Abstract
Mutations in the biosynthesis or signaling pathways of gibberellin (GA) can cause dwarfing phenotypes in plants, and the use of such mutations in plant breeding was a major factor in the success of the Green Revolution. DELLA proteins are GA signaling repressors whose functions are conserved in different plant species. Recent studies show that GA promotes stem growth by causing degradation of DELLA proteins via the ubiquitin-proteasome pathway. The most widely utilized dwarfing alleles in wheat (Triticum aestivum; e.g. Rht-B1b and Rht-D1b) encode GA-resistant forms of a DELLA protein that function as dominant and constitutively active repressors of stem growth. All of the previously identified dominant DELLA repressors from several plant species contain N-terminal mutations. Here we report on a novel dwarf mutant from Brassica rapa (Brrga1-d) that is caused by substitution of a conserved amino acid in the C-terminal domain of a DELLA protein. Brrga1-d, like N-terminal DELLA mutants, retains its repressor function and accumulates to high levels, even in the presence of GA. However, unlike wild-type and N-terminal DELLA mutants, Brrga1-d does not interact with a protein component required for degradation, suggesting that the mutated amino acid causes dwarfism by preventing an interaction needed for its degradation. This novel mutation confers nondeleterious dwarf phenotypes when transferred to Arabidopsis (Arabidopsis thaliana) and oilseed rape (Brassica napus), indicating its potential usefulness in other crop species.
Dwarfism is a desirable characteristic for many agricultural plants. In grain crops, dwarfism can reduce lodging and increase harvest index, and the breeding of dwarf wheat (Triticum aestivum) and rice (Oryza sativa) cultivars was a major factor in the success of the Green Revolution (Khush, 2001). Dwarfism is often caused by mutations in genes controlling the biosynthesis or signaling pathway of the plant hormone GA (Peng et al., 1999; Hedden, 2003; Sun and Gubler, 2004). The most widely utilized semi-dwarf wheat cultivars in agriculture contain the Rht-B1b or Rht-D1b allele that encodes a mutant form of a DELLA protein, a GA signaling repressor (Peng et al., 1999; Hedden, 2003).
DELLA proteins encoded by Rht and its orthologs in Arabidopsis (Arabidopsis thaliana; GAI, RGA, RGL1, and RGL2), maize (Zea mays; d8), grape (Vitis vinifera; VvGAI), barley (Hordeum vulgare; SLN1), and rice (SLR1) have conserved function as repressors of GA signaling (Sun and Gubler, 2004). Five DELLA protein genes (GAI, RGA, RGL1, RGL2, and RGL3) are present in Arabidopsis, and with the exception of RGL3, these genes have been shown to share partially overlapping roles in repressing GA-regulated plant growth and development (Thomas and Sun, 2004). Genetic analysis indicates that RGA and GAI are the major repressors that modulate GA-mediated vegetative growth and floral induction; RGL2 is important in regulating seed germination; and RGL1 and RGL2, along with RGA, control flower development (Dill and Sun, 2001; King et al., 2001; Lee et al., 2002; Cheng et al., 2004; Tyler et al., 2004). In contrast, rice and barley each contains only one DELLA protein gene (SLR1 and SLN1, respectively). Loss-of-function mutations in these single genes affect all GA-regulated processes in these plants (Ikeda et al., 2001; Chandler et al., 2002).
The DELLA designation refers to the first of two amino acid sequences in the N-terminal domain that are conserved among all DELLA proteins. These DELLA proteins belong to a subfamily of the GRAS (for GAI, RGA, and SCARECROW) family of proteins that regulate diverse aspects of plant development (Pysh et al., 1999; Tian et al., 2004). All GRAS proteins share a conserved C-terminal GRAS domain, but their N termini are more divergent. The GRAS domain contains two Leu heptad repeat motifs, presumably for protein-protein interactions. In addition, one or two putative nuclear localization signals are present in some of the GRAS members, suggesting that they may function as transcriptional regulators (Pysh et al., 1999). Indeed, several DELLA proteins, when fused with the green fluorescence protein, are localized to the nuclei in transgenic plants (Silverstone et al., 2001; Fleck and Harberd, 2002; Gubler et al., 2002; Itoh et al., 2002; Wen and Chang, 2002).
Recent studies show that GA derepresses its signaling pathway by inducing degradation of the DELLA proteins (Gomi and Matsuoka, 2003) and that this proteolysis event is targeted by a ubiquitin E3 ligase complex SCFSLY1/GID2 to the 26S proteasome (McGinnis et al., 2003; Sasaki et al., 2003). In wild-type plants, DELLA repressors are degraded in the presence of GA, and GA-promoted growth occurs (Silverstone et al., 2001; Gubler et al., 2002; Itoh et al., 2002). Importantly, the N-terminal DELLA domain (including the DELLA and VHYNP motifs and nearby sequences) is essential for controlling DELLA protein stability by GA. Deletion and specific point mutations within the DELLA domain cause the DELLA protein to be resistant to GA-dependent degradation (Dill et al., 2001; Gubler et al., 2002; Itoh et al., 2002) and confer dominant, GA-insensitive dwarf phenotypes (Peng et al., 1997, 1999). In contrast, all of the previously identified mutations in the C-terminal GRAS domain of DELLA proteins are loss-of-function and cause recessive slender phenotypes in several plant species, suggesting that this C-terminal domain is important for its repressor function (Peng et al., 1997; Gubler et al., 2002; Itoh et al., 2002; Dill et al., 2004).
We previously characterized a semidominant, GA-insensitive dwarf mutant, dwf2, of Brassica rapa, which significantly reduced the height of cultivated oilseed B. rapa in field experiments (Muangprom and Osborn, 2004; Fig. 1). Our comparative mapping analysis with the related model organism Arabidopsis identified a homolog of Arabidopsis DELLA protein gene RGA as a candidate for DWF2. In this article, we report on the isolation and characterization of this B. rapa dwarf gene. We show that a single nucleotide substitution in the C-terminal GRAS domain of an RGA homolog causes the dominant dwarf phenotype, and it appears to do so by a novel mechanism involving the prevention of an interaction with an F-box protein required for DELLA protein degradation. This work reports a gain-of-function mutation in the GRAS domain that results in a constitutively active DELLA repressor.
Figure 1.
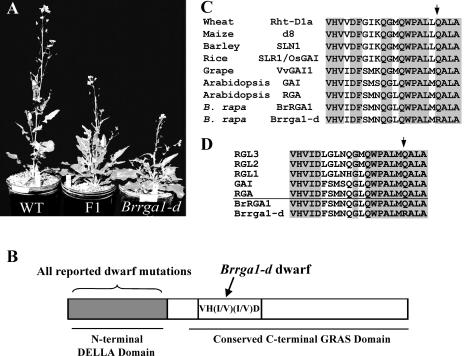
Mutation of the conserved Q residue in the VHIID region of BrRGA1 causes dwarfism. A, Phenotypes of near-isogenic B. rapa plants: homozygous wild-type BrRGA1 (WT), heterozygous BrRGA1/Brrga1-d (F1), and homozygous Brrga1-d. B, Structure of DELLA proteins showing positions of mutations causing dwarfism. Mutations in the N terminus result in dwarf plants in wheat (Rht-B1b, Rht-D1b), maize (D8-Mp1, D8-1, D8-2023), Arabidopsis (gai, rga-Δ17, rgl1-Δ17), barley (Sln1d), rice (slrΔDELLA), and grape (Vvgai1; Peng et al., 1999; Dill et al., 2001; Boss and Thomas, 2002; Chandler et al., 2002; Itoh et al., 2002; Wen and Chang, 2002), while Brrga1-d dwarf B. rapa is caused by a mutation in the C-terminal region. C and D, Amino acid sequence alignment comparing VHIID regions of Brrga1-d and BrRGA1 with those of other DELLA proteins functioning as GA signaling repressors from different plant species (C) or with all five Arabidopsis DELLA proteins that control diverse processes of plant growth and development (D). Arrows indicate position of the mutated amino acid in Brrga1-d.
RESULTS
Comparative Fine Mapping and Cloning of BrRGA1 and BrRGA2
We previously mapped DWF2 to the bottom of the B. rapa linkage group R6 in a region having homology to the top of Arabidopsis chromosome 2 (Muangprom and Osborn, 2004). The alignment suggested that DWF2 is homologous to RGA in Arabidopsis; however, we were unable to detect RFLPs for the BrRGA sequence or for marker sequences that are similar to Arabidopsis sequences flanking RGA, in order to test for cosegregation of DWF2 and BrRGA1. In this study, we observed complete cosegregation of the BrRGA1 sequence (detected by using single strand conformation polymorphism analyses [SSCP]; see “Materials and Methods”) with alleles at the DWF2 locus in 410 backcross (BC5) plants. In addition, 2 B. rapa sequences, which are similar to the Arabidopsis sequences flanking the RGA locus (At2g01560, 5.0 kb from RGA; and At2g01720, 61 kb from RGA), were analyzed by SSCP in the BC5 population, and they were located 1.96 and 0.50 cM on either side of BrRGA1/DWF2.
The results from SSCP gels indicated that the BrRGA PCR primers amplified at least two different products. Only one of the products was polymorphic in the BC5 population for which the detected alleles showed perfect cosegregation with plant height. The other product showed no polymorphism. Sequence analysis indicated that both PCR products showed highest sequence similarity to AX081276, a genomic sequence from oilseed rape (Brassica napus), and for genes with defined functions both showed highest homology to Arabidopsis RGA. We named the PCR product that cosegregated with DWF2 as BrRGA1 and the other as BrRGA2. Using thermal asymmetrical interlaced-PCR, we were able to amplify complete coding sequence and 5′ and 3′ untranslated regions of both genes.
The deduced BrRGA1 and BrRGA2 proteins are 573 and 579 amino acids, respectively. These two proteins contain all conserved regions as defined previously for DELLA protein (Sun and Gubler, 2004). The BrRGA1 showed 77% (456/591) amino acid identities and 80% (479/591) similarities to BrRGA2. Using BLAST to search the Plant Protein database (http://www.arabidopsis.org/Blast/), BrRGA1 showed the highest amino acid sequence homology to CAC3312, a predicted protein product of AX081276 from oilseed rape, with 90% identities and 90% (521/573) similarities and showed 76% (446/583) identities and 80% (470/583) similarities to RGA.
Molecular Analysis of the dwf2 (Brrga1-d) Mutant Allele
To verify that DWF2 is BrRGA1, we analyzed DNA sequence of BrRGA1 in the dwf2 mutant and identified a single nucleotide substitution changing a conserved Q-to-R in the C-terminal GRAS domain near the VH(I/V)(I/)D motif. Based on these results and the results from transgenic analyses presented below, we renamed DWF2 as BrRGA1 and the dwf2 allele as Brrga1-d. The Brrga1-d mutant is a semidominant and GA-insensitive dwarf (Fig. 1; Muangprom and Osborn, 2004) whose phenotype is similar to the gain-of-function DELLA gene mutants in other species (Thomas and Sun, 2004). This mutation is novel because all previously reported semidominant, GA-insensitive dwarfs in other species are caused by mutations in the N-terminal domain of the DELLA protein (Fig. 1B). This Q residue (Q328 in BrRGA1 and Q341 in AtRGA) is likely to be important for the DELLA protein function because it is absolutely conserved in all known DELLA repressors from different plant species (Fig. 1, C and D; Silverstone et al., 1998; Peng et al., 1999; Boss and Thomas, 2002; Chandler et al., 2002; Lee et al., 2002; Wen and Chang, 2002; Tyler et al., 2004).
Brrga1-d Affects Feedback Regulation of a GA Biosynthetic Gene
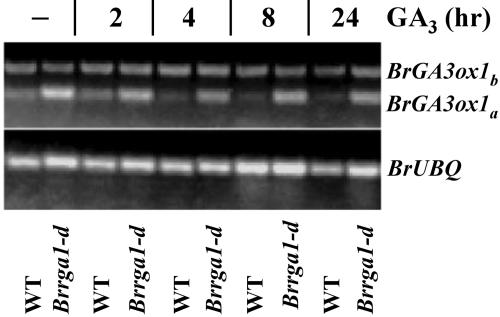
Expression of some of the GA biosynthetic genes (e.g. GA 3-oxidase [GA3ox]) is affected by activity of the GA response pathway via a feedback mechanism (Olszewski et al., 2002). Previous studies reported that DELLA motif deletion mutants, gai-1 and rga-Δ17, exhibit reduced feedback inhibition of the GA biosynthetic gene AtGA3ox1 expression in response to GA (Cowling et al., 1998; Dill et al., 2001). To determine if Brrga1-d also affects the feedback inhibition of GA3ox expression, degenerate primers designed for conserved sequences of Arabidopsis and pea (Pisum sativum) GA3ox were used to amplify two putative GA3ox cDNAs from B. rapa by reverse transcription (RT)-PCR. DNA sequence analysis showed that both PCR products have highest sequence similarity to Arabidopsis GA3ox1 (At1g15550), suggesting that these two cDNAs are Brassica GA3ox (named BrGA3ox1a and BrGA3ox1b). RNA-blot analysis showed that transcript levels of BrGA3ox1a were higher in Brrga1-d than in wild-type shoots of 2-week-old plants (Fig. 2). Furthermore, GA application reduced the amount of this transcript in wild-type but not in Brrga1-d shoots. In contrast, expression of BrGA3ox1b was not responsive to GA treatment (Fig. 2), consistent with previous findings that only a subset of the GA3ox genes is under feedback regulation (Olszewski et al., 2002).
Figure 2.
Brrga1-d affects feedback regulation of a putative BrGA3ox gene. Transcript levels of BrGA3ox1 detected by RT-PCR in shoots of 2-week-old Brrga1-d and wild-type (WT) plants with no GA (−) and 2, 4, 8, and 24 h after GA application. The pair of primers used for RT-PCR amplified 2 products (BrGA3ox1a and BrGA3ox1b), both of which show highest homology to At1g15550 (Arabidopsis GA3ox) with score 343 and E-value of 5e-094 for BrGA3ox1a, and score 325 and E-value of 1e-088 for BrGA3ox1b.
Brrga1-d Protein Is Resistant to GA-Induced Degradation
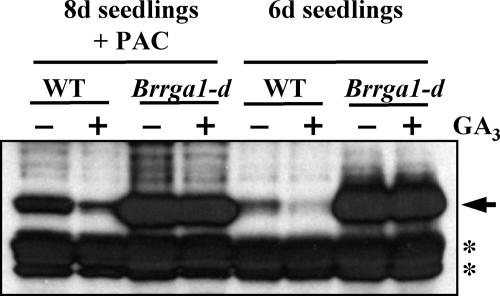
GA promotes its signaling pathway by causing proteolysis of the DELLA proteins. Previous studies showed that gain-of-function mutations in the DELLA motif of RGA, SLR1, and SLN1 (rga-Δ17, slr1ΔDELLA, sln1d) not only confer a GA-insensitive dwarf phenotype but also allow the mutant proteins to be resistant to GA-induced degradation (Dill et al., 2001; Gubler et al., 2002; Itoh et al., 2002). Immunoblot analysis using polyclonal anti-AtRGA antibodies was performed to examine the level and GA responsiveness of Brrga1-d in comparison to the wild-type BrRGA1. Consistent with the previous findings, we showed that GA induced BrRGA1 degradation, whereas treatment of the GA biosynthesis inhibitor paclobutrazol (PAC) resulted in an elevated accumulation of BrRGA1 in 8-d-old wild-type seedlings (Fig. 3). Interestingly, Brrga1-d protein accumulated to much higher levels than did BrRGA1, and this mutant protein is resistant to GA-induced degradation (Fig. 3).
Figure 3.
Brrga1-d is resistant to GA-induced degradation. Immunoblot analysis using purified anti-RGA polyclonal antibodies on protein extracted from 6-d-old seedlings or 8-d-old seedlings pretreated with GA biosynthesis inhibitor, PAC, of Brrga1-d and wild-type B. rapa plants with (+) and without (−) GA treatment. Protein bands indicated with an arrow are BrRGA1 or Brrga1-d proteins, whereas those marked with an asterisk (*) represent nonspecific immunoreactive proteins.
To determine whether BrRGA1 expression at mRNA level is different in wild-type and Brrga1-d plants, we measured the levels of the BrRGA1 mRNA in a number of tissues, including shoot of 2-week-old seedling, and leaf, stem, open flower, bud, and siliques of 4-week-old plants. Using semiquantitative RT-PCR method, we found that BrRGA1 transcripts in wild-type and Brrga1-d plants are present at similar levels in all tissues tested (data not shown), supporting the idea that the function of this gene is mainly regulated at the protein level.
Q341-to-R Mutation in AtRGA Confers a Semidwarf Phenotype in Arabidopsis
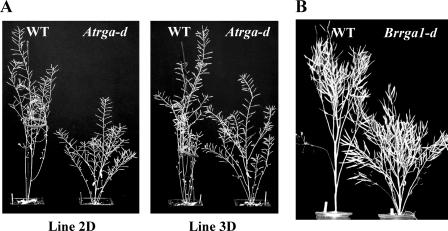
To investigate the effect of mutating the conserved Q in the DELLA proteins of other species, we generated transgenic Arabidopsis plants expressing Atrga-d with an identical mutation as in Brrga1-d under the native AtRGA promoter. Approximately 60% of the T1 plants (22 total) containing the mutant Atrga-d construct had plant heights that were 65% to 80% of wild-type plants (data not shown). T2 plants carrying homozygous Atrga-d mutant transgene were dwarfed compared to T2 segregants lacking the transgene (Fig. 4A).
Figure 4.
Plants containing the Brrga1-d mutation are dwarf. A, Arabidopsis ecotype Wassilewskija is dwarfed by expressing a mutated Atrga transgene with the Brrga1-d mutation (Atrga-d). Primary transformants (T1 plants) were allowed to set seed (by self-pollination), and progeny (T2 plants) from two independent transgenic lines (2D and 3D) are shown. Left, T2 plants lacking the transgene. Right, T2 plants that are homozygous for the transgene. B, Hybrid oilseed rape is dwarfed by having the Brrga1-d allele transferred by interspecific hybridization of B. rapa and B. oleracea, embryo rescue, chromosome doubling, and backcrossing to the parents of a hybrid combination (A. Muangprom, I. Mauriera, and T.C. Osborn, unpublished data). Left, BrRGA1 wild-type hybrid. Right, Brrga1-d semidwarf hybrid.
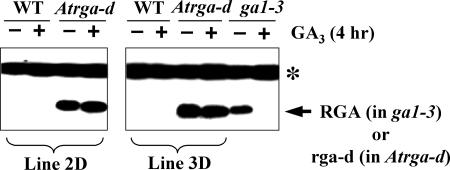
Immunoblot analysis using polyclonal anti-RGA antibodies showed that the level of the repressor protein in T2 segregants containing Atrga1-d was much higher than that in T2 segregants containing only RGA (Fig. 5). In addition, the mutant repressor protein did not disappear with GA application (Fig. 5). These results confirm the constitutive, suppressive function of the GA-resistant Atrga-d mutant protein, and they indicate that the effect of this Q-to-R mutation in DELLA proteins is conserved in different plant species.
Figure 5.
Atrga-d is resistant to GA-induced degradation. Immunoblot results showing the levels of AtRGA or Atrga-d proteins in plants that were described in Figure 4A with (+) and without (−) GA application. Plants transformed with the wild-type transgene did not show accumulation of the wild-type repressor protein (data not shown). The GA-deficient mutant ga1-3 that accumulates higher amounts of AtRGA than wild type was used as a control. Protein bands indicated with arrows are AtRGA or Atrga-d proteins, whereas those marked with an asterisk (*) represent nonspecific immunoreactive proteins.
Atrga1-d Does Not Interact with SLY1 or sly1-d in Yeast Two-Hybrid Assay
Recent studies suggest that the E3 ubiquitin ligase complex SCFGID2/SLY1 targets DELLA proteins for GA-induced degradation by the 26S proteasome (Itoh et al., 2003; Thomas and Sun, 2004). Using yeast two-hybrid assays, all five Arabidopsis DELLA proteins were shown to interact with the Arabidopsis F-box protein SLY1 (Dill et al., 2004; Fu et al., 2004; Tyler et al., 2004). Moreover, the GRAS domain is required for SLY1 binding, whereas the DELLA motif is not (Dill et al., 2004). We found that the mutant Atrga-d did not interact with SLY1 in the yeast two-hybrid system (Table I). Recently, a gain-of-function sly1-d mutation was shown to enhance the interaction of sly1-d with the DELLA proteins leading to a significant reduction of both wild-type and GA-resistant rga-Δ17 proteins, explaining why sly1-d suppressed the dwarf phenotype of rga-Δ17 (Dill et al., 2004; Fu et al., 2004). However, the mutant Atrga-d protein did not interact with sly1-d in the yeast cells (Table I), further supporting the idea that the high accumulation of mutant repressor protein Atrga-d is due to loss of interaction with SLY1.
Table I.
The Arabidopsis rga-d mutation abolishes the interaction with SLY1 and sly1-d in yeast two-hybrid assays
| DB Fusion | AD Fusion | His− Media + 3-ATa | β-Gal Unitsb |
|---|---|---|---|
| mm | |||
| SLY1 | AtRGA | 5 | 0.3 ± 0.0 |
| sly1-d | AtRGA | 60 | 186.5 ± 8.7 |
| SLY1 | Atrga-d | − | <0.1 |
| sly1-d | Atrga-d | − | <0.1 |
| LexA | AtRGA | − | <0.1 |
| LexA | Atrga-d | − | <0.1 |
| SLY1 | Gal4 | − | <0.1 |
| sly1-d | Gal4 | − | <0.1 |
| LexA | Gal4 | 1 | <0.1 |
The relative growth on His− plates containing 3-AT (0, 1, 2, 5, 10, 30, and 60 mm). Each value indicates the highest concentration of 3-AT in which the yeast was able to grow. Dash indicates no growth on 0 mm 3-AT. The experiments were repeated once and the same results were obtained.
For each pairwise combination, three individual colonies were used to measure β-gal activity; the average ± se is shown.
DISCUSSION
Using comparative mapping analysis with the related model organism Arabidopsis, we identified a homolog of Arabidopsis RGA (BrRGA1) to be DWF2 in B. rapa (Muangprom and Osborn, 2004; this report). Surprisingly, we found that a novel Q-to-R mutation near the VH(I/V)(I/V)D motif in the C-terminal GRAS domain of BrRGA1 results in the GA-insensitive dwarf (Brrga1-d; Fig. 1). A mutation in the Arabidopsis RGA gene (Atrga-d) that is identical to that in Brrga1-d also confers a dwarf phenotype in transgenic Arabidopsis, indicating that this conserved Q residue has a similar role in DELLA proteins of different species.
Interestingly, while homozygous transgenic plants expressing rga-Δ17 are extremely dwarf and sterile (Dill et al., 2001), homozygous transgenic Atrga-d plants are semidwarf and fully fertile (Fig. 4A). The phenotype of the Atrga-d plants is consistent with the semidwarf phenotype of the Brrga1-d plants. It is likely that this Q-to-R mutation not only makes the DELLA proteins GA-resistant, but also may reduce the specific repressor activity of the DELLA proteins and therefore has a milder dwarfing effect than the N-terminal mutation. These results suggest that this Q-to-R mutation may be useful in agriculture. We transferred the Brrga1-d allele to canola (B. napus) hybrids, which are often tall and susceptible to lodging, and found that it significantly reduced plant height (Fig. 4B) and lodging in field experiments without affecting seed yield (A. Muangprom, I. Mauriera, and T.C. Osborn, unpublished data).
Degradation of regulatory proteins by the proteasome is important for controlling developmental processes in eukaryotes (Hellmann and Estelle, 2002; Smalle and Vierstra, 2004). Recent studies suggest that the E3 ubiquitin ligase complex SCFGID2/SLY1 targets DELLA proteins for GA-induced degradation and that proteolysis of DELLA is initiated by GA-dependent phosphorylation and finished by the SCFGID2/SLY1-proteasome pathway (McGinnis et al., 2003; Sasaki et al., 2003; Dill et al., 2004; Fu et al., 2004; Gomi et al., 2004). The N-terminal DELLA domain is known to be important for GA-induced degradation of AtRGA, SLR1, or SLN1 (Dill et al., 2001; Gubler et al., 2002; Itoh et al., 2002). This domain, however, is not required for SLY1 interaction in the yeast two-hybrid assay (Dill et al., 2004). It was hypothesized that the DELLA domain may be important for GA-induced phosphorylation of DELLA proteins by an unidentified protein kinase. Here, we demonstrated that the conserved Q residue near the VH(I/V)(I/V)D motif in the GRAS domain is also needed for degradation. But how does this conserved Q affect the degradation processes? In rice, GID2 (SLY1 ortholog) was shown to interact only with phosphorylated, but not with unphosphorylated, SLR1 (RGA ortholog; Gomi et al., 2004). The Q-to-R mutation in Atrga-d is located near putative kinase target sites (S277, T352, T356, and S396). Thus, this mutation may inhibit interaction with SLY1 by preventing phosphorylation of the nearby residue(s). Alternatively, the Q-to-R mutation may alter protein conformation in a way that prevents its interaction with SLY1.
Several lines of evidence from previous studies suggest that the C terminus of the GRAS domain is essential for the repressive function and for GA-induced degradation of DELLA proteins. Mutant proteins of Arabidopsis rga-1 or barley sln1c having small C-terminal truncations (67 or 18 amino acids, respectively) remain stable after GA treatment (Gubler et al., 2002; Dill et al., 2004), suggesting that they have lost an interaction domain needed for degradation. Concordantly, deletion of the last 64 amino acids from the C terminus of GAI (GAI-NT2) completely abolishes its interaction with SLY1 or sly1-d in yeast (Dill et al., 2004). Although rga-1 and sln1c accumulate GA-resistant proteins, they have slender phenotypes, indicating they also lack repressive function. Therefore, the newly identified Q-to-R mutation in Brrga1-d/Atrga-d is unique. Unlike the previously characterized C-terminal mutations, it retains the suppressive function, although it prevents GA-induced degradation of the mutant DELLA protein by abolishing interaction with the F box protein. This Q-to-R mutation could be useful to gain more insight on GA regulation in plant development, especially on the mechanism of GA-induced DELLA protein degradation. It also may be useful for reducing plant height in a wide range of crop species.
MATERIALS AND METHODS
Plant Material
dwarf2 was originally identified as a spontaneous mutant of rapid cycling Brassica rapa (Zanewich et al., 1991). For mapping, the dwf2 allele (renamed Brrga1-d in this paper) was backcrossed 5 times into the nondwarf cv R500 as described previously (Muangprom and Osborn, 2004). The segregating BC5 generation and progeny of BC5S2 plants that were either homozygous tall (wild-type) or dwarf (Brrga1-d) were used for this study.
Comparative Fine Mapping and Cloning of BrRGA1 and BrRGA2
A mapping population consisting of 410 BC5 plants was used to identify the candidate gene controlling the dwarf phenotype as described previously (Muangprom and Osborn, 2004). Arabidopsis (Arabidopsis thaliana) gene sequences surrounding the RGA locus were obtained from the MATDB database (http://mips.gsf.de/proj/thal/db/index.html, as accessed during 2001–2002) and used to search a Brassica oleracea database (The Arabidopsis Information Resource [TAIR], www.arabidopsis.org/Blast/; and The Institute for Genomic Research [TIGR], www.tigr.org/tdb/e2k1/bog1/). The resulting sequences showing highest homology to the Arabidopsis sequences were used to design primers for SSCP. In the case that no homology was found, the Arabidopsis gene sequences themselves were used. All the primers used in this study were designed using Primer 3 in Biology WorkBench (http://biowb.sdsc.edu/CGI/BW.cgi). The BrRGA1 marker was amplified using AX910 (5′-CTTGCCCTTACCTCAAGTTC-3′) and AX1270 (5′-TCGAAAACAGAGTTAACCGCCA-3′) primers derived from sequences in AX081276, a genomic sequence from oilseed rape (Brassica napus) that showed highest homology to Arabidopsis RGA with a score of 1,078 (E-value of 0.0). The Brassica rapa sequence similar to At2g01720 was amplified using degenerate gene-specific primers (5′-ATCTCCAGATCGTAAAYGCNGA-3′ and 5′-AGTTGCCAGNGCYTGNACCAT-3′) and another B. rapa sequence similar to At2g01560 was amplified using primers (5′-CCGCATCTCTCTGTCATCGT-3′ and 5′-TTGGGTTTGGTTTCTTTCAGCA-3′) derived from jbofi80tf, a B. oleracea clone that showed highest homology to At2g01560 (score 534, E-value of 3.9e-29). PCR reactions were performed using a 10-μL reaction. dCTP was used at one-half the concentration of other dNTPs, and 0.1 μL 32P dCTP (10 μci/μL) was added in each reaction. For polymorphism detection, SSCP analysis was performed using 0.5× mutation detection enhancement (MDE, CAMBREX Bioscience Rockland, Rockland, ME) gels and polymorphisms were detected using autoradiography by exposing gels at −80°C for 4 h or at room temperature for 16 to 18 h.
Using two pairs of primers, AX49 (5′-CAGATCAGAAATGAAGAGGGAT-3′) and AX 1270, and AX910 and AX1740 (5′-TTCCAAGCGGAGGTGGTTAT-3′), derived from AX081276, we were able to amplify most of the coding sequence of the Brrga1-d and BrRGA1 using genomic DNA isolated from dwf2 and wild-type plants. The AX49 and AX1270 primers did not amplify BrRGA2. The AX910 and AX1740 primers amplified both genes but only BrRGA1 contained the polymorphism site.
The completed sequences of BrRGA1 and BrRGA2 were obtained using thermal asymmetrical interlaced-PCR (Liu and Whittier, 1995). Two rounds of PCR amplifications were used to isolate 5′ and 3′ of BrRGA sequences. The first PCR reactions were conducted using partial degenerate primers AD-2 (5′-AGWGNAGWANCAWAGG-3′) and BrRGAr199 (5′-TTTCAACGCAACCTCAGCCA-3′) to obtain 5′ sequences, and BrRGAf1543 (5′-GTTCGGTTCGTCCGGTTTTG-3′) and AD-1 (5′-TGWGNAGWANCASAGA-3′) to obtain 3′ sequences. The resulting PCR products were used as templates for the second round of PCR using BrRGAr100 (5′-TTTACCAAACACCGCAGGGG-3′) and AD-2, or BrRGAf1610 (5′-TTGGCTTTGTTTAATGGAGGCG-3′) and AD-1, for 5′or 3′, respectively. The PCR reactions were performed as previously reported (Schomberg et al., 2003) and the PCR products were gel purified, cloned, and sequenced. The resulting sequences were used to design primers, 5′2 to 2 (5′-AGGCCTAGTTCACTTCAAAGGTTA-3′) and 3′31 to 34 (5′-TCACCGGTTCGGTCCGGGTTT-3′), to amplify BrRGA1, and 5′F32 (5′-TGGAAAAGCATCTTCAGTTCTCCA-3′) and 3′R2632 (5′-TCACCGGTTCGGTCCGGGTTT-3′) to amplify BrRGA2 using genomic DNA from both dwf2 and wild-type plants as templates. The resulting PCR products were gel purified and cloned. For BrRGA1 or BrRGA2, at least eight clones were sequenced from each genotype to obtain the complete sequences of these genes.
RNA Analysis
Plants were grown in a growth chamber at 22°C under a 16-h-light/8-h-dark cycle. Total RNA was isolated from shoots of 2-week-old plants treated with water, or 5 μL of 1 mm GA3, for various times. RNA isolation and RT-PCR were performed as described (Schomberg et al., 2003). Degenerate primers GA3ox492 (5′-TCACCATCAYYGGMTCRCCTC-3′) and GA3ox840 (5′-AGATCATCGCGAAWWACYTGTA-3′), designed from conserved sequences of Arabidopsis and pea GA3ox, were used to amplify B. rapa GA3ox. The reactions were amplified for 30 cycles and the PCR products were sequenced to confirm that they were parts of GA3ox. For loading control and testing DNA contamination, BrUBQ691 (5′-CGGATCAGCAGAGGTTGATCTT-3′) and BrUBQ1250 (5′-CCTGCAGTTGACAGCCCTTGG-3′) were used because these primers provided products with different sizes for the genomic DNA and cDNA. The reactions were amplified for 20 cycles.
Immunoblot Analysis
B. rapa total proteins were isolated from 6-d-old seedlings after 3 h of treatment with water or with 100 μm GA3, or from 8-d-old seedlings pretreated with 100 μm PAC for 4 d before water or 100 μm GA3 application for 3 h. The protein blot was probed with affinity-purified anti-AtRGA antibodies from a rabbit (DU176). Arabidopsis total proteins were isolated from rosette leaves of mature plants after 4 h of treatment with water or with 20 μL of 1 mm GA3. The blot was probed with unpurified anti-AtRGA antibodies. Immunoblot analysis was performed as described previously (Silverstone et al., 2001).
Site-Directed Mutagenesis and Plant Transformation
PCR-based overlap extension mutagenesis (Ho et al., 1989) was used to generate the 1-bp substitution (identical to the mutation in Brrga1-d) in the Arabidopsis RGA gene in pRG123, in which the AtRGA gene (with a KpnI site and a BamHI site immediately upstream and downstream of the RGA open reading frame) was expressed under its own promoter (1.6-kb 5′ UTR and 0.9-kb 3′ UTR). The mutant PCR product and pRG123 were digested with KpnI and BamHI, and the mutant PCR product was used to replace wild-type AtRGA in pRG123. Because BamHI site in pRG123 erased the stop codon of wild-type AtRGA, EcoRI fragment containing the stop codon of wild-type AtRGA from pRG102 (Silverstone et al., 2001) was used to replace the same fragment in the pRG123 containing the wild-type and mutant Atrga-d to create PRGA∷AtRGA and PRGA∷Atrga-d, respectively. The coding regions were analyzed by DNA sequencing to ensure that no mutations were introduced during amplification. The PRGA∷Atrga-d or PRGA∷AtRGA fragments were placed into the binary vector pPZP221B (Kang et al., 2001) at the PstI and XbaI sites. The constructs were transferred into Agrobacterium tumefaciens and transformed into Arabidopsis Wassilewskija ecotypes.
Yeast Two-Hybrid Assays
AtRGA and Atrga-d were fused to the Gal4 activation domain. SLY1 and sly1-d were fused to the LexA DNA-binding domain. The complete coding sequences of Atrga-d were amplified from PRGA∷Atrga-d as described for AtRGA (Dill et al., 2004). Similar levels of AtRGA and Atrga-d proteins expressed in yeasts were determined by immunoblot analysis. Plasmid constructs and yeast two-hybrid assays were performed as described previously (Dill et al., 2004).
Distribution of Materials
Upon requests, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AY928549 for BrRGA1 and AY928550 for BrRGA2.
Acknowledgments
We thank Mark Doyle, Ayala Most, Peizhen Yang, Joseph Walker, Lewis Lukens, Josh Udall, Pablo Quijada, and Ivan Maureira for technical assistance, and Richard Amasino for helpful discussion.
This work was supported by the U.S. Department of Agriculture/Initiative for Future Agriculture and Food Systems (grant to T.C.O.), by Bayer CropScience (grant to T.C.O.), and by the National Science Foundation (grant nos. IBN–0078003 and IBN–0235656 to T.-p.S.). A.M. was supported in part by a scholarship from the Royal Thai government.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.057646.
References
- Boss PK, Thomas MR (2002) Association of dwarfism and floral induction with a grape ‘green revolution’ mutation. Nature 416: 847–850 [DOI] [PubMed] [Google Scholar]
- Chandler PM, Marion-Poll A, Ellis M, Gubler F (2002) Mutants at the Slender1 locus of barley cv Himalaya: molecular and physiological characterization. Plant Physiol 129: 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Qin L, Lee S, Fu X, Richards DE, Cao D, Luo D, Harberd NP, Peng J (2004) Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 131: 1055–1064 [DOI] [PubMed] [Google Scholar]
- Cowling RJ, Kamiya Y, Seto H, Harberd NP (1998) Gibberellin dose-response regulation of GA4 gene transcript levels in Arabidopsis. Plant Physiol 117: 1195–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill A, Jung H-S, Sun T-p (2001) The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc Natl Acad Sci USA 98: 14162–14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill A, Sun T-p (2001) Synergistic de-repression of gibberellin signaling by removing RGA and GAI function in Arabidopsis thaliana. Genetics 159: 777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill A, Thomas SG, Hu J, Steber CM, Sun T-p (2004) The Arabidopsis F-box protein SLEEPY1 targets GA signaling repressors for GA-induced degradation. Plant Cell 16: 1392–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck B, Harberd NP (2002) Evidence that the Arabidopsis nuclear gibberellin signalling protein GAI is not destabilized by gibberellin. Plant J 32: 935–947 [DOI] [PubMed] [Google Scholar]
- Fu X, Richards DE, Fleck B, Xie D, Burton N, Harberd NP (2004) The Arabidopsis mutant sleepy1gar2-1 protein promotes plant growth by increasing the affinity of the SCFSLY1 E3 ubiquitin ligase for DELLA protein substrates. Plant Cell 16: 1406–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi K, Matsuoka M (2003) Gibberellin signalling pathway. Curr Opin Plant Biol 6: 489–493 [DOI] [PubMed] [Google Scholar]
- Gomi K, Sasaki A, Itoh H, Ueguchi-Tanaka M, Ashikari M, Kitano H, Matsuoka M (2004) GID2, an F-box subunit of the SCF E3 complex, specifically interacts with phosphorylated SLR1 protein and regulates the gibberellin-dependent degradation of SLR1 in rice. Plant J 37: 626–634 [DOI] [PubMed] [Google Scholar]
- Gubler F, Chandler P, White R, Llewellyn D, Jacobsen J (2002) GA signaling in barley aleurone cells: control of SLN1 and GAMYB expression. Plant Physiol 129: 191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P (2003) The genes of the Green Revolution. Trends Genet 19: 5–9 [DOI] [PubMed] [Google Scholar]
- Hellmann H, Estelle M (2002) Plant development: regulation by protein degradation. Science 297: 793–797 [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Morton RM, Pullen JK, Pease LR (1989) Site directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77: 51–59 [DOI] [PubMed] [Google Scholar]
- Ikeda A, Ueguchi-Tanaka M, Sonoda Y, Kitano H, Koshioka M, Futsuhara Y, Matsuoka M, Yamaguchi J (2001) slender rice, a constitutive gibberellin response mutant is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell 13: 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Matsuoka M, Steber CM (2003) A role for the ubiquitin-26S-proteasome pathway in gibberellin signaling. Trends Plant Sci 8: 492–497 [DOI] [PubMed] [Google Scholar]
- Itoh H, Ueguchi-Tanaka M, Sato Y, Ashikari M, Matsuoka M (2002) The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 14: 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BH, Busse JS, Dickey C, Rancour DM, Bednarek SY (2001) The Arabidopsis cell plate-associated dynamin-like protein, ADL1Ap, is required for multiple stages of plant growth and development. Plant Physiol 126: 47–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khush GS (2001) Green revolution: the way forward. Nat Rev Genet 2: 815–822 [DOI] [PubMed] [Google Scholar]
- King K, Moritz T, Harberd N (2001) Gibberellins are not required for normal stem growth in Arabidopsis thaliana in the absence of GAI and RGA. Genetics 159: 767–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Cheng H, King KE, Wang W, He Y, Hussain A, Lo J, Harberd NP, Peng J (2002) Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev 16: 646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-G, Whittier RF (1995) Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25: 674–681 [DOI] [PubMed] [Google Scholar]
- McGinnis KM, Thomas SG, Soule JD, Strader LC, Zale JM, Sun T-p, Steber CM (2003) The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15: 1120–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muangprom A, Osborn TC (2004) Characterization of a dwarf gene in Brassica rapa, including the identification of a candidate gene. Theor Appl Genet 108: 1378–1384 [DOI] [PubMed] [Google Scholar]
- Olszewski N, Sun T-p, Gubler F (2002) Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell (Suppl) 14: S61–S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP (1997) The Arabidopsis GAI gene defines a signalling pathway that negatively regulates gibberellin responses. Genes Dev 11: 3194–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, et al (1999) ‘Green Revolution’ genes encode mutant gibberellin response modulators. Nature 400: 256–261 [DOI] [PubMed] [Google Scholar]
- Pysh LD, Wysocka-Diller JW, Camilleri C, Bouchez D, Benfey PN (1999) The GRAS gene family in Arabidopsis: sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J 18: 111–119 [DOI] [PubMed] [Google Scholar]
- Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong D-H, An G, Kitano J, Ashikari M, et al (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299: 1896–1898 [DOI] [PubMed] [Google Scholar]
- Schomberg FM, Bizzell CM, Lee DJ, Zeevaart JAD, Amasino RA (2003) Overexpression of a novel class of gibberellin 2-oxidases decreases gibberellin levels and creates dwarf plants. Plant Cell 15: 151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone AL, Ciampaglio CN, Sun T-p (1998) The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 10: 155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone AL, Jung H-S, Dill A, Kawaide H, Kamiya Y, Sun T-p (2001) Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 13: 1555–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Vierstra RD (2004) The ubiquitin 26s proteasome proteolytic pathway. Annu Rev Plant Biol 55: 555–590 [DOI] [PubMed] [Google Scholar]
- Sun T-p, Gubler F (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol 55: 197–223 [DOI] [PubMed] [Google Scholar]
- Thomas SG, Sun T-p (2004) Update on gibberellin signaling. A tale of the tall and the short. Plant Physiol 135: 668–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Wan P, Sun S, Li J, Chen M (2004) Genome-wide analysis of the GRAS gene family in rice and Arabidopsis. Plant Mol Biol 54: 519–532 [DOI] [PubMed] [Google Scholar]
- Tyler L, Thomas SG, Hu J, Dill A, Alonso JM, Ecker JR, Sun T-p (2004) DELLA proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol 135: 1008–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C-K, Chang C (2002) Arabidopsis RGL1 encodes a negative regulator of gibberellin responses. Plant Cell 14: 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanewich KP, Rood SB, Southworth CE, Williams PH (1991) Dwarf mutants of Brassica: responses to applied gibberellins and gibberellin content. J Plant Growth Regul 10: 121–127 [Google Scholar]