Abstract
Arabidopsis (Arabidopsis thaliana) mutants lacking the tonoplastic malate transporter AttDT (A. thaliana tonoplast dicarboxylate transporter) and wild-type plants showed no phenotypic differences when grown under standard conditions. To identify putative metabolic changes in AttDT knock-out plants, we provoked a metabolic scenario connected to an increased consumption of dicarboxylates. Acidification of leaf discs stimulated dicarboxylate consumption and led to extremely low levels of dicarboxylates in mutants. To investigate whether reduced dicarboxylate concentrations in mutant leaf cells and, hence, reduced capacity to produce OH− to overcome acidification might affect metabolism, we measured photosynthetic oxygen evolution under conditions where the cytosol is acidified. AttDT::tDNA protoplasts showed a much stronger inhibition of oxygen evolution at low pH values when compared to wild-type protoplasts. Apparently citrate, which is present in higher amounts in knock-out plants, is not able to replace dicarboxylates to overcome acidification. To raise more information on the cellular level, we performed localization studies of carboxylates. Although the total pool of carboxylates in mutant vacuoles was nearly unaltered, these organelles contained a lower proportion of malate and fumarate and a higher proportion of citrate when compared to wild-type vacuoles. These alterations concur with the observation that radioactively labeled malate and citrate are transported into Arabidopsis vacuoles by different carriers. In addition, wild-type vacuoles and corresponding organelles from AttDT::tDNA mutants exhibited similar malate channel activities. In conclusion, these results show that Arabidopsis vacuoles contain at least two transporters and a channel for dicarboxylates and citrate and that the activity of AttDT is critical for regulation of pH homeostasis.
In plant cells, excess malate is stored within the large, central vacuole. NMR studies demonstrated that newly synthesized malate first accumulates in the cytoplasm and, after reaching a certain threshold concentration, is subsequently transported into the vacuole, leading to a rapid exchange between the cytosol and the organelle (Gout et al., 1993). This observation, however, does not correspond to the classic storage theory, where a solute is stored within the vacuole until it is utilized by cellular processes, but implies that vacuolar malate accumulation is a complex process comprising synthesis and degradation, energization status of the vacuole, and modulation of transport.
Vacuolar malate transport has already been investigated in detail using flux analysis, membrane potential- and pH-dependent fluorescence probes, and electrophysiological analysis (Martinoia et al., 1985; White and Smith, 1989; Ratajczak et al., 1994; Cerana et al., 1995; Pei et al., 1996; Pantoja and Smith 2002; Hafke et al., 2003). According to these studies, malate uptake is energized by vacuolar proton pumps, and the driving force is the resulting electrochemical potential difference between the cytosol and the vacuole. Depending on the system analyzed, the apparent Km values ranged between 1.5 and more than 10 mm, and cross-inhibition studies using radiolabeled malate and citrate suggested that dicarboxylates, as well as citrate, pass the vacuolar membrane by the same transporter (White and Smith, 1989; Rentsch and Martinoia, 1991).
Electrophysiological measurements showed that a malate channel is present in the vacuolar membrane of both C3 and Crassulacean acid metabolism species (Cerana et al., 1995; Pei et al., 1996; Cheffings et al., 1997; Pantoja and Smith, 2002; Hafke et al., 2003). The malate currents observed are strongly inward rectifying, thus favoring the movement of malate from the cytosol into the vacuole. Recently, it was shown that macroscopic currents observed on Kalanchoë daigremontiana vacuoles can be attributed to the activity of a small 3-pS malate-selective channel (Hafke et al., 2003), and the observation that channel density and open probability suffice for the required nocturnal malate transport in this species led to the assumption that the channel described might correspond to the malate carrier identified in flux experiments (Hafke et al., 2003).
Recently, we identified the vacuolar malate transporter from Arabidopsis (Arabidopsis thaliana) on the molecular level (AttDT [A. thaliana tonoplast dicaboxylate transporter]; Emmerlich et al., 2003). It turned out that this carrier is an ortholog of the renal Na+-dicarboxylate symporter present in proximal tubules of mammalian kidneys. Homozygous knock-out mutants lacking AttDT (AttDT::tDNA) contained less malate in leaves, and the tonoplastic malate transport activity was reduced to less than 20% of that observed on wild-type vacuoles.
Malate is involved in the tricarboxylate and glyoxylate cycle, acts as temporary storage of CO2 in C4 and Crassulacean acid metabolism plants, and is closely linked to phosphoenolpyruvate (PEP) and pyruvate-utilizing pathways through PEP carboxylase and malic enzyme. Furthermore, malate can be utilized as an osmoticum and as a counter ion for potassium or sodium (Martinoia and Rentsch, 1994). Since malate is at the branching point of a wide number of metabolic pathways, the cytosolic concentration is well controlled and was reported to range between 1 and 3 mm in the dark and 2 and 5 mm in the light (Gerhardt et al., 1987; Chang and Roberts, 1991).
In a previous report, we identified the vacuolar malate transporter and the corresponding knock-out mutant plant. Surprisingly, we did not observe a clear phenotypic alteration of the knock-out plant grown under standard conditions. Given the high importance of malate in plant metabolism, we questioned whether mutants deleted in the vacuolar malate transporter AttDT are still able to exhibit regulatory processes depending upon a nonperturbed malate homeostasis. In this context, we focused on regulation of cytosolic pH, as this process is known to interact closely with carboxylate metabolism (Smith and Raven, 1979; Davies, 1986; Kurdjian and Guern, 1989). For this analysis, we revealed the effect of alterations of pH on the expression of the AttDT gene in leaf cells; quantified the pH-controlled levels of various carboxylates in leaf discs; determined the oxygen evolution of wild-type and AttDT::tDNA protoplasts, depending upon changing pH values; and measured the carboxylate fluxes across the tonoplast using either radioactively labeled metabolites or patch clamping.
From the results presented, we conclude that the tonoplastic malate transporter AttDT is critical for regulation of pH homeostasis under certain conditions, and Arabidopsis vacuoles contain at least two types of carrier proteins and a channel for transport of dicarboxylates and citrate.
RESULTS
Effect of Acidification on the Level of Various Carboxylates in Leaf Discs
To gain more information on the implication of carboxylates for pH homeostasis in Arabidopsis, we quantified the levels of the three major Arabidopsis carboxylic acids (malate, fumarate, and citrate; Chia et al., 2000), dependent upon changing pH. For this, we prepared leaf discs at the end of the light period and subsequently incubated them for 24 h in the dark in MES-buffered medium at various pH values (Fig. 1). Our basic rationale for this approach was that incubation of leaf discs for 24 h at low pH values provokes lowered cytosolic pH values and an increased consumption of malate. We were interested in learning how AttDT knock-out mutants, which contain lower malate content (Emmerlich et al., 2003), would behave under such stress conditions and more about regulatory processes.
Figure 1.
Levels of malate (A), fumarate (B), and citrate (C) in Arabidopsis leaf discs incubated at various pH values. Arabidopsis discs from wild types or knock-out mutants have been prepared from leaves at the end of the light period and incubated for 24 h in the dark in MES-buffered water. Data are the mean ± se of four independent measurements.
At the end of the light period, malate levels were 9.4 μmol/g fresh weight (FW) for wild-type and 4.1 μmol/g FW for mutant plants. After 24 h of incubation at pH 7, wild-type leaf discs contained 5.7 μmol/g FW, whereas corresponding knock-out samples contained only 3.0 μmol/g FW (Fig. 1A). A successive decrease of the external pH value correlated in both plant lines with a decrease of leaf malate levels, finally reaching 2.2 μmol/g FW at pH 3.7 in wild-type and less than 0.5 μmol/g FW in knock-out leaf discs (Fig. 1A). Similarly, fumarate levels in wild-type tissue were 17.5 μmol/g FW at pH 7 and decreased by lowering the external pH to about 9 μmol/g FW at pH 3.7 (Fig. 1B). As seen for malate, fumarate was also lower in knock-out leaf discs incubated at pH 7 (2.7 μmol/g FW), and was reduced to 1.3 μmol/g FW at pH 3.7 (Fig. 1B). The corresponding fumarate levels at the end of the light phase were 20.3 (se ± 3.2) μmol/g FW in wild-type and 6.7 (se ± 1.1) μmol/g FW in knock-out leaves.
Interestingly, citrate is the sole carboxylic acid measured that is higher in knock-out than in wild-type leaf discs. Wild-type leaf discs incubated at pH 7 contained citrate levels of 9.5 μmol/g FW, whereas in corresponding knock-out tissue, citrate was present at a concentration of 13.5 μmol/g FW (Fig. 1C). The corresponding citrate levels at the end of the light phase were 8.7 (se ± 0.8) μmol/g FW in wild-type and 13.0 (se ± 0.3) μmol/g FW in knock-out leaves. It appears remarkable that lowering the pH in the incubation medium did not provoke similar relative citrate changes in both plant lines (Fig. 1C).
Effect of Acidification on Photosynthetic Oxygen Evolution by Wild-Type or Knock-Out Protoplasts
Photosynthetic oxygen evolution is a complex process dependent upon a close interaction of a wide number of metabolic processes located in the chloroplasts and cytosol and optimal maintenance of enzyme activity (Walker, 1992). Therefore, the ability to release photosynthetically derived oxygen by protoplasts can be taken as a simple and reliable criterion for controlled cellular pH homeostasis.
To analyze whether knock-out mesophyll cells differ in their ability to cope with increased cytosolic acidification when compared with wild-type cells, we prepared protoplasts from both genotypes and incubated them in MES-buffered medium harboring, in addition, 0.1 mm benzoic acid. Benzoic acid is a weak acid (pK 4.2) able to pass the plasma membrane in the protonated form. After entering the cell, the proton is released and acidifies the cytosol (Lambert and Statford, 1998). This effect allows the immediate acidification of the cytosol that is required to study the effect of a pH shift on photosynthesis in protoplasts.
Protoplasts from wild-type plants and knock-out mutants exhibit at pH 7.2 nearly the same rate of oxygen evolution, namely, 67 μmol/mg chl h−1 and 63 μmol/mg chl h−1, respectively (Fig. 2). Both types of protoplasts showed a reduced rate of oxygen evolution in response to acidification of the incubation medium (Fig. 2). However, acidification inhibited photosynthesis of knock-out protoplasts significantly stronger than in protoplasts from wild-type plants (Fig. 2). For example, at pH 4.3, knock-out protoplasts produced only about 26% of oxygen released by wild-type cells, and at pH 4.1, knock-out protoplasts were unable to perform photosynthesis, whereas wild-type protoplasts still exhibited substantial net oxygen production (Fig. 2). These results clearly underscore that knock-out protoplasts exhibited an increased sensitivity to cytosolic acidification.
Figure 2.
Effect of lowering the pH on photosynthetic oxygen release by wild-type or knock-out protoplasts. Protoplasts isolated from both plant lines were incubated in MES-buffered incubation medium in the presence of 0.1 mm benzoic acid. Data are the mean ± se of four individual measurements.
Subcellular Localization of Carboxylic Acids in Arabidopsis Mesophyll Cells
As reported above, total levels of carboxylic acids in wild-type and knock-out leaf tissues differ significantly. In addition, the data presented reveal that acidification correlates with an increased consumption of dicarboxylic acids, but the data do not provide information on their subcellular distribution. Assuming that the vacuole accounts for about 80% of the cell volume, it must be suggested that either the AttDT::tDNA mutant exhibits an extremely high cytosolic malate concentration or that, despite the fact that the mutant is lacking the vacuolar malate transporter AttDT, malate is still partially localized within the vacuole.
To answer this question, we isolated Arabidopsis mesophyll protoplasts from wild-type or AttDT::tDNA plants and compared malate, fumarate, and citrate contents in both intact protoplasts and corresponding vacuoles. For better comparison of these metabolite data with the photosynthetic performance of corresponding plant lines (Fig. 2), we isolated protoplasts in the morning and subsequently enriched the vacuoles by a mild hypo-osmotic treatment and flotation of intact vacuoles on a Percoll gradient (for details, see Emmerlich et al., 2003; Frangne et al., 2002). The levels of organic acids are given per unit of the vacuolar marker enzyme α-mannosidase. Activities of this enzyme are known to exhibit only minimal fluctuations even under different growth conditions (E. Martinoia, unpublished data) and allow comparison, on one hand, between different plants and, on the other hand, between the vacuole and the intact protoplast (Boller and Kende, 1979).
As observed for entire leaf discs (Fig. 1), mesophyll protoplasts isolated from wild-type plants and AttDT::tDNA mutants contain more fumarate than malate (Fig. 3A, left). As in entire leaves, citrate content in isolated protoplasts is higher than malate content, and the total malate and fumarate levels in knock-out protoplasts are significantly lower than in corresponding wild-type protoplasts (Fig. 3A, left). Slight differences in the relative ratios between the individual carboxylic acids in leaf discs and protoplasts probably derive from the fact that the former have been incubated for 24 h in the dark, whereas the latter have been purified in the morning from intact leaves. Alternatively, this ratio could also be slightly different between mesophyll and epidermis cells.
Figure 3.
Vacuolar proportion of malate, fumarate, and citrate in wild types and AttDT::tDNA mutants. A, Carboxylic acid content in protoplasts (left) and carboxylic acid content in vacuoles (right). B, Vacuolar proportions of malate, fumarate, and citrate in relation to protoplast content. C, Cytosolic content of carboxylates based on α-mannosidase activity. Data are the mean ± se of three different experiments with four replicates each.
Interestingly, vacuoles prepared from homozygous AttDT knock-out plants contained much lower, but still considerable, amounts of malate and fumarate (Fig. 3A, right). Calculation of the percentage of vacuolar carboxylate revealed that wild-type plant vacuoles contained 56.8% ± 8.8% malate and 35.3% ± 4.6% fumarate (Fig. 3B), whereas AttDT::tDNA vacuoles contained 25.1% ± 6.0% of the cellular malate and 26.8% ± 3.2% of the cellular fumarate (Fig. 3B). It should be mentioned that malate and fumarate content measured in vacuoles from AttDT::tDNA mutants cannot be due to contamination with intact protoplasts or other organelles, which was less than 5% (data not shown).
In contrast to dicarboxylates, the vacuolar proportion of citrate was slightly increased in vacuoles isolated from AttDT::tDNA mutants, namely, 73.2% ± 8.6% and 80.3% ± 14.8% for wild-type and mutant plants, respectively (Fig. 3A, right, and B). However, considering the higher citrate content in AttDT::tDNA protoplasts (Fig. 3A, left), the vacuolar amount of citrate in mutants is about 1.6-fold that detected in wild-type plants (Fig. 3A, right).
Calculation of the cytosolic carboxylic acid concentrations, based on α-mannosidase activity, revealed that mutant plants not only exhibit decreased vacuolar malate and fumarate levels but also, surprisingly, decreased cytosolic concentrations of these dicarboxylates (Fig. 3C). In remarkable contrast to this, the increase in cellular citrate in knock-out lines is mainly due to the higher vacuolar concentration of citrate, whereas the cytosolic citrate concentrations are similar in both plant genotypes (Fig. 3C).
Respiratory Activities of Arabidopsis Wild-Type and AttDT Knock-Out Leaf Discs
In Figure 3, we show altered subcellular levels of the three main carboxylic acids in knock-out mutants. To reveal whether these alterations might correlate with changing respiratory activities in corresponding tissues, we prepared leaf discs 2 h after the end of the light period and analyzed the rates of CO2 release and oxygen consumption by Warburg manometry.
As shown, knock-out leaf discs released CO2 at a rate that was 33.6% (±5.8%) faster than observed on wild-type leaf discs (Fig. 4A). In combination with the measured rates of oxygen consumption (data not shown), we not only observed increased respiratory activity in knock-out tissues but also calculated an increased respiratory quotient. Respiratory quotients above 1 indicate partial use of carboxylic acids as substrates for mitochondrial ATP synthesis. Wild-type leaf discs exhibited a respiratory quotient of 1.13 (±0.03), whereas knock-out leaf discs exhibited a respiratory quotient of 1.26 (±0.03; Fig. 4B).
Figure 4.
Respiratory activity and respiratory quotient of wild-type and knock-out leaf tissue. Leaf discs from wild-type plants or knock-out lines were prepared from intact leaves 2 h after the end of the light period. CO2 release and oxygen consumption were measured in a Warburg device. A, CO2 release. B, Respiratory quotient (CO2/O2). Data are the mean ± se of four individual measurements.
Effect of Acidification on the Expression of the AttDT Gene
To gain insight into the putative interaction between pH control and the expression of the vacuolar dicarboxylate transporter gene AttDT, we prepared leaf discs from Arabidopsis at the end of the light period and incubated these in MES-buffered medium at various pH values for 24 h in the dark. Subsequently, total RNA was isolated and northern-blot analysis was conducted using radioactively labeled AttDT cDNA.
Incubation of leaf discs at pH 7 did not alter the level of AttDT mRNA when compared with the content of this mRNA species at the end of the light period (Fig. 5). By contrast, acidification of the medium below pH 6 strongly induced accumulation of AttDT mRNA in leaf discs, reaching a maximum at pH 4 (Fig. 5). These results clearly reveal a positive correlation between acidification of the incubation medium and accumulation of AttDT mRNA.
Figure 5.
Northern-blot analysis of AttDT mRNA accumulation in response to decreasing external pH values. Arabidopsis wild-type discs have been prepared from leaves at the end of the light period and incubated for 24 h (in the dark) in MES-buffered water. Subsequently, total RNA was purified and northern-blot analysis was conducted using radioactively labeled AttDT cDNA. Control samples were prepared from leaves at the end of the light period. A representative blot is shown; rRNA is presented as information on loading.
Transport of Carboxylates into Isolated Arabidopsis Vacuoles
In conclusion, the data above indicate that vacuoles of AttDT::tDNA plants still possess transport systems for both dicarboxylates and citrate (Fig. 3, A–C) and that mutants are still able to mobilize most of the endogenous dicarboxylic acids upon demand induced by acidification (Fig. 1, A and B). For dicarboxylates, it is still a matter of debate whether the transport activity observed in flux experiments corresponds to the malate channel activity observed by electrophysiological techniques (see introduction).
The high citrate levels in knock-out vacuoles were surprising, since kinetic data raised by use of radioactively labeled carboxylates indicated that both citrate and malate are transported through the same carrier system into the vacuole (Oleski et al., 1987; Rentsch and Martinoia, 1991). The malate channel described also exhibited a citrate permeability that was, however, much lower compared to malate. To explain our unexpected observations of increased citrate and reduced malate levels in knock-out vacuoles (Fig. 1, A and C), we performed (1) transport studies comparing citrate uptake in vacuoles from wild-type and mutant plants; and (2) electrophysiological experiments to reveal whether the vacuolar malate channel described is still present in AttDT::tDNA mutants.
Uptake experiments using 250 μm radioactively labeled malate or citrate showed, similar to our previous publication (Emmerlich et al., 2003), that vacuolar malate uptake capacity is strongly decreased in the AttDT::tDNA mutant (Fig. 6). In contrast to this, citrate transport into vacuoles from knock-out lines and wild-type plants occurred at similar rates (Fig. 6). These results clearly demonstrate that, despite the cross-inhibition of malate and citrate observed in flux experiments (Emmerlich et al., 2003), malate and citrate transport across the tonoplast from Arabidopsis mesophyll cells occurs through separate transporters.
Figure 6.
[14C]Malate and [14C]citrate uptake into vacuoles isolated from wild types and AttDT::tDNA mutants. [14C]Malate or [14C]citrate was given at a concentration of 250 μm. Uptake was allowed for 10 min and was linear within this time (data not shown). Data are the mean ± se of three independent experiments. For details, see also Emmerlich et al. (2003). WT, Wild type.
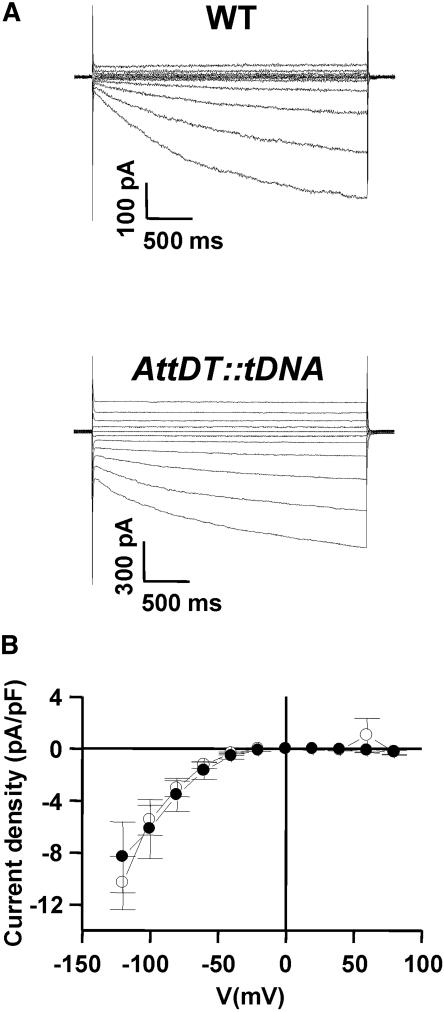
Robust whole-vacuole macroscopic currents could be detected by the patch-clamp technique on vacuoles isolated from Arabidopsis mesophyll cells in the presence of a buffer containing l-(−)-malic acid and 1,3-bis[tris(hydroxymethyl) methylamino] propane (BTP; Fig. 7A). These currents displayed a large time-dependent component that activated at negative transmembrane potentials (please note that the convention proposed by Bertl et al. [1992] is adopted) and a smaller time-independent component present at both positive and negative voltages. While the time-dependent component was very stable (up to 1 h or more), the time-independent current was maximal immediately after the break-in, displayed almost linear current-voltage relationships, and decreased progressively during the registration. In some cases, this component disappeared in a few minutes.
Figure 7.
Membrane currents across the tonoplast of mesophyll cells from wild types or AttDT::tDNA mutants recorded in a whole-vacuole configuration. A, Current response to voltage steps from the holding potential (0 mV) to a series of test voltages of 3-s duration in steps of 20 mV between −120 and +80 mV in vacuoles from wild-type plants and from plants lacking the malate transporter (AttDT::tDNA). B, Current-voltage relationship of the time-dependent component. Current density (pA/pF) was obtained by dividing the mean value of the current in the last 20 ms by the membrane capacitance measured through the compensation circuit of the patch-clamp amplifier (white circles, wild-type mean ± se, n = 4; black circles, AttDT::tDNA mean ± se, n = 3). Data were corrected for the instantaneous current and/or leakage. Pipette solution (inside vacuole) contained 16 mm BTP, 10 mm malate, 3 mm MgCl2, 1 mm CaCl2 (pH 7.5 with BTP) and the bath (cytosolic side) solution contained 160 mm BTP, 100 mm malate, 3 mm MgCl2, 1 mm CaCl2, 1 mm EGTA/2 mm TRIS (pH 7.4 with BTP). Osmolarity of the patch solution was adjusted to 440 mosmol kg−1 with sorbitol.
The labile time-independent component did not display any evident ionic selectivity and could not be studied in detail. Therefore, we concentrated our analysis on the time- and voltage-dependent current. The strongly rectifying properties of this component are summarized in the current density plot (pA/pF) obtained by dividing the mean value of the current in the last 20 ms by the tonoplast capacitance (Fig. 7B, white circles).
To gain more information on the nature of this current, we performed a tail analysis in asymmetric ionic solution (malate2−out/malate2−in = 100/10), which displayed a reversal potential of about +10 mV (Fig. 8, A and B), i.e. close to the theoretical potential of +23 mV expected for malate2− at these ionic activities (Hafke et al., 2003) and significantly different from the Nernst potential expected for BTPH+ (VNernst = −63 mV; Hafke et al., 2003). This observation suggests that the time-dependent current originates from inward-rectifying malate channels. Furthermore, after replacing malate by fumarate, we observed that this current was increased by a factor of about 2 and that addition of 10 mm citrate determined an almost complete inhibition of both the malate and the fumarate currents (data not shown).
Figure 8.
Determination of the reversal potential of the time-dependent current in vacuoles from wild-type plants. A, Top, Representative tail currents elicited by a tail pulse from −50 to +40 mV (at 10-mV increments) following a conditioning prepulse to −120 mV; bottom, the same tail currents as in the top image plotted at a larger time and current resolution. B, Value of the instantaneous tail currents is plotted as a function of the tail potential. The reversal potential (about +10 mV) is very close to the theoretical potential for malate and substantially different from the Nernst potential for BTPH+.
In sum, these observations correspond to the vacuolar malate channel characteristics described for Arabidopsis cell cultures and other plants and strongly indicate that the currents observed are mediated by malate moving from the cytoplasm into the vacuole. To investigate now whether the malate channel involved corresponds to the malate transporter AttDT, we additionally patched vacuoles isolated from homozygous AttDT::tDNA deletion mutants. Interestingly, for unknown reasons, mutant vacuoles appeared to be less stable in this particular experimental setup when compared to wild-type vacuoles. Nevertheless, mutant vacuoles exhibited very similar currents and current density characteristics of the time-dependent component, as observed on wild-type vacuoles (Fig. 7B, black circles). Furthermore, replacement of malate by fumarate results in increased currents as observed for wild-type vacuoles, and citrate, given at a concentration of 10 mm, completely inhibited malate and fumarate currents, as observed in wild-type plants (data not shown). These results show that AttDT::tDNA plants still possess the vacuolar malate channel and indicate that AttDT does not correspond to the vacuolar malate channel.
DISCUSSION
Recently, we identified AttDT as a vacuolar malate transporter and showed that Arabidopsis plants lacking in AttDT exhibited only a small residual malate transport activity and significantly reduced total malate levels in leaf tissues (Emmerlich et al., 2003). Since malate homeostasis is thought to be a crucial element for maintaining a functional primary metabolism in plants, we were surprised that homozygous AttDT::tDNA plants did not exhibit strong phenotypic alterations when compared to wild types.
It is known from experiments on other species that cellular carboxylate metabolism, especially the malate metabolism, is important for regulation of the cytosolic pH (Smith and Raven, 1979). To reveal whether Arabidopsis plants lacking the vacuolar malate transporter differ in their ability to cope with altered pH values, we first checked the involvement of carboxylates in pH homeostasis in this species. The observation that incubation of leaf discs at low pH led to an increased consumption of malate and fumarate, but not of citrate, in wild-type plants (Fig. 1, A–C) is in full accordance with our knowledge of regulation of cytosolic pH homeostasis. Acidification activates malate-degrading malic enzyme and inhibits PEP carboxylase (Davies, 1986). By these opposite effects, the enhanced malate degradation correlates with the net production of OH−, thus compensating for an increased proton concentration by acidification. The simultaneous decrease of malate and fumarate (Fig. 1, A and B) is probably due to the enzyme connecting both metabolites directly. The enzyme involved, fumarase, catalyzes a reaction close to thermodynamic equilibrium.
Interestingly, conversion of malate (and fumarate) to pyruvate not only provides the required OH− ions (Smith and Raven, 1979), but also supplies mitochondria with the substrate required for ATP synthesis. By doing so, mobilization of dicarboxylates in leaf cells also provides the energy for P-type ATPases involved in pumping protons against an existing pH gradient. Therefore, the increased energy consumption at the expense of dicarboxylates probably allows the challenged cells to arrest the cytosolic pH relatively close to the optimal value.
In contrast to malate and fumarate, degradation of citrate does not occur in low-pH-challenged leaf discs (Fig. 1C). Although the exact reasons for this discrepancy are difficult to analyze, we speculate that tight control of the metabolic flux within the citric acid cycle, and the involvement of several enzymes for conversion of citrate to malate (Lehninger et al., 1994), might prevent the efficient use of this metabolite for regulation of intracellular pH homeostasis.
As indicated above, the regulatory action of dicarboxylic acids for pH homeostasis ultimately depend upon the presence of these compounds in the cytosol and upon the concentrations of malate and fumarate in the vacuole, which can be used if required in the cytosol. Therefore, the observation that knock-out mesophyll protoplasts are more sensitive to acidification (Fig. 2) concurs with the observation that both the vacuolar as well as the cytosolic malate and fumarate concentrations were lowered in AttDT::tDNA mutants (Fig. 3A, right, and C). Obviously, a disturbed tonoplastic malate exchange as present in AttDT knock-out plants (Fig. 6; Emmerlich et al., 2003) provokes so far unknown regulatory reactions at the expense of cytosolic energy equivalents.
This assumption is further reinforced by the demonstration that radioactively labeled malate fed into mutant leaf discs entered the Krebs cycle much faster than in wild-type tissues (Emmerlich et al., 2003). Moreover, the observation that AttDT::tDNA leaf discs exhibited both an increased respiratory activity (Fig. 4A) and an increased respiratory quotient (Fig. 4B) clearly demonstrates the accelerated use of cytosolic carboxylic acids as an energy source in mutant tissue.
We showed that AttDT is able to catalyze malate import into isolated vacuoles (Fig. 6). In addition, the observation that malate feeding into leaf discs promotes expression of this AttDT (Emmerlich et al., 2003) further underscores this function. However, acidification also induces expression of the AttDT gene (Fig. 5) and correlates with a dramatic decrease of total cellular dicarbonic acid levels (Fig. 1, A and B). AttDT, therefore, may also play a role in malate export. Thus, our observation might explain the rapid cycling of malate between the cytosol and the vacuole observed in NMR studies (Gout et al., 1993). As a consequence of our data, one has to hypothesize that the malate transporter AttDT is less rectifying than the tonoplastic malate channel and that AttDT allows malate to be exported but also to enter the vacuole at lower membrane potential differences. An alternative explanation of the observed up-regulation of AttDT would be that, if flux into or out of the vacuole is increased, the whole vacuolar malate transfer machinery is induced. In any case, the strong decrease of malate and fumarate observed during the acidification experiments indicates AttDT is not the sole vacuolar malate exporter.
Compartmentation analysis revealed that vacuolar concentrations as well as vacuolar proportions of both dicarboxylates were lower in AttDT::tDNA mutants when compared to wild-type plants (Fig. 3A, right, and C). This indicates that both dicarboxylates cross the tonoplast using the same transporter. In addition, this observation may be explained by our finding that a malate transporter (Fig. 6) and a malate channel (Figs. 7 and 8) are present in the tonoplast and exhibit slightly different affinities for both dicarboxylates. However, it must be assumed that the channel does not exhibit sufficient activity to accumulate dicarboxylates at concentrations required for proper metabolic functioning and therefore cannot fully compensate for the absence of AttDT.
The evidence that two dicarboxylate translocating systems exist in the tonoplast may also explain the differences observed concerning the affinity and the relative permeability of the vacuolar transport system when dicarboxylate transport was investigated on different systems (Martinoia and Ratajczak, 1997). Vacuolar vesicles have a much higher surface-to-volume ratio than intact vacuoles. Consequently, due to the different surface-to-volume ratio and a continuous leak of protons and ions through the tonoplast, generation of the electromotive force is faster in vesicles and energy loss by leaking compounds is compensated more efficiently. It is therefore likely that transport experiments using vesicles favor the transfer of dicarboxylates through the channel, which is strongly rectifying and starts to open at membrane potentials higher than 40 to 50 mV. By contrast, intact vacuoles are less energized and the predominant dicarboxylate transport system is the transporter (Emmerlich et al., 2003; Fig. 6).
In contrast to malate and fumarate, citrate content was increased in mutant leaves (Fig. 3A). This result was unexpected since, from flux analysis using radiolabeled malate and citrate, it has been suggested that both carboxylates cross the tonoplast using the same transporter (Oleski et al., 1987; White and Smith, 1989; Rentsch and Martinoia, 1991). By contrast, patch-clamp experiments revealed that citrate permeability was much lower compared to malate permeability. The demonstration that citrate mainly accumulates in the vacuole (Fig. 3A, right), that knock-out vacuoles contain about 1.6-fold more citrate than wild-type vacuoles (Fig. 3A, right), and the flux analysis on isolated wild-type vacuoles (Fig. 6) strongly support our assumption that AttDT is not the main tonoplast citrate carrier. This conclusion does not necessarily contradict our previous observation that malate transport by AttDT is inhibited by citrate (Emmerlich et al., 2003); it just indicates that kinetic experiments with closely related compounds have to be interpreted carefully. It has to be assumed that vacuolar malate transporters can bind both carboxylates, but transport is restricted to the smaller class of compounds.
CONCLUSION
In total, our observations indicate that AttDT does not correspond to the vacuolar malate channel and explain why substantial amounts of malate and fumarate can be found in AttDT::tDNA vacuoles (Fig. 3B). In addition, we clearly revealed that AttDT is critical for pH homeostasis under the conditions tested. The two dicarboxylate transport systems (transporter and channel) in concert with the so far unknown citrate transporter allow the plant cell to regulate the storage and possibly the release of citrate and malate independently. Furthermore, regulation of energization across the tonoplast could also lead to a preferential import or export of malate or fumarate. This gives the plant an important flexibility to adapt the metabolism to particular situations.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Wild-type plants and AttDT::tDNA plants were grown under short-day conditions in a growth chamber as described previously (Emmerlich et al., 2003). All experiments have been conducted with the mutant line AttDT::tDNA1-1. We already showed that effects provoked by deletion of the vacuolar dicarboxylate transporter are specifically linked to the t-DNA insertion into the AttDT gene (Emmerlich et al., 2003). Plants used for patch-clamp studies have been grown in an 8-h light phase.
Isolation of Protoplasts and Quantification of Photosynthetic Oxygen Evolution
Protoplasts from wild-type and knock-out leaves were isolated as in Stitt et al. (1982). Photosynthesis was measured in a Clark oxygen electrode as in Herold et al. (1981). Incubation media were buffered with MES (20 mm) at various pH values; benzoic acid was present at a concentration of 0.1 mm.
Warburg Manometry
CO2 release and oxygen consumption were measured in a Warburg device (Braun-GmbH, Melsungen, Germany). Leaf discs were prepared 2 h after the end of the light period from intact plants and respiratory activity (CO2 release and oxygen consumption) was quantified for the next 2 h.
Extraction of Total RNA and Northern Blotting
RNA isolation and northern-blot analysis were performed using standard procedures (Sambrook et al., 1989) from the kits and materials described by Emmerlich et al. (2003).
Quantification of Carboxylates
Fifty to 100 mg of leaf material were rapidly frozen in liquid nitrogen. One milliliter of water (about 80°C) was added and the probes were heated for 8 min at 95°C in an Eppendorf thermo-incubator. After centrifugation (5 min, 12,000g), the supernatant was used to determine the carboxylates. Malate, fumarate, and citrate were quantified spectrophotometrically in a combined assay according to Passonneau and Lowry (1993). Recovery experiments revealed that about 95% of each carboxylate was recovered during the extraction.
α-Mannosidase Assay
α-Mannosidase was measured using p-nitrophenyl mannopyranoside as substrate (Boller and Kende, 1979).
Isolation of Vacuoles, Localization Studies, and [14C]Carboxylate Flux Experiments
For flux analysis and localization experiments, protoplasts and vacuoles were isolated as described (Frangne et al., 2002). For localization experiments, α-mannosidase and carboxylates were determined in the protoplast and vacuole fractions. Vacuolar proportions were measured assuming that α-mannosidase is completely localized in the vacuole (Boller and Kende, 1979).
For patch-clamp experiments, protoplasts were loaded on a cover slide. After the cells were attached to the bottom, vacuoles were released by incubating the protoplasts in 160 mm BTP, 100 mm malic acid, 3 mm MgCl2 (adjusted to pH 7.4 with BTP), and 8 mm EDTA. The osmolarity was adjusted to 440 mosmol kg−1 with sorbitol.
Patch Clamping and Data Acquisition
Solutions for patch clamping were prepared according to Hafke et al. (2003). Osmolarity of the bath and the pipette solution were adjusted to 440 mmol kg−1. Seal was accomplished at more than 2 to 5 GOhm and whole cell was made by zap application. Patch clamps were made using a HEKA EPC10 amplifier (HEKA Electronic, Lambrecht, Germany). Data acquisition and analysis were made using PULSE (HEKA Electronic). Patch pipettes were prepared from gas chromatography glass (Harvard Apparatus, Edenbridge, UK) by DMZ-Universal puller (Zeiss-Instruments GmbH, Jena, Germany). Vacuoles with 25- to 37-μm diameter were selected. Voltage refers to the cytoplasmic side of the vacuole while the vacuolar side is at the ground (Bertl et al., 1992).
Solution for Patch Clamping
Unless otherwise indicated, the pipette solution (inside vacuole) contained 16 mm BTP, 10 mm malate, 3 mm MgCl2, 1 mm CaCl2 (pH 7.5 with BTP) and the bath (cytosolic side) solution contained 160 mm BTP, 100 mm malate, 3 mm MgCl2, 1 mm CaCl2, 1 mm EGTA/2 mm TRIS (pH 7.4 with BTP). Osmolarity of the patch solution was adjusted to 440 mosmol kg−1 with sorbitol.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number AJ223445.
This work was supported by the Deutsche Forschungsgemeinschaft, Graduate Research School 845 (to the laboratory of H.E.N.); by the European Union project Novel Ion Channels in Plants (NICIP; EU HPRN–CT–00245; BBW 01.0598 to F.G. and E.M.); and by the Italy-Switzerland Consiglio Nazionale delle Ricerche/Swiss National Foundation bilateral cooperation program. E.M. was financially supported by the Swiss National Foundation.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.058453.
References
- Bertl AE, Blumwald R, Coronado R, Eisenberg G, Findlay G, Gradmann B, Hille K, Kohler K, Kolb HA, MacRobbie E, et al (1992) Electrical measurements on endomembranes. Science 258: 873–874 [DOI] [PubMed] [Google Scholar]
- Boller T, Kende H (1979) Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol 63: 1123–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerana R, Giromini L, Colombo R (1995) Malate-regulated channels permeable to anions in vacuoles of Arabidopsis thaliana. Aust J Plant Physiol 22: 115–121 [Google Scholar]
- Chang K, Roberts JKM (1991) Cytoplasmic malate levels in maize root tips during K+ ion uptake determined by 13C-NMR spectroscopy. Biochim Biophys Acta 1092: 29–34 [DOI] [PubMed] [Google Scholar]
- Cheffings CM, Pantoja O, Ashcroft FM, Smith JAC (1997) Malate transport and vacuolar ion channels in CAM plants. J Exp Biol 48: 623–631 [DOI] [PubMed] [Google Scholar]
- Chia DW, Yoder TJ, Reiter W-D, Gibson SI (2000) Fumaric acid: an overlooked form of fixed carbon in Arabidopsis and other plant species. Planta 211: 743–751 [DOI] [PubMed] [Google Scholar]
- Davies DD (1986) The fine control of cytosolic pH. Physiol Plant 67: 702–706 [Google Scholar]
- Emmerlich V, Linka N, Reinhold T, Hurth MA, Traub M, Martinoia E, Neuhaus HE (2003) The plant homolog to the human sodium/dicarboxylic cotransporter is the vacuolar malate carrier. Proc Natl Acad Sci USA 100: 11122–11126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangne N, Eggmann T, Koblischke C, Weissenböck G, Martinoia E, Klein M (2002) Flavone glucoside uptake into barley mesophyll and Arabidopsis cell culture vacuoles. Energization occurs by H+-antiport and ATP-binding cassette-type mechanisms. Plant Physiol 128: 726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R, Stitt MN, Heldt HW (1987) Subcellular metabolite levels in spinach leaves. Regulation of sucrose synthesis during diurnal alterations in photosynthesis. Plant Physiol 83: 339–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout E, Bligny R, Pascal N, Douce R (1993) 13C nuclear magnetic resonance studies of malate and citrate synthesis and compartmentation in higher plant cells. J Biol Chem 268: 3986–3992 [PubMed] [Google Scholar]
- Hafke JB, Hafke Y, Smith JAC, Lüttge U, Thiel G (2003) Vacuolar malate uptake is mediated by an anion-selective inward rectifier. Plant J 35: 116–128 [DOI] [PubMed] [Google Scholar]
- Herold A, Leegood RC, McNeil PH, Robinson SP (1981) Accumulation of maltose during photosynthesis in protoplasts isolated from spinach leaves treated with mannose. Plant Physiol 67: 85–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdjian A, Guern J (1989) Intracellular pH: measurement and importance in cell activity. Annu Rev Plant Physiol Plant Mol Biol 40: 271–303 [Google Scholar]
- Lambert BJ, Statford M (1998) Weak-acid preservatives: modelling microbial inhibition and response. J Appl Microbiol 86: 157–164 [DOI] [PubMed] [Google Scholar]
- Lehninger AL, Nelson DL, Cox MM (1994) Prinzipien der Biochemie. Spektrum Akademischer Verlag, Heidelberg
- Martinoia E, Flügge UI, Kaiser G, Heber U, Heldt HW (1985) Energy dependent uptake of malate into vacuoles isolated from barley mesophyll protoplasts. Biochim Biophys Acta 806: 311–319 [Google Scholar]
- Martinoia E, Ratajczak R (1997) Transport of organic molecules across the tonoplast. In A Leigh, D Sanders, eds, The Plant Vacuole, Advances in Botanical Research. Academic Press, San Diego, pp 365–400
- Martinoia E, Rentsch D (1994) Malate compartimentation—responses to a complex metabolism. Annu Rev Plant Physiol Plant Mol Biol 45: 447–467 [Google Scholar]
- Oleski N, Mahdavi P, Bennett AB (1987) Transport properties of the tomato fruit tonoplast. II. Citrate transport. Plant Physiol 84: 997–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoja O, Smith JAC (2002) Sensitivity of the plant vacuolar malate channel to pH, Ca2+ and anion-channel blockers. J Membr Biol 186: 31–42 [DOI] [PubMed] [Google Scholar]
- Passonneau LV, Lowry OH (1993) Enzymatic Analysis. A Practical Approach. Humana Kozak Press, Totowa, NJ
- Pei Z-M, Ward JM, Harper JF, Schroeder JI (1996) A novel chloride channel in Vicia faba guard cell vacuoles activated by the serine/threonine kinase, CDPK. EMBO J 15: 6564–6574 [PMC free article] [PubMed] [Google Scholar]
- Ratajczak R, Kemna I, Lüttge U (1994) Characteristics, partial purification and reconstitution of the vacuolar malate transporter of the CAM plant Kalanchoë daigremontiana Hamet et Perrier de la Bâthie. Planta 195: 226–236 [Google Scholar]
- Rentsch D, Martinoia E (1991) Citrate transport into barley mesophyll vacuoles—comparison with malate uptake activity. Planta 184: 532–537 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Smith FA, Raven JA (1979) Intracellular pH and its regulation. Annu Rev Plant Physiol 30: 289–311 [Google Scholar]
- Stitt M, McC Lilley R, Heldt HW (1982) Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol 70: 971–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D (1992) Energy, Plant, and Man. Packard Publishing, Chichester, UK
- White PJ, Smith JAC (1989) Proton and anion transport at the tonoplast in Crassulacean-acid-metabolism plants: specificity of the malate-influx system in Kalanchoë daigremontiana. Planta 179: 265–274 [DOI] [PubMed] [Google Scholar]