We provide evidence that the peroxisomal ATP-binding cassette (ABC) transporter COMATOSE (CTS) is involved in the biosynthesis of jasmonic acid (JA) in Arabidopsis (Arabidopsis thaliana) leaves. Basal JA levels were greatly reduced but not completely abolished in two cts mutant alleles, and JA production in response to wounding showed slower kinetics and reached a lower level than wild type. We propose the operation of two parallel pathways for peroxisomal import of jasmonate precursors, a route that requires CTS function and a parallel leak pathway involving anion trapping.
It is well established that the bioactive, lipid-derived compounds collectively known as jasmonates play important roles in plant defense, metabolism, and development (Farmer and Ryan, 1992; Creelman and Mulpuri, 2002). A biosynthetic pathway for JA was proposed in 1983 by Vick and Zimmerman (1983), in which JA is formed from the unsaturated fatty acid, linolenic acid (18:3), an abundant octadecanoid in higher plant membranes. In Arabidopsis, a parallel, hexadecanoid pathway based on the 16:3 fatty acid is also present (Weber et al., 1997). Most of the enzymes involved in the 18:3 pathway have now been identified by a combination of classic biochemical and genetic approaches (Creelman and Mulpuri, 2002; Turner et al., 2002). Following phospholipase-dependent release of 18:3 from chloroplast membrane lipids, molecular oxygen is introduced by 13-lipoxygenase activity to generate 13-hydroperoxy octadecatrienoic acid. Allene oxide synthase catalyzes the formation of an unstable intermediate, 12,13-epoxy-octadecatrienoic acid, which is converted to (9S,13S)-12-oxo-phytodienoic acid (OPDA) by allene oxide cyclase. The synthesis of OPDA occurs in the chloroplast, and in Arabidopsis more than 90% of OPDA is esterified to chloroplast galactolipids (Stelmach et al., 2001). In subsequent steps, OPDA is reduced to 3-oxo-2-(2′-pentenyl)-cyclopentane-1-octanoic acid (OPC:8), which is then shortened by three rounds of peroxisomal β-oxidation to yield JA. The involvement of specific isoforms of acyl-CoA oxidase (ACX1) and thiolase (KAT2) in the β-oxidation steps of wound-induced leaf JA biosynthesis has recently been demonstrated (Cruz Castillo et al., 2004). Thus, JA biosynthesis is initiated in the chloroplast and terminates in the peroxisome. Although Arabidopsis has at least three isoforms of 12-oxo-phytodienoate reductase, only OPR3 reduces the 9S,13S-stereoisomer of OPDA, the biological precursor of JA (Schaller et al., 2000). Since OPR3 is located in the peroxisome (Strassner et al., 2002), it is clear that OPDA or other intermediates in JA synthesis must be transported from the chloroplast to the peroxisome, but the nature of these transport steps is unknown. It has generally been assumed that OPDA is freely membrane permeable because of its structural similarity to fatty acids, but since OPDA is an important signaling molecule in its own right (Blechert et al., 1999; Stintzi et al., 2001), it is plausible that control of its movement between subcellular compartments would be highly regulated (Stenzel et al., 2003). This could implicate membrane-bound transporters in the chloroplast and/or peroxisome.
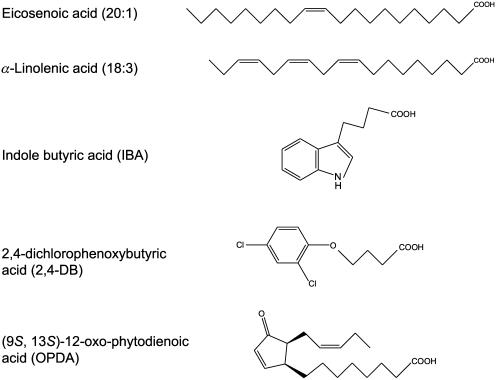
A role in regulating fatty acid or fatty acyl-CoA import into the peroxisome has recently been demonstrated for the CTS ABC transporter (Footitt et al., 2002), also known as PXA1 and PED3 (Zolman et al., 2001; Hayashi et al., 2002). cts mutant seeds retain oil bodies, accumulate very long-chain fatty acyl-CoAs, and do not germinate in the absence of classical seed dormancy-breaking treatments (Footitt et al., 2002). Moreover, cts mutant seedlings cannot establish in the absence of Suc. Interestingly, alleles of cts were identified independently in screens for resistance to indole butyric acid (IBA) and the synthetic auxin precursor 2,4-dichlorophenoxy butyric acid (2,4-DB; Zolman et al., 2001; Hayashi et al., 2002). In wild-type Arabidopsis, these compounds are converted to bioactive auxins by β-oxidation. Mutation of CTS blocks β-oxidation of IBA and 2,4-DB, suggesting that CTS is also able to transport (or regulate the transport of) these compounds. This implies that the substrate specificity of CTS is quite broad. Many, but not all, ABC transporters are able to accept a variety of structurally quite diverse compounds (Theodoulou, 2000). Common features of the potential CTS substrates are an acyl chain of at least four carbons, terminating in a carboxyl group that is potentially esterified to CoA (Fig. 1). Examination of the structure of IBA and 2,4-DB suggests that a ring structure also is accommodated by the transporter. Given the structural similarities of these molecules to OPDA, which is derived from linolenic acid (Fig. 1), we hypothesized that CTS might also mediate the transport of this molecule and thus participate in JA biosynthesis.
Figure 1.
Chemical structures of potential CTS substrates. Free acids are shown, but their CoA esters are also possible substrates.
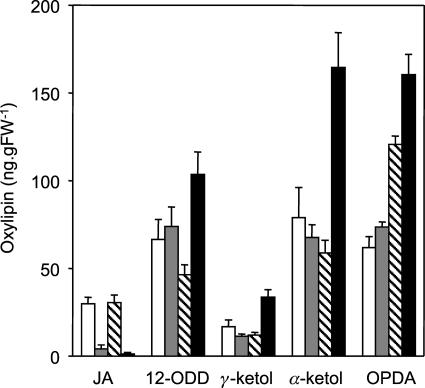
Levels of JA and oxylipin intermediates were measured in two cts mutant alleles and their respective wild types by liquid chromatography-mass spectrometry (Fig. 2). The basal level of JA was around 30 ng g−1 fresh weight (FW) in wild-type plants. cts mutant alleles contained considerably less JA than wild type: 4.084 ± 2.346 and 1.236 ± 0.885 ng g−1 FW in cts1 and cts2, respectively. OPDA levels were similar in cts1 and Landsberg erecta (Ler), which might reflect feedback inhibition of the pathway, as is the case for OPR3 (Mussig et al., 2000). In agreement with this, unwounded opr3 leaves were found to contain less free OPDA than expected by the block in OPR3 (Stenzel et al., 2003). However, OPDA was increased significantly in cts2 relative to the wild type, Wassilewskija (Ws). Since both cts1 and cts2 are effectively null alleles, this suggests possible ecotype-specific differences in the regulation of the JA pathway that are not related to CTS. Interestingly, in cts2, the levels of 13-hydroxy-12-oxo-octadecadienoic acid (α-ketol) and 9-hydroxy-12-oxo-octadecadienoic acid (γ-ketol) were elevated relative to the wild type. An increase in these compounds, which are formed by nonenzymic hydrolysis of unstable allene oxides (Grechkin et al., 1991), is indicative of an increased ability to oxidize 18:3 (Weber et al., 1997). In a previous report, α-ketol formation increased in response to dnOPDA treatment of Arabidopsis (Weber et al., 1997), suggesting that the increase in ketols in cts2 might reflect the elevated levels of OPDA in this mutant.
Figure 2.
cts mutants are JA deficient. Measurement of JA and oxylipin intermediates in cts mutants and respective wild types is shown. White bars, Wild-type Ler; gray bars, cts1; hatched bars, wild-type Ws; black bars, cts2. Values are means ± se from eight independent samples, each of which was analyzed in duplicate. Surface-sterilized seeds of the mutants cts1 and cts2 were plated on Gamborg's B5 salts, 0.7% (w/v) agarose, pH 5.8, for 4 d at 22°C in continuous light (150 μmol m−2 s−1) to confirm the phenotype. They were then transferred to plates, as above, supplemented with 0.5% (w/v) Suc and nicked to allow germination. Both mutants and wild-type seeds were stratified on the same media in the dark for 2 d at 4°C followed by germination for 7 d at 22°C. Seedlings were then transferred to soil in cellular trays and grown for 13 d under a long-day cycle (22°C, 16 h light/18°C, 8 h dark) at 150 to 170 μmol m−2 s−1, 60% relative humidity. For each mutant and wild type, there were 8 trays of 20 seedlings; from each tray, two replicate samples of rosette leaf were harvested, weighed, and immediately frozen in liquid nitrogen. Oxylipins were quantified from 200 mg of tissue that was ground to powder and extracted overnight in 70:30 (v/v) acetone:50 mm citric acid with 20 ng prostaglandin A1 (Sigma, St. Louis) added as an internal standard. Oxylipins were extracted from the aqueous phase by partitioning 3 times with diethyl ether, evaporating to dryness, and resuspending in 60% methanol prior to HPLC tandem mass spectrometry analysis. Separation was achieved using a LUNA C18(2) 150 × 2-mm column (Phenomenex, Torrance, CA) and a methanol-water-0.2% formic acid gradient. Eluted compounds were fragmented by targeted tandem mass spectrometry using an LCQ mass spectrometer (Thermo Separation Products, Riviera Beach, FL), and the daughter ions identified and quantified using response factors calibrated between prostaglandin A1 and authentic oxylipin standards (Larodan, Malmo, Sweden).
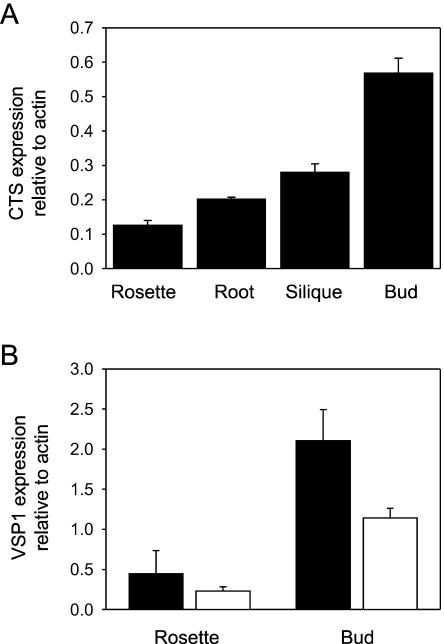
Analysis of the Arabidopsis genome indicates that CTS is a single gene (Sánchez-Fernández et al., 2001) and is therefore likely to participate in β-oxidation throughout the plant. In accordance with this, CTS is expressed in all tissues (http://www.cbs.umn.edu/arabidopsis). Quantitative reverse transcription-PCR, shown in Figure 3A, demonstrated that floral tissues contain about 2-fold more CTS transcript than siliques and 4- to 5-fold more than roots or rosette leaves. In order to relate these findings to a potential role in JA biosynthesis, the expression of a JA-responsive gene was examined in the cts1 mutant. Arabidopsis vegetative storage protein 1 (VSP1) is expressed preferentially in flowers and buds and only at very low levels in other tissues (Utsugi et al., 1996, 1998). VSP1 expression is induced in vegetative tissue by methyl JA and coronatine in a COI1-dependent manner (Benedetti et al., 1995) and is also induced by wounding (Berger et al., 1995, Utsugi et al., 1998). Moreover, VSP1 is constitutively expressed in the cev1 mutant that has constitutively active jasmonate- and ethylene-signaling pathways (Ellis and Turner, 2001). Thus, VSP1 was selected as a well-characterized JA-responsive gene whose expression might be detected in unwounded or untreated tissue. Primers specific for VSP1 (At5g24780) were designed and the levels of transcript measured by quantitative PCR in samples grown under identical conditions to those used for the experiment shown in Figure 2. In agreement with previous reports, VSP1 was expressed at low levels in vegetative tissue but was highly expressed in floral tissue, and the level was decreased by almost one-half in flowers of the cts1 mutant (Fig. 3B). As the floral expression of VSP1 is known to be COI-dependent (Benedetti et al., 1995), this is consistent with the fact that cts mutants exhibit reduced JA.
Figure 3.
Transcript levels of CTS and VSP in tissues measured by quantitative real-time PCR. A, Expression of CTS relative to actin in wild-type Ler plants. B, Expression of VSP1 relative to actin in wild-type Ler (black bars) and cts1 mutant plants (white bars). Plants were grown under the same conditions as the samples for basal JA analysis (Fig. 2). Tissues were harvested, weighed, and frozen immediately in liquid nitrogen. RNA was extracted from 100-mg tissue using an RNeasy mini kit and treated with RNase-free DNase I (Qiagen, Westburg, The Netherlands). Total RNA (3 μg) was reverse transcribed with Superscript II reverse transcriptase (Invitrogen Life Technologies, Paisley, UK) using a mixture of oligo(dT)15 and random hexamer primers. PCR reactions were carried out in triplicate on three independent RNA samples for each tissue, using the 300-nmol primers QCTSF1 (5′-GAGATTAGGCATGGCACGTT-3′) and QCTSR1 (5′-GTCGCATTTGTGCATTCATC-3′) or VSP1right (5′-GGCACCGTGTCGAAGTTTAT-3′) and VSP1left (5′-CTCTTGGTCGCTACGGTCTC-3′). Identical reactions were carried out using the 300-nmol primers ACT2F (5′-CTCAGGTATCGCTGACCGTA-3′) and ACT2R (5′-GAGATCCACATCTGCTGGAAT-3′). PCR cycling conditions were 95°C, 3 min, followed by 40 cycles of 95°C, 15 s; 60°C, 30 s. Data were analyzed using the Icycler instrument software (Bio-Rad, Hemel Hempstead, UK), which produced cycle threshold values and relative PCR product levels calculated from the standard curve. Values obtained for CTS or VSP1 were divided by those obtained from actin in the same sample to correct for any variation in the amount of input cDNA and are presented as expression relative to actin.
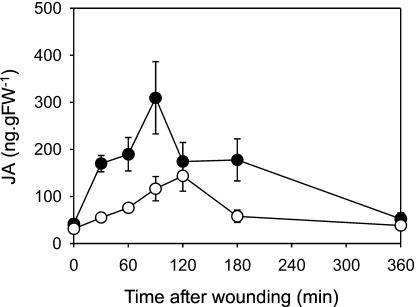
The induction of JA biosynthesis by wounding is well studied, and it is becoming evident that developmentally regulated JA biosynthesis and wound-induced JA biosynthesis occur via distinct but overlapping pathways (Turner et al., 2002). We therefore examined the role of CTS in wound-induced JA formation. In this experiment, basal JA levels were not significantly different in Ws and cts2 (41.404 ± 11.000 and 31.065 ± 8.636 ng g−1 FW, respectively). This may reflect the fact that plants were grown under short days, compared to the long-day growth conditions, in the experiment reported in Figure 2, and that the plants were of a different developmental age. However, a marked difference was seen following wounding: In wild-type leaves, wounding induced an increase in JA that peaked 90 min after treatment at approximately 310 ng g−1 FW (Fig. 4). The kinetics of this response are consistent with published reports (McConn et al., 1997; Weber et al., 1997; Stenzel et al., 2003; Cruz Castillo et al., 2004). Although JA increased in response to wounding of cts2 leaves, the response was slower, with JA peaking 2 h after wounding at a lower level of 144 ng g−1 FW.
Figure 4.
Time course of JA accumulation in cts2 mutant and wild type following wounding of leaves. Arabidopsis plants were grown and wounded as described by Weber et al. (1997). Briefly, plants were grown under short-day conditions (9 h light; 150 μmol m−2 s−1 at 23°C, 70% relative humidity). The apical halves of rosette leaves from 4-week-old plants were wounded with forceps and kept under light (150 μmol m−2 s−1) for up to 3 h. JA was quantified as described in the legend to Figure 2. White symbols, cts2; black symbols, Ws. Values are means ± se of five independent determinations.
Taken together, these data suggest a possible role for CTS in the peroxisomal import of JA precursors. The simplest interpretation of this finding is that CTS mediates ATP-dependent transport of OPDA or OPDA-CoA into the peroxisome. However, until CTS has been reconstituted for transport studies, other hypotheses cannot be ruled out; for example, CTS may regulate the activity of another transport system since dual roles as primary transporters and transport regulators have been established for a range of different ABC proteins (Higgins, 1995). It is also not clear whether free OPDA or a CoA-esterified form is transported into the peroxisome. Cytosolic addition of a large, polar CoA moiety to OPDA would render it membrane impermeable in the absence of a transporter. While Arabidopsis contains a large family of acyl-CoA synthetases (ACS) potentially capable of forming OPDA-CoA esters (Shockey et al., 2002, 2003), OPR3 accepts free OPDA as a substrate in vitro, which is consistent with the possibility that activation occurs following this reduction step in the peroxisome. It was concluded that the peroxisomal very long-chain ACSs LACS6 and LACS7 do not participate in jasmonate biosynthesis since the lacs6/lacs7 double knockout is not male sterile (Fulda et al., 2004). However, the CTS-dependent pathway is intact in this double mutant. A cts/lacs6/lacs7 triple mutant would resolve this issue. Alternatively, other members of the ACS family might catalyze the activation of OPDA or OPC:8.
It is evident from the data in Figures 2 to 4 that CTS contributes both to basal and wound-induced JA biosynthesis in leaves and that, potentially, it can fulfill this role in other tissues. However, since cts mutants contain low but measurable JA levels, CTS is not absolutely required for JA synthesis. Therefore, there must be an alternative route for uptake of OPDA into the peroxisome, either an unidentified transporter or a passive mechanism. Two physicochemical parameters largely define the membrane transport properties of weak acids such as OPDA: lipophilicity, as measured by the octanol-water partition coefficient (log Kow), and acid strength, expressed as the pKa. Although values for OPDA have not been published, Dathe et al. (1993) demonstrated that JA is a weak acid with a pKa of 4.5 and a log Kow of 1.76. Thus, depending on the proton concentration, JA exists in two forms, the lipophilic free acid and the essentially membrane-impermeable anion. In the absence of specific transport proteins, these physicochemical parameters predict that JA distributes according to the ion trap concept, leading to accumulation in alkaline compartments and depletion in acidic compartments. Peroxisomal pH measurements have not been published for plants, but use of targeted pH reporters indicated a pH of 8.2 in mammalian peroxisomes (Dansen et al., 2000) and the basic pI of plant peroxisomal lumen proteins suggests that plant peroxisomes are also likely to be basic compartments. Since cytosolic pH is in the range of 7.2 to 7.4 in plants (Kurkdjian and Guern, 1989), weak acids are predicted to be anion trapped in peroxisomes. The structure of OPDA suggests that it will have a very similar pKa to JA, but the longer acyl chain dictates that it is significantly more lipophilic, with an estimated log Kow of 4.26 for the uncharged form (addition of 0.5 log Kow unit for each extra CH2 group). Thus, OPDA is likely to be ion trapped in peroxisomes but with relatively low efficiency, since the anionic form is sufficiently lipophilic to cross membranes, albeit to a lesser extent than the free acid. Subsequent reduction to OPC:8 would increase the chemical gradient and help to drive transport into the peroxisome. This passive mechanism could constitute a leak pathway for OPDA import, permitting JA biosynthesis in the absence of CTS (Fig. 5). This would explain the low basal level of JA in cts mutant alleles. The relative contribution of the two import pathways would be predicted to differ at different developmental stages and in response to different stimuli, depending not only on the level of CTS transport activity but also on the prevailing pH gradient between the cytosol and the peroxisome lumen. The response to wounding in cts2, in which basal JA is unchanged but where induction of JA synthesis is delayed and of reduced magnitude, is thus also consistent with anion trapping of a jasmonate precursor.
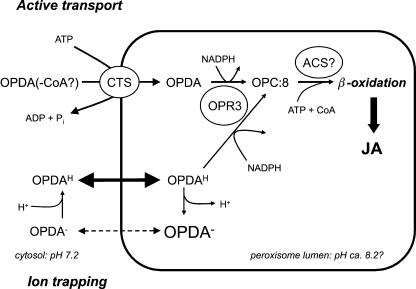
Figure 5.
Model showing parallel pathways for import of jasmonate precursors into peroxisomes. In the CTS-dependent pathway, OPDA or its CoA ester is transported into the peroxisome in an ATP-dependent manner (active transport). It is possible that OPDA-CoA might be cleaved by peroxisomal thioesterase prior to reduction (Tilton et al., 2004; data not shown). Alternatively, OPDA-free acid (OPDAH) is membrane permeable and can enter the peroxisome without the need for a transport protein. The peroxisome lumen is predicted to be more basic than the cytosol; therefore, a higher proportion of OPDA exists in the dissociated form (OPDA−), which has much lower membrane permeability, resulting in ion trapping. In this pathway, the partitioning of OPDA between cytosol and peroxisome depends on the pH gradient between the two compartments. Inside the peroxisome, OPDA is reduced to OPC:8 by the action of OPR3, using NADPH as a cofactor. By analogy with fatty acids, OPC:8 would require CoA esterification prior to β-oxidation. The ACSs responsible for this hypothetical reaction are currently unknown.
What are the other physiological consequences of compromised JA synthesis in cts mutants? Typically, Arabidopsis mutants defective in JA biosynthesis or perception are male sterile (Feys et al., 1994; McConn and Browse, 1996; Sanders et al., 2000; Stintzi and Browse, 2000; Ishiguro et al., 2001). cts mutants, notably, are not male sterile. We propose that sufficient JA for male fertility is produced via the leak pathway in cts mutants. In agreement with this, McConn and Browse (1996) demonstrated that the threshold level of the JA precursor 18:3 for male fertility was less than 5% of total fatty acids (compared with 44% in wild type), strongly suggesting that JA can be reduced without resulting in male sterility. Previous work has shown that CTS is required for germination and seedling establishment (Footitt et al., 2002). The consequence of the involvement of CTS in JA biosynthesis as well as the established role in fatty acid transport suggest a metabolic and signaling role for CTS. The requirement for CTS in germination and seedling establishment can be interpreted in this light. The requirement for classic dormancy-breaking treatments to induce germination in cts suggests an involvement for CTS in endogenous signaling at the initiation of germination, whereas the requirement for CTS in seedling establishment represents the expression of the metabolic function of CTS. Involvement of CTS in the biosynthesis of JA suggests that CTS may have more subtle or as yet undefined functions during plant growth and begs the question of other substrates of CTS.
Acknowledgments
F.L.T wishes to thank Dr. Richard Bromilow for constructive discussions about physicochemical parameters.
This work was supported by a grant-in-aid from the Biotechnology and Biological Sciences Research Council, UK, to Rothamsted Research and by grant number 24P19770 to A.B.
References
- Benedetti CE, Xie D, Turner JG (1995) COI-1 dependent expression of an Arabidopsis vegetative storage protein in flowers and siliques and in response to coronatine or methyl jasmonate. Plant Physiol 109: 567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S, Bell E, Sadka A, Mullet JE (1995) Arabidopsis thaliana Atvsp is homologous to soybean VspA and VspB genes encoding vegetative storage protein acid phosphatases and is regulated similarly by methyl jasmonate, wounding, sugars, light and phosphate. Plant Mol Biol 27: 933–942 [DOI] [PubMed] [Google Scholar]
- Blechert S, Bockelmann C, Füßlein M, von Schrader T, Stelmach BA, Niesel U, Weiler EW (1999) Structure-activity analyses reveal the existence of two separate groups of active octadecanoids in elicitation of the tendril-coiling response of Bryonia dioica Jacq. Planta 207: 470–479 [Google Scholar]
- Creelman RA, Mulpuri R (2002) The oxylipin pathway in Arabidopsis. In CR Somerville, EM Meyerozitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, pp 1–24, doi/10.1199/tab.0012, http://www.aspb.org/publications/arabidopsis/ [DOI] [PMC free article] [PubMed]
- Cruz Castillo M, Martínez C, Buchala A, Métraux J-P, León J (2004) Gene-specific involvement of β-oxidation in wound-activated responses in Arabidopsis. Plant Physiol 135: 85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansen TB, Wirtz KWA, Wanders RJA, Pap EHW (2000) Peroxisomes in human fibroblasts have a basic pH. Nat Cell Biol 2: 51–53 [DOI] [PubMed] [Google Scholar]
- Dathe W, Kramell H-M, Daeter W, Kramell R, Slovik S, Hartung W (1993) Uptake of jasmonic acid and related compounds by mesophyll protoplasts of the barley leaf. J Plant Growth Regul 12: 133–140 [Google Scholar]
- Ellis C, Turner JG (2001) The Arabidopsis cev1 mutant has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell 13: 1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA (1992) Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase-inhibitors. Plant Cell 4: 129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJF, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6: 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt S, Slocombe SP, Larner V, Kurup S, Wu Y, Larson T, Graham I, Baker A, Holdsworth M (2002) Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J 21: 2912–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda M, Schnurr J, Abbadi A, Heinz E, Browse J (2004) Peroxisomal acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana. Plant Cell 16: 394–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grechkin AN, Kuramshin RA, Safonova EY, Latypov SK, Ilyasov AV (1991) Formation of ketols from linolenic acid 13-hydroperoxide via allene oxide. Evidence for two distinct mechanisms of allene oxide hydrolysis. Biochim Biophys Acta 1086: 317–325 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Nito K, Takei-Hoshi R, Yagi M, Kondo M, Suenaga A, Yamaya T, Nishimura M (2002) Ped3p is a peroxisomal ATP-binding cassette transporter that might supply substrates for fatty acid β-oxidation. Plant Cell Physiol 43: 1–11 [DOI] [PubMed] [Google Scholar]
- Higgins CF (1995) The ABC of channel regulation. Cell 82: 693–696 [DOI] [PubMed] [Google Scholar]
- Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K (2001) The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronized pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13: 2191–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkdjian A, Guern J (1989) Intracellular pH: measurement and importance in cell activity. Annu Rev Plant Physiol Plant Mol Biol 40: 271–303 [Google Scholar]
- McConn M, Browse J (1996) The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell 8: 403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M, Creelman RA, Bell E, Mullet JE, Browse J (1997) Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA 94: 5473–5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussig C, Biesgen C, Lisso J, Uwer U, Weiler EW, Altmann T (2000) A novel stress-inducible 12-oxophytodienoate reductase from Arabidopsis thaliana provides a potential link between Brassinosteroid action and jasmonic-acid synthesis. J Plant Physiol 157: 143–152 [Google Scholar]
- Sánchez-Fernández R, Davies TG, Coleman JO, Rea PA (2001) The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J Biol Chem 276: 30231–30244 [DOI] [PubMed] [Google Scholar]
- Sanders PS, Lee PY, Biesgen C, Boone JD, Beals TP, Weiler E, Goldberg RB (2000) The Arabidopsis DELAYED DEHISCENCE1 gene encodes and enzyme in the jasmonic acid synthesis pathway. Plant Cell 12: 1041–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller F, Biesgen C, Mussig C, Altmann T, Weiler E (2000) 12-Oxophytodienoate reductase 3 (OPR3) is the isoenzyme involved in jasmonate biosynthesis. Planta 210: 979–984 [DOI] [PubMed] [Google Scholar]
- Shockey JM, Fulda MS, Browse JA (2002) Arabidopsis contains nine long-chain acyl-coenzyme A synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol 129: 1710–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockey JM, Fulda MS, Browse J (2003) Arabidopsis contains a large superfamily of acyl-activating enzymes. Phylogenetic and biochemical analysis reveals a new class of acyl-coenzyme A synthetases. Plant Physiol 132: 1065–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmach BA, Müller A, Henning P, Gebhardt S, Schubert-Zsilavecz M, Weiler EW (2001) A novel class of oxylipins, sn1-O-(12-oxophytodienoyl)-sn2-O-(hexadecatrienoyl)-monogalactosyl diglyceride, from Arabidopsis thaliana. J Biol Chem 276: 12832–12838 [DOI] [PubMed] [Google Scholar]
- Stenzel I, Hause B, Miersch O, Kurz T, Maucher H, Weichert H, Ziegler J, Feussner I, Wasternack C (2003) Jasmonate biosynthesis and the allene oxide cyclase family of Arabidopsis thaliana. Plant Mol Biol 51: 895–911 [DOI] [PubMed] [Google Scholar]
- Stintzi A, Browse J (2000) The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc Natl Acad Sci USA 97: 10625–10630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A, Weber H, Reymond P, Browse J, Farmer EE (2001) Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc Natl Acad Sci USA 98: 12837–12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassner J, Schaller F, Frick UB, Howe GA, Weiler EW, Amrhein N, Macheroux P, Schaller A (2002) Characterization and cDNA-microarray expression analysis of 12-oxophytodienoate reductases reveals differential roles for octadecanoid biosynthesis in the local versus the systemic wound response. Plant J 32: 585–601 [DOI] [PubMed] [Google Scholar]
- Theodoulou FL (2000) Plant ABC transporters. Biochim Biophys Acta 1465: 79–103 [DOI] [PubMed] [Google Scholar]
- Tilton GB, Shockey JM, Browse J (2004) Biochemical and molecular characterization of ACH2, an acyl-CoA thioesterase from Arabidopsis thaliana. J Biol Chem 279: 7487–7494 [DOI] [PubMed] [Google Scholar]
- Turner JG, Ellis C, Devoto A (2002) The jasmonate signal pathway. Plant Cell (Suppl) 14: S153–S164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsugi S, Sakamoto W, Murata M, Motoyashi F (1998) Arabidopsis thaliana vegetative storage protein (VSP) genes: gene organisation and tissue specific expression. Plant Mol Biol 38: 565–576 [DOI] [PubMed] [Google Scholar]
- Utsugi S, Sakamoto W, Ogura Y, Murata M, Motoyoshi F (1996) Isolation and characterisation of cDNA clones corresponding to the genes expressed preferentially in floral organs of Arabidopsis thaliana. Plant Mol Biol 32: 759–765 [DOI] [PubMed] [Google Scholar]
- Vick BA, Zimmerman DC (1983) The biosynthesis of jasmonic acid: a physiological role for plant lipoxygenase. Biochem Biophys Res Commun 111: 470–477 [DOI] [PubMed] [Google Scholar]
- Weber H, Vick BA, Farmer EE (1997) Dinor-oxo-phytodienoic acid—a new hexadecanoid signal in the jasmonate family. Proc Natl Acad Sci USA 94: 10473–10478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman B, Silva ID, Bartel B (2001) The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid β-oxidation. Plant Physiol 127: 1266–1278 [PMC free article] [PubMed] [Google Scholar]