ABSTRACT
A major challenge faced by bacteria is infection by bacteriophage (phage). Abortive infection is one strategy for combating phage in which an infected cell kills itself to limit phage replication, thus protecting neighboring kin. One class of abortive infection systems is the cyclic oligonucleotide based anti-phage signaling system (CBASS) which relies on two core enzymatic activities; an oligo-nucleotide cyclase that is activated following phage infection and a cyclic-oligo-nucleotide sensitive effector whose activity kills the infected cell. However, the mechanisms behind the deployment and activation of these lethal CBASS systems prior to and following infection have largely remained a mystery. While exploring unique genomic features of the current pandemic Vibrio cholerae biotype El Tor for clues underlying its pandemic success we found its CBASS was spuriously activated by the folate biosynthesis inhibitor sulfamethoxazole, but only after the population had reached a high-cell density. This population density-dependent activity revealed that transcription of both the oligo-nucleotide cyclase, dncV, and the CBASS phospholipase effector, capV, is enhanced at high-cell density by quorum sensing. Taken together, these results demonstrate that the V. cholerae CBASS is deployed when the environment is densely populated and activated in response to a perturbation in folate biosynthesis.
IMPORTANCE
To counteract infection with phage, bacteria have evolved a myriad of molecular defense systems. Some of these systems initiate a process called abortive infection, in which the infected cell kills itself to prevent phage propagation. However, such systems must be inhibited in the absence of phage infection to prevent spurious death of the host. Here, we show that the cyclic oligonucleotide based anti-phage signaling system (CBASS) accomplishes this by sensing intracellular folate molecules and only expressing this system in a group. These results enhance our understanding of the evolution of the seventh Vibrio cholerae pandemic and more broadly how bacteria defend themselves against phage infection.
KEYWORDS: cyclic GMP-AMP, CD-NTases, phage, quorum sensing, folate
INTRODUCTION
The diarrheal disease cholera, caused by the Gram-negative bacterium Vibrio cholerae, spreads through the consumption of contaminated food and water (1). Of the seven recorded cholera pandemics, the classical biotype is believed to have caused at a minimum both the fifth (1881–1896) and sixth (1899–1923) pandemics, whereas the El Tor biotype is responsible for initiating and perpetuating the seventh pandemic (1961–today) (2, 3). While strains of the classical biotype are now rarely encountered in environmental and clinical settings, numerous assays have been developed to help distinguish V. cholerae isolates as belonging to either the classical or El Tor biotypes (4, 5). These include disparate growth on citrate, hemolytic activity, casein proteolysis, production of acidic or neutral byproducts from growth on glucose, differences in virulence gene allele sequences and expression, and disparate sensitivities to the cationic antimicrobial peptide polymyxin B and folate biosynthesis inhibitors, such as sulfamethoxazole (SMX).
Two of the largest genetic differences between the V. cholerae biotypes are the Vibrio Seventh Pandemic Islands 1 and 2 (VSP-1 and VSP-2) genomic islands that are defining features of the El Tor biotype and absent in the classical biotype (3, 6, 7). VSP-1 and VSP-2, which collectively contain ~36 genes, are hypothesized to have played a critical role in the pandemic evolution of the El Tor biotype and many recent studies have begun to explore the biological functions they encode. The predominant function of these islands appears to be protection against invasive biological elements as two phage defense systems, AvcID (8, 9) and a Type II cyclic oligonucleotide based anti-phage signaling system (CBASS) (10 – 13), are encoded on VSP-1 while VSP-2 encodes the ddmABC operon, which inhibits plasmid acquisition, plasmid stability, and phage invasion (14, 15). In addition to biological defense, a three-gene operon on VSP-2 that is encoded in some strains of El Tor mediates aerotaxis in response to zinc (16). Outside of these four systems, little is known of the function of the VSP islands or their contribution to the emergence of the El Tor biotype.
The VSP-1 CBASS encompasses a four gene operon [vc0178(capV)-vc0179(dncV)-vc0180(cap2)-vc0181(cap3)]. In this system, via an unknown mechanism, phage infection stimulates DncV, a member of the CD-NTase family of enzymes (17), to synthesize 3′3′ cyclic GMP-AMP (cGAMP) (11, 18). cGAMP then allosterically activates the phospholipase CapV, which rapidly degrades the infected cell’s own membrane (10). Cap2 enhances the production of cGAMP by post-translationally modifying the C-terminus of DncV in a manner analogous to ubiquitination (12, 13). Conversely, Cap3 suppresses DncV activity by precisely proteolyzing this same C-terminal modification (12, 13). Ultimately, activation of VSP-1 CBASS rapidly kills the infected cell to restrict phage propagation and protect neighboring kin from further phage predation, a mechanism called abortive infection (11). Based on the observations that DncV binds folates (19), which inhibit its cyclase activity, and that treatment of Escherichia coli expressing V. cholerae dncV with antifolate antibacterial agents, including SMX, leads to an increase in intracellular cGAMP (19), it can be hypothesized that the concentration and composition of intracellular folates plays an important role in the regulation of V. cholerae CBASS abortive infection. While CBASS systems are widely encoded in bacterial genomes (11, 17, 20), we are just beginning to learn how these lethal population-level phage defense systems are controlled.
Bacteria sense their population density through quorum sensing (QS). Based on the constituency and abundance of bacteria in the environment, a bacterium uses QS to enact gene expression patterns that promote or discourage participation in coordinated population-level behaviors [reviewed in references (21, 22)]. Bacteria assess the local population density and composition by producing, secreting, and detecting small molecules called autoinducers (AIs) whose environmental concentrations are a proxy for bacterial abundance. In V. cholerae, QS gene expression programs for low-cell density (LCD) and high-cell density (HCD) are regulated by the transcription factors LuxO and HapR, respectively. At LCD, when the environmental concentrations of AIs are low, LuxO is active and hapR mRNA is degraded. As AI concentrations increase in the environment, a proxy for an increasing population density, LuxO activity is inhibited via dephosphorylation, driving hapR expression and enabling induction of the HCD gene expression regulon. The dichotomous QS-dependent activities of LuxO and HapR are primarily responsible for the differential regulation of more than 500 V. cholerae genes (23). These population-dependent changes in transcription determine whether V. cholerae pursue behavioral strategies best suited for a solitary or group lifestyle at LCD or HCD.
To better understand the contribution of the VSP islands to the evolution of the seventh V. cholerae pandemic, we explored if the VSP islands in the El Tor biotype were responsible for some of the well-known phenotypic differences between strains of the classical and El Tor biotypes. We report that El Tor’s sensitivity to the folate biosynthesis inhibitor SMX is dependent on the VSP-1 encoded Type-II CBASS. This sensitivity results from the spurious activation of DncV and the subsequent activation of the phospholipase CapV. Furthermore, during these studies, we found that the expression and activity of dncV and capV are induced at HCD by the V. cholerae QS pathway, consistent with its function as a population-level phage defense mechanism. Our findings identify both transcriptional and post-transcriptional mechanisms that lead to the deployment and activation of the El Tor V. cholerae CBASS.
RESULTS
VSP-1 and VSP-2 do not impact metabolic phenotypes commonly used to distinguish the El Tor and classical V. cholerae biotypes
We examined whether the VSP-1 and VSP-2 islands contributed to phenotypic behaviors commonly attributed to the El Tor biotype by performing several biotyping assays comparing classical strain O395 (24) and the El Tor strain C6706str2 (C6706) (25) with those of single VSP island mutants (ΔVSP-1 and ΔVSP-2) and a double VSP island mutant (ΔVSP1/2). O395 is known to be deficient in both protease and hemolysin production in comparison to C6706, as demonstrated on casein milk agar and blood agar plates, respectively (4). Simple streaks of these strains on milk agar and sheep blood agar confirmed differential degradation of casein and hemolytic activities between O395 and C6706, as indicated by the absence and presence of a zone of clearing on each medium, respectively (Fig. 1A and B). We found that deletion of one or both VSP islands did not affect either activity as all three mutants phenocopied the parental C6706 strain (Fig. 1A and B). Similarly, classical O395 cannot utilize citrate as a sole carbon source (26) or grow on MacConkey agar, while C6706 can do both. Again, we found that the VSP islands do not contribute to these two phenotypes as all three mutants grew comparably to the parental C6706 strain on these media (Fig. 1C and D). Finally, El Tor strains are known to produce acetoin upon fermentation of glucose, while classical strains do not (27). Using the Voges-Proskauer assay to measure acetoin by the generation of a red color, we found supernatants of C6706 produced a deep red color, indicative of acetoin production, while classical O395 was weakly pink (Fig. 1E). Production of acetoin was not grossly impacted upon deletion of either or both VSP islands (Fig. 1E) as these strains also produced a deep red color like the parental C6706. While casein degradation in milk agar and production of the fermentative bioproduct acetoin have been linked to QS (4, 28) and the inability of O395 to perform these functions is likely due, in part, to its non-functional hapR allele (29), we nonetheless demonstrate the VSP islands do not contribute to these phenotypes in C6706.
Fig 1.

The VSP islands do not contribute to common V. cholerae biotyping phenotypes or virulence in a murine model of cholera. Differential biotyping phenotypes between classical O395, El Tor C6706, and C6706 VSP island mutants (ΔVSP-1, ΔVSP-2, and ΔVSP-1/2) demonstrating strain-specific (A) proteolysis of casein on milk agar, (B) hemolytic activity on blood agar, (C) growth on citrate minimal medium agar, (D) growth on matched LB and MacConkey agar plates, and (E) production of acetoin detected by Voges-Proskauer Assay. All images are representative of three independent experimental replicates. (F) In vivo competition between a 1:1 mixture of C6706 ΔVSP1/2 and C6706 ΔlacZ in an infant mouse model of cholera. Intestinal colony-forming units (CFUs) were enumerated using blue-white screening 20 h after oral gavage. N = 8 mice and statistical significance was determined using a one-sample t test and a hypothetical mean log10 C.I. = 0. The hypothetical mean is represented by a dotted line. The calculated mean log10 C.I. is represented by a solid line. ns = not significant.
The VSP-1 and VSP-2 islands do not contribute to El Tor C6706 colonization in an infant mouse model
Subtle differences in the sequences of the virulence gene alleles ctxB and tcpA between strains of the El Tor and classical biotypes have been used for biotyping novel V. cholerae isolates (4). The classical biotype is also more permissive in its in vitro expression of the V. cholerae virulence regulon compared to the El Tor biotype (30), which anecdotally causes less severe cholera in humans (31). To determine whether the VSP islands collectively contribute to in vivo colonization we performed a competition infection between C6706 and ΔVSP-1/2 in the infant mouse model of cholera. Despite the previous attribution of dncV, the CBASS cGAMP synthase located in VSP-1, to colonization (18), we found no competitive defect in the ability of the double VSP island mutant to colonize the infant mouse intestinal tract (Fig. 1F). The discrepancy between this finding and (18) may be attributed to unknown epistatic relationships between dncV and other VSP encoded genes which obscure this colonization defect in our study, genetic differences between laboratory lineages of C6706 (32), or specific conditions not replicated in our study. Nevertheless, our result suggests that the collective impact of the VSP islands on colonization in the infant mouse model of cholera is negligible.
VSP-1 increases sensitivity to sulfamethoxazole
In addition to different metabolic and virulence characteristics, different susceptibilities to antibiotics have also been observed between classical and El Tor strains. One such antibiotic is the cationic antimicrobial peptide polymyxin B, which disrupts the outer membrane of Gram-negative bacteria. Indeed, as previously observed in pandemic V. cholerae biotypes (33, 34), we found that classical O395 was more sensitive to polymyxin B than El Tor C6706 with half maximal inhibitory concentrations (IC50) of 0.6 and 18.4 µg/mL, respectively (Fig. S1; Table S1). However, deletion of VSP-1, VSP-2, or both islands did not grossly impact El Tor’s susceptibility to polymyxin B (Fig. S1; Table S1) demonstrating the VSP islands do not contribute to this biotype-specific phenotype.
In contrast to polymyxin B, strains of the El Tor biotype have been shown to exhibit greater sensitivity to antifolate antimicrobials, such as SMX, than those of the classical biotype (5). SMX impairs folate biosynthesis by inhibiting the activity of dihydropteroate synthase. After treating our strains with a concentration gradient of SMX and measuring culture optical densities after 24 h, we found C6706 was profoundly more sensitive to SMX (IC50 36.5 µg/mL) than classical O395 (IC50 230.7 µg/mL) (Fig. 2A; Fig. S2A; Table S1). Surprisingly, both ΔVSP-1 and ΔVSP-1/2 phenocopied O395’s SMX resistance while the ΔVSP-2 mutant retained the parental C6706 SMX sensitivity (Fig. 2A; Fig. S2A; Table S1). Hypothesizing SMX sensitivity could be attributed to VSP-1, we reintroduced VSP-1 into the ΔVSP-1/2 mutant on a single-copy cosmid (pVSP-1) and found this was sufficient to restore SMX sensitivity (Fig. 2B; Fig. S2B). Provision of pVSP-1 to O395 also increased its sensitivity to SMX (Fig. 2B; Fig. S2C). Additionally, providing pVSP-1 to E. coli BL21(DE3) also led to increased SMX sensitivity (Fig. 2B), though to a lesser degree than seen in O395 as the strain was already fairly sensitive to this antibiotic (Fig. S2D). Taken together, these results demonstrate the disparity in V. cholerae biotype-specific sensitivities to SMX (5) is the result of a factor encoded on VSP-1.
Fig 2.

VSP-1 encoded CBASS is responsible for V. cholerae biotype-specific SMX sensitivity. Twenty-four hours planktonic antibiotic sensitivity assays were performed in a variety of SMX concentration gradients. Heatmaps (A), (B), and (D) represent relative growth for each strain calculated using culture optical densities and the equation (OD600 SMX treatment/OD600 untreated) and reported as a color-coded mean % of N = 3 biological replicates. The IC50 for all strains in (A) are presented in Table S1. Scatter plots corresponding to (A), (B), and (D) are presented in (Fig. S2A through F). (C) Cartoon depiction of the VSP-1 genomic island. Dotted lines indicate partial VSP-1 truncations. Gray chevrons highlight the four gene VSP-1 CBASS operon. Numerals 1–5 indicate the location of a mutation identified in each of the five respective V. cholerae isolates found to have spontaneously evolved SMX resistance.
Sulfamethoxazole sensitivity requires the CBASS genes dncV and capV
The VSP-1 island of V. cholerae C6706 contains 12 known or predicted genes (Fig. 2C). To identify which genes are responsible for SMX sensitivity, we challenged three partial VSP-1 island mutants (ΔavcD-vc0176, ΔvspR-cap3, and Δvc0182-vc0185) with a gradient of SMX concentrations and found only the ΔvspR-cap3 mutant was more resistant to SMX (Fig. 2D; Fig. S2E). The five genes missing in this mutant include the negative transcriptional regulator of CBASS, vspR (18), and the four gene CBASS (dncV-cap3) (11) (Fig. 2C). It was previously shown that folates are allosteric regulators of DncV which suppress cGAMP synthesis in vitro (19) and that cGAMP is required for the activation of the lethal phospholipase CapV (10). We therefore hypothesized that inhibition of folate biosynthesis by SMX could alleviate the folate-dependent repression of DncV activity and lead to CapV-dependent cell death. In support of this hypothesis, deletion of either dncV or capV was sufficient to enhance resistance to SMX in these conditions, while loss of vspR, cap2, or cap3 was not (Fig. 2D; Fig. S2F).
We also performed a screen for V. cholerae isolates that could spontaneously evolve resistance to SMX on solid agar in the presence of filter discs infused with 40 mg/mL SMX. Five colonies were selected from the zones of inhibition around three discs, and each was found to grow in the presence of 50 µg/mL SMX in liquid LB, a concentration impermissible for growth of WT V. cholerae C6706 (Fig. S2G). We performed whole-genome sequencing on these five spontaneous SMX -resistant isolates which revealed each one had independently evolved SMX resistance by mutation of either capV or dncV (Table S2; Fig. 2C). This result reinforced our earlier observation that targeted deletion of capV and dncV was linked to SMX resistance (Fig. 2D; Fig. S2F).
Sulfamethoxazole activates DncV cGAMP synthesis
It was previously reported that the addition of either sulfonamide or trimethoprim, another antifolate antibacterial agent, to E. coli ectopically expressing dncV from a high-copy plasmid enhanced the catalytic activity of DncV (19). To test whether SMX treatment might induce cGAMP synthesis by DncV in V. cholerae expressed from its native genetic context, we back-diluted stationary phase cultures of C6706 and ΔcapV 1:1,000 in fresh medium, allowed them to recover for approximately one hour, introduced 100 µg/mL SMX or a DMSO control, and measured culture optical density over the course of 6 h. Concurrent with monitoring culture density, we also measured the intracellular concentration of cGAMP using UPLC-MS/MS, but this measurement was only performed in the ΔcapV cultures where detectable levels of cGAMP can accumulate without inducing CapV-dependent cell lysis (10). Surprisingly, C6706 cultures treated with SMX only exhibited a growth defect after 2 h of exposure to the antibiotic (Fig. 3A). This growth defect is a consequence of CapV activity as there was no difference in growth between ΔcapV cultures treated with and without SMX (Fig. 3A). Similarly, cGAMP was only found in ΔcapV cultures challenged with SMX and was not detected until more than 2 h after its addition (Fig. 3A). While these results demonstrate that SMX stimulates DncV synthesis of cGAMP in its native environment, they also reveal a profound delay exists between introduction of the antibiotic and evidence of DncV activity under these experimental conditions.
Fig 3.
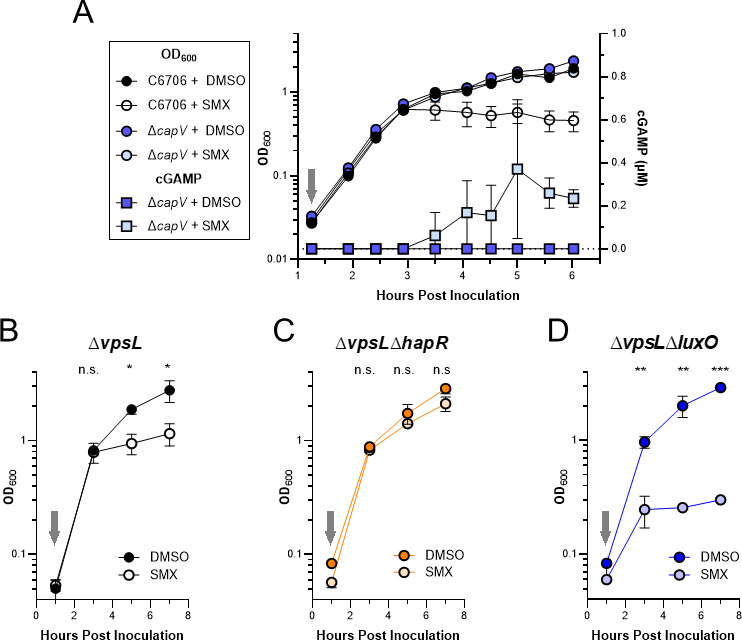
Culture density-dependent sensitivity to SMX reveals quorum sensing regulation of VSP-1 CBASS. (A) Growth curves (OD600, left y-axis) of WT C6706 and ΔcapV cultures treated without (+DMSO) and with 100 µg/mL SMX (+SMX). Intracellular cGAMP (µM, right y-axis) measured by UPLC-MS/MS in the SMX treated and untreated ΔcapV cultures. Growth curves of (B) quorum fluent ΔvpsL, (C) LCD-locked ΔvpsLΔhapR, and (D) HCD-locked ΔvpsLΔluxO cultures treated without (+DMSO) and with 100 µg/mL SMX (+SMX). Gray arrows indicate addition of 100 µg/mL SMX or DMSO. N = 3 biological replicates and error bars represent standard deviation. Statistical significance calculated using an unpaired T test with the Holm-Šídák method (*P < 0.05, **P < 0.005, ***P < 0.0005), n.s. = not significant.
QS contributes to CBASS-dependent SMX sensitivity
Hypothesizing the 2-h delay in SMX-dependent growth inhibition and concomitant synthesis of cGAMP (Fig. 3A) was related to QS, we challenged QS mutants locked in LCD or HCD gene expression with SMX and monitored culture densities over time. Because LCD QS mutants have a propensity to form biofilms, which could interfere with measuring optical density, we utilized QS mutants derived from the biofilm-null C6706 ΔvpsL background (35). Importantly, there is no difference between C6706 and ΔvpsL when challenged with SMX or DMSO in these conditions (Fig. S3). As previously seen for C6706 (Fig. 3A), the ΔvpsL cultures with an intact QS system experienced a similar 2-h delay in growth inhibition in response to SMX (Fig. 3B). In agreement with our hypothesis that QS induces CBASS, the LCD-locked ΔvpsLΔhapR strain was tolerant of SMX (Fig. 3C), while the HCD-locked ΔvpsLΔluxO was hypersensitive to SMX with an abbreviated temporal delay in growth inhibition (Fig. 3D). Taken together, these results support the argument that QS contributes to the regulation of CBASS.
QS regulates transcription of the V. cholerae CBASS operon
To determine if QS regulation of CBASS was at the level of transcription induction, we first monitored the abundance of capV and dncV transcripts in cultures of V. cholerae C6706 over time using RT-qPCR. Three hours following inoculation and corresponding with ~0.6 OD600, a culture density consistent with the transition from LCD to HCD (36), the abundance of each transcript abruptly increased ~8-fold relative to their initial levels and remained high for the duration of the experiment (Fig. 4A). Notably, the increased abundance of both capV and dncV transcripts 3-h post-inoculation precedes both the SMX-dependent detection of cGAMP in the ΔcapV mutant and growth inhibition by SMX of quorum fluent strains in previous experiments (Fig. 3A and B; Fig. S3A).
Fig 4.

QS regulates expression of V. cholerae CBASS. (A) Growth curve (OD600, left y-axis, solid line) of V. cholerae C6706 and the corresponding fold-change in transcript abundance (right-axis) of capV and dncV, relative to the initial 2 h time point, measured using RT-qPCR. N = 2 biological replicates and error bars represent standard deviation. (B) Relative transcript abundance of capV, dncV, and hapR measured by qRT-PCR in ΔcsqAΔluxS grown in the presence (+) and absence (−) of exogenous autoinducers (AIs). N = 3 biological replicates and error bars represent standard error of the mean. (C) % relative luminescence units of the indicated strains normalized to the mean lum/OD600 of ∆vpsL∆luxO maintaining the luminescent transcriptional reporter pPCBASS::lux. Population densities are low-cell density (LCD) and high-cell density (HCD). N = 3 biological replicates and error bars represent standard deviation. Statistical significance calculated using an unpaired t test with the Holm-Šídák method (*P < 0.05). This method was only used between strains at the same population density and the lack of graphical comparison indicates no statistical difference observed. (D) Relative luminescence (lum/OD600) of E. coli maintaining the luminescent transcriptional reporter pPCBASS::lux and the Ptac inducible hapR plasmid (pHapR) or a vector control (pVector2) grown in the presence of 6.25 µM IPTG. N = 3 biological replicates and error bars represent standard deviation. Statistical significance calculated using an unpaired t test with the Holm-Šídák method (**P < 0.005, ***P < 0.0005), n.s. = not significant. (E) Intracellular cGAMP measured using UPLC-MS/MS in the quorum fluent ΔcapV and LCD-locked ΔcapVΔcsqAΔluxS strains grown ~24 h in the presence of 100 µg/mL SMX. N = 4 biological replicates and error bars represent standard deviation. Statistical significance calculated using an unpaired t test (*P < 0.05).
To confirm the observed increase in capV and dncV transcripts were the result of HCD gene expression, we measured their abundance in a strain of C6706 (ΔcsqAΔluxS) (37) which is incapable of producing the two primary V. cholerae AIs, CAI-2, and AI-1. In monoculture, ΔcsqAΔluxS is locked in LCD gene expression, regardless of the population density, but can be converted to HCD gene expression by the introduction of exogenous AIs. Using RT-qPCR, we found when ΔcsqAΔluxS was grown in the presence of exogenous AIs there was at least a sixfold greater abundance of capV and dncV transcripts than when grown in their absence (Fig. 4B). As a control, the abundance of hapR transcript also increased upon AI addition indicating the cultures had been converted to HCD gene expression (Fig. 4B).
To better understand how QS was regulating the abundance of CBASS transcripts, we constructed a luminescent transcriptional plasmid reporter containing a 913 nt region 5′ of the capV translational start site with the CBASS promoter (pPCBASS::lux) and measured luminescence generated by biofilm-null ΔvpsL QS mutants at LCD and HCD. The HCD-locked strain, ΔvpsLΔluxO was the most luminescent at both densities, whereas the LCD-locked strain, ΔvpsLΔhapR, was sparingly luminescent regardless of the culture density (Fig. 4C). The quorum fluent strain, ΔvpsL, resembled ΔvpsLΔhapR at LCD and ΔvpsLΔluxO at HCD (Fig. 4C). These results indicated that transcription initiation of the CBASS promoter is positively regulated at HCD.
The transcription factor primarily responsible for HCD gene expression in V. cholerae is HapR (29). To determine if HapR could enhance expression of the CBASS promoter independent of additional V. cholerae genes, we measured the luminescent output of pPCBASS::lux in a heterologous E. coli host and provided hapR in trans on an inducible plasmid (pHapR). The relative luminescence of E. coli cultures maintaining pHapR was significantly greater than the vector control (pVector2) following introduction of the inducer (Fig. 4D). Finally, we tested whether the HCD-dependent transcriptional activation of the CBASS operon resulted in increased synthesis of cGAMP in cultures grown in the presence of SMX. To avoid CBASS-dependent SMX toxicity, we measured the intracellular concentration of cGAMP in the quorum fluent ΔcapV and LCD-locked ΔcapVΔcsqAΔluxS strains grown for approximately 24 h in the presence of 100 µg/mL SMX. This analysis revealed that in the presence of SMX, the LCD-locked ΔcapVΔcsqAΔluxS strain exhibited a significant reduction in intracellular cGAMP relative to the quorum fluent ΔcapV strain when both strains were grown to HCD (Fig. 4E). In total, our data indicate CBASS transcription, and therefore activity, is induced at HCD in V. cholerae C6706 by HapR.
HapR-dependent induction of CBASS transcription contributes to phage defense
QS induction of the CBASS operon suggests that this system would exhibit higher levels of phage defense at HCD. However, we have not identified a condition in which the V. cholerae CBASS operon protects against infection by the three major V. cholerae lytic phage ICP-1, ICP-2, and ICP-3 (38). Thus, we are unable to test this prediction in V. cholerae expressing CBASS from its native genomic context. To circumvent this challenge, we examined if the V. cholerae CBASS operon provided phage defense when expressed from a low-copy cosmid (pVSP-1) in E. coli to the lytic phage T2 in shaking liquid cultures. In these conditions, pVSP-1 alone provided only minimal enhancement of E. coli growth when challenged with T2 at low multiplicities of infection (MOIs) (Fig. 5A; Fig.S4). However, HapR expression substantially enhanced protection by pVSP-1 to T2 phage infection, demonstrating that QS enhancement of CBASS expression leads to greater phage defense (Fig. 5A; Fig. S4).
Fig 5.

HapR enhances VSP-1 mediated phage defense in E. coli. (A) Growth of E. coli containing either pHapR induced with 10 µM IPTG and pVSP-1 with their associated vector controls after overnight growth with T2 phage is shown as a mean percent of the uninfected culture from N = 3 biological replicates. mean % presented numerically and by heatmap for each condition. Scatter plot of data presented in Fig. S4. (B) Model of folate (by SMX) and QS (by HapR) regulation of CBASS activity and expression. At HCD, HapR induces transcription of the CBASS operon. Inhibition of folate biosynthesis by SMX alleviates the folate-dependent non-competitive inhibition of DncV leading to synthesis of cGAMP and activation of the phospholipase CapV. CBASS activity ultimately culminates in abortive infection that thwarts phage predation by limiting phage replication. Solid arrows and brakes indicate regulatory mechanisms that influence CBASS activity addressed in this study. Black hatched arrows and brakes indicate mechanisms known to contribute to CBASS activity but were not found to significantly contribute to QS or folate mechanisms illuminated in this study. Red brake represents a hypothesized phage-dependent disturbance in folates which is sensed by DncV to initiate abortive infection by the V. cholerae CBASS. Created with BioRender.com.
DISCUSSION
The earliest strains of the El Tor biotype were first encountered during the period of 1897–1938 and considered non-pathogenic enteric commensals (3). Through genotypic analysis of early and modern El Tor strains, Hu et al. (3) traced the evolutionary linage of the El Tor biotype from non-pathogenic commensal to pandemic scourge in six phases. While these phases include the predictable acquisition of key virulence factors including tcpA, toxT, and cholera toxin, the fifth stage (1925–1954) is primarily defined by El Tor’s acquisition of VSP-1 and VSP-2 from unknown origins. While it has been hypothesized that acquisition of VSP-1 and VSP-2 potentiated El Tor’s pandemicity and rise to global dominance in the seventh pandemic, we are just beginning to understand their utility and the functions they encode.
Our analyses show that VSP-1 and VSP-2 do not contribute to previously identified metabolic differences between El Tor and Classical biotypes nor do they collectively influence colonization in an infant mouse infection model. Rather, we demonstrate that the increased sensitivity to the antibiotic SMX in the model El Tor strain C6706 is due to the spurious activation of the abortive infection anti-phage Type-II CBASS encoded on VSP-1. Upon exposure to SMX, inhibition of folate biosynthesis de-represses DncV which leads to the synthesis of cGAMP (Fig. 5B). This increase of intracellular cGAMP allosterically activates the phospholipase CapV leading to the degradation of bacterial membranes and cell death (10) (Fig. 5B). We note that a recent study independently reached the same conclusion finding that the CBASS system of V. cholerae strain N16961 drove sensitivity to SMX (39). N16961 is a natural locked LCD mutant of V. cholerae with a non-functional hapR, and thus the role of QS in CBASS activity could not be observed in this study.
In response to increased usage of SMX to treat cholera infections, SMX resistance has been observed in 79% of O1/O139 isolates (40). However, such resistance is not due to the loss of CBASS function but rather the acquisition of other resistance mechanisms like the SXT integrative conjugative element (41, 42). This is interesting in that all five of the spontaneous SMX-resistant clones identified in our study contained mutations in dncV or capV. While acquisition of mutations in CBASS appear to be the most common route for evolving SMX resistance in vitro the fact that El Tor strains retain CBASS function and VSP-1 (43), while acquiring resistance to SMX via alternate mechanisms argues that the VSP-1 island continues to play an important role in the fitness of circulating El Tor biotype strains. One such role could be enhanced resistance to phage predation which has been hypothesized to contribute to the global rise of the El Tor biotype and displacement of the classical biotype prior to the seventh pandemic (44).
The link between SMX exposure and the activation of the VSP-1 CBASS is likely the result of non-competitive inhibition of DncV by folate molecules. The x-ray crystal structure of DncV unexpectedly revealed a molecule of 5-methyl-tetrahydrate folate bound on the opposite face of the active site (19). Further study revealed that the in vitro cyclase activity of purified DncV was inhibited by the addition of various folate molecules (19). Additionally, introduction of the folate biosynthesis inhibitors sulfonamide and trimethoprim to E. coli expressing recombinant DncV led to an increase in the intracellular concentration of cGAMP relative to untreated cells (19). DncV belongs to the CD-NTase family of cyclic oligo-nucleotide synthases, which are often allosterically regulated [reviewed in reference (45)]. For example, metazoan cGAS only synthesizes 2′3′ cyclic GMP-AMP when bound to dsDNA as this is a biological signal for viral invasion or genome instability (46, 47), while the homolog cGLR1 from Drosophila melanogaster responds to dsRNA (48). Activation of DncV in V. cholerae by SMX indicates folates likely allosterically inhibit this CD-NTase in its native host.
Given that DncV initiates an abortive infection program (49), and its activity is inhibited by folates (19), we hypothesize that disruption of folate metabolism is a cellular indication of phage infection. In support of this idea, phage often encode nucleotide biosynthetic enzymes and extensively remodel cellular nucleotide pools, which can in turn alter folate metabolism. For example, the bacteriophage T4 encoded protein 55.1 forms a complex with E. coli FolD, a bifunctional enzyme that catalyzes interconversion of 5,10-methylene-tetrahydrofolate and 10-formyl-tetrahydrofolate, and induces hypersensitivity to trimethoprim (50). Additionally, the T4 strain, T4D, has been shown to shift the metabolism of folate compounds during infection in E. coli [reviewed in reference (51)]. T4 phage has also been shown in numerous studies to be susceptible to CBASS anti-phage activities (11 – 13). Our results support a model (Fig. 5B) that depletion of folates upon phage infection is the activation signal for DncV to initiate abortive infection, though this remains to be formally tested. It is also unclear whether folates facilitate or antagonize the dichotomous activities of Cap2 and Cap3 which enhance and suppress DncV activity, respectively, through post-translation modification of its C-terminus (12, 13). Under the conditions we tested, activation by folate depletion is indifferent to the post-translational modification state of DncV as loss of either cap2 or cap3 did not substantially affect SMX sensitivity (Fig. 2D; Fig. S2F).
Our studies of SMX activation of DncV showed a consistent delay in cell killing, which we determined is due to the QS-dependent transcription of CBASS by the HCD transcriptional regulator HapR (Fig. 5B). In agreement with this, RNA-seq analysis of V. cholerae C6706 demonstrated that CBASS transcripts were significantly more abundant at HCD than LCD (23). QS regulation appears to be another mechanism to restrict the activity of the VSP-1 CBASS system to the most appropriate conditions, thereby preventing spurious activation of DncV and CapV and unnecessary cell death. Identification of the VSP-1 encoded transcription factor VspR (Fig. 2C and 5B) was pivotal to the discovery of DncV (18). ChIP analysis revealed VspR was associated with DNA sequences found within the CBASS locus and vspR mutants contained a greater abundance of CBASS gene transcripts, implicating it as a negative transcriptional regulator of CBASS (18). Contrary to our expectation, under our experimental conditions, vspR did not contribute to CBASS-dependent SMX sensitivity (Fig. 2D; Fig. S2F). If and how VspR and QS regulation of CBASS overlap remains to be explored.
The role of QS in defense against phage has been previously described [reviewed in reference (52)] and some notable examples include the regulation of phage receptor abundance in E. coli (53) as well as hemagglutinin protease production in El Tor V. cholerae (54), CRISPR-Cas expression in Pseudomonas aeruginosa (55), and the hapR independent HCD-regulation of the El Tor VSP-II encoded ddmABC anti-phage and plasmid defense system (15). From an ecological perspective, reserving the expression and activity of the VSP-1 CBASS to situations where El Tor V. cholerae would find itself in an environment densely populated with kin fits with its biological function of preventing phage infection by abortive replication. HCD populations are environments where phage will be the most prevalent and bacteria at greater risk of infection. At LCD, when neighbors are scarce, abortive infection is unlikely to be an evolutionarily advantageous strategy and infected cells may instead rely on non-lethal phage defense mechanisms. Though this remains to be formally tested, one scenario in which El Tor V. cholerae defends against phage could be a reliance of the non-lethal depletion of nucleotides by the avcID system (8, 9) at LCD and the CBASS system at HCD, both of which are encoded in VSP-1. Although the molecular mechanism of CBASS is now well characterized (56), the regulation of such systems and their contribution to bacterial evolution and environmental adaptation is just beginning to be described. CBASS is widely conserved in bacteria (20) and whether QS regulation of such systems is commonplace should be further investigated.
MATERIALS AND METHODS
Growth conditions and media
All strains of V. cholerae and E. coli were grown in LB broth-Miller media (NEOGEN) at 35°C with aeration, unless otherwise stated. When noted, antibiotic selection was utilized at the following concentrations: streptomycin (50 µg/mL), chloramphenicol (10 µg/mL when used alone or 5 µg/mL when used with another antibiotic), kanamycin (100 µg/mL when used alone; 50 µg/mL when used with another antibiotic) and tetracycline (5 µg/mL). IPTG was used at 6.25–10 μM as indicated. E. coli BW29427, a diaminopimelic acid (DAP) auxotroph, was additionally supplemented with 300 µg/mL DAP and used for the conjugative transfer of vectors and cosmids to all V. cholerae strains presented in this work.
Cloning and strain construction
All gene deletions from the V. cholerae genome were performed using the vector pKAS32 (57). Deletion constructs were cloned using a three-piece Gibson Assembly composed of ~1 kb homologous sequences both 5′ and 3′ of the genomic region to be removed and linear pKAS32 double digested with KpnI and SacI. Mutants were obtained through allelic exchange (57) and verified by Sanger sequencing. All mutants utilized in this study were complete deletions of the genomic regions of interest except for ΔvspR, where the vspR codons 1, 2, and 6 were all mutated into stop codons. The pPCBASS::lux plasmid was generated by amplification of 913 n.t. upstream of capV and Gibson assembly into BamHI/SpeI digested pBBR-lux (58) using the primers Lux_CBASSpr_FW and Lux_CBASSpr_RV, 5′.
Metabolic growth assays
Using an inoculating loop, overnight cultures were applied as a single streak on the surface of agar plates and incubated at 35°C for 24 h. Images were taken of plates using an iPhone. For the casein hydrolysis protease assay, milk agar plates were prepared according to reference (4) and contained 20.0 g/L dry skim milk and 9.2 g/L brain-heart infusion and 15 g/L agar. Blood agar plates were prepared according to reference (59) using Mueller Hinton Broth in 1.5% agar with 5% sheep blood. Citrate minimal medium agar was prepared according to reference (4). For comparing growth on MacConkey agar, an overnight culture of each strain was normalized to an OD600 of 0.5 and serial diluted 10-fold in phosphate-buffered saline to 10−7. About 2.5 µL of each dilution was plated on both LB and MacConkey agar plates and incubated ~16 h at 35°C and imaged with an iPhone. For the Voges-Proskauer (VP) Assay, each strain was inoculated in 3 mL of Methyl Red-Voges-Proskauer (MR-VP) broth medium (4) from a plate and incubated overnight. Then, 130 µL of 5% (wt/vol) α-naphthol and 43 µL of 1 M potassium hydroxide were added to 1 mL aliquots of the overnight cultures and allowed color to develop over 48 h at room temperature.
Infant mouse competition assay
Infant mice were infected as described previously (60, 61). Briefly, 3- to 5-day-old CD-1 neonate mice (Charles River, Wilmington, MA, USA) were orogastrically inoculated with approximately 106 total colony-forming unit (CFU) 2 h after separation from dam mice. ΔVSP-1/2 was co-inoculated 1:1 with a ΔlacZ C6706str2 strain to allow for differentiation by blue-white screening upon recovery. Mice were maintained at 30°C until euthanasia 20-h post-inoculation. To enumerate V. cholerae CFU, intestinal segments (small intestine and the large intestine plus cecum) were homogenized, serially diluted, and plated on LB + 0.1 mg/mL streptomycin, 0.08 mg/mL 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal). Blue and white colony counts were used to determine the competitive index (C.I.) for ΔVSP-1/2 using the following equation: C.I. = (CFU ΔVSP-1/2intestine/CFU ΔlacZ intestine)/(CFU ΔVSP-1/2inoculum/CFU ΔlacZ inoculum). The fitness of the ΔlacZ strain was previously shown to have no colonization defect relative to the parental C6706str2 (60). All animal experiments in this study were approved by the Institutional Animal Care and Use Committee at Michigan State University.
Polymyxin B IC50
For the polymyxin B resistance assay, 2 mL LB cultures were incubated for 16 h at 35°C with aeration. Cultures were then diluted 1:100 in fresh LB, aliquoted into 96-well plates (COSTAR), and challenged with a fourfold serial dilution of polymyxin B (Sigma) from 55.5 to 0.014 μg/mL. Plates were incubated for 20 h without aeration at 35°C and the culture absorbance was measured at 600 nm using an Envision 2105 Multimode Plate Reader (PerkinElmer) and percent growth for each biological replicate was calculated by dividing the absorbance 600 nm of polymyxin B treated wells by the absorbance of the untreated control well. The reported mean percent growth and standard deviation were calculated from three biological replicates for each polymyxin B concentration. IC50 was calculated using a non-linear regression analysis performed using GraphPad Prism version 9.5.0.
Sulfamethoxazole IC50 and challenges
Overnight cultures were grown in the presence of streptomycin unless pLAFR-derived cosmids were being maintained, in which case tetracycline was supplemented in place of streptomycin. Sulfamethoxazole (10 mg/mL) stocks were prepared in DMSO, diluted in LB to concentrations 15× greater than the final desired concentration, and multichannel pipetted into clear polystyrene 96-well plates (COSTAR) in 10 µL aliquots. Overnight cultures were diluted 1:10,000 in fresh media supplemented with the same antibiotic selection as the overnight media. One hundred and forty microliters of the diluted cultures were multichannel pipetted into 96-well plates preloaded with sulfamethoxazole. Plates were wrapped in parafilm and incubated at 35°C with aeration for 24 h. Culture absorbance was measured at 600 nm using an Envision 2105 Multimode Plate Reader (PerkinElmer). The percent growth for each biological replicate was calculated by dividing the mean absorbance of technical replicate treatment wells (N = 2–4) by the mean absorbance of technical replicate untreated control wells (N = 2–4). The reported mean percent growth and standard error mean were calculated from three biological replicates for each concentration. IC50 was calculated using a non-linear regression analysis performed using GraphPad Prism version 9.5.0.
Spontaneous SMX-resistant isolates of V. cholerae C6706
V. cholerae C6706 was streaked on an LB plate from a freezer stock and incubated at 30°C overnight. One colony was inoculated into 3 mL of LB broth and cultured at 37°C until OD600 ~0.3. About 500 µL of this culture was transferred into a 2-mL screw cap tube, mixed with 300 µL 80% glycerol and stored at −80°C. And 100 µL of this culture was spread plated onto an LB plate, followed by application of three sterile filter paper disks onto the plate and finally 10 µL 40 mg/mL SMX was pipetted onto each disk. The plate was incubated at 30°C for 2 days before small colonies appeared in the zones of inhibition. Five such colonies were selected to inoculate into LB broth, cultured for 5 h at 37°C and saved as glycerol stocks before further testing. For SMX resistance testing, single colonies from all five potential SMXR mutant isolates, as well as the parent strain, were inoculated into a 96-well plate containing 150 µL LB with and without 50 µg/mL SMX. Three colonies were used for each isolate and the plate was incubated in a plate reader at 30°C for 20 h with constant shaking and OD600 was monitored every 0.5 h. For DNA extraction, one colony from each strain was cultured in 3 mL LB broth at 30°C overnight, and 1.5 mL culture was used for DNA extraction using Wizard Genomic DNA Purification kit (Promega). The resulting DNA samples from the five SMXR isolates, as well as the parent V. cholerae C6706 strain, were sequenced at the SeqCenter (Pittsburgh, PA, USA) on an Illumina NovaSeq 6000 sequencer, producing 2 × 151 bp paired-end reads. Variant calling was performed with BreSeq v.0.38.1 using default parameters against a reference genome (NCBI accession number: GCA_015482825.1). The genome sequences of the 5 SMXR isolates were compared against the genome sequence of the parental strain to identify the mutations underlying spontaneous SMX resistance.
Growth curves and cGAMP quantification by UPLC-MS/MS following sulfamethoxazole challenge
Overnight cultures were started in triplicate from freezer stocks and grown in LB supplemented with streptomycin. Cultures were inoculated 1:1,000 in 50 mL fresh media containing selection in 125 mL flasks and grown for 15 min before being divided equally into two sister cultures. Sister cultures were grown for one additional hour before being sampled to assess culture growth by measuring absorbances at 600 nm and CFU enumeration by serial dilution plating. Immediately following the initial sampling, sister cultures were challenged with either 100 µg/mL SMX or a DMSO vehicle control and further sampling continued at ~30-min intervals for the duration of the experiment. We elected to challenge these strains with 100 µg/mL SMX as this was sufficient to induce CBASS-dependent SMX sensitivity in C6706 and below the IC50 of ΔVSP-1 (Fig. 2A; Fig. S2A; Table S1), which has an analogous SMX resistance phenotype to the ΔcapV strain (Fig. 2D; Fig. S2F).
For the purposes of measuring intracellular cGAMP, additional aliquots of ΔcapV sister cultures (±100 µg/mL sulfamethoxazole) were removed at all time points during this experiment and similarly analyzed as previously described (10). About 1-mL culture aliquots were pelleted at 15k × g in microcentrifuge tubes for 1 min, supernatants were removed by aspiration, and pelleted cells were suspended in 200 µL of ice-cold extraction buffer [acetonitrile, methanol, HPLC-grade water, formic acid (2:2:1:0.02, vol/vol/vol/vol)], and stored at −20°C overnight. Extracts were centrifugation at 15k × g for 1 min, to remove cellular debris, and the resulting clarified extracts were transferred to a new microcentrifuge tube and dried in a SpeedVac. Desiccated extracts were dissolved in 100 µL of HPLC-grade water and loaded into glass sample vials for UPLC-MS/MS analysis using an Acquity Ultra Performance LC system (Waters) coupled with a Quattro Premier XE mass spectrometer (Waters). Chromatography and multiple reaction monitoring parameters were performed as previously described (10). A cGAMP standard curve was generated using a twofold serial dilution of cGAMP (Axxora) in HPLC-grade water spanning 1.9–125 nM. Intracellular concentrations of cGAMP were calculated by dividing the total moles of cGAMP in a sample by the product of the enumerated CFU in each sample and a standard cell volume of 6.46 × 10−16 L (10, 62).
Growth curves of sulfamethoxazole-treated quorum sensing and CBASS mutants
Overnight cultures were diluted to an OD600 of 0.01 in 6 mL fresh LB medium, recovered for 1 h at 37°C with aeration, and split into paired test tubes. Within a pair of cultures, one was challenged with 100 µg/mL sulfamethoxazole, dissolved in DMSO, while the second culture was challenged with DMSO vehicle control. Cultures were incubated at 37°C with aeration and the culture OD600 was measured at the times presented for the duration of each experiment. The mean and standard deviation of biological triplicate samples for all strains and treatments are reported.
Time course gene expression using RT-qPCR
Biological duplicate overnight cultures were started from freezer stock in LB and back diluted 1:10,000 into 250 mL LB in 1 L flasks and grown at 35°C with aeration. Cultures were sampled 2, 2.5, 3, 4, and 5 h post-inoculation in 50, 30, 1, 0.5, and 0.5 mL aliquots, respectively, and the cells were pelleted by centrifugation. Cell pellets were suspended in 1 mL TRIzol Reagent (Thermo Fischer) and RNA was purified following the manufacturer’s specifications. Following the manufacturer recommendations, 5 µg of RNA was treated with TURBO Dnase (Ambion) and cDNA was generated using SuperScript III (Thermo Fischer). SYBR Green PCR Master Mix (Thermo Fischer) was used according to the manufacturer’s recommendations in 25 µL reactions containing 6.25 ng cDNA template (no reverse-transcription controls used 6.25 ng DNAse-treated RNA template) and a final primer concentration of 100 nM. qRT-PCRs reactions were performed in technical duplicate using a StepOnePlus real-time PCR system (Thermo Fisher Scientific). Gene expression was calculated using ΔCT relative to the gyrA housekeeping gene and comparative ΔΔCT was determined by comparison of each time point to the ΔCT of the initial 2 h sample (~0.05 OD600).
Gene expression in response to exogenous autoinducers by RT-qPCR
Colonies of ΔcsqAΔluxS V. cholerae were inoculated into LB and incubated with aeration at 30°C for 16 h. Each culture was then diluted 1:500—once into fresh LB supplemented autoinducers (5 µM CAI-1 and 5 µM AI-2) and once into fresh LB without autoinducers. These cultures were incubated with aeration at 30°C for 2 h, at which point the OD600 measurements for all cultures were confirmed to be similar (OD600 = 0.4–0.45). The cells were lysed in TRIzol Reagent (Invitrogen), and then RNA was extracted using the Direct-zol RNA Microprep kit (Zymo Research). RNA was then treated with 0.5 µL TURBO DNase (Invitrogen) at 37°C for 90 min, then an additional 0.5 µL TURBO DNAse was added and the samples were incubated for an additional 90 min at 37°C. cDNA was synthesized from total RNA using the SuperScript III Reverse Transcriptase kit (Invitrogen). For each sample, RT-qPCR was performed on 50 ng of cDNA using the SYBR Select Master Mix kit (Applied Biosystems) and 250 nM of each primer. Expression levels were calculated for each target gene by normalizing to the housekeeping gene recA, then the relative fold expression was calculated by comparing the target gene expression in the presence of autoinducers to the absence of autoinducers and reported as the mean of measurements obtained from three biological replicates.
Luminescent reporter assays
To assess QS induction of CBASS transcription, overnight cultures of ΔvpsL, ΔvpsLΔhapR, and ΔvpsLΔluxO (35) containing pPCBASS::lux (pKAD1) inoculated from individual colonies (n = 3) grown in LB in glass test tubes were back diluted 1:1,000 in 3 mLs of LB with chloramphenicol and grown with shaking at 35°C. At 2 h, to measure low cell density, and 20 h, to measure high cell density, the bioluminescence and OD600 of 200 mL of each culture were transferred to a solid black 96-well plate and quantified on an Envision 2105 Multimode Plate Reader (PerkinElmer). Relative light units were determined by dividing the bioluminescence by the OD600, and each was normalized to the mean relative light units of the locked high cell density ΔvpsLΔluxO mutant at the corresponding time point.
Measurement of intracellular cGAMP in response to SMX at LCD and HCD
Four independent 2 mL LB cultures with 50 µg/mL streptomycin were inoculated from isolated colonies and grown overnight at 35°C with shaking overnight. The next day, 25 mL LB with 50 µg/mL streptomycin in flasks was inoculated 1:1,000 from the overnight cultures and grown at 35°C with shaking for 1 h before the addition of 100 µg/mL SMX. Cultures were then grown until the next day (approximately 24 h total) at 35°C with shaking. An aliquot (1.8 mL) of each stationary phase culture was acquired and the cells were harvested, extracted, and analyzed by UPLC-MS/MS as described above.
pHapR and pVSP-1 in E. coli
Three independent 2 mL overnight cultures of E. coli DH10B with pPCBASS::lux (pKAD1) with pVector2 (pEVS141) (63) or pHapR (pSLS13) (64) grown in LB with chloramphenicol (5 µg/mL) and kanamycin (50 µg/mL) were back diluted 1:100 in the same media. About 150 µL of each culture was placed in a solid black 96-well plate and incubated without shaking at 35°C. After 1.5 h, HapR production was induced with 6.25 µg/mL IPTG, and bioluminescence and OD600 were measured on an Envision 2105 Multimode Plate Reader (PerkinElmer) hourly.
Phage challenge assay
Overnight cultures of E. coli DH10B with pVector (pLAFR) (65) or pVSP-1 (10) combined with pVector2 (pEVS141) (63) or pHapR (pSLS13) (64) were grown in 2 mL LB with 50 µg/mL kanamycin and 5 µg/mLtetracycline overnight at 35°C with shaking. Each culture was back diluted 1:1,000 in 2 mL of LB with the same antibiotics +10 µM IPTG and grown 2–3 h with shaking at 35°C before addition of T2 phage at the indicated MOI, followed by overnight growth with shaking at 35°C. The OD600 was measured the following day (~20 h) on an Envision 2105 Multimode Plate Reader (PerkinElmer), and the OD600 of each culture was normalized to the uninfected control and reported as percent growth.
ACKNOWLEDGMENTS
This research was supported and funded by NIH grants GM139537 and AI158433 to C.M.W. and R01AI121337 to W.L.N. G.B.S. was funded by a Dr. Collett Endowed Fellowship for Discovery Fund, Department of Microbiology & Immunology, University of Michigan, and NIH training grant T32 AI007528.
Contributor Information
Christopher M. Waters, Email: watersc3@msu.edu.
Michael T. Laub, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.00875-23.
Fig. S1-S4.
Tables S1-S5.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Clemens JD, Nair GB, Ahmed T, Qadri F, Holmgren J. 2017. Cholera. Lancet 390:1539–1549. doi: 10.1016/S0140-6736(17)30559-7 [DOI] [PubMed] [Google Scholar]
- 2. Siddique AK, Cash R. 2014. Cholera outbreaks in the classical biotype era. Curr Top Microbiol Immunol 379:1–16. doi: 10.1007/82_2013_361 [DOI] [PubMed] [Google Scholar]
- 3. Hu D, Liu B, Feng L, Ding P, Guo X, Wang M, Cao B, Reeves PR, Wang L. 2016. Origins of the current seventh cholera pandemic. Proc Natl Acad Sci U S A 113:E7730–E7739. doi: 10.1073/pnas.1608732113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Son MS, Taylor RK. 2011. Genetic screens and biochemical assays to characterize Vibrio cholerae O1. Curr Protoc Microbiol. doi: 10.1002/9780471729259.mc06a02s22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Northrup RS, Doyle MA, Feeley JC. 1972. In vitro susceptibility of El Tor and classical Vibrio cholerae strains to trimethoprim and sulfamethoxazole. Antimicrob Agents Chemother 1:310–314. doi: 10.1128/AAC.1.4.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dziejman M, Balon E, Boyd D, Fraser CM, Heidelberg JF, Mekalanos JJ. 2002. Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc Natl Acad Sci U S A 99:1556–1561. doi: 10.1073/pnas.042667999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Shea YA, Finnan S, Reen FJ, Morrissey JP, O’Gara F, Boyd EF. 2004. The Vibrio seventh pandemic Island-II is a 26·9 KB genomic Island present in Vibrio cholerae El Tor and O139 serogroup isolates that shows homology to a 43·4 KB genomic Island in V. Vulnificus. Microbiology (Reading) 150:4053–4063. doi: 10.1099/mic.0.27172-0 [DOI] [PubMed] [Google Scholar]
- 8. Hsueh BY, Severin GB, Elg CA, Waldron EJ, Kant A, Wessel AJ, Dover JA, Rhoades CR, Ridenhour BJ, Parent KN, Neiditch MB, Ravi J, Top EM, Waters CM. 2022. Phage defence by deaminase-mediated depletion of deoxynucleotides in bacteria. Nat Microbiol 7:1210–1220. doi: 10.1038/s41564-022-01162-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tal N, Millman A, Stokar-Avihail A, Fedorenko T, Leavitt A, Melamed S, Yirmiya E, Avraham C, Brandis A, Mehlman T, Amitai G, Sorek R. 2022. Bacteria deplete deoxynucleotides to defend against bacteriophage infection. Nat Microbiol 7:1200–1209. doi: 10.1038/s41564-022-01158-0 [DOI] [PubMed] [Google Scholar]
- 10. Severin GB, Ramliden MS, Hawver LA, Wang K, Pell ME, Kieninger A-K, Khataokar A, O’Hara BJ, Behrmann LV, Neiditch MB, Benning C, Waters CM, Ng W-L. 2018. Direct activation of a phospholipase by cyclic GMP-AMP in El Tor Vibrio Cholerae. Proc Natl Acad Sci U S A 115:E6048–E6055. doi: 10.1073/pnas.1801233115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen D, Melamed S, Millman A, Shulman G, Oppenheimer-Shaanan Y, Kacen A, Doron S, Amitai G, Sorek R. 2019. Cyclic GMP-AMP signalling protects bacteria against viral infection. Nature 574:691–695. doi: 10.1038/s41586-019-1605-5 [DOI] [PubMed] [Google Scholar]
- 12. Ledvina HE, Ye Q, Gu Y, Sullivan AE, Quan Y, Lau RK, Zhou H, Corbett KD, Whiteley AT. 2023. An E1-E2 fusion protein primes antiviral immune signalling in bacteria. Nature 616:319–325. doi: 10.1038/s41586-022-05647-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jenson JM, Li T, Du F, Ea C-K, Chen ZJ. 2023. Ubiquitin-like conjugation by bacterial cGAS enhances anti-phage defence. Nature 616:326–331. doi: 10.1038/s41586-023-05862-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jaskólska M, Adams DW, Blokesch M. 2022. Two defence systems eliminate plasmids from seventh pandemic Vibrio cholerae. Nature 604:323–329. doi: 10.1038/s41586-022-04546-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Hara BJ, Alam M, Ng W-L. 2022. The Vibrio cholerae seventh pandemic Islands act in tandem to defend against a circulating phage. PLoS Genet 18:e1010250. doi: 10.1371/journal.pgen.1010250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murphy SG, Johnson BA, Ledoux CM, Dörr T. 2021. Vibrio cholerae’s mysterious seventh pandemic Island (VSP-II) encodes novel Zur-regulated zinc starvation genes involved in chemotaxis and cell congregation. PLoS Genet 17:e1009624. doi: 10.1371/journal.pgen.1009624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whiteley AT, Eaglesham JB, de Oliveira Mann CC, Morehouse BR, Lowey B, Nieminen EA, Danilchanka O, King DS, Lee ASY, Mekalanos JJ, Kranzusch PJ. 2019. Bacterial cGAS-like enzymes synthesize diverse nucleotide signals. Nature 567:194–199. doi: 10.1038/s41586-019-0953-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davies BW, Bogard RW, Young TS, Mekalanos JJ. 2012. Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell 149:358–370. doi: 10.1016/j.cell.2012.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu D, Wang L, Shang G, Liu X, Zhu J, Lu D, Wang L, Kan B, Zhang J-R, Xiang Y. 2014. Structural biochemistry of a Vibrio cholerae dinucleotide cyclase reveals cyclase activity regulation by folates. Mol Cell 55:931–937. doi: 10.1016/j.molcel.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 20. Millman A, Melamed S, Amitai G, Sorek R. 2020. Diversity and classification of cyclic-oligonucleotide-based anti-phage signalling systems. Nat Microbiol 5:1608–1615. doi: 10.1038/s41564-020-0777-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ng W-L, Bassler BL. 2009. Bacterial quorum-sensing network architectures. Annu Rev Genet 43:197–222. doi: 10.1146/annurev-genet-102108-134304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Papenfort K, Bassler BL. 2016. Quorum sensing signal-response systems in gram-negative bacteria. Nat Rev Microbiol 14:576–588. doi: 10.1038/nrmicro.2016.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Papenfort K, Förstner KU, Cong J-P, Sharma CM, Bassler BL. 2015. Differential RNA-Seq of Vibrio cholerae identifies the VqmR small RNA as a regulator of biofilm formation. Proc Natl Acad Sci U S A 112:E766–75. doi: 10.1073/pnas.1500203112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herrington DA, Hall RH, Losonsky G, Mekalanos JJ, Taylor RK, Levine MM. 1988. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med 168:1487–1492. doi: 10.1084/jem.168.4.1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thelin KH, Taylor RK. 1996. Toxin-coregulated pilus, but not mannose-sensitive Hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect Immun 64:2853–2856. doi: 10.1128/iai.64.7.2853-2856.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brumfield KD, Carignan BM, Ray JN, Jumpre PE, Son MS. 2017. Laboratory techniques used to maintain and differentiate biotypes of Vibrio cholerae clinical and environmental isolates. J Vis Exp, no. 123 :55760.:55760. doi: 10.3791/55760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoon SS, Mekalanos JJ. 2006. 2,3-Butanediol synthesis and the emergence of the Vibrio cholerae El Tor biotype. Infect Immun 74:6547–6556. doi: 10.1128/IAI.00695-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hawver LA, Giulietti JM, Baleja JD, Ng W-L. 2016. Quorum sensing coordinates cooperative expression of pyruvate metabolism genes to maintain a sustainable environment for population stability. mBio 7:e01863-16. doi: 10.1128/mBio.01863-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A 99:3129–3134. doi: 10.1073/pnas.052694299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. DiRita VJ, Neely M, Taylor RK, Bruss PM. 1996. Differential expression of the ToxR regulon in classical and E1 Tor biotypes of Vibrio cholerae is due to biotype-specific control over toxT expression. Proc Natl Acad Sci U S A 93:7991–7995. doi: 10.1073/pnas.93.15.7991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaper JB, Morris JG, Levine MM. 1995. Cholera. Clin Microbiol Rev 8:48–86. doi: 10.1128/CMR.8.1.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stutzmann S, Blokesch M. 2016. Circulation of a quorum-sensing-impaired variant of Vibrio cholerae strain C6706 masks important phenotypes. mSphere 1:1–10. doi: 10.1128/mSphere.00098-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hankins JV, Madsen JA, Giles DK, Brodbelt JS, Trent MS. 2012. Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in gram-positive and gram-negative bacteria. Proc Natl Acad Sci U S A 109:8722–8727. doi: 10.1073/pnas.1201313109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matson JS, Yoo HJ, Hakansson K, Dirita VJ. 2010. Polymyxin B resistance in El Tor Vibrio cholerae requires lipid acylation catalyzed by Msbb. J Bacteriol 192:2044–2052. doi: 10.1128/JB.00023-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Waters CM, Lu W, Rabinowitz JD, Bassler BL. 2008. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic Di-GMP levels and repression of vpsT. J Bacteriol 190:2527–2536. doi: 10.1128/JB.01756-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303–314. doi: 10.1016/s0092-8674(02)00829-2 [DOI] [PubMed] [Google Scholar]
- 37. Hammer BK, Bassler BL. 2007. Regulatory small RNAs circumvent the conventional quorum sensing pathway in pandemic Vibrio cholerae. Proc Natl Acad Sci U S A 104:11145–11149. doi: 10.1073/pnas.0703860104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seed KD, Bodi KL, Kropinski AM, Ackermann H-W, Calderwood SB, Qadri F, Camilli A. 2011. Evidence of a dominant lineage of Vibrio cholerae-specific lytic bacteriophages shed by cholera patients over a 10-year period in Dhaka. mBio 2:e00334-10. doi: 10.1128/mBio.00334-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brenzinger S, Airoldi M, Ogunleye AJ, Brochado AR. 2023. The antiphage defense system CBASS controls resistance and enables killing by antifolate antibiotics in Vibrio Cholerae. Microbiology. Microbiology, Microbiology. doi: 10.1101/2023.02.27.530311 [DOI] [PubMed]
- 40. Liu C, Wang Y, Azizian K, Omidi N, Hassan Kaviar V, Kouhsari E, Maleki A. 2022. Antimicrobial resistance in Vibrio cholerae O1/O139 clinical isolates: a systematic review and meta-analysis. Expert Rev Anti Infect Ther 20:1217–1231. doi: 10.1080/14787210.2022.2098114 [DOI] [PubMed] [Google Scholar]
- 41. Wozniak RAF, Fouts DE, Spagnoletti M, Colombo MM, Ceccarelli D, Garriss G, Déry C, Burrus V, Waldor MK. 2009. Comparative ICE genomics: insights into the evolution of the SXT/R391 family of ices. PLoS Genet 5:e1000786. doi: 10.1371/journal.pgen.1000786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bhotra T, Das MM, Pal BB, Singh DV. 2016. Genomic profile of antibiotic resistant, classical ctxB positive Vibrio cholerae O1 Biotype El Tor isolated in 2003 and 2005 from Puri, India: a retrospective study. Indian J Med Microbiol 34:462–470. doi: 10.4103/0255-0857.195356 [DOI] [PubMed] [Google Scholar]
- 43. Grim CJ, Choi J, Chun J, Jeon Y-S, Taviani E, Hasan NA, Haley B, Huq A, Colwell RR. 2010. Occurrence of the Vibrio cholerae seventh pandemic VSP-I Island and a new variant. OMICS 14:1–7. doi: 10.1089/omi.2009.0087 [DOI] [PubMed] [Google Scholar]
- 44. Zahid MSH, Waise Z, Kamruzzaman M, Ghosh AN, Nair GB, Khairul Bashar SAM, Mekalanos JJ, Faruque SM. 2011. An experimental study of phage mediated bactericidal selection & emergence of the El Tor Vibrio cholerae. Indian J Med Res 133:218–224. [PMC free article] [PubMed] [Google Scholar]
- 45. Kranzusch PJ. 2019. cGAS and CD-Ntase enzymes: structure, mechanism, and evolution. Curr Opin Struct Biol 59:178–187. doi: 10.1016/j.sbi.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gao P, Ascano M, Wu Y, Barchet W, Gaffney BL, Zillinger T, Serganov AA, Liu Y, Jones RA, Hartmann G, Tuschl T, Patel DJ. 2013. Cyclic [G(2',5')pA(3',5')P] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 153:1094–1107. doi: 10.1016/j.cell.2013.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Civril F, Deimling T, de Oliveira Mann CC, Ablasser A, Moldt M, Witte G, Hornung V, Hopfner K-P. 2013. Structural mechanism of cytosolic DNA sensing by cGAS. Nature 498:332–337. doi: 10.1038/nature12305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hartmann R, Justesen J, Sarkar SN, Sen GC, Yee VC. 2003. Crystal structure of the 2’-specific and double-stranded RNA-activated interferon-induced antiviral protein 2’-5’-oligoadenylate synthetase. Mol Cell 12:1173–1185. doi: 10.1016/s1097-2765(03)00433-7 [DOI] [PubMed] [Google Scholar]
- 49. Cohen D, Melamed S, Millman A, Shulman G, Oppenheimer-Shaanan Y, Kacen A, Doron S, Amitai G, Sorek R. 2019. Cyclic GMP-AMP signalling protects bacteria against viral infection. Nature 574:691–695. doi: 10.1038/s41586-019-1605-5 [DOI] [PubMed] [Google Scholar]
- 50. Mattenberger Y, Mattson S, Métrailler J, Silva F, Belin D. 2011. 55.1, a gene of unknown function of phage T4, impacts on Escherichia coli folate metabolism and blocks DNA repair by the NER. Mol Microbiol 82:1406–1421. doi: 10.1111/j.1365-2958.2011.07897.x [DOI] [PubMed] [Google Scholar]
- 51. Green JM, Matthews RG. 2007. Folate biosynthesis, reduction, and polyglutamylation and the interconversion of folate derivatives. EcoSal Plus 2. doi: 10.1128/ecosalplus.3.6.3.6 [DOI] [PubMed] [Google Scholar]
- 52. Wang Y, Dai J, Wang X, Wang Y, Tang F. 2022. Mechanisms of interactions between bacteria and bacteriophage mediate by quorum sensing systems. Appl Microbiol Biotechnol 106:2299–2310. doi: 10.1007/s00253-022-11866-6 [DOI] [PubMed] [Google Scholar]
- 53. Høyland-Kroghsbo Nina Molin, Maerkedahl RB, Svenningsen SL. 2013. A quorum-sensing-induced bacteriophage defense mechanism. mBio 4:e00362-12. doi: 10.1128/mBio.00362-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hoque MM, Naser IB, Bari SMN, Zhu J, Mekalanos JJ, Faruque SM. 2016. Quorum regulated resistance of Vibrio cholerae against environmental bacteriophages. Sci Rep 6:37956. doi: 10.1038/srep37956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Høyland-Kroghsbo N.M, Paczkowski J, Mukherjee S, Broniewski J, Westra E, Bondy-Denomy J, Bassler BL. 2017. Quorum sensing controls the Pseudomonas aeruginosa CRISPR-Cas adaptive immune system. Proc Natl Acad Sci U S A 114:131–135. doi: 10.1073/pnas.1617415113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Duncan-Lowey B, Kranzusch PJ. 2022. CBASS Phage defense and evolution of antiviral nucleotide signaling. Curr Opin Immunol 74:156–163. doi: 10.1016/j.coi.2022.01.002 [DOI] [PubMed] [Google Scholar]
- 57. Skorupski K, Taylor RK. 1996. Positive selection vectors for allelic exchange. Gene 169:47–52. doi: 10.1016/0378-1119(95)00793-8 [DOI] [PubMed] [Google Scholar]
- 58. Lenz DH, Miller MB, Zhu J, Kulkarni RV, Bassler BL. 2005. CsrA and three redundant small RNAs regulate quorum sensing in Vibrio cholerae. Mol Microbiol 58:1186–1202. doi: 10.1111/j.1365-2958.2005.04902.x [DOI] [PubMed] [Google Scholar]
- 59. Gerhardt P, Murray R. E, Wood WA, Krieg NR.. 1994. Methods for general and molecular bacteriology, 1st ed. American Society for Microbiology, Washington, D. C. [Google Scholar]
- 60. Van Alst AJ, DiRita VJ. 2020. Aerobic metabolism in Vibrio cholerae is required for population expansion during infection. mBio 11:e01989-20. doi: 10.1128/mBio.01989-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Anthouard R, DiRita VJ. 2013. Small-molecule inhibitors of toxT expression in Vibrio cholerae. mBio 4:MBio doi: 10.1128/mBio.00403-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Massie JP, Reynolds EL, Koestler BJ, Cong J-P, Agostoni M, Waters CM. 2012. Quantification of high-specificity cyclic diguanylate signaling. Proc Natl Acad Sci U S A 109:12746–12751. doi: 10.1073/pnas.1115663109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dunn AK, Millikan DS, Adin DM, Bose JL, Stabb EV. 2006. New rfp- and pES213-derived tools for analyzing symbiotic Vibrio fischeri reveal patterns of infection and lux expression in situ. Appl Environ Microbiol 72:802–810. doi: 10.1128/AEM.72.1.802-810.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Svenningsen SL, Waters CM, Bassler BL. 2008. A negative feedback loop involving small RNAs accelerates Vibrio cholerae’s transition out of quorum-sensing mode. Genes Dev 22:226–238. doi: 10.1101/gad.1629908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Friedman AM, Long SR, Brown SE, Buikema WJ, Ausubel FM. 1982. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene 18:289–296. doi: 10.1016/0378-1119(82)90167-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1-S4.
Tables S1-S5.


