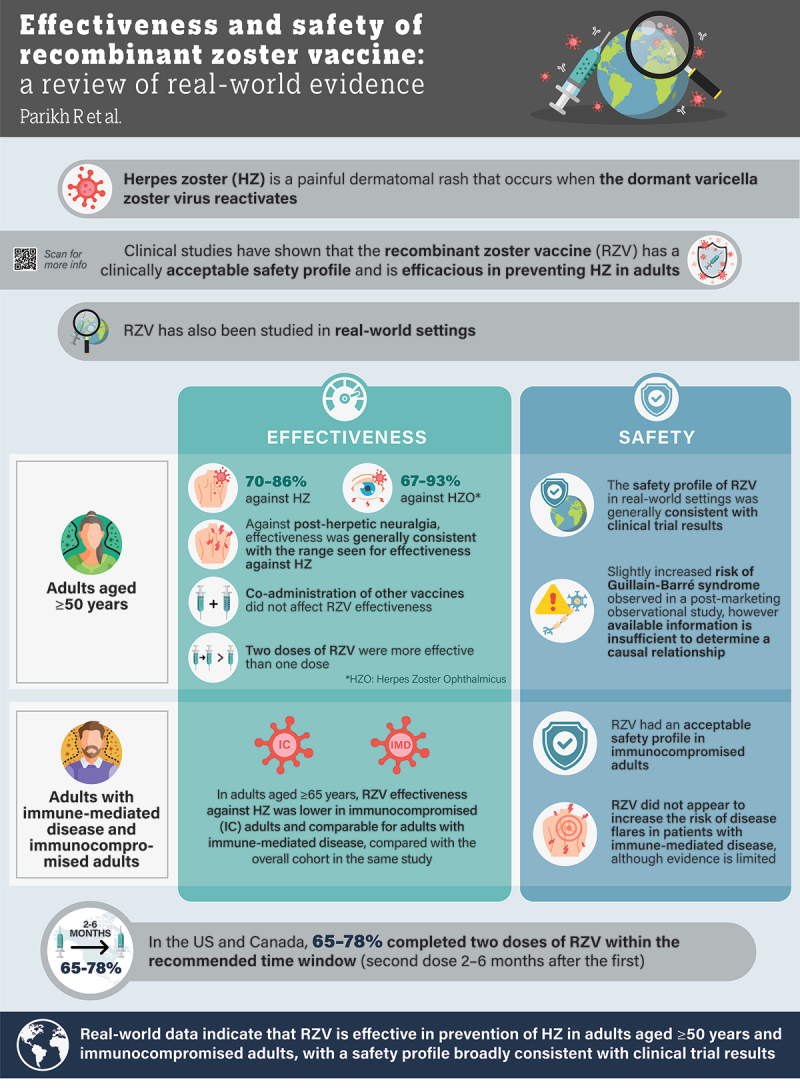
ABSTRACT
The recombinant zoster vaccine (RZV) was licensed in the US for prevention of herpes zoster (HZ) in 2017. We conducted a literature search (January 1, 2017–August 1, 2023) using PubMed, Embase, and Scopus to consolidate the real-world evidence related to RZV. Overall, RZV effectiveness against HZ was high across the studied populations in real-world settings, including adults aged ≥ 50 years and patients aged ≥ 18 years with immunodeficiency or immunosuppression. Effectiveness was higher with two doses versus one dose, especially in elderly people and immunocompromised individuals. The safety profile of RZV was broadly consistent with that established in clinical trials. RZV does not appear to increase the risk of disease flares in patients with immune-mediated diseases. Approximately two-thirds of individuals received a second RZV dose within 2–6 months after the first dose. Collectively, RZV effectiveness against HZ was high, and these real-world studies reaffirm its favorable benefit–risk profile.
KEYWORDS: Autoimmune disease, completion rate, effectiveness, herpes zoster, immunodeficiency, immunosuppression, real-world evidence, recombinant zoster vaccine, post-herpetic neuralgia, safety
Plain Language Summary
What is the context?
Herpes zoster is a common and painful rash that develops following reactivation of latent (meaning silent or dormant) varicella zoster virus, which is the virus that causes the common childhood illness chickenpox. The recombinant zoster vaccine (RZV) was first approved for the prevention of herpes zoster in the USA and Canada in 2017 and has since been approved in the European Union and various other countries. The approval was based on the results of large clinical trials. Since its launch over 5 years ago, evidence for RZV use in real-world settings has been collected; the benefits of real-world studies include large sample sizes, more diverse populations, and the ability to identify rare side effects.
What is new?
We provide a review of real-world studies, which have shown that RZV is effective across the studied populations, including in adults aged 50 years and above and in patients with immunodeficiencies (i.e., those who have a decreased ability to fight infections or other diseases) or receiving immunosuppressive therapies (treatments that lower the activity of the body’s immune system). The safety profile of RZV in real-world studies was generally consistent with that seen in clinical trials.
What is the impact?
These studies show the effectiveness and well-tolerated safety profile of RZV in real-world settings.
GRAPHICAL ABSTRACT
Introduction
Herpes zoster (HZ) is a common, painful, blistering dermatomal rash that arises following reactivation of latent varicella zoster virus (VZV), primarily in people aged ≥ 50 years1,2 and in immunocompromised individuals.3–5 HZ, with or without its attendant complications, places a substantial burden on patients and healthcare systems.1,2,6
The adjuvanted recombinant zoster vaccine (RZV; Shingrix®, GSK) was first approved in 2017, in Canada and the US, and then in subsequent years in other countries, including the European Union in March 2018.6–8 Initially, RZV was indicated for HZ prevention in adults aged ≥ 50 years, based on phase III clinical trials (ZOE-50 and ZOE-70) in patients aged ≥ 50 years and ≥ 70 years, respectively.9,10 These studies demonstrated high vaccine efficacy rates (97.2% [95% confidence interval (CI): 93.7–99.0] in adults aged ≥ 50 years [mean follow-up 3.2 years] and 91.3% [95% CI: 86.8–94.5] in adults aged ≥ 70 years [mean follow-up 3.7 years]), with mild-to-moderate, transient, injection-site and systemic reactions commonly reported.9,10 Supported by data from several studies in immunocompromised patients,11–15 the indication was expanded in the European Union in August 2020 to include adults aged ≥ 18 years at increased risk of HZ, and in the US in July 2021 to include adults aged ≥ 18 years at increased risk of HZ because of immunodeficiency or immunosuppression caused by known disease or therapy.7,8,16
Collection and analysis of real-world evidence is critical in understanding vaccine effectiveness and safety; indeed, a key advantage of real-world studies is the heterogeneity of the populations involved (i.e., there are few exclusions compared with clinical trials). Further, in several real-world studies, the large sample sizes have enabled the identification of rare adverse events (AEs) and the detection of events to an extent that would not be possible in clinical trials.2,17 Following the initial approval of RZV, several studies reporting real-world evidence for RZV were published. However, a comprehensive review of these studies is required. The principal aims of this review were, therefore, to identify and summarize studies reporting real-world evidence on the effectiveness and safety of RZV across various adult populations (including, but not limited to, adults aged ≥ 50 years, adults who received prior zoster vaccine live [ZVL] vaccination, and adults aged ≥ 18 years with immunodeficiency or immunosuppression caused by known disease or therapy) and studies investigating completion rates for the recommended two-dose administration regimen of RZV. Due to the broad scope of this review and the resulting heterogeneity in the methodology of the real-world studies, a meta-analysis was not conducted. In parallel, a separate literature review was conducted summarizing the clinical studies of RZV (available at: doi).
Methods
We conducted a targeted literature search of articles published between January 1, 2017, and August 1, 2023, using the Embase, Scopus, and National Library of Medicine PubMed databases. Search terms comprised “recombinant zoster vaccine” and terms related to real-world evidence or other topics of focus for this review (“real world evidence,” “post surveillance,” “licensure,” “observational study,” “cohort,” “case control study,” “case report,” “claims,” “health records,” “experience,” “series completion,” and “data mining”). Case reports were subsequently excluded, as in all instances the identified publications described three or fewer patients. The studies identified through this literature search were manually reviewed for relevance: the abstracts of identified studies were read in detail and additional papers selected from reference lists of the initially identified papers. Studies were included if they examined the safety or effectiveness of RZV against HZ, post-herpetic neuralgia (PHN), or HZ ophthalmicus (HZO) or of RZV series completion in a real-world setting in adult populations of any age in any country; distinct clinical trials were excluded, as were papers without an English language abstract.
Effectiveness of RZV
In total, seven primary studies18–24 that examined the real-world effectiveness of RZV in various populations (Table 1) and a meta-analysis25 of two of these studies18,19 were identified. Six studies identified HZ based on diagnosis codes from the International Classification of Diseases, ninth or tenth version (ICD-9 or ICD-10),18–23 and six studies reported the median duration of follow-up, which was approximately 3–24 months in vaccinated groups.18–23 As the initial indication in the US for RZV was in adults aged ≥ 50 years, which was followed by a recommendation from the Advisory Committee on Immunization Practices in 2017,7,8,16,26 most studies (n = 6)18–23 focused on RZV use in this age group; however, one study focused on RZV use in adults aged ≥ 65 years.
Table 1.
Overview of real-world evidence investigating the effectiveness of the recombinant zoster vaccinea.
| HZ incidence per 100,000 person-years (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|
| Publication | Study type/period | Median (IQR) follow-up, months | Population | Sample size | Unvaccinated | Vaccinated | Adjusted or estimated vaccine effectiveness,b % (95% CI)c |
| Patients aged ≥ 50 years | |||||||
| Sun et al. Vaccine 202120 | Retrospective cohort study using EHR data from Kaiser Permanente Hawaii, USAd Jan 2018–Dec 2019 |
24 (24–24)[vaccinated] 24 (14.1–24.0)[unvaccinated] |
Aged ≥ 50 years | RZV – two doses: 11,864 Unvaccinated: 66,492 |
1063.3 (1006–1122.8) | Two doses: 325.6 (217.4–464.4)f | Two doses: 83.5 (74.9–89.2)f |
| Aged 50–59 years | Vaccinated: 196 PY Unvaccinated: 49,449 PY |
944.4 (861.3–1032.7) | Two doses: 0f | Two doses: 100f | |||
| Aged 60–69 years | Vaccinated: 717 PY Unvaccinated: 42,592 PY |
1037.7 (944–1137.5) | Two doses: 557.7 (173.1–1295.3)f | Two doses: 67.7 (11.8–88.1)f | |||
| Aged 70–79 years | Vaccinated: 4537 PY Unvaccinated: 17,914 PY |
1194.6 (1041.6–1361.9) | Two doses: 286.5 (157.6–471.7)f | Two doses: 83.3 (70.1–90.7)f | |||
| Aged ≥ 80 years | Vaccinated: 2841 PY Unvaccinated: 9764 PY |
1536.2 (1303.3–1795.4) | Two doses: 352.0 (176.4–617.3)f | Two doses: 86.4 (73.5–93.0)f | |||
| Aged ≥ 50 years with prior ZVL vaccinatione | Vaccinated: 184 PY Unvaccinated: 3695 PY |
703.6 (466.6–1009.7) | Two doses: 543.8 (31–2392.2)f | Two doses: 61.1 (–124.9–93.3)f | |||
| Aged ≥ 50 years with no prior ZVL vaccinatione | Vaccinated: 8107 PY Unvaccinated: 116,024 PY |
1074.8 (1016.2–1135.5) | Two doses: 320.7 (212.7–460.2)f | Two doses: 83.9 (75.2–89.5)f | |||
| Incidence of HZO | Vaccinated: 8404 PY Unvaccinated: 120,739 PY |
72.1 (58.0–88.3) | 11.9 (0.7–52.3)f | Two doses: 93.3 (48.7–99.1)f | |||
| Sun et al. Clin Infect Dis 202118 | Retrospective cohort study using claims and EHR data from OptumLabs Data Warehouse, USAd Jan 2018–Dec 2019 |
7.0 (2.8–13.0)[vaccinated] | Aged ≥ 50 years | RZV – two doses: 173,745 Unvaccinated: 4,596,074 |
893.1 (886.2–900.0) | Two doses: 258.8 (230.6–289.4)f | Two doses: 85.5 (83.5–87.3) |
| Aged 50–59 years | Vaccinated: 2019 PY Unvaccinated: 2,252,215 PY |
684.8 (674.1–695.7) | Two doses: <544.8 (282.9–933.0)f,g | Two doses: 85.6 (53.3–95.6) | |||
| Aged 60–69 years | Vaccinated: 22,934 PY Unvaccinated: 2,040,881 PY |
848.9 (836.4–861.7) | Two doses: >148.3 (103.8–203.8)f,g | Two doses: 87.7 (82.5–91.4)f | |||
| Aged 70–79 years | Vaccinated: 65,423 PY Unvaccinated: 1,926,358 PY |
1034.1 (1019.8–1048.5) | Two doses: 246.1 (210.0–286.1)f | Two doses: 86.5 (83.9–88.6)f | |||
| Aged ≥ 80 years | Vaccinated: 24,750 PY Unvaccinated: 965,456 PY |
1191.0 (1169.4–1212.9) | Two doses: 371.7 (300.8–452.9)f | Two doses: 80.3 (75.1–84.3)f | |||
| Aged ≥ 50 years with prior ZVL vaccinationh | NR | 754.5 (718.4–791.7) | Two doses: 239.4 (142.8–371.8)f | Two doses: 84.8 (75.3–90.7)f | |||
| Aged ≥ 50 years with no prior ZVL vaccinationh | NR | 908.1 (895.0–921.3) | Two doses: 260.9 (205.9–325.0)f | Two doses: 87.1 (83.4–89.9)f | |||
| Izurieta et al. Clin Infect Dis 202119 | Cohort study using Medicare claims and enrollment database analysis, USAd Nov 2017–Oct 2019 |
~~2.9 (one dose) ~~7.1 (two doses) |
Aged ≥ 65 years | RZV – one dose: 1,498,275 RZV – two doses: 1,006,446 Unvaccinated: 15,589,546 |
1032 (1028–1036) | One dose: 450 (431–469) Two doses: 309 (295–323) |
One dose: 56.9 (55.0–58.8) Two doses: 70.1 (68.6–71.5) |
| Aged 65–79 years | RZV – one dose: 1,222,612 RZV – two doses: 821,731 Unvaccinated: 11,486,608 |
1010 (1005–1014) | One dose: 429 (408–449) Two doses: 298 (282–313) |
One dose: 58.6 (56.5–60.7) Two doses: 70.6 (68.9–72.1) |
|||
| Aged ≥ 80 years | RZV – one dose: 275,663 RZV – two doses: 184,715 Unvaccinated: 4,102,938 |
1103 (1094–1111) | One dose: 538 (491–585) Two doses: 357 (322–391) |
One dose: 50.9 (46.2–55.1) Two doses: 68.5 (65.1–71.6) |
|||
| Aged ≥ 65 years with prior ZVL within 5 years of RZV | RZV – one dose: 269,852 RZV – two doses: 175,367 Unvaccinated: 2,075,196 |
852 (842–862) | One dose: 420 (376–463) Two doses: 315 (281–350) |
One dose: 51.0 (45.4–56.0) Two doses: 63.0 (58.3–67.2) |
|||
| Aged ≥ 65 years with no prior ZVL within 5 years of RZV | RZV – one dose: 1,128,423 RZV – two doses: 831,079 Unvaccinated: 13,514,350 |
1060 (1056–1064) | One dose: 456 (435–477) Two doses: 307 (292–323) |
One dose: 57.6 (55.4–59.6) Two doses: 71.1 (69.5–72.6) |
|||
| Aged ≥ 65 years and immunocompromised | RZV – one dose: 60,600 RZV – two doses: 40,442 Unvaccinated: 746,654 |
1715 (1690–1740) | One dose: 1034 (892–1175) Two doses: 585 (489–681) |
One dose: 37.0 (27.4–45.4) Two doses: 64.1 (57.2–69.8) |
|||
| Aged ≥ 65 years and not immunocompromised | RZV – one dose: 1,437,675 RZV – two doses: 966,004 Unvaccinated: 14,842,892 |
1001 (997–1005) | One dose: 424 (406–443) Two doses: 297 (283–311) |
One dose: 58.4 (56.4–60.3) Two doses: 70.5 (69.0–72.0) |
|||
| Aged ≥ 65 years with autoimmune disease | RZV – one dose: 92,069 RZV – two doses: 61,999 Unvaccinated: 886,123 |
1489 (1468–1509) | One dose: 625 (535–715) Two doses: 444 (377–512) |
One dose: 57.7 (50.9–63.6) Two doses: 68.0 (62.3–72.8) |
|||
| Incidence of HZO | RZV – one dose: 487,000 PY RZV – two doses: 618,000 PY Unvaccinated: 25,235,000 PY |
77 (75–78) | 40 (34–45) 25 (21–29) |
One dose: 44.7 (36.0–52.3) Two doses: 66.8 (60.7–72.0) |
|||
| Incidence of PHN | RZV – one dose: 295,000 PY RZV – two doses: 240,000 PY Unvaccinated: 19,857,000 PY |
99 (97–100) | 44 (37–52) 23 (17–29) |
One dose: 51.4 (42.0–59.2) Two doses: 76.0 (68.4–81.8) |
|||
| Bruxvoort et al. Open Forum Infect Dis 202221 | Retrospective cohort study using EHR data from Kaiser Permanente Southern California, USAd Apr 2018–Sept 2019 |
≥ 12 | Aged ≥ 50 years, with or without concomitant vaccination | RZV – two doses, with concomitant vaccinei: 12,898 RZV – two doses, without concomitant vaccinei: 28,353 |
NA | Two doses, with concomitant vaccinei: 220 (160–300) Two doses, without concomitant vaccinei: 340 (290–400) |
Adjusted HR: 0.75 (0.53–1.08)i |
| Khan et al. Clin Gastroenterol Hepatol 202222 | Retrospective cohort study using Veterans Affair Healthcare System data, USAd Jan 2018–Oct 2020 |
NR | Aged 50–60 years, with IBD | RZV – one dose: 358 RZV – two doses: 655 Unvaccinated: 5995 |
393 | One dose: 179 Two doses: 0 |
One dose: HR for HZ = 0.44 (95% CI: 0.06–3.17) One-dose VE: 56%j Two doses: HR for HZ = 0 (95% CI: 0–0) Two-dose VE: 100%j |
| Aged >60 years, with IBD | RZV – one dose: 1518 RZV – two doses: 4220 Unvaccinated: 20,554 |
457 | One dose: 248 Two doses: 180 |
One dose: HR for HZ = 0.52 (95% CI: 0.24–1.10) One-dose VE: 48.0%j Two doses: HR for HZ = 0.39 (95% CI: 0.19–0.80) Two-dose VE: 61.0%j |
|||
| Lu et al. Ophthalmol 202123 | Retrospective, observational cohort study using the OptumLabs Data Warehouse, USAd Jan 2018–Dec 2019 |
730 days (730–730) [vaccinated] | Aged ≥ 50 years | RZV – two doses: 177,289 Unvaccinated: 4,665,290 Vaccinated: 117,517 PY Unvaccinated: 7,374,053 PY |
76.7 (95% CI: 74.7–78.7) | 25.5 (95% CI: 17.4–35.8) | VE against HZO: 89.1% (95% CI: 82.9–93.0) |
| Patients aged ≥ 18 years undergoing HSCT | |||||||
| Baumrin et al. Blood Adv 202124 | Single-center prospective observational cohort study at Dana–Farber Cancer Institute, USA Dec 2018–Jun 2020 |
8.6 (5.7–11.5)[total vaccinated cohort] | Allogeneic HSCT recipients aged ≥ 18 years, 9–24 months after transplant | RZV – at least one dose: 158 RZV – two doses: 150 |
NA | At least one dose: 3669 Two doses only: 2834 HZ incidence was 4/157 in patients receiving at least dose and 3/144 in patients receiving two doses |
NA |
aExcluding case reports.
bAdjusted or estimated VE; for full details of the statistical methodology used, please refer to the relevant original study.
cUnless stated otherwise.
dHZ diagnosed according to International Classification of Diseases, 9th or 10th version (ICD-9 or ICD-10).
ePrior ZVL within 1 year of the index date, which was defined as the date on which both of the following were met: 1) the patient reached age ≥ 50 years in 2018 or 2019, and met age eligibility criteria for RZV; 2) the patient had at least 365 days of continuous enrollment in the Kaiser Permanente Hawaii database prior to becoming age-eligible for the RZV vaccine.
fAll patients received two doses; patients receiving a single dose were excluded.
gThe analysis reported these values using < or > symbols for reasons of patient privacy.
hPrior ZVL within 5 years of the index date, which was defined as the date on which both of the following were met: 1) the patient reached age 50 years in 2018 or 2019, and met age eligibility criteria for RZV; 2) the patient had at least 365 days of continuous enrollment in the OptumLabs Data Warehouse.
iConcomitant vaccination defined as any other vaccine received on the same day as either the first or second dose of RZV (most common types were influenza vaccines [65.9%] and pneumococcal vaccines [20.2%]). After adjusting for clinical and demographic covariates, the difference between the group with vs. without concomitant vaccination was not statistically significant (adjusted hazard ratio 0.75 [95% CI: 0.53–1.08]).
jVE calculated as (1 – HR) * 100%.
CI, confidence interval; EHR, electronic health record; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation; HZ, herpes zoster; HZO, herpes zoster ophthalmicus; IBD, inflammatory bowel disease; IQR, interquartile range; NA, not applicable; NR, not reported; PHN, post-herpetic neuralgia; PY, person-years; RZV, recombinant zoster vaccine; VE, vaccine effectiveness; ZVL, zoster vaccine live.
Adults aged ≥ 50 years
Two large retrospective cohort studies, both of which excluded immunocompromised individuals, investigated the effectiveness of RZV in general populations aged ≥ 50 years.18,20 These studies included analyses using electronic health record (EHR) data from Kaiser Permanente Hawaii (KPH; N = 78,356 individuals included in the study) and from the OptumLabs® (Optum Inc., Cambridge, MA, USA) Data Warehouse (OLDW), which includes claims and EHR data for individuals enrolled in commercial insurance, Medicare Advantage, or Medicare Part D (N = 4,769,819 adults included in the study).18,20 The number of adults who received the recommended two doses of RZV was 11,864 in the KPH study and 173,745 in the OLDW study.18,20 Compared with unvaccinated individuals, vaccine effectiveness against HZ was reported as 83.5% (95% CI: 74.9–89.2) and 85.5% (95% CI: 83.5–87.3), respectively, in the two studies (Table 1).18,20
In addition, a cohort study conducted by the US Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) investigated RZV effectiveness in Medicare beneficiaries aged ≥ 65 years.19 The study, which used information from Medicare Parts A, B, and D (i.e., fee-for-service data), included data from approximately 15.5 million unvaccinated individuals and approximately 1 million individuals who had received two RZV doses. Vaccine effectiveness against HZ was estimated as 70.1% (95% CI: 68.6–71.5) for individuals receiving two doses and was similar regardless of whether individuals received the second dose within 6 months of the first (median time from first to second dose 90 days, vaccine effectiveness 70.0% [95% CI: 68.4–71.5]) or ≥ 6 months after the first dose (median time from first to second dose 230 days, vaccine effectiveness 71.7% [95% CI: 66.1–76.3]).19 Although some benefit was evident with a second RZV dose given more than 6 months after the first,19 these findings should be interpreted with caution as the median time from the first to second dose in this group was 7.7 months, and in a previous clinical trial (ZOSTER-026), noninferiority of immune response was not demonstrated for a two-dose, 0- and 12-month, RZV schedule (compared with a two-dose, 0- and 2-month, schedule).27 A systematic review and meta-analysis25 combined the data from the study by Izurieta et al.19 and the OLDW study by Sun et al.18 Using a random-effects meta-analysis model, the pooled vaccine effectiveness of RZV against HZ in adults aged ≥ 50 years was calculated as 79.2% (95% CI: 57.6 − 89.7).25 The differences in the study populations of these two studies should be noted. Izurieta et al.19 used data from adults aged ≥ 65 years, whereas Sun et al.18 used data from adults aged ≥ 50 years; furthermore, immunocompromised individuals were included in the former study but not in the latter.
Overall, vaccine effectiveness appeared lower in the Izurieta et al. study (70.1%; 95% CI: 68.6–71.5)19 than in pivotal clinical trials9 and other studies in individuals aged ≥ 50 years included in the KPH and OLDW databases (vaccine effectiveness: 83.5% [95% CI: 74.9–89.2] and 85.5% [95% CI: 83.5–87.3], respectively).18,20 Among the three real-world studies, lower vaccine effectiveness in the Izurieta et al. study19 compared with the other two studies18,20 could perhaps be attributed to the older age of the population (≥ 65 years vs. ≥ 50 years) and non-exclusion of immunocompromised individuals. In addition, key potential contributors to effectiveness – efficacy differences (in the three real-world studies vs. pivotal clinical trials) included ascertainment factors linked to healthcare pursuit, diagnosis, coding (such as differential outcome misclassification and false-positive cases), residual confounding factors due to unbalanced cohorts and a less restrictive eligibility criteria allowing for a wider and more heterogeneous group of patients with comorbid or underlying conditions to be included in the real-world studies.28 Moreover, HZ case identification varied in real-world studies (primarily by ICD codes) compared with pivotal trials (primarily polymerase chain reaction confirmation) which can impact the specificity of HZ case identification and resultant efficacy/effectiveness.9,10,19 HZO has been reported in 10–20% of HZ cases,1,29 and several real-world studies have assessed the effectiveness of RZV in preventing HZO (Table 1).19,20,23 Among KPH members aged ≥ 50 years, RZV vaccine effectiveness after two doses was 93.3% (95% CI: 48.7–99.1) for preventing HZO.20 In addition, in an analysis of OLDW data for adults aged ≥ 50 years, two doses of RZV had a vaccine effectiveness of 89.1% (95% CI: 82.9–93.0) for HZO.23 Further, the Medicare fee-for-service beneficiary analysis previously discussed reported that two RZV doses were associated with a vaccine effectiveness of 66.8% (95% CI: 60.7–72.0) for HZO in adults aged ≥ 65 years.19 Within each of these studies, results suggested that RZV had comparable efficacy in preventing both HZ and HZO.
PHN is the most common complication of HZ, seen in 10–34% of cases,30 with a greater incidence in individuals aged ≥ 60 years and in patients with immunosuppression.3–5,31 In the previously mentioned analysis of Medicare beneficiaries aged ≥ 65 years, RZV effectiveness in preventing PHN (within 90–180 days of HZ onset) was 76.0% (95% CI: 68.4–81.8) for two doses of RZV compared with the unvaccinated cohort (Table 1).19 Effectiveness against PHN was consistent with effectiveness against HZ (70.1% [95% CI: 68.6–71.5]) in the same analysis.19
Analysis of age subgroups
The effectiveness of RZV was explored in distinct age subgroups in three of the cohort studies (Table 1).18–20 Two of the studies reported generally consistent RZV effectiveness across studied age groups,19,20 in line with efficacy findings in analyses of the pivotal clinical trials.9,10 The OLDW real-world evidence study suggested slightly lower efficacy in patients aged ≥ 80 years, but vaccine effectiveness remained high in this subgroup (80.3% [95% CI: 75.1–84.3]).18 Collectively, these results support the real-world effectiveness of RZV in adults aged ≥ 50 years.
Adults with prior zoster vaccine live vaccination
The three cohort studies described above also investigated RZV effectiveness in individuals who had received prior ZVL, either within the prior year or within the prior 5 years.18–20 Among participants who received two RZV doses, HZ incidence rates appeared to be similar regardless of whether participants had received ZVL in the prior 5 years (Table 1), thus indicating the benefit of revaccination with RZV against HZ, in spite of previous ZVL vaccination.18,19 Izurieta et al.19 reported RZV effectiveness of 63.0% (95% CI: 58.3–67.2) within 5 years of ZVL receipt, and the corresponding value reported by Sun et al.,18 based on OLDW data, was 84.8% (95% CI: 75.3–90.7). The abovementioned meta-analysis of these two studies suggested that RZV was 75.5% (95% CI: 41.5 − 89.7) effective in preventing HZ in participants who had received ZVL within the prior 5 years.25 Based on KPH data, Sun et al.20 reported RZV effectiveness of 61.1% (95% CI: −124.9–93.3) within 1 year of ZVL receipt. The very wide CIs in that report demonstrate the uncertainty in the estimate, as might be expected given the small sample size.20 Indeed, results of the KPH study should be interpreted cautiously, as the number of participants who received two doses of RZV following ZVL was very small (n = 296), providing only 184 person-years (PY) of follow-up data and further limiting the ability to accurately evaluate vaccine effectiveness; although the incidence of HZ was similarly low in patients who received RZV versus those who did not (543.8 [95% CI: 31.0–2392.2] vs. 703.6 [95% CI: 466.6–1009.7] per 100,000 PY), only 3,695 PY of follow-up data were available for RZV-unvaccinated individuals (Table 1).20 The potential for unmeasured confounding may also have influenced this result, which is not unexpected as individuals who received RZV soon after ZVL may have been at increased risk of developing HZ. Furthermore, when interpreting vaccine effectiveness in individuals who have previously received ZVL, it should be noted that these patients will likely have some protection from their previous ZVL vaccination, which can impact the effectiveness of RZV. Overall, available real-world data indicate that there is clinical benefit of revaccination against HZ with RZV in patients previously vaccinated with ZVL.
Adults with immune-mediated diseases
Patients with autoimmune disease often receive immunosuppressive medication, which increases their risk of HZ. The effectiveness of RZV in patients with autoimmune diseases was investigated by Izurieta et al. in Medicare beneficiaries aged ≥ 65 years.19 Autoimmune diseases included conditions such as multiple sclerosis, psoriasis, psoriatic arthritis, rheumatoid arthritis, systemic lupus erythematosus, and ulcerative colitis.19 RZV effectiveness was 68.0% (95% CI: 62.3–72.8) in patients with autoimmune diseases, which was similar to the effectiveness in the overall cohort in this analysis (70.1% [95% CI: 68.6–71.5]) (Table 1).19 Although Izurieta et al. did not include patients with Crohn’s disease in the autoimmune disease subgroup,19 other studies have assessed vaccine effectiveness in patients with inflammatory bowel disease (IBD), including a retrospective cohort study using Veterans Affairs Healthcare System data from patients with IBD. This study reported RZV vaccine effectiveness of 100% (95% CI: 100–100) after two doses in patients with IBD aged 50–60 years, and 61% (95% CI: 20–81) after two doses in patients aged >60 years; however, the sample size for the two-dose vaccinated group was small for those aged 50–60 years (715 PY of follow-up; Table 1).22 Although a pooled post hoc analysis of patients with immune-mediated diseases in the ZOE-50 and ZOE-70 clinical trials reported higher RZV efficacy in this subgroup (90.5% [95% CI: 73.5–97.5]), these trials excluded immunosuppressed patients.32 Overall, these real-world findings support the effectiveness of RZV in patients with immune-mediated diseases.
Immunocompromised adults
In clinical trials, RZV vaccine efficacy was reported as 68.2% (95% CI: 55.6–77.5) in immunocompromised adults aged ≥ 18 years undergoing autologous hematopoietic stem cell transplantation (HSCT)11 and 87.2% (95% CI: 44.3–98.6) in a post hoc analysis in patients with hematologic malignancies.15 In real-world settings, a single-center, prospective, observational, cohort study in allogeneic HSCT recipients aged ≥ 18 years reported an HZ incidence of 37.3 per 1000 PY in patients who received at least one RZV dose, and 28.3 per 1000 PY in patients who received two doses (Table 1).24 Although this study lacked an unvaccinated comparator group, the authors noted that the rates of HZ breakthrough with two RZV doses were low compared with historic controls.24 Moreover, in the phase III, randomized, placebo-controlled study of RZV referenced above, in a total of 1846 patients aged ≥ 18 years who had undergone recent autologous HSCT, the incidence of HZ in the modified total vaccinated cohort (patients who received two vaccine doses and did not develop HZ within 1 month of the second dose) was 30.0 per 1000 PY for RZV and 94.3 per 1000 PY for placebo (incidence rate ratio 0.32 [95% CI: 0.22–0.44]; vaccine efficacy 68.2% [95% CI: 55.6–77.5]);11 thus, in immunocompromised adults, real-world data24 appear to be consistent with clinical trial data.11
The Medicare beneficiary analysis referred to earlier investigated RZV effectiveness in a subgroup of patients considered to be immunocompromised.19 This subgroup included patients with human immunodeficiency virus (HIV)/acquired immune deficiency syndrome, hematologic or solid malignancies, immune deficiencies, transplants and related conditions, and rheumatologic and inflammatory conditions, as well as those undergoing dialysis or with intermediate conditions (defined as conditions viewed unlikely to be immunocompromising but included for optional use by users seeking to be as inclusive as possible). RZV effectiveness after two doses was only slightly lower in immunocompromised versus immunocompetent patients (Table 1).19
Adults receiving concomitant vaccination
Real-world data have also been used to investigate the effects of concomitant administration of other vaccines alongside RZV. In a retrospective cohort study using EHR data from Kaiser Permanente Southern California (KPSC), the HZ incidence in adults aged ≥ 50 years who received RZV with concomitant vaccination was not higher than in adults who received RZV without concomitant vaccination, suggesting that vaccine effectiveness was not impacted by concomitant vaccination (HZ incidence 2.2 vs. 3.4 per 1000 PY).21 In this study, concomitant vaccination was defined as any other vaccine received on the same day as either the first or second dose of RZV, with influenza vaccines (65.9% of participants) and pneumococcal vaccines (20.2% of participants) most commonly received.21 The hazard ratio, adjusted for clinical and demographic variables, was 0.75 (95% CI: 0.53–1.08) for individuals who received RZV with rather than without concomitant vaccination (Table 1). These data complement prior clinical trial evidence indicating that the immune responses of RZV and influenza vaccine are not impacted by co-administration.33
One versus two doses
In pivotal efficacy studies in older adults and immunocompromised individuals, RZV was administered as a two-dose vaccine.9–11 Two studies were identified that explored RZV effectiveness after one or two doses.19,22 In both studies, the two-dose versus one-dose regimen was consistently associated with greater effectiveness across all studied subgroups (Table 1).19,22 The lower effectiveness of one dose was more pronounced with increasing age and in immunocompromised patients; vaccine effectiveness against PHN was 51.4% (95% CI: 42.0–59.2) for one dose of RZV and 76.0% (95% CI: 68.4–81.8) for two doses of RZV.19 These findings underscore the importance of completing the two-dose series.
Real-world safety of RZV vaccination
Besides safety evaluation in clinical trials, the safety of RZV vaccination has been extensively studied in regulatory- and manufacturer-led, post-licensure surveillance studies6,34,35 and other studies.24,36–48 These studies focused on RZV safety in specific populations and explored key areas of interest, such as the incidence of Guillain–Barré syndrome (GBS).6,36,46–48
Adults aged ≥ 50 years
An initial post-licensure safety surveillance report for RZV was conducted by the FDA and CDC using data obtained from October 2017 through June 2018 from the Vaccine Adverse Event Reporting System (VAERS), which collates reports from healthcare providers, vaccine manufacturers, and the public.34 This study encompassed the first 8 months of RZV use in the USA, during which approximately 3.2 million RZV doses were distributed. At the time of the analysis, RZV was licensed for use only in adults aged ≥ 50 years, although a small proportion of the AEs reported (0.6%) were in adults aged <50 years. Overall, the reporting rate was 136 per 100,000 RZV doses distributed, with pyrexia, injection-site pain, and injection-site erythema the most common; the reported signs or symptoms were similar regardless of whether RZV was administered alone or combined with other vaccines. Vaccination errors accounted for 5% of reports, with most such instances (62%) related to administration errors (e.g., subcutaneous rather than intramuscular administration). Serious AEs were rare (4 per 100,000 doses), and overall, the safety profile of RZV was consistent with clinical trial results. The authors concluded that these initial data were reassuring and suggested that healthcare providers should counsel patients to expect self-limiting local adverse reactions, to encourage completion of the two-dose RZV regimen.34
The manufacturer of RZV (GSK) subsequently published an analysis of worldwide safety data from the GSK safety database, which included spontaneous reports of AEs, received either directly or via indirect routes (e.g., the scientific literature), in individuals given RZV.6 Compared with the VAERS study, the manufacturer-led analysis included a longer monitoring period (October 2017 through February 2019), during which approximately 9.3 million doses of RZV were distributed. Where age was known, almost all reports (97.2%) were from adults aged ≥ 50 years. In total, 15,638 reports were received: symptoms related to vaccine reactogenicity (e.g., injection-site reactions, pyrexia, chills, fatigue, etc.) were most common, with a reporting rate of approximately 50 reports per 100,000 doses distributed; such symptoms typically lasted 3–4 days after vaccination. Most reports were non-serious (95%); among serious reports, the most common events were HZ (28% of reports), pyrexia (10%), pain in an extremity (9%), and pain (8%). Overall, 5.1% described symptoms potentially linked to severe reactogenicity (e.g., decreased mobility of injected arm or extensive swelling of the injected arm); when described, these events occurred within the first few days after vaccination and generally lasted 3–4 days, although in rare cases, symptoms persisted for one week or more. A total of 865 case reports listed 837 HZ events and 50 HZ-related complications; generally, laboratory corroboration of HZV (i.e., HZV-positive polymerase chain reaction, culture, immunohistochemical staining, or other testing) was not reported. In total, 22.9% of reports outlined vaccination errors, which included product preparation or reconstitution errors (29.7%), inappropriate or incomplete course of administration (26.7%), incorrect administration route (16.4%), and storage inconsistencies (12.9%); most reports of vaccination errors were not accompanied by symptoms (82.7%). Observed-to-expected analyses for certain outcomes (i.e., total mortality, and the two most commonly reported potential immune-mediated diseases, GBS and Bell’s palsy) and data-mining analyses for all reported AEs did not reveal any unanticipated patterns. Overall, the manufacturer-led analysis demonstrated that the safety profile of RZV was consistent with that observed in clinical trials conducted prior to licensing.6
Following reports in Germany of vesicular and bullous cutaneous eruptions (compatible with HZ rash) occurring in temporal association with RZV vaccination, GSK conducted a focused analysis of the worldwide safety database referred to above to investigate the incidence of events suggestive of HZ or non-HZ vesicular and bullous cutaneous eruptions.35 At the time of this analysis, approximately 2.5 years of reporting data were available, and over 32 million RZV doses had been distributed. In total, 2423 reports describing HZ or HZ complications were identified, of which 645 described possible lack of efficacy. The reporting rate of vaccination failure was low (2.0 cases per 100,000 RZV doses distributed) and in line with the high efficacy of RZV in adults aged ≥ 50 years demonstrated in clinical trials.9,10 A total of 1928 reports met criteria for possible VZV reactivation (HZ or HZ complications with an onset within 30 days post vaccination). An observed-to-expected analysis demonstrated that, generally, the observed incidence of those cases was below the background incidence in the general population. Additionally, 810 reports of non-HZ vesicular and bullous cutaneous eruptions were identified. These included injection-site rashes, attributed to the vaccine’s reactogenicity, and non-injection-site hypersensitivity rashes, in a population (older adults) that has a higher prevalence of, and susceptibility for, dermatologic disorders in general35 and for hypersensitivity rashes including vesicular, blistering, or pustular rashes as clinical manifestations. Discussion of potential underlying pathophysiologic mechanisms (immune exhaustion or cytokine-mediated reactivation) supported the medical review of the reports retrieved and did not raise safety concerns.35
In contrast to the abovementioned studies analyzing passive reports submitted to the regulators and the manufacturer, another study conducted active post-licensure surveillance with proactive capture and rapid analysis of the EHR data from the CDC Vaccine Safety Datalink (VSD) framework.48 This approach allowed for near–real-time sequential monitoring to overcome issues, such as underreporting, that are linked to passive report databases. The prospective cohort study followed individuals aged ≥ 50 years enrolled in seven VSD-data –contributing healthcare systems in the US from January 2018, when RZV was introduced among this population, through December 2019. Within that period, a total of 647,833 RZV doses were administered. Primary outcomes evaluated included acute myocardial infarction, stroke, supraventricular tachycardia, polymyalgia rheumatica, convulsions, Bell’s palsy, optic ischemic neuropathy, giant cell arteritis, anaphylaxis, and GBS. Secondary outcomes included systemic and local reactions occurring within 1–7 days, as well as gout, myocarditis, pericarditis, and several eye-related diseases, diagnosed within 1–42 days. ZVL recipients (2013–2017) served as historical controls for primary outcomes, whereas non-RZV vaccine recipients who had an annual well-person visit in 2018−2019 served as controls for both primary and secondary outcomes. No sustained increased risk was reported for any primary outcomes. An increase was reported for four secondary outcomes, including increased risk of reactions (systemic reactions, adjusted relative risk [aRR] = 1.17 [95% CI: 1.10 − 1.24]; local reactions, aRR = 2.75 [95% CI: 2.14 − 3.54]; combined “any reaction” group, aRR = 1.27 [95% CI: 1.20 − 1.34]), and a slight increase in gout (aRR = 1.08 [95% CI: 1.08 − 1.14]).48
The safety of RZV vaccination was also assessed in a data-mining study using IBM® MarketScan® (IBM Corp., Armonk, NY, USA) data from commercially insured people in the USA; the data covered the administration of approximately 1 million RZV doses in adults aged ≥ 50 years, from January 2018 through May 2020.36 The analysis assessed whether various health outcomes were associated with RZV vaccination, through evaluation of temporal clustering of outcomes identified via ICD-10 Clinical Modification (ICD-10-CM) codes. In the first few days after vaccination, clustering of ICD-10-CM codes relating to the following were identified: unspecified AEs, complications, or reactions to immunization or other medical substances or care; fever; unspecified allergy; syncope and collapse; cellulitis; myalgia; and dizziness and giddiness. These associations were considered to reflect the known safety profile of RZV and the outcomes typically reported after injected vaccinations.36 A subsequent publication by the same authors applied a modified statistical approach using the same database and including a similar number of RZV doses.47 This study employed a sequential analysis using the tree-based scan statistic to identify AEs in days 1−28 versus 29−56 following RZV vaccination. Statistically significant signals emerged only for unspecified AEs or complications in days 1−28; the majority (90% of unique cases within signals) were reported within the first week following vaccination. Based on previous case-by-case investigations of similar signals, the authors speculated that most of the cases might relate to nonserious AEs, such as injection-site reactions, headache, fever, and fatigue.47
A retrospective cohort study using EHR data from KPSC reported the incidence of various reactions in over 30,000 adults aged ≥ 50 years receiving at least one dose of RZV.37 Among these adults, very few experienced medically attended local reactions 1–7 days after the first dose (0.2% in adults receiving only one dose, or two doses), medically attended systemic reactions (approximately 0.5%), or medically attended pain (approximately 0.3%); these events did not influence whether individuals completed the two-dose series.37
Overall, the abovementioned reports in adults aged ≥ 50 years indicate that the real-world safety profile of RZV is consistent with that seen in clinical studies and with that described in the Prescribing Information for RZV.7,8 A summary of these studies is provided in Table 2. The incidence of GBS in the abovementioned studies is discussed in a later section of this article.
Table 2.
Overview of real-world evidence investigating the incidence of selected adverse eventsa after administration of the recombinant zoster vaccine.
| Safety dataa |
|||||
|---|---|---|---|---|---|
| Publication | Study type/period | Population | Sample | Outcome | Results |
| Adults aged ≥ 50 yearsb | |||||
| Hesse et al. MMWR Morb Mortal Wkly 201934 | Retrospective analysis of VAERS data Oct 2017−Jun 2018 |
US population | ~3.2 million RZV doses | Total reports of AEs, N (%) n per 100,000 doses distributed |
4381 (100.0) 136 |
| Serious AEs, n (%) | 130 (3.0) | ||||
| Fever, n (%) | 1034 (23.6) | ||||
| Injection-site pain, n (%) | 985 (22.5) | ||||
| Injection-site erythema, n (%) | 880 (20.1) | ||||
| Tavares-Da-Silva et al. Vaccine 20206 | Retrospective analysis of the GSK safety database Oct 2017−Feb 2019 |
Worldwide population | 9,323,118 RZV doses | Total reports of AEs, N (%) n per 100,000 doses distributed |
15,638 (100) 167.7 |
| Serious AEs, n (%) | 741 (4.7) | ||||
| Fever, n (%) | 1658 (10.6) | ||||
| Injection-site reactions, n (%) | 2849 (61.4) | ||||
| Injection-site pain, n (%) | 1699 (10.9) | ||||
| Nelson et al. Am J Epidemiol 202348 | Prospective analysis of VSD data Jan 2018−Dec 2019 |
US adults ≥ 50 years enrolled in 7 VSD-data-contributing healthcare systems | End-of-surveillance analysis: RZV recipients: n = 647,307 first and second doses; “well-visit” comparators:c n = 1,086,206 | Diagnoses consistent with systemic reactions: RZV group, n (rate per 10,000 dosesd) RZV vs “well-visit” group, aRRd (95% CI) |
2202 (31.49) 1.17 (1.10−1.24) |
| Diagnoses consistent with local reactions: RZV group, n (rate per 10,000 dosesd) RZV vs “well-visit” group, aRRd (95% CI) |
202 (2.69) 2.75 (2.14−3.54) |
||||
| Any adverse reaction diagnosis: RZV group, n (rate per 10,000 dosesd) RZV vs “well-visit” group, aRRd (95% CI) |
2497 (35.63) 1.27 (1.20−1.34) |
||||
| Urgent or emergency healthcare visit for any reason RZV group, n (rate per 10,000 dosesd) RZV vs “well-visit” group, aRRd (95% CI) |
2541 (36.93) 1.01 (0.96−1.07) |
||||
| Pirrotta et al. Drug Saf 202135 | Retrospective analysis of the GSK safety database Oct 2017−Apr 2020 |
Worldwide population | 32,597,779 RZV doses | Reports of AEs suggestive of HZ or HZ complications, n | 2423 |
| Reports of possible VZV reactivation,e n | 1928 | ||||
| Reports of vaccination failure, n | 645 | ||||
| Unique reports of AEs suggestive of other vesicular and bullous cutaneous eruptions (non-HZ), n | 810 | ||||
| Injection-site eruptions, n | 74 | ||||
| Non-injection-site eruptions, n | 557 | ||||
| Yih et al. Am J Epidemiol 202236 | Retrospective analysis of the IBM MarketScan database Jan 2018−May 2020 |
Commercially insured US adults aged ≥ 50 years | 1,014,329 RZV doses | ICD-10 Diagnosis Description (post-vaccination risk window detected): | Cases, n in 56-day follow-up; n in risk window (excess cases per 100,000 doses) |
| Dizziness and giddiness (days 1−8) | 1448; 251 (7.8) | ||||
| Poisoning by, adverse effect of, and underdosing of diuretics and other and unspecified drugs, medicaments, and biological substances (days 1−4)f | 187; 68 (5.9) | ||||
| Fever of other and unknown origin (days 1−2) | 973; 82 (5.1) | ||||
| Syncope and collapse (days 1−3) | |||||
| Fever, unspecified (days 1−2) | 908; 74 (4.5) | ||||
| Adverse effects of other vaccines and biological substances (days 1−3)f | 40; 33 (3.2) | ||||
| Yih et al. Am J Epidemiol 202347 | Retrospective analysis of the IBM MarketScan database Jan 2018−May 2020 |
Commercially insured US adults aged ≥ 50 years | 999,876 RZV doses | ICD-10 Diagnosis Descriptionf (days 1−28 post vaccination) |
Cases, n (attributable risk per 100,000 doses): |
| Adverse effect of other vaccines and biological substances | 37 (3.7) | ||||
| Other complications following immunization, not classified elsewhere | 27 (2.6) | ||||
| Poisoning by, adverse effect of, and underdosing of viral vaccines | 24 (2.4) | ||||
| Adverse effect of other viral vaccines | 23 (2.3) | ||||
| Ackerson et al. Vaccine 202137 | Retrospective cohort study Apr–Nov 2018 |
Kaiser Permanente Southern California members aged ≥ 50 years | 31,120 individuals who received at least one RZV dose: n = 10,222 first dose only n = 20,898 second dose completed |
Days 1−7 after first RZV dose: Medically attended local reactions First dose only group, n (%) Second dose completed group, n (%) |
21 (0.2) 32 (0.2) |
| Medically attended systemic reactions First dose only group, n (%) Second dose completed group, n (%) |
55 (0.5) 85 (0.4) |
||||
| Medically attended pain First dose only group, n (%) Second dose completed group, n (%) |
32 (0.3) 50 (0.2) |
||||
| Immunocompromised adults aged ≥ 18 years | |||||
| Baumrin et al. Blood Adv 202124 | Prospective cohort study Dec 2018−Jun 2020 |
Allogeneic hematopoietic cell transplantation recipients | 158 patients who received at least one RZV doseg | Days 1−7 after vaccination:h All solicited AEs Any grade, n/N (%) Grade 3, n/N (%) |
139/151 (92.1) 49/151 (32.5) |
| Injection-site AEs Any grade, n/N (%) Grade 3, n/N (%) |
131/150 (87.3) 28/150 (18.7) |
||||
| Pain Any grade, n/N (%) Grade 3, n/N (%) |
129/150 (86.0) 24/150 (16.0) |
||||
| Days 1−30 after vaccination:h All unsolicited AEs, n/N (%) Related to trial intervention, n/N (%) |
11/150 (7.3) 6/150 (4.0) |
||||
| Serious AEs, n/N (%) | 2/150 (1.3) | ||||
| Barghash et al. J Heart Lung Transplant 202038 | Retrospective cohort study Sep 2018−Jun 2020 |
Heart transplant recipients | 65 patients who received at least one RZV dose: n = 65 first dose n = 46 second dose |
All AEs After first dose, n (%) After second dose, n (%) |
23 (35.4) 13 (28.3) |
| Injection-site reactions After first dose, n (%) After second dose, n (%) |
19 (29.2) 13 (28.3) |
||||
| Fever After first dose, n (%) After second dose, n (%) |
1 (1.5) 1 (2.2) |
||||
| Gastrointestinal side effects After first dose, n (%) After second dose, n (%) |
1 (1.5) 0 (0) |
||||
| Headache After first dose, n (%) After second dose, n (%) |
1 (1.5) 0 (0) |
||||
aThe safety data listed for each study are not exhaustive; a selection of key outcomes is presented.
bThe vast majority of cases involved individuals aged ≥ 50 years, as this was the age group for which RZV was indicated at the time of the data collection. In some studies, nevertheless, a small fraction of cases came from individuals aged <50 years.
cIndividuals who had not received RZV, had an annual well-person healthcare visit during the RZV uptake period, and had been vaccinated against influenza during the year before their visit.
dPropensity-score –adjusted for age, sex, study site, a dermatology visit, an optometry visit, and prior zoster vaccine live vaccination.
eWhen considering an RF of 75%, the observed number of possible VZV reactivations was lower than the expected number in all countries. The observed number of possible VZV reactivations was higher than the expected number for RFs of <10% for worldwide and the USA, <56% for Canada, and <53% for Germany. The RF is the proportion of possible VZV reactivations reported among all those events that actually occurred in the vaccinated general population within the risk period, regardless of the causality.
fThe authors noted that previous studies examining similar nonspecific post-vaccination signals case by case using similar methodology found that the majority of the cases experienced conditions such as injection-site reactions, fever, fatigue, and headache.
gThe majority of the participants had co-administered vaccines (94% at first RZV dose, 84% at second RZV dose).
hThe data are reported as n/N. The total N varied, as participants with missing symptom diaries for the first or second RZV dose were removed unless a grade 3 event was reported.
AE, adverse event; aRR, adjusted relative risk; CI, confidence interval; HZ, herpes zoster; RF, reported fraction; RZV, recombinant zoster vaccine; US, United States; VAERS, Vaccine Adverse Event Reporting System; VSD, Vaccine Safety Datalink; VZV, varicella zoster virus.
Immunocompromised adults aged ≥ 18 years
In clinical trials, an acceptable safety profile for RZV was shown in patients aged ≥ 18 years undergoing autologous HSCT,11 people living with HIV,12 patients with solid13 or hematologic15 malignancies, and patients after renal transplantation.14
Data from two real-world, single-center, US studies24,38 (Table 2) complement the clinical trial findings. Baumrin et al.24 conducted a small, prospective, observational study exploring the safety of RZV in 158 allogeneic HSCT recipients aged ≥ 18 years who received RZV 9–24 months after transplantation at the Dana–Farber Cancer Institute (Boston, MA, USA); most of these patients received other vaccines co-administered with RZV. AEs were reported by 92.1% of patients, including injection-site AEs (87.3% of patients), most commonly pain. Serious AEs were reported in two patients (1.3%). Four of 157 (2.5%) patients experienced an episode of HZ; one case (0.6%, 1/109 PYs) was fatal, presenting as disseminated vesicular rash, pneumonitis, and hepatitis in a cord blood recipient. There was no difference in the incidence of graft-versus-host disease, disease relapse, or death in patients receiving RZV versus historic controls. The authors concluded that RZV had an acceptable safety profile and was well tolerated in allogeneic HSCT recipients.24 Barghash et al.38 conducted a small, retrospective study at Mount Sinai Hospital (New York, NY, USA) in 65 immunosuppressed adults who had previously received a heart transplant and received RZV between September 2018 and June 2020. Chart reviews identified AEs in 35.4% of patients after the first RZV dose and in 28.3% after the second dose; the AEs were typically injection-site reactions (29.2% and 28.2% of patients after first and second RZV dose, respectively). The authors reported no evidence of increased allograft rejection after the vaccinations.38
Adults with immune-mediated diseases
RZV safety has also been investigated in real-world studies in patients with immune-mediated diseases, many of whom were receiving immunosuppressive therapies, as summarized below and in Table 3. Besides AE incidence, these studies investigated the theoretical concern that vaccination could be associated with an increased risk of disease flares due to vaccine adjuvants.39,40
Table 3.
Overview of real-world evidence investigating the incidence of adverse events and disease flares after administration of the recombinant zoster vaccine in patients with immune-mediated diseases.
| Publication | Study type/period | Follow-up | Population | Sample size | Principal underlying conditions, n (%) | Concomitant medication, n (%) | Flare incidencea | AE incidencea,b |
|---|---|---|---|---|---|---|---|---|
| Gupta et al. J Clin Rheumatol 202245 | Medical records review Jan 2018–Mar 2020 |
3 months | Adults with rheumatic disease | RZV – one dose: 31 RZV – two doses: 34 |
RA, 20 (31) Polymyalgia rheumatica, 11 (17) Sjögren syndrome, 6 (9) SLE, 5 (8) PsA, 5 (8) Scleroderma, 4 (6) IBD arthropathy, 3 (5) AS, 2 (3) Other, 8 (15) |
Biologic DMARD, 16 (24.6) Nonbiologic DMARD, 29 (44.6) No DMARD, 20 (30.8) |
Baseline period: n = 8 (12.3%) After RZV: n = 3 (9.2%) |
Minor, systemic AEs: n = 4 (6.2%) |
| Venerito et al. Int J Mol Sci 202349 | Prospective study at the RA clinic of a tertiary center Feb−Jun 2022 |
3 months | Patients with RA on JAK inhibitors or biologic DMARD who received two doses of RZV | JAK inhibitors: n = 26 Biologic DMARD: n = 26 | RA, 52 (100) | JAK inhibitors, 26 (50) Biologic DMARD, 26 (50) |
After RZV: n = 0 | Local site reactions n = 45 (86.5%) Most frequent systemic AEs: fatigue (n = 13, 25%); fever (n = 11, 21.2%); headache (n = 11, 21.2%) No serious AEs |
| Khan et al. J Crohns Colitis 202242 | Retrospective cohort study using Veterans Affairs Healthcare System data Study period NR |
90 days | Patients with IBD | RZV – 1677 Unvaccinated matched controls –1677 |
IBD, 1677 (100) | Mesalamine, 1450 (86) Anti-TNF agents, 109 (7) Thiopurines, 105 (6) Anti-TNF and thiopurines, 6 (<1) Vedolizumab, 7 (<1) |
Cumulative 90-day incidence of IBD flare: 1.2% (vaccinated) vs. 1.0% (unvaccinated); p = 0.503 | NR |
| Lenfant et al.Rheumatology (Oxford) 202143 | Single-center review of electronic medical records Feb 2018–Mar 2020 |
Median 36 weeks | Adults with (n = 359) or without (n = 263) IMID | RZV – one dose: 146 RZV – two doses: 476 | RA, 88 (25)c Vasculitis, 50 (14)c PMR, 29 (8)c Gout, 28 (8)c SLE, 24 (7)c |
Non-biologic DMARD, 176 (49) Glucocorticoids, 125 (35) Biologic DMARD, 68 (19) Other biologics, 47 (13) |
In the IMID subgroupd
n = 59/359 (16.4%): RA, 21/88 (24%) Vasculitis, 5/50 (10%) PMR, 5/29 (17%) Gout, 5/28 (18%) SLE, 4/24 (17%) |
In the entire population, n = 54 (8.7%): most frequently, injection-site reactions (n = 19, 3%); general fatigue (n = 12, 2%); myalgia (n = 12; 2%); and fever (n = 6; 1%) |
| Leung et al.Arthritis Rheumatol 202241e | SCCS analysis of medical claims data from the IBM MarketScan (patients aged 50–64 years; 2017–2019) and Centers for Medicare and Medicaid Services Medicare (patients aged ≥ 65 years; 2017–2020) databases | ≥ 6 months | Aged ≥ 50 years, with IMID | At least one RZV dose: MarketScan, n = 7207; Medicare, n = 72,468 | Marketscan: RA, 3415 (47.4) PsO, 1039 (14.4) PsA, 992 (13.8) IBD, 1477 (20.5) UC, 916 (12.7) CD, 594 (8.2) SLE, 507 (7.0) AxSpA, 216 (3.0) AS, 166 (2.3) Medicaid: RA, 45,851 (63.3) IBD, 12,679 (17.5) UC, 7416 (10.2) PsA, 6678 (9.2) PsO, 5993 (8.3) CD, 5384 (7.4) SLE, 4205 (5.8) AxSpA, 86 (<1) AS, 72 (<1) |
NR | At least one RZV dose: risk window – MarketScan 9%, Medicare 11–12%; control window –MarketScan 10%, Medicare 13%f | NR |
| Raza et al. South Med J 202244 | Single-center, retrospective study 2018 |
4.78 months after second vaccine | Patients with rheumatic disease who received two doses of RZV | N = 47 | RA, 36 (76.6) SLE, 5 (10.6) Fibromyalgia, 3 (6.4) Vasculitis, 2 (4.2) Sjögren syndrome, 2 (4.2) |
Conventional DMARD, 28 (59) Biologic/targeted DMARD, 23 (49) Both conventional and biologic/targeted DMARD, 12 (25) |
Four events of disease flare occurred at subsequent post-vaccination follow-up visits | Mild AEs: 6.4% |
| Satyam et al. Dig Dis Sci 202039 | Prospective, observational study at tertiary IBD referral center Feb 2018–Jul 2019 |
Median (IQR) 207 days (169–304.5) days | Patients with IBD | At least one RZV dose: n = 67 | UC, n = 28 CD, n = 39 | Prednisone, 7 (10.4) Immunomodulator (6-mercaptopurine, azathioprine, or methotrexate), 11 (16.4) Anti-TNF inhibitor, 9 (13.4) Vedolizumab, 15 (22.4) Ustekinumab, 9 (13.4) Tofacitinib, 3 (4.5) |
1/67 (1.5%) | Local AEs: 74.6% Systemic AEs: 56.7% |
| Stevens et al. ACR Open Rheumatol 202040 | Retrospective chart review at a rheumatology outpatient center Feb 2018–Feb 2019 |
Mean (range): 13.2 (1.0–50.0) weeks | Patients with RA or other SRD | At least one RZV dose: n = 403 | RA, n = 239 (59.3) SRD, n = 164 (40.7) PsA, n = 28 (6.9) SLE, n = 16 (3.9) Spondylitis, n = 12 (2.9) Sjögren syndrome, n = 12 (2.9) Giant cell arteritis, n = 8 (1.9) Other, n = 88 (21.8) |
Any immunosuppressant, n = 316 (78.4) MTX, n = 143 (35.5) Prednisone, n = 106 (26.3) Tofacitinib, n = 52 (12.9) TNF inhibitor, n = 105 (26.1) Other biologic, n = 49 (12.2) Other immunosuppressant, n = 49 (12.2) |
After first RZV dose: 23 (5.7%) After second RZV dose: 5 (2.3%) After both RZV doses: 1 (0.2%) |
Mild AEs: n = 51 (12.7%) After first RZV dose: 43 (10.7%) After second RZV dose: 12 (5.4%) After both RZV doses: n = 4 (<1%) |
aUnless otherwise stated, n = number of patients.
bAfter RZV.
cUnderlying conditions in the IMID subgroup.
dMultivariable analysis revealed that glucocorticoid use at the time of RZV dosing was the only factor significantly associated with flares (odds ratio 2.31; 95% CI: 1.30–4.10; p = .004).
eFlare rate for each IMID can be found in Leung et al. Arthritis Rheumatol 2022.41
fFor specific values for flare incidence according to IMID, the reader is referred to the original Leung et al. paper.41
AE, adverse event; AS, ankylosing spondylitis; axSpA, axial spondyloarthritis; CD, Crohn’s disease; DMARD, disease-modifying antirheumatic drug; IBD, inflammatory bowel disease; IMID, immune-mediated inflammatory disease; JAK, Janus kinase; MTX, methotrexate; NR, not recorded; PMR, polymyalgia rheumatica; PsA, psoriatic arthritis; pts, patients; PsO, psoriasis; RA, rheumatoid arthritis; RZV, recombinant zoster vaccine; SCCS, self-controlled case series; SLE, systemic lupus erythematosus; SRD, systemic rheumatic disease; TNF, tumor necrosis factor; UC, ulcerative colitis.
A self-controlled risk interval study was conducted using claims data from IBM MarketScan and Centers for Medicare & Medicaid Services (Baltimore, MD, USA) databases (2018–2019).41 The self-controlled risk interval design was selected to investigate whether a temporal association exists between RZV administration and disease flares in older adults with immune-mediated inflammatory diseases. The IBM MarketScan database included patients for whom outpatient pharmaceutical claims were available; patients were covered by employer-sponsored insurance each year from US states. The Centers for Medicare & Medicaid Services database included patients with enrollment in Medicare Parts A (hospital insurance), B (outpatient medical insurance), and D (prescription drug coverage). The study investigated the incidence of disease flares among patients aged ≥ 50 years with immune-mediated inflammatory diseases who received at least one RZV dose. The study population was stratified across databases according to age, such that 7207 adults aged 50–64 years with employer-sponsored insurance coverage were included from the MarketScan database and 72,468 Medicare fee-for-service beneficiaries aged ≥ 65 years were included from the Medicare database. Patients had various diseases; the most common included rheumatoid arthritis, psoriasis, psoriatic arthritis, ulcerative colitis, Crohn’s disease, and systemic lupus erythematosus. No increase in the risk of flares was identified in the self-controlled case-series analysis: that is, the incidence of flares was similar during a control period (98–140 days) prior to vaccination and during follow-up (1–42 days) post vaccination. In patients aged ≥ 65 years, 12% had flares after the first RZV dose, and 11% after the second dose, compared with 13% who experienced flares during the period prior to vaccination; in patients aged 50–64 years, 9% developed flares in the period following the first or second doses of RZV, compared with 10% during the period prior to vaccination (Table 3).41
Two studies specifically investigated RZV safety in patients with IBD, primarily focusing on the incidence of disease flares.39,42 A retrospective cohort study, using data from the Veterans Affairs Healthcare System for patients aged ≥ 50 years with IBD, explored the incidence of disease flares (by chart review) in patients receiving RZV (N = 1677) compared with matched, unvaccinated patients.42 No difference was found in the incidence of IBD flares within 90 days after vaccination between the RZV and unvaccinated groups: flares were reported in 1.2% versus 1.0% of patients, respectively (Table 3).42 A prospective, observational, single-center study at Boston Medical Center (Boston, MA, USA) investigated the safety of RZV in 67 patients with IBD who received at least one RZV dose.39 Two-thirds of patients were immunosuppressed. Eleven patients (16.4%) were on an immunomodulator (6-mercaptopurine, azathioprine, or methotrexate), 9 (13.4%) were on a tumor necrosis factor inhibitor, 15 (22.4%) were on vedolizumab, 9 (13.4%) were on ustekinumab, and 3 (4.5%) were on tofacitinib. Rates of injection-site reactions were considered similar to those in the general population (Table 3). A disease flare was reported in only one patient in this study, and the authors concluded that the study suggests that rates of IBD flare are not increased by RZV administration.39 However, the study was uncontrolled and without a comparator group.
Besides studies in IBD populations, several studies explored RZV safety in patients with rheumatologic disorders.40,43–45,49 Although these studies were uncontrolled and without a comparator arm, they nonetheless highlight real-world experience with RZV. The incidence of disease flares with RZV vaccination was investigated in a single-center study, conducted at the rheumatology outpatient center at Brigham and Women’s Hospital (Boston, MA, USA), in 403 patients with rheumatoid arthritis or other systemic rheumatic diseases who were vaccinated between February 2018 and February 2019.40 Most patients (78.4%) were receiving immunosuppressive medications. AEs were reported in 12.7% of patients, most commonly injection-site reactions; all AEs were mild and were consistent with the known safety profile of RZV. Disease flares 12 weeks post vaccination were identified in 6.7% of patients; all flares were mild and responded to treatment with glucocorticoids without requiring changes in immunosuppressive therapy (Table 3). The authors noted that the incidence of flares was lower than the background incidence in a previous analysis conducted at the same institution prior to the availability of RZV.40,50
A retrospective study, using EHR data from the Cleveland Clinic Rheumatology Department (Cleveland, OH, USA), investigated the safety of RZV in patients aged ≥ 18 years who received at least one dose of RZV from February 2018 to March 2020.43 In the total rheumatology clinic population studied who received at least one dose of RZV (N = 622), 8.7% of patients reported AEs following RZV vaccination, most commonly local reactions. Among the total of 622 patients, 359 had immune-mediated inflammatory diseases, most frequently rheumatoid arthritis (25% of patients). Disease flares following RZV vaccination were reported in 16% of the 359 patients; 31% of the flares were related to a change in treatment, and 25% of patients with flares required alteration of immunosuppressive therapy. Multivariable analysis revealed that flares were significantly more likely (odds ratio 2.31; p = .004) in patients using glucocorticoids at the time of RZV administration. As short-term glucocorticoid therapy is typically used to control active disease, the authors suggested that it may be prudent to delay RZV vaccination until low disease activity is achieved. However, the study lacked a comparator group, so a definitive causal link between RZV vaccination and disease flares could not be confirmed.43
Two small, single-center studies explored the safety of RZV in rheumatology patients, most of whom were receiving disease-modifying antirheumatic drugs.44,45 In the first study (N = 47), three (6.4%) patients self-reported AEs after RZV vaccination but all were mild; four patients (8.5%) had disease flares at subsequent follow-up, but these flares were not reported immediately after vaccination and were considered likely related to the natural disease course and not to RZV vaccination.44 In the second study (N = 65), self-reported AEs after vaccination occurred in four (6.2%) patients, but none were severe; the incidence of disease flares was 5.6 per 100 PY in the pre-vaccination baseline period, compared with 2.1 per 100 PY in the post-vaccination follow-up period.45 A third small study followed rheumatology patients receiving Janus kinase inhibitors or one of the biologic agents rituximab and abatacept at the rheumatoid arthritis clinic of a tertiary center in Italy.49 A total of 52 patients, aged 18−85 years, received two RZV doses 1 month apart and were followed up for AE detection for 7 days after immunization. No flares were detected within the total duration of the study (3 months’ follow-up). Injection-site reactions, such as swelling and redness, were reported by 86.5% of patients, and fatigue was the most reported systemic AE (25%).49
Collectively, these studies suggest that RZV is well tolerated in real-world clinical practice in patients with immune-mediated diseases, including those receiving concomitant immunosuppressive therapy. Although evidence is limited and it is difficult to compare these studies directly (as the definition of disease flare may vary between studies and flare cases may not have been chart reviewed), RZV does not appear to increase the risk of disease flares. Additional research on this topic is required.
Guillain–barré syndrome
Individuals who develop HZ have an elevated risk of also developing GBS,51 and studies have evaluated the potential risk of GBS following RZV. In the GSK safety database analysis discussed earlier, 17 cases of GBS were reported, representing a reporting rate of 0.18 per 100,000 RZV doses distributed.6 The authors evaluated this reporting rate further using an observed-to-expected analysis and found that the observed number of cases of GBS following RZV was not greater than expected in the patient population.6
A data-mining study using IBM MarketScan data for commercially insured people in the USA aged ≥ 50 years, and including approximately 1 million RZV doses administered from January 2018 through May 2020, found no association between RZV and GBS.36 Nine cases of GBS were identified in this sample, of which four, after further review, were considered likely not true new-onset GBS cases.36 Subsequently, a sequential data-mining analysis of this database by the same authors reported no signals for GBS on days 8−21 following RZV vaccination.47
Initial post-licensure safety surveillance by the CDC using the VSD database identified an elevated incidence of GBS following RZV.46 Subsequently, a US FDA- and CDC-led analysis of the Medicare claims database investigated the incidence of GBS following RZV vaccination in adults aged ≥ 65 years (1,318,004 doses in 849,397 patients), using data up to February 2020.46 In this self-controlled case series, the authors identified an increased risk of GBS in the risk versus control window (relative risk 2.84; 95% CI: 1.53–5.27; p = .001); that is, a small excess of approximately 3 cases per million RZV doses during the 42-day period following vaccination in the Medicare population.46 A self-controlled case-series analysis of data from two US claims databases suggested a confounding effect of HZ episodes on the potential causal association between RZV vaccination and the risk of GBS.52 Nonetheless, the RZV label was updated, with the FDA noting a potentially increased risk of GBS following RZV vaccination in post-marketing reports while also acknowledging that the available evidence was insufficient to establish a causal relationship.8,53
In an active post-licensure study using data from the VSD framework, a preliminary signal for GBS was observed in RZV recipients compared with historical ZVL recipients (aRR = 5.25; p = .02) but waned over time (aRR = 1.24; p > .05).48 Following chart review, the aRR for confirmed GBS in RZV recipients versus historical ZVL recipients ranged from 1.04 (95% CI: 0.14−7.74) to 1.56 (95% CI: 0.18−18.62).48
Overall, GBS following RZV administration is a rare event. Some post-marketing data suggest a potentially increased risk of GBS. However, collective evidence is insufficient to determine a causal association between RZV and GBS; even if it is confirmed, this relatively low risk is to be balanced with the health benefits of RZV vaccination.54
Two-dose RZV series completion rates
Overall, 14 real-world studies investigated completion rates for the RZV two-dose series (Table 4).19,38,39,41,45,55–63 These studies were all conducted in the US, with the exception of a single study in Canada.57 Although most of the studies did not report mean or median times to two-dose RZV completion, five studies reported these times as approximately 86–140 days (Table 4).38,41,59,61,62 Importantly, the recommended RZV dosage schedule comprises a second dose administered 2–6 months after the first (or 1–2 months later for individuals who are or will be immunodeficient or immunosuppressed and who would benefit from a shorter vaccination schedule).8
Table 4.
Overview of real-world evidence investigating series completion rate for the recombinant zoster vaccinea.
| Publication | Study type/period | Population | Two-dose series completion rate |
|---|---|---|---|
| Patients aged ≥ 50 years | |||
| Patterson et al. Hum Vaccin Immunother 202155b and Patterson et al. J Am Pharm Assoc 202256c |
Retrospective study of IQVIA prescription claims and medical claims datasets, USA Oct 2017–Sep 2019 |
Aged ≥ 50 years (N = 7,097,441) | Second dose within 60 days: 3%55 Second dose within 90 days: 36%55 Second dose within 2–6 months: 67.6%56 Second dose within 6 months: 70.4%56 Second dose within 12 months: 81.8%56 |
| McGirr et al. Vaccine 202157 | Retrospective study of IQVIA prescription database, Canada Jan 2017–May 2019 |
Aged ≥ 50 years (6-months’ follow-up: N = 155,747; 12-months’ follow-up: N = 55,524) |
6-month follow-up cohort: Second dose within 2–6 months: 65.0% 12-month follow-up cohort: Second dose within 2–6 months: 66.8% Second dose within 2–12 months: 74.9% |
| Izurieta et al. Clin Infect Dis 202119 | Cohort study using Medicare claims and enrollment database analysis, USA Nov 2017–Oct 2019 |
Aged ≥ 65 years (N = 1,006,446) |
Second dose within 6 months: 78% Second dose within 12 months: 86% |
| LaMori et al. Vaccine 202258 | Retrospective study using EHR data from Optum Clinformatics Data Mart, USA Apr 2017–Mar 2021 |
Aged ≥ 50 years (N = 726,352) | Second dose within 2–6 months: 71.8% Second dose within 6 months: 72.3% Second dose within 24 months: 86.2% |
| Leung et al. Vaccine 202262 | Retrospective study of IQVIA PharMetrics Plus and IBM MarketScan databases, USA Apr 2017–Dec 2021 |
Aged 50–64 years (IQVIA 6-month follow-up: N = 1,106,956; MarketScan 6-month follow-up: N = 788,831; IQVIA 12-month follow-up: N = 868,619; MarketScan 12-month follow-up: N = 594,987)d |
IQVIA 6-month follow-up cohort: 67% (median dose interval: 89 days) MarketScan 6-month follow-up cohort: 71% (median dose interval: 90 days) IQVIA 12-month follow-up cohort: 78% MarketScan 12-month follow-up cohort: 82% |
| Gatwood et al. Am J Prev Med 202259 | Prospective quality improvement program conducted at a US pharmacy chain Nov 2018–2021 |
Aged ≥ 50 years (N = 113,441) | Without clinical nudge: 71.9% (mean days to completion: 109.8)e With clinical nudge: 75.2% (mean days to completion: 93.3)e |
| Gatwood et al. Vaccine 202361f | Prospective quality improvement program conducted at a US pharmacy chain Jan 2019–Mar 2020 |
Aged ≥ 50 years (N = 220,966) |
Prior to text messagingg being launched:h 83.9% (mean days to completion: 88.6) After text messaging being launched: With text message: 88.3% (mean days to completion: 86.2) Without text message: 85.3% (mean days to completion: 94.4) |
| Ackerson et al. Vaccine 202137 | Retrospective cohort study using EHR data from Kaiser Permanente Southern California, USA Apr 2018–Nov 2018 |
Aged ≥ 50 years (N = 31,120) | Second dose within 9 months: 67.2% |
| Fix et al. Vaccine 202363 | Retrospective cohort study of IBM MarketScan database, USA Jan 2018−Dec 2019 |
Aged 50−64 years (N = 4,678,729) |
Second dose within 6 months: 88.6% (average dose interval: 3.7 months) Second dose within study period: 89.5% |
| Special patient populations | |||
| Gupta et al. J Clin Rheumatol 202245 | Medical records review using University of Texas Southwestern Medical Center EHR data, USA Jan 2018–Mar 2020 |
Aged ≥ 18 years with established rheumatic disease (N = 65) | 52.3%a |
| Leung et al. Arthritis Rheumatol 202241 | Self-controlled case-series analysis using claims data from IBM MarketScan, USA (2017–2019) and Centers for Medicare and Medicaid Services Medicare databases, USA (2017–2020) |
Aged ≥ 50 years with immune-mediated inflammatory disorders (N = 216,199) | Aged 50–64 years: 76.6% (median dose interval: 100 days) Aged ≥ 65 years: 85.4% (median dose interval: 98 days) |
| Satyam et al. Dig Dis Sci 202039 | Prospective, observational, single-center study at Boston Medical Center, USA Feb 2018–Jul 2019 |
Patients with IBD (N = 67) | 82%a |
| Barghash et al. J Heart Lung Transplant 202038 | Retrospective single-center study, USA Sept 2018–June 2020 |
Adults who had previously received a heart transplant (N = 65) | 70.8% (average dose interval: 4.6 months) |
| Porter et al. J Am Pharm Assoc 202160 | Retrospective cohort study in an HIV/infectious disease clinic Jul 2018–Dec 2018 |
HIV-positive patients aged ≥ 50 years, without immunocompromise (N = 119) Pharmacist-directed pilot program (n = 35) Usual HZ education (n = 84) |
Pharmacist-driven RZV administration program: 66% (p < .001 vs. standard care) Standard care: 23% |
aNo timescale reported.
bPaper described the same study as in footnote c and evaluated cumulative RZV uptake, second-dose completion levels, and adherence to dosing recommendations in the US.
cPaper evaluated completion rates and adherence in the overall US adult population aged ≥ 50 years and in subpopulations of interest (e.g., immunocompromised individuals, African Americans), and evaluated factors affecting completion rates and adherence.
dSome individuals were included in both the 6-month and 12-month cohorts, but the cohorts do not completely overlap because of different enrollment requirements described in the study.
eClinical nudge consisted of an alert triggered ≥ 8 weeks after the initial RZV dose and a prompt to proactively contact such patients. The alert appeared in the pharmacy electronic dashboard each time eligible patients visited the pharmacy until either the second dose was given or 6 months after the first dose had passed.
fPaper described the second phase of the program first implemented in November 2018 and described in Gatwood et al. Am J Prev Med 2022.59 The second phase included additional initiatives (text messaging) that were launched on September 18, 2019.
gPatients received a text message ≥ 8 weeks after the initial RZV dose in addition to the clinical nudge described in footnote e. Messages were sent once monthly until either the second dose was given or 6 months after the first dose had passed.
hWith clinical nudge in place, as described in footnote e.
EHR, electronic health record; HIV, human immunodeficiency virus; HZ, herpes zoster; IBD, inflammatory bowel disease; RZV, recombinant zoster vaccine.
In general population studies conducted in adults aged ≥ 50 years, two-dose completion rates within 2–6 months of the first dose ranged from 65% to 78%.19,38,55–58,62,63 Completion rates assessed over longer periods (up to 24 months after the first dose) ranged from 67% to 89.5% (Table 4).19,37,55–58,62,63 In studies assessing completion rates at multiple time points, completion rates tended to increase with increased duration of follow-up, as expected (Table 4).19,55–58,62,63 In special populations, such as those with immune-mediated inflammatory disorders, completion rates were broadly similar to those in the general population (Table 4).38,39,41,45,60
In the US, a large retrospective pharmacy and medical claims analysis extracted data from the IQVIA LRx Longitudinal Pharmacy and Dx Medical Claims Databases. Annually, the LRx database includes almost 4 billion adjudicated electronic prescription claims, and monthly, the Dx database includes approximately 1 billion non-adjudicated claims for professional fees from >870,000 practitioners.56 Overall, data were collated for >7 million adults aged ≥ 50 years with an RZV claim between October 1, 2017, and September 30, 2019; a total of 1,225,088 individuals had data available for 12 months after the index date. The RZV second-dose completion rate was 70.4% at 6 months and 81.8% at 12 months, median time to the second dose was 4.08–5.13 months, and adherence to receiving the second dose 2–6 months after the first dose was 67.6%.56 Another large retrospective US study using the IQVIA PharMetrics Plus and IBM MarketScan databases for adults aged 50−64 years reported similar completion rates62 (Table 4). Moreover, Fix et al. retrospectively analyzed data from January 2018 through December 2019 using the IBM MarketScan database.63 In a cohort of over 4.5 million enrollees aged 50−64 years, 89.5% of the individuals who received a first RZV dose completed the two-dose series. Among those who received both doses, 88.6% did so within the recommended 2–6 months.63 A cohort study conducted by the US FDA and CDC investigated RZV effectiveness among Medicare beneficiaries aged ≥ 65 years.19 The study included data from approximately 15.5 million unvaccinated individuals and approximately 1 million individuals who had received two RZV doses, among whom the second RZV dose was given by 6 months after the first dose in 78% of individuals and by 12 months in 86% of individuals.19
In Canada, data on RZV use were retrospectively collected from the IQVIA LRx Longitudinal Prescription Database, which includes patient-level data for >70% of prescriptions dispensed at approximately 6000 Canadian retail pharmacies.57 The RZV second-dose completion rate was 65.0% within 2–6 months of the first dose and 74.9% within 2–12 months of the first dose. However, the authors mentioned the potential for some immunizations not to be recorded in the pharmacy-based database: that is, in some regions, nurses can immunize patients without a prescription, and some patients may purchase prescriptions at sources other than retail pharmacies or receive drugs during hospital inpatient stays. Thus, the recorded completion rates in the database may have underestimated the actual completion rates in the real-life setting, although this is unlikely to have been a major difference.57
The abovementioned real-world data suggest that two-dose RZV completion rates are generally high. However, up to one-fifth of vaccinees do not receive a second dose and up to one-third of individuals may not receive the second RZV dose within the 2- to 6-month period after the first dose, as recommended for healthy adults aged 50 years and above.8,19,55,56,62,63 As explained previously, in the study conducted by the US FDA and CDC investigating RZV effectiveness among Medicare beneficiaries aged ≥ 65 years,19 effectiveness with two doses was higher than with one dose; although effectiveness remained comparable for individuals who received their second dose beyond 6 months, as the median time from the first to second dose was 230 days, this should be interpreted with caution given the findings in ZOSTER-026.27 Collectively, available data suggest that there is a need to ensure that patients receive two doses of RZV within the recommended schedule.
Beyond counseling and standard follow-up, several real-world studies explored potential strategies to improve adherence.59–61,64 For example, pharmacist-driven RZV administration programs were suggested to increase completion rates versus standard provider-directed RZV education.60 Using alerts within pharmacy electronic databases to prompt pharmacists to engage with individuals requiring a second dose of RZV was also reported to improve completion rates and shorten the time to completion.59 The addition of a patient-facing nudge via text messaging achieved further benefits.61 Finally, receiving a pharmacist phone call was reported to significantly increase the likelihood of individuals returning for a second RZV dose.64
Conclusions and future directions
The real-world evidence summarized in this review complements and supports the clinical trial data previously accrued for RZV. Overall, RZV effectiveness against HZ was high across the studied populations in real-world settings, including in adults aged ≥ 50 years and in patients aged ≥ 18 years at increased risk of HZ because of immunodeficiency or immunosuppression.18,19,21–24 Besides the high RZV efficacy in phase III clinical trials,9–11 the real-world effectiveness results for RZV are reassuring, as these results were obtained in a more heterogeneous population. Further, the safety profile of RZV in post-licensing surveillance database analyses and other real-world safety studies was broadly consistent with the safety profile of RZV established in clinical trials.6,24,34–49 Collectively, these real-world studies reaffirm the favorable benefit–risk profile of the vaccine.
The real-world studies reported in this review also highlight areas requiring further attention. Most of the studies were conducted in adults aged ≥ 50 years, and in the US, RZV only recently (mid-2021) gained approval for use in adults aged ≥ 18 years with immunodeficiency or immunosuppression due to disease or therapy.8,16 Therefore, a need exists for further real-world evidence for RZV in adults aged ≥ 18 years, with increased focus on effectiveness and safety in at-risk populations (e.g., people living with HIV, people with autoimmune diseases), the concurrent use of specific immunosuppressive therapies, and longer-term follow-up for the assessment of RZV effectiveness. Strategies to increase RZV two-dose completion rates could be another future focus for healthcare providers. In addition, there is a current paucity of real-world evidence from outside the USA. Data from other countries may help enhance understanding of the clinical profile of RZV in diverse populations.
Acknowledgments
Medical writing support was provided by Stavroula Bitsi, PhD, and Alex Coulthard, BSc, of Apollo, OPEN Health Communications, funded by GSK, in accordance with Good Publication Practice 3 (GPP) guidelines (www.ismpp.org/gpp-2022).
Funding Statement
GSK Biologicals SA funded this review and all costs associated with its development and publishing.
Disclosure statement
All authors are employees of and hold stock/stock options in GSK.
Author contributions
All authors were involved in the planning, discussion, and interpretation of the data. All reviewed and revised the manuscript, and approved the final manuscript as submitted.
Data availability statement
The data summarized in this review are from published articles and are publicly available.
References
- 1.Kawai K, Gebremeskel BG, Acosta CJ.. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiore J, Co-van der Mee MM, Maldonado A, Glasser L, Watson P. Safety and reactogenicity of the adjuvanted recombinant zoster vaccine: experience from clinical trials and post-marketing surveillance. Ther Adv Vaccines Immunother. 2021;9:25151355211057479. doi: 10.1177/25151355211057479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patil A, Goldust M, Wollina U. Herpes zoster: a review of clinical manifestations and management. Viruses. 2022;14(2):192. doi: 10.3390/v14020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson RW, Levin MJ. Herpes zoster and its prevention by vaccination. Interdiscip Top Gerontol Geriatr. 2020;43:131–22. doi: 10.1159/000504484. [DOI] [PubMed] [Google Scholar]
- 5.McKay SL, Guo A, Pergam SA, Dooling K. Herpes zoster risk in immunocompromised adults in the United States: a systematic review. Clin Infect Dis. 2020;71(7):e125–e34. doi: 10.1093/cid/ciz1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tavares-Da-Silva F, Co MM, Dessart C, Hervé C, López-Fauqued M, Mahaux O, Van HL, Stegmann JU. Review of the initial post-marketing safety surveillance for the recombinant zoster vaccine. Vaccine. 2020;38(18):3489–500. doi: 10.1016/j.vaccine.2019.11.058. [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency . Shingrix EPAR – Product Information. Last updated 2021 Oct 13 [accessed 2022 Oct]. https://www.ema.europa.eu/documents/product-information/shingrix-epar-product-information_en.pdf.
- 8.US Food & Drug Administration . SHINGRIX (Zoster Vaccine Recombinant, Adjuvanted), suspension for intramuscular injection. Prescribing Information. Revised 2021 Jul [accessed 2022 Oct]. https://www.fda.gov/media/108597/download.
- 9.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Díez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–32. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 11.Bastidas A, de la Serna J, El Idrissi M, Oostvogels L, Quittet P, López-Jiménez J, Vural F, Pohlreich D, Zuckerman T, Issa NC, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA. 2019;322(2):123–33. doi: 10.1001/jama.2019.9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkowitz EM, Moyle G, Stellbrink HJ, Schürmann D, Kegg S, Stoll M, El Idrissi M, Oostvogels L, Heineman TC . Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis. 2015;211(8):1279–87. doi: 10.1093/infdis/jiu606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vink P, Delgado MI, Maximiano AC, Rubio-Viqueira B, Jung KH, Rodriguez MJF, Grande E, Marrupe GD, Lowndes S, Puente J, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: a randomized trial. Cancer. 2019;125(8):1301–12. doi: 10.1002/cncr.31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vink P, Ramon Torrell JM, Sanchez Fructuoso A, Kim SJ, Kim SI, Zaltzman J, Ortiz F, Campistol Plana JM, Fernandez Rodriguez AM, Rebollo Rodrigo H, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in chronically immunosuppressed adults following renal transplant: a phase 3, randomized clinical trial. Clin Infect Dis. 2020;70(2):181–90. doi: 10.1093/cid/ciz177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dagnew AF, Ilhan O, Lee WS, Woszczyk D, Kwak JY, Bowcock S, Sohn SK, Rodriguez MG, Chiou TJ, Quiel D, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19(9):988–1000. doi: 10.1016/s1473-3099(19)30163-x. [DOI] [PubMed] [Google Scholar]
- 16.Anderson TC, Masters NB, Guo A, Shepersky L, Leidner AJ, Lee GM, Kotton CN, Dooling KL. Use of recombinant zoster vaccine in immunocompromised adults aged ≥ 19 years: recommendations of the advisory committee on immunization practices - United States, 2022. Morb Mortal Wkly Rep. 2022;71(3):80–4. doi: 10.15585/mmwr.mm7103a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sturkenboom M, Bahri P, Chiucchiuini A, Grove KT, Hahné S, Khromava A, Kokki M, Kramarz P, Kurz X, Larson HJ, et al. Why we need more collaboration in Europe to enhance post-marketing surveillance of vaccines. Vaccine. 2020;38 Suppl 2():B1–B7. doi: 10.1016/j.vaccine.2019.07.081. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Kim E, Kong CL, Arnold BF, Porco TC, Acharya NR. Effectiveness of the recombinant zoster vaccine in adults aged 50 and older in the United States: a claims-based cohort study. Clin Infect Dis. 2021;73(6):949–56. doi: 10.1093/cid/ciab121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izurieta HS, Wu X, Forshee R, Lu Y, Sung HM, Agger PE, Chillarige Y, Link-Gelles R, Lufkin B, Wernecke M, et al. Recombinant zoster vaccine (Shingrix): real-world effectiveness in the first 2 years post-licensure. Clin Infect Dis. 2021;73(6):941–8. doi: 10.1093/cid/ciab125. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Jackson K, Dalmon CA, Shapiro BL, Nie S, Wong C, Arnold BF, Porco TC, Acharya NR. Effectiveness of the recombinant zoster vaccine among Kaiser Permanente Hawaii enrollees aged 50 and older: a retrospective cohort study. Vaccine. 2021;39(29):3974–82. doi: 10.1016/j.vaccine.2021.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruxvoort KJ, Qian L, Wu J, Florea A, Ackerson B, Sy LS, Vega DL, Takhar H, Tseng HF. Herpes zoster following recombinant zoster vaccine with or without concomitant vaccination. Open Forum Infect Dis. 2022;9(3):ofac011. doi: 10.1093/ofid/ofac011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan N, Wang L, Trivedi C, Pernes T, Patel M, Xie D, Yang YX. Efficacy of recombinant zoster vaccine in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2022;20(7):1570–8.e1571. doi: 10.1016/j.cgh.2021.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Lu A, Sun Y, Porco TC, Arnold BF, Acharya NR. Effectiveness of the recombinant zoster vaccine for herpes zoster ophthalmicus in the United States. Ophthalmology. 2021;128(12):1699–707. doi: 10.1016/j.ophtha.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumrin E, Izaguirre NE, Bausk B, Feeley MM, Bay CP, Yang Q, Ho VT, Baden LR, Issa NC. Safety and reactogenicity of the recombinant zoster vaccine after allogeneic hematopoietic cell transplantation. Blood Adv. 2021;5(6):1585–93. doi: 10.1182/bloodadvances.2020003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mbinta JF, Nguyen BP, Awuni PMA, Paynter J, Simpson CR. Post-licensure zoster vaccine effectiveness against herpes zoster and postherpetic neuralgia in older adults: a systematic review and meta-analysis. Lancet Healthy Longev. 2022;3(4):e263–e75. doi: 10.1016/s2666-7568(22)00039-3. [DOI] [PubMed] [Google Scholar]
- 26.Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103–8. doi: 10.15585/mmwr.mm6703a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lal H, Poder A, Campora L, Geeraerts B, Oostvogels L, Vanden AC, Heineman TC. Immunogenicity, reactogenicity and safety of 2 doses of an adjuvanted herpes zoster subunit vaccine administered 2, 6 or 12 months apart in older adults: results of a phase III, randomized, open-label, multicenter study. Vaccine. 2018;36(1):148–54. doi: 10.1016/j.vaccine.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Harpaz R. The effectiveness of recombinant zoster vaccine: observations in the wild. Clin Infect Dis. 2021;73(6):957–60. doi: 10.1093/cid/ciab130. [DOI] [PubMed] [Google Scholar]
- 29.Kong CL, Thompson RR, Porco TC, Kim E, Acharya NR. Incidence rate of herpes zoster ophthalmicus: a retrospective cohort study from 1994 through 2018. Ophthalmology. 2020;127(3):324–30. doi: 10.1016/j.ophtha.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh CH, Chang KS, Huang SS, Tsay SL, Tsai JM, Wang YJ. Comparing prodrugs with acyclovir for treating postherpetic neuralgia among herpes zoster patients: a systematic review and meta-analysis. Healthcare (Basel). 2022;10(7):1181. doi: 10.3390/healthcare10071181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gruver C, Guthmiller KB. Postherpetic neuralgia. Treasure Island (FL): StatPearls; 2022. PMID: 29630250. https://www.ncbi.nlm.nih.gov/books/NBK493198/. [Google Scholar]
- 32.Dagnew AF, Rausch D, Hervé C, Zahaf T, Levin MJ, Schuind A. Efficacy and serious adverse events profile of the adjuvanted recombinant zoster vaccine in adults with pre-existing potential immune-mediated diseases: a pooled post hoc analysis on two parallel randomized trials. Rheumatol (Oxford). 2021;60(3):1226–33. doi: 10.1093/rheumatology/keaa424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarz TF, Aggarwal N, Moeckesch B, Schenkenberger I, Claeys C, Douha M, Godeaux O, Grupping K, Heineman TC, Fauqued ML, et al. Immunogenicity and safety of an adjuvanted herpes zoster subunit vaccine coadministered with seasonal influenza vaccine in adults aged 50 years or older. J Infect Dis. 2017;216(11):1352–61. doi: 10.1093/infdis/jix481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hesse EM, Shimabukuro TT, Su JR, Hibbs BF, Dooling KL, Goud R, Lewis P, Ng CS, Cano MV. Postlicensure safety surveillance of recombinant zoster vaccine (Shingrix) – United States, October 2017–June 2018. MMWR Morb Mortal Wkly Rep. 2019;68(4):91–4. doi: 10.15585/mmwr.mm6804a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pirrotta P, Tavares-Da-Silva F, Co M, Lecrenier N, Hervé C, Stegmann JU. An analysis of spontaneously reported data of vesicular and bullous cutaneous eruptions occurring following vaccination with the adjuvanted recombinant zoster vaccine. Drug Saf. 2021;44(12):1341–53. doi: 10.1007/s40264-021-01118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yih WK, Kulldorff M, Dashevsky I, Maro JC. A broad safety assessment of the recombinant herpes zoster vaccine. Am J Epidemiol. 2022;191(5):957–64. doi: 10.1093/aje/kwac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ackerson B, Qian L, Sy LS, Bruxvoort K, Wu J, Luo Y, Diaz-Decaro J, Talarico C, Tseng HF. Completion of the two-dose recombinant zoster vaccine series in adults 50 years and older. Vaccine. 2021;39(6):926–32. doi: 10.1016/j.vaccine.2020.12.076. [DOI] [PubMed] [Google Scholar]
- 38.Barghash MH, Taimur S, Rana M, Behar J, Mancini DM. Recombinant herpes zoster vaccine after heart transplantation: a single-center experience. J Heart Lung Transplant. 2020;39(12):1501–3. doi: 10.1016/j.healun.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Satyam VR, Li PH, Reich J, Qazi T, Noronha A, Wasan SK, Farraye FA. Safety of recombinant zoster vaccine in patients with inflammatory bowel disease. Dig Dis Sci. 2020;65(10):2986–91. doi: 10.1007/s10620-019-06016-4. [DOI] [PubMed] [Google Scholar]
- 40.Stevens E, Weinblatt ME, Massarotti E, Griffin F, Emani S, Desai S. Safety of the zoster vaccine recombinant adjuvanted in rheumatoid arthritis and other systemic rheumatic disease patients: a single center’s experience with 400 patients. ACR Open Rheumatol. 2020;2(6):357–61. doi: 10.1002/acr2.11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung J, Anderson TC, Dooling K, Xie F, Curtis JR. Recombinant zoster vaccine uptake and risk of flares among older adults with immune-mediated inflammatory diseases in the US. Arthritis Rheumatol. 2022;74(11):1833–41. doi: 10.1002/art.42261. [DOI] [PubMed] [Google Scholar]
- 42.Khan N, Trivedi C, Aberra F, Pernes T, Yang YX. Safety of recombinant zoster vaccine in patients with inflammatory bowel disease. J Crohns Colitis. 2022;16(9):1505–7. doi: 10.1093/ecco-jcc/jjac040. [DOI] [PubMed] [Google Scholar]
- 43.Lenfant T, Jin Y, Kirchner E, Hajj-Ali RA, Calabrese LH, Calabrese C. Safety of recombinant zoster vaccine: a retrospective study of 622 rheumatology patients. Rheumatol (Oxford). 2021;60(11):5149–57. doi: 10.1093/rheumatology/keab139. [DOI] [PubMed] [Google Scholar]
- 44.Raza S, Acharya S, Howard G, Pattanaik D. Safety of recombinant zoster vaccine in rheumatology patients. South Med J. 2022;115(2):125–8. doi: 10.14423/smj.0000000000001354. [DOI] [PubMed] [Google Scholar]
- 45.Gupta S, Arasaratnam RJ, Solow EB, Bajaj P. A medical records review study assessing safety of zoster vaccine recombinant, adjuvanted in patients with rheumatic disease. J Clin Rheumatol. 2022;28(2):e528–e31. doi: 10.1097/rhu.0000000000001790. [DOI] [PubMed] [Google Scholar]
- 46.Goud R, Lufkin B, Duffy J, Whitaker B, Wong HL, Liao J, Lo AC, Parulekar S, Agger P, Anderson SA, et al. Risk of Guillain-Barré syndrome following recombinant zoster vaccine in Medicare beneficiaries. JAMA Intern Med. 2021;181(12):1623–30. doi: 10.1001/jamainternmed.2021.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yih WK, Kulldorff M, Dashevsky I, Maro JC. Sequential data-mining for adverse events after recombinant herpes zoster vaccination using the tree-based scan statistic. Am J Epidemiol. 2023;192(2):276–82. doi: 10.1093/aje/kwac176. [DOI] [PubMed] [Google Scholar]
- 48.Nelson JC, Ulloa-Pérez E, Yu O, Cook AJ, Jackson ML, Belongia EA, Daley MF, Harpaz R, Kharbanda EO, Klein NP, et al. Active postlicensure safety surveillance for recombinant zoster vaccine using electronic health record data. Am J Epidemiol. 2023;192(2):205–16. doi: 10.1093/aje/kwac170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venerito V, Stefanizzi P, Cantarini L, Lavista M, Galeone MG, Di Lorenzo A, Iannone F, Tafuri S, Lopalco G. Immunogenicity and safety of adjuvanted recombinant zoster vaccine in rheumatoid arthritis patients on anti-cellular biologic agents or JAK inhibitors: a prospective observational study. Int J Mol Sci. 2023;24(8):6967. doi: 10.3390/ijms24086967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bykerk VP, Shadick N, Frits M, Bingham CO 3rd, Jeffery I, Iannaccone C, Weinblatt M, Solomon DH. Flares in rheumatoid arthritis: frequency and management. A report from the BRASS registry. J Rheumatol. 2014;41(2):227–34. doi: 10.3899/jrheum.121521. [DOI] [PubMed] [Google Scholar]
- 51.Anderson TC, Leung JW, Harpaz R, Dooling KL. Risk of Guillain–Barré syndrome following herpes zoster, United States, 2010–2018. Hum Vaccin Immunother. 2021;17(12):5304–10. doi: 10.1080/21645515.2021.1985890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haguinet F, Pirrotta P, Bauchau V. Evaluation of the potential confounding effect of shingles on the risk of Guillain-Barré syndrome after vaccination with the recombinant zoster vaccine. ISPE Annual Meeting Abstracts. 2021. [accessed 2023 Feb 2]. https://onlinelibrary.wiley.com/doi/10.1002/pds.5305.
- 53.FDA . FDA requires a warning about Guillain-Barré syndrome (GBS) be included in the prescribing information for Shingrix. 2021. Mar 24 [accessed 2022 Dec 19]. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-requires-warning-about-guillain-barre-syndrome-gbs-be-included-prescribing-information-shingrix.
- 54.Janusz CB, Anderson TC, Leidner AJ, Lee GM, Dooling K, Prosser LA. Projected risks and health benefits of vaccination against herpes zoster and related complications in US adults. Hum Vaccin Immunother. 2022;18(5):2060668. doi: 10.1080/21645515.2022.2060668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patterson BJ, Chen CC, McGuiness CB, Glasser LI, Sun K, Buck PO. Early examination of real-world uptake and second-dose completion of recombinant zoster vaccine in the United States from October 2017 to September 2019. Hum Vaccin Immunother. 2021;17(8):2482–7. doi: 10.1080/21645515.2021.1879579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patterson BJ, Chen CC, McGuiness CB, Ma S, Glasser LI, Sun K, Buck PO. Factors influencing series completion rates of recombinant herpes zoster vaccine in the United States: a retrospective pharmacy and medical claims analysis. J Am Pharm Assoc (2003). 2022;62(2):526–36.e510. doi: 10.1016/j.japh.2021.11.010. [DOI] [PubMed] [Google Scholar]
- 57.McGirr A, Bourgoin T, Wortzman M, Millson B, McNeil SA. An early look at the second dose completion of the recombinant zoster vaccine in Canadian adults: a retrospective database study. Vaccine. 2021;39(25):3397–403. doi: 10.1016/j.vaccine.2021.04.053. [DOI] [PubMed] [Google Scholar]
- 58.LaMori J, Feng X, Pericone CD, Mesa-Frias M, Sogbetun O, Kulczycki A. Real-world evidence on adherence and completion of the two-dose recombinant zoster vaccine and associated factors in U.S. adults, 2017–2021. Vaccine. 2022;40(15):2266–73. doi: 10.1016/j.vaccine.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 59.Gatwood J, Brookhart A, Kinney O, Hagemann T, Chiu CY, Ramachandran S, Hohmeier KC. Clinical nudge impact on herpes zoster vaccine series completion in pharmacies. Am J Prev Med. 2022;63(4):582–91. doi: 10.1016/j.amepre.2022.04.018. [DOI] [PubMed] [Google Scholar]
- 60.Porter AM, Fulco PP. Impact of a pharmacist-driven recombinant zoster vaccine administration program. J Am Pharm Assoc (2003). 2021;61(2):e136–e9. doi: 10.1016/j.japh.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 61.Gatwood J, Brookhart A, Kinney O, Hagemann T, Chiu CY, Ramachandran S, Gravlee E, Hohmeier K. Impact of patient and provider nudges on addressing herpes zoster vaccine series completion. Vaccine. 2023;41(3):778–86. doi: 10.1016/j.vaccine.2022.12.016. [DOI] [PubMed] [Google Scholar]
- 62.Leung J, Gray EB, Anderson TC, Sharkey SM, Dooling K. Recombinant zoster vaccine (RZV) second-dose series completion in adults aged 50–64 years in the United States. Vaccine. 2022;40(50):7187–90. doi: 10.1016/j.vaccine.2022.10.065. [DOI] [PubMed] [Google Scholar]
- 63.Fix J, Vielot NA, Lund JL, Weber DJ, Smith JS, Hudgens MG, Becker-Dreps S. Patterns of use of recombinant zoster vaccine among commercially-insured immunocompetent and immunocompromised adults 50–64 years old in the United States. Vaccine. 2023;41(1):49–60. doi: 10.1016/j.vaccine.2022.10.076. [DOI] [PubMed] [Google Scholar]
- 64.Tyler R, Kile S, Strain O, Kennedy CA, Foster KT. Impact of pharmacist intervention on completion of recombinant zoster vaccine series in a community pharmacy. J Am Pharm Assoc (2003). 2021;61(4s):S12–S6. doi: 10.1016/j.japh.2020.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data summarized in this review are from published articles and are publicly available.


