Abstract
Plant cysteine (Cys) synthesis can occur in three cellular compartments: the chloroplast, cytoplasm, and mitochondrion. Cys formation is catalyzed by the enzyme O-acetylserine(thiol)lyase (OASTL) using O-acetylserine (OAS) and sulfide as substrates. To unravel the function of different isoforms of OASTL in cellular metabolism, a transgenic approach was used to down-regulate specifically the plastidial and cytosolic isoforms in potato (Solanum tuberosum). This approach resulted in decreased RNA, protein, and enzymatic activity levels. Intriguingly, H2S-releasing capacity was also reduced in these lines. Unexpectedly, the thiol levels in the transgenic lines were, regardless of the selected OASTL isoform, significantly elevated. Furthermore, levels of metabolites such as serine, OAS, methionine, threonine, isoleucine, and lysine also increased in the investigated transgenic lines. This indicates that higher Cys levels might influence methionine synthesis and subsequently pathway-related amino acids. The increase of serine and OAS points to suboptimal Cys synthesis in transgenic plants. Taking these findings together, it can be assumed that excess OASTL activity regulates not only Cys de novo synthesis but also its homeostasis. A model for the regulation of Cys levels in plants is proposed.
Cys is the first committed molecule in plant metabolism that contains both sulfur and nitrogen, and, thus, the regulation of its biosynthesis is of utmost importance for the synthesis of a number of essential metabolites in plant pathways (Hesse et al., 2004). Cys is incorporated into proteins and glutathione (GSH) directly or serves as a sulfur donor for the synthesis of S-containing compounds such as Met and its derivatives S-adenosylmethionine and S-methylmethionine, and many secondary compounds such as S-methylcysteine, S-alkylcysteine, glucosinolates, and phytoalexins (Schmidt and Jäger, 1992; Ravanel et al., 1998; Matthews, 1999; Hesse and Hoefgen, 2003). Furthermore, Cys acts as a general catalyst in redox reactions through the nucleophilic properties of its sulfur atom, utilizing dithiol-disulfide interchange, as displayed in the thioredoxin and the glutaredoxin systems (Schürmann and Jacquot, 2000; Jacquot et al., 2002).
Cys is formed from two substrates, sulfide and activated Ser, as a carbon backbone and is catalyzed by the enzyme O-acetylserine(thiol)lyase (OASTL), which transfers sulfide to O-acetylserine (OAS) to form Cys. The activated Ser, OAS, is synthesized by Ser acetyltransferase (SAT). In plants, OASTL has been shown to be present in 100- to 400-fold excess over SAT (Schmidt and Jäger, 1992; Höfgen et al., 2001). There is biochemical and molecular evidence that in plants, SAT and OASTL are associated in a multienzyme complex called Cys synthase, first described in Salmonella typhimurium and Escherichia coli and later in Arabidopsis (Arabidopsis thaliana; Kredich, 1996; Bogdanova et al., 1997; Wirtz et al., 2001; Berkowitz et al., 2002). The current model of Cys formation proposes that in the formed complex of OASTL and SAT, OASTL is virtually inactive but causes the stabilization of SAT, while SAT is only active when bound in the complex. OAS formed in the complex now decreases the binding affinity of both enzymes, and OASTL is released to convert OAS to Cys; thus, the dissociation serves to control OAS synthesis. However, the concentration of OASTL is far in excess of SAT, and the free OASTL is responsible for the production of Cys (Hawkesford, 2000; Saito, 2000; Berkowitz et al., 2002; Hell et al., 2002).
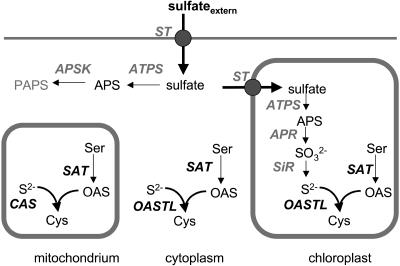
Cys synthesis occurs at several subcellular locations, each of which has its own enzyme isoforms, at least in Arabidopsis with its gene family consisting of seven isoforms (Jost et al., 2000; Höfgen et al., 2001). There, the presence of isoforms in the cytosol, the plastids, and mitochondria suggests that the ability to form Cys is essential for all compartments active in protein biosynthesis. However, their respective contributions to the net Cys synthesis and any functional interactions that may occur between these subcellular locations are unknown. Interestingly, only Arabidopsis seems to possess a mitochondrial localized OASTL (Hesse et al., 1999), while in other plants such as spinach (Spinacia oleracea) and potato (Solanum tuberosum), β-cyanoalanine synthase (CAS) substitutes for this function (Fig. 1; Saito et al., 1994; Warrilow and Hawkesford, 1998, 2000; Hatzfeld et al., 2000; Maruyama et al., 2001). In this context, it is important to note that in Arabidopsis mitochondria, a CAS exists additionally to OASTL (Hatzfeld et al., 2000).
Figure 1.
Sulfur metabolism compartmentation of Cys biosynthesis. External sulfate is taken up through members of a multigene family of sulfate transporters. The inert sulfate is activated by covalent binding to ATP to form adenosine-5′-phosphosulfate (APS) either in the cytosol or plastid. In the cytosol, APS can be phosphorylated to phosphoadenosine-5′-phosphosulfate (PAPS). In chloroplasts, sulfate bound in APS is reduced to sulfide via sulfite and subsequently transferred to activated Ser (OAS) to form Cys. Cys formation takes place in three cellular compartments: chloroplasts, but also cytosol and mitochondria. In these compartments, both SAT and OASTL isoforms are present but the reductive component of the pathway is missing. In potato, the function of OASTL in mitochondria is probably substituted by CAS. ATPS, ATP sulfurylase; APSK, APS kinase; APR, APS reductase; ST, sulfate transporter; SiR, sulfite reductase; ser, serine; S2−, sulfide; SO32−, sulfite.
To promote our understanding of the role of the different OASTL isoforms, we initiated a transgenic approach of producing potato plants with reduced enzymatic activities for OASTL via antisense-mediated inhibition of previously cloned endogenous potato OASTL isoforms (Hesse and Höfgen, 1998). Several plants with respectively decreased activity could be identified. Interestingly, these plants showed no gross alterations of growth or yield under normal growth conditions. It could be demonstrated that the reduction of OASTL enzyme activity in transgenic potato plants resulted in increased thiol levels. These data provide evidence that both isoforms are able to substitute for each other in function, probably due to the transfer of S intermediates between cellular compartments, and indicate that excess OASTL is responsible for the regulation of Cys homeostasis.
RESULTS
Engineering and Screening of Plants with Reduced OASTL Enzyme Activity
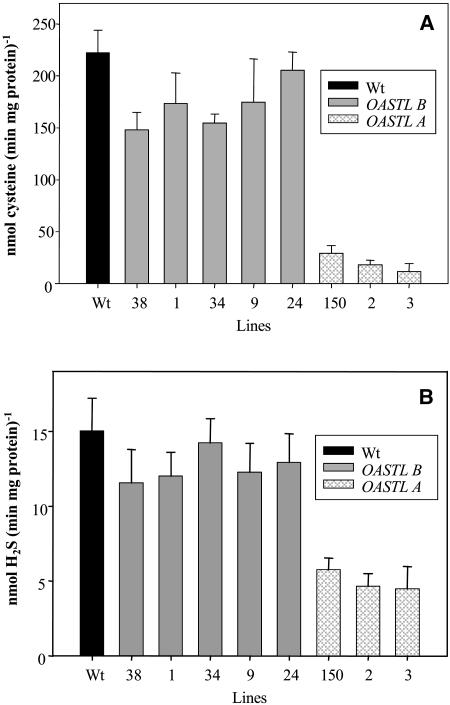
To decrease the activity of OASTL, potato plants were transformed with the vector pBinAR harboring a cDNA encoding a sequence from either the StOASTL A or the StOASTL B gene (Hesse and Höfgen, 1998) in reverse orientation with respect to the cauliflower mosaic virus 35S promoter. After regenerating 80 independent transgenic plants/construct, lines were selected with respect to reduced OASTL activity and H2S-releasing activity levels. Eight lines were chosen for detailed analysis based on their reduced activity levels (Fig. 2A). Greenhouse-grown plant material was evaluated and scored based on macroscopic phenotypic alterations. Transgenic plants were phenotypically indistinguishable from wild-type plants (data not shown). OASTL activity was determined in crude protein extracts of the transgenic lines 1, 9, 24, 34, and 38 expressing the plastidial antisense construct and in transgenic lines 2, 3, and 150 expressing the cytosolic antisense construct and compared with control. Wild-type extracts revealed an OASTL activity in crude extracts of about 220 nmol (milligrams protein × minute)−1. For the plastidial antisense lines, the activities were down to 150 nmol (milligrams protein × minute)−1 (line 38), 160 nmol (milligrams protein × minute)−1 (line 34), 170 nmol (milligrams protein × minute)−1 (lines 1 and 9), and 210 nmol (milligrams protein × minute)−1 (line 24; Fig. 2A). A higher reduction was observed for cytosolic antisense lines, down to 10 nmol (milligrams protein × minute)−1 (line 3), 15 nmol (milligrams protein × minute)−1 (line 2), and 20 nmol (milligrams protein × minute)−1 (line 150). The relative decrease of OASTL in plastidial antisense lines as compared with wild type is clearly smaller than in transgenic plants expressing the cytosolic antisense construct. This observation might indicate the different amounts of enzyme in the different cellular compartments or that the reduced plastidial activity is compensated in part by other OASTL isoforms.
Figure 2.
OASTL and l-Cys desulfhydrase activity in transgenic potato plants. Total extracts of soluble proteins were prepared and the OASTL (A) and l-Cys desulfhydrase (B) activities were determined in spectrophotometric assays by measuring either the formation of Cys (A) or by measuring the formation of H2S from Cys (B). Activities are given as nanomoles (minutes × milligrams protein)−1.
l-Cys desulfhydrase activity was determined in the same extracts of soluble proteins used for the measurement of OASTL activity as described in Figure 2A. The l-Cys desulfhydrase activity was determined by measuring the formation of H2S from Cys (Fig. 2B). In parallel to the reduction of OASTL activity in the OASTL antisense plants, l-Cys desulfhydrase activity was reduced. In the transgenic plants carrying the OASTL antisense construct against the cytosolic OASTL, the effects were more pronounced than in the plastidial antisense plants. It was shown previously in in vitro experiments that the recombinant purified OASTL B-protein catalyzed the formation of Cys from OAS and H2S, but also the formation of H2S from Cys. In a molar ratio, the enzyme formed about 25 times more Cys than H2S per milligram protein during the same incubation time, suggesting H2S release as a side reaction of the Cys synthase reaction (Burandt et al., 2001). Thus, in vitro, the reaction of OASTL is a net H2S-consuming reaction. The results shown in Figure 2B indicate that, also in vitro, the OASTL proteins catalyze the release of H2S from Cys. However, in comparison to the reduction in OASTL activity (Fig. 2A), the level of reduction in H2S-releasing activity is not as strong. Therefore, one can assume that H2S-releasing activity resulted not exclusively from the side activity of OASTL proteins but additionally from other enzymes such as true l-Cys desulfhydrase proteins. The results indicate that a l-Cys desulfhydrase protein is also localized in the cytosol.
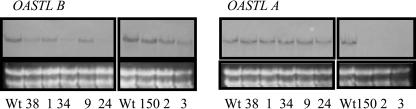
To further test whether the reduced OASTL activity resulted from a decreased endogenous transcript amount, total RNA from the selected plants was isolated and screened for OASTL expression. For all investigated transgenic lines, a substantial reduction in their transcript levels in comparison to wild-type plants was observed, while RNA levels of the second, not antisensed, isoform were not affected by the manipulation (Fig. 3).
Figure 3.
Northern-blot analysis of transgenic potato plants. The leaf material of five transgenic plants of the same line was combined and total RNA was extracted. A total of 15 μg RNA was loaded in each lane and blotted and probed with digoxigenin-labeled cDNAs of plastidic and cytosolic OASTL B and A, respectively. To prove equal loading of the extracted RNA, the ethidium bromide-stained RNA is shown at the bottom. The wild-type OASTL transcripts have a size of about 1.6 kb.
Measurements of OASTL Protein Content in Transgenic Potato Lines and OASTL Activity in a Native Gel
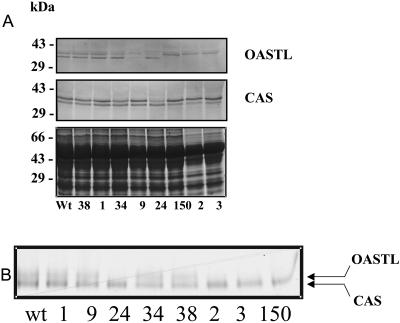
Protein extracts from leaves of the selected lines of OASTL antisense plants were subjected to protein analysis with an antiserum directed to OASTL protein (Fig. 4A). In the wild-type potato plants, the antiserum detected proteins with sizes of approximately 34 kD and 36 kD, respectively. This is in agreement with the predicted sizes of the cytosolic OASTL and the mature plastidial OASTL proteins, respectively, as deduced from the sequence analysis. Immunoblotting revealed a slight decrease in OASTL protein levels for the plastidial antisense plants (top band) but a significant decrease in the bottom band corresponding to cytosolic OASTL isoform in comparison to control plants. As judged by the immunoblot experiments, the level of OASTL protein was correlated only to the RNA blot for the cytosolic antisense plants, indicating that repression of OASTL transcript led to a reduced availability of the corresponding mRNA for translation. Using a second antibody directed against CAS, it was revealed that the content of this protein is not affected by decreases in either OASTL. Moreover, due to the similarity between OASTL and CAS at the protein level, a cross detection of the OASTL antiserum cannot be excluded and would explain the lower reduction level of OASTL in the plastidial antisense plants.
Figure 4.
Protein-blot analysis and activity staining of OASTL and CAS contents. A, For the protein blot-analysis, total protein extracts were prepared, separated on SDS-gels, and blotted onto nitrocellulose membranes. Monospecific antibodies recognizing the OASTL and CAS proteins, respectively, were used for the immunoreaction. The Coomassie-stained gel loaded with the same samples is shown in the lowest section to demonstrate loading of equal protein amounts. B, After native PAGE with 150 μg protein/lane, the gel was subjected to activity staining.
To further demonstrate that reductions in OASTL protein levels correlate with decreases in specific OASTL activity in leaves, protein extracts were separated on a native gel and analyzed for OASTL activity (Fig. 4B). In agreement with the data obtained from RNA and protein-blot analysis, the transgenic plants showed lower activities. The other activity seen can be assigned as CAS (Fig. 4B, bottom band).
Considering these results, we conclude that repression of the OASTL gene by antisense inhibition resulted in alterations of OASTL protein levels, which were in accordance to the corresponding protein quantities and enzyme activities in both transgenic lines.
Effect of OASTL Antisense Inhibition on Metabolite Levels in Source Leaves
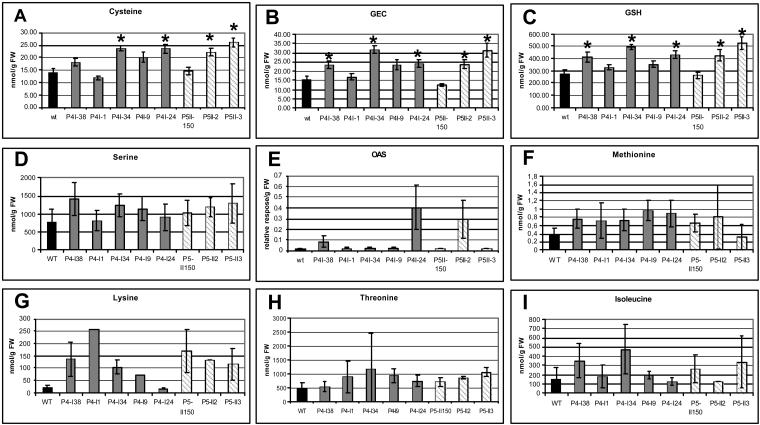
The effect of a decreased expression of the OASTL gene on the amounts of thiols and Asp-derived amino acid compounds was tested. Following the commonly held assumption that the de novo synthesis of amino acids in higher plants occurs in the chloroplasts, source leaf tissues were analyzed for soluble metabolites using HPLC (Kreft et al., 2003) and gas chromatography-mass spectrometry (GC-MS)-based technology (Roessner et al., 2000, 2001). Analyzing the thiol levels revealed that in all transgenic plants, thiols such as Cys, γ-glutamylcysteine (GEC), and GSH increased significantly (Fig. 5, A–C). There is no direct correlation between the reduction of the OASTL activity and thiol content, but generally, these metabolites have higher levels in transgenic plants than in wild-type control plants. Furthermore, plants with higher Cys levels also have higher levels of GEC and GSH. For example, line 34 contained 1.7-fold more Cys, 2.0-fold more GEC, and 1.8-fold more GSH. Line 3 had comparable increases in Cys (1.9-fold), GEC (2.0-fold), and GSH (1.9-fold). It is important to note that almost all lines accumulated Ser (except line 1), the immediate precursor of OAS production (Fig. 5D). This is of importance because lines 38, 24, and 2 showed elevated but not significantly elevated levels of OAS (Fig. 5E). Since this intermediate is hardly detectable in leaves of wild-type plants, its degree of accumulation could not be determined in a practical manner. An increase of SAT protein was not detected (data not shown). Furthermore, although statistically not significant, all levels of amino acids of the Asp family were increased (Fig. 5, F–I), Met (Fig. 5F), which receives its sulfide moiety from Cys, especially benefits from the increased Cys levels and accumulated up to 2-fold. The increase might initiate internal pathway regulation that explains the increase of other amino acids such as Lys (Fig. 5G), Thr (Fig. 5H), and Ile (Fig. 5I). Other metabolites were not affected by the antisense approach (data not shown).
Figure 5.
Impact of reduced OASTL activity on the level of thiols, selected amino acids, and OAS. Metabolites were extracted from leaf tissues of 8-week-old plants. Cys (A), GEC (B), and GSH (C) were determined by HPLC analysis with monobrombimane derivatization and fluorescence detection. Amounts of thiols are given in nanomoles per gram fresh weight (FW) and represent mean values ± sd (n = 5). Differences between wild-type and transgenic plants analyzed using Student's t test were statistically significant (asterisks, P < 0.05). Ser (D), Met (F), Lys (G), Thr (H), and Ile (I) were determined by HPLC analysis with O-phtaldialdehyde derivatization and fluorescence detection. Amounts of amino acids are given in nanomoles per gram FW and represent mean values ± sd (n = 5). E, OAS was measured from leaf material using GC-MS. Relative response is determined as ratio area metabolite/area internal standard. Data are presented as mean ± sd of five individual plants per line, one measurement per plant.
DISCUSSION
Cys represents a key compound with several cellular functions being a proteinogenic amino acid, sulfur donor, or part of protective metabolites (Hesse et al., 2004). The multitude of implementations in the cellular network makes it necessary to regulate the synthesis and cellular homeostasis tightly. Cys synthesis can be regulated at several steps (Hesse et al., 2004). Most of our understanding on this particular step is based on studies of E. coli (Kredich et al., 1969) and later studies showing that related regulatory networks are present in higher plants (Hesse et al., 2004). Recent efforts have identified the SAT/OASTL complex as a suitable regulator of Cys synthesis. The current model states that SAT is active in the complex, while OASTL is inactive, resulting in OAS formation and disruption of the complex, followed by Cys synthesis (Hesse et al., 2004). On the other hand, excess OASTL in transgenic tobacco (Nicotiana tabacum) plants revealed a more tolerant phenotype when exposed to oxidative stress but with a minimal increase in thiols under normal growth conditions (Noji et al., 2001; Youssefian et al., 2001; Sirko et al., 2004). In this paper, an antisense-mediated down-regulation of OASTL activity was used to misbalance the SAT/OASTL ratio to investigate the particular roles of OASTL isoforms in Cys formation and homeostasis. A set of transgenic plants was investigated expressing antisense RNA for the cytosolic and the plastidial isoform, respectively. Surprisingly, no effect on the growth habit of the transgenic lines could be observed, although OASTL activities were maximally reduced to 5% in crude extracts of transgenic plants. Generally, the reduction obtained with the cytosolic isoform was higher than for the plastidial one. An explanation for this result remains to be found. Constructs were made with the full-length cDNAs and the impact of antisense down-regulation cannot be predicted. However, this finding might indicate that the effectiveness of the cytosolic antisense RNA is higher than the plastidial RNA with its extension of the plastidial-targeting coding sequence, even though they share 68.1% identity at the nucleic acid level. Another explanation could be that the molar ratio between cytosolic and plastidial OASTL activity is displaced in potato toward higher cytosolic activity as occurs in pea (Pisum sativum; Lunn et al., 1990). The down-regulation of OASTL activity is clearly a result of the antisense approach. RNA-blot analysis revealed a specific reduction in OASTL RNA levels of the antisensed isoform in transgenic plants corroborated by the enzyme activity measurements, protein-blot analysis, and the zymogram.
OASTL catalyzes the incorporation of reduced sulfur into organic compounds. Modifying the enzyme activity at such a critical position in a pathway should therefore lead to alterations in metabolic turnover and affect levels of the respective biosynthetic end product. In this respect, changes in OASTL activity levels are likely to result in alterations of soluble Cys contents. Such a possibility has not been investigated in Arabidopsis or other plants. Overexpression of OASTL in Arabidopsis and tobacco has been shown to result in only moderate increases in Cys and GSH levels, though these increases led to augmented tolerance to oxidative stress or cadmium exposure (Harada et al., 2001; Noji et al., 2001; Youssefian et al., 2001; Sirko et al., 2004). Earlier studies revealed that OASTL is in excess (up to 400-fold) over the amount required to provide the overall flux of the pathway and is therefore not limiting to the rate of Cys synthesis in plants (Schmidt and Jäger, 1992; Ruffet et al., 1995; Sirko et al., 2004). Taking this into consideration, one would expect that Cys synthesis would not be affected or even reduced by decreases in expression. It is surprising then that the reduction of OASTL activity and subsequent increases in the SAT/OASTL ratio in potato provoked a substantial and statistically significant increase in Cys levels (and in GEC and GSH) in a manner similar to that seen in transgenic plants overexpressing SAT (Blaszczyk et al., 1999; Harms et al., 2000; Noji and Saito, 2002; Wirtz and Hell, 2003). Here, the SAT/OASTL ratio was elevated, resulting in higher thiol levels, which indicate that OAS is limiting. Taking these results together, one has to conclude that the formation of the SAT/OASTL complex might have two functions: the activation of SAT to form OAS and the protection of Cys homeostasis by binding OASTL, which is inactive and thus inhibiting the OASTL-driven reverse reaction (Fig. 6). Both the synthesis of OAS as Cys precursor and the reduced degrading activity of OASTL cause the increase in soluble thiols. This might also explain why SAT, as a more N-related enzyme, is up-regulated by S deprivation (Takahashi et al., 1997; Hirai et al., 2003; Nikiforova et al., 2003). Nonetheless, the reduced capability to produce Cys apparently seems to generate a bottleneck in the Cys pathway, which is indicated by the accumulation of OAS and the precursor Ser. An increase in SAT protein was not observed. A plausible explanation for this finding could be the fact that the reduction in OASTL protein resulted in fewer binding partners for SAT, which causes the increase in Ser levels. If SAT is bound to OASTL, OAS can be synthesized but no longer used for Cys formation because of the reduction of free OASTL protein (Fig. 6). The increase in Cys levels has no effect on cellular sulfate levels (data not shown; Hesse et al., 1997) but does influence Met levels in transgenic plants. Virtually all the transgenic plants have higher Met levels than wild-type plants. Other amino acids of the Asp family are also increased by the manipulation. This can be explained by the internal regulation of the pathway (Hesse and Hoefgen, 2003). Increasing Met levels might result in more S-adenosylmethionine (not determined), which positively regulates Thr synthase activity. The investigated transgenic plants have increased Thr but also Ile levels. Remarkably, Lys levels are also increased, which cannot be explained by the current regulation model of the pathway. Moreover, a positive correlation between free Lys and Met could be observed for Arabidopsis plants modified in Lys metabolism (Zhu and Galili, 2004). Other nonpathway-related amino acids or metabolites did not show significant alterations in leaves.
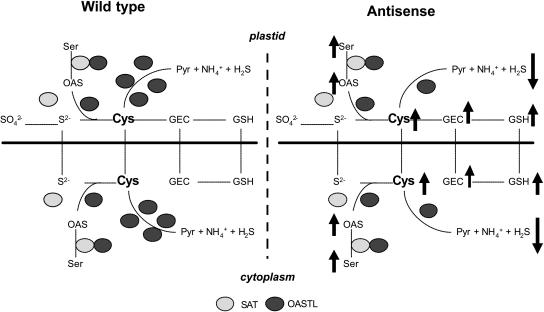
Figure 6.
Comparative model of Cys biosynthesis control in potato wild-type and transgenic plants. In wild-type plants, Cys homeostasis is controlled by the formation of the SAT/OASTL complex and the excess of free OASTL protein being able to catalyze the reverse reaction. In transgenic potato, however, the catabolic activity is reduced resulting in increased thiol levels independent from the down-regulated OASTL isoform. Precursor levels of Cys synthesis such as Ser and OAS increase in their amounts, too. Furthermore, OASTL isoforms are able to substitute each other by exchange of metabolites via the chloroplast membrane. Thus, in potato, the relative enzymatic excess of OASTL over SAT controls the Cys status. Pyr, Pyruvate; S2−, sulfide; SO42−, sulfate; SO32−, sulfite; Ser, serine. Arrows indicate the increase and decrease, respectively, of metabolites.
The increase in Cys and GSH levels in both types of antisense plants indicate that sulfur-related metabolites are exchanged between compartments, at least between chloroplasts and cytosol. Although the activity of the plastidial isoform is down-regulated, thiol levels were increased. It has to be assumed that sulfide, although very reactive, is able to cross the plastidial membrane, suggesting that both isoforms can substitute for each other (Fig. 6). Other compounds such as GEC must also cross organelle membranes, since GSH synthetase is localized in both cytosol and chloroplasts while GEC synthetase occurs only in plastids (Cobbett et al., 1998; The Arabidopsis Genome Initiative, 2000; Noctor et al., 2002; Hartmann et al., 2003). Through this interorganelle transfer, the plant cell is able to keep Cys homeostasis constant, although it is questionable how the cells sense the Cys content in each compartment. In this context, it is of interest why OASTL protein is in such an excess over SAT. Apparently, from the obtained results, it can be assumed that the excess of OASTL is, under normal conditions, responsible for both de novo synthesis and breakdown of Cys to keep Cys or reduced sulfur levels in balance (Fig. 6). The breakdown of Cys, the inverse reaction of OASTL (Burandt et al., 2002), results in pyruvate, ammonia, and H2S production. The latter is volatile and could possibly be used as latent natural pathogen protection. A reduced H2S-releasing capacity in the transgenic potato plants was observed; it would be interesting to investigate if these plants are less resistant to pathogen infections. An H2S-releasing activity of true Cys desulfhydrase proteins is probably induced only in certain physiological states. There are a number of Cys desulfhydrase candidates in the Arabidopsis genome such as NifS-like proteins (Leon et al., 2002). However, the percentage of the turnover of Cys to H2S catalyzed by desulfhydrases and by the side reaction of OASTL proteins is not known.
The presented data provide evidence for a more complex regulation of Cys synthesis and homeostasis. The formation of the SAT/OASTL complex is likely part of the regulatory cascade. The obtained data indicate that other players are involved in the regulation of Cys biosynthesis in plants and that the current models require adjustments.
MATERIALS AND METHODS
Generation of Transgenic Potato Lines
Potato (Solanum tuberosum; Saatzucht Lange AG, Bad Schwartau, Germany) OASTL isoforms (Hesse and Höfgen, 1998) were cut from the pBluescript SK− as Asp718/BamHI fragment for the cytosolic OASTL isoform (1.308 bp; asOAS-TL A) and the XhoI/BamHI fragment for the plastidial OASTL isoform (1.404 bp; asOAS-TL B) and cloned in their reverse orientations to the cauliflower mosaic virus 35S promoter into the vector pBinAR-Kan (Höfgen and Willmitzer, 1990) previously cut with Asp718/BamHI and BamHI/SalI, respectively, to generate antisense constructs for plant transformation. The transformation of potato by Agrobacterium tumefaciens (Rocha-Sosa et al., 1989) using the strain C58C1/pGV2260 (Deblaere et al., 1985) was carried out as described by Dietze et al. (1995). Transgenic plants were selected on medium containing kanamycin (10 mg L−1). The resulting transgenic plants were planted in soil and grown in the greenhouse under a 16-h-light, 8-h-dark regime at 20°C. Leaf material was screened for OASTL activity according to Gaitonde (1967).
Plant Cultivation
Transgenic OASTL antisense plants were propagated in tissue culture along with potato wild-type plants and transferred into soil after 2 weeks of cultivation. The rooted shoots were planted in small pots and grown in the phytotron with a light regime of 200 to 250 μmol s−1 m−1 (16 h/8 h) under a hood to retain high air humidity. After 2 weeks, plants were transferred into pots with a diameter of 20 cm and cultivated in a greenhouse providing nearly natural light conditions with an approximately 16-h-light/8-h-dark period plus natural sunlight. Light intensity and temperature were dependent on environmental conditions, but light did not fall below 250 to 300 μmol photons m−2 s−1, and temperature did not sink below 18°C. Leaf material was harvested from greenhouse-grown plants after approximately 8 weeks of cultivation, before the onset of flowering. Leaf discs were excised from tissues of similar developmental stage. Transition to the reproductive stage could usually be observed only in plants older than 10 weeks. All plant material was sampled in the morning and immediately frozen in liquid nitrogen before storage at −80°C.
RNA Extraction and Northern-Blot Analysis
Total RNA was extracted essentially as described by Chomczynski and Sacchi (1987). RNA samples (15 μg) were separated on 1% denaturing agarose-formaldehyde gels. Equal loading was controlled by staining the gels with ethidium bromide. After RNA transfer onto nylon membranes, filters were probed with digoxigenin-labeled cDNA fragments obtained by PCR encoding the respective mature OASTL proteins. For the PCR-labeling reaction of OASTL A, primer P95 (5′-GGATCCGCGGGGGAAAAGAATGGAA-3′) and P96 (5′-GACGTCTCAAGGCTCCACAGTCAT-3′) were used. For OASTL B, primer P97 (5′-GGATCCGCAGTGTCTGTACCAACGAAA-3′) and P98 (5′-GACGTCTCACAATTCTGGCTTCAT-3′) with the respective cDNA clone as template were used (Hesse and Höfgen, 1998). A colorimetric detection method with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate as substrates for alkaline phosphatase was applied.
SDS-PAGE and Western Blotting
For the determination of protein steady-state levels, 100 mg plant material was mortared to a fine powder in liquid nitrogen. Protein estimation was done according to Bradford (1976) using bovine serum albumin as a standard. A total of 500 μL of sample buffer (56 mm Na2CO3, 56 mm dithiothreitol, 2% SDS, 12% Suc, and 2 mm EDTA) was added, samples were incubated for 15 min at 95°C, and cell debris was removed by centrifugation. About 10 μg of the total protein supernatant was subjected to SDS-PAGE (Laemmli, 1970) and blotted (Sambrook et al., 1989). Antibodies directed against purified spinach (Spinacia oleracea) OASTL, purified spinach CAS (Hatzfeld et al., 2000), and SAT (Saito et al., 1995) were used for the immunodetection. It is not known which OASTL/CAS isoforms were used for raising the antibodies because the N-terminal amino acid sequences of the purified proteins used for immunization have not been determined. A colorimetric detection method with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate as substrates for alkaline phosphatase was applied.
Activity Staining
Extracts of soluble proteins were prepared using 100 mg frozen plant material and 250 μL 20-mm Tris-HCl, pH 8.0. Of each sample, 150 μg protein was separated by native PAGE (8%) at a constant current of 25 mA at 4°C. After electrophoresis, the gel was immersed for 3 min at 30°C in the reaction mix (2.5 mm KCN, 2.5 mm l-Cys, and 25 mm CAPS buffer, pH 10.0), which had been prewarmed at 30°C. The incubation was stopped by adding 0.2 mm lead acetate, pH 4.0. The appearance of brown bands revealed the position of the enzymes in the gel (Akopyan et al., 1975).
Determination of Enzyme Activity
Extracts of soluble proteins were prepared using 100 mg frozen plant material and 1 mL 20-mm Tris-HCl, pH 8.0. The mixture was further homogenized and centrifuged for 10 min at 13,000g. The assay for OASTL activity contained in a total volume of 1 mL: 5 mm OAS, 5 mm Na2S, 33.4 mm dithiotreitol, 100 mm Tris-HCl, pH 7.5, and 50 μL enzyme extract (Schmidt, 1990). The reaction was initiated by the addition of Na2S and incubated for 30 min at 37°C after which the reaction was terminated by adding 1 mL acidic ninhydrin reagent (0.8% ninhydrin [w/v] in 1:4 concentrated HCl:HOAc) to determine the Cys concentration (Gaitonde, 1967). The samples were heated at 100°C for 10 min to allow color development and cooled on ice. The color complex was stabilized by adding 900 μL 70% ethanol to 100-μL samples, then the A560. Solutions with different concentrations of Cys were prepared, treated in the same way as the assay samples, and used for the quantification of the enzymatically formed Cys.
l-Cysteine desulfhydrase activity was measured by the release of sulfide from Cys. The assay for measuring l-Cys desulfhydrase activity contained in a total volume of 1 mL: 100 mm Tris-HCl, pH 8.0, 2.5 mm dithiothreitol, 0.8 mm l-Cys, and 100 μL enzyme extract. After 15 min at 37°C, the reaction was terminated by adding 100 μL of 30 mm FeCl3 dissolved in 1.2 n HCl and 100 μL 20 mm N,N-dimethyl-p-phenylenediamine dihydrochloride dissolved in 7.2 n HCl (Siegel, 1965). The formation of methylene blue was determined at 670 nm in a spectrophotometer. Solutions with different concentrations of Na2S were prepared, treated in the same way as the assay samples, and used for the quantification of the enzymatically formed H2S.
Extraction and Analysis of Soluble Thiol Compounds
Individual soluble thiols were determined as the sum of their reduced and oxidized forms. One hundred milligrams of fresh ground leaf material was added to 100 mg of polyvinylpolypyrrolidone (previously washed with 0.1 m HCl) and 1 mL of 0.1 m HCl. The samples were shaken for 60 min at room temperature. After centrifugation (15 min at 13,000g; 4°C), the supernatants were frozen at −20°C until reduction/derivatization. Thiols were reduced by incubation with 10 mm dithiothreitol for 40 min at room temperature and derivatized for 15 min in the dark according to Hell and Bergmann (1990) or Kreft et al. (2003). Column eluent was monitored by fluorescence detection (λex 380/λem 480). Mixed standards treated exactly as the sample supernatants were used as a reference for the quantification of Cys and GSH content.
OAS Measurement by GC-MS
For GC-MS analysis, polar metabolite fractions were extracted from 60 mg frozen plant material and ground to a fine powder with MeOH/CHCl3. The fraction of polar metabolites was prepared by liquid partitioning into water as described earlier (Roessner et al., 2000; Wagner et al., 2003). Metabolite samples were derivatized by methoxyamination, using a 20-mg mL−1 solution of methoxyamine hydrochloride in pyridine, and subsequent trimethylsilylation, with N-methyl-N-(trimethylsilyl)-trifluoroacetamide (Roessner et al., 2000). A C12, C15, C19, C22, C28, C32, C36 n-alkane mixture was used for the determination of retention time indices (Wagner et al., 2003). Ribitol was added for internal standardization. Samples were injected in splitless mode (1 μL/sample) and analyzed using a quadrupole-type GC-MS system (MD800, ThermoQuest, Manchester, UK). The level of OAS was determined as relative-response ratios of peak areas of this compound to peak area of internal standard (ribitol), normalized with respect to the fresh weight of the sample. The chromatograms and mass spectra were evaluated using MASSLAB software (ThermoQuest).
Extraction and Analysis of Soluble Amino Acids
Soluble amino acids were determined following a modified protocol from Scheible et al. (1997). Leaf tissues (about 100 mg/plant) were ground to a fine powder in liquid nitrogen in a bead mill and extracted 3 times for 20 min at 80°C: once with 400 μL of 80% (v/v) aqueous ethanol (buffered with 2.5 mm HEPES-KOH, pH 7.5) and 10 μL of 20-μmol l-nor-Val (as an internal standard), once with 400 mL of 50% (v/v) aqueous ethanol (buffered as before), and once with 200 μL of 80% (v/v) aqueous ethanol. Between the extraction steps, the samples were centrifuged for 10 min at 13,000g, and the supernatants were collected. The combined ethanol/water extracts were stored at −20°C or directly subjected to reversed phase-HPLC using an ODS column (Hypersil C18; 150- × 4.6-mm i.d.; 3 μm; Knauer, Berlin) connected to an HPLC system (Dionex, Idstein, Germany). Amino acids were measured by precolumn derivatization with orthophthaldehyde in combination with fluorescence detection (Lindroth and Mopper, 1979) as described by Kreft et al. (2003). Peak areas were integrated using Chromeleon 6.30 software (Dionex) and subjected to quantification by means of calibration curves made from standard mixtures.
Acknowledgments
We thank Romy Ackermann for performing the potato transformations, the gardeners for excellent greenhouse work, and Josef Bergstein for photographical assistance. We thank P. von Trzebiatowski and J. Volker for their excellent technical assistance. We are grateful to Prof. Dr. A. Schmidt, Hannover, for helpful discussions. We thank Dr. P. Burandt, Hannover, for the rescreening of the different transgenic potato lines. We thank Prof. Dr. K. Saito for providing the antibodies for SAT and Megan McKenzie for carefully revising this manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (project SCHM 307/15–3 to A.R., J.P.), the European Union (grant nos. Bio4CT 97–2182 and QLRT–2000–00103), and the Max-Planck Society (K.R., R.H., H.H.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.057125.
References
- Akopyan TN, Braunstein AE, Goryachenkova EV (1975) Beta-cyanoalanine synthase: purification and characterization. Proc Natl Acad Sci USA 72: 1617–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz O, Wirtz M, Wolf A, Kuhlmann J, Hell R (2002) Use of bio-molecular interaction analysis to elucidate the regulatory mechanism of the cysteine synthase complex from Arabidopsis thaliana. J Biol Chem 277: 30629–30634 [DOI] [PubMed] [Google Scholar]
- Blaszczyk A, Brodzik R, Sirko A (1999) Increased resistance to oxidative stress in transgenic tobacco plants overexpressing bacterial serine acetyltransferase. Plant J 20: 237–243 [DOI] [PubMed] [Google Scholar]
- Bogdanova N, Hell R (1997) Cysteine synthesis in plant: protein-protein interactions of serine acetyltransferase from Arabidopsis thaliana. Plant J 11: 252–262 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Burandt P, Schmidt A, Papenbrock J (2001) Cysteine synthesis and cysteine desulfuration in Arabidopsis plants at different developmental stages and light conditions. Plant Physiol Biochem 39: 861–870 [Google Scholar]
- Burandt P, Schmidt A, Papenbrock J (2002) Three O-acetylserine(thiol)lyase from Arabidopsis catalyse cysteine synthesis and cysteine desulfuration at different pH values. J Plant Physiol 159: 111–119 [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single step method of RNA isolation by guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159 [DOI] [PubMed] [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B (1998) The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. Plant J 16: 73–78 [DOI] [PubMed] [Google Scholar]
- Deblaere R, Bytebier B, de Greve H, Debroek F, Schell J, van Montagu M, Leemanns J (1985) Efficient octopine Ti plasmid-derived vectors of Agrobacterium mediated gene transfer to plants. Nucleic Acids Res 13: 4777–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietze J, Blau A, Willmitzer L (1995) Agrobacterium-mediated transformation of potato (Solanum tuberosum). In I Potrykus, G Spangenberg, eds, Gene Transfer to Plants XXII. Springer-Verlag, Berlin, pp 24–29
- Gaitonde MK (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other natural occurring amino acids. Biochem J 104: 627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada E, Choi YE, Tsuchisaka A, Obata H, Sano H (2001) Transgenic tobacco plants expressing a rice cysteine synthase gene are tolerant to toxic levels of cadmium. J Plant Physiol 158: 655–661 [Google Scholar]
- Harms K, von Ballmoos P, Brunold C, Höfgen R, Hesse H (2000) Expression of a bacterial serine acetyltransferase in transgenic potato plants leads to increased levels of cysteine and glutathione. Plant J 22: 335–343 [DOI] [PubMed] [Google Scholar]
- Hartmann TJ, Fricker MD, Rennenberg H, Meyer AJ (2003) Cell-specific measurement of cytosolic glutathione in poplar leaves. Plant Cell Environ 26: 965–973 [DOI] [PubMed] [Google Scholar]
- Hatzfeld Y, Maruyama A, Schmidt A, Noji M, Ishizawa K, Saito K (2000) Beta-cyanoalanine synthase is a mitochondrial cysteine synthase-like protein in spinach and Arabidopsis thaliana. Plant Physiol 123: 1163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkesford MJ (2000) Plant responses to sulfur deficiency and the genetic manipulation of sulfate transporters to improve S-utilization efficiency. J Exp Bot 51: 131–138 [PubMed] [Google Scholar]
- Hell R, Bergmann L (1990) Glutamylcysteine synthetase in higher plants: catalytic properties and subcellular localization. Planta 180: 603–612 [DOI] [PubMed] [Google Scholar]
- Hell R, Jost R, Berkowitz O, Wirtz M (2002) Molecular and biochemical analysis of the enzymes of cysteine biosynthesis in the plant Arabidopsis thaliana. Amino Acids 22: 245–257 [DOI] [PubMed] [Google Scholar]
- Hesse H, Höfgen R (1998) Isolation of cDNAs encoding cytosolic (accession no. AF044172) and plastidic (accession no. AF044173) cysteine synthase isoforms from Solanum tuberosum. Plant Physiol 116: 1604 [Google Scholar]
- Hesse H, Höfgen R (2003) Molecular aspects of methionine biosynthesis in Arabidopsis and potato. Trends Plant Sci 8: 259–262 [DOI] [PubMed] [Google Scholar]
- Hesse H, Lipke J, Altmann T, Höfgen R (1997) Expression analysis and subcellular localization of cysteine synthase isoforms from Arabidopsis thaliana. In WJ Cram, LJ De Kok, I Stulen, C Brunold, H Rennenberg, eds, Sulfur Metabolism in Higher Plants. Buckhuys Publishers, Leiden, The Netherlands, pp 227–230
- Hesse H, Lipke J, Altmann T, Höfgen R (1999) Molecular cloning and expression analysis of mitochondrial and plastidic isoforms of cysteine synthase (O-acetylserine(thiol)lyase) from Arabidopsis thaliana. Amino Acids 16: 113–131 [DOI] [PubMed] [Google Scholar]
- Hesse H, Nikiforova V, Gakière B, Hoefgen R (2004) Molecular analysis and control of cysteine biosynthesis: integration of nitrogen and sulfur metabolism. J Exp Bot 55: 1283–1292 [DOI] [PubMed] [Google Scholar]
- Hirai YM, Fujiwara T, Awazuhara M, Kimura T, Masaaki N, Saito K (2003) Global expression profiling of sulfur-starved Arabidopsis by DNA macroarray reveals the role of O-acetyl-L-serine as a general regulator of gene expression in response to sulfur nutrition. Plant J 33: 651–663 [DOI] [PubMed] [Google Scholar]
- Höfgen R, Kreft O, Willmitzer L, Hesse H (2001) Manipulation of thiol contents in plants. Amino Acids 20: 291–299 [DOI] [PubMed] [Google Scholar]
- Höfgen R, Willmitzer L (1990) Biochemical and genetic analysis of different patatin isoforms expressed in various organs of potato (Solanum tuberosum). Plant Sci 66: 221–230 [Google Scholar]
- Jacquot J-P, Gelhaye E, Rouhier N, Corbier C, Didierjean C, Aubry A (2002) Thioredoxins and related proteins in photosynthetic organisms: molecular basis for thiol dependent regulation. Biochem Pharmacol 64: 1065–1069 [DOI] [PubMed] [Google Scholar]
- Jost R, Wirtz M, Berkowitz O, Hopkins L, Hawkesford M, Hell R (2000) Genomic and functional analysis of the O-acetylserine (thiol) lyase gene family involved in cysteine biosynthesis in Arabidopsis thaliana. Gene 253: 237–247 [DOI] [PubMed] [Google Scholar]
- Kredich NM (1996) Biosynthesis of cysteine. In FC Neidhardt, R Curtiss, JL Ingraham, ECC Lin, KB Low, B Magasanik, WS Reznikoff, M Riley, M Schaechter, E Umberger, eds, Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. ASM Press, Washington DC, pp 514–527
- Kredich NM, Becker MA, Tomkins GM (1969) Purification and characterization of cysteine synthetase, a bifunctional protein complex, from Salmonella typhimurium. J Biol Chem 244: 2428–2439 [PubMed] [Google Scholar]
- Kreft O, Höfgen R, Hesse H (2003) Functional analysis of cystathionine c-synthase in genetically engineered potato plants. Plant Physiol 131: 1843–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Leon S, Touraine B, Briat JF, Lobreaux S (2002) The AtNFS2 gene from Arabidopsis thaliana encodes a NifS-like plastidial cysteine desulfurase. Biochem J 366: 557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindroth P, Mopper K (1979) High performance liquid chromatographic determination of subpicomole amounts of amino acids by precolumn fluorescence derivatization with o-phthaldialdehyde. Anal Chem 51: 1667–1674 [Google Scholar]
- Lunn JE, Droux M, Martin J, Douce R (1990) Localization of ATP-sulfurylase and O-acetylserine(thiol)lyase in spinach leaves. Plant Physiol 94: 1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama A, Saito K, Ishizawa K (2001) Beta-cyanoalanine synthase and cysteine synthase from potato: molecular cloning, biochemical characterization, and spatial and hormonal regulation. Plant Mol Biol 46: 749–760 [DOI] [PubMed] [Google Scholar]
- Matthews BF (1999) Lysine, threonine and methionine biosynthesis. In BK Singh, ed, Plant Amino Acids: Biochemistry and Biotechnology. Dekker, New York, pp 205–225
- Nikiforova V, Freitag J, Kempa S, Adamik M, Hesse H, Hoefgen R (2003) Transcriptome analysis of sulfur depletion in Arabidopsis thaliana: Interlacing of biosynthetic pathways provides response specificity. Plant J 33: 633–650 [DOI] [PubMed] [Google Scholar]
- Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot 53: 1283–1304 [DOI] [PubMed] [Google Scholar]
- Noji M, Saito K (2002) Molecular and biochemical analysis of serine acetyltransferase and cysteine synthase towards sulfur metabolic engineering in plants. Amino Acids 22: 231–243 [DOI] [PubMed] [Google Scholar]
- Noji M, Saito M, Nakamura M, Aono M, Saji H, Saito K (2001) Cysteine synthase overexpression in tobacco confers tolerance to sulfur-containing environmental pollutants. Plant Physiol 126: 973–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanel S, Gakiere B, Job D, Douce R (1998) The specific features of methionine biosynthesis and metabolism in plants. Proc Natl Acad Sci USA 95: 7805–7812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Sosa M, Sonnewald U, Frommer W, Stratmann M, Schell J, Willmitzer L (1989) Both developmental and metabolic signals activate the promoter of the class I patatin gene. EMBO J 8: 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, Willmitzer L, Fernie AR (2001) Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell 13: 11–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Wagner C, Kopka J, Trethewey RN, Willmitzer L (2000) Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J 23: 131–142 [DOI] [PubMed] [Google Scholar]
- Ruffet ML, Lebrun M, Droux M, Douce R (1995) Subcellular distribution of serine acetyltransferase from Pisum sativum and characterization of an Arabidopsis thaliana putative cytosolic isoform. Eur J Biochem 227: 500–509 [DOI] [PubMed] [Google Scholar]
- Saito K (2000) Regulation of sulfate transport and synthesis of sulfur-containing amino acids. Curr Opin Plant Biol 3: 188–195 [PubMed] [Google Scholar]
- Saito K, Tatsuguchi K, Takagi Y, Murakoshi I (1994) Isolation and characterization of a cDNA that encodes a putative mitochondrion-localizing isoform of cysteine synthase (O-acetyserine(thiol)lyase) from Spinacea oleracea. J Biol Chem 269: 28187–28192 [PubMed] [Google Scholar]
- Saito K, Yokoyama H, Noji M, Murakoshi I (1995) Molecular cloning and characterization of a plant serine acetyltransferase playing a regulatory role in cysteine biosynthesis from watermelon. J Biol Chem 270: 16321–16326 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
- Scheible W, Gonzales-Fontes A, Morcuende R, Lauerer M, Geiger M, Glaab J, Gojon A, Schulze E, Stitt M (1997) Tobacco mutants with a decreased number of functional Nia genes compensate by modifying the diurnal regulation of transcription, post-translational modification and turnover of nitrate reductase. Planta 203: 304–319 [DOI] [PubMed] [Google Scholar]
- Schmidt A (1990) Sulfur metabolism. D. Cysteine synthase. In PJ Lea, ed, Methods in Plant Biochemistry. Academic Press, London, pp 349–354
- Schmidt A, Jäger K (1992) Open questions about sulfur metabolism in plants. Annu Rev Plant Physiol Plant Mol Biol 43: 325–349 [Google Scholar]
- Schürmann P, Jacquot J-P (2000) Plant thioredoxin systems revisited. Annu Rev Plant Physiol Plant Mol Biol 51: 371–400 [DOI] [PubMed] [Google Scholar]
- Siegel M (1965) A direct microdetermination for sulfide. Anal Biochem 11: 126–132 [DOI] [PubMed] [Google Scholar]
- Sirko A, Blaszczyk A, Liszewska F (2004) Overproduction of SAT and/or OASTL in transgenic plants: a survey of effects. J Exp Bot 55: 1881–1888 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yamazaki M, Sasakura N, Watanabe A, Leustek T, de Almeida Engler J, Engler G, Van Montagu M, Saito K (1997) Regulation of cysteine biosynthesis in higher plants: a sulfate transporter induced in sulfate-starved roots plays a central role in Arabidopsis thaliana. Proc Natl Acad Sci USA 94: 11102–11107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 [DOI] [PubMed] [Google Scholar]
- Wagner C, Sefkow M, Kopka J (2003) Construction and application of a mass spectral and retention time index database generated from plant GC/EI-TOF-MS metabolite profiles. Phytochemistry 62: 887–900 [DOI] [PubMed] [Google Scholar]
- Warrilow AGS, Hawkesford MJ (1998) Separation, subcellular location and influence of sulfur nutrition on isoforms of cysteine synthase in spinach. J Exp Bot 49: 1625–1636 [Google Scholar]
- Warrilow AGS, Hawkesford MJ (2000) Cysteine synthase substrate specificities classify the mitochondrial isoform as a cyanoalanine synthase. J Exp Bot 51: 985–993 [DOI] [PubMed] [Google Scholar]
- Wirtz M, Berkowitz O, Droux M, Hell R (2001) The cysteine synthase complex from plants: Mitrochondrial serine acetyltransferase from Arabidopsis thaliana carries a bifunctional domain for catalysis and protein-protein interaction. Eur J Biochem 268: 686–693 [DOI] [PubMed] [Google Scholar]
- Wirtz M, Hell R (2003) Production of cysteine for bacterial and plant biotechnology: application of cysteine feedback-insensitive isoforms of serine acetyltransferase. Amino Acids 24: 195–203 [DOI] [PubMed] [Google Scholar]
- Youssefian S, Nakamura M, Orudgev E, Kondo N (2001) Increased cysteine biosynthesis capacity of transgenic tobacco overexpressing an O-acetylserine(thiol) lyase modifies plant responses to oxidative stress. Plant Physiol 126: 1001–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Galili G (2004) Lysine metabolism is concurrently regulated by synthesis and catabolism in both reproductive and vegetative tissues. Plant Physiol 135: 129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]