Signaling through the redox active molecule hydrogen peroxide (H2O2) is important for several processes in plants, such as stomatal closure, root growth, gravitropism, and responses to pathogen challenge (Neill et al., 2002; Laloi et al., 2004). Although oxidative modification of reactive Cys residues within proteins has been suggested as a means by which H2O2 signaling can activate responses such as gene expression and reversible protein phosphorylation (Cooper et al., 2002; Danon, 2002), the linkage of H2O2 perception to intracellular signaling remains to be elucidated. Here, we report genetic and physiological data that demonstrate a previously uncharacterized function for the Arabidopsis (Arabidopsis thaliana) ethylene receptor ETR1, that of mediating H2O2 signaling in stomatal guard cells. Stomata in the loss-of-function etr1-7 mutant do not close in response to H2O2, and mutation of a Cys residue in the N-terminal region of ETR1 disrupts H2O2 signaling in both plants and in yeast (Saccharomyces cerevisiae).
Large-scale analyses of H2O2-modulated gene expression in Arabidopsis and tobacco have shown that expression of genes encoding elements of both two-component signal transduction pathways and ethylene signaling are up-regulated by exogenous H2O2 (Desikan et al., 2001; Vandenabeele et al., 2003), suggesting that these phenomena may be linked. His kinases (HKs) are part of two-component systems that transduce environmental signals into cellular responses. Some of them are known to function as cytokinin and ethylene receptors in plants (Hwang et al., 2002). Hybrid HKs consist of an N-terminal signal input domain (with some having hydrophobic transmembrane regions, such as ETR1), a HK domain, and a C-terminal response regulator domain. During typical HK signaling, the HK domain is autophosphorylated on a His residue, with subsequent transfer of the phosphate group onto an Asp residue in the response regulatory domain of the same protein. A subsequent relay of phosphotransfer reactions occurs downstream of HK, effecting various signaling processes (Hwang et al., 2002). However, HK activity may not be required for all downstream responses (Wang et al., 2003).
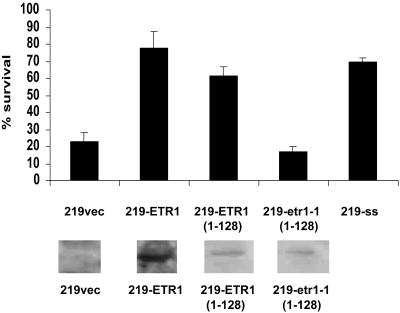
In yeast, two-component signaling systems function as H2O2 sensors (Singh, 2000; Buck et al., 2001). As part of a study to determine potential functions for plant HKs in H2O2 signaling, we focused on the ethylene receptor ETR1. ETR1 is a well-characterized hybrid HK in Arabidopsis and one for which extensive genetic, physiological, and biochemical analyses have demonstrated its function as an ethylene receptor (Guo and Ecker, 2004). Although ETR1 does have HK activity, such activity is not required for ethylene responses (Wang et al., 2003). The yeast TM219 mutant lacking a functional SLN1-SSK1 two-component system has enhanced susceptibility to growth inhibition by H2O2 (Singh, 2000). This system was used to determine if ETR1 could function in yeast to mediate oxidative stress responses. Transformation of TM219 with SLN1 and SSK1 together increased survival following exposure to H2O2 to a level comparable to that of the wild type (Fig. 1). Transformation of TM219 with full-length ETR1 resulted in a similar effect (Fig. 1), indicating that ETR1 can indeed function in yeast to mediate H2O2 responses. ETR1 is membrane-located in yeast (Fig. 1), but the particular membrane has not been identified. To determine if the N-terminal sensing domain of ETR1 was required for H2O2 responsiveness, TM219 was transformed with N-terminal constructs (containing the first 128 amino acids) of ETR1 containing either a wild-type Cys-65 or a Cys-65Tyr mutation in the second hydrophobic domain of ETR1 (as in the etr1-1 mutant). Only the construct containing the Cys-65 residue was able to increase survival following exposure to H2O2 (Fig. 1), indicating that the N-terminal domain of ETR1 is sufficient and that the Cys-65 residue is required for rescuing sensitivity to H2O2 in yeast. The mechanism by which ETR1 can restore H2O2 perception in TM219 is not known, although these data indicate that the HK domain of ETR1 is not required, but that the Cys-65 is essential.
Figure 1.
Yeast mutants lacking a functional HK that are more susceptible to H2O2 can be complemented with ETR1. The sln1::ssk1 mutant yeast strain TM219 (MATα ura3, leu2, trp1, his3, sln1::URA3, ssk1::LEU2) was grown and maintained in YPD medium (1% yeast extract, 2% bactopeptone, and 2% dextrose). For transformation of TM219, cells from an overnight culture were used to inoculate 100 mL of liquid YPD and incubated at 30°C until log phase. The cells were recovered by centrifugation (4,500g for 5 min), washed in 5 mL of 1 × LiAc/TE mix (100 mm lithium acetate, pH 7.5, 10 mm Tris-HCl, and 1 mm EDTA), and resuspended in 1 mL LiAc/TE. The plasmid DNA to be transformed (<5 μg) and 50 to 100 μg of sonicated salmon sperm DNA were mixed with 100 μL of yeast suspension and 700 μL of sterile 40% PEG4000 and incubated for 30 min at 30°C. The cells were heat shocked for 15 min at 42°C in the presence of 88 μL of DMSO and subjected to a pulse spin before resuspending in 0.2 mL of TE. The transformed cells were selected on appropriate selection plates. The expression of ETR1 in TM219 was confirmed by western blotting using anti-ETR1 (C-terminal) or anti-GST antibodies (Insight Biotechnology, Wembley, UK), as described (Rodriguez et al., 1999). For H2O2 sensitivity tests, log-phase cells were treated with H2O2 (1.25 mm) for 30 min and plated out at various dilutions on YPD agar plates. After incubation for 3 d at 30°C, the number of colonies was counted and percent survival calculated by comparing untreated versus treated cultures. 219vec, TM219 transformed with pYCDE2 vector alone; 219-ETR1, 219 with pYCDE2 containing full-length ETR1; 219-ETR1(1-128), 219 with pYCDE2 containing N-terminal region (1–128) of ETR1 fused to GST; 219-etr1-1(1-128), 219 transformed with pYCDE2 containing the Cys-65 mutation in N-terminal region of ETR1 fused to GST; 219ss, 219 with SLN1 and SSK1 plasmids as a positive control. Data represent the mean ± se from four independent experiments. Sections below indicate the expression of ETR1 in the 219 transformants, as determined by western blotting of yeast membranes isolated as described (Rodriguez et al., 1999), using an anti-ETR1 (C-terminal) antibody for the full-length ETR1 transformant or an anti-GST antibody for the N-terminal transformants.
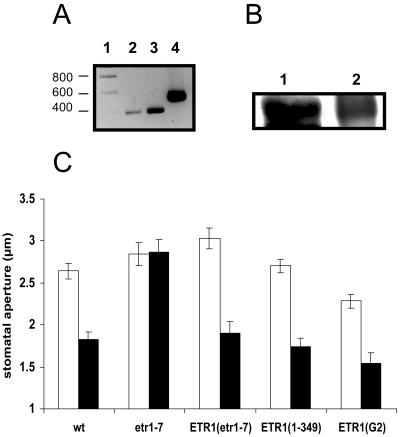
Various etr1 mutants of Arabidopsis have been used to demonstrate the role of ETR1 in ethylene signaling (Schaller and Kieber, 2002; Guo and Ecker, 2004). We exploited some of these mutants to show that ETR1 is also required for a different process, the well-characterized H2O2 signaling response of stomatal closure. To confirm that ETR1 is expressed in guard cells, both reverse transcription (RT)-PCR and western blotting were performed on guard cell-enriched fragments, indicating that ETR1 is expressed in guard cells (Fig. 2, A and B). The etr1-1 mutant contains a Cys-65Tyr mutation in the second hydrophobic domain of the transmembrane region, whereas the etr1-3 mutant has an Ala-31Val mutation in the first hydrophobic domain (Chang et al., 1993). The etr1-1 mutant is ethylene-insensitive in terms of the classic ethylene response, the so-called triple response, and the etr1-3 mutant also has very much reduced ethylene sensitivity (Hall et al., 1999). The etr1-7 mutant was created by mutagenizing a population of etr1-1 plants and is a loss-of-function allele with a stop codon at Trp-74 in the second hydrophobic domain, although it is ethylene responsive (Hua and Meyerowitz, 1998). To determine the effects of H2O2 on stomatal closure in wild type and etr1 mutants, leaves were treated with exogenous H2O2 and the resulting stomatal apertures measured. As reported previously (Pei et al., 2000), exposure of wild-type Arabidopsis leaves to H2O2 induced stomatal closure. However, the loss-of-function mutant etr1-7 was insensitive to H2O2 (Fig. 2), indicating that stomatal closure in response to H2O2 requires a functional ETR1 protein. To confirm this, etr1-7 plants complemented with a wild-type full-length ETR1 gene (Gamble et al., 2002) were tested for H2O2-induced stomatal closure; sensitivity to H2O2 was fully restored (Fig. 2).
Figure 2.
ETR1 is expressed in wild-type Arabidopsis guard cells and is required for H2O2-induced closure. A, RT-PCR of RNA extracted from guard cells (lane 2) and whole leaves (lane 3). Lane 4, Genomic DNA positive control; lane 1, DNA marker (indicated in basepairs). For RNA extractions, frozen leaf material was blended in a Waring blender (3 × 15 s) in water (2.5 g: 20 mL containing 1.5% TRIzol reagent [Invitrogen, Paisley, UK]) and ice, and the homogenate filtered through a 100-μm mesh (Spectramesh, VWR International, Poole, UK). This was repeated a further four times in the same mixture. The guard cell-enriched epidermal fragments (>95% guard cells, as assessed by FDA/DAPI staining; Hey et al., 1997) were then homogenized in TRIzol reagent (1 mL) with glass beads in a Fastprep bead beater (Fisher, Loughborough, UK) to break open the guard cells. RNA was extracted following the TRIzol RNA extraction procedure provided by the manufacturers. Reverse transcription was performed on DNAsed RNA, with PCR primers designed against sequences unique to ETR1; forward primer was ETR1F (5′-GTTTGTGAATCTGATGGAGGG-3′) and reverse primer was ETR1R (5′-GTTGTTTTGTGAATTTCTCG-3′). Genomic DNA was used as a control for the PCR to check that the RT products were from cDNA, and the PCR product subsequently sequenced. B, Western blot of guard cell proteins. Lane 1, Guard cell proteins; lane 2, yeast proteins. Guard cell-enriched epidermal fragments were prepared as above (but minus TRIzol) and the proteins extracted in extraction buffer (100 mm HEPES, pH 7.5, 5 mm EDTA, 5 mm EGTA, 10 mm DTT, 10 mm Na3VO4, 10 mm NaF, 50 mm α-glycerophosphate, 1 mm PMSF, 5 μg mL−1 aprotinin, and 5 μg mL−1 leupeptin) in the Fastprep bead beater, centrifuged for 2 × 20 min at 15,500g at 4°C, and the supernatant concentrated using Microcon (VWR International) spin columns. Yeast proteins were isolated from cells expressing full-length ETR1, as described (Rodriguez et al., 1999). Proteins were prepared for SDS-PAGE by incubating at 37°C for 1 h in SDS buffer without DTT and electrophoresed on a 7.5% SDS-polyacrylamide gel. Western blotting was performed using an anti-ETR1 (C terminus) antibody (Insight Biotechnology) and detected using enhanced chemiluminescence (GE Healthcare, Bucks, UK). C, The loss-of-function etr1-7 mutant is insensitive to H2O2. Arabidopsis leaves were floated for 3 h under continuous illumination (200–250 μE m−2 s−1) in MES/KCl buffer (5 mm KCl/10 mm MES/50 μm CaCl2, pH 6.15). Once the stomata were fully open, leaves were treated with H2O2 for a further 3 h. The leaves were subsequently homogenized individually in a Waring blender for 30 s and the epidermal fragments collected on a 100-μm nylon mesh (SpectraMesh). Stomatal apertures from epidermal fragments were then measured using a calibrated light microscope attached to an imaging system (Leica QWin software, Leica, Milton Keynes, UK). Stomatal closure response to H2O2 (100 μm) in wild type (wt); etr1-7; etr1-7 complemented with full-length ETR1 (ETR1[etr1-7]); etr1-7 complemented with ETR1 truncated at 349 (ETR1[1-349]); etr1-7 complemented with ETR1 containing a mutation in the G2 box of the HK domain (ETR1[G2]). White bars, control; black bars, H2O2. Data are expressed as mean ± se (n = 60 guard cells) from three independent experiments.
The function of various ETR1 domains in guard cell-H2O2 signaling was then assessed by utilizing etr1-7 plants complemented with the HK inactive G2 mutant or a truncated ETR1 (1-349). The mutation in the G2 box of ETR1 results in expression of a protein containing the HK domain, but in which there is no HK activity, whereas the 1-349 mutation results in a truncated protein lacking the HK domain (Gamble et al., 2002). Stomata of both these mutants responded to H2O2 and stomatal closure resulted (Fig. 2), indicating that the N-terminal region of ETR1 is sufficient for this response, and that neither the presence nor function of the HK domain is required for H2O2-induced closure. This is unlike the situation for ethylene signaling, where the presence but not the function of the HK domain in ETR1 is essential for a response (Gamble et al., 2002).
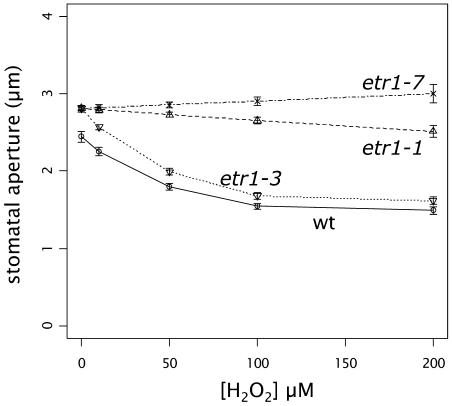
We investigated stomatal responses to H2O2 in the etr1-1 and etr1-3 mutants, both of which have mutations in the N-terminal transmembrane region. Similar to etr1-7, etr1-1 stomata were essentially insensitive to a range of concentrations of H2O2 (Fig. 3). On the other hand, the response of the etr1-3 mutant closely matched that of the wild type at all concentrations of H2O2 tested (Fig. 3). Cys-65 resides in the second hydrophobic domain of ETR1 and is essential for ethylene signaling (Schaller and Bleecker, 1995; Rodriguez et al., 1999). The etr1-3 mutant contains an Ala-31Val point mutation and has severely reduced responses to ethylene (Hall et al., 1999; data not shown). Thus, we demonstrate here that the ethylene-insensitive mutants etr1-1 and etr1-3 have different responses to H2O2. The etr1-1 mutant is insensitive, whereas the etr1-3 responds to H2O2 like wild type. These data suggest that the Cys-65 residue is pivotal to H2O2 responses in Arabidopsis guard cells.
Figure 3.
Cys-65 of ETR1 is required for H2O2-induced stomatal closure. Leaves of wild-type (○), etr1-7 (×), etr1-1 (▵), and etr1-3 (▿) plants were incubated in the light to induce stomatal opening, followed by exposure to H2O2 at the indicated concentrations, and stomatal apertures measured after 3 h. The data were obtained from four to five independent experiments (n = 100 guard cells per data point). The raw data were analyzed by model selection using Generalized Linear modeling with a Gamma response and inverse link, and the calculated apertures and error bars are shown.
In summary, our data demonstrate an unexpected role for ETR1, that of mediating stomatal closure in response to H2O2. Until now, ETR1 has been associated solely with ethylene perception and signaling. Our discovery that ETR1 can, in fact, mediate cellular responses to two different signaling molecules, namely ethylene and H2O2, indicates multiple functions for a single protein, as suggested recently for other plant receptors and enzymes (Szekeres, 2003; Moore, 2004). Moreover, it is possible that ETR1 could act as a central node mediating cross-talk between ethylene and H2O2 signaling, although whether such shared responses occur in other cells in addition to guard cells remains to be determined.
Acknowledgments
We thank E. Schaller (Dartmouth College, Hanover, NH) for the ETR1 full-length construct and for seeds of etr1-7 plants complemented with ETR1 constructs; T. Bleecker's laboratory (University of Wisconsin, Madison, WI) for the ETR1 and etr1-1 N-terminal region constructs; E. Meyerowitz (California Insitute of Technology, Pasadena, CA) for etr1-7 seeds; H. Saito (University of Tokyo, Tokyo) for the TM219 yeast mutant; and J. Gray and colleagues (University of Sheffield, Sheffield, UK) for advice on RNA and protein isolation from Arabidopsis guard cells.
This work was supported by the Biotechnology and Biological Sciences Research Council, UK.
References
- Buck V, Quinn J, Pino TS, Martin H, Saldanha J, Makino K, Morgan BA, Millar JBA (2001) Peroxide sensors for the fission yeast stress-activated mitogen-activated protein kinase pathway. Mol Biol Cell 12: 407–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM (1993) Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science 262: 539–544 [DOI] [PubMed] [Google Scholar]
- Cooper CE, Patel RP, Brookes PS, Darley-Usmar VM (2002) Nanotransducers in cellular redox signalling: modification of thiols by reactive oxygen and nitrogen species. Trends Biochem Sci 27: 489–492 [DOI] [PubMed] [Google Scholar]
- Danon A (2002) Redox reactions of regulatory proteins: Do kinetics promote specificity? Trends Biochem Sci 27: 197–203 [DOI] [PubMed] [Google Scholar]
- Desikan R, A-H Mackerness S, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble RL, Qu X, Schaller GE (2002) Mutational analysis of the ethylene receptor ETR1. Role of the histidine kinase domain in dominant ethylene insensitivity. Plant Physiol 128: 1428–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ecker JR (2004) The ethylene signalling pathway: new insights. Curr Opin Plant Biol 7: 40–49 [DOI] [PubMed] [Google Scholar]
- Hall AE, Chen QG, Findell JL, Schaller GE, Bleecker AB (1999) The relationship between ethylene binding and dominant insensitivity conferred by mutant forms of the ETR1 ethylene receptor. Plant Physiol 121: 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey SJ, Bacon A, Burnett E, Neill SJ (1997) Abscisic acid signal transduction in epidermal cells of Pisum sativum L. Argenteum: both dehydrin mRNA accumulation and stomatal responses require protein phosphorylation and dephosphorylation. Planta 202: 85–92 [Google Scholar]
- Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Hwang I, Chen H-C, Sheen J (2002) Two-component signal transduction pathways in Arabidopsis. Plant Physiol 129: 500–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloi C, Apel K, Danon A (2004) Reactive oxygen signalling: the latest news. Curr Opin Plant Biol 7: 323–328 [DOI] [PubMed] [Google Scholar]
- Moore BD (2004) Bifunctional and moonlighting enzymes: lighting the way to regulatory control. Trends Plant Sci 9: 221–228 [DOI] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Hancock JT (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5: 388–395 [DOI] [PubMed] [Google Scholar]
- Pei Z-M, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Rodriguez FI, Esch JJ, Hall AE, Binder BM, Schaller GE, Bleecker AB (1999) A copper co-factor for the ethylene receptor ETR1 from Arabidopsis. Science 283: 996–998 [DOI] [PubMed] [Google Scholar]
- Schaller GE, Bleecker AB (1995) Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science 270: 1809–1811 [DOI] [PubMed] [Google Scholar]
- Schaller GE, Kieber JJ (2002) Ethylene. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society for Plant Biologists, Rockville, MD, pp 1–2
- Singh KK (2000) The Sacccharomyces cerevisiae SLN1P-SSK1P two-component system mediates response to oxidative stress and in an oxidant-specific fashion. Free Radic Biol Med 29: 1043–1050 [DOI] [PubMed] [Google Scholar]
- Szekeres M (2003) Brassinosteroid and systemin: two hormones perceived by the same receptor. Trends Plant Sci 8: 102–104 [DOI] [PubMed] [Google Scholar]
- Vandenabeele S, Van Der Kelen K, Dat J, Gadjev I, Boonefaes T, Morsa S, Rottiers P, Slooten L, van Montagu M, Zabeau M, et al (2003) A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco. Proc Natl Acad Sci USA 100: 16113–16118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Hall A, O'Malley R, Bleecker AB (2003) Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission. Proc Natl Acad Sci USA 100: 352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]