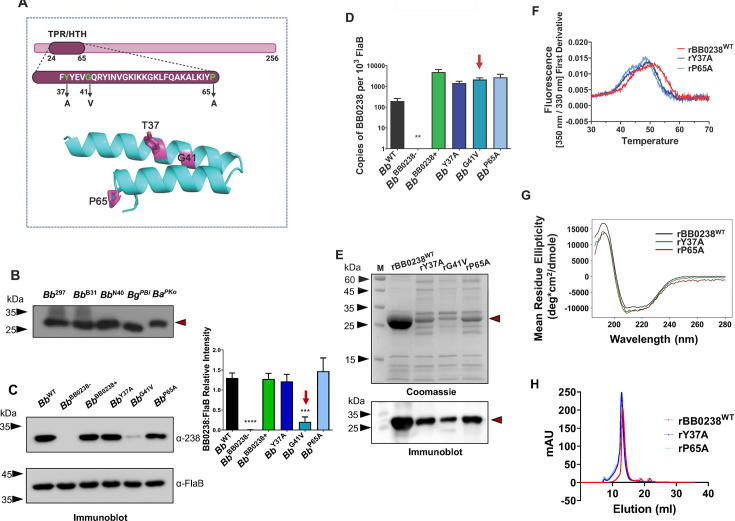
Fig 2.
Characterization of TPR-like/HTH motif in BB0238. (A) Location of the TPR-like/HTH motif and amino acid sequence. Residues relevant to the point mutants are shown in green within the context of the HTH motif (upper panel). Replaced residues are highlighted in magenta in the lower panel; Tyr37, Gly41, and Pro65 correspond to residues Tyr43, Gly47, and Pro71 in B. burgdorferi 297. (B) Conservation of BB0238. Equal amounts of lysates from B. burgdorferi isolates 297 (Bb 297), B31 (Bb B31), and N40 (Bb N40), as well as B. garinii isolate PBi (Bg PBi) and B. afzelii isolate PKo (Ba PKo), were immunoblotted against BB0238 antibody raised against the recombinant protein from B. burgdorferi B31. Position of BB0238 is shown by red arrow. The molecular weight markers (kDa) are indicated by black arrowheads. (C) Mutations in the TPR-like/HTH motif destabilize BB0238. Lysates of B. burgdorferi 297 (Bb WT), bb0238 mutant (Bb BB0238−), bb0238 complement (Bb BB0238+), and bb0238 point mutants Y37A (Bb Y37A), G41V (Bb G41V), and P65A (Bb P65A) were detected using antiserum against BB0238 (upper left panel). Antiserum against FlaB was used as a loading control (lower left panel). The right panel denotes the densiometric analysis of BB0238 protein shown in the left panel. The red arrow highlights significantly low Bb G41V transcript levels. ***P < 0.01. (D) bb0238 transcript levels. The mRNA levels for isolates, as in panel C, were measured using RT-qPCR. The bb0238 transcript levels do not significantly change between isolates, even in Bb G41V (P > 0.05), except for the null mutant Bb BB0238- where it is undetectable (**). (E) Recombinant BB0238 proteins in E. coli. Isolated proteins in gels were either stained with Coomassie brilliant blue or immunoblotted with anti-BB0238 antibody. (F) NanoDSF thermal profiles for rBB0238WT, as well as rY37A and rP65A point mutants, were determined via the first derivative of intrinsic fluorescence emission ration (350 nm/330 nm). Thermal transition (Tm) decreased significantly (P < 0.0001) with rY37A and rP65A. (G) Mutations in TPR-like/HTH motif do not alter the secondary structure of BB0238. Far-ultraviolet (UV) circular dichroism (CD) spectra of rBB0238WT (black), rY37A (green), and rP65A (red) from 200 to 260 nm are similar in all cases. (H) Quaternary structures of rBB0238WT, rY37A, and rP65A were examined via size exclusion chromatography. The elution profile for rBB0238WT depicted the dimerization of wild-type protein (red). Slightly earlier elution profiles of rY37A and rP65A (dark blue and light blue, respectively) reflected a size change in the dimeric state of the point mutants.