Abstract
Post-COVID conditions (PCCs) are common and have significant morbidity. Risk factors for PCC include advancing age, female sex, obesity, and diabetes mellitus. Little is known about treatment, inflammation, and PCC. Among 882 individuals with confirmed SARS-CoV-2 infection participating in a randomized trial of COVID-19 convalescent plasma (CCP) vs control plasma with available biospecimens and symptom data, the association between early CCP treatment, cytokine levels, and PCC was evaluated. Cytokine and chemokine levels were assessed at baseline, day 14, and day 90 using a multiplexed sandwich immunoassay (Meso Scale Discovery). Presence of any self-reported PCC symptoms was assessed at day 90. Associations between CCP treatment, cytokine levels, and PCC were examined using multivariate logistic regression models. One third of the 882 participants had day 90 PCC symptoms, with fatigue (14.5%) and anosmia (14.5%) being most common. Cytokine levels decreased from baseline to day 90. In a multivariable analysis, female sex (adjusted odds ratio [AOR] = 2.69 [1.93–3.81]), older age (AOR = 1.32 [1.17–1.50]), and elevated baseline levels of IL-6 (AOR = 1.59 [1.02–2.47]) were independently associated with development of PCC. Those who received early CCP treatment (≤5 days after symptom onset) compared to late CCP treatment had statistically significant lower odds of PCC.
IMPORTANCE
Approximately 20% of individuals infected with SARS-CoV-2 experienced long-term health effects, as defined PCC. However, it is unknown if there are any early biomarkers associated with PCC or whether early intervention treatments may decrease the risk of PCC. In a secondary analysis of a randomized clinical trial, this study demonstrates that among outpatients with SARS-CoV-2, increased IL-6 at time of infection is associated with increased odds of PCC. In addition, among individuals treated early, within 5 days of symptom onset, with COVID-19 convalescent plasma, there was a trend for decreased odds of PCC after adjusting for other demographic and clinical characteristics. Future treatment studies should be considered to evaluate the effect of early treatment and anti-IL-6 therapies on PCC development.
KEYWORDS: COVID-19, COVID-19 serotherapy, post-COVID condition (PCC), post-acute sequelae of COVID (PASC), interleukin-6, cytokines, chemokines
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 600 million people worldwide and more than 97 million people in the United States (1). Many survivors of coronavirus disease 2019 (COVID-19) report long-term health effects, including persistent symptoms, the development of new comorbidities, and exacerbation of underlying pre-existing conditions. The medical community has formally recognized the long-term health effects of COVID-19 by establishing a diagnosis of post-COVID conditions (PCCs). The World Health Organization (WHO) (2) and U.S. Centers for Disease Control and Prevention (CDC) (3) differ in the definition of the timeline of this diagnosis, but both require that patients have sustained health conditions after resolution of acute COVID-19. According to the WHO, the diagnosis is a history of COVID-19 at least 3 months prior who have had symptoms for at least 2 months that an alternative diagnosis cannot explain (2). The CDC defines PCC as symptoms persisting beyond 4 weeks after acute infection (4).
Although the true incidence of PCC is unknown due to differences in study populations and methodologies, its clinical impact is substantial. Recent data suggest that the prevalence is approximately 20%, with the most commonly reported symptoms being fatigue, shortness of breath, and cognitive dysfunction (5) with higher rates among those >65 years of age (6). Previous studies have demonstrated several host factors that are associated with the development of PCC, including the severity of acute COVID-19, female sex (7 – 9), advanced age (6, 10, 11), being unvaccinated (11, 12), and presence of pre-COVID comorbidities.
Early treatment for COVID-19 is recommended to prevent hospitalization and death. While a recent observational study has suggested decreased PCC with early treatment with nirmatrelvir (13), the relationship between early effective COVID therapy and the development of PCC has been unclear. In addition to demographic factors and comorbidities, inflammation appears to play a critical role in COVID-19 prognosis. Individuals with elevated levels of IL-6 and C-reactive protein are more likely to have more severe disease and an increased risk of hospitalization during the acute phase (14 – 18), particularly among males (19). Few studies have evaluated whether cytokines are associated with PCC (20 – 26), and the limited data demonstrate inconsistent results. Some studies have shown an association between PCC and IL-6 (20, 22), while others have not (24, 25). This is likely due to study limitations, including small sample sizes, lack of prospective monitoring of symptoms and sample collection, and inconsistent timing in the disease course in relation to cytokine evaluation.
To investigate these relationships, we analyzed prospectively collected data from participants enrolled in a randomized outpatient treatment trial of COVID-19 convalescent plasma (CCP) to identify factors associated with the development of PCC, including early treatment and biomarkers measured early in the course of disease.
MATERIALS AND METHODS
Participants
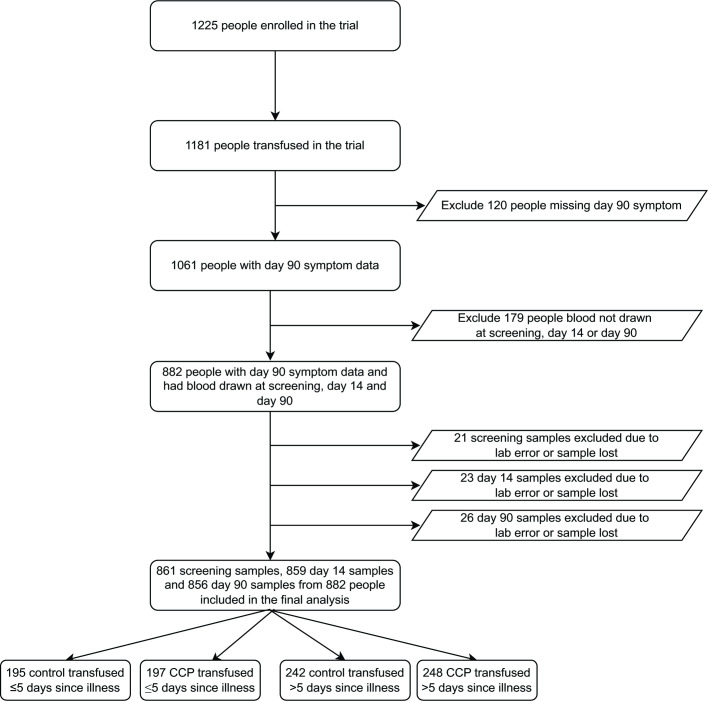
The Convalescent Plasma to Limit SARS-CoV-2 Associated Complications (CSSC-004) trial was a double-blind, multicenter, randomized, controlled trial investigating the use of CCP for the prevention of hospitalization among outpatients when compared to control plasma, as previously described (27). The trial recruited 1,225 symptomatic adult outpatients with acute SARS-CoV-2 infection from 3 June 2020 to 1 October 2021 at 23 sites. Of these, 1,181 participants received either CCP or control plasma transfusion. Trial participants were transfused within 9 days of symptom onset. Follow-up clinic visits were conducted at days 14, 28, and 90 post-transfusion. For this analysis, we restricted the study sample to those with blood drawn at screening, day 14, and day 90, and complete symptom data on day 90. Subsequently, we excluded 21 screening samples, 23 day 14 samples, and 26 day 90 samples, due to inadequate sample volume, laboratory error in cytokine measurement, or missing results leading to exclusion of the sample. These lost samples were from different individuals at each time point, so these individuals were included in the analyses at other time points, when data were available.
Outcomes
The primary outcome was PCC, which was defined as the presence of any self-reported symptom (cough, fatigue, shortness of breath, headache, neurologic changes, loss of taste, loss of smell, nausea, vomiting, diarrhea, runny/stuffy nose, myalgias, sore throat, chills, fever, or skin manifestations) at the 90-day visit. All CSSC-004 trial participants were asked about the presence and severity of symptoms at days 0, 1, 3, 5, 7, 10, 14, 28, and 90 through a structured, self-report form administered via phone (days 1, 3, 5, 7, and 10) and at in-person visits (days 0, 14, 28, and 90).
Variable definition
Age was modeled as a continuous variable in regressions. Sex was defined as biologic sex assigned at birth (male/female). Race (Black, American Indian/Alaskan Native [AI/AN], white, multiple, or other race] and ethnicity (Hispanic, non-Hispanic) were self-reported at enrollment. Body mass index (BMI) was calculated using height and weight at the screening visit. Calendar time was used as a surrogate for SARS-CoV-2 variant (transfusions prior to 15 June 2021 were classified as occurring during the pre-Alpha/Alpha wave, and those from 15 June 2021 to 1 October 2021 were classified as occurring during the Delta wave). Baseline comorbidities were collected through self-report and abstracted from medical records at enrollment. Treatment was categorized into receipt of early control plasma (≤5 days post-symptom onset), late receipt of control plasma (>5 days post-symptom onset), early receipt of CCP (≤5 days post-symptom onset), and late receipt of CCP (>5 days post-symptom onset)—a timing threshold consistent with the original trial analysis (27). Vaccination status was defined as fully vaccinated (i.e., more than 2 weeks from the second mRNA vaccine or 2 weeks from the first vector vaccine), partially vaccinated (i.e., receipt of one mRNA vaccine or less than 2 weeks from a vector vaccine), or unvaccinated.
Cytokine measurement
Blood was collected in ethylenediaminetetraacetic acid (EDTA) tubes, and plasma was separated and isolated within 2–8 hours after collection by centrifugation and stored at –80°C until cytokines were measured.
Custom multiplexed sandwich immunoassays using MULTI-ARRAY electrochemiluminescence detection technology (Meso Scale Discovery, Gaithersburg, MD, USA) were used for the quantitative evaluation of 21 different human cytokine and chemokine analytes in plasma samples following the manufacturer’s instructions. The cytokines and chemokines were selected based on previous SARS-CoV-2 studies (28 – 30). Analytes were considered “detectable” if both runs of each sample had a signal greater than the analyte- and plate-specific lower limit of detection (i.e., 2.5 standard deviations of the plate-specific blank). Analyte concentrations (pg/mL) from both runs of each analyte were averaged for analysis. Cytokine values below the lower limit of detection or above the upper limit of detection were estimated by the multiplex assay using extrapolation from the standard curve. Values above or below the fit curve range were reported missing by the assay.
Statistical analysis
Values that were not reported because they were above or below the fit curve were imputed using a single stochastic draw from the tail of a truncated log10-normal distribution fit to the detectable values of each cytokine. For those below fit curve range, values were randomly drawn from the extrapolated lower tails (from 0 to the minimum available value) of the distribution for each analyte. For those above fit curve range, values were randomly drawn from the extrapolated upper tails (from the maximum available value to 10 times the available maximum) of the distribution for each analyte. Cytokine values were log10 transformed, and the outliers (Q3 + 1.5 × interquartule range or Q1 − 1.5 × interquartule range) from each visit were excluded to avoid potential lab error.
Two-tailed χ2 tests were performed to compare the baseline characteristics between treatment arms and PCC groups. Fisher’s exact test was used to compare across race categories due to a small number of participants in some racial groups. Spaghetti plots were produced to evaluate the trajectory of each analyte from screening to day 14 to day 90. Wilcoxon rank sum tests were used to compare the cytokine levels at screening between non-PCC and PCC groups. Similar to our previous treatment analyses and consistent with previously established risk factors for PCC, logistic regressions adjusted for the baseline covariates including age (continuous), sex (male vs female), race (white vs non-white), BMI (<30 vs ≥30), vaccine status (partially or fully vs unvaccinated), and diabetes (no vs yes) were used to evaluate the association between each cytokine (log10 transformed values) and PCC. Benjamini-Hochberg correction with a false discovery rate of 0.05 was conducted to control for multiple comparisons across all the cytokines. Univariate and multivariate logistic regressions were modeled to estimate the potential association of baseline characteristics, IL-6, and CCP on the impact of PCC. We conducted post hoc separate analyses for IL-6 to evaluate its association with SARS-CoV-2 viral load and major symptoms. Missing data on covariates were handled using available case method to include all cases where the variable(s) of interested were observed for each model. All analyses were performed in R 4.2.1 (R Core Team, Vienna, Austria).
RESULTS
Study population
Of 1,181 transfused trial participants, 589 were randomized to receive control plasma and 592 were randomized to receive CCP, with 257 (43.4%) receiving CCP within 5 days of symptom onset (Fig. 1). A total of 1,061 participants returned for day 90 visits, of whom 882 had biorepository plasma samples and symptom data at baseline, day 14 and day 90. There were no significant differences in baseline characteristics, comorbidities, or COVID-19 vaccine status among those included in this study sampling compared to the entire clinical trial population (Table S1).
Fig 1.
Study population.
The median age of the 882 subjects was 43 years, with 299 (33.9%) ≥50 years. Five hundred six (57.4%) female and 116 Black (13.2%) participants were included. There were no differences by trial treatment group (Table 1) or between those with and without PCC symptom data (Tables S2 and S3). The geometric mean of SARS-CoV-2 viral load was similar comparing control to CCP, while different comparing early to late transfusion of plasma. Demographic factors were also similar among those included in this analysis compared to the entire trial population (Table S1).
TABLE 1.
Characteristics of the study population by treatment group a
| Characteristic | Overall, N = 882 | Control ≤5 days, n = 195 | CCP ≤5 days, n = 197 | Control >5 days, n = 242 | CCP >5 days, n = 248 |
|---|---|---|---|---|---|
| Age (%) | |||||
| 18–29 | 162 (18.4) | 38 (19.5) | 37 (18.8) | 37 (15.3) | 50 (20.2) |
| 30–49 | 421 (47.7) | 91 (46.7) | 102 (51.8) | 117 (48.3) | 111 (44.8) |
| 50–64 | 247 (28.0) | 55 (28.2) | 49 (24.9) | 75 (31.0) | 68 (27.4) |
| ≥65 | 52 (5.9) | 11 (5.6) | 9 (4.6) | 13 (5.4) | 19 (7.7) |
| Sex (%) | |||||
| Female | 506 (57.4) | 108 (55.4) | 107 (54.3) | 156 (64.5) | 135 (54.4) |
| Male | 376 (42.6) | 87 (44.6) | 90 (45.7) | 86 (35.5) | 113 (45.6) |
| Race (%) | |||||
| Asian | 28 (3.2) | 11 (5.6) | 4 (2.0) | 2 (0.8) | 11 (4.4) |
| Black | 116 (13.2) | 19 (9.7) | 26 (13.2) | 29 (12.0) | 42 (16.9) |
| American Indian/Alaska Native |
15 (1.7) | 4 (2.1) | 3 (1.5) | 3 (1.2) | 5 (2.0) |
| White | 706 (80.0) | 154 (79.0) | 159 (80.7) | 207 (85.5) | 186 (75.0) |
| Mixed/other | 17 (1.9) | 7 (3.6) | 5 (2.5) | 1 (0.4) | 4 (1.6) |
| Ethnicity (%) | |||||
| Hispanic/Latino | 109 (12.4) | 29 (14.9) | 25 (12.7) | 24 (9.9) | 31 (12.5) |
| Non-Hispanic/Latino | 773 (87.6) | 166 (85.1) | 172 (87.3) | 218 (90.1) | 217 (87.5) |
| BMI (%) | |||||
| <30 | 522 (59.2) | 109 (55.9) | 115 (58.4) | 141 (58.3) | 157 (63.3) |
| 30–34.9 | 179 (20.3) | 45 (23.1) | 41 (20.8) | 47 (19.4) | 46 (18.5) |
| ≥35 | 141 (16.0) | 32 (16.4) | 35 (17.8) | 40 (16.5) | 34 (13.7) |
| Missing | 40 (4.5) | 9 (4.6) | 6 (3.0) | 14 (5.8) | 11 (4.4) |
| Hypertension (%) | |||||
| No | 675 (76.5) | 145 (74.4) | 148 (75.1) | 195 (80.6) | 187 (75.4) |
| Yes | 207 (23.5) | 50 (25.6) | 49 (24.9) | 47 (19.4) | 61 (24.6) |
| Diabetes (%) | |||||
| No | 810 (91.8) | 182 (93.3) | 182 (92.4) | 224 (92.6) | 222 (89.5) |
| Yes | 72 (8.2) | 13 (6.7) | 15 (7.6) | 18 (7.4) | 26 (10.5) |
| Anxiety (%) | |||||
| No | 822 (93.2) | 179 (91.8) | 182 (92.4) | 231 (95.5) | 230 (92.7) |
| Yes | 60 (6.8) | 16 (8.2) | 15 (7.6) | 11 (4.5) | 18 (7.3) |
| Vaccine status (%) | |||||
| Unvaccinated | 688 (78.0) | 148 (75.9) | 154 (78.2) | 154 (78.2) | 192 (77.4) |
| Partially | 55 (6.2) | 12 (6.2) | 11 (5.6) | 11 (5.6) | 16 (6.5) |
| Fully | 139 (15.8) | 35 (17.9) | 32 (16.2) | 32 (16.2) | 40 (16.1) |
| Baseline C-reactive protein (%) |
|||||
| Normal (≤1.0 mg/dL) | 662 (75.1) | 146 (74.9) | 144 (73.1) | 180 (74.4) | 192 (77.4) |
| Abnormal (>1.0 mg/dL) | 216 (24.5) | 49 (25.1) | 53 (26.9) | 61 (25.2) | 53 (21.4) |
| Missing | 4 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 3 (1.2) |
| COVID-19 wave (%) | |||||
| Pre-Alpha/Alpha | 704 (79.8) | 152 (77.9) | 161 (81.7) | 195 (80.6) | 196 (79.0) |
| Delta | 178 (20.2) | 43 (22.1) | 36 (18.3) | 47 (19.4) | 52 (21.0) |
| SARS-CoV-2 viral load, geometric mean copies (95% CI) | 7,248 (5,909, 8,889) | 3,041 (2,197, 4,208) | 29,121 (18,489, 45,866) | 13,774 (8,665, 21,896) | 3,387 (2,404, 4,770) |
CCP, COVID-19 convalescent plasma. PCC, post COVID-19 conditions at day 90.
There were 590 (66.9%) individuals without PCC and 292 (33.1%) individuals with PCC at day 90 (Table 2). The most common reported symptoms were fatigue (14.5%), anosmia (14.5%), and ageusia (10.0%) (Table S4). Forty-eight participants (5.4%) had both fatigue and anosmia; 80 participants had one or the other, but not both.
TABLE 2.
Baseline characteristics of the study population by PCC a
| Characteristic | Overall, N = 882 | Non-PCC, n = 590 | PCC, n = 292 | P b value |
|---|---|---|---|---|
| Age (%) | ||||
| 18–29 | 162 (18.4) | 134 (22.7) | 28 (9.6) | <0.001 |
| 30–49 | 421 (47.7) | 274 (46.4) | 147 (50.3) | |
| 50–64 | 247 (28.0) | 156 (26.4) | 91 (31.2) | |
| ≥65 | 52 (5.9) | 26 (4.4) | 26 (8.9) | |
| Sex (%) | ||||
| Female | 506 (57.4) | 301 (51.0) | 205 (70.2) | <0.001 |
| Male | 376 (42.6) | 289 (49.0) | 87 (29.8) | |
| Race (%) | ||||
| Asian | 28 (3.2) | 24 (4.1) | 4 (1.4) | 0.061 |
| Black | 116 (13.2) | 74 (12.5) | 42 (14.4) | |
| American Indian/Alaska Native | 15 (1.7) | 11 (1.9) | 4 (1.4) | |
| White | 706 (80.0) | 466 (79.0) | 240 (82.2) | |
| Mixed/other | 17 (1.9) | 15 (2.5) | 2 (0.7) | |
| Ethnicity (%) | ||||
| Hispanic/Latino | 109 (12.4) | 66 (11.2) | 43 (14.7) | 0.163 |
| Non-Hispanic/Latino | 773 (87.6) | 524 (88.8) | 249 (85.3) | |
| BMI (%) | ||||
| <30 | 522 (59.2) | 365 (61.9) | 157 (53.8) | 0.042 |
| 30–34.9 | 179 (20.3) | 119 (20.2) | 60 (20.5) | |
| ≥35 | 141 (16.0) | 81 (13.7) | 60 (20.5) | |
| Missing | 40 (4.5) | 25 (4.2) | 15 (5.1) | |
| Hypertension (%) | ||||
| No | 675 (76.5) | 461 (78.1) | 214 (73.3) | 0.130 |
| Yes | 207 (23.5) | 129 (21.9) | 78 (26.7) | |
| Diabetes (%) | ||||
| No | 810 (91.8) | 550 (93.2) | 260 (89.0) | 0.045 |
| Yes | 72 (8.2) | 40 (6.8) | 32 (11.0) | |
| Anxiety (%) | ||||
| No | 822 (93.2) | 557 (94.4) | 265 (90.8) | 0.059 |
| Yes | 60 (6.8) | 33 (5.6) | 27 (9.2) | |
| Vaccine status (%) | ||||
| Unvaccinated | 688 (78.0) | 454 (76.9) | 234 (80.1) | 0.488 |
| Partially | 55 (6.2) | 37 (6.3) | 18 (6.2) | |
| Fully | 139 (15.8) | 99 (16.8) | 40 (13.7) | |
| Baseline C-reactive protein (%) | ||||
| Normal (≤1.0 mg/dL) | 662 (75.1) | 452 (76.6) | 210 (71.9) | 0.086 |
| Abnormal (>1.0 mg/dL) | 216 (24.5) | 137 (23.2) | 79 (27.1) | |
| Missing | 4 (0.5) | 1 (0.2) | 3 (1.0) | |
| Treatment group (%) | ||||
| Control ≤5 days since symptom onset | 195 (22.1) | 131 (22.2) | 64 (21.9) | 0.244 |
| CCP ≤5 days since symptom onset | 197 (22.3) | 143 (24.2) | 54 (18.5) | |
| Control >5 days since symptom onset | 242 (27.4) | 156 (26.4) | 86 (29.5) | |
| CCP >5 days since symptom onset | 248 (28.1) | 160 (27.1) | 88 (30.1) | |
| COVID-19 wave (%) | ||||
| Pre-Alpha/Alpha | 704 (79.8) | 462 (78.3) | 242 (82.9) | 0.133 |
| Delta | 178 (20.2) | 128 (21.7) | 50 (17.1) | |
| SARS-CoV-2 viral load, geometric mean copies (95% CI) | 7,248 (5,909, 8,889) | 7,208 (5,613, 9,256) | 7,329 (5,136, 10,457) | 0.943 |
PCC, post-COVID-19 condition.
P values for all variables except race and SARS-CoV-2 viral load were calculated using two-tail χ2 test. Two-tail Fisher’s exact test was used for race due to small cell numbers, and Wilcoxon rank sum test was used for the continuous variable SARS-CoV-2 viral load. P values <0.05 are in bold.
Soluble markers of inflammation
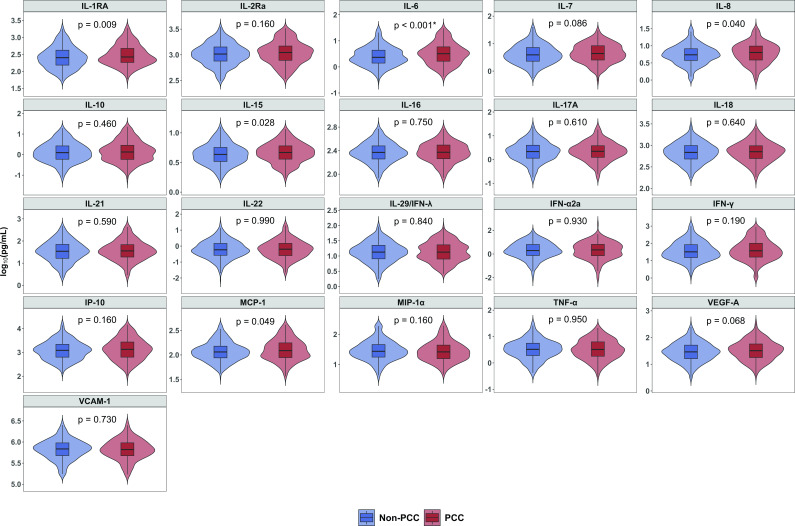
Levels of most cytokines decreased over time from baseline screening to day 90 (Fig. 2; Fig. S1). Of the 21 cytokines evaluated, IL-1RA, IL-6, IL-8, IL-15, and MCP-1 were elevated at baseline among those with PCC compared to participants who did not develop PCC (Fig. 3; Fig. S2). Elevated levels of IL-6 were significantly associated with PCC after correcting for multiple comparisons. In a multivariable model adjusting for age, sex, race, BMI, diabetes, vaccine status, and plasma transfused, an elevated level of IL-6 at baseline was associated with the presence of PCC (adjusted odds ratio [AOR] = 1.59, 95% CI = 1.02–2.47) (Table S5).
Fig 2.
Trajectory of the cytokines during study period. Abbreviations: PCC, post-COVID-19 condition; SCR, screening visit; D14, day 14 visit; D90, day 90 visit. Note: each dot represents a sample, and each line represents a person.
Fig 3.
Comparison of cytokines at screening between study participants with PCC and without PCC. PCC, post-COVID-19 condition. Note: P values were determined by Wilcoxon rank sum test. *IL-6 was significantly different after adjusting multiple comparison using Benjamini-Hochberg correction with a false discovery rate of 0.05.
Baseline factors associated with PCC
In multivariable analysis, (N = 882) older age, female sex, and baseline elevated IL-6 were associated with PCC (Table 3). There was no difference in the effect of early vs late control plasma or late CCP on the odds of PCC; however, there was a trend for early treatment with CCP to have the lowest odds of PCC (AOR = 0.75 [0.46, 1.23]) compared to early administration of control plasma. (Table 3). Similarly, when evaluating only those who received CCP, there was a statistically significant decreased odds of PCC in those who were treated within 5 days of symptom onset (AOR = 0.60 [0.38, 0.95]) compared to those who received late CCP treatment (Table 4). There was no statistically significant interaction between CCP treatment and IL-6. Similar trends, although not significant, were seen among the full trial population seen at day 90 (N = 1,061) (Tables S6A and S6B).
TABLE 3.
Logistic regression model assessing demographic and clinical factors and PCC among the study population
| Characteristic | OR (95% CI), N = 882 | |||
|---|---|---|---|---|
| Univariate | Model 1 | Model 2 | ||
| Age, scaled to 10 years | 1.31 (1.18, 1.46) | 1.35 (1.20, 1.51) | 1.32 (1.17, 1.50) | |
| Sex | ||||
| Male | Ref. | Ref. | Ref. | |
| Female | 2.26 (1.68, 3.06) | 2.38 (1.74, 3.28) | 2.69 (1.93, 3.81) | |
| Race | ||||
| White | Ref. | Ref. | Ref. | |
| Non-white | 0.84 (0.58, 1.20) | 0.81 (0.55, 1.20) | 0.85 (0.56, 1.28) | |
| BMI b | ||||
| <30 | Ref. | Ref. | Ref. | |
| ≥30 | 1.39 (1.04, 1.87) | 1.21 (0.88, 1.66) | 1.19 (0.85, 1.67) | |
| Vaccine status | ||||
| None | Ref. | Ref. | Ref. | |
| Partially or fully | 0.83 (0.58, 1.16) | 0.87 (0.60, 1.25) | 0.87 (0.59, 1.26) | |
| Diabetes | ||||
| No | Ref. | Ref. | Ref. | |
| Yes | 1.69 (1.03, 2.75) | 1.24 (0.72, 2.14) | 1.23 (0.69, 2.21) | |
| Plasma transfused | ||||
| Control ≤5 days since symptom onset | Ref. | Ref. | Ref. | |
| CCP ≤5 days since symptom onset | 0.77 (0.50, 1.19) | 0.85 (0.53, 1.34) | 0.75 (0.46, 1.23) | |
| Control >5 days since symptom onset | 1.13 (0.76, 1.68) | 1.08 (0.70, 1.67) | 1.05 (0.66, 1.67) | |
| CCP >5 days since symptom onset | 1.13 (0.76, 1.68) | 1.30 (0.85, 1.99) | 1.21 (0.77, 1.92) | |
| Log10 IL-6, pg/mL | – | – | 1.59 (1.02, 2.47) | |
P values <0.05 are highlighted in bold. Model 1 includes age, sex, race, BMI, vaccine status, diabetes, and the time between CCP transfusion and illness onset. Model 2 additionally includes log10 IL-6.
BMI, body mass index; CCP, COVID-19 convalescent plasma; PCC, post-COVID-19 condition at day 90; Ref., reference group; –, not applicable.
TABLE 4.
Logistic regression model assessing demographic and clinical factors and PCC among the people transfused with CCP a
| Characteristic | OR (95% CI), n = 445 | |||
|---|---|---|---|---|
| Univariate | Model 1 | Model 2 | ||
| Age, scaled to 10 yr | 1.30 (1.13, 1.51) b | 1.36 (1.16, 1.60) | 1.33 (1.12, 1.58) | |
| Sex | ||||
| Male | Ref. | Ref. | Ref. | |
| Female | 1.96 (1.30, 2.98) | 2.32 (1.49, 3.66) | 2.54 (1.59, 4.14) | |
| Race | ||||
| White | Ref. | Ref. | Ref. | |
| Non-white | 0.82 (0.49, 1.33) | 0.78 (0.45, 1.31) | 0.72 (0.41, 1.24) | |
| BMI | ||||
| <30 | Ref. | Ref. | Ref. | |
| ≥30 | 1.49 (0.98, 2.25) | 1.45 (0.92, 2.26) | 1.30 (0.81, 2.09) | |
| Vaccine status | ||||
| None | Ref. | Ref. | Ref. | |
| Partially or fully | 0.71 (0.42, 1.15) | 0.69 (0.40, 1.16) | 0.71 (0.40, 1.20) | |
| Diabetes | ||||
| No | Ref. | Ref. | Ref. | |
| Yes | 1.41 (0.72, 2.71) | 0.95 (0.44, 1.99) | 1.10 (0.49, 2.44) | |
| CCP transfused | ||||
| >5 days since symptom onset | Ref. | Ref. | Ref. | |
| ≤5 days since symptom onset | 0.69 (0.46, 1.03) | 0.64 (0.41, 0.98) | 0.60 (0.38, 0.95) | |
| Log10 IL-6, pg/mL | – | – | 1.91 (1.03, 3.56) | |
BMI, body mass index; CCP, COVID-19 convalescent plasma; PCC, post-COVID-19 condition at day 90; Ref., reference group; –, not applicable.
P values <0.05 are highlighted in bold. Model 1 includes age, sex, race, BMI, vaccine status, diabetes, and the time between CCP transfusion and illness onset. Model 2 additionally includes log10 IL-6.
DISCUSSION
This study is among the first to show elevation of IL-6 at infection was associated with PCC and that early treatment with CCP for COVID-19 trended toward a lower odds of PCC. Notably, greater levels of IL-6 at baseline were associated with the presence of persistent symptoms at day 90, while early treatment with CCP was associated with lower odds of PCC. Finally, consistent with other studies, advanced age (10), diabetes mellitus, and higher BMI were associated with PCC (6, 11, 31).
Approximately one third of participants with outpatient SARS-CoV-2 infection diagnosed early in the pandemic continued to have symptoms 90 days after infection. The most common symptoms at day 90 were less likely to be respiratory, and more consistent with neurologic injury including fatigue, loss of smell, and loss of taste (12). A recent study of EHR data from the VA showed that treatment with nirmatrelvir decreased the risk of PCC (13); while another study of online participants demonstrated no benefit to nirmatrelvir with either the prevalence or severity of PCC (32). Studies of remdesivir have also been had conflicting data as the randomized SOLIDARITY trial did not show a benefit of remdesivir against PCC (33), while an observational study in Italy demonstrated a positive effect of remdesivir on PCC (34). CCP has been shown to reduce hospitalizations and prevent morbidity and mortality when provided early and at high-titer (35 – 42). In this early treatment trial, CCP reduced COVID-19-related hospitalizations by 54% and demonstrated a trend toward lower odds of PCC overall, and even lower PCC odds among those treated with CCP within 5 days (27). Given that CCP functions primarily as an antiviral therapy, this observation is consistent with results of small molecule antivirals and suggests that antibody therapies could reduce PCC incidence when instituted early in the course of COVID-19. The observation that early antibody administration was associated with reduced likelihood of PCC is consistent with the finding that vaccines that elicit antibodies to SARS-CoV-2 also reduce PCC (43). With larger sample size, it may be possible to identify patient populations or clinical phenotypes of PCC that may benefit from early treatment to prevent PCC. Additional studies are needed to better understand the impact of early treatment with monoclonals, CCP, remdesivir, and other oral antivirals among those with outpatient SARS-CoV-2 infection.
IL-6 plays a critical role during infections; this proinflammatory cytokine leads to an acute-phase response, B-cell maturation and also T-cell expansion (44). Notably, elevated levels of IL-6 at screening were associated with the development of PCC after adjusting for demographic factors and comorbidities. While cytokine levels decreased from baseline screening to day 90, they remained elevated during the COVID-19 recovery phase, which likely contributes to PCC.
This study had several notable strengths. First, the study population was large, geographically and demographically diverse, and entirely outpatient (27). Second, biospecimens and data on symptoms were actively ascertained in real time from participants through in-person visits early in the onset of illness and on day 90 after transfusion. In addition to the presence or absence of symptoms over time, we collected samples at each visit to allow for cytokine analysis. Finally, study data on symptoms were collected uniformly across all sites, including pre-Omicron COVID-19 variants, thus increasing the generalizability of our findings.
This study has several important limitations. First, participants were asked about 17 symptoms identified early in the trial as important symptoms of COVID-19. During the pandemic, knowledge of PCC expanded to include more neurocognitive symptoms; however, we could not conduct additional validated measurements of sleep or neurologic function as many participants had completed follow-up. Also, while day 90 follow-up was excellent (90%), it was not complete. However, those who did not complete the study did not differ demographically from those who did. Also, samples were not available from all patients at all visits. Sometimes during the sample collection for each patient, insufficient samples were collected due to participant specific factors (patient became pre-syncopal or vein collapsed), or rarely a loss of specimen between the collection site and the laboratory. Any loss of specimen should be considered at random. In addition, COVID-19 has disproportionately affected the elderly and people of color. Within our sample, only a small proportion were age >65 years or of minority ethnicity or race, though the proportions were similar to those in the general American population. Fatigue was a common complaint during the pandemic. Our study did not have an uninfected control group to ascertain if our reported rate of fatigue was different than in the general population, and fatigue was not measured using a standardized reporting instrument for quantification. Our study was also limited by sample size in trying to understand the impact of early treatment on the various clinical phenotypes associated with PCC. We could not further delineate PCC phenotype beyond the presence or absence of symptoms at a fixed time. More inclusive phenotyping including exacerbation of a chronic condition or development of a new comorbid condition during recovery may yield different results. Also, this study was done early in the pandemic when relatively few participants were fully vaccinated. Results may differ among participants who have immunity from prior infection or vaccination.
In summary, this study demonstrated high rates of PCC symptoms at day 90, particularly among those with higher baseline levels of IL-6 at the time of infection or who did not receive early CCP treatment. Future studies might examine the impact of anti-IL6 agents and the development of PCC among outpatients. Despite increasing vaccination uptake, PCC risk does not disappear, and IL-6 modulation may be a possible therapeutic intervention to reduce the burden of long-term symptoms among those with SARS-CoV-2 infection.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the plasma donors and study participants who generously gave of their time and biological specimens to the CSSC-001 and CSSC-004 trials and the passionate study personnel who facilitated these studies.
This study was funded principally by the US Department of Defense’s (DOD) Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense (JPEO-CBRND), in collaboration with the Defense Health Agency (DHA) (contract number: W911QY2090012) with additional support from Bloomberg Philanthropies, State of Maryland, the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID) 3R01AI152078-01S1 and 3R01AI120938-05S1, National Institute on Drug Abuse (NIDA) F31DA054849, NIH National Center for Advancing Translational Sciences (NCATS) U24TR001609, Division of Intramural Research NIAID NIH, Mental Wellness Foundation, Moriah Fund, Octapharma, HealthNetwork Foundation, and the Shear Family Foundation. The study sponsors did not contribute to the study design, the collection, analysis, and interpretation of data, or the decision to submit this manuscript for publication.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Kelly Gebo- Consults for the Aspen Institute, Teach for America, served as a non-paid member of scientific advisory board for Pfizer and writes COVID management guidelines for UpToDate which are out of scope of paper. Sonya L. Heath- Nothing to disclose. Yuriko Fukuta- Nothing to disclose. Xianming Zhu- Nothing to disclose. Sheriza Baksh- Nothing to disclose. Alison G. Abraham- Consultant for Implementation Group Inc, Hirslanden Klinik, Zurich CH, & ELSEVIER. Feben Habtehyimer- Nothing to disclose. David Shade- Nothing to disclose. Jessica Ruff- Nothing to disclose. Malathi Ram- Nothing to disclose. Oliver Laeyendecker- Nothing to disclose. Reinaldo E. Fernandez- Nothing to disclose. Eshan U. Patel- Nothing to disclose. Owen R. Baker- Nothing to disclose. Shmuel Shoham- Served on a CCP guideline panel. Edward R. Cachay- has received unrestricted research grants from Gilead and Merck paid to the Regents of the University of California. He also participated in an advisory board to Theratechnologies for an unrelated topic. Judith S. Currier- Consulted for Merck and Company in 2021, currently not on any guidelines panel. Jonathan M. Gerber- Nothing to disclose. Thomas J. Gniadek- Currently employed by Fenwal, Inc., a Fresenius Kabi Company. Barry Meisenberg- Nothing to disclose. Donald N. Forthal- nothing to disclose. Laura L. Hammitt- research funding to my institution from AstraZeneca, CDC, Merck, NIH, and Pfizer. Moises A. Huaman reports contracts from Gilead Sciences Inc, Insmed Inc, AN2 Therapeutics, Inc to the University of Cincinnati, outside the submitted work. Adam Levine- Nothing to disclose. Giselle S. Mosnaim- Received research grant support from Teva, Alk-Abello, and Genentech and currently receives research grant support from Novartis, GlaxoSmithKline, and Sanofi-Regeneron. Serves as Immediate Past President of the American Academy of Allergy Asthma and Immunology and Co-Chair of the Continuous Assessment Program Examination for the American Board of Allergy and Immunology Bela Patel- Part of COVID trials and PAH trials, no disclosures relevant to CP. James H. Paxton- Research funding from MindRhythm, Inc. Jay S. Raval- Consultant and Advisor with Sanofi Genzyme; Board of Directors Member with the American Society for Apheresis, no overlap with CP. Catherine G. Sutcliffe- Nothing to disclose. Shweta Anjan- Nothing to disclose. Seble Kassaye- Helped to produce educational materials related to HIV with Integritas Communications, LLC and Vindico Medical Education, LLC. Janis E. Blair- Nothing to disclose. Karen Lane- nothing to disclose. Nichol A. McBee- Nothing to disclose. Amy L. Gawad- Nothing to disclose. Piyali Das- Nothing to disclose. Sabra L. Klein- Nothing to disclose. Andrew Pekosz- Nothing to disclose. Arturo Casadevall- Serves on the scientific advisory board of SAB Therapeutics. Evan M. Bloch- reports personal fees and non-financial support from Terumo BCT, Abbott Laboratories, Tegus and UptoDate, outside of the submitted work. E.M.B. is a member of the United States Food and Drug Administration (FDA) Blood Products Advisory Committee. Served on a CCP guideline panel Daniel Hanley- reports personal fees from Neurelis, Neurotrope, and medicolegal consulting. Aaron A.R. Tobian- Served on a CCP guideline panel. David J. Sullivan- Founder and Board member with stock options (macrolide for malaria). D.J.S. reports AliquantumRx, Hemex Health malaria diagnostics consulting and royalties for malaria diagnostic test control standards to Alere- all outside of submitted work.
Contributor Information
Kelly A. Gebo, Email: kgebo@jhmi.edu.
Aaron A. R. Tobian, Email: atobian1@jhmi.edu.
Suresh Mahalingam, Griffith University-Gold Coast Campus, Gold Coast, Australia .
ETHICS APPROVAL
All study activities were approved by the Johns Hopkins University single Institutional Review Board, the Navajo Nation Human Research Review Board, the Indian Health Service National Institutional Review Board, and the Human Research Protection Office of the United States Department of Defense. All study activities followed the Declaration of Helsinki, the Good Clinical Practice guidelines of the International Conference on Harmonization, and all applicable regulatory requirements. Written informed consent was obtained from all study participants.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.00618-23.
Supplemental tables and figures.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Centers for Disease Control and Prevention . COVID data tracker. Available from: https://covid.cdc.gov/covid-data-tracker/#datatracker-home. Accessed 11 March 2023
- 2. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV, WHO Clinical Case Definition Working Group on Post-COVID-19 Condition . 2022. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . Long COVID or post-COVID conditions. Available from: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects. Accessed 11 March 2023
- 4. Centers for Disease Control and Prevention . Post-COVID conditions: Overview for Healthcare providers. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html. Accessed 11 March 2023
- 5. Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, Lekoubou A, Oh JS, Ericson JE, Ssentongo P, Chinchilli VM. 2021. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open 4:e2128568. doi: 10.1001/jamanetworkopen.2021.28568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention . 2020. Post–COVID conditions among adult COVID-19 survivors aged 18–64 and ≥65 years — United States. Available from: https://www.cdc.gov/mmwr/volumes/71/wr/mm7121e1.htm. Retrieved 11 Mar 2023.
- 7. Tenforde MW, Devine OJ, Reese HE, Silk BJ, Iuliano AD, Threlkel R, Vu QM, Plumb ID, Cadwell BL, Rose C, Steele MK, Briggs-Hagen M, Ayoubkhani D, Pawelek P, Nafilyan V, Saydah SH, Bertolli J. 2023. Point prevalence estimates of activity-limiting long-term symptoms among United States adults ≥1 month after reported severe acute respiratory syndrome coronavirus 2 infection, 1 November 2021. J Infect Dis 227:855–863. doi: 10.1093/infdis/jiac281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang X, Wang F, Shen Y, Zhang X, Cen Y, Wang B, Zhao S, Zhou Y, Hu B, Wang M, Liu Y, Miao H, Jones P, Ma X, He Y, Cao G, Cheng L, Li L. 2021. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open 4:e2127403. doi: 10.1001/jamanetworkopen.2021.27403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sneller MC, Liang CJ, Marques AR, Chung JY, Shanbhag SM, Fontana JR, Raza H, Okeke O, Dewar RL, Higgins BP, Tolstenko K, Kwan RW, Gittens KR, Seamon CA, McCormack G, Shaw JS, Okpali GM, Law M, Trihemasava K, Kennedy BD, Shi V, Justement JS, Buckner CM, Blazkova J, Moir S, Chun T-W, Lane HC. 2022. A longitudinal study of COVID-19 sequelae and immunity: baseline findings. Ann Intern Med 175:969–979. doi: 10.7326/M21-4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ayoubkhani D, Khunti K, Nafilyan V, Maddox T, Humberstone B, Diamond I, Banerjee A. 2021. Post-covid syndrome in individuals admitted to hospital with COVID-19: retrospective cohort study. BMJ 372:693. doi: 10.1136/bmj.n693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Azzolini E, Levi R, Sarti R, Pozzi C, Mollura M, Mantovani A, Rescigno M. 2022. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA 328:676–678. doi: 10.1001/jama.2022.11691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ayoubkhani D, Bermingham C, Pouwels KB, Glickman M, Nafilyan V, Zaccardi F, Khunti K, Alwan NA, Walker AS. 2022. Trajectory of long Covid symptoms after COVID-19 vaccination: community based cohort study. BMJ 377:e069676. doi: 10.1136/bmj-2021-069676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xie Y, Choi T, Al-Aly Z. 2022. Nirmatrelvir and the Risk of Post-Acute Sequelae of COVID-19. Infectious Diseases (except HIV/AIDS). doi: 10.1101/2022.11.03.22281783 [DOI]
- 14. Del Valle DM, Kim-Schulze S, Huang H-H, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU, Xie H, Patel M, Tuballes K, Van Oekelen O, Rahman A, Kovatch P, Aberg JA, Schadt E, Jagannath S, Mazumdar M, Charney AW, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, Gnjatic S. 2020. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 26:1636–1643. doi: 10.1038/s41591-020-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lavillegrand J-R, Garnier M, Spaeth A, Mario N, Hariri G, Pilon A, Berti E, Fieux F, Thietart S, Urbina T, Turpin M, Darrivere L, Fartoukh M, Verdonk F, Dumas G, Tedgui A, Guidet B, Maury E, Chantran Y, Voiriot G, Ait-Oufella H. 2021. Elevated plasma IL-6 and CRP levels are associated with adverse clinical outcomes and death in critically ill SARS-CoV-2 patients: Inflammatory response of SARS-CoV-2 patients. Ann. Intensive Care 11:9. doi: 10.1186/s13613-020-00798-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharifpour M, Rangaraju S, Liu M, Alabyad D, Nahab FB, Creel-Bulos CM, Jabaley CS, Emory COVID-19 Quality & Clinical Research Collaborative . 2020. C-reactive protein as a prognostic indicator in hospitalized patients with COVID-19. PLoS One 15:e0242400. doi: 10.1371/journal.pone.0242400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L, Muñoz-Ruiz M, McKenzie DR, Hayday TS, Francos-Quijorna I, Kamdar S, Joseph M, Davies D, Davis R, Jennings A, Zlatareva I, Vantourout P, Wu Y, Sofra V, Cano F, Greco M, Theodoridis E, Freedman JD, Gee S, Chan JNE, Ryan S, Bugallo-Blanco E, Peterson P, Kisand K, Haljasmägi L, Chadli L, Moingeon P, Martinez L, Merrick B, Bisnauthsing K, Brooks K, Ibrahim MAA, Mason J, Lopez Gomez F, Babalola K, Abdul-Jawad S, Cason J, Mant C, Seow J, Graham C, Doores KJ, Di Rosa F, Edgeworth J, Shankar-Hari M, Hayday AC. 2020. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med 26:1623–1635. doi: 10.1038/s41591-020-01186-5 [DOI] [PubMed] [Google Scholar]
- 18. Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, Zhang P, Liu X, Gao G, Liu F, Jiang Y, Cheng X, Zhu C, Xia Y. 2020. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerging Microbes & Infections 9:1123–1130. doi: 10.1080/22221751.2020.1770129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scully EP, Schumock G, Fu M, Massaccesi G, Muschelli J, Betz J, Klein EY, West NE, Robinson M, Garibaldi BT, Bandeen-Roche K, Zeger S, Klein SL, Gupta A. 2021. Sex and gender differences in testing, hospital admission, clinical presentation, and drivers of severe outcomes from COVID-19. Open Forum Infect Dis 8:fab448. doi: 10.1093/ofid/ofab448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peluso MJ, Lu S, Tang AF, Durstenfeld MS, Ho H, Goldberg SA, Forman CA, Munter SE, Hoh R, Tai V, Chenna A, Yee BC, Winslow JW, Petropoulos CJ, Greenhouse B, Hunt PW, Hsue PY, Martin JN, Daniel Kelly J, Glidden DV, Deeks SG, Henrich TJ. 2021. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome Coronavirus 2 infection. J Infect Dis 224:1839–1848. doi: 10.1093/infdis/jiab490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peluso MJ, Deitchman AN, Torres L, Iyer NS, Munter SE, Nixon CC, Donatelli J, Thanh C, Takahashi S, Hakim J, Turcios K, Janson O, Hoh R, Tai V, Hernandez Y, Fehrman EA, Spinelli MA, Gandhi M, Trinh L, Wrin T, Petropoulos CJ, Aweeka FT, Rodriguez-Barraquer I, Kelly JD, Martin JN, Deeks SG, Greenhouse B, Rutishauser RL, Henrich TJ. 2021. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep 36:109518. doi: 10.1016/j.celrep.2021.109518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Littlefield KM, Watson RO, Schneider JM, Neff CP, Yamada E, Zhang M, Campbell TB, Falta MT, Jolley SE, Fontenot AP, Palmer BE. 2022. SARS-CoV-2-specific T cells associate with inflammation and reduced lung function in pulmonary post-acute Sequalae of SARS-CoV-2. PLoS Pathog 18:e1010359. doi: 10.1371/journal.ppat.1010359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, Li S, Hong S, Zhang R, Xie J, Kornilov SA, Scherler K, Pavlovitch-Bedzyk AJ, Dong S, Lausted C, Lee I, Fallen S, Dai CL, Baloni P, Smith B, Duvvuri VR, Anderson KG, Li J, Yang F, Duncombe CJ, McCulloch DJ, Rostomily C, Troisch P, Zhou J, Mackay S, DeGottardi Q, May DH, Taniguchi R, Gittelman RM, Klinger M, Snyder TM, Roper R, Wojciechowska G, Murray K, Edmark R, Evans S, Jones L, Zhou Y, Rowen L, Liu R, Chour W, Algren HA, Berrington WR, Wallick JA, Cochran RA, Micikas ME, Wrin T, Petropoulos CJ, Cole HR, Fischer TD, Wei W, Hoon DSB, Price ND, Subramanian N, Hill JA, Hadlock J, Magis AT, Ribas A, Lanier LL, Boyd SD, Bluestone JA, Chu H, Hood L, Gottardo R, Greenberg PD, Davis MM, Goldman JD, Heath JR, ISB-Swedish COVID-19 Biobanking Unit . 2022. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 185:881–895. doi: 10.1016/j.cell.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Queiroz MAF, Neves PFM das, Lima SS, Lopes J da C, Torres MK da S, Vallinoto IMVC, Bichara CDA, Dos Santos EF, de Brito MTFM, da Silva ALS, Leite M de M, da Costa FP, Viana M de N do S de A, Rodrigues FBB, de Sarges KML, Cantanhede MHD, da Silva R, Bichara CNC, van den Berg AVS, Veríssimo A de OL, Carvalho M da S, Henriques DF, Dos Santos CP, Nunes JAL, Costa IB, Viana GMR, Carneiro FRO, Palacios VR da CM, Quaresma JAS, Brasil-Costa I, Dos Santos EJM, Falcão LFM, Vallinoto ACR. 2022. Cytokine profiles associated with acute COVID-19 and long COVID-19 syndrome. Front Cell Infect Microbiol 12:922422. doi: 10.3389/fcimb.2022.922422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Swank Z, Senussi Y, Alter G, Walt DR. 2022. Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. Pathology. doi: 10.1101/2022.06.14.22276401 [DOI] [PMC free article] [PubMed]
- 26. Durstenfeld MS, Peluso MJ, Kaveti P. 2022. Inflammation during early post-acute COVID-19 is associated with reduced exercise capacity and long COVID symptoms after 1 year. medRxiv [Google Scholar]
- 27. Sullivan DJ, Gebo KA, Shoham S, Bloch EM, Lau B, Shenoy AG, Mosnaim GS, Gniadek TJ, Fukuta Y, Patel B, Heath SL, Levine AC, Meisenberg BR, Spivak ES, Anjan S, Huaman MA, Blair JE, Currier JS, Paxton JH, Gerber JM, Petrini JR, Broderick PB, Rausch W, Cordisco M-E, Hammel J, Greenblatt B, Cluzet VC, Cruser D, Oei K, Abinante M, Hammitt LL, Sutcliffe CG, Forthal DN, Zand MS, Cachay ER, Raval JS, Kassaye SG, Foster EC, Roth M, Marshall CE, Yarava A, Lane K, McBee NA, Gawad AL, Karlen N, Singh A, Ford DE, Jabs DA, Appel LJ, Shade DM, Ehrhardt S, Baksh SN, Laeyendecker O, Pekosz A, Klein SL, Casadevall A, Tobian AAR, Hanley DF. 2022. Early outpatient treatment for COVID-19 with Convalescent plasma. N Engl J Med 386:1700–1711. doi: 10.1056/NEJMoa2119657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fernández-Ruiz M, Humar A, Baluch A, Keshwani S, Husain S, Kumar D. 2015. Baseline serum interleukin-6 to interleukin-2 ratio is associated with the response to seasonal trivalent influenza vaccine in solid organ transplant recipients. Vaccine 33:7176–7182. doi: 10.1016/j.vaccine.2015.10.134 [DOI] [PubMed] [Google Scholar]
- 29. Bonny TS, Patel EU, Zhu X, Bloch EM, Grabowski MK, Abraham AG, Littlefield K, Shrestha R, Benner SE, Laeyendecker O, Shoham S, Sullivan D, Quinn TC, Casadevall A, Pekosz A, Redd AD, Tobian AAR. 2021. Cytokine and chemokine levels in Coronavirus disease 2019 convalescent plasma. Open Forum Infect Dis 8:faa574. doi: 10.1093/ofid/ofaa574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karaba AH, Zhu X, Benner SE, Akinde O, Eby Y, Wang KH, Saraf S, Garonzik-Wang JM, Klein SL, Bailey JR, Cox AL, Blankson JN, Durand CM, Segev DL, Werbel WA, Tobian AAR. 2022. Higher proinflammatory cytokines are associated with increased antibody titer after a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients. Transplantation 106:835–841. doi: 10.1097/TP.0000000000004057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vimercati L, De Maria L, Quarato M, Caputi A, Gesualdo L, Migliore G, Cavone D, Sponselli S, Pipoli A, Inchingolo F, Scarano A, Lorusso F, Stefanizzi P, Tafuri S. 2021. Association between long COVID and overweight/obesity. J Clin Med 10:4143. doi: 10.3390/jcm10184143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Durstenfeld MS, Peluso MJ, Lin F, Peyser ND, Isasi C, Carton TW, Henrich TJ, Deeks SG, Olgin JE, Pletcher MJ, Beatty AL, Marcus GM, Hsue PY. n.d. Association of Nirmatrelvir/Ritonavir Treatment with Long COVID Symptoms in an Online Cohort of Non-Hospitalized Individuals Experiencing Breakthrough SARS-CoV-2 Infection in the Omicron Era. Infectious Diseases (except HIV/AIDS). doi: 10.1101/2023.03.02.23286730 [DOI]
- 33. Nevalainen OPO, Horstia S, Laakkonen S, Rutanen J, Mustonen JMJ, Kalliala IEJ, Ansakorpi H, Kreivi H-R, Kuutti P, Paajanen J, Parkkila S, Paukkeri E-L, Perola M, Pourjamal N, Renner A, Rosberg T, Rutanen T, Savolainen J, Haukka JK, Guyatt GH, Tikkinen KAO, Solidarity Finland Investigators . 2022. Effect of remdesivir post hospitalization for COVID-19 infection from the randomized SOLIDARITY Finland trial. Nat Commun 13:6152. doi: 10.1038/s41467-022-33825-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boglione L, Meli G, Poletti F, Rostagno R, Moglia R, Cantone M, Esposito M, Scianguetta C, Domenicale B, Di Pasquale F, Borrè S. 2022. Risk factors and incidence of long-COVID syndrome in hospitalized patients: does remdesivir have a protective effect. QJM 114:865–871. doi: 10.1093/qjmed/hcab297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Körper S, Grüner B, Zickler D, Wiesmann T, Wuchter P, Blasczyk R, Zacharowski K, Spieth P, Tonn T, Rosenberger P, Paul G, Pilch J, Schwäble J, Bakchoul T, Thiele T, Knörlein J, Dollinger MM, Krebs J, Bentz M, Corman VM, Kilalic D, Schmidtke-Schrezenmeier G, Lepper PM, Ernst L, Wulf H, Ulrich A, Weiss M, Kruse JM, Burkhardt T, Müller R, Klüter H, Schmidt M, Jahrsdörfer B, Lotfi R, Rojewski M, Appl T, Mayer B, Schnecko P, Seifried E, Schrezenmeier H. 2022. One-year follow-up of the CAPSID randomized trial for high-dose convalescent plasma in severe COVID-19 patients. J Clin Invest 132:e163657. doi: 10.1172/JCI163657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, van Buskirk C, Grossman BJ, Joyner M, Henderson JP, Pekosz A, Lau B, Wesolowski A, Katz L, Shan H, Auwaerter PG, Thomas D, Sullivan DJ, Paneth N, Gehrie E, Spitalnik S, Hod EA, Pollack L, Nicholson WT, Pirofski L, Bailey JA, Tobian AAR. 2020. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest 130:2757–2765. doi: 10.1172/JCI138745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, Wiggins CC, Bruno KA, Klompas AM, Lesser ER, Kunze KL, Sexton MA, Diaz Soto JC, Baker SE, Shepherd JRA, van Helmond N, Verdun NC, Marks P, van Buskirk CM, Winters JL, Stubbs JR, Rea RF, Hodge DO, Herasevich V, Whelan ER, Clayburn AJ, Larson KF, Ripoll JG, Andersen KJ, Buras MR, Vogt MNP, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, Paneth NS, Fairweather D, Wright RS, Casadevall A. 2021. Convalescent plasma antibody levels and the risk of death from COVID-19. N Engl J Med 384:1015–1027. doi: 10.1056/NEJMoa2031893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Estcourt LJ, Cohn CS, Pagano MB, Iannizzi C, Kreuzberger N, Skoetz N, Allen ES, Bloch EM, Beaudoin G, Casadevall A, Devine DV, Foroutan F, Gniadek TJ, Goel R, Gorlin J, Grossman BJ, Joyner MJ, Metcalf RA, Raval JS, Rice TW, Shaz BH, Vassallo RR, Winters JL, Tobian AAR. 2022. Clinical practice guidelines from the association for the advancement of blood and biotherapies (AABB): COVID-19 convalescent plasma. Ann Intern Med 175:1310–1321. doi: 10.7326/M22-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Senefeld JW, Johnson PW, Kunze KL, Bloch EM, van Helmond N, Golafshar MA, Klassen SA, Klompas AM, Sexton MA, Diaz Soto JC, Grossman BJ, Tobian AAR, Goel R, Wiggins CC, Bruno KA, van Buskirk CM, Stubbs JR, Winters JL, Casadevall A, Paneth NS, Shaz BH, Petersen MM, Sachais BS, Buras MR, Wieczorek MA, Russoniello B, Dumont LJ, Baker SE, Vassallo RR, Shepherd JRA, Young PP, Verdun NC, Marks P, Haley NR, Rea RF, Katz L, Herasevich V, Waxman DA, Whelan ER, Bergman A, Clayburn AJ, Grabowski MK, Larson KF, Ripoll JG, Andersen KJ, Vogt MNP, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, Buchholtz ZA, Pletsch MC, Wright K, Greenshields JT, Joyner MJ, Wright RS, Carter RE, Fairweather D. 2021. Access to and safety of COVID-19 convalescent plasma in the United States expanded access program: A national registry study. PLoS Med 18:e1003872. doi: 10.1371/journal.pmed.1003872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tobian AAR, Cohn CS, Shaz BH.. COVID-19 convalescent plasma. Blood 2022; 140(3): 196-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, Chen J, Lopez BV, Eagar TN, Yi X, Zhao P, Rogers J, Shehabeldin A, Joseph D, Masud F, Leveque C, Olsen RJ, Bernard DW, Gollihar J, Musser JM. 2021. Significantly decreased mortality in a large cohort of Coronavirus disease 2019 (COVID-19) patients transfused early with convalescent plasma containing high-titer anti-severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) spike protein IgG. Am J Pathol 191:90–107. doi: 10.1016/j.ajpath.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Casadevall A, Pirofski L. 2020. The convalescent sera option for containing COVID-19. J Clin Invest 130:1545–1548. doi: 10.1172/JCI138003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, Canas LS, Graham MS, Klaser K, Modat M, Murray B, Kerfoot E, Chen L, Deng J, Österdahl MF, Cheetham NJ, Drew DA, Nguyen LH, Pujol JC, Hu C, Selvachandran S, Polidori L, May A, Wolf J, Chan AT, Hammers A, Duncan EL, Spector TD, Ourselin S, Steves CJ. 2022. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID symptom study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis 22:43–55. doi: 10.1016/S1473-3099(21)00460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jones SA, Jenkins BJ. 2018. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol 18:773–789. doi: 10.1038/s41577-018-0066-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental tables and figures.