ABSTRACT
Cryptococcosis remains a significant threat to human health. While the popular C57BL/6J mouse model of cryptococcosis has some advantages, there are some serious shortcomings that limit the ability of researchers to address the disease. Since humans are resistant to environmental cryptococcal infection until rendered immunodeficient while C57BL/6J mice are innately susceptible, we screened 15 inbred mouse strains for resistance to Cryptococcus infection. The SJL/J mouse strain was unusually resistant to several virulent strains of Cryptococcus while the closely related FVB/J strain was susceptible. The infection pathobiology in SJL/J mice and susceptible mice was similar for the first 7–10 days then markedly diverged toward pathogen clearance. Interferon-gamma (IFN-γ) expression was critical for SJL/J resistance while tumor necrosis factor-alpha was also important. Both CD4 and CD8 T cells produced IFN-γ and were collectively critical. While IL-17 and associated cytokines were expressed in SJL/J mice, IL-17 inhibition did not affect the outcome of infection. Unlike the C57BL/6J, infected SJL/J mice failed to express Th2/atopy-type cytokines even when rendered susceptible by IFN-γ blockade or infection with an alternative fatal cryptococcal strain virulent in SJL/J. These data suggest that the atopic-type response typically associated with the C57BL/6J model is not critical for susceptibility. The productive immune response in SJL/J mice resulted in immune memory that protected mice from reinfection. The SJL/J cryptococcal resistance phenotype was associated with a strong genetic linkage from regions located in chromosomes 2 and 11 suggesting strain-specific modifiers contribute to disease severity. Thus, SJL/J mice are an exciting alternative animal model for cryptococcal pathobiology.
IMPORTANCE
Cryptococcosis studies often utilize the common C57BL/6J mouse model. Unfortunately, infection in these mice fails to replicate the basic course of human disease, particularly hampering immunological studies. This work demonstrates that SJL/J mice can recapitulate human infection better than other mouse strains. The immunological response to Cryptococcus infection in SJL/J mice was markedly different from C57BL/6J and much more productive in combating this infection. Characterization of infected mice demonstrated strain-specific genetic linkage and differential regulation of multiple important immune-relevant genes in response to Cryptococcus infection. While our results validate many of the previously identified immunological features of cryptococcosis, we also demonstrate limitations from previous mouse models as they may be less translatable to human disease. We concluded that SJL/J mice more faithfully recapitulate human cryptococcosis serving as an exciting new animal model for immunological and genetic studies.
KEYWORDS: complement, Cryptococcus neoformans, Th1/Th2, atopic immunity, QTL analysis
INTRODUCTION
Infection with Cryptococcus species remains a major health concern. Even with a gold-standard treatment regimen, patients still experience mortality rates as high as 30% (1, 2). Thus, more effective treatment options are needed. While antifungal small-molecule drugs have always been the backbone of cryptococcosis therapy, additional modalities such as vaccination or immune modulation might be exciting treatment options (3 – 7). However, limitations in our knowledge of anticryptococcal immunity make the development of immune-focused treatments challenging.
Most people seroconvert to express cryptococcus-specific antibodies during childhood without serious illness (8, 9), suggesting that humans encounter this fungus relatively frequently. This also implies that most healthy humans respond to cryptococcal infection with effective immune responses and eventually develop some immunological memory of cryptococcal antigens. However, we have limited data about this host’s protective immune response from clinical studies as most clinical cases of cryptococcosis are highly associated with immune deficiency. Cryptococcosis was once considered an AIDS-defining illness (10 – 12) and is still strongly associated with late-stage AIDS. As other immunodeficiencies have become more prevalent, the association with cryptococcal infection with some specific immune deficiencies has also become clear (13 – 15). Of note, T-cell deficiency or disruption seems to be a common feature of Cryptococcus-associated immune deficiencies (16). Thus, the epidemiology of cryptococcosis suggests that T-cell-based immunity is critical for host protection and that immunocompetent humans are generally resistant to cryptococci but the exact immunological mechanisms of immunity are unclear.
Many laboratories have utilized animal models for molecular insight into the immunology and pathobiology of cryptococcal infection. While various animal models have been used (17), the C57BL/6J mouse infection model has become the most common, probably due to relatively easy access to genetic and reagent scientific tools. Pulmonary delivery of Cryptococcus yeasts, typically by intratracheal injection, intranasal administration, or intrapharyngeal aspiration, is thought to model the natural route of infection, inhalation of environmental yeast or spores. While scientific models attempt to mimic natural infection, we must acknowledge that differences in the size of inoculum and routes of infection may alter the observed level of resistance to infection. Alternatively, some groups choose to bypass the initial pulmonary phase of infection by injecting mice with cryptococci intravenously. In laboratory mice, pulmonary delivery of highly virulent Cryptococcus strains, such as C. neoformans strain H99 (ATCC 208821), results in a progressive and fatal infection characterized by fungal dissemination to the brain and, in some mouse strains, a markedly Th2- and atopy-dominated immune response which is not productive in clearing the microbe (18). On the other hand, less virulent Cryptococcus strains usually fail to produce a significant infection in mice and elicit only a minor immune response. A few moderate virulence strains, such as Cryptococcus neoformans strain D52 (ATCC 24067), cause a more chronic and eventually resolving infection in C57BL/6J mice that can be worsened by interfering with host protective factors, such as depleting T cells. However, even in these moderate virulence models, atopic-type immunity persists in C57BL/6J and other susceptible mice (19 – 25) which makes studies of productive immunity difficult. Taken together, the C57BL/6J mouse model can recapitulate cryptococcal dissemination into the CNS from a pulmonary initial infection and general T-cell involvement. However, data derived from the manipulation of host immune factors in these models are difficult to parse due to the nonproductive nature of the cellular immune response in these mice. Furthermore, it is unclear whether this type of nonproductive cellular immune response has any relevance during human infections. The constitution and mechanisms of the initial protective immune response as well as subsequent memory responses have been particularly difficult to probe using these C57BL/6J-based models.
While the C57BL/6J model may have some serious limitations, inbred mice are nonetheless still much more tractable and logistically advantageous compared to other animal models. Thus, an alternative inbred mouse that better models cryptococcal infection in humans is needed. In this study, we sought a relatively common inbred mouse strain that was naturally resistant to infection with highly virulent strains of Cryptococcus but that could be rendered susceptible to infection by experimental manipulation, such as the depletion of T cells. We found that SJL/J mice fit these parameters as they are naturally resistant to pulmonary cryptococcal infection but can be rendered susceptible.
It should be noted that SJL/J mice have some noted immunological and pathological variations compared to other strains of inbred mice. Some SJL/J mice develop spontaneous cancers and some muscular dystrophy-type symptoms, although much later in life than the mice used in the studies presented here (26, 27). SJL/J strain mice have also been extensively used in autoimmune studies, especially experimental autoimmune encephalomyelitis (EAE) (28, 29). Although SJL/J mice are notably more susceptible to EAE, wild-type SJL/J mice do not develop high frequencies of EAE without further experimental induction, such as autoantigen and adjuvant sensitization or transgene expression. Thus, SJL/J strain mice seem to be an intriguing model to study immune regulation.
The data presented here extend previous work showing that SJL/J mice are resistant to C. neoformans infection when infected intravenously (30) or using a less virulent C. neoformans strain (31). Using this SJL/J model, we demonstrate some important immunological differences between SJL/J mice and other mouse strains including adaptive immune resistance to repeat infection. Interestingly, we found that SJL/J mouse resistance to cryptococcal infection has a tractable genetic component with contributing regions on chromosomes 2 and 11. Overall, we consider SJL/J mice to be an exciting alternative strain to use for cryptococcal disease modeling.
RESULTS
C57BL/6J mice infected with highly virulent C. neoformans do not replicate the T-cell dependence observed in human infection cases
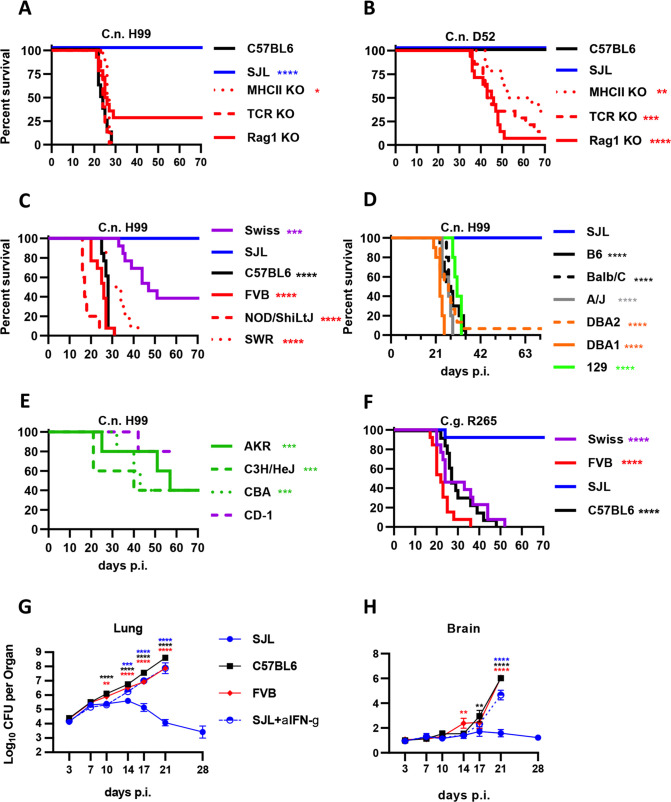
While substantial progress has been made by many groups using the C57BL/6J cryptococcosis mouse model, including ourselves, there are substantial limitations to this model. For example, the composition, characteristics, and mechanisms of protective immunity are particularly difficult to probe. To demonstrate this, we examined cryptococcal infections in wild-type C57BL/6J (Jax 000664) mice as well as genetically deficient mice derived from C57BL/6J. Mice deficient in MHCII (MHCII KO) lack CD4+ T cells, those deficient in the T-cell receptor (TCR KO) lack all T cells, and mice deficient in RAG1 (Rag1 KO) lack T and B cells. These mice were separately infected with two strains of C. neoformans: a highly virulent strain called H99 which is the most commonly used strain in the cryptococcal research field (Fig. 1A) and a moderately virulent strain D52 (Fig. 1B). While human infection is highly associated with AIDS and other functional T-cell immunodeficiencies, mice lacking T cells or even all lymphocytes on the C57BL/6J background have no difference in survival between wild-type and mutant strains when infected with the H99 strain indicating that T cells have no protective effect in this model (Fig. 1A). By contrast, these same mouse strains infected with the moderately virulent D52 strain show a substantial loss of protection in the T-cell-deficient strains (Fig. 1B).
Fig 1.
SJL/J strain mice are uniquely resistant to cryptococcal infection using highly virulent strains. Various strains of laboratory mice were infected with 5,000 yeast cells of indicated Cryptococcus strains. C57BL/6J (black line), SJL/J (blue), MHC class II KO (dotted dark red), TCR KO (dashed red), and Rag1 KO (solid red) mice were infected with (A) high virulence Cryptococcus neoformans strain H99 and (B) moderate virulence C. neoformans strain D52. For (A) and (B), statistical comparisons indicated strain compared to C57BL/6J mice. N = 8 mice per condition for C57BL/6J and SJL/J and 12–15 mice per condition for the knockout strains divided between two independent experiments. (C) Swiss-Webster (purple), SJL/J (blue), C57BL/6J (black), FVB/J (red), NOD/SHiLtJ (red dashed), and SWR/J (red dotted) mice were infected with high virulence C. neoformans strain H99. (D) SJL/J (blue), C57BL/6J (black), Balb/C (black dashed), A/J (gray), DBA2 (orange dashed), DBA1 (orange), and 129S1/SvImJ (129; green) mice were infected with high virulence C. neoformans strain H99. (E) AKR (green), C3H/HeH (green dashed), CBA (green dotted), and CD-1 mice (purple dashed) mice were infected with high virulence C. neoformans strain H99. For (D) and (E), N = 5 mice per group except C57BL/6J (20) and SJL/J (15). Several similar experiments were combined for these plots. (F) Swiss-Webster (purple), SJL/J (blue), C57BL/6J (black), and FVB/J (red) mice were infected with high virulence C. gattii strain R265. For (C) and (F), N = 10–13 mice per group divided into two independent experiments, except N = 5 for NOD/SHiLtJ. For (C)–(F), statistical comparisons are indicated strain compared to SJL/J mice. For these survival experiments, the mice in (A), (B), (C), and (F) are combined data from females and males with no notable differences observed between females and males, whereas (D) and (E) were performed with female mice. SJL/J (blue), C57BL/6J (black), FVB/J (red), and SJL/J mice dosed with anti-IFN-γ neutralizing monoclonal antibody (blue dashed) were infected with C. neoformans strain H99. At the indicated intervals following infection, lungs (G) and brains (H) were collected and fungal loads were measured. Statistical comparisons are the indicated (color matched) condition compared to SJL/J mice at the same timepoint. For (G) and (H), N = 16 total mice per group per timepoint with four total experimental repeats except for N = 8 with two experimental repeats for 17 days p.i.
SJL/J mice are highly resistant to infection with highly virulent Cryptococcus strains
Since serious limitations exist for exclusively using the C57BL/6J mice and the D52 C. neoformans strain including the ability to measure surrogate survival endpoints, we sought to identify a mouse strain that better replicates human cryptococcosis using the fully virulent H99 strain C. neoformans. Swiss-Webster mice were moderately resistant to infection with virulent C. neoformans H99 (Fig. 1C). Swiss-Webster mice are a genetically heterogeneous population of mice outbred from a contained mouse population now maintained by commercial breeders making further study of these mice difficult. However, many inbred strains were derived from the Swiss-Webster pool and are readily available from commercial sources. We, therefore, examined several Swiss-Webster-derived inbred strains for their susceptibility to C. neoformans H99. While FVB/J (Jax 001800), NOD/ShiLtJ (Jax 001976), and SWR/J (Jax 000689) mice were susceptible to C. neoformans H99 similar to C57BL/6J, the SJL/J (Jax 000686) strain was completely resistant to the infection with no deaths observed (Fig. 1C). SJL/J resistance to C. neoformans H99 appears to be highly unusual as no other tested mouse strain showed equivalent levels of resistance (Fig. 1D and E). This was in contrast to previous data using intravenous infection with a different C. neoformans strain showing high, but not unique, resistance in SJL/J mice (30). Resistance of SJL/J strain to Cryptococcus does not seem to be specific to the H99 strain since very similar resistance levels were observed when infected with Cryptococcus gattii strain R265, also a highly virulent strain belonging to a distinct lineage (Fig. 1F).
Cryptococcal infection in SJL/J and susceptible mice begins similarly but SJL/J mice later diverge and clear the infection
To investigate the trajectory of infection during C. neoformans H99 infection, fungal loads in the lungs and brains of pulmonary-infected mice were carefully monitored at several timepoints in the SJL/J strain, the related but susceptible FVB/J inbred strain, as well as the C57BL/6J strain. All infected mice showed a rapid early fungal expansion from the time of infection to 7 days post-infection (Fig. 1G). There seems to be a slowdown in fungal growth in all strains between days 7 and 14 post-infection, with a more pronounced plateau observed in the SJL/J mice with significant differences emerging on days 10 and 14 post-infection between SJL/J and the FVB/J and C57BL/6J mice (Fig. 1G). After day 14, fungal loads in SJL/J mice start to decrease markedly while explosive fungal growth continues in the C57BL/6J and FVB/J mice. The fungal loads observed in the SJL/J mice at 17 and 21 days post-infection were drastically reduced compared to FVB/J and C57BL/6J (Fig. 1G). Dissemination in the brain was also drastically reduced in SJL/J mice compared to C57BL/6J and FVB/J mice (Fig. 1H). The general trajectory of cryptococcal lung fungal loads seems to be consistent between fungal strains in SJL/J mice since Guillot et al. (31) and Carroll et al. (32) showed similar trends in fungal loads while utilizing the D52 strain of C. neoformans to infect SJL/J mice. That said, infection with the more virulent cryptococcal strain here does seem to result in an extended early period before fungal loads diverge in SJL/J mice vs susceptible strains.
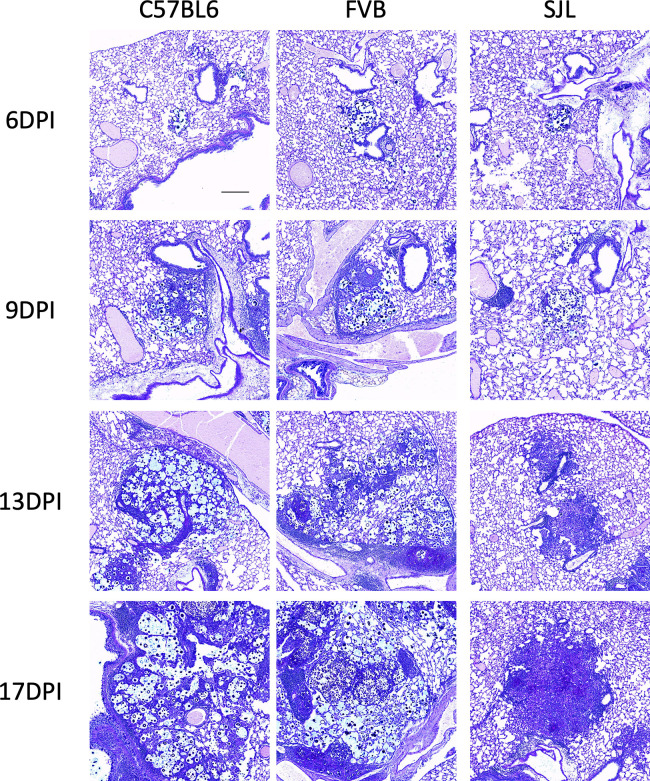
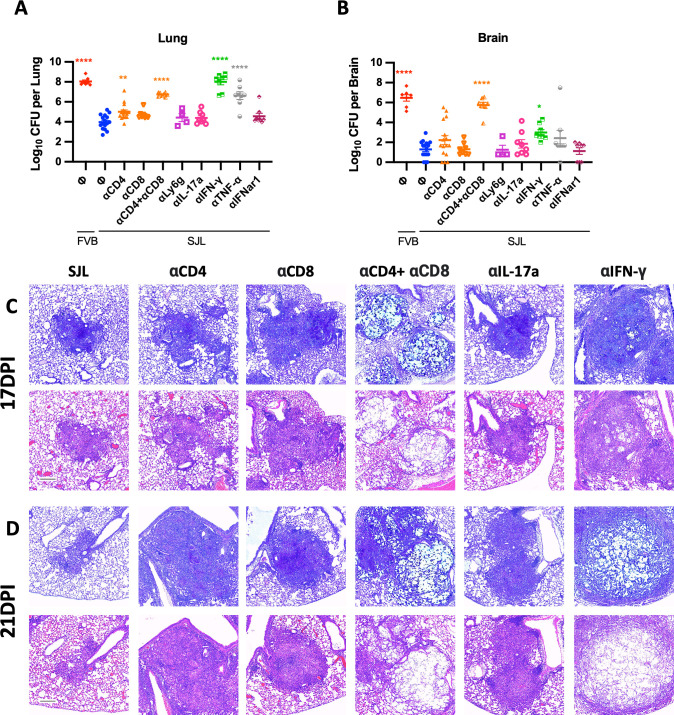
To monitor the infectious nodule properties and the general effectiveness of the immune response, lung sections were also acquired at corresponding times after infection. At 6 days post-infection, infectious loci appeared small and similar in all strains (Fig. 2; 6 dpi) with comparable abundance (Fig. S1, 6 dpi). Lung fungal nodules at day 9 appeared larger than day 6 with more cellularity but remained similarly sized in all examined strains (Fig. 2, 9 dpi; Fig. S1, 9 dpi). By 13 days post-infection, however, differences start to appear between the examined mouse strains in lung nodules. Lung nodules in the C57B/6J and FVB/J mice appear to be mostly fungal (Fig. 2, 13 dpi; note the blue staining marking the fungal products). While some host cell recruitment is evident, the fungal organism appears to be incompletely contained in FVB and C57BL6 lungs (Fig. 2, 13 dpi). By contrast, fungal nodules in SJL/J lungs demonstrate markedly better containment with more cellularity (Fig. 2, 13 dpi; note the dark purple color marking the nuclei of recruited cells). Consistent with this, lung nodules at 17 days post-infection in C57BL/6J and FVB/J mice were very large with failed containment of fungal cells. Nodules in SJL/J lungs appear almost as large but are mostly composed of host cells with only a few remaining fungal elements (Fig. 2, 17 dpi). In both the fungal load and histology, cryptococcal infection in SJL/J mice appears to be similar to FVB/J and C57BL/6J at early stages but pathobiology diverges toward clearance in SJL/J mice sometime between days 7 and 14 post-infection.
Fig 2.
Histological analysis shows that cryptococcal infection in SJL/J mice is similar to susceptible strains early but diverges late. Lung sections from C57BL/6J, FVB/J, and SJL/J mice infected with C. neoformans strain H99 at indicated times post-infection. Sections stained with alcian blue, which stains Cryptococcus yeast blue, and PAS, which stains host mucin and microbial carbohydrate bright pink. Note that structures that stain with both alcian blue and PAS appear dark blue or purple. DPI indicates days post-infection. The scale bar indicates 200 µm.
Robust Th1- and Th17-type cellular immunity in SJL/J mice contrasts against Th2-type immunity in C57BL/6J and FVB/J mice
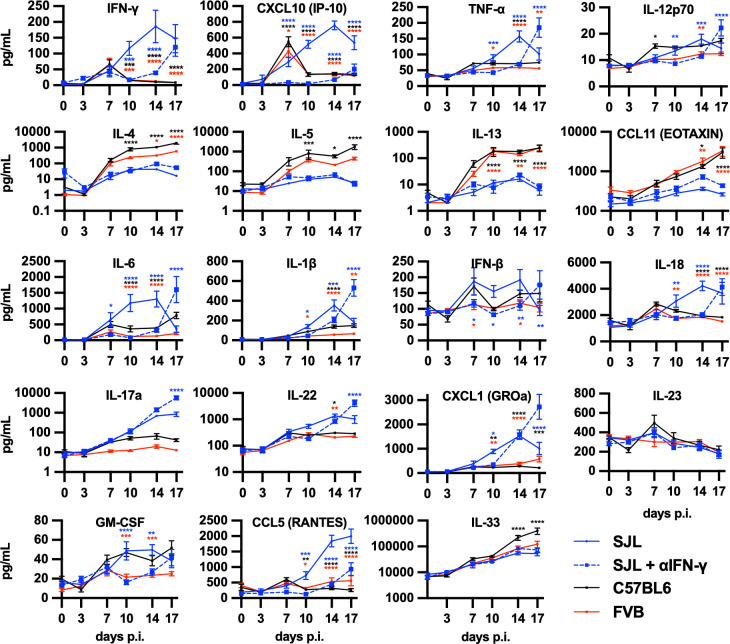
Since immune function has been highly linked to cryptococcal infection outcomes (19 – 22), we hypothesized that immune function may be relevant to the cryptococcal resistance displayed by SJL/J mice. Therefore, we measured lung cytokine and chemokine levels in C. neoformans H99-infected SJL/J, FVB/J, and C57BL/6J mice. Th1-associated cytokines and chemokines, interferon-gamma (IFN-γ), CXCL10, and tumor necrosis factor-alpha (TNF-α), were all elevated in SJL/J lungs 10 and 14 days post-infection (Fig. 3, top row). Similarly, several innate- or inflammatory-type cytokines and chemokines were also elevated in SJL/J mice: interleukin (IL)-6, IL-1β, IL-18, granulocyte macrophage colony-stimulating factor (GM-CSF), and CCL5 (Fig. 3). SJL/J mice also significantly upregulated IL-17a and the associated cytokine IL-22 and chemokine CXCL1 (Fig. 3, 4th row). By contrast, Th2-associated cytokines and chemokines (IL-4, IL-5, IL-13, and CCL11) were elevated in C57BL/6J mice (Fig. 3, 2nd row). Interestingly, FVB/J mice also showed elevated Th2 cytokines but to a lesser extent than C57BL/6J mice.
Fig 3.
SJL/J mice infected with cryptococci induce a robust cytokine response not featuring Th2-associated cytokines. Cytokine and chemokine levels in lung homogenates from SJL/J (blue), C57BL/6J (black), FVB/J (red), and SJL/J mice dosed with anti-IFN-γ neutralizing monoclonal antibody (blue dashed) mice infected with C. neoformans strain H99 at the indicated timepoints. Statistical comparisons are the indicated (color matched) condition compared to SJL/J mice at the same timepoint. N = 8 total mice over two independent experiments.
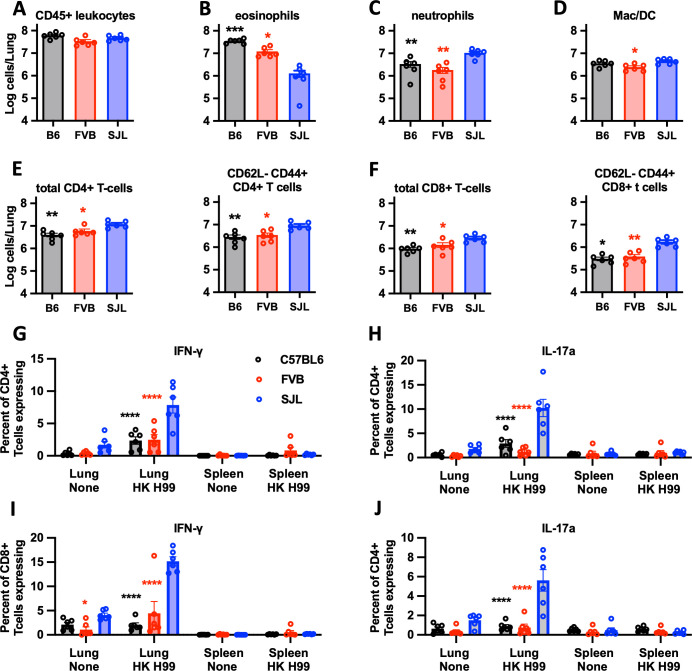
Immunophenotyping of leukocytes isolated from infected lungs showed a similar level of overall cellular recruitment between SJL/J, FVB/J, and C57BL/6J mice (Fig. 4A). Consistent with our cytokine data, SJL/J lungs recruited fewer eosinophils and more neutrophils, CD4+ T cells, and CD8+ T cells (Fig. 4B through F). Furthermore, activated subpopulations (CD62L− and CD44+) of both CD4+ and CD8+ T cells were also more prevalent in the lungs of infected SJL/J mice (Fig. 4E and F). Restimulated CD4+ and CD8+ T cells produced IFN-γ and IL-17a strongly suggesting a role for T cells in the secretion of these cytokines (Fig. 4G through J). Together, these data are consistent with a robust pulmonary Th1 and a possible Th17 response in SJL/J mice which contrasts with the Th2-dominated response in the C57BL/6J- and FVB/J-susceptible strains.
Fig 4.
Immunophenotyping of lung leukocytes from cryptococcus-infected SJL/J, FVB/J, and C57BL/6J mice. (A–F) Indicated populations measured from lung leukocyte preparations acquired 14 days post-infection in C56BL/6J (black/gray), FVB/J (red), and SJL/J (blue) mice infected with C. neoformans H99. (G–J) Percentage of CD4+ T cells (G and H) and CD8+T cells (I and J) isolated from lungs or spleens expressing IFN-γ (G and I) or IL-17a (H and J) following in vitro stimulation with heat-killed C. neoformans H99. Statistical comparisons are the indicated (color matched) condition compared to SJL/J mice. N = 6 mice per group combined from two independent experiments.
SJL/J resistance to Cryptococcus depends on IFN-γ, TNF-α, and T cells
To determine which of the many immune factors differentially regulated in SJL/J mice are critical for their resistance to highly virulent C. neoformans strain H99, cytokines and immune cells were depleted or blocked using monoclonal antibodies. In contrast to previous work utilizing C57BL/6J mice infected with less virulent cryptococci (23 – 25) which showed strong CD4+ T-cell dependence, depletion of CD4+ T cells alone in SJL/J increased fungal loads only slightly in the lungs but not in the brains 21 days post-infection (Fig. 5A and B). Similarly, single depletion of CD8+ T cells also did not impact fungal loads (Fig. 5A and B) unlike previous models (24, 25, 33) which showed some effects of single CD8 depletion. Interestingly, fungal nodules in CD4- or CD8-depleted SJL/J lungs did appear larger than in control SJL/J lungs (Fig. 5C and D; Fig. S2) suggesting a possible subtle immune effect of these perturbations. However, like previous models (24, 25), simultaneous depletion of both CD4+ and CD8+ cells resulted in a marked increase in fungal loads in the lungs (Fig. 5A) and especially the brain (Fig. 5B) as well as a distinct loss of fungal containment in lung sections (Fig. 5C and D; Fig. S2). Thus, resistance to virulent C. neoformans in SJL/J mice depends on T cells but the CD4+ and CD8+ compartments seem somewhat redundant. Depletion of neutrophils did not alter fungal loads (Fig. 5A and B).
Fig 5.
CD4+ T cells, CD8+ T cells, IFN-γ, and TNF-α are all critical for the effective SJL/J response to cryptococcal infection. Day 21 lung fungal loads (A) and brain fungal loads (B) from SJL/J mice dosed with the indicated monoclonal antibodies as well as control FVB/J mice infected with C. neoformans H99. Statistical comparisons are the indicated condition compared to SJL/J mice not dosed with antibodies (blue circles). Data are combined from several similar experiments with at least N = 8 over two experiments for each antibody depletion condition except n = 4 with one experiment for anti-Ly6C. Lung histological sections from similarly treated mice at (C) 17 and (D) 21 days post-infection. For each timepoint, the top rows are alcian blue stained sections and the bottom row sections are stained with H&E. For all images, the scale bar indicates 200 µm.
We then investigated the role of cytokines previously described as associated with cryptococcal immunity. Despite moderate expression of IFN-β (Fig. 3) and our previous work demonstrating the efficacy of exogenous type I IFN on cryptococcal infection (3 – 5), type 1 IFN receptor blockade in SJL/J did not change lung or brain fungal loads (Fig. 5A and B) suggesting that endogenous type 1 IFN has little role in this model. SJL/J mice expressed high levels of both IFN-γ and IL-17a in the lungs during critical periods of infection (Fig. 3). IL-17 blockade did not change lung or brain fungal loads (Fig. 5A and B) or drastically alter lung nodule pathology (Fig. 5C and D; Fig. S2). In contrast, depletion of the Th1-associated cytokines IFN-γ and TNF-α resulted in highly elevated lung fungal loads (Fig. 5A) and moderately elevated brain fungal loads (Fig. 5B) with more substantial effects dependent on IFN-γ compared to TNF-α. Furthermore, the depletion of IFN-γ resulted in a fungal load kinetic very similar to that of the susceptible strains of mice, FVB/J and C57BL/6J (Fig. 1G and H). Consistent with the importance of IFN-γ, lung nodules in IFN-γ-depleted mice showed a profound loss of fungal control (Fig. 5C and D; Fig. S2). Together, these data suggest that SJL/J strain resistance to cryptococcal infection is highly dependent on Th1-associated cytokines but not on those associated with Th17.
Blockade of IFN-γ does not result in an upregulation of Th2-associated factors in SJL/J mice
Because IFN-γ was critical to cryptococcal immunity in the SJL/J strain, we also examined cytokine and chemokine expression in SJL/J mice dosed with the IFN-γ neutralizing monoclonal antibody. IFN-γ antibody-dosed SJL/J mice had markedly reduced the levels of IFN-γ and the associated cytokines, IL-12, TNF-α, and CXCL10, up through day 14 post-infection consistent with strong target neutralization and confirming the dependence of this entire pathway on IFN-γ (Fig. 3, top row). While IL-17a expression was unaffected by IFN-γ neutralization, the innate and inflammatory cytokines, IL-6, IL-1β, IL-18, GM-CSF, and CCL5, were markedly depressed at 10 and 14 days post-infection by blocking IFN-γ (Fig. 3). We also observed a sharp increase in many of these inflammatory cytokines late in the infection at 17 days post-infection correlating to the uncontrolled fungal outgrowth (Fig. 3). Surprisingly, IFN-γ blockade did not result in any significant increase in the expression of the Th2-type cytokines, IL-4, IL-5, IL-13, or CCL11 (Fig. 3, 2nd row). These data indicate that while blockade of IFN-γ results in profound susceptibility to C. neoformans H99 infection in SJL/J mice, this susceptibility is not correlated with a reversal of the immune polarization type. Instead, blocking IFN-γ seems to prevent the initiation of productive cytokine response.
Fatal cryptococcal infection in SJL/J mice still does not result in Th2-associated cytokines
During preliminary experiments, we noted a VGI strain Cryptococcus gattii strain (C.g. VGI) originally isolated from a GM-CSF autoantibody-positive patient (34) that has substantially higher virulence in SJL/J mice than the classical high virulence H99 strain (Fig. S3). Overall, C.g. VGI strain induces a progressive cryptococcosis in SJL/J mice roughly similar to disease induced by other virulent strains in other mouse models. In addition, we analyzed lung cytokine levels in C.g. VGI-infected SJL/J mice compared to C. neoformans H99-infected SJL/J and C57BL/6J mice. Unlike C57BL/6J mice infected with C. neoformans H99, C.g. VGI-infected SJL/J mice did not upregulate the Th2-associated cytokines and chemokines IL-4, IL-5, IL-13, and CCL11 (Fig. S4, 2nd row). In fact, the immune response in C.g. VGI -infected SJL/J mice was similar to C. neoformans H99-infected SJL/J mice with high levels of IFN-γ-associated, Th17-associated, and inflammatory-associated cytokines with, perhaps, a slight delay in the induction of some cytokines (Fig. S4). Thus, while C.g. VGI infection leads to a progressive infection in SJL/J mice, the immune response in SJL/J mice was not dominated by the Th2-associated response often correlated with mouse susceptibility.
Previous C. neoformans infection in SJL/J mice results in protection from fatal secondary infection
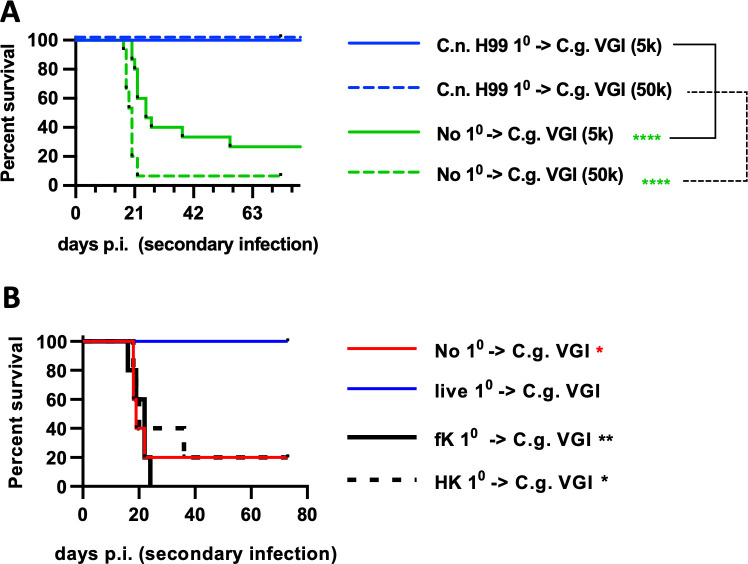
Since the above data demonstrated that T cells have such a significant role in the SJL/J immunity to cryptococci and are strongly implicated by the epidemiology of human disease (10 – 12, 16), we hypothesized that the SJL/J adaptive immune response would provide long-lasting immunity to cryptococcal infection. SJL/J mice were initially infected with live C. neoformans H99 and then allowed to clear this primary infection. As above, all SJL/J mice survived and recovered from this infection. After 60 days, these recovered mice were challenged with a C. gattii strain C.g. VGI. All recovered SJL/J mice survived the C.g. VGI rechallenge while about 70% of naïve SJL/J mice reached the endpoint when challenged with 5,000 C.g. VGI and about 95% reached the endpoint when challenged with 50,000 C.g. VGI (Fig. 6A). To test whether live infection was required for this protective effect, SJL/J mice were again infected with live H99 or dosed with heat- or formalin-killed H99 cells and then allowed to recover before re-infecting with C.g. VGI as previously. While the SJL/J mice infected with live cryptococci were protected as above, mice dosed with the killed H99 yeast cells succumbed to the secondary infection similar to naïve mice (Fig. 6B). Thus, SJL/J mice develop a robust memory-like response to cryptococcal infection that is effective in protecting the mice against infection by related strains. Interestingly, this protective memory-like response seemed to require live primary infection. Overall, these data demonstrate that SJL/J mice develop a robust anti-cryptococcal response that is comparatively effective against primary infection and that resolves into protective memory. This immune phenotype seems to generally mimic the situation in healthy humans.
Fig 6.
Recovered SJL/J mice are immune to fatal secondary infection. (A) Survival plot of SJL/J mice infected with C. neoformans H99 (blue lines) or left uninfected (“No 10”; green lines) then allowed to clear infection for 60 days then reinfected with 5,000 (solid lines) or 50,000 (dashed lines) C.g. VGI as indicated. N = 15–19 mice per group combined from three independent experiments. (B) SJL/J mice were dosed with live, formalin-killed (“fK”), or heat-killed (“HK”) C. neoformans H99 or left uninfected and then reinfected with 50,000 C.g. VGI. For B, N = 5 mice per group. Statistical comparisons are the indicated condition compared to SJL/J mice infected with live C. neoformans H99 and then reinfected.
QTL analysis of cryptococcal resistance to infection demonstrates strain-specific response with genetic linkage in two chromosomes
The genetic basis for resistance and susceptibility to cryptococci has proven mostly elusive; yet, the above data showed that Swiss-Webster-derived inbred SJL/J mice are substantially more resistant to C. neoformans H99 infection and respond to cryptococcal infection with a substantially different immune response compared to susceptible mouse strains. Thus, we hypothesized that SJL/J resistance to cryptococcal infection might be associated with strain-specific genetic modifiers. First, resistant SJL/J mice were crossed with the Swiss-Webster-derived susceptible FVB/J strain generating F1 hybrids of SJL × FVB. Similar to the SJL mice, the SJL × FVB F1 hybrids were mostly resistant to C. neoformans H99 infection although more F1 mice in experiment 2 appear to have succumbed to infection (Fig. 7B), suggesting that SJL/J resistance to C. neoformans H99 infection appeared to be dominant. To further characterize these findings, SJL × FVB F1 hybrid mice were crossed to FVB/J mice generating about 200 FVB × SJL N2 hybrid mice. These mice were infected with C. neoformans H99. About 20% of FVB × SJL N2 hybrid mice were resistant to the infection (up to 100 days post-infection) while about 80% succumbed to infection at roughly similar kinetics to susceptible strains (Fig. 7C). These data confirm that there is a genetic component associated with resistance to infection and suggest that multiple loci are involved. To map loci linked to SJL/J cryptococcal resistance, genomic DNA from the FVB × SJL N2 hybrid cohort (n = 208) was submitted for genotyping. QTL analysis showed significant linkage in chromosomes 2 (logarithm of the odds [LOD] score = 3.4) and 11 (LOD score = 5.1) (Fig. 7D) indicating the involvement of multiple loci in cryptococcal resistance. In post hoc analysis, grouping FVB × SJL N2 mice by SNP genotype at the loci of the chromosome 2 and 11 linkage peaks showed that those mice with SJL-type alleles at both the chromosome 2 and 11 linkage peaks were substantially more resistant to H99 infection than those with FVB/J type alleles at either or both of those loci (Fig. 7E). Interestingly, the mice with SJL-type alleles at both the chromosome 2 and 11 linkage peaks showed the highest resistance but were not as resistant as the SJL × FVB F1 hybrid mice indicating the potential involvement of other loci. Also, those mice with only one SJL-type allele at the loci of interest were not substantially protected confirming that both loci are involved in the resistance to infection.
Fig 7.
SJL/J resistance to cryptococcal infection is a heritable quantitative trait linked to regions on chromosomes 2 and 11. (A) Cartoon depicting breeding strategies and summarizing outcomes. Initially, SJL/J mice were crossed with FVB strain mice generating the SJL × FVB F1 mice (top row) used in (B). Some of these SJL × FVB F1 mice were backcrossed with FVB/J mice generating SJL × FVB N2 mice used in C and D. (B) Survival plots of two experimental repeats of SJL × FVB F1 (purple), FVB/J (red), SJL/J (blue), and C57BL/6J mice (black) infected with C. neoformans H99. For experiment 1, F1 mice were bred in-house, N = 5 for FVB, SJL, and C57BL/6J and 51 for SJLxFVB F1. For experiment 2, F1 mice were bred under contract by Taconic, N = 5 for FVB, SJL, and C57BL/6J and 20 for SJL × FVB F1. (C) Survival plot of SJL × FVB N2 (purple), FVB/J (red), SJL/J (blue), and C57BL/6J mice (black) infected with C. neoformans H99. N = 10 for FVB, SJL, and C57BL/6J and 208 for SJL × FVB N2. (D) Logarithm of odds plot of linkage analysis from SNP genotyping of the SJL × FVB N2 mice depicted in C. (E) Replot of survival of the SJL × FVB N2 data in (C). Mice were grouped by genotype of the SNPs corresponding to the LOD peaks on chromosomes 2 and 11 depicted in (D). Statistical comparisons in (E) are the indicated condition compared to mice expressing the SJL/J variants at both loci (Blue). These experiments used mixed female and male mice.
Areas of linkage associated with those peaks were estimated as positions 26,997,973 to 62,510,985 on chromosome 2 and positions 21,636,240 to 59,157,913 on chromosome 11. Upon examination of genes located within this chromosome 2 region, we found that the susceptible FVB/J mice carry the “Hc0” allele in complement component c5 and are thus c5 deficient. To confirm the c5 status of the relevant mouse, strains c3 and c5 levels in serum samples were compared. SJL/J mice express robust levels of c5 (Fig. S5A) while the FVB/J and the related SWR/J mice expressed no c5. On the other hand, all tested strains produced c3 indicating c5 loss in FVB is a specific deletion and not a broad loss of complement in the samples (Fig. S5B). Thus, these data suggest that the linkage of this region on chromosome 2 to SJL/J resistance to infection relative to FVB/J mice is probably in large part due to this c5 allelic difference.
By contrast, our examinations of the linked region on chromosome 11 found no major coding changes in any contained genes. Interestingly, the Th2 cytokine master locus is within the linked chromosome 11 region. Thus, the linked region contains the conventional Th2 cytokine genes for IL-4, IL-5, and IL-13 as well as other immune-related genes such as IRF1, IL-12b (p40), IL-3, and GM-CSF. While no major coding differences were found in these genes between FVB, C57BL/6J, and SJL/J, substantially different regulation of these genes was observed since SJL/J mice produced lower levels of IL-4, IL-5, and IL-13 during infection (Fig. 3). That said, levels of these cytokines in SJL/J mice were well above detection limit levels indicating that SJL/J mice differentially regulate these cytokines but are not null for these gene products. Also, previous literature has shown that SJL/J mice are fully capable of mounting a Th2 response (35). Taken together, these data suggest that SJL/J resistance to cryptococcal infection may be linked to genetically encoded regulation of immune factors located on chromosome 11.
DISCUSSION
Unlike many other commonly used mouse strains, we show that SJL/J mice are highly resistant to infection with virulent cryptococcal strains, particularly the common H99 strain, which are fatal to other strains of mice. These data build on previous work showing SJL/J mice have increased resistance to less virulent strains (30 – 32). Importantly, SJL/J mice can be rendered susceptible by inducing immunodeficiencies similar to those observed in cryptococcosis patients. Like humans, SJL/J mice also develop a protective memory response to cryptococcal infection. Utilizing this novel model, our data show that even when SJL/J mice are infected with a highly virulent cryptococcal strain to which they are susceptible, SJL/J mice do not express significant Th2-associated cytokine polarization. In addition, the SJL/J strain survival advantage against fatal cryptococcal infection allowed clear binary phenotype selection, this approach resulted in clear genetic linkage areas on chromosomes 2 and 11. Thus, data presented here demonstrate that SJL/J mice infected with virulent Cryptococcus strains are an alternative and, in some ways, a superior murine model for experimental cryptococcosis.
The data described above agree with and extend the descriptive immunological data put forward by Guillot et al. (31) using the less virulent D52 strain of C. neoformans. While some differences exist between these data sets in terms of timepoints, techniques, and analytes measured, our data and the data by Guillot et al (31) largely agree. Both data sets demonstrate elevated inflammatory innate immune type cytokines and chemokines (IL-6, IL-1β, and CCL5) in SJL/J mice. Our data also show elevated CCL5 and IL-18 while Guillot et al. did not detect a difference. The data by Guillot et al., however, show some other elevated innate immune type factors: IL-1Ra CCL2, CCL3, and CCL4. Importantly, both sets show a substantially larger Th1-type cytokine response and lower Th2-type cytokine response in SJL/J mice than in C57BL/6J. For example, IFN-γ, IL-12, and TNF-α are elevated while IL-4 is depressed in SJL/J mice in both our data (Fig. 3) and in Guillot et al. We also show CXCL10, an IFN-γ-associated chemokine is elevated and IL-5, IL-13, and CCL11 are lower in SJL/J mice. Correspondingly, eosinophil recruitment was reduced, and neutrophil recruitment was elevated in both studies [Fig. 4 and (31)]. We were also able to demonstrate the evidence of a Th17-type response in SJL/J mice with elevated IL-17a and IL-22 (Fig. 3) with confirmed IL-17a production by T cells (Fig. 4). In addition, both data sets demonstrate an increase in CXCL1 (CXCL2 was also elevated in Guillot et al.); chemokines often associated with Th17 responses. Thus, these data sets agree and suggest that SJL/J mice develop a Th1, and probably a Th17 response, against pulmonary C. neoformans infection while C57BL6 and other susceptible mouse strains develop a Th2-dominated response. While these conclusions largely confirm the current paradigm of Th1 correlating to resistance to cryptococcal infection and Th2 correlating to susceptibility (18), it should be noted that in these cases mouse strain differences dominated over Cryptococcus strain differences in terms of immunophenotype, further underlining the importance of mouse strain selection for Cryptococcal experimentation.
In addition to the descriptive immunity described above, our data mechanistically confirm the roles of IFN-γ , TNF-α, and T cells in protective immunity against C. neoformans in SJL/J mice. However, our data also raise interesting questions concerning the role of Th2-associated atopic-type immunity in clinical cryptococcosis. Extreme Th2 polarization and associated atopic phenotypes are perhaps the dominant immunological feature of C57BL/6J mice infected with highly virulent strains of Cryptococcus (18 – 25). By contrast, we demonstrate that SJL/J mice do not express a Th2 cytokine-dominated response to cryptococcal infection (Fig. 3). Furthermore, even when SJL/J mice are rendered susceptible to infection by blocking IFN-γ using a monoclonal antibody, they do not re-polarize toward Th2 immunity (Fig. 3). This is substantially different from studies utilizing more moderately virulent cryptococcal strains and conventional mouse strains. In those settings, interfering with IFN-γ, or other Th1-associated factors, results in infected mice dramatically increasing expression of IL-4, IL-5, IL-13, or other Th2-associated factors (36 – 41) often with associated eosinophilia and other atopic phenotypes. The data presented here also demonstrate that SJL/J mice do not induce pronounced Th2 polarization when infected with a cryptococcal strain to which the mice are susceptible (Fig. S4) further demonstrating that survival-level susceptibility does not correlate to Th2 polarization in SJL/J mice and that Th2 polarization is not required for susceptibility to cryptococci. These data suggest an alternative model of susceptibility to cryptococcosis different from the commonly held belief that Th1 vs Th2 polarization is the sole immunological determinant in the mouse models of cryptococcosis. Instead, the data in Fig. 3; Fig. S4 demonstrate an alternative mode of susceptibility characterized by a delay in the induction of an immune response that appears similar in polarization to a productive response. Future studies will be necessary to determine whether other models show this mode of susceptibility and to determine which of these models, or both, are relevant to human disease. That said, the Th1 vs Th2 polarization dichotomy may still have an important role in clinical cryptococcosis and has an undisputed role in understanding the critical immunological pathways that determine outcomes of infection in many other strains of mice. Another consideration is that some sex differences have been observed in SJL mice (42, 43). While the major survival resistance vs susceptibility phenotypes were confirmed in both sexes, the immunology studies presented above were performed in female mice which have been shown to tend toward Th1 in some studies (43). Overall, we suggest that SJL/J mice may be an interesting scientific tool to reexamine the role of Th2 immunity in cryptococcal infection.
Immune memory is an important component of the human response to cryptococcal infection since most people acquire Cryptococcus reactive antibodies as children (8, 9) and the loss of T-cell immunity in advanced AIDS remains the leading risk factor for cryptococcosis (10 – 12). While immunological memory to cryptococcus infection has been difficult to study in most mouse models, our data show that SJL/J mice develop a robust protective memory response. Further study is certainly required to demonstrate if SJL/J mice develop specific memory antibodies and T-cell responses similar to humans. SJL/J memory responses may be an exciting tool to study the role and antigen specificity of B or T cells in productive anticryptococcal immune responses.
Several previous studies have attempted to genetically map chromosomal loci linked to resistance to Cryptococcal infection in mice. Qureshi and his coworkers published a series of important papers by crossing relatively resistant CBA/J mice with susceptible strains, C57BL6/J (44, 45) and C3H/HeN (32), infecting the F2 progeny with D52, a cryptococcal strain of low virulence and using fungal load as the analyzed trait. These studies demonstrated areas linked with lower fungal loads on chromosomes 1 and 9 when comparing CBA/J to C3H/HeN (32) and chromosome 3 and two regions on chromosome 17 when comparing CBA/J to C57BL6/J (44). A follow-up study demonstrated that replacing the C57BL6/J Cnes2 locus with the CBA/J allele results in improved fungal resistance and important changes to immune phenotypes underlining the importance of the Cnes2 region (45). By contrast, when C57BL6/J mice were crossed with the relatively resistant BALB/C mice by Chen et al. (20), an entirely blended immunological and fungal burden phenotype was observed upon infection with D52 strain, and no genetic linkage was reported. Finally, Rhodes et al. showed data consistent with resistance to intravenous cryptococcal infection being a dominant and monogenic trait by cross-breeding BIO.A (resistant) and A/WySn (susceptible) mice but they did not link this trait to any locus. In addition, they analyzed many of these inbred strains, including SJL/J, as well as their crossed mice and noted that the c5 allele status on chromosome 11 highly correlated to susceptibility (30). Unlike these previous studies, we utilized the SJL/J mice crossed with susceptible FVB mice and infected the progenies with a highly virulent H99 strain, which allowed us to utilize survival for genetic linkage studies. The data presented here demonstrate that SJL/J resistance to virulent cryptococcal infection has a genetic component as was associated with a significant strain-specific linkage in chromosomes 2 and 11. Thus, genetic approaches have demonstrated regions on chromosomes 1, 2, 9, 11, and 17 important for cryptococcal infection but none of these studies identified a common region other than Rhodes et al.’s observation on c5 (30) and our own. Certainly, these differences in mouse and Cryptococcus strains may impact the regions that are critical between any two strains and the effect of random recombination sites and rates could certainly impact these types of experiments even when large cohorts are used. It is interesting to note that all these linkage experiments utilize some type of pulmonary delivery and the Qureshi studies in fact selected on pulmonary fungal loads suggesting that the regions linked to resistance may be more specific to the lung phase of infection while other genes and loci may be important for other phases of infection.
While we cannot rule out the involvement of other genes within the region of linkage on mouse chromosome 2, the complement c5 gene is an especially compelling candidate gene on chromosome 2 for resistance to cryptococcal infection. Previous cryptococcal infection studies have also implicated the complement pathway as important for anticryptococcal immunity. In addition, it should be noted that male SJL/J mice express extraordinarily high levels of complement c5 (42). However, resistance to cryptococcal infection was evident in both sexes of mice suggesting that resistance was not only due to this male exclusive higher c5 expression level. While a few previous studies have implicated complement c5 (30, 46, 47), many more studies have focused on the role of opsonization of the cryptococcal yeast by c3 complexes (48 – 52). However, c3-mediated phagocytosis of encapsulated live cryptococcal yeast is not particularly efficient due to the expression of mitigating virulence factors (53). The data presented here show that c5 deficiency contributes to cryptococcal susceptibility independent of c3 status ( Fig. S5), suggesting that the role of complement in Cryptococcus may extend beyond opsonization. Following activation, the complement cascade cleaves c5 into c5a, an important inflammatory mediator, and c5b, which initiates membrane attack complex formation (54). Thus, we hope future studies will better elucidate the roles of these important functions of c5 cleavage products in anticryptococcal immunity.
In contrast to the H0 c5 null allele in FVB/J mice, we were unable to pinpoint any similar major coding changes within the implicated region on chromosome 11. Notably, several important immune genes are grouped within the linked region on chromosome 11 controlled by the intronic Th2-LCR expression control region (55, 56) and the differential regulation of these genes was demonstrated during cryptococcal infection (Fig. 3; Fig. S4). Intriguingly, humans express a similar Th2 control region with a high level of synteny to the mouse region on human chromosome 5. While future studies will be required to determine whether non-coding SJL/J alleles contribute to differential Th2 cytokine expression and cryptococcal resistance, we propose SJL/J mice may prove to be a useful tool for the study of this important genetic region.
While much work remains to be done, we view the productive and robust immune response generated by SJL/J mice to be the most striking difference between SJL/J mice and more conventional animal models. We hope other groups will explore the utility of this novel model.
MATERIALS AND METHODS
Mice
All laboratory mouse housing, breeding, and procedures were conducted according to the Institutional Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases approved animal protocols (LCIM-5e and CMB-15). Studies were performed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Other than for breeding, mice from separate strains, sexes, and suppliers were separately housed.
All wild-type mouse strains were purchased from The Jackson Laboratory (Bar Harbor, ME) except CD-1 mice as well as a few C57BL/6, SJL, and FVB mice were acquired from Taconic. No differences in cryptococcal infection were observed between corresponding wild-type C57BL/6, SJL, and FVB mice from different suppliers. All knockout strains were acquired by NIAID supply agreement with Taconic. Experiments were started with mice aged 8- to 12-week old. Some experiments were performed with separately housed males and females as indicated in the figure legends, whereas the remaining experiments were performed with female mice.
Generation of FVBXSJL animals
All animal procedures were performed following the NIH ACUC guidelines. All breeding at NIH for this work was under the animal study proposal (ASP) CMB15. Five 5- to 6-week-old FVBN females were super-ovulated by intraperitoneal injection of 5 I.U. of pregnant mare serum gonadotropin. Approximately 48 hours later, 5 I.U. of human chorionic gonadotropin (HCG) was injected and the females were mated individually with the 8- to 12-week-old SJL/J males. After overnight, the vaginal plugs were checked and the pregnant females were allowed to stay together with the males. The pups were delivered after 19–20 days of mating. The pups were weaned after 21 days. The male and female pups were separated and housed as five pups per cage.
We then kept 10 of the male offspring (F1) to make new pairings. At the age of 8–10 weeks, these males were housed individually and were used for further breeding. Ten 4–5-week-old FVBN females were super-ovulated as above and were placed with the males immediately following the HCG injection. The copulatory plugs were checked the following morning. The new-born pups from this mating were then used for the experiment.
Cryptococcal strains
Cryptococcus neoformans H99 (ATCC 208821), C. neoformans D52 (ATCC 24067), Cryptococcus gattii R265 (ATCC-MYA-4093), and C. gattii VGI (previously described in (34) as strain CH18) were maintained and cultured as previously (3 – 5). Briefly, aliquots of frozen stocks were thawed for each experiment for overnight 30°C culture in yeast extract–peptone–dextrose (YPD; MP Biomedicals, Santa Ana, CA). Rinsed yeast were diluted in sterile PBS to deliver 5,000 yeast per mouse in 20 µL (except for Fig. 6 where mice were infected with the indicated dose). Mice were infected as previously (3 – 5) by intrapharyngeal aspiration.
Mouse tissue collection, processing, and analysis
At each predetermined day post-infection, mice were euthanized by CO2, and lungs and brains were collected. For some experiments, serum samples were also collected by retro-orbital bleed under anesthesia. For colony-forming unit (CFU) analysis, lung and brain samples were homogenized by probe homogenizer. Colony forming units were measured by dilution plating onto YPD plates. Cytokine analysis lung homogenate samples were cleared by centrifugation. Cytokines and chemokines were measured by the Procartaplex Luminex panel (Thermo-Fisher) according to the manufacturer’s instructions. Histological tissue sections were fixed in formalin. Tissue samples were embedded in paraffin, sectioned, and stained at Histoserv, Inc. (Germantown, MD).
For monoclonal antibody administration, all antibodies were purchased from BioXcell (Lebanon, NH) and administered IP. CD4 (clone GK1.5) and CD8 (53–6.72) T-cell depleting antibodies were administered at 100 µg per dose started 8 days before infection with double doses given on the day before and after infection. Neutrophil-depleting antibody (1A8) was given at 300 µg per dose started 6 days before infection. IFN-γ (XMG1.2), TNF-α (XT3.11), IL-17 (17F3), and IFNar1 (MAR1-5A3) antibodies were dosed at 250 µg per dose and started the day before infection. All antibodies were dosed twice per week after infection.
Leukocyte isolation
Leukocytes in the lungs and spleen were isolated as described in the previous study (1, 2). Lungs were digested with gentleMACS™ lung dissociator system (Milteni Biotec) using collagenase IV (Worthington) and DNase I (Worthington). Lung leukocytes were enriched with Percoll gradient (30%, 44%, and 70%) centrifugation. The spleen was mashed on a 70-µm cell strainer, and red blood cells were lysed with RBC lysis buffer (BioLegend). Cells were stained with acridine orange-propidium iodine and counted using a Cellometer K2 cytometer (Nexcelom).
T-cell restimulation assay
This assay was performed as described in the previous study (57). Lung and spleen leukocytes (1 × 106 cells/well) were cultivated for 17 hours with heat-killed H99-derived acapsular strain Hcap59∆ (multiplicity of infection [MOI] = 1) (58) or C. albicans SC5314 (MOI = 1). Cells were treated with the protein transport inhibitor cocktail (Thermofisher Scientific) for the last 3 hours of culture to stop cytokine release. Intracellular cytokines in T cells were detected with flow cytometry.
Flow cytometry
All procedures were performed as described in the previous study (57). Lung and spleen leukocytes were stained with the following mAb clones: Ly6C (HK1.4), CD3ε (145–2C11), B220 (RA3-6B2), CD45 (30-F11), CD206 (C068C2), CD86 (GL-1), CD11c (N418), Ly6G (1A8), CD11b (M1/70), Siglec-F (E50-2440), CD80 (16–10A1), CD62L (MEL-14), CD44, (IM7) CD8a (53–6.7), CD4 (GK1.5), TCR-β (H57-597), IFNγ (XMG1.2), and IL-17A (TC11-18H10.1). Flow data were acquired using BD Symphony A5 flow cytometer (BD Bioscience) and analyzed using FlowJo software (Tree Star Inc.).
QT linkage analysis
Mice were infected with H99, and tail samples were reserved at the endpoint of euthanasia. To map loci linked to SJL/J cryptococcal resistance, genomic DNA from the entire FVB x SJL N2 hybrid cohort was isolated and submitted for GIGA-MUGA SNP mapping (Neogen) (59). Analysis was performed as previously described (60). A total of 16,380 genome-wide informative SNPs between the two strains were obtained. One QTL genome scan was performed using R/qtl (61) selecting susceptibility vs resistance (mouse survival) to infection (Binary) as phenotype. Two regions, one in chromosome 2 (LOD = 3.4) and one in chromosome 11 (LOD = 5.1) showed significant LOD scores (>3).
Statistics
Statistical analysis was performed in GraphPad Prism (San Diego, CA, USA). Survival plots were compared pairwise as indicated using log-rank testing. Time course CFU, cytokine, and T-cell restimulation data were analyzed by two-way ANOVA followed by multiple comparison corrected testing (Dunnett method) as indicated in the figures and legends. Other data were analyzed by one-way ANOVA followed by multiple comparison corrected testing (Dunnett method) as indicated in the figures and legends.
ACKNOWLEDGMENTS
This work was supported by a research fund from the intramural program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Contributor Information
K. J. Kwon-Chung, Email: jkchung@niaid.nih.gov.
J. Andrew Alspaugh, Duke University Hospital, Durham, North Carolina, USA .
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.02123-23.
Figures S1-S5.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Zhao Y, Ye L, Zhao F, Zhang L, Lu Z, Chu T, Wang S, Liu Z, Sun Y, Chen M, Liao G, Ding C, Xu Y, Liao W, Wang L. 2023. Cryptococcus neoformans, a global threat to human health. Infect Dis Poverty 12:20. doi: 10.1186/s40249-023-01073-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ssebambulidde K, Anjum SH, Hargarten JC, Chittiboina P, Shoham S, Seyedmousavi S, Marr KA, Hammoud DA, Billioux BJ, Williamson PR. 2022. Treatment recommendations for non-HIV associated cryptococcal meningoencephalitis including management of post-infectious inflammatory response syndrome. Front Neurol 13:994396. doi: 10.3389/fneur.2022.994396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis MJ, Martin RE, Pinheiro GM, Hoke ES, Moyer S, Mayer-Barber KD, Chang YC, Kwon-Chung KJ. 2022. MDA5 signaling induces type 1 IFN- and IL-1-dependent lung vascular permeability which protects mice from opportunistic fungal infection. Front Immunol 13:931194. doi: 10.3389/fimmu.2022.931194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davis MJ, Moyer S, Hoke ES, Sionov E, Mayer-Barber KD, Barber DL, Cai H, Jenkins L, Walter PJ, Chang YC, Kwon-Chung KJ. 2019. Pulmonary iron limitation induced by exogenous type I IFN protects mice from Cryptococcus gattii independently of T cells. mBio 10:e00799-19. doi: 10.1128/mBio.00799-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sionov E, Mayer-Barber KD, Chang YC, Kauffman KD, Eckhaus MA, Salazar AM, Barber DL, Kwon-Chung KJ. 2015. Type I IFN induction via poly-ICLC protects mice against cryptococcosis. PLoS Pathog 11:e1005040. doi: 10.1371/journal.ppat.1005040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ueno K, Tsuge S, Shimizu K, Miyazaki Y. 2023. Promising whole-cell vaccines against cryptococcosis. Microbiol Immunol 67:211–223. doi: 10.1111/1348-0421.13056 [DOI] [PubMed] [Google Scholar]
- 7. Oliveira LVN, Wang R, Specht CA, Levitz SM. 2021. Vaccines for human fungal diseases: close but still a long way to go. NPJ Vaccines 6:33. doi: 10.1038/s41541-021-00294-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deshaw M, Pirofski LA. 1995. Antibodies to the Cryptococcus neoformans capsular glucuronoxylomannan are ubiquitous in serum from HIV+ and HIV- individuals. Clin Exp Immunol 99:425–432. doi: 10.1111/j.1365-2249.1995.tb05568.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abadi J, Pirofski L. 1999. Antibodies reactive with the cryptococcal capsular polysaccharide glucuronoxylomannan are present in sera from children with and without human immunodeficiency virus infection. J Infect Dis 180:915–919. doi: 10.1086/314953 [DOI] [PubMed] [Google Scholar]
- 10. Kwon-Chung KJ, Sorrell TC, Dromer F, Fung E, Levitz SM. 2000. cryptococcosis: clinical and biological aspects. Med Mycol 38 Suppl 1:205–213. [PubMed] [Google Scholar]
- 11. Grant IH, Armstrong D. 1988. Fungal infections in AIDS. Infect Dis Clin N Am 2:457–464. doi: 10.1016/S0891-5520(20)30198-7 [DOI] [PubMed] [Google Scholar]
- 12. Mitchell TG, Perfect JR. 1995. Cryptococcosis in the era of AIDS--100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev 8:515–548. doi: 10.1128/CMR.8.4.515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pappas PG. 2013. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc 124:61–79. [PMC free article] [PubMed] [Google Scholar]
- 14. Wu B, Liu H, Huang J, Zhang W, Zhang T. 2009. Pulmonary cryptococcosis in non-AIDS patients. Clin Invest Med 32:E70–E77. doi: 10.25011/cim.v32i1.5090 [DOI] [PubMed] [Google Scholar]
- 15. Lionakis MS. 2019. Primary Immunodeficiencies and invasive fungal infection: when to suspect and how to diagnose and manage. Curr Opin Infect Dis 32:531–537. doi: 10.1097/QCO.0000000000000593 [DOI] [PubMed] [Google Scholar]
- 16. Elsegeiny W, Marr KA, Williamson PR. 2018. Immunology of cryptococcal infections: developing a rational approach to patient therapy. Front Immunol 9:651. doi: 10.3389/fimmu.2018.00651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Normile TG, Bryan AM, Del Poeta M. 2020. Animal models of Cryptococcus neoformans in identifying immune parameters associated with primary infection and reactivation of latent infection. Front Immunol 11:581750. doi: 10.3389/fimmu.2020.581750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olszewski MA, Zhang Y, Huffnagle GB. 2010. Mechanisms of cryptococcal virulence and persistence. Future Microbiol 5:1269–1288. doi: 10.2217/fmb.10.93 [DOI] [PubMed] [Google Scholar]
- 19. Arora S, Olszewski MA, Tsang TM, McDonald RA, Toews GB, Huffnagle GB. 2011. Effect of cytokine interplay on macrophage polarization during chronic pulmonary infection with Cryptococcus neoformans. Infect Immun 79:1915–1926. doi: 10.1128/IAI.01270-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen G, McNamara DA, Hernandez Y, Huffnagle GB, Toews GB, Olszewski MA. 2008. Inheritance of immune polarization patterns is linked to resistance versus susceptibility to Cryptococcus neoformans in a mouse model. Infect Immun 76:2379–2391. doi: 10.1128/IAI.01143-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Traynor TR, Kuziel WA, Toews GB, Huffnagle GB. 2000. Ccr2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J Immunol 164:2021–2027. doi: 10.4049/jimmunol.164.4.2021 [DOI] [PubMed] [Google Scholar]
- 22. Miyagi K, Kawakami K, Kinjo Y, Uezu K, Kinjo T, Nakamura K, Saito A. 2005. CpG oligodeoxynucleotides promote the host protective response against infection with Cryptococcus neoformans through induction of interferon-gamma production by CD4+ T cells. Clin Exp Immunol 140:220–229. doi: 10.1111/j.1365-2249.2005.02772.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mody CH, Lipscomb MF, Street NE, Toews GB. 1990. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. J Immunol 144:1472–1477. [PubMed] [Google Scholar]
- 24. Huffnagle GB, Yates JL, Lipscomb MF. 1991. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med 173:793–800. doi: 10.1084/jem.173.4.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hill JO, Harmsen AG. 1991. Intrapulmonary growth and dissemination of an avirulent strain of Cryptococcus neoformans in mice depleted of CD4+ or CD8+ T cells. J Exp Med 173:755–758. doi: 10.1084/jem.173.3.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ponzio NM, David CS, Shreffler DC, Thorbecke GJ. 1977. Properties of reticulum cell sarcomas in SJL/J mice. V. nature of reticulum cell sarcoma surface antigen which induces proliferation of normal SJL/J T cells. J Exp Med 146:132–145. doi: 10.1084/jem.146.1.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bittner RE, Anderson LV, Burkhardt E, Bashir R, Vafiadaki E, Ivanova S, Raffelsberger T, Maerk I, Höger H, Jung M, Karbasiyan M, Storch M, Lassmann H, Moss JA, Davison K, Harrison R, Bushby KM, Reis A. 1999. Dysferlin deletion in SJL mice (SJL-Dysf) defines a natural model for limb girdle muscular dystrophy 2B. Nat Genet 23:141–142. doi: 10.1038/13770 [DOI] [PubMed] [Google Scholar]
- 28. Pöllinger B, Krishnamoorthy G, Berer K, Lassmann H, Bösl MR, Dunn R, Domingues HS, Holz A, Kurschus FC, Wekerle H. 2009. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J Exp Med 206:1303–1316. doi: 10.1084/jem.20090299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenberg NL, Ringel SP, Kotzin BL. 1987. Experimental autoimmune myositis in SJL/J mice. Clin Exp Immunol 68:117–129. [PMC free article] [PubMed] [Google Scholar]
- 30. Rhodes JC, Wicker LS, Urba WJ. 1980. Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect Immun 29:494–499. doi: 10.1128/iai.29.2.494-499.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guillot L, Carroll SF, Homer R, Qureshi ST. 2008. Enhanced innate immune responsiveness to pulmonary Cryptococcus neoformans infection is associated with resistance to progressive infection. Infect Immun 76:4745–4756. doi: 10.1128/IAI.00341-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carroll SF, Lafferty EI, Flaczyk A, Fujiwara TM, Homer R, Morgan K, Loredo-Osti JC, Qureshi ST. 2012. Susceptibility to progressive Cryptococcus neoformans pulmonary infection is regulated by loci on mouse chromosomes 1 and 9. Infect Immun 80:4167–4176. doi: 10.1128/IAI.00417-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mody CH, Chen GH, Jackson C, Curtis JL, Toews GB. 1993. Depletion of murine CD8+ T cells in vivo decreases pulmonary clearance of a moderately virulent strain of Cryptococcus neoformans. J Lab Clin Med 121:765–773. [PubMed] [Google Scholar]
- 34. Saijo T, Chen J, Chen SC, Rosen LB, Yi J, Sorrell TC, Bennett JE, Holland SM, Browne SK, Kwon-Chung KJ. 2014. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio 5:e00912–14. doi: 10.1128/mBio.00912-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Filbey KJ, Grainger JR, Smith KA, Boon L, van Rooijen N, Harcus Y, Jenkins S, Hewitson JP, Maizels RM. 2014. Innate and adaptive type 2 immune cell responses in genetically controlled resistance to intestinal helminth infection. Immunol Cell Biol 92:436–448. doi: 10.1038/icb.2013.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Decken K, Köhler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately MK, Alber G. 1998. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun 66:4994–5000. doi: 10.1128/IAI.66.10.4994-5000.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoag KA, Lipscomb MF, Izzo AA, Street NE. 1997. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol 17:733–739. doi: 10.1165/ajrcmb.17.6.2879 [DOI] [PubMed] [Google Scholar]
- 38. Olszewski MA, Huffnagle GB, McDonald RA, Lindell DM, Moore BB, Cook DN, Toews GB. 2000. The role of macrophage inflammatory protein-1 alpha/CCL3 in regulation of T cell-mediated immunity to Cryptococcus neoformans infection. J Immunol 165:6429–6436. doi: 10.4049/jimmunol.165.11.6429 [DOI] [PubMed] [Google Scholar]
- 39. Arora S, Hernandez Y, Erb-Downward JR, McDonald RA, Toews GB, Huffnagle GB. 2005. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J Immunol 174:6346–6356. doi: 10.4049/jimmunol.174.10.6346 [DOI] [PubMed] [Google Scholar]
- 40. Herring AC, Falkowski NR, Chen G-H, McDonald RA, Toews GB, Huffnagle GB. 2005. Transient neutralization of tumor necrosis factor alpha can produce a chronic fungal infection in an immunocompetent host: potential role of immature dendritic cells. Infect Immun 73:39–49. doi: 10.1128/IAI.73.1.39-49.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Milam JE, Herring-Palmer AC, Pandrangi R, McDonald RA, Huffnagle GB, Toews GB. 2007. Modulation of the pulmonary type 2 T-cell response to Cryptococcus neoformans by intratracheal delivery of a tumor necrosis factor alpha-expressing adenoviral vector. Infect Immun 75:4951–4958. doi: 10.1128/IAI.00176-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lynch DM, Kay PH. 1995. Studies on the polymorphism of the fifth component of complement in laboratory mice. Exp Clin Immunogenet 12:253–260. [PubMed] [Google Scholar]
- 43. Hussain S, Kirwin SJ, Stohlman SA. 2011. Increased T regulatory cells lead to development of Th2 immune response in male SJL mice. Autoimmun 44:219–228. doi: 10.3109/08916934.2010.519746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carroll SF, Loredo Osti JC, Guillot L, Morgan K, Qureshi ST. 2008. Sex differences in the genetic architecture of susceptibility to Cryptococcus neoformans pulmonary infection. Genes Immun 9:536–545. doi: 10.1038/gene.2008.48 [DOI] [PubMed] [Google Scholar]
- 45. Shourian M, Flaczyk A, Angers I, Mindt BC, Fritz JH, Qureshi ST. 2015. The Cnes2 locus on mouse chromosome 17 regulates host defense against cryptococcal infection through pleiotropic effects on host immunity. Infect Immun 83:4541–4554. doi: 10.1128/IAI.00697-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun D, Zhang M, Liu G, Wu H, Zhu X, Zhou H, Shi M. 2016. Real-time imaging of interactions of neutrophils with Cryptococcus neoformans demonstrates a crucial role of complement C5A-C5Ar signaling. Infect Immun 84:216–229. doi: 10.1128/IAI.01197-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lovchik JA, Lipscomb MF. 1993. Role for C5 and neutrophils in the pulmonary intravascular clearance of circulating Cryptococcus neoformans. Am J Respir Cell Mol Biol 9:617–627. doi: 10.1165/ajrcmb/9.6.617 [DOI] [PubMed] [Google Scholar]
- 48. Sun D, Shi M. 2016. Neutrophil swarming toward Cryptococcus neoformans is mediated by complement and leukotriene B4. Biochem Biophys Res Commun 477:945–951. doi: 10.1016/j.bbrc.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mershon-Shier KL, Vasuthasawat A, Takahashi K, Morrison SL, Beenhouwer DO. 2011. In vitro C3 deposition on cryptococcus capsule occurs via multiple complement activation pathways. Mol Immunol 48:2009–2018. doi: 10.1016/j.molimm.2011.06.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luo Y, Cook E, Fries BC, Casadevall A. 2006. Phagocytic efficacy of macrophage-like cells as a function of cell cycle and Fcgamma receptors (Fcgammar) and complement receptor (CR)3 expression. Clin Exp Immunol 145:380–387. doi: 10.1111/j.1365-2249.2006.03132.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gates MA, Kozel TR. 2006. Differential localization of complement component 3 within the capsular matrix of Cryptococcus neoformans. Infect Immun 74:3096–3106. doi: 10.1128/IAI.01213-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zaragoza O, Taborda CP, Casadevall A. 2003. The efficacy of complement-mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Eur J Immunol 33:1957–1967. doi: 10.1002/eji.200323848 [DOI] [PubMed] [Google Scholar]
- 53. Park YD, Shin S, Panepinto J, Ramos J, Qiu J, Frases S, Albuquerque P, Cordero RJB, Zhang N, Himmelreich U, Beenhouwer D, Bennett JE, Casadevall A, Williamson PR. 2014. A role for LHC1 in higher order structure and complement binding of the Cryptococcus neoformans capsule. PLoS Pathog 10:e1004037. doi: 10.1371/journal.ppat.1004037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dobó J, Kocsis A, Dani R, Gál P. 2022. Proprotein convertases and the complement system. Front Immunol 13:958121. doi: 10.3389/fimmu.2022.958121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Williams A, Lee GR, Spilianakis CG, Hwang SS, Eisenbarth SC, Flavell RA. 2013. Hypersensitive site 6 of the Th2 locus control region is essential for Th2 cytokine expression. Proc Natl Acad Sci USA 110:6955–6960. doi: 10.1073/pnas.1304720110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee GR, Fields PE, Griffin TJ, Flavell RA. 2003. Regulation of the Th2 cytokine locus by a locus control region. Immunity 19:145–153. doi: 10.1016/s1074-7613(03)00179-1 [DOI] [PubMed] [Google Scholar]
- 57. Ueno K, Urai M, Sadamoto S, Shinozaki M, Takatsuka S, Abe M, Otani Y, Yanagihara N, Shimizu K, Iwakura Y, Shibuya K, Miyazaki Y, Kinjo Y. 2019. A dendritic cell-based systemic vaccine induces long-lived lung-resident memory Th17 cells and ameliorates pulmonary mycosis. Mucosal Immunol 12:265–276. doi: 10.1038/s41385-018-0094-4 [DOI] [PubMed] [Google Scholar]
- 58. Huston SM, Ngamskulrungroj P, Xiang RF, Ogbomo H, Stack D, Li SS, Timm-McCann M, Kyei SK, Oykhman P, Kwon-Chung KJ, Mody CH. 2016. Cryptococcus gattii capsule blocks surface recognition required for dendritic cell maturation independent of internalization and antigen processing. J Immunol 196:1259–1271. doi: 10.4049/jimmunol.1501089 [DOI] [PubMed] [Google Scholar]
- 59. Morgan AP, Fu CP, Kao CY, Welsh CE, Didion JP, Yadgary L, Hyacinth L, Ferris MT, Bell TA, Miller DR, Giusti-Rodriguez P, Nonneman RJ, Cook KD, Whitmire JK, Gralinski LE, Keller M, Attie AD, Churchill GA, Petkov P, Sullivan PF, Brennan JR, McMillan L, Pardo-Manuel de Villena F. 2015. The mouse universal genotyping array: from substrains to subspecies. G3 (Bethesda) 6:263–279. doi: 10.1534/g3.115.022087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rodriguez-Gil JL, Watkins-Chow DE, Baxter LL, Elliot G, Harper UL, Wincovitch SM, Wedel JC, Incao AA, Huebecker M, Boehm FJ, Garver WS, Porter FD, Broman KW, Platt FM, Pavan WJ. 2020. Genetic background modifies phenotypic severity and longevity in a mouse model of niemann-pick disease type C1. Dis Model Mech 13. doi: 10.1242/dmm.042614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Broman KW, Wu H, Sen S, Churchill GA. 2003. R/qtl: QTL mapping in experimental crosses. Bioinf 19:889–890. doi: 10.1093/bioinformatics/btg112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S5.