Abstract
The chloroplast enzyme phosphoribulokinase (PRK; EC 2.7.1.19) is part of the Calvin cycle (reductive pentose phosphate pathway) responsible for CO2 fixation in photosynthetic organisms. In green algae and vascular plants, this enzyme is light regulated via reversible reduction by reduced thioredoxin. We have sequenced and characterized the gene of the PRK from the marine diatom Odontella sinensis and found that the enzyme has the conserved cysteine residues necessary for thioredoxin-dependent regulation. Analysis of enzymatic activity of partially purified diatom enzyme and of purified protein obtained by native overexpression in Escherichia coli, however, revealed that under natural redox conditions the diatom enzyme is generally active. Treatment of the enzyme with strong oxidants results in inhibition of the enzyme, which is reversible by subsequent incubation with reducing agents. We determined the redox midpoint potentials of the regulatory cysteine in the PRK from O. sinensis in comparison to the respective spinach (Spinacia oleracea) enzyme and found a more positive redox potential for the diatom PRK, indicating that in vivo this enzyme might not be regulated by thioredoxin. We also demonstrate that in protease-treated diatom plastids, activities of enzymes of the oxidative pentose phosphate pathway are not detectable, thus reducing the need for a tight regulation of the Calvin cycle in diatoms. We discuss our results in the context of rearrangements of the subcellular compartmentation of metabolic pathways due to the peculiar evolution of diatoms by secondary endocytobiosis.
The enzyme phosphoribulokinase (PRK; EC 2.7.1.19) is found in most phototrophic organisms. Next to Rubisco, it is one of the key enzymes of the reductive pentose phosphate pathway as it catalyzes the only reaction by which Calvin cycle intermediates can be provided for further CO2 fixation. The kinase catalyzes the in-line transfer of the γ-phosphoryl group from ATP to the C-1 hydroxyl of Ru-bP, thereby forming Ru-1,5-bP, the acceptor molecule for CO2. Class I and II PRK enzymes are known (Martin and Schnarrenberger, 1997). Class I enzymes are found in protobacteria and in some cyanobacteria (Badger et al., 2002), while Class II PRK is found in cyanobacteria, in eukaryotic algae, and in land plants. The PRK of the latter group typically is a dimer of identical 40-kD subunits, which is located in the stroma of the chloroplasts (Porter et al., 1986). In plants, PRK activity is linked to the light-driven electron transport at the thylakoid membrane via the ferredoxin/thioredoxin system (Wolosiuk and Buchanan, 1978; Buchanan, 1980; Porter et al., 1988; Jacquot et al., 1997). During illumination, plastidic thioredoxins are constantly reduced by the ferredoxin-thioredoxin oxidoreductase. The reduced form of thioredoxin is then able to reduce an intramolecular regulatory disulfide of the oxidized, inactive PRK, resulting in an immediate activation of the kinase (Hirasawa et al., 1999). Higher plant PRKs possess four Cys; the regulatory disulfide of PRK is formed by the two N-terminal Cys (Cys-16 and Cys-55 in spinach [Spinacia oleracea]; Porter et al., 1988; Porter and Hartman, 1990). Several plastidic enzymes in plants are light regulated via thioredoxin (for review, see Ruelland and Miginiac-Maslow, 1999), but it is an exceptional feature of the PRK that both regulatory Cys are located in the active site. Apparently, Cys-55 has a modest facilitative role in catalysis (Porter et al., 1988; Porter and Hartman, 1990). A systematic investigation on the oxidation-reduction midpoint potentials (Em) of the disulfide/dithiol couple of enzymes regulated by thioredoxin revealed that PRK possesses the most positive potential (−290 mV for spinach PRK; Hirasawa et al., 1998, 1999) and therefore is easier to activate than the other light-regulated enzymes of the Calvin cycle. It has been demonstrated that in the green alga Chlamydomonas reinhardtii the PRK can form regulatory complexes with the enzyme glyceraldehyde phosphate dehydrogenase (GAPDH) and a small protein named CP12 (Wedel et al., 1997; Mouche et al., 2002; Lebreton et al., 2003). Recently it has been shown that the PRK from the cyanobacterium Synechococcus sp. PCC 7042 is permanently active but can be inactived by strong oxidants (Kobayashi et al., 2003).
A tight regulation of Calvin cycle enzymes via the ferredoxin/thioredoxin system is essential, as this pathway constitutes the interface between the light reactions at the thylakoid membranes and the utilization of the produced metabolites for other biosynthetic purposes. For example, the oxidative pentose phosphate pathway (OPP) in land plant plastids (Schnarrenberger et al., 1973, 1995) converts hexose phosphates into pentose phosphates generating NADPH. These reactions run contrary to that of the Calvin cycle (which is also termed reductive pentose phosphate pathway); having both pathways active at the same time in the same compartment would result in a futile cycle with a net consumption of three ATP per fixed CO2. Consequently, some Calvin cycle enzymes are activated in the light as described above, while the Glc-6-P dehydrogenase (G6PDH), catalyzing the first oxidative reaction of the OPP, is inhibited after reduction by thioredoxin (Wenderoth et al., 1997).
Diatoms (Bacillariophyceae) contribute significantly to the total O2 evolution and primary biomass production in oceans and other aquatic ecosystems (Falkowski et al., 1998). However, little is known about photosynthesis of diatoms or other chromophytic chlorophyll a/c-containing algae and biochemical investigations on diatom photosynthesis are still fragmentary. The studies so far mainly focused on the characterization of components of the photosynthetic apparatus like the light-harvesting complexes (Bhaya and Grossman, 1993; Büchel, 2003), the photosystems (Lavaud et al., 2002), or the chloroplast ATP synthase (Pancic et al., 1990), and they indicated a high similarity to the respective structures of other algae and higher plants.
With the exception of the plastid-encoded genes for the large and small subunits of the Rubisco and the GAPDH (Hwang and Tabita, 1991; Liaud et al., 2000), there are practically no reports about Calvin cycle enzymes in these organisms. Furthermore, it is still unclear how light regulation of photosynthetic enzymes in chromophytic plastids occurs. In contrast to the respective enzymes of green algae and higher plants, the γ-subunit of the chloroplast ATPase of the diatom Odontella sinensis does not have the regulatory Cys residues (Pancic and Strotmann, 1993), which are involved in covalent redox modulation of enzymatic land plants. Also, there is no redox-regulated malate dehydrogenase isoform present in diatom plastids (Ocheretina et al., 2000).
In this study, we investigated the redox state of the PRK in diatom plastids under different redox conditions. We determined the redox properties of the heterologously expressed and purified PRK from the diatom O. sinensis. Using the redox poising technique (Hutchison and Ort, 1995; Hirasawa et al., 1998), we determined the redox midpoint potential (Em) of the diatom PRK in comparison to the spinach enzyme. We also show that there is apparently no OPP in diatom plastids.
RESULTS
Features of the PRK from O. sinensis
We cloned and sequenced the complete gene encoding the PRK of the diatom O. sinensis from a cDNA library using degenerate primers, which were derived from homologous Prk regions of other organisms. To verify this sequence, we amplified the identical genomic DNA fragment from nuclear DNA of O. sinensis, showing that there is no intron in the entire nuclear Prk gene in O. sinensis. Comparison of the derived amino acid sequence with PRK sequences from land plants, cyanobacteria, red algae, and other diatoms revealed a sequence similarity of 60% to 80% identical amino acids. As in most other plastid PRKs, the O. sinensis enzyme contains four Cys residues present in the primary sequence (Fig. 1; positions 19, 63, 224, and 236). The first two residues, located in the N-terminal part of the polypeptide, are in conserved positions and are found in all sequences analyzed so far. It has been shown that the first two Cys are responsible and indispensable for the redox regulation in land plant plastids (Porter et al., 1988; Porter and Hartmann, 1990; Brandes et al., 1996). Interestingly, although the Cys in the C-terminal region apparently are conserved throughout land plants, red algae, and even xanthophytes (which, like diatoms, evolved by secondary endocytobiosis), the other two Cys found in the centric diatom O. sinensis and the pennate Phaeodactylum tricornutum do not match these positions; moreover, even within the diatoms there are variations as the PRK from the centric Thalassiosira pseudonana does not have Cys at all in this region (Fig. 1).
Figure 1.
Alignment of domains of PRKs from different organisms. Odo sin, O. sinensis (accession no. Y08610); Phae tr, P. tricornutum (diatom, EST sequence data at http://avesthagen.sznbowler.com/); Tha ps, T. pseudonana (diatom, genome sequence data at http://genome.jgi-psf.org/thaps1/thaps1.home.html); Vau lit, Vaucheria litoralis (Xanthophyte, AF336986); Galdsu, G. sulphuraria (red alga, AJ012719); Syncyst, Synechocystis PCC6803 (MM77134); Chl rei, C. reinhardtii (M36123); Pis sat, Pisum sativum (Y11248); Ara th, Arabidopsis thaliana (X58149); and Spin ol, spinach (X07654). Numbers above the sequences and at the end of the lines indicate the respective amino acid positions. A, N-terminal region with the regulatory Cys residues. B, C-terminal regions containing additional Cys residues.
The PRK protein from O. sinensis contains a bipartite presequence (data not shown), which is structurally very similar to other plastid-targeting presequences in diatoms (Kroth, 2002). Typical for such presequences is an N-terminal signal peptide with a basic amino acid residue and a hydrophobic domain, followed by a domain showing features of transit peptides of land plant plastid preproteins (Kilian and Kroth, 2005). By gel filtration experiments, we found that the PRKs from different diatoms (O. sinensis, Coscinodiscus granii, and P. tricornutum) are homodimers with an apparent molecular mass of 44 kD/subunits on SDS gels (data not shown). This is identical to PRK enzymes from land plants but differs from previously published data for the PRK of the haptophyte Heterosigma carterae (Hariharan et al., 1998).
PRK Enzyme Activity in Diatom Stromal Extracts
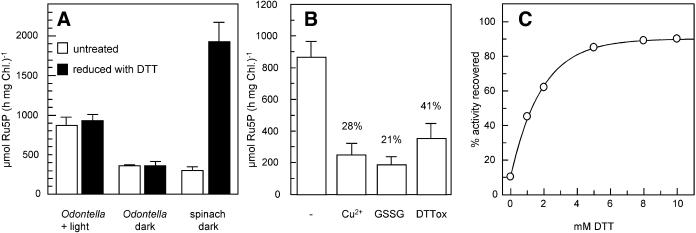
In previous works, it has been shown that the enzymatic activity of PRK from land plants is strongly dependent on the redox state and that a full activation of PRK in vivo is found only in illuminated plants (Wedel and Soll, 1998). We analyzed the PRK activity as described in “Materials and Methods” in crude stromal extracts of purified plastids isolated either from the diatoms O. sinensis and C. granii or from spinach. Both algae and plants were either kept in the dark or illuminated for several hours before plastid preparation. The plastids were broken osmotically to isolate stromal extracts, after they had routinely been checked for integrity by measuring light-driven oxygen evolution using an oxygen electrode. In agreement with previous publications (Wolusiuk and Buchanan, 1978; Gardemann et al., 1983), we were able to increase the PRK activity from dark-treated spinach plants about 5-fold by incubation with reduced dithiothreitol (DTT; Fig. 2A). In the diatom plastids, no such difference was found: After incubation with DTT we obtained the same enzymatic activity as in the control reaction without DTT. However, PRK activities in O. sinensis were 2.5 times lower in plastids isolated from algae incubated in the dark for at least 12 h compared to preilluminated algae (Fig. 2A). The decreased activity in dark-adapted diatom plastids can probably be attributed to reduced enzyme levels. Semiquantitative western-blot analyses support this view, showing the enzyme levels increasing by a factor of 2 to 3 after onset of illumination (data not shown).
Figure 2.
Effects of reducing and oxidizing conditions on the enzymatic activity of the PRK from O. sinensis and spinach stromal extracts. A, Stromal extracts were prepared as described in “Materials and Methods” from plants/algae kept for several hours in the dark or in the light. PRK activity from diatoms generally was not affected by treatment with 50 mm reduced DTT (black columns); in light-treated diatoms, there was a generally higher PRK activity. In a control preparation from spinach plastids, treatment with 50 mm DTT resulted in a strong increase of PRK activity. The bars represent the sd from three independent experiments. B, Inactivation of PRK from O. sinensis stromal extracts by incubation with 5 mm CuCl2 (final concentration), 125 mm GSSG, 125 mm oxidized DTT. The first column represents the control reactions, the number above the other columns represent the residual activity. C, Reductive reactivation of PRK from O. sinensis stromal extracts that had been inactivated with DTNB. After reduction with reduced DTT, about 90% of the original activity was recovered after 10 min.
As the diatom PRK possesses Cys residues located in the same position as the regulatory Cys in land plant PRKs, we checked whether the enzymatic PRK activity in O. sinensis was in any way influenced by oxidation or by reduction. We isolated crude stromal extracts from previously illuminated diatoms and treated them with oxidizing agents like copper in the presence of oxygen, oxidized glutathione (GSSG), or oxidized DTT and found a decrease of PRK activity to different degrees (Fig. 2B). The degree of inhibition corresponds to the redox potential of the oxidizing agents (DTT −327 mV; Lees and Whitesides, 1993; GSSG −250 mV; Lundström and Holmgren, 1983). Furthermore, PRK activity was completely abolished by treatment of the stromal extract with the strong oxidant dithiobisnitro-benzoic acid (DTNB, Ellman's reagent; Brandes et al., 1996). Enzymatic activity of oxidized inactivated PRK could be restored by treatment with reducing agents (Fig. 2C), thus indicating that PRK inhibition was not due to unspecific oxidation and inactivation of the enzyme.
Production and Purification of Recombinant Diatom PRK
To exclude unknown interfering factors or proteins within the stromal extract and to investigate the redox regulation of PRK in detail, we fused the cDNA encoding the mature part of the PRK protein from O. sinensis to a gene coding for an intein tag (intein plus a chitin-binding domain) for affinity chromatography. The putative N terminus of the mature O. sinensis PRK protein (position 43) was inferred by comparison of the complete amino acid sequence of the PRK with (1) other mature PRK sequences (Milanez and Mural, 1988; Hariharan et al., 1998) and (2) known cleavage motifs in diatom plastid preproteins (Kroth, 2002). The PRK was expressed in Escherichia coli as soluble protein but only in low amounts. This was not surprising, as poor expression levels of heterologous PRKs in bacterial expression systems have been described before (Brandes et al., 1996). The protein was loaded on chitin beads, and PRK was eluted after intein-mediated cleavage overnight induced by DTT. This way, we obtained pure PRK protein without any additional tag. We were able to increase the expression slightly by the addition of l-Ara and glycerol to the bacterial growth media according to Milanez et al. (1991) and by growing the bacteria at 16°C. The PRK purified by this procedure had a high specific activity (300 units mg−1), which is comparable to the enzyme purified from spinach (Gardemann et al., 1983; Table I). Vmax and the respective Km values for ATP and Ru5P were rather similar to the enzyme from spinach and the cyanobacterium Synechocystis PCC7942 but differed markedly from data published for the haptophyte alga H. carterae (Hariharan et al., 1998).
Table I.
Enzymatic properties of the PRK from O. sinensis and from other organisms
| O. sinensis | Spinacha | H. carteraeb | Syn 7942c | |
|---|---|---|---|---|
| Vmax (units mg−1) | 300 ± 14 | 300 | 218 | n.d.d |
| Km ATP (μm) | 84 ± 6.5 | 65 | 208 | 90 |
| Km Ru5P (μm) | 118 ± 13 | 66 | 226 | n.d. |
Spinach according to Gardemann et al. (1983) and Geck and Hartman (2000).
H. carterae according to Hariharan et al. (1998).
According to Kobayashi et al. (2003).
Not determined.
Analysis of Diatom PRK Inactivation by DTNB
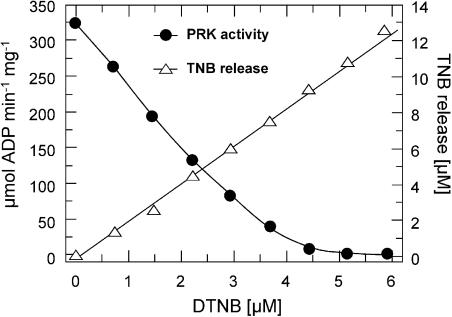
Disulphide bridge formation requires a close proximity of both sulfhydryl groups involved. Such proximity has been successfully demonstrated for PRK from spinach utilizing the dithiol DTNB (Porter et al., 1988; Porter and Hartman, 1990). DTNB reacts with Cys groups by releasing one molecule of TNB and leaving a second molecule TNB covalently bound to the Cys. If another reduced Cys is present in close proximity to this protein-bound TNB, both can react, resulting in the formation of a disulfide bridge between the Cys residues and the release of the second TNB molecule (Riddles et al., 1979). We titrated purified recombinant O. sinensis PRK (5.7 μm) with DTNB. Determination of the activity revealed a direct correlation between the DTNB concentration and the degree of inactivation. Total inactivation was observed at equimolar concentrations of DTNB and the respective PRK subunit concentration. At 4.5 μm DTNB, we observed only 2% of the former activity, while a complete inhibition was observed at 5.5 μm DTNB (Fig. 3). To check that the inactivation was not due to incorporation of the reagent, we examined the inactivated enzyme after gel filtration and dialysis for A412. The data confirmed that TNB was not incorporated in the enzyme. The release of 2 m equivalents TNB/mol of DTNB during the inhibition is consistent with the oxidative formation of a single disulfide per subunit of PRK. To test whether the disulfide is formed inter- or intramolecularly, we determined the apparent Mr of the DTNB oxidized enzyme by SDS-PAGE under nonreducing conditions. No high Mr bands were detected (data not shown), indicating the formation of intramolecular disulfide bonds only.
Figure 3.
Stoichiometry of the redox reaction of diatom PRK with DTNB. Overexpressed and purified PRK from O. sinensis (5.7 μm) was oxidized stepwise with DTNB, and the amount of released TNB was monitored spectroscopically at λ = 412 nm (white triangles). In parallel, the activity of the PRK was measured in an enzymatic test (black circles). The data clearly show that two molecules TNB are released per molecule DTNB, indicating that DTNB reacts with two Cys in close proximity by forming a disulfide. The reactions were incubated at 15°C to stabilize the diatom enzyme.
Determination of the Midpoint Redox Potential of the PRK from Diatoms
As the PRK from O. sinensis apparently can be modulated reversibly by oxidation, we need to question why the enzyme is permanently active in stromal extracts. Different secondary or tertiary structure might be responsible for conformational changes that results in a different redox potential of the diatom enzyme compared to land plant enzymes. Therefore, we determined the midpoint redox potential of the PRKs from spinach and O. sinensis by redox titration (redox poising; Hutchison and Ort, 1995). In this procedure, the fully oxidized enzyme is incubated at various ambient redox conditions (Eh) defined by mixtures of reduced and oxidized DTT, followed by measurement of the enzymatic activity. As DTT can be oxidized by dissolved oxygen, nitrogen-saturated buffers were used and incubations performed under a nitrogen atmosphere. Testing our system with purified spinach PRK, we determined a redox Em of −289 ± 4 mV (data not shown), which is close to the published value of 295 ± 10 mV for PRK isolated from spinach plastids (Hirasawa et al., 1998) and 290 ± 10 mV for the respective recombinant protein (D. Knaff, personal communication).
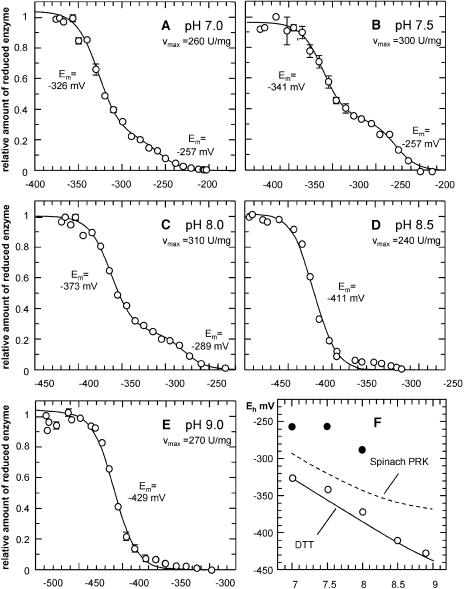
Optimization of the protocol for the recombinant diatom PRK revealed that the enzymatic activity was not stable when the enzyme was incubated at 25°C; therefore, we incubated at 10°C with various DTTox/red ratios. Under anaerobic conditions, we observed stable PRK activity for 2 h or more. This was sufficient to achieve redox equilibrium between DTT and the enzyme, a process that takes up to 30 min (this is about 2 times faster than observed for the spinach enyzme). We have determined the Em values for the PRK at various pH values (Fig. 4). The maximum activity obtained at the lowest Eh values was identical to the maximum activity of the completely reduced purified enzyme. In contrast to the results of the spinach enzyme (not shown), we found that the curves did not fit exactly to a Nernst equation for a two-electron single-component redox reaction. Especially at pH 7.5 and 8.0, we obtained a biphasic curve resulting in the calculation of 2 putative Em values. Plotting the Em values of the spinach and the diatom PRK as a function of the pH shows that the obtained Em of the diatom PRK are either above or below the value of spinach PRK. Two Em values indicate that either additional Cys within the PRK might form disulphide bridges or that one of the Em values represents the formation of artificial mixed dimers of enzyme and DTT. Therefore, we also plotted the respective values of DTT, which were very similar to the more negative Em values of the diatom PRK. This indicates that the higher value of −257 mV, pH 7.0, in fact represents the actual Em value of the diatom PRK at pH 7.
Figure 4.
Redox titration of purified recombinant O. sinensis PRK at different pH values. A to E, Oxidized PRK (0.5 μm) was incubated for 75 min at 10°C in oxygen-free buffers (150 mm each) of indicated pH. Eh values were adjusted by different ratios of reduced and oxidized DTT (80 mm total concentration). Enzymatic activity as a measure for reduced enzyme is plotted as a function of the Eh value. Each data point was obtained in two independent experiments. Experimental data were fitted to the Nernst equation for a two-component two-electron redox system. F, Em versus pH values. From the biphasic redox titrations, two midpoint redox potentials (Em) were obtained. The lower values (white circles) apparently can be related to the formal standard redox potential 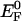 of DTT; thus, the higher values (black circles) represent the actual Em values of the O. sinensis PRK. The broken line represents the Em values of spinach PRK calculated from Em and pKa values of the regulatory cysteins (Hirasawa et al., 1999).
of DTT; thus, the higher values (black circles) represent the actual Em values of the O. sinensis PRK. The broken line represents the Em values of spinach PRK calculated from Em and pKa values of the regulatory cysteins (Hirasawa et al., 1999).
There Is Apparently No OPP in Diatom Plastids
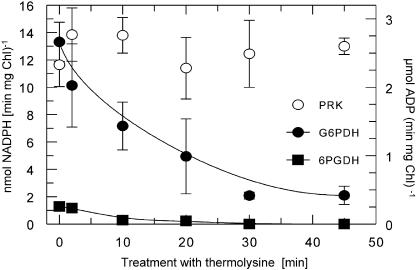
To analyze the redox regulation properties of enzymes of the OPP, we measured the activity of 2 key enzymes, the G6PDH and the 6-phosphogluconate dehydrogenase (6PGDH). We purified intact plastids from the diatoms O. sinensis and C. granii and found low respective activities in the stromal extracts. Both enzymes apparently were not affected by changing redox conditions (data not shown). This result indicated that we might have measured the cytosolic isoenzymes attached to the outside of the envelope membranes of the plastids; therefore, we treated the plastids with the protease thermolysin (Cline et al., 1984) prior to hypotonic lysis (Fig. 5). After incubating the plastids for 45 min with thermolysin, the activity of G6PDH was drastically reduced, whereas there was no measurable 6PGDH activity. As a control, we measured the activity of the stromal PRK (there is no known cytosolic PRK isoenzyme), which remained constant over the whole time period. As PRK from O. sinensis is sensitive to thermolysin, these results clearly indicate that there is apparently no G6PDH and 6PGDH activity measurable in the diatom plastids. Accordingly, we could not identify any plastidic isoforms of G6PDH and 6PGDH (as defined by the possession of a plastid-targeting presequence) in the genome of the diatom T. pseudonana (Armbrust et al., 2004).
Figure 5.
Enzymatic activities of the OPP enzymes G6PDH (black circles) and 6PGDH (black squares) in isolated plastids from the diatom C. granii. Plastids were pretreated with the protease thermolysin for the time periods indicated on the x axis. After incubation, the protease was inactivated by the addition of excess EGTA compared to the Ca2+ concentration and the plastids were broken osmotically. Enzymatic analysis of the stromal extracts demonstrates that the G6PDH and the 6PGDH get degraded by thermolysin treatment, which indicates that the measured activity is due to cytosolic contamination. As a control, the stromal PRK (white circles) is not degraded by thermolysin.
DISCUSSION
We found the PRK from O. sinensis to be active in diatom plastids independent of whether the algae were illuminated prior to isolation or whether they were kept in the dark (Fig. 2). This was surprising as the respective enzyme from land plants is usually inactive and gets activated in the light via reduction by reduced thioredoxin (Anderson, 1973; Gardemann et al., 1983). From the nucleotide sequence, it was obvious that the respective Cys residues involved in redox regulation of the land plant enzymes (Porter et al., 1988) are present in the diatom PRK. The distance and the amino acid sequence between the regulatory Cys are strongly conserved within land plants and green algae (see also Fig. 1). For the PRK of O. sinensis, however, we found an insertion of 5 amino acids between Cys 19 and 63. The PRK sequences of the rhodophyte Galdieria sulphuraria and of some cyanobacteria show deletions between the regulatory Cys. Interestingly, the enzymes from cyanobacteria (Kobayashi et al., 2003) and from G. sulphuraria are also possibly not redox regulated (R. Scheibe, personal communication). In contrast to thioredoxin, where the redox active Cys are already in proximity at the level of the primary enzyme structure, the orientation of regulatory Cys of PRK is dependent on the protein fold. Therefore, it is possible that the insertion in the diatom enzyme affects the distance between the regulatory Cys. Enzymatic analyses support this view: We were able to show reversible inactivation of the enzyme by strong oxidants and to recover activity by subsequent reduction of the enzyme. Oxygen, which is believed to inactivate the land plant PRK, apparently is not strong enough to form a disulfide bridge, thus leaving the enzyme in a permanently active mode. There are reports that algae and cyanobacteria accumulate higher concentrations of H2O2 (Badger et al., 2000); according to our data, this is probably not true for diatom plastids.
A likely reason for the lost ability of the diatom PRK to be regulated by thioredoxin could be the apparently missing OPP in diatom plastids. In land plants, both reductive and oxidative pentose phosphate pathways have to be controlled strictly to prevent an energy-consuming futile cycle. Since the oxidative pathway is missing in diatom plastid, such a stringent control appears to be no longer necessary.
Previous work on light regulation of diatom enzymes indicated that thioredoxin might not play a role in diatom plastids. Several enzymes in diatoms, like the chloroplast ATPase (Pancic and Strotmann, 1993), the plastidic malate dehydrogenase (Ocheretina et al., 2000; R. Mertens and P.G. Kroth, unpublished data), and the GAPDH (Liaud et al., 2000), are not affected by the oxidation or reduction simply because they do not possess the respective Cys residues. Our finding that the PRK from O. sinensis potentially can be regulated by oxidation/reduction now rules out the possibility that redox regulation of PRK might have been developed in plastids of the green algae and land plant clade only, but rather was invented before the green and red algal lineages diverged. We tried to purify thioredoxin from diatom plastids by affinity chromatography, so far without success. However, we were recently able to identify a gene for a plastid thioredoxin of the f-type in the genome database of the diatom T. pseudonana, thus also indicating that redox regulation by thioredoxin might occur in diatom plastids. So what are the actual targets? In our first experiments, we also tested the redox regulation of the Fru-1,6-bisphosphatase (FbPase) and the sedoheptulose-1,7-bisphosphatase in stromal extracts of diatom plastids (data not shown). For both enzymes, we found a strong reductive activation with similar activation kinetics as found for spinach FbPase (Hertig and Wolosiuk, 1983), indicating that the FbPase might be redox regulated in diatoms in vivo.
Another question is how diatoms survive without plastidic oxidative pentose pathway. In land plants, the OPP is closely connected to the biosynthesis of fatty acids (Jensen, 1985) and to the shikimate pathway (Farr et al., 1994) by supplying NADPH and sugar phosphates in the dark. As diatom plastids do not accumulate starch (they store the β-glucan chrysolaminarin in cytosolic vacuoles instead), sugar phosphates have to be imported into their plastids in the dark as well, making an OPP more or less useless. Reduction equivalents might easily be imported into the plastids by shuttle systems known from land plant plastids (Eicks et al., 2002).
Diatoms and other algae-like euglenophytes (brown algae, etc.) are supposed to have evolved by so-called secondary endocytobiosis. Substantial evidence suggests that all plastids can be traced back to a single primary endocytobiosis, which led to the evolution of plastids in green algae, land plants, red algae, and glaucophytes; (see Delwiche and Palmer, 1997; Martin et al., 1998; Moreira et al., 2000), where a cyanobacterium had been taken up by a heterotrophic host and was transformed into a plastid. In secondary endocytobiosis, complete eukaryotic algae (unicellular ancestors of modern red and green algae) were taken up by eukaryotic host cells and were reduced to plastids. This process drastically increased the complexity of the resulting cells, as the genes for complete pathways like the Calvin cycle had to be transferred from the vanishing nucleus of the endosymbiont to the nucleus of the secondary host (Kroth, 2002; Kilian and Kroth, 2005). As the modification of plastid targeting sequences is a complex process, it is unclear yet how these additional gene transfers affected the general metabolic pathways in diatoms. For example, in this paper, we show that one important pathway, the OPP, apparently is missing in diatom plastids. This might not be the only relocated pathway in diatoms: Liaud et al. (2000) demonstrated that isoforms of the glycolytic enzymes triosephophate isomerase and GAPDH are targeted into the mitochondria. Future analysis of the T. pseudonana genome will reveal how extensive genetic and enzymatic rearrangements might have occurred as a result of secondary endocytobiosis.
MATERIALS AND METHODS
Materials
If not stated otherwise, all chemicals used were p.a. grade. Odontella sinensis was cultured according to Pancic et al. (1990) in artificial seawater at 16°C and a light intensity of 15 μmol photons m−2 s−1 in a 14-/10-h-light/-dark cycle. Spinach (Spinacia oleracea) var Polka was grown at 20°C and a 12-/12-h-light/-dark cycle at a light intensity of 300 μmol photons m−2 s−1. Plastids from O. sinensis were isolated according to Wittpoth et al. (1998) and purified either on linear 40% to 80% Percoll gradients or on a 40-Percoll cushion as described. Plastids from spinach leaves were isolated based on the method of Heldt and Sauer (1971) with some modifications. Leaves were harvested 2 to 3 h after the light was switched on and homogenized in isolation medium A (360 mm sorbitol, 15 mm MES buffer, pH 6.5, 5 mm MgCl2, 10 mm NaCl). After filtration, the plastids were sedimented by centrifugation (2,500g for 90 s) and resuspended in medium B (360 mm sorbitol, 50 mm HEPES buffer, pH 7.6, 1 mm MgCl2, 1 mm MnCl2, 2 mm EDTA, 10 mm NaCl, 0.8 mm KH2PO4). Plastids were pelleted (1,000g, 60 s) and again washed and pelleted (3,000g, 120 s). If further purification was necessary, intact plastids were purified by centrifugation through a Percoll cushion as described for the O. sinensis plastids (Wittpoth et al., 1998). For determination of chlorophyll concentrations, diatom plastids were diluted 100-fold in 90% acetone and spinach plastids in 80% acetone. Calculation of chlorophyll contents of diatom plastids was based on extinction coefficients from Jeffrey and Humphrey (1975): x μg/mL Chl = (11.47 E664 − 0.4 E630) + (24.36 E630 − 3.73 E664). For spinach plastids, the following equation was used: x μg Chl/mL = 20.2 E645 + 8.02 E630. The integrity of the isolated plastids was routinely checked by measuring carbonate-dependent oxygen evolution of the organelles utilizing a Clark-type oxygen electrode (Hansatech, King's Lynn, UK) as described (Wittpoth et al., 1998).
Cloning of the PRK Gene
The gene encoding the PRK was cloned from a cDNA library of the diatom O. sinensis (Pancic and Strotmann, 1993) by PCR utilizing degenerate primers that were deduced from known Prk sequences.
Measurement of Enzymatic Activities (PRK, G6PDH, and 6PGDH)
PRK activity of stromal extracts or purified protein was measured according to Racker (1957) in a coupled-optical test. PRK was added to a buffer consisting of 50 mm Bicine (KOH), pH 7.95, 40 mm KCl, 10 mm MgCl2, 2 units mL−1 l-lactate dehydrogenase, 3 units mL−1 pyruvat kinase, 0.2 mm NADH, 1 mm ATP, 1 mm d-ribulose-5-P (Ru5P). For some assays, Ru5P was produced in the cuvette (incubation for 5 min before start of the assay) from ribose-5-P (1 mm) by phosphoriboisomerase (2 units mL−1). G6PDH and 6PGDH were measured directly by the production of NADPH from the respective sugar phosphates. The assays consist of 50 mm Tricine, pH 8, 10 mm MgCl2, and 0.4 mm NADP and the sugar phosphates at a concentration of 2 mm.
Reduction of PRK in Stromal Extracts
Stromal extracts were obtained from purified plastids by pelleting the plastids at 1,000g for 3 min and resuspension in 50 mm Bicine-KOH, pH 8.0. After incubation for 15 min on ice, the thylakoid membranes were removed by centrifugation at 20,000g for 15 min. Stromal fractions equivalent to 150 to 250 μg chlorophyll were incubated for 10 min at room temperature with 50 mm reduced DTT plus 10 mm MgCl2, 5 mm CuCl2, 125 mm GSSG, and 125 mm oxidized DTT.
Overexpression and Purification of PRK in Escherichia coli
The PRK gene was cloned into the vector pTYB1 (New England Biolabs, Beverly, MA). We amplified the region encoding the mature part of the PRK by PCR creating new NdeI (5′) and SapI (3′) restriction sites and ligated this fragment into the vector resulting in an in-frame fusion to a reading frame encoding an intein tag and a chitin-binding domain. This vector PTYmatPRK was transformed into E. coli strain BL21 (DE3) codonplus (Stratagene, La Jolla, CA). The bacteria were grown in Luria-Bertani medium including 0.2 Ara and 10% (v/v) glycerol at 30°C up to an optical density of 0.7, and then cooled to 16°C. After addition of isopropylthio-β-galactoside (final concentration 0.7 mm), the cells were grown for a further 16 h at 16°C, then harvested by centrifugation for 10 min at 10,000g at 4°C.
Sixty grams of cells were suspended in buffer (20 mm HEPES/KOH, pH 7.85; 1 mm NaCl, 1 mm EDTA, 6 mm MgCl2, and 1 mm phenlymethane sulfonfluorid) in a glass/teflon homogenizer. After addition of Dnase I, the cells were ruptured in a French press (Aminco International, Lake Forrest, CA) with a constant pressure of 1,200 psi. Cell debris was removed by centrifugation (15 min, 6,000g, 4°C). The supernatant was again centrifuged (30 min, 12,000g, 4°C) and the supernatant used for further purification by affinity chromatography on chitin agarose. After addition of 0.1% (v/v) Triton X-100, the extract was filtered through a 0.2-μm nylon membrane and loaded onto a column (2.2 × 5 cm) containing 20 mL chitin agarose beads. The flow through fraction was checked by western blots to ensure proper binding of the fusion protein. The column was washed with 300 mL washing buffer A (20 mm HEPES/KOH, pH 7.85, 1 m NaCl, 1 mm EDTA, 0.1% Triton X-100), followed by washing with 60 mL washing buffer B (20 mm HEPES/KOH, pH 7.5, 50 mm NaCl, 1 mm EDTA). The column was then washed with 60 mL of the same buffer containing 60 mm DTT, sealed, and stored at 4°C for 16 to 20 h. During this, the reducing conditions resulted in a protein splicing releasing the mature part of the PRK while the intein tag remains bound to the column. Elution of the PRK by 20 mm HEPES, pH 7.5, 50 mm NaCl; 1 mm EDTA resulted in a sharp peak fraction containing PRK protein with a purity of approx 95%. Further purification was achieved by ion-exchange chromatography on a prepacked Resource Q column (2 mL, Amersham, Buckinghamshire, UK) equilibrated in 20 mm BisTris propane, pH 7.5, 1 mm EDTA, and 50 mm NaCl. After a washing step to decrease unspecific binding, the PRK was eluted in a linear NaCl gradient (50–1,000). The eluate was concentrated by high pressure filtration, dialyzed 4 times against storage buffer (50 mm Bicine/KOH, pH 8.0, and 20% [v/v] glycerol) and stored at −80°C.
Preparation of PRK from Spinach
Chloroplasts from 2 kg of spinach were prepared as described above. The plastids were then broken by addition of 300 to 400 mL of 50 mm Bicine/KOH, pH 8.0, 2.5 mm EDTA, 1 mm phenlymethane sulfonfluorid, 5 mm DTT, 5 mm MgCl2, and 10 mm NaCl for 10 min on ice. The suspension was then centrifuged for 20 min at 12,000g. The second pellet obtained from a fractionated ammonium sulfate precipitation (35% and 65%) was resuspended in 50 mm Bicine/KOH, pH 8.0, 1 mm EDTA, and 10 mm DTT (BED) buffer and diluted to a maximum current of 5 mS/cm. Further purification of the PRK was performed according to Brandes et al. (1996): The proteins were loaded onto a Toyopearl Super-Q650m ion-exchange column and eluted by a 50 to 500 mm NaCl gradient in BED buffer. The fractions containing PRK activity were pooled and precipitated by adding 65% (w/v) ammonium sulfate. For the next purification step by gel exclusion chromatography the protein was dissolved in BED buffer containing 150 mm NaCl and loaded in a small volume onto a 300-mL BioGel A5m column. Again, PRK-containing fractions were pooled and concentrated by ammonium sulfate precipitation. After resuspension the protein was dialyzed against BED buffer and the solution adjusted to pH 6.5 by addition of crystalline Bicine, followed by loading on a Reactive Red affinity chromatography column. After washing with 50 mm Bicine/KOH, pH 6.5, and 10 mm DTT, the column was washed with 10 mm sodium-phosphate, pH 6.8, and the protein eluted with 10 mm sodium-phosphate pH 7.2, 10 mm DTT, and 5 mm ATP. The peak fractions were dialyzed and stored at −80°C.
Overexpression and Purification of Thioredoxin
Spinach thioredoxins f and m were expressed from plasmids Trxf and Trxm (Stumpp et al., 1999) that were kindly provided by Dr. Toru Hisabori (Tokyo Institute of Technology). Plasmids were expressed in E. coli strain BL21 (DE3) in Luria-Bertani media and purified by hydrophobic interaction chromatography as described in Stumpp et al. (1999).
Protein and Substrate Concentrations
The concentration of purified PRK and thioredoxin was determined by the Bradford Assay (Bio-Rad Laboratories, Hercules, CA). Substrates were measured directly in a photometer: ATP (λ = 259 nm, ɛ = 15.4 mmol−1 cm−1), TNB2− (412 nm, ɛ = 14.15 mmol−1 cm−1), NADH and NADPH (340 nm, ɛ = 6.22 mmol−1 cm−1), and oxidized DTT (310 nm, ɛ = 0.11 mmol−1 cm−1).
Inactivation of PRK by DTNB
PRK from Odontella was inhibited by DTNB according to Porter et al. (1988). Purified enzyme (5.7 μg) was incubated with stepwise increasing concentrations of DTNB. After each addition, we waited until absorption at λ = 412 nm was stable (approximately 5 min) and removed a sample for measuring the activity of the PRK. At the end of the titration, TNB was removed by gel filtration and dialysis and the protein was analyzed at 412 nm for the incorporation of TNB into the protein. One aliquot was subjected to SDS-PAGE under nonreducing conditions.
Determination of Redox Potentials
Solutions of defined pre-equilibrated redox potentials (Eh) were generated in oxygen-free buffer according to Hutchison and Ort (1995) by mixing different amounts of reduced and oxidized DTT. Fully oxidized spinach PRK (1 μm) was incubated in a volume of 100 μL MOPS buffer (100 mm, pH 7) with a defined Eh at a total DTT concentration of 80 mm. After incubation for 120 min at 25°C samples were withdrawn to determine Eh and the PRK activity as a measure for the reduced enzyme concentration. PRK from Odontella (0.5 μm) was incubated accordingly for 75 min at 10°C in buffers (150 mm) at various pH values (pH 7 and 7.5: MOPS buffer; pH 8: Bicine buffer; pH 8.5 and 9: BisTris propane buffer) and analyzed as described. Experimental data were fitted to the Nernst equation for a two-electron single-component system (spinach) or a two-electron two-component system (Odontella).
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number Y08610.
Acknowledgments
The authors thank Prof. Dr. Heinrich Strotmann for support and helpful discussions, Prof. Dr. David Knaff for helpful suggestions, Prof. Dr. Toru Hisabori and Dr. Johann Lavaud for critical comments on the manuscript, and Renate Thelen and Katharina Weyrauch for help in cloning the Prk gene.
This work was supported by the University of Konstanz, the Deutsche Forschungsgemeinschaft (Transregio-SFB TR1, project Kr 1661–2, grant to P.G.K.), and by the European community (MARGENES, grant no. QLRT–2001–01226 to P.G.K.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.055285.
References
- Anderson LE (1973) Regulation of the pea leaf ribulose-5-phosphate kinase activity. Biochim Biophys Acta 321: 484–488 [DOI] [PubMed] [Google Scholar]
- Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam NH, Zhou S, Allen AE, Apt KE, Bechner M, et al (2004) The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306: 79–86 [DOI] [PubMed] [Google Scholar]
- Badger MR, Hanson D, Price GD (2002) Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria. Funct Plant Biol 29: 161–173 [DOI] [PubMed] [Google Scholar]
- Badger MR, von Caemmerer S, Ruuska S, Nakano H (2000) Electron flow to oxygen in higher plants and algae: rates and control of direct photoreduction (Mehler reaction) and rubisco oxygenase. Philos Trans R Soc Lond B Biol Sci 355: 1433–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaya D, Grossman AR (1993) Characterization of gene clusters encoding the fucoxanthin chlorophyll proteins of the diatom Phaeodactylum tricornutum. Nucleic Acids Res 21: 4458–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes HK, Hartman FC, Lu T-YS, Larimer FW (1996) Efficient expression of the gene for spinach phosphoribulokinase in Pichia pastoris and utilization of the recombinant enzyme to explore the role of regulatory cysteinyl residues by site-directed mutagenesis. J Biol Chem 271: 6490–6496 [DOI] [PubMed] [Google Scholar]
- Buchanan BB (1980) Role of light in the regulation of chloroplast enzymes. Annu Rev Plant Physiol 31: 341–374 [Google Scholar]
- Büchel C (2003) Fucoxanthin-chlorophyll proteins in diatoms: 18 and 19 kDa subunits assemble into different oligomeric states. Biochemistry 42: 13027–13034 [DOI] [PubMed] [Google Scholar]
- Cline K, Werner-Washburne M, Lubben TH, Keegstra K (1984) Thermolysin is a suitable protease for probing the surface of intact pea plastids. Plant Physiol 75: 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche CF, Palmer JD (1997) The origin of plastids and their spread via secondary symbiosis. Plant Syst Evol 11: 53–86 [Google Scholar]
- Eicks M, Maurino V, Knappe S, Flügge UI, Fischer K (2002) The plastidic pentose phosphate translocator represents a link between the cytosolic and the plastidic pentose phosphate pathways in plants. Plant Physiol 128: 512–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski PG, Barber RT, Smetacek V (1998) Biogeochemical controls and feedbacks on ocean primary production. Science 281: 200–205 [DOI] [PubMed] [Google Scholar]
- Farr TJ, Huppe HC, Turpin DH (1994) Coordination of chloroplastic metabolism in N-limited Chlamydomonas reinhardtii by redox modulation: The activation of phosphoribulosekinase and glucose-6-phosphate dehydrogenase is relative to the photosynthetic supply of electrons. Plant Physiol 105: 1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardemann A, Stitt M, Heldt HW (1983) Control of CO2 fixation: regulation of spinach ribulose-5-phosphate kinase by stromal metabolite levels. Biochim Biophys Acta 722: 51–60 [Google Scholar]
- Geck MK, Hartman FC (2000) Kinetic and mutational analyses of the regulation of phosphoribulokinase by thioredoxins. J Biol Chem 275: 18034–18039 [DOI] [PubMed] [Google Scholar]
- Hariharan T, Johnson PJ, Cattolico RA (1998) Purification and characterization of phosphoribulokinase from the marine chromophytic alga Heterosigma carterae. Plant Physiol 117: 321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt HW, Sauer F (1971) The inner membrane of the chloroplast as the site of specific metabolite transport. Biochim Biophys Acta 234: 83–91 [DOI] [PubMed] [Google Scholar]
- Hertig CM, Wolosiuk RA (1983) Studies on the hysteretic properties of chloroplast fructose-1,6-bisphosphatase. J Biol Chem 258: 984–989 [PubMed] [Google Scholar]
- Hirasawa M, Brandes HK, Hartman FC, Knaff DB (1998) Oxidation-reduction properties of the regulatory site of spinach phosphoribulokinase. Arch Biochem Biophys 350: 127–131 [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Schürmann P, Jacquot JP, Manieri W, Jacquot P, Keryer E, Hartman FC, Knaff DB (1999) Oxidation-reduction properties of chloroplast thioredoxins, ferredoxin:thioredoxin reductase, and thioredoxin f-regulated enzymes. Biochemistry 38: 5200–5205 [DOI] [PubMed] [Google Scholar]
- Hutchison RS, Ort DR (1995) Measurement of equilibrium midpoint potentials of thiol/disulfide regulatory groups in thioredoxin activated chloroplast enzymes. Methods Enzymol 252: 220–228 [DOI] [PubMed] [Google Scholar]
- Hwang S-R, Tabita FR (1991) Cotranscription, deduced primary structure, and expression of the chloroplast-encoded rbcL and rbcS genes of the marine diatom Cylindrotheca sp. strain N1. J Biol Chem 266: 6271–6279 [PubMed] [Google Scholar]
- Jacquot J-P, Lancelin J-M, Meyer Y (1997) Thioredoxins: structure and function in plant cells. New Phytol 136: 543–570 [DOI] [PubMed] [Google Scholar]
- Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz (BPP) 167: 191–194 [Google Scholar]
- Jensen RA (1985) The shikimate/arogenate pathway: link between carbohydrate metabolism and secondary metabolism. Physiol Plant 66: 164–168 [Google Scholar]
- Kilian O, Kroth PG (2005) Identification and characterization of a new conserved motif within the presequence of proteins targeted into complex diatom plastids. Plant J 41: 175–183 [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Tamoi M, Iwaki T, Shigeoka S, Wadano A (2003) Molecular characterization and redox regulation of phosphoribulokinase from the cyanobacterium Synechococcus sp. PCC 7942. Plant Cell Physiol 44: 269–276 [DOI] [PubMed] [Google Scholar]
- Kroth PG (2002) Protein transport into secondary plastids and the evolution of primary and secondary plastids. Int Rev Cytol 221: 191–255 [DOI] [PubMed] [Google Scholar]
- Lavaud J, van Gorkom HJ, Etienne AL (2002) Photosystem II electron transfer cycle and chlororespiration in planktonic diatoms. Photosynth Res 74: 49–57 [DOI] [PubMed] [Google Scholar]
- Lebreton S, Graciet E, Gontero B (2003) Modulation, via protein-protein interactions, of glyceraldehyde-3-phosphate dehydrogenase activity through redox phosphoribulokinase regulation. J Biol Chem 278: 12078–12084 [DOI] [PubMed] [Google Scholar]
- Lees WJ, Whitesides GM (1993) Equilibrium constants for thiol-disulfide interchange reactions: a coherent, corrected set. J Org Chem 58: 642–647 [Google Scholar]
- Liaud MF, Lichtle C, Apt K, Martin W, Cerff R (2000) Compartment-specific isoforms of TPI and GAPDH are imported into diatom mitochondria as a fusion protein: evidence in favor of a mitochondrial origin of the eukaryotic glycolytic pathway. Mol Biol Evol 17: 213–223 [DOI] [PubMed] [Google Scholar]
- Lundström J, Holmgren A (1993) Determination of the reduction-oxidation potential of the thioredoxin-like domains of protein disulfide isomerase from the equilibrium with glutathion and thioredoxin. Biochemistry 32: 6649–6655 [DOI] [PubMed] [Google Scholar]
- Martin W, Schnarrenberger C (1997) The evolution of the Calvin cycle from prokaryotic to eukaryotic chromosomes: a case study of functional redundancy in ancient pathways through endosymbiosis. Curr Genet 32: 1–18 [DOI] [PubMed] [Google Scholar]
- Martin W, Stoebe B, Goremykin V, Hansmann S, Hasegawa M, Kowallik KV (1998) Gene transfer to the nucleus and the evolution of chloroplasts. Nature 393: 162–165 [DOI] [PubMed] [Google Scholar]
- Milanez S, Mural RJ (1988) Cloning and sequencing of cDNA encoding the mature form of phosphoribulokinase from spinach. Gene 66: 55–63 [DOI] [PubMed] [Google Scholar]
- Milanez S, Mural RJ, Hartman FC (1991) Roles of cysteinyl residues of phosphoribulokinase as examined by site-directed mutagenesis. J Biol Chem 266: 10694–10699 [PubMed] [Google Scholar]
- Moreira D, Le Guyader H, Philippe H (2000) The origin of red algae and the evolution of chloroplasts. Nature 405: 69–72 [DOI] [PubMed] [Google Scholar]
- Mouche F, Gontero B, Callebaut I, Mornon JP, Boisset N (2002) Striking conformational change suspected within the phosphoribulokinase dimer induced by interaction with GAPDH. J Biol Chem 277: 6743–6749 [DOI] [PubMed] [Google Scholar]
- Ocheretina O, Haferkamp I, Tellioglu H, Scheibe R (2000) Light-modulated NADP-malate dehydrogenases from mossfern and green algae: insights into evolution of the enzyme's regulation. Gene 258: 147–154 [DOI] [PubMed] [Google Scholar]
- Pancic PG, Kowallik KV, Strotmann H (1990) Characterization of CF1 from the diatom Odontella sinensis. Bot Acta 103: 274–280 [Google Scholar]
- Pancic PG, Strotmann H (1993) Structure of the nuclear encoded gamma subunit of CF0CF1 of the diatom Odontella sinensis including its presequence. FEBS Lett 320: 61–66 [DOI] [PubMed] [Google Scholar]
- Porter MA, Hartman FC (1990) Exploration of the function of a regulatory sulfhydryl of phosphoribulokinase from spinach. Arch Biochem Biophys 281: 330–334 [DOI] [PubMed] [Google Scholar]
- Porter MA, Milanez S, Stringer CD, Hartman FC (1986) Purification and characterization of ribulose-5-phosphate kinase from spinach. Arch Biochem Biophys 245: 14–23 [DOI] [PubMed] [Google Scholar]
- Porter MA, Stringer CD, Hartman FC (1988) Characterization of the regulatory thioredoxin site of phosphoribulokinase. J Biol Chem 263: 123–129 [PubMed] [Google Scholar]
- Racker E (1957) The reductive pentose phosphate cycle. I. Phosphoribulokinase and ribulose diphosphate carboxylase. Arch Biochem Biophys 69: 300–310 [DOI] [PubMed] [Google Scholar]
- Riddles WP, Blakeley RL, Zerner B (1979) Ellmann's reagent: 5,5′-Dithiobis (nitrobenzoic acid): a reexamination. Anal Biochem 94: 75–81 [DOI] [PubMed] [Google Scholar]
- Ruelland E, Miginiac-Maslow M (1999) Regulation of chloroplast enzyme activities by thioredoxin: activation or relief from inhibition? Trends Plant Sci 4: 136–141 [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C, Flechner A, Martin W (1995) Enzymatic evidence for a complete oxidative pentose phosphate pathway in chloroplasts and an incomplete pathway in the cytosol of spinach leaves. Plant Physiol 108: 609–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C, Oeser A, Tolbert NE (1973) Two isoenzymes each of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in spinach leaves. Arch Biochem Biophys 154: 438–448 [DOI] [PubMed] [Google Scholar]
- Stumpp MT, Motohashi K, Hisabori T (1999) Chloroplast thioredoxin mutants without active-site cysteines facilitate the reduction of the regulatory disulphide bridge on the γ-subunit of chloroplast ATP synthase. Biochem J 341: 157–163 [PMC free article] [PubMed] [Google Scholar]
- Wedel N, Soll J (1998) Evolutionary conserved light regulation of Calvin cycle activity by NADPH-mediated reversible phosphoribulokinase/CP12/glyceraldehyde-3-phosphate dehydrogenase complex dissociation. Proc Natl Acad Sci USA 95: 9699–9704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedel N, Soll J, Paap BK (1997) CP12 provides a new mode of light regulation of Calvin cycle activity in higher plants. Proc Natl Acad Sci USA 94: 10479–10484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderoth I, Scheibe R, von Schaewen A (1997) Identification of the cysteine residues involved in redox modification of plant plastidic glucose-6-phosphate dehydrogenase. J Biol Chem 272: 26985–26990 [DOI] [PubMed] [Google Scholar]
- Wittpoth C, Kroth PG, Weyrauch K, Kowallik KV, Strotmann H (1998) Functional characterization of isolated plastids from two marine diatoms. Planta 206: 79–85 [Google Scholar]
- Wolosiuk RA, Buchanan BB (1978) Regulation of chloroplast phosphoribulokinase by the ferredoxin/thioredoxin system. Arch Biochem Biophys 189: 97–101 [DOI] [PubMed] [Google Scholar]