Abstract
Context/objective:
Assessed feasibility and potential effectiveness of using a novel robotic upright stand trainer (RobUST) to deliver postural perturbations or provide assistance-as-needed at the trunk while individuals with spinal cord injury (SCI) performed stable standing and self-initiated trunk movements. These tasks were assessed with research participants’ hands on handlebars for self-balance assistance (hands on) and with hands off (free hands).
Design:
Proof of concept study.
Participants:
Four individuals with motor complete (n = 3) or incomplete (n = 1) SCI who were not able to achieve independent standing and presented a neurological lesion level ranging from cervical 4 to thoracic 2.
Outcome measures:
Ground reaction forces, trunk displacement, and electromyography activity of trunk and lower limb muscles.
Results:
Research participants received continuous pelvic assistance via RobUST, and manual trainer assistance at the knees to maintain standing. Participants were able to attempt all tasks. Free hands trunk perturbations resulted in greater load bearing-related sensory information (73% ipsilateral vertical loading), trunk displacement (57%), and muscle activation compared to hands on. Similarly, free hands stable standing with RobUST assistance-as-needed resulted in 8.5% larger bodyweight bearing, 112% larger trunk movement velocity, and higher trunk muscles activation compared to standing with hands on. Self-initiated trunk movements controlled by hands on showed 116% greater trunk displacement, 10% greater vertical ground reaction force, and greater ankle muscle activation compared to free hands.
Conclusion:
RobUST established a safe and challenging standing environment for individuals with SCI and has the potential to improve training paradigms and assessments of standing postural control.
Keywords: Spinal cord injury, Standing, Postural control, Robotics, Force field, Perturbation
Introduction
An injury to the human spinal cord can lead to motor deficits including the inability to walk, stand, and generate volitional movements. Postural control, which is a foundational ability to generate effective motor outputs, is also drastically impaired after spinal cord injury (SCI).1–4 Whether the SCI results in a motor complete (no clinically detectable motor function below the level of injury) or motor incomplete classification (some motor function below the level of injury), the spinal circuitry controlling posture and locomotion typically remains intact below the level of injury, and can generate activation patterns related to the peripheral sensory information provided.5–9.
Research on animal models shows postural control deficits to be related to the extent and location of SCI,10 highlighting the importance of supraspinal inputs for postural control.11–15 Complete spinal cats can achieve effective weight bearing during standing,16 but cannot control balance.17 Rabbits with incomplete SCI are unable to generate effective postural corrections in response to platform tilts.10,18 However, with the addition of spinal cord epidural stimulation, studies in spinalized and/or decerebrated animal models,19–22 which rely entirely on the integration of limb-related somatosensory information to adjust balance,22 provided evidence that the spinal circuitry retains unexpected capability to regain partial postural control.23,24 In humans with incomplete SCI who could achieve independent standing, standing postural training with visual feedback showed the potential to improve postural control during stable and dynamic standing.25 With epidural stimulation, we have observed that self-initiated bodyweight shifting resulted in meaningful lower limb activation pattern modulation after a motor complete and sensory incomplete SCI,26 suggesting that the human spinal circuitry can interpret posture-related sensory information.
These findings support the view that dedicated technology for postural training and testing of individuals with SCI would be beneficial for exploiting the postural control potential that conceivably resides in the human lumbosacral circuitry. Recently, a novel cable-driven robotic upright stand trainer (RobUST) has been designed and validated in able-bodied adults to provide trunk perturbations and standing balance assistance-as-needed through trunk and pelvic force fields at ROAR Laboratory (Columbia University).27,28 RobUST has also been shown to be a potential training device for upright postural control in one individual with SCI resulting in complete functional independence and the ability to stand and ambulate independently.29
The purpose of this proof-of-concept study was to assess the feasibility and potential effectiveness of using RobUST for trunk perturbation and assistance-as-needed in non-ambulatory individuals with chronic SCI (at least 1 year post-injury) that are not able to achieve independent standing. Additionally, we investigated how self-assistance for balance provided by the participants’ hands placed on fixed handlebars affected postural control during these motor tasks.
Materials and methods
Participants
Four individuals with motor complete (n = 3) or incomplete (n = 1) SCI, classified following the International Standards for Neurological Classification of Spinal Cord Injury using the American Spinal Injury Association (ASIA) Impairment Scale (AIS),30 were recruited for this study. These individuals presented a neurological level of the lesion ranging from C (cervical) 4 to T (thoracic) 2 and were not able to achieve independent standing (Table 1). Inclusion and exclusion criteria are reported in the Supplemental Methods.
Table 1.
Characteristics of the research participants.
| Pub ID | Sex | Body mass (kg) | Age (yrs) | Time since injury (yrs) | Injury level | AIS grade | L LE motor score | R LE motor score |
|---|---|---|---|---|---|---|---|---|
| A121 | M | 99.7 | 52 | 4.4 | C4 | B | 0 | 0 |
| A103 | M | 68.0 | 38 | 3.8 | T2 * | A | 0 | 0 |
| C43 | F | 70.3 | 33 | 17.7 | C4 | C | 5 | 12 |
| A124 | M | 68.0 | 27 | 4.8 | T1 | A | 0 | 0 |
Pub ID: publication identifier; Injury level: neurological level of the lesion. C: cervical; T: thoracic. L: left; R: right; LE: lower extremity. Injury grade and lower extremity motor score were assessed using the American Spinal Injury Association (ASIA) Impairment Scale (AIS). * Extensive hardware consisting of screws and rod fusion that begin at C4 and continue through T8.
The experimental protocol was approved by the Institutional Review Board at the University of Louisville (IRB #17.0587) and was in accordance with the declaration of Helsinki. All participants provided written informed consent prior to participating in this study. Research participants and other persons appearing in the Supplemental Videos and Figures included in this manuscript gave written informed consent and granted full permission for their image to be used in publication.
Experimental protocol
RobUST was operated in the perturbation modality to assess the effects of (i) trunk perturbations on standing postural control with free hands or with hands placed on fixed handlebars (hands on) to assist balance control (Supplemental video 1). RobUST was operated in the force field (FF) modality to provide trunk assistance-as-needed during (ii) stable standing (Supplemental video 2) and (iii) self-initiated trunk movements (Supplemental video 3) performed with free hands. Stable standing and self-initiated trunk movements were also performed without RobUST assistance at the trunk and with hands on, to mimic a typical standing environment for SCI individuals. Participants underwent an acclimation session for each standing condition prior to data collection. Visual feedback was provided during all sessions using a monitor (Figure 1C) that displayed the participant's transverse plane trunk position in relation to the circular boundary depicted on the 2-D plot.
Figure 1.
Left: Robotic upright stand trainer (RobUST) setup and components (left): (a) aluminum frame, (b) motion capture cameras, (c) visual feedback, (d) electrical box, (e) computer and controller setup, (f) force platforms, (g) motors/encoders. Right: representative SCI individual standing with RobUST: knees extension is manually assisted by a trainer, while RobUST applies constant force to assist hips extension, and provides a force field at the trunk to assist-as-needed.
Robotic stand trainer
The RobUST used in this study has been previously described in detail.27 The device consists of an aluminum frame with 12 mounted motors (Maxon Motor, Switzerland) for controlling forces applied by cables to human participants (Figure 1).
The cables are routed from the motors through pulleys and connected to a dedicated harness at the trunk and pelvis. Dedicated sensors measure the tension applied at each cable. The platform is equipped with ten infrared cameras (Vicon Bonita 10; Denver, CO), which track trunk and pelvis motion. This system allows the study of two different standing paradigms resulting from the application of forces at the trunk to (a) elicit postural perturbations or (b) provide FF to assist-as-needed. In the RobUST FF mode, a virtual circular boundary is programed around the individual (Supplemental Figure 1).28,31 Participants can move freely within the FF boundary, where a low-level (19 N) constant force was present to optimize the functioning of the device. A restoring force is applied when the trunk moves beyond the FF boundary, bringing it back within the boundary, closer to the neutral standing position.
Experimental procedures
The RobUST tasks were performed overground, with dedicated harness on participant’s trunk and pelvis. The trunk belt (width: 11 cm) was positioned with its top margin below the axilla, and shoulder straps connected to the trunk belt. The pelvic belt was centered on the anterior superior iliac spines and was secured by additional thigh straps. Manual trainer assistance at the knees was provided to maintain knee extension and avoid buckling. After the RobUST cables were attached to the harness, a constant force of 80N was applied to facilitate appropriate hip extension and anterior tilt, with additional manual trainer assistance provided as needed. This constant force was found to be optimal for assisting pelvis stability while allowing some pelvic displacement when higher forces came into play, such as strong muscle spasms or manual trainer repositioning.
Trunk perturbations
The four RobUST trunk motors exerted a low-level constant force (30 N) that provided appropriate cables tension to deliver controlled perturbations without hindering or promoting trunk movement. Perturbations were characterized by a trapezoidal force with 0.15s rise time, 0.8s constant time, and 0.15s fall time. Perturbation magnitude was selected during the acclimation session and was relative to the participant’s body weight (BW). Perturbation magnitudes equal to 10, 15, and 20%BW were attempted, and the magnitude ensuring a safe and challenging environment was selected. Specifically, the goal was to induce meaningful trunk movement while allowing the participant to control, at least partially, the displacement.
During data collection, four perturbations per direction (front (F), back (B), right (R), left (L)) were randomly delivered under two standing conditions: free hands (n = 16 perturbations) or hands on (n = 16 perturbations). Perturbation onset was preceded by a 3s countdown, and its direction was not disclosed to the participant. The perturbation was deemed successful if the participant was independently able to bring the trunk back to the neutral upright standing position or independently maintain the trunk posture for at least 3 s after the end of a perturbation. During the free hands condition, the perturbation was considered unsuccessful if participant used their upper limbs to grasp the handlebars for external support. Assistance was provided between 3 and 10 s after the perturbation by a trainer, if needed, to bring the trunk back to the center position. Participants were urged to contribute as much as possible to this task. Participants A121 and A103 were not able to maintain trunk control with free hands prior to the perturbation onset. Therefore, external manual assistance was provided by a trainer to avoid trunk collapse while allowing perturbation-elicited trunk displacement. Perturbation control was considered unsuccessful in these instances.
Stable standing and self-initiated trunk movements
During stable standing, participants were instructed to stand as stable as possible for one minute. Dynamic standing postural control was also assessed during antero-posterior (AP), medio-lateral (ML), and circular self-initiated trunk movements. The goal for the first two tasks was to achieve the largest AP or ML peak-to-peak trunk displacement. During circular movements, participants were instructed to cover as much area as possible with their trunk in a circular fashion.
Stable standing and self-initiated trunk movements were performed under two different conditions: (a) trunk assistance-as-needed provided by RobUST FF and free hands (FF-free hands); (b) hands on handlebars and no RobUST FF assistance at the trunk (hands on-No FF), to mimic a typical standing environment.26,32,33 The RobUST FF characteristics for trunk assistance-as-needed were determined as detailed in the Supplemental Methods. Three attempts for each trunk movement and condition were performed. The need for manual trainer assistance at the trunk resulted in a failed attempt. FF-free hands attempts that resulted in the placement of upper limbs on the handlebars were also considered failed attempts. Overall, 11% of the self-initiated trunk movement trials were rejected for further analysis for these reasons.
Data acquisition
Motor force, kinematic data, force plates data, and electromyography (EMG) of the following trunk and lower limb muscles were collected as detailed in the Supplemental Methods. EMG activity of right (R) upper trapezius (UT), rectus abdominis (RA), external oblique (OB), erector spinae (ES), adductor (AD), vastus lateralis (VL), medial hamstring (MH), tibialis anterior (TA), medial gastrocnemius (MG) was recorded.
Data analysis
A low-pass digital filter was applied to motor, force plate, and handlebar force data with a cut-off frequency of 25 Hz (postural perturbation data) or 10 Hz (stable standing and self-initiated trunk movement data).34,35 Kinematic data of the trunk geometric center27 were low-pass filtered (6 Hz) in Vicon Nexus. A band-pass digital filter (10-500 Hz) was applied to EMG data.
Trunk perturbations
The instantaneous resultant perturbation force applied at the trunk was calculated considering the force generated by the four trunk motors and the related angles resulting from trunk geometric center, pulleys, and horizontal axis.
Perturbation onset was defined as the time at which the resultant perturbation force was greater than the mean baseline force plus 3 times the standard deviation. Four time windows were defined to assess the time course of postural responses to trunk perturbations: (i) baseline (tb), 150–50 ms before perturbation onset; (ii) t1, 70–270 ms after perturbation onset; (iii) t2, 350–700 ms after perturbation onset; (iv) tc, time window customized for each research participant. tb, t1 and t2 were previously defined to assess earlier responses to postural perturbation and stabilizing reaction responses in able-bodied individuals.34,36 tc was consistent across conditions and defined retrospectively via visual inspection of the data to capture the late EMG responses generated by lower limb muscles between 700 ms and 5s after perturbation onset. tc was set as 2.1-5.0s for A121, 0.85-2.85s for A103, 0.85-1.70s for C43, and 0.85-1.60s for A124.
Trunk displacement was calculated as the total distance traveled by the geometric center from perturbation onset to the end of each time window. Peak vertical and horizontal (i.e. resultant magnitude of Fx and Fy) forces were calculated to characterize ground reaction forces. EMG signal was rectified and filtered by a 25 Hz low-pass filter to calculate peak EMG.
Stable standing
Mean trunk velocity was calculated as the total distance traveled in the transverse plane by the trunk geometric center divided by the standing attempt duration. Mean vertical forces were calculated from force plates and handlebars data. Integrated EMG normalized by standing attempt duration was calculated and reported for the muscles that showed activation during stable standing in at least one of the four research participants.
During stable standing with RobUST FF and free hands, the duration of assistance for trunk control was defined as the total time during which at least one of the four trunk motors was activated to generate a restoring force (FF-on-duration).
Self-initiated trunk movements
For each movement direction and condition, the attempt with greater trunk displacement was considered for further analysis (see Supplemental Methods for calculation details). RobUST FF-on-duration, peak vertical, and horizontal ground reaction forces were calculated. Additionally, integrated EMG normalized by standing attempt duration was calculated and reported for the muscles that showed activation during self-initiated trunk movements in at least one of the research participants.
The magnitude of the difference in kinetic, kinematic, and EMG outcomes between motor tasks practiced with free hands or hand on was assessed by Cohen's effect size (d).37 In particular, differences resulting in medium (0.50-0.79) or large (≥0.80) effect sizes were considered relevant.
Results
Trunk perturbations
Trunk perturbations magnitude ranged between 15%BW (participant C43) and 20%BW (participants A121, A103, A124). The perturbations elicited kinetic, kinematic, and EMG responses that were safely controlled by the participants during free hands and hands on conditions, with additional manual assistance provided by a trainer (manual trainer assistance) as needed (Figure 2, Supplemental Video 1). With hands on the handlebars, all participants successfully controlled all perturbations. The two participants that were able to maintain independent trunk control with free hands showed different success rates: 33% for C43 (achieved during back and left perturbations) and 100% for A124.
Figure 2.
Representative trunk perturbations with free hands or hands on handlebars applied on participants A121 and A124. Pert: perturbation resultant force; HB Fz: vertical force applied to the handlebar; antero-posterior (AP) or medio-lateral (ML) displacement of the trunk geometric center; R: right; UT: upper trapezius; TA: tibialis anterior; MG: medial gastrocnemius; ES: erector spinae; MH: medial hamstrings; vertical (z), medio-lateral (x) and antero-posterior (y) force (F) of the right force plate. Gray, shaded area reflects a period of standing with hands on the handlebars to reposition the trunk after the perturbation.
Trunk perturbations generally resulted in greater kinetic, kinematic, and EMG responses when the individuals were standing with free hands compared to hands on (Figures 2 and 3). Vertical loading on the lower limb ipsilateral to the perturbation was 25.8% greater than baseline during free hands condition compared to 12% greater than baseline during hands on (d = 2.83). Horizontal ground reaction force was 30% greater than baseline during free hands compared to 20% greater than baseline during hands on (d = 1.24). Unloading on the lower limb contralateral to the perturbation was also 42% greater during free hands (d = 1.51) (Figure 3). Trunk displacement was on average 57% greater with free hands (d = 0.82).
Figure 3.
Effects of trunk perturbations with free hands or hands on fixed handlebars. Fz (vertical ground reaction force) and Fh (horizontal ground reaction force), expressed as percent change from baseline (quiet standing immediately prior to perturbation), are reported for the side ipsilateral (Ipsi) to the perturbation, contralateral (Contra) to the perturbation, and for the anterior and posterior (AP) perturbations pooled together. Peak EMG normalized by baseline value is reported for each perturbation direction (L: left; R: right; B: back; F: front) for the following muscles: upper trapezius, UT; erector spinae, ES; rectus abdominis, RA; external oblique, OB; adductor, AD; vastus lateralis, VL; tibialis anterior, TA; medial gastrocnemius, MG. Data are shown as mean ± standard deviation. Medium (0.50-0.79, *) or large (≥ 0.80, **) effect size is reported.
The magnitude of the difference in peak EMG responses between free hands and hands on varied across muscles and perturbation directions. Medium and large differences primarily coincided with greater peak EMG observed with free hands as compared to hands on (Figure 3). It is also interesting to note that participant A124, who successfully controlled all perturbations with free hands, was the only individual demonstrating early activation (t1) of trunk muscles (Supplemental Figure 2).
Stable standing
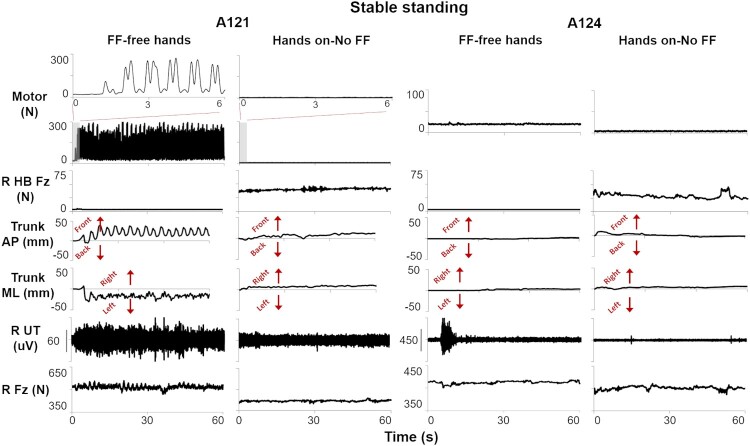
All individuals maintained trunk control during standing with hands on-No FF. While performing stable standing with free hands, individuals A121 and A103 required activation of RobUST FF to maintain the trunk in upright position hands (A121, Figure 4). These two individuals demonstrated a forward trunk displacement when the trainer’s manual assistance was removed. RobUST actively pulled the trunk toward the center position, and then stopped applying force until the trunk moved again outside of the FF boundary (Figure 4; Supplemental Video 2). Conversely, participants C43 and A124 performed stable standing with FF-free hands without RobUST FF activation as they remained within the circular boundary for the entire trial duration.
Figure 4.
Kinetic, kinematic, and electromyography (EMG) time series data for two participants (A121 and A124) during stable standing with (a) RobUST force field and free hands (FF-free hands), or (b) hands on handlebars without RobUST FF (hands on-No FF). RobUST FF was activated when A121 trunk moved beyond the force field boundary. HB Fz: vertical force applied to the handlebar; antero-posterior (AP) or medio-lateral (ML) displacement of the trunk geometric center; R: right; UT: upper trapezius; Fz: force plate vertical force.
Stable standing with RobUST FF and free hands resulted in 8.5% larger weight bearing (d = 1.19), larger trunk mean velocity (d = 0.96), and larger activation of representative trunk muscles as compared to standing with hands on (Figure 5). Conversely, no trends between the two standing conditions were found in lower limb muscles, as exemplified for VL, TA, and MG (Figure 5).
Figure 5.
Effects of RobUST force field and free hands (FF-free hands), or hands on handlebars without RobUST force field (hands on-No FF) on stable standing characteristics. Fz: combined force plate mean vertical force, expressed as percent body weight (%BW). Mean Vel.: mean velocity of the trunk geometric center. iEMG: integrated EMG normalized by baseline (quiet sitting) values is reported for right UT: upper trapezius; OB: external oblique; ES: erector spinae; VL: vastus lateralis; MG: medial gastrocnemius; TA: tibialis anterior. Data are shown as mean ± standard deviation. Medium (0.50-0.79, *) or large (≥ 0.80, **) effect size is reported.
Self-initiated trunk movements
All participants were able to perform the self-initiated trunk movements while standing with hands on-No FF (i.e. Supplemental Video 3). During FF-free hands, participants C43 and A124 performed two of the three movements without FF activation (ML and circle for C43; AP and ML for A124). The remaining task was performed while interacting with RobUST FF, which was active for 2.1 s (C43) or 1.8 s (A124, see Supplemental Video 3). Individuals A121 and A103 required RobUST FF activation throughout the entire duration of all FF-free hands attempts.
Trunk displacement was notably greater with large effect sizes in all movement tasks controlled by hands on the handlebars (AP, d = 3.53, ML, d = 2.27; Circle, d = 2.24). Medium and large differences in vertical peak force primarily coincided with greater values observed for hands on without RobUST FF (Figure 6). Similarly, increased horizontal peak forces were found during hands on-No FF conditions. On the other hand, during FF-free hands, greater UT activation (46%) was observed across movement directions as compared to hands on-No FF (AP, d = 1.38; ML, d = 1.08; Circle, d = 0.56). No consistent activation trends were detected for the other trunk and thigh muscles assessed in this study. Conversely, representative distal muscles (TA and MG) tended to show greater activity during hands on-No FF.
Figure 6.
Kinetic, kinematic and EMG variables assessed during self-initiated antero-posterior (AP), medio-lateral (ML) and circular (Circle) trunk movements performed with RobUST force field and free hands (FF-free hands), or hands on handlebars without RobUST force field (hands on-No FF). R: right force plate; L: left force plate. Fz (vertical ground reaction force) and Fh (horizontal ground reaction force) are expressed as percent body weight (%BW). iEMG: integrated EMG normalized by baseline (quiet sitting) values is reported for right UT: upper trapezius; ES: erector spinae; OB: external oblique; TA: tibialis anterior; MG: medial gastrocnemius; VL: vastus lateralis. Data are shown as mean ± standard deviation. Medium (0.50-0.79, *) or large (≥ 0.80, **) effect size is reported.
Discussion
Standing is one of the primary tasks practiced by individuals with SCI to improve functional recovery. SCI participants, particularly those with impaired trunk control, generally place their upper limbs on dedicated devices (i.e. standing apparatus or walkers) to self-assist postural control.32,33,38–40 In the present study, a robotic-enhanced standing environment was utilized in which free hands upright postural control could be practiced and investigated among individulas with SCI who were unable to stand independently. The study demonstrated the feasibility of using the RobUST to promote standing and self-initiated trunk movements without the use of upper limbs for balance control in this population. The RobUST was also effective in delivering precise perturbations that participants could attempt to control with free hands and with hands on handlebars.
Appropriate lower limb kinetic responses to RobUST trunk perturbations (i.e. leg loading in response to ipsilateral perturbations, and leg unloading following contralateral perturbations) were augmented during free hands as compared to hands on (Figures 2 and 3). Horizontal forces, which also play an important role in postural response generation, and trunk displacement followed the same trend of greater magnitude with free hands. This is important because somatosensory information plays a major role in the generation and control of postural responses,41–43 and because leg loading is a crucial determinant of the neuromuscular activation pattern characteristics after SCI. For example, increasing weight bearing during stepping on a treadmill with body weight support facilitates the generation of appropriate muscle activation patterns, while lower weight bearing leads to abnormal locomotor patterns.8,44 Research on animal models also showed that sensory information associated with changes in limb loading due to platform tilting is important to elicit postural-related neuromuscular responses.10,19 Similarly, the larger kinematic- and kinetic-related sensory information observed with free hands in this study was more often accompanied by greater peak activation of representative trunk and lower limb muscles (Figures 2 and 3).
Stable standing with RobUST FF for assistance-as-needed and free hands resulted in larger bodyweight bearing, larger mean trunk velocity, and higher activation of representative trunk muscles (Figures 4 and 5). These findings agree with previous observations in abled-bodied individuals during independent standing which demonstrated that providing mechanical stabilization with upper limbs leads to altered postural strategies that reduce or even suppress trunk and lower limb neuromuscular responses.28,45–48 Similarly, the use of upper limbs on parallel bars for assisting postural control can impair gait patterns in paralyzed individuals.49 As expected, self-initiated trunk movements showed much greater trunk displacement with hands on to lead dynamic postural control (Figure 6). This coincided with a trend of higher vertical peak force and higher activation of representative ankle plantar and dorsi flexors. However, UT was generally more active with free hands, conceivably reflecting its volitional engagement to contribute to the dynamic postural control required by these motor tasks.50.
These observations support the view that the human spinal circuitry can generate postural responses after SCI, even though postural control is severely impaired.10,18 It is therefore important to find appropriate strategies to capitalize on this capability. From this perspective, the regular standing environment for SCI individuals, which includes self-assistance for balance provided by the participants’ upper limbs placed on assistive devices, appears suboptimal for assessment and training of standing postural control. RobUST provides a more functional standing environment with enriched postural-related sensory information that can be used by non-ambulatory SCI individuals. Clinically, providing an environment that is as sensory rich as possible, i.e. least amount of external and self-assistance, may be a beneficial facet of postural rehabilitation by augmenting postural responses and activating the nervous system below the level of injury. Future studies should be aimed at expanding RobUST-mediated postural perturbations and assistance-as-needed at the pelvis, as well as implementing RobUST for postural training in individuals with different injury severity, including motor complete SCI individuals receiving spinal cord stimulation, in order to assess the potential of postural control recovery in paralyzed individuals.
Supplementary Material
Acknowledgments
This work was supported by the New York State Department of Health under Grant C31290GG. We thank the research volunteers for their valuable contribution to this study. We also gratefully acknowledge our research staff for their contribution to data collection, and our training staff for their support of the research volunteers; Dr Sarah Wagers medical oversight; Yukishia Austin, Lynn Robbins, and Kristen Johnson for medical management.
Authors declare no competing interests. The datasets generated during and/or analyzed during the current study are available from the corresponding author through material transfer agreement upon reasonable request.
Funding Statement
This work was supported by the New York State Spinal Cord Injury Research Board under Grant C31290GG.
Disclosure statement
Conflict of interest No potential conflict of interest was reported by the author(s).
References
- 1.Horak F, Macpherson J.. Postural orientation and equilibrium. In: S J, R L, (eds.) Handbook of physiology. New York: Oxford University Press; 1996. p. 255–292. [Google Scholar]
- 2.Macpherson J, Deliagina T, Orlovsky G.. Control of body orientation and equilibrium in vertebrates. In: Stein P, Grillner S, Selverston A, Stuart D, (eds.) Neurons, networks and motor behavior. Cambridge, MA: MIT Press; 1997. p. 257–267. [Google Scholar]
- 3.Orlovsky T, Orlovskiĭ GN, Deliagina T, Grillner S.. Neuronal control of locomotion: from mollusc to man. Oxford: Oxford University Press; 1999. [Google Scholar]
- 4.Massion J, Dufosse M.. Coordination between posture and movement: why and how? Physiology 1988;3(3):88–93. [Google Scholar]
- 5.Edgerton VR, Tillakaratne NJK, Bigbee AJ, de Leon RD, Roy RR.. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci 2004;27:145–167. [DOI] [PubMed] [Google Scholar]
- 6.Grillner S. Neurobiological bases of rhythmic motor acts in vertebrates. Science 1985;228(4696):143–149. [DOI] [PubMed] [Google Scholar]
- 7.Hubli M, Dietz V.. Concurrent neuromechanical and functional gains following upper-extremity power training post-stroke. J Neuroeng Rehabil 2013;10(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR.. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol 1997;77(2):797–811. [DOI] [PubMed] [Google Scholar]
- 9.Roy RR, Harkema SJ, Edgerton VR.. Basic concepts of activity-based interventions for improved recovery of motor function after spinal cord injury. Arch Phys Med Rehabil 2012;93(9):1487–1497. [DOI] [PubMed] [Google Scholar]
- 10.Lyalka VF, Orlovsky GN, Deliagina TG.. Impairment of postural control in rabbits with extensive spinal lesions. J Neurophysiol 2009;101(4):1932–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musienko PE, Zelenin PV, Lyalka VF, Orlovsky GN, Deliagina TG.. Postural performance in decerebrated rabbit. Behav Brain Res 2008;190(1):124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deliagina T, Popova L, Grant G.. The role of tonic vestibular input for postural control in rats. Arch Ital Biol 1997;135(3):239–261. [PubMed] [Google Scholar]
- 13.Deliagina TG, Zelenin PV, Orlovsky GN.. Physiological and circuit mechanisms of postural control. Curr Opin Neurobiol 2012;22(4):646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macpherson JM, Fung J, Jacobs R.. Postural orientation, equilibrium, and the spinal cord. Adv Neurol 1997;72:227–232. [PubMed] [Google Scholar]
- 15.Mori S, Matsuyama K, Kohyama J, Kobayashi Y, Takakusaki K.. Neuronal constituents of postural and locomotor control systems and their interactions in cats. Brain Dev 1992;14:S109–S120. [PubMed] [Google Scholar]
- 16.De. Leon R, Hodgson J, Roy R, Edgerton VR.. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J Neurophysiol 1998;80(1):83–91. [DOI] [PubMed] [Google Scholar]
- 17.Macpherson JM, Fung J.. Weight support and balance during perturbed stance in the chronic spinal cat. J Neurophysiol 1999;82(6):3066–3081. [DOI] [PubMed] [Google Scholar]
- 18.Lyalka VF, Zelenin PV, Karayannidou A, Orlovsky GN, Grillner S, Deliagina TG.. Impairment and recovery of postural control in rabbits with spinal cord lesions. J Neurophysiol 2005;94(6):3677–3690. [DOI] [PubMed] [Google Scholar]
- 19.Musienko PE, Zelenin PV, Orlovsky GN, Deliagina TG.. Facilitation of postural limb reflexes with epidural stimulation in spinal rabbits. J Neurophysiol 2010;103(2):1080–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavrov I, Gerasimenko Y, Burdick J, Zhong H, RR Roy, VR Edgerton. Integrating multiple sensory systems to modulate neural networks controlling posture. J Neurophysiol 2015;114(6):3306–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musienko PE, Gorskiĭ OV, Kilimnik VA, Kozlovskaia IB, Courtine G, Edgerton VR, et al. [Neuronal control of posture and locomotion in decerebrated and spinalized animals]. Ross Fiziol Zh Im I M Sechenova 2013;99(3):392–405. [PubMed] [Google Scholar]
- 22.Musienko PE, Courtine G, Tibbs JE, Kilimnik VA, Savochin A, Garfinkel A, et al. Somatosensory control of balance during locomotion in decerebrated cat. J Neurophysiol 2012;107(8):2072–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyalka VF, Hsu L-J, Karayannidou A, Zelenin PV, Orlovsky GN, Deliagina TG.. Facilitation of postural limb reflexes in spinal rabbits by serotonergic agonist administration, epidural electrical stimulation, and postural training. J Neurophysiol 2011;106(3):1341–1354. [DOI] [PubMed] [Google Scholar]
- 24.Musienko PE, Gorskii OV, Kilimnik VA, Kozlovskaya IB, Courtine G, Edgerton VR, et al. Regulation of posture and locomotion in decerebrate and spinal animals. Neurosci Behav Physiol 2015;45(2):229–237. [Google Scholar]
- 25.Sayenko DG, Alekhina MI, Masani K, Vette A, Obata H, Popovic M, et al. Positive effect of balance training with visual feedback on standing balance abilities in people with incomplete spinal cord injury. Spinal Cord 2010;48(12):886–893. [DOI] [PubMed] [Google Scholar]
- 26.Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. The Lancet 2011;377(9781):1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan M, Luna T, Santamaria V, Omofuma I, Martelli D, Rejc E, et al. Stand trainer With applied forces at the pelvis and trunk: response to perturbations and assist-As-needed support. IEEE Trans Neural Syst Rehabil Eng 2019;27(9):1855–1864. [DOI] [PubMed] [Google Scholar]
- 28.Luna TD, Santamaria V, Omofumal I, Khan MI, Agrawal SK. editors. Control mechanisms in standing while simultaneously receiving perturbations and active assistance from the robotic upright stand trainer (RobUST)). International conference for biomedical robotics and biomechatronics (BioRob); 2020. New York, USA: IEEE. [Google Scholar]
- 29.Santamaria V, Luna TD, Agrawal SK.. Feasibility and tolerance of a robotic postural training to improve standing in a person with ambulatory spinal cord injury. Spinal Cord Series and Cases 2021;7(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burns S, Biering-Sørensen F, Donovan W, Graves D, Jha A, Johansen M, et al. Using the spinal cord injury common data elements. Top Spinal Cord Inj Rehabil 2012;18(1):23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santamaria V, Luna T, Khan M, Agrawal S.. The robotic trunk-support-trainer (TruST) to measure and increase postural workspace during sitting in people with spinal cord injury. Spinal Cord Series and Cases 2020;6(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rejc E, Angeli CA, Harkema SJ.. Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PLOS ONE 2015;10(7):e0133998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med 2018;379(13):1244–1250. [DOI] [PubMed] [Google Scholar]
- 34.Carpenter MG, Allum JH, Honegger F.. Directional sensitivity of stretch reflexes and balance corrections for normal subjects in the roll and pitch planes. Exp Brain Res 1999;129(1):93–113. [DOI] [PubMed] [Google Scholar]
- 35.Leonard JA, Brown RH, Stapley PJ.. Responses of neurons in chinchilla auditory cortex to frequency-modulated tones. J Neurophysiol 2009;101(4):2017–2033. [DOI] [PubMed] [Google Scholar]
- 36.Henry SM, Fung J, Horak FB.. Emg responses to maintain stance during multidirectional surface translations. J Neurophysiol 1998;80(4):1939–1950. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan GM, Feinn R.. Using effect size—or Why thePvalue Is Not enough. J Grad Med Educ 2012;4(3):279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sayenko DG, Rath M, Ferguson AR, Burdick JW, Havton LA, Edgerton VR, et al. Self-assisted standing enabled by non-invasive spinal stimulation after spinal cord injury. J Neurotrauma 2019;36(9):1435–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang SR, Nandor MJ, Kobetic R, Foglyano KM, Quinn RD, Triolo RJ.. Real-time computer-based visual feedback improves visual acuity in downbeat nystagmus – a pilot study. J Neuroeng Rehabil 2016;13(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gill ML, Grahn PJ, Calvert JS, Linde MB, Lavrov IA, Strommen JA, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med 2018;24(11):1677–1682. [DOI] [PubMed] [Google Scholar]
- 41.Deliagina TG, Beloozerova IN, Popova LB, Sirota MG, Swadlow HA, Grant G, et al. Role of different sensory inputs for maintenance of body posture in sitting rat and rabbit. Motor Control 2000;4(4):439–452. [DOI] [PubMed] [Google Scholar]
- 42.Beloozerova IN, Zelenin PV, Popova LB, Orlovsky GN, Grillner S, Deliagina TG.. Postural control in the rabbit maintaining balance on the tilting platform. J Neurophysiol 2003;90(6):3783–3793. [DOI] [PubMed] [Google Scholar]
- 43.Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol 2002;88(3):1097–1118. [DOI] [PubMed] [Google Scholar]
- 44.Finch L, Barbeau H, Arsenault B.. Influence of body weight support on normal human gait: development of a gait retraining strategy. Phys Ther 1991;71(11):842–855. [DOI] [PubMed] [Google Scholar]
- 45.Horak FB, Henry SM, Shumway-Cook A.. Postural perturbations: new insights for treatment of balance disorders. Phys Ther 1997;77(5):517–533. [DOI] [PubMed] [Google Scholar]
- 46.Clapp S, Wing AM.. Light touch contribution to balance in normal bipedal stance. Exp Brain Res 1999;125(4):521–524. [DOI] [PubMed] [Google Scholar]
- 47.Jeka JJ. Light touch contact as a balance Aid. Phys Ther 1997;77(5):476–487. [DOI] [PubMed] [Google Scholar]
- 48.Dickstein R, Shupert CL, Horak FB.. Fingertip touch improves postural stability in patients with peripheral neuropathy. Gait Posture 2001;14(3):238–247. [DOI] [PubMed] [Google Scholar]
- 49.Visintin M, Barbeau H.. The effects of parallel bars, body weight support and speed on the modulation of the locomotor pattern of spastic paretic gait. A preliminary communication. Spinal Cord 1994;32(8):540. [DOI] [PubMed] [Google Scholar]
- 50.Jacobs J, Horak F.. Cortical control of postural responses. J Neural Transm 2007;114(10):1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.