Abstract
Maltose is the major form of carbon exported from the chloroplast at night as a result of transitory starch breakdown. Maltose exists as an α- or β-anomer. We developed an enzymatic technique for distinguishing between the two anomers of maltose and tested the accuracy and specificity of this technique using β-maltose liberated from maltoheptose by β-amylase. This technique was used to investigate which form of maltose is present during transitory starch degradation in bean (Phaseolus vulgaris), wild-type Arabidopsis (Arabidopsis thaliana), two starch deficient Arabidopsis lines, and one starch-excess mutant of Arabidopsis. In Phaseolus and wild-type Arabidopsis, β-maltose levels were low during the day but were much higher at night. In Arabidopsis plants unable to metabolize maltose due to a T-DNA insertion in the gene for the cytosolic amylomaltase, (Y. Lu, T.D. Sharkey [2004] Planta 218: 466–473) levels of α- and β-maltose were high during both the day and night. In starchless mutants of Arabidopsis, total maltose levels were low and almost completely in the α-form. We also found that the subcellular concentration of β-maltose at night was greater in the chloroplast than in the cytosol by 278 μm. We conclude that β-maltose is the metabolically active anomer of maltose and that a sufficient gradient of β-maltose exists between the chloroplast and cytosol to allow for passive transport of maltose out of chloroplasts at night.
In leaves, transitory starch is formed in the chloroplasts during the day and broken down at night. Transitory starch acts as an energy reserve, providing the plant with carbohydrate during the night when sugars cannot be made by photosynthesis, and as an overflow, allowing photosynthesis to go faster than Suc synthesis during the day (Ludewig et al., 1998).
It has long been known that maltose is an intermediate in the breakdown of storage starch, for example during malting, but the importance of maltose in transitory starch degradation has only recently been proven. Early reports of significant maltose levels in leaves undergoing transitory starch breakdown (Levi and Gibbs, 1976; Peavey et al., 1977; Kruger and ap Rees, 1983; Neuhaus and Schulte, 1996; Servaites and Geiger, 2002) were counterbalanced by reports concluding that phosphorolytic starch breakdown was the predominant pathway in leaves (Heldt et al., 1977; Stitt et al., 1978; Stitt and ap Rees, 1979, 1980; Stitt and Heldt, 1981).
The form of carbon exported from the chloroplast at night was reexamined in light of accumulating genetic and biochemical evidence against phosphorolytic degradation for export. We found that at least two-thirds of the carbon exported from dark-adapted, isolated chloroplasts was in the form of maltose (Weise et al., 2004). The maltose is likely used by an amylomaltase enzyme (Chia et al., 2004; Lu and Sharkey, 2004) in metabolism that may be similar to that used by Escherichia coli (Boos and Shuman, 1998).
Maltose transport across the chloroplast membrane has been known for some time (Rost et al., 1996) and recently the maltose transporter was identified. The knockout of this transporter results in plants with a starch-excess phenotype and very high levels of maltose (Nittylä et al., 2004). However, when the subcellular distribution of maltose was examined, we found a higher concentration of maltose in the cytosol than the chloroplast (Weise et al., 2004). These observations indicated that maltose transport could be active, but if this were true it would eliminate the energetic advantage of exporting maltose (Weise and Sharkey, 2005).
Maltose is made in the chloroplast by β-amylase (EC 3.2.1.2), which releases maltose from the nonreducing ends of α-1,4-glucan chains with a degree of polymerization of 4 or greater. There are nine genes encoding β-amylase-like proteins annotated in the Arabidopsis (Arabidopsis thaliana) genome (Zeeman et al., 2004). Four of these have putative chloroplast targeting transit peptide sequences. Lao et al. (1999) confirmed the targeting of one β-amylase by producing a β-amylase-green fluorescent protein fusion. When a potato chloroplast-targeted isoform of β-amylase was repressed by RNA antisense, the plants accumulated excess starch (Scheidig et al., 2002).
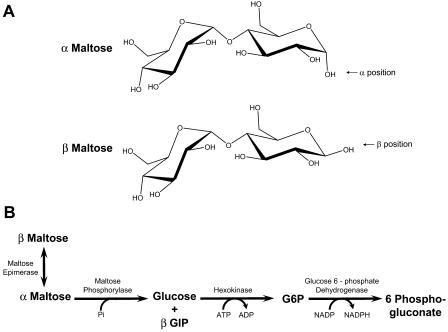
β-Amylase is an inverting enzyme, meaning that it makes the β-anomer of maltose (Fig. 1A). The spontaneous interconversion between the α- and β-forms is a slow process. We calculated a half-life of over 90 min, or a rate constant of 0.007 min−1, from data in Shirokane and Suzuki (1995). Because this interconversion is slow, the anomeric form of maltose could be important during starch breakdown.
Figure 1.
The α- and β-anomers of maltose and the pathway for the enzymatic determination of α- and β-maltose. A, α and β refer to the position of the hydroxide of the hemiacetal at the reducing end of the molecule. In the α-anomer, the OH attached to the anomeric carbon is on the opposite side of the sugar ring from the CH2OH group. In the β-anomer, the OH is on the same side of the ring as the CH2OH group. B, Maltose epimerase catalyzes the interconversion of α- and β-maltose. Therefore, it is the strict specificity of maltose phosphorylase for α-maltose and the order in which these enzymes are added to the assay buffer that make the determination of α- and β-maltose possible.
We developed a technique for distinguishing between the two anomers of maltose and tested the technique using maltose liberated from maltoheptose by β-amylase (Fig. 1B). This technique was used to investigate which form of maltose is present in bean (Phaseolus vulgaris), wild-type Arabidopsis, two starch deficient Arabidopsis lines, and one starch-excess mutant of Arabidopsis during transitory starch degradation. We also determined the subcellular concentrations of α- and β-maltose in the dark. We conclude that β-maltose is the metabolically active anomer of maltose and that a sufficient gradient of β-maltose exists between the chloroplast and cytosol to allow for the passive transport.
RESULTS
β-Maltose Determination Method Validation
Commercially available maltose normally exists as a mixture of anomeric forms (Fig. 2A). Maltose phosphorylase has a strict specificity for α-maltose; when added to a reaction mixture by itself, it will react only with α-maltose (Shirokane et al., 2000). Upon adding maltose phosphorylase to a reaction mixture containing commercially obtained maltose, an initial rise in absorbance, reflecting reduction of NADP to NADPH, was seen. The change in optical density (OD) then slowed to a rate that may reflect the uncatalyzed epimerization of β-maltose to α-maltose. Maltose epimerase catalyzes the interconversion of α- and β-maltose until they reach equilibrium. Adding maltose epimerase to the reaction mixture with maltose phosphorylase caused a second increase in OD of about the same magnitude as the first change (Fig. 2A). This indicates that commercial maltose is approximately 1:1 α-:β-maltose. Plant extracts could not substitute for maltose epimerase, indicating no detectable maltose epimerase activity in plants under the conditions we used.
Figure 2.
Reaction curves for the validation of the α- and β-maltose NADP-linked spectrophotometric assay. A, Sequential measurement of α- and β-maltose was achieved by adding maltose phosphorylase, then maltose epimerase to a reaction mixture that contained 650 pmol of commercially obtained maltose. B, β-Maltose produced by the action of β-amylase on 125 pmol maltoheptose (G7) was not acted on by maltose phosphorylase until maltose epimerase was added. A change in OD is observed only when maltose epimerase is added to the reaction mixture, demonstrating that maltose phosphorylase is specific for the α-anomer. In both A and B, Glc-6-P dehydrogenase and hexokinase were already added to the reaction mixture. MPL, Maltose phosphorylase; MER, maltose epimerase; β-Amy, β-amylase. Horizontal bar = 2 min in both A and B; vertical bar = 0.00092 OD units or 100 pmol under our assay conditions in both A and B.
We were unable to find a source for anomerically pure maltose. To confirm the specificity of our NADPH-linked spectrophotometric assay for α- and β-maltose, we used β-amylase to generate β-maltose from maltoheptose. β-Amylase converts one molecule of maltoheptose (G7) to two molecules of β-maltose and one molecule of maltotriose. When β-amylase was added to the reaction mixture containing maltoheptose, maltose phosphorylase, hexokinase, and Glc-6-P dehydrogenase, no change in the OD was detected, confirming that β-amylase does not produce α-maltose. When maltose epimerase was added, an increase in absorbance of NADPH proportional to twice the maltoheptose concentration was detected (Fig. 2B).
α- and β-Maltose in Leaves of Bean and Arabidopsis
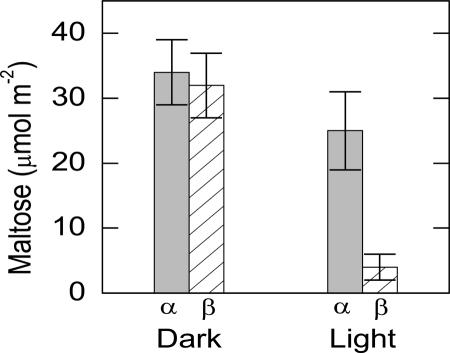
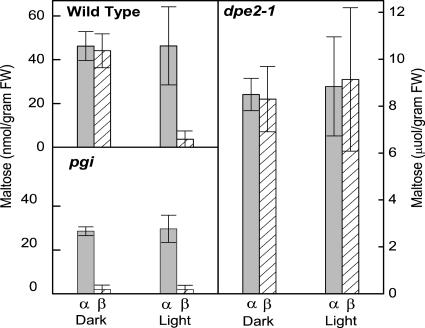
Maltose was extracted from leaf punches taken in the middle of the light period and the middle of the dark period. In bean plants, α-maltose levels were similar in light and dark, but β-maltose levels were high only in the dark (Fig. 3). β-Maltose levels were 28 μmol m−2 higher in the middle of the dark period than in the middle of the light period. Similar results were obtained from wild-type Arabidopsis, with β-maltose levels 40 nmol g−1 fresh weight (FW) higher in the middle of the dark period (Fig. 4).
Figure 3.
Diel changes in α- and β-maltose leaves in bean. Light samples were taken 6 h after the beginning of the 12-h-light period. Dark samples were taken 6 h after the beginning of the dark period. The leaf weight was 180 g m−2 so 30 μmol m−2 is equal to 167 nmol g−1. Values are mean ± se (n = 5).
Figure 4.
Diel changes in α- and β-maltose leaves in wild-type Arabidopsis, dpe2-1 (knockout of cytosolic amylomaltase), and pgi (knockout of plastidic pgi). The dpe2-1 plants are unable to metabolize maltose. The pgi knockout plants are unable to make starch. Light samples were taken 6 h after the beginning of the 12-h-light period. Dark samples were taken 6 h after the beginning of the dark period. Values are mean ± se (n = 5).
We also assayed maltose from Arabidopsis plants in which the cytosolic amylomaltase enzyme was disrupted by a T-DNA insertion (dpe2-1; Lu and Sharkey, 2004 ). This plant had very high levels of both α- and β-maltose during the light and dark periods (Fig. 4). In Arabidopsis plants unable to make starch due to knockouts in phosphoglucoisomerase (pgi), total maltose levels were low and almost completely in the α-form (Fig. 4). Any epimerization that might have occurred during processing of the sample would have converted α- to β-maltose, in this case causing the ratio to approach 1:1. The same results were obtained with the starchless mutant deficient in phosphoglucomutase (pgm; data not shown).
α- and β-Maltose Concentrations in the Chloroplast and Cytosol of Bean
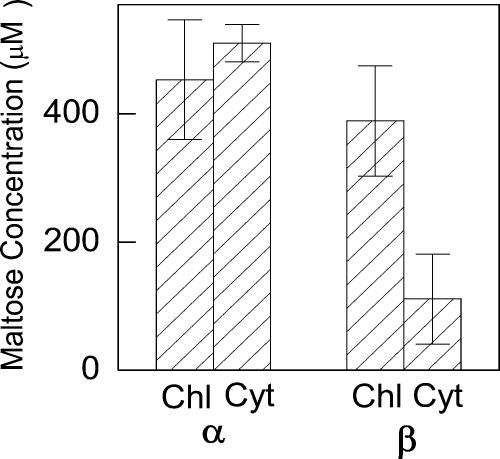
In the dark, the concentration of α-maltose was the same in the chloroplast and cytosol (Fig. 5); however, the concentration of β-maltose was greater in the chloroplast than in the cytosol by 278 μm (Fig. 5). During the day, α-maltose concentrations and distributions were the same as at night, while β-maltose concentrations were below our detection limit, 12 nmol g−1 dry weight, with the nonaqueous fractionation procedure used here (data not shown).
Figure 5.
α- and β-maltose concentrations in bean at night. Samples were taken 6 h after the beginning of the 12-h-dark period. Chl, Chloroplast; Cyt, cytosol. Concentrations were obtained using the volumes for spinach cells. Values are mean ± se (n = 4).
DISCUSSION
We found that during the dark period when transitory starch is being broken down, β-maltose was present, but it was absent in the light or in plants that could not break down starch (Figs. 3 and 4). The method described here depends on there being no epimerase (mutarotase) in plants. If there were, then we would see only equilibrium ratios of the anomeric forms of maltose in our extracts. Because the day samples have ratios that are far from equilibrium, very little interconversion can have occurred during processing of the samples. We conclude that maltose exported from the chloroplast at night is β-maltose, the product of chloroplastic β-amylase. A low level of maltose is always present in Arabidopsis mutants unable to make starch (Weise et al., 2004). However, in these plants, the maltose is almost entirely in the α-form. The source of this low level of α-maltose is unclear. It could be explained by the activity of cytosolic α-amylase. There are three genes that encode α-amylase-like proteins in the Arabidopsis genome, At1g76130, At1g69830, and At4g25000, and one of these has a putative transit peptide sequence for chloroplast localization, At1g69830 (Zeeman et al., 2004). Perhaps one of the cytosolic α-amylases is working on a cytosolic polyglucan (Yang and Steup, 1990) to generate the low levels of α-maltose.
In the dpe2-1 plants, the gene encoding cytosolic amylomaltase is disrupted by a T-DNA insertion. Amylomaltase is believed to catalyze the addition of polyglucan to maltose, releasing a Glc in the process (Lu and Sharkey, 2004). The dpe2 plants are unable to metabolize maltose and thus accumulate maltose to a high concentration (Chia et al., 2004; Lu and Sharkey, 2004). These plants have roughly equal levels of α- and β-maltose both day and night. In the pgm and pgi plants, amylomaltase is presumably still active and this is why no β-maltose is found in these plants.
The equal levels of α- and β-maltose in the dpe2-1 mutant combined with the low levels of β-maltose observed in the wild type during the day leads us to hypothesize that the amylomaltase enzyme in the cytosol uses β-maltose exclusively. This hypothesis presumes that maltose epimerase activity is absent in both the stroma and cytosol. There are no known maltose epimerases in plants and our finding of anomeric ratios far from equilibrium confirm that maltose must not have come into contact with epimerase during processing. The only hexose epimerase in plants is aldose 1-epimerase, but this enzyme is specific for Glc and Gal with poor catalytic efficiency toward maltose (Bailey et al., 1967). Hexokinase is not specific for the anomeric form of Glc and the spontaneous rate of anomeric interconversion of Glc-6-P is very rapid (Bailey et al., 1968). Therefore, the anomeric form of Glc is not important to metabolism.
A gradient of β-maltose from inside the chloroplast to the cytosol is present at night. This allows for the hypothesis of passive transport of maltose during starch to Suc conversion. In our previous investigation (Weise et al., 2004), we did not find a gradient in total maltose to drive passive export of maltose from chloroplasts at night. Active transport would eliminate any energetic advantage in maltose export over export of Glc or triose phosphates (Weise and Sharkey, 2005). The energetic cost of starch conversion to Suc at night can vary from 2 to 3 ATP/Suc synthesized (counting the UTP used by UDP-Glc pyrophosphorylase as an ATP). If Glc alone were exported, this would require two ATPs for the hexokinase activity in the cytosol plus the UTP. If the energy in the Glc-Glc bond of β-maltose can be preserved, then the export of β-maltose would incur an energetic cost that is one-third less than export of Glc alone or the active transport of maltose.
In conclusion, we have demonstrated a simple and robust method for the detection of the α- and β-forms of maltose. The β-anomer of maltose shows a diel change, with β-maltose present only during starch degradation at night and absent in starchless mutants. When maltose metabolism is blocked, β-maltose levels are high. Therefore, β-maltose is the metabolically active form of maltose during transitory starch degradation and conversion to Suc. The difference in distribution of the two maltose anomers between the chloroplast and the cytosol resolves the previous question of why more maltose was seen in the cytosol than the chloroplast. There is a substantial gradient of β-maltose from the chloroplast to the cytosol at night.
MATERIALS AND METHODS
Plant Materials and Growing Conditions
Bean (Phaseolus vulgaris) cv Linden; wild-type Arabidopsis (Arabidopsis thaliana) of the ecotype Wassilewskija; the starch-excess mutant dpe2-1, deficient in amylomaltase, genetic background Wassilewskija (Lu and Sharkey, 2004); and 2 starchless mutants, pgi1-1, deficient in the plastidic isozyme of pgi, genetic background Columbia (Yu et al., 2000), and stf1, deficient in the plastidic isozyme of phosphoglucomutase, genetic background ecotype Landsberg erecta (Kofler et al., 2000), were used. All plants were grown under a 12-h photoperiod, 400 μmol photons m−2 s−1. A Conviron (Pembina, ND) growth chamber with cool-white fluorescent tubes supplemented with 6 60-W incandescent lamps was used. The temperature was 24°C during the light period and 18°C during the dark period. Humidity was maintained at a minimum of 60%. Plants used in experiments were between 3 and 5 weeks old.
Maltose Extraction
Leaf punches (3.4 cm2) were taken from bean leaves 6 h after the beginning of the 12-h-light period and 6 h after the beginning of the dark period. Leaf punches were quickly placed into a 1.5-mL microfuge tube and plunged into liquid nitrogen. Leaf material was ground to a powder while frozen and 300 μL of 3.5% perchloric acid was added to the leaf powder to stop all metabolism and extract the soluble carbohydrates. Leaf powder and perchloric acid were thoroughly mixed by vortexing the microfuge tubes. Tubes were then centrifuged for 5 min and 200 μL of the perchloric acid was carefully pipetted off and saved. The perchloric acid was neutralized and brought to a pH of 7 by adding approximately 68 μL of neutralizing buffer containing 2 m KOH, 150 mm HEPES, and 10 mm KCl. The sample was frozen and then thawed to precipitate salts, centrifuged, and the supernatant was placed in a new microfuge tube. For Arabidopsis, 2 to 4 leaves were taken (0.06–0.2 g FW) 6 h after the beginning of the 12-h-light period and 6 h after the beginning of the dark period. Leaves were quickly placed into a 1.5-mL microfuge tube and plunged into liquid nitrogen. Microfuge tubes were then weighed and further treatment was as described for bean leaf punches.
α- and β-Maltose Assays
Maltose determinations were made using NADP-linked assays (Lowry and Passonneau, 1972) in a Sigma ZFP 22 dual wavelength filterphotometer (Sigma Instrument, Berlin). A 50 mm KH2PO4 assay buffer, pH 7.4, containing 20 mm KCl, 10 mm MgCl2, 2 mm EDTA, 500 μm NADP, 500 μm ATP, 0.25% Triton X-100, 1 unit mL−1 Glc-6-P dehydrogenase was prepared at the beginning of each day and kept on ice. A total of 800 μL of assay buffer was added to a self-masking cuvette along with 5 to 10 μL of plant sample. The cuvette was then placed in the spectrophotometer for 7 min before beginning the assay to allow the mixture to come to room temperature. The reaction was started by adding 1 unit of hexokinase to the cuvette. We used 334 nm for the measuring wavelength and 405 for the control wavelength in the dual wavelength measurement system to allow very sensitive measurements. The extinction coefficient of NADPH at 334 nm is 6,180 OD M−1 cm−1.
All chemicals and enzymes, with the exception of maltose phosphorylase and maltose epimerase, were purchased from Sigma-Aldrich (St. Louis). Maltose epimerase and maltose phosphorylase were purchased from Kikkoman (Tokyo). These enzymes allow accurate and precise measurements of maltose, even in the presence of micromolar levels of Suc, using the reactions shown in Figure 1B (Shirokane et al., 2000). Maltose phosphorylase has a strict specificity for α-maltose. Four units of maltose phosphorylase were added to the cuvette to determine the amount of α-maltose present in the sample. Maltose epimerase catalyzes the interconversion of α- and β-maltose (Shirokane and Suzuki, 1995). Four units of maltose epimerase were added to the cuvette after all the α-maltose had been removed by maltose phosphorylase and subsequent reactions. By adding maltose phosphorylase and maltose epimerase sequentially, accurate and precise measurements of both the α- and β-anomers of maltose were possible. When maltose epimerase was added before maltose phosphorylase, no signal was detected. Upon adding maltose phosphorylase, a signal that corresponded to the total amount of maltose was seen.
Nonaqueous Fractionation
Nonaqueous fractionation of leaf material was carried out using methods similar to those of Gerhardt and Heldt (1984) as modified (Sharkey and Vanderveer, 1989) and described briefly here. Two leaflets from bean (3–4 g FW) were taken 6 h after the beginning of the 12-h-light period and 6 h after the beginning of the dark period. Leaflets were immediately frozen in liquid nitrogen. Frozen leaves were then ground in a mortar and pestle and kept frozen during the grinding process by the addition of liquid nitrogen. A leaf material/liquid nitrogen slurry was then poured into a 50-mL Falcon tube and a 70-μm nylon cell strainer was quickly attached to the top of the tube with Parafilm to prevent leaf material from escaping due to the boiling of the liquid nitrogen. After most of the liquid nitrogen had boiled off, the Falcon tube was placed into a specially made refrigerated freeze drying apparatus, with a glass cold finger placed in a dry ice ethanol bath. For the first 3 h of the drying process, the dryer was kept at room temperature. After 3 h, the sample was brought to −20°C and dried for 3 to 4 d until the pressure dropped to 4 to 5 Pa. The refrigeration was then turned off and the sample was allowed to come to room temperature under vacuum for one additional day to avoid condensation on the sample when the vacuum was broken.
The sample was then brought to atmospheric pressure by adding dry nitrogen gas. Next, 15 mL of dry heptane/tetrachloroethylene, density 1.32 g mL−1, was added to the tube. The sample was then placed in an aluminum cold block, previously chilled to −20°C, and sonicated with a Branson Bio cell disruptor 250 with a micro horn (Danbury, CT) at a power level of 5.5 and 40% duty cycle. The sample was sonicated for 1 min, allowed to recool for 1 min, and then sonicated a second minute. After sonification, the suspension was filtered though 105-micron nylon mesh into a new 50-mL Falcon tube. The retentate was rinsed once with 10 mL of dry heptane/tetrachloroethylene, density 1.32 g mL−1, and a second time with 10 mL of dry heptane alone. The filtrate was centrifuged at slow speed, <2,000g, for 7 min in a clinical centrifuge. The supernatant was then decanted and discarded and the pellet was resuspended in 4 mL of dry heptane/tetrachloroethylene, density 1.32 g mL−1. The resuspended sample was then placed on a gradient consisting of 6 density steps, 4 mL each, from 1.6 g mL−1 to 1.35 g mL−1. The remainder of the sample was used to assay for total enzyme activity and metabolite levels. The gradient was centrifuged in a swinging bucket rotor at 4,000g for 1.5 h.
After centrifugation, the gradient was divided into five 5- to 7-mL fractions. From each fraction, 1 mL was taken for enzyme assays with the remainder being used for α- and β-maltose assays. Heptane was added to each sample, and samples were centrifuged. The supernatant was decanted and the pellets were allowed to dry overnight under reduced pressure in a desiccator containing paraffin and silica gel. After the samples were dried, they were weighed so that results from the enzyme and metabolite portions of each part of the gradient could be related to each other.
Marker Enzyme Assays
For enzyme assays, the samples were resuspended in 750 μL of 50 mm HEPES, pH 7.8, 10 mm dithiothreitol (DTT), 5 mm MgCl2, 1 mm EDTA, 20% glycerol, and 1% (v/v) protease inhibitor cocktail (Sigma P 9599). The samples were briefly sonicated. The samples were then centrifuged for 5 min in a microcentrifuge. The supernatant was used for enzyme assays and the pellet was used for Chl determinations. All manipulations were carried out on ice.
NADP-dependent glyceraldehyde 3-P dehydrogenase (GAPDH) was used for the chloroplast marker. GAPDH was assayed at room temperature by adding 2 to 10 μL of extract to 800 μL of 100 mm Bicine, pH 7.8, 30 mm DTT, 20 mm MgCl2, 20 mm KCl, 2 mm EDTA, 1 mm ATP, 300 μm NADPH, and 0.15 units 3-phosphoglycerate kinase. The sample was incubated on the bench for 10 min prior assaying to allow the DTT to activate the GAPDH. After activation, the sample was place in a spectrophotometer and the reaction was started by adding 3-phosphoglyceric acid to give a final concentration of 4 mm. The decrease in absorbance as NADPH was oxidized to NADP was read at 334 nm.
Phosphoenolpyruvate (PEP) carboxylase was used as the cytosol marker. PEP carboxylase was assayed at room temperature by adding 5 μL of extract to 250 μL of 100 mm Bicine, pH 7.8, 20 mm MgCl2, 20 mm KCl, 3.15 mm NaHCO3, with 1.18 Ci mol−1 NaH14CO3, 2 mm EDTA, 466 μm NADH, and 8 units malate dehydrogenase. The reaction was started by adding PEP to give a final concentration of 2 mm. After 5 min, the reaction was stopped by adding 500 μL of 2 n HCl. The samples were dried overnight. The next day, 100 mL of water and 3 mL of Bio-Safe II scintillation cocktail were added to each sample. After vortexing, the samples were counted in a scintillation counter.
Chlorophyll was determined by adding 1 mL of 95% ethanol to the pellet, briefly sonicating, and then centrifuging for 2 min in a microcentrifuge. The absorbance at 654 was read and converted to chlorophyll using the equations in Wintermans and DeMots (1965).
Previous work found no maltose in the vacuole (Weise et al., 2004); therefore, data analysis was done assuming two compartments (Gerhardt and Heldt, 1984; Sharkey and Vanderveer, 1989). Other compartments of leaf cells are small; for example, the mitochondrial compartment is estimated to be less than 1% of the volume of spinach leaf cells (Winter et al., 1994). We used compartment volumes for spinach (Spinacia oleracea) to estimate concentrations (Winter et al., 1994). Maltose was measured after extraction of the metabolite fraction in 300 to 500 μL 3.5% perchloric acid. Further treatment was as described above for maltose extraction.
Supplementary Material
This work was supported by the Chemical Sciences, Geosciences and Biosciences Division, U.S. Department of Energy (grant nos. DE–FG02–99ER20345 and DE–FG02–04ER15565).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.055996.
References
- Bailey JM, Fishman PH, Pentchev PG (1967) Studies on mutarotases I. Purification and properties of a mutarotase from higher plants. J Biol Chem 242: 4263–4269 [PubMed] [Google Scholar]
- Bailey JM, Fishman PH, Pentchev PG (1968) Studies on mutarotases. II. Investigations of possible rate-limiting anomerizations in glucose metabolism. J Biol Chem 243: 4827–4831 [PubMed] [Google Scholar]
- Boos W, Shuman H (1998) Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol Mol Biol Rev 62: 204–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia T, Thorneycraft D, Chapple A, Messerli G, Chen J, Zeeman S, Smith SM, Smith AM (2004) A cytosolic glycosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. Plant J 37: 853–863 [DOI] [PubMed] [Google Scholar]
- Gerhardt R, Heldt HW (1984) Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in nonaqueous media. Plant Physiol 75: 542–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt HW, Chon CJ, Maronde D, Herold A, Stankovic ZS, Walker DA, Kraminer A, Kirk MR, Heber U (1977) Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol 59: 1146–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler H, Häusler RE, Schulz B, Gröner F, Flügge U-I, Weber A (2000) Molecular characterisation of a new mutant allele of the plastid phosphoglucomutase in Arabidopsis, and complementation of the mutant with the wild-type cDNA. Mol Gen Genet 263: 978–986 [DOI] [PubMed] [Google Scholar]
- Kruger NJ, ap Rees T (1983) Maltose metabolism by pea chloroplasts. Planta 158: 179–184 [DOI] [PubMed] [Google Scholar]
- Lao NT, Schoneveld O, Mould RM, Hibberd JM, Gray JC, Kavanaugh TA (1999) An Arabidopsis gene encoding a chloroplast-targeted β-amylase. Plant J 20: 519–527 [DOI] [PubMed] [Google Scholar]
- Levi C, Gibbs M (1976) Starch degradation in isolated chloroplasts. Plant Physiol 57: 933–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV (1972) A Flexible System of Enzymatic Analysis. Academic Press, Orlando, FL, pp 1–291
- Lu Y, Sharkey TD (2004) The role of amylomaltase in maltose metabolism in the cytosol of photosynthetic cells. Planta 218: 466–473 [DOI] [PubMed] [Google Scholar]
- Ludewig F, Sonnewald U, Kauder F, Heineke D, Geiger M, Stitt M, Müller-Röber BT, Gillissen B, Kühn C, Frommer WB (1998) The role of transient starch in acclimation to elevated atmospheric CO2. FEBS Lett 429: 147–151 [DOI] [PubMed] [Google Scholar]
- Neuhaus HE, Schulte N (1996) Starch degradation in chloroplasts isolated from C3 or CAM (crassulacean acid metabolism)-induced Mesembryanthemum crystallinum L. Biochem J 318: 945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nittylä T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC (2004) A novel maltose transporter is essential for starch degradation in leaves. Science 303: 87–89 [DOI] [PubMed] [Google Scholar]
- Peavey DG, Steup M, Gibbs M (1977) Characterization of starch breakdown in the intact spinach chloroplast. Plant Physiol 60: 305–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost S, Frank C, Beck E (1996) The chloroplast envelope is permeable for maltose but not for maltodextrins. Biochim Biophys Acta 1291: 221–227 [DOI] [PubMed] [Google Scholar]
- Scheidig A, Fröhlich A, Schulze S, Lloyd JR, Kossmann J (2002) Downregulation of a chloroplast-targeted β-amylase leads to a starch-excess phenotype in leaves. Plant J 30: 581–591 [DOI] [PubMed] [Google Scholar]
- Servaites JC, Geiger DR (2002) Kinetic characteristics of chloroplast glucose transport. J Exp Bot 53: 1581–1591 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Vanderveer PJ (1989) Stromal phosphate concentration is low during feedback limited photosynthesis. Plant Physiol 91: 679–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokane Y, Ichikawa K, Suzuki M (2000) A novel enzymatic determination of maltose. Carbohydr Res 329: 699–702 [DOI] [PubMed] [Google Scholar]
- Shirokane Y, Suzuki M (1995) A novel enzyme, maltose 1-epimerase from Lactobacillus brevis IFO 3345. FEBS Lett 367: 177–179 [DOI] [PubMed] [Google Scholar]
- Stitt M, ap Rees T (1979) Capacities of pea chloroplasts to catalyse the oxidative pentose phosphate pathway and glycolysis. Phytochemistry 18: 1905–1911 [Google Scholar]
- Stitt M, ap Rees T (1980) Carbohydrate breakdown by chloroplasts of Pisum sativum. Biochim Biophys Acta 627: 131–143 [DOI] [PubMed] [Google Scholar]
- Stitt M, Bulpin PV, ap Rees T (1978) Pathway of starch breakdown in photosynthetic tissues of Pisum sativum. Biochim Biophys Acta 544: 200–214 [DOI] [PubMed] [Google Scholar]
- Stitt M, Heldt HW (1981) Physiological rates of starch breakdown in isolated intact spinach chloroplasts. Plant Physiol 68: 755–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise SE, Sharkey TD (2005) Energetics of carbon export from the chloroplast at night. In Photosynthesis: Fundamental Aspects to Global Perspectives, Proceedings of the 13th International Congress on Photosynthesis, August 29–September 3, 2004. Allen Press, Lawrence, KS, (in press)
- Weise SE, Weber A, Sharkey TD (2004) Maltose is the major form of carbon exported from the chloroplast at night. Planta 218: 474–482 [DOI] [PubMed] [Google Scholar]
- Winter H, Robinson DG, Heldt HW (1994) Subcellular volumes and metabolite concentrations in spinach leaves. Planta 193: 530–535 [Google Scholar]
- Wintermans JGFM, DeMots A (1965) Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta 109: 448–453 [DOI] [PubMed] [Google Scholar]
- Yang Y, Steup M (1990) Polysaccharide fraction from higher plants which strongly interacts with the cytosolic phosphorylase isozyme. Plant Physiol 94: 960–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Lue WL, Wang SM, Chen J (2000) Mutation of Arabidopsis plastid phosphoglucose isomerase affects leaf starch synthesis and floral initiation. Plant Physiol 123: 319–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC, Smith SM, Smith AM (2004) The breakdown of starch in leaves. New Phytol 163: 247–261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.