Abstract
Uncoordinated protein 45A (UNC-45A) is the only known ATP-independent microtubule (MT)-severing protein. Thus, it severs MTs via a novel mechanism. In vitro and in cells, UNC-45A–mediated MT severing is preceded by the appearance of MT bends. While MTs are stiff biological polymers, in cells, they often curve, and the result of this curving can be breaking off. The contribution of MT-severing proteins on MT lattice curvature is largely undefined. Here, we show that UNC-45A curves MTs. Using in vitro biophysical reconstitution and total internal fluorescence microscopy analysis, we show that UNC-45A is enriched in the areas where MTs are curved versus the areas where MTs are straight. In cells, we show that UNC-45A overexpression increases MT curvature and its depletion has the opposite effect. We also show that this effect occurs is independent of actomyosin contractility. Lastly, we show for the first time that in cells, Paclitaxel straightens MTs, and that UNC-45A can counteracts the MT-straightening effects of the drug.
Keywords: UNC-45A, microtubules, microtubule curvature
Microtubules (MTs) are stiff biological polymers (1, 2), yet, they often curve, and the result of this curving can be breaking (3, 4). MT curvature is regulated by mechanical interactions with other cellular filaments, cell membranes, and cellular organelles, exposure to MT-targeting agents, and interaction with microtubule-associated proteins (MAPs). Regarding the effect of MT-targeting agents on MT curvature, in vitro studies on the structure of Taxol-MT have demonstrated its impact on both individual protofilaments and the overall lattice of MT. Taxol straightens the protofilaments, while simultaneously enhancing heterogeneity and flexibility within the MT lattice (5, 6). With regards to the effect of MAPs on MT curvature, in vitro studies have demonstrated that the MT plus-end destabilizing proteins kinesin-13 and kinesin-1 stabilize the αβ-tubulin curved conformation and lock the MT lattice in a curved conformation, respectively (7, 8), while the MT stabilizing protein Tau distorts the αβ-tubulin within MT filaments and increases MT curvature (9). The contribution of MT-severing proteins on MT curvature has never been described.
The uncoordinated protein 45 (UNC-45) plays a significant role in various organisms due to its wide distribution and evolutionary conservation (10, 11). In invertebrates, there exists a single isoform of UNC-45 that regulates the stability and functioning of myosin. Vertebrates, on the other hand, have two isoforms of UNC45: UNC45B, found exclusively in muscle cells, and UNC45A, expressed in all cell types (12). Despite its conservation throughout evolution, the precise functions of UNC-45 are not yet fully understood. UNC-45A comprises four distinct domains, namely an N-terminal domain that contains three tetratricopeptide repeat sequences. We recently made the discovery that the N-terminal domain of UNC-45A is essential for MT binding (13). Additionally, there is a central domain whose function remains largely unknown, a neck domain that has recently been proposed as necessary for the oligomerization of UNC-45 (14) and a C-terminal UNC-45/CRO1/She4p domain that plays a critical role in interacting with myosin II (15, 16).
In mammalian cells UNC-45A exhibits a dual role that is not mutually exclusive, functioning as a regulator of both myosin and MT activities. With regards to the latter, UNC-45A is a centrosome- (17, 18) and mitotic-spindle–associated protein in cancer cells (17, 19), and is enriched in MT dense areas of nervous system and ciliated epithelium (20). UNC-45A exhibits upregulated expression in cancer cells, wherein it antagonizes the MT-stabilizing properties of Paclitaxel, enabling cells to undergo division via multipolar spindles despite the presence of the drug. Conversely, the inhibition of UNC-45A reestablishes the susceptibility of cancer cells to Paclitaxel, thereby restoring their sensitivity to the drug (19). Mechanistically, our recent findings have shown that UNC-45A is a MT-severing protein in both in vitro and in living cells and that this effect is independent of myosin (21, 22, 23, 24). What makes this particularly intriguing is that, unlike all other known MT-severing proteins, UNC-45A lacks an ATPase domain and affects MT stability independent of ATP (13).
Because, we have observed the appearance of MT bends and kinks prior to UNC-45A-mediated MT severing in both in vitro and living cell experiments (13), here we wanted to ascertain the position of UNC-45A with respect to MT curvature and determine if UNC-45A expression affects MT curvature in cells. We found that UNC-45A enriched in the areas where MTs are curved versus the areas where MT is straight. We also show that UNC-45A overexpression increases the curvature of both perinuclear and peripheral MTs. This effect of UNC-45A on MT curvature is independent of actomyosin contractility because UNC-45A binds to MTs independent of its myosin II binding domain and acts on MT even in the presence of the myosin II inhibitor blebbistatin (13, 19). Furthermore, here we show that actomyosin contractility does not affect perinuclear MT curvature. Lastly, we show that Paclitaxel straightens MTs in cells and that UNC-45A counteracts the MT-straightening effects of Paclitaxel. Taken together, our studies support the role of the MT-severing protein UNC-45A as a regulator of MT curvature in cells including in cells exposed to the MT-targeting agent Paclitaxel.
Results
UNC-45A signal increases in the curved region of MTs and in Taxol-stabilized GTP MTs
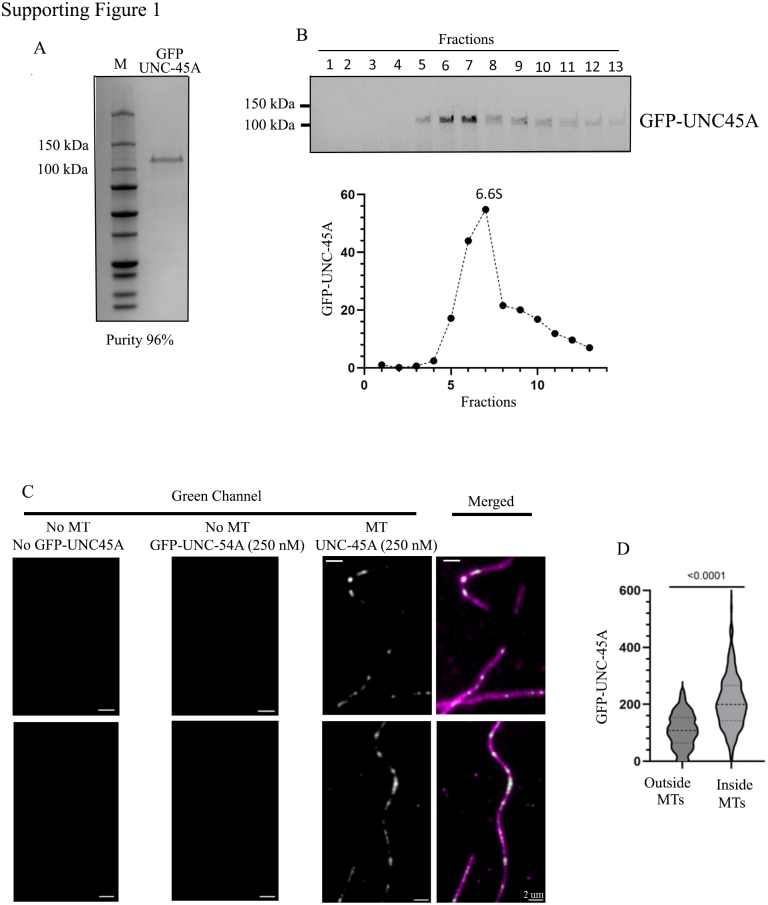
We have recently shown exposure to UNC-45A kinks in vitro MTs and that MTs’ break and depolymerization follow these kinks (13). We have also demonstrated that MTs in UNC-45A–overexpressing cells (mouse rat fibroblasts, RFL-6) break and these breakages are preceded by MT bending at the breaking site (13). While performing these experiments, we noticed that MTs in UNC-45A–overexpressing cells appeared more curved overall than control cells. Thus, we sought to determine the localization of UNC-45A with respect to MT curvature. For this, we used the in vitro system because it allows for the evaluation of UNC-45A with respect to MT curvature in the absence of other cellular factors and MTs with a broad range of curvatures and because it allows evaluating single MTs in the absence of the MTs crowdedness typical of cells. Guanosine-5’-[(α,β)-methyleno] triphosphate (GMPCPP)-stabilized and rhodamine-labeled MTs were further stabilized with 10 μM Taxol as we have previously described (13, 19) and gently pipetted in a perfusion chamber so that a subset of the MTs would curve naturally while binding to the surface of the chamber. Next, we introduced 250 nM of GFP-UNC-45A and imaged the localization of UNC-45A to MT curvature using total internal fluorescence (TIRF) microscopy as we have previously described (13, 19) (Fig. 1A). As shown in Figure 1, B and C, we found that UNC-45A is enriched in the areas where MTs are curved versus the areas where MT is straight. To provide additional evidence of UNC-45A’s preference for curved MTs, we conducted a comparative analysis of GFP-UNC-45A signal intensity between two types of in vitro MTs: rhodamine-labeled GMPCPP MTs and Taxol-stabilized GTP MTs. Previous studies have demonstrated that these two MT types exhibit distinct characteristics in terms of stiffness and curvature with rhodamine-labeled GMPCPP MTs being more rigid and predominantly straight, and Taxol-stabilized GTP MTs being less rigid and more curved (1, 25). We found that UNC-45A signal is enriched in the Taxol-stabilized GTP MTs versus the GMPCPP MTs (Fig. 1, D–M). All in vitro experiments were performed with recombinant GFP-UNC-45A that was at least 95% pure (Fig. S1A). Glycerol gradient sedimentation of purified GFP-UNC-45A indicates that over 75% of the protein introduced in the TIRF chamber is in the monomeric form (Fig. S1B). GFP brightness analysis indicates that the signal is significantly stronger on MTs than areas outside of them (Fig. S1, C and D).
Figure 1.
UNC-45A is enriched in the curved regions of MTs and in Taxol-stabilized GTP MTs.A, representative images of GFP-UNC-45A (green) binding to straight and curved rhodamine-labeled Taxol-stabilized GMPCPP MTs (magenta). B, left, GFP-UNC-45A signal intensity (expressed as arbitrary fluorescence intensity, AFU). Right, background signal intensity of green channel (expressed as AFU) nearby MT curves plotted against MT curvature. Sixteen MTs were analyzed for a total of 137 measurements from three different chambers. Single measurements are shown as gray dots, and average intensity and SDs are shown as black dots with error bars. C, left, GFP-UNC-45A signal intensity (expressed as AFU) categorized into three MT curvature k groups (0 < k < 0.3 rad/μm, 0.3 ≤ k < 0.6 rad/μm, and 0.6 ≤ k rad/μm). Right, background fluorescence intensity of green channel (expressed as AFU) nearby MT curves are categorized into three MT curvature k groups (0 < k < 0.3 rad/μm, 0.3 ≤ k < 0.6 rad/μm, and 0.6 ≤ k rad/μm). D, representative images of GFP-UNC-45A (green) binding to rhodamine-labeled GMPCPP MT (magenta). E, GFP-UNC-45A signal intensity (expressed as AFU). F, background signal intensity of green channel (expressed as AFU) nearby MT curves plotted against GMPCPP MT curvature. Twenty MTs were analyzed for a total of 245 measurements from three different chambers. G, GFP-UNC-45A signal intensity (expressed as AFU) categorized into three MT curvature k groups (0 < k < 0.2 rad/μm, 0.2 ≤ k < 0.4 rad/μm, and 0.4 ≤ k rad/μm). H, background fluorescence intensity of green channel (expressed as AFU) nearby MT curves are categorized into three MT curvature k groups (0 < k < 0.2 rad/μm, 0.2 ≤ k < 0.4 rad/μm, and 0.4 ≤ k rad/μm). I, representative images of GFP-UNC-45A (green) binding to rhodamine-labeled Taxol stabilized GTP MT (magenta). J, GFP-UNC-45A signal intensity (expressed as AFU). K, Background signal intensity of green channel (expressed as AFU) nearby MT curves plotted against Taxol-stabilized GTP MT curvature. L, GFP-UNC-45A signal intensity (expressed as AFU) categorized into three MT curvature k groups (0 < k < 0.4 rad/μm, 0.4 ≤ k < 0.8 rad/μm, and 0.8 ≤ k rad/μm). M, background fluorescence intensity of green channel (expressed as AFU) nearby MT curves are categorized into three MT curvature k groups (0 < k < 0.4 rad/μm, 0.4 ≤ k < 0.8 rad/μm, and 0.8 ≤ k rad/μm). All statistical significances of difference were assessed with an unpaired two-tailed Student's t test. GMPCPP, guanosine-5’-[(α,β)-methyleno] triphosphate; MT, microtubule; UNC-45A, uncoordinated protein-45A.
UNC-45A overexpression increases MT curvature in the peripheral area of the cells
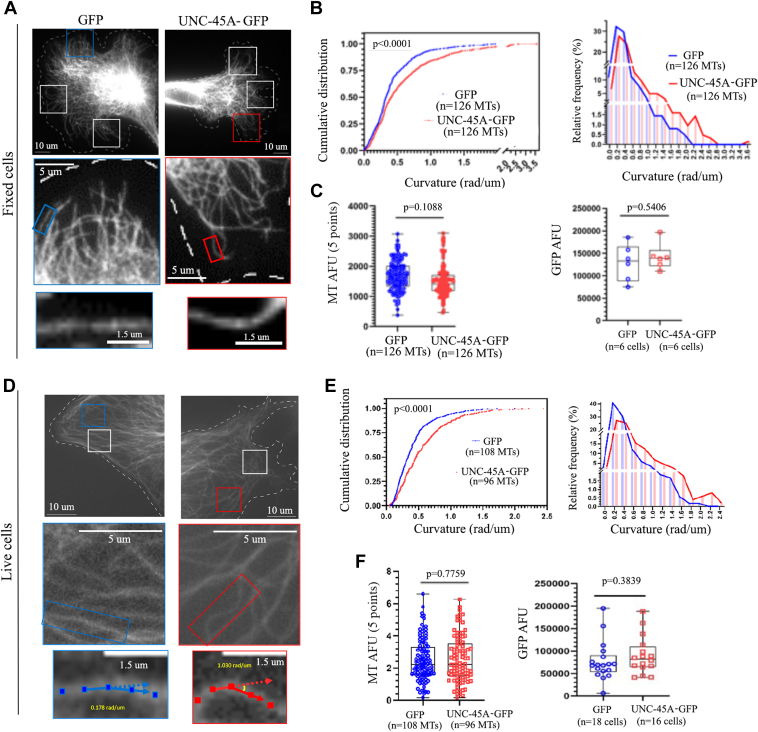
We investigated the influence of UNC-45A on MT curvature in the peripheral region of the cells. This area is commonly examined when studying morphological alterations in MTs, as it offers convenient visualization, enables the selection of individual MTs, and facilitates their analysis (3). To this end, RFL-6 cells were lentivirally infected with either GFP (control) or UNC-45A-GFP and 12 h post infection cells were fixed, stained with anti-alpha-tubulin to visualize MTs (Fig. 2A) and subjected to immunofluorescence microscopy. For each condition (cells expressing GFP and UNC45A-GFP), we assessed MT curvature in visually extracted single MTs using a semiautomated MT tracking algorithm in MATLAB. This method was previously described in our work (3, 26, 27). We focused on individual MTs that were neither crossing nor bundling and were at least 2 μm away from the cell edge. To quantify the curvature, we selected five points with the highest curvature within each condition. Using these parameters and analysis method, we found that MTs in UNC-45A-GFP–overexpressing cells were more curved than control GFP-overexpressing cells (Fig. 2B). All measurements were taken in MTs with similar fluorescent intensity (Fig. 2C, left) and in cells expressing equal amounts of GFP (Figs. 2C, right and S2A).
Figure 2.
Overexpression of UNC-45A increases MT curvature in the peripheral area of cells.A, top two panels, representative images of MTs visualized via anti-alpha-tubulin staining in fixed GFP and UNC-45A-GFP–overexpressing RFL-6 cells. The white dotted lines indicate cell edges. Images were taken with the same exposure time. Three representative regions of interest (ROI) are shown. Middle two panels, close-up of one of representative areas shown in the top panels. Bottom two panels, representative magnified images region of single MT shown in middle panels where five curvature values were obtained. B, left, cumulative distribution of MT curvature calculated using the five curvature points per condition. The mean curvature values and SDs of GFP and UNC-45A-GFP were 0.421 rad/m 0.341 and 0.581 rad/m 0.509, respectively. Right, histogram of MT curvature distribution shown in B, left. C, left, quantification of MT fluorescence intensity (arbitrary fluorescence units, AFUs) along the length of the measured MTs. n represents number of total MTs evaluated per condition. Right, quantification of GFP mass (AFUs) in GFP and UNC-45A-GFP–overexpressing cells. D, top two panels, representative images of MTs visualized with Deep Red in live GFP and UNC-45A-GFP–overexpressing RFL-6 cells. The white dotted lines indicate cell edges. Images were taken using the same exposure time. Two representative regions of interest (ROI) are shown. Middle two panels, close-up of one of representative areas shown in the top panels. Bottom two panels, representative magnified images region of single MT shown in middle panels where five curvature values were obtained. For each MT, dots were placed every 0.5 μm for 2.5 μm long to record x-y coordinates. Yellow numbers indicate calculated curvature values at middle point using two adjacent points along MT. E, left, cumulative distribution of MT curvature calculated using the five curvature points per condition. The mean curvature values and SDs of GFP and UNC-45A-GFP were 0.415 rad/m 0.303 and 0.571 rad/m 0.407, respectively. Right, histogram of MT curvature distribution shown in E, left. F, left, quantification of MT fluorescence intensity (AFUs) along the length of the measured MTs. n represents number of total MTs evaluated per condition. Right, quantification of GFP mass (AFUs) in GFP and UNC-45A-GFP–overexpressing cells. All statistical significances of difference were assessed with an unpaired two-tailed Student's t test. MT, microtubule.
Next, our aim was to verify whether UNC-45A overexpression in living cells also results in an increase in MT curvature, similar to what we observed in fixed cells (Fig. 2, A–C). However, the semiautomated MT tracking algorithm we employed for fixed cells is not applicable to live cell imaging, especially when using fluorescent microscopy (3, 26, 27), which is essential for our experiments. Therefore, our initial goal was to confirm that the manual quantification of MT curvature using the click-point method, as utilized in our previous work (3, 26, 27), yields consistent results with the semiautomated approach. As depicted in Fig. S2, B–D, both automated and manual quantification of MT curvature in GFP and UNC-45A-GFP–overexpressing cells yielded comparable results in fixed cell samples. Thus, we performed time-lapse microscopy of Tubulin Tracker Deep Red-labeled MTs in RFL-6 cells lentivirally infected with either GFP (control) or UNC-45A-GFP (Fig. 2D). We found that UNC-45A-GFP–overexpressing cells had MTs that were significantly more curved than in control cells (Fig. 2E). We took all measurements in MTs with similar fluorescent intensity (Fig. 2F, left panel) and in cells expressing equal amounts of GFP (Figs. 2F, right panel and S2E). Taken together, this suggests UNC-45A may act on MT curvature in cells.
UNC-45A overexpression increases MT curvature in the perinuclear area of the cells
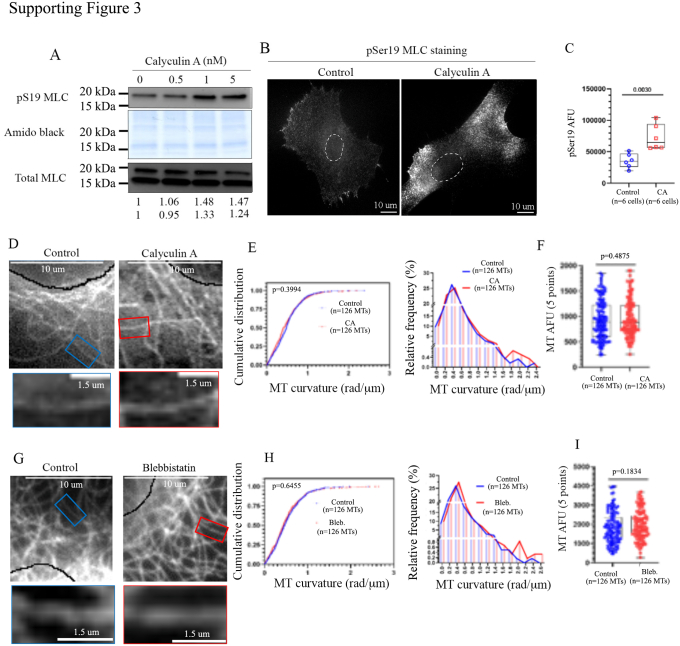
In the above-described set of experiments, we evaluated MT curvature in GFP, and UNC-45A-GFP–expressing cells in MTs at the cell periphery. This is commonly done because it allows easy visualization, selection, and analysis of individual MTs (3). However, this cell area is subjected to intense actomyosin contractility, which can affect MT curvature (28). To demonstrate UNC-45A’s influence MT curvature independently of actomyosin, we next focused on examining its effect on a distinct subset of cellular MTs. Specifically, we investigated the MTs located in the perinuclear region of cells. This region was chosen because previous research has suggested that it experiences fewer actomyosin-generated forces in certain cell types (28). By concentrating on this area, we aimed to establish a clearer understanding of UNC-45A’s role in shaping MT curvature, free from potential confounding influences of actomyosin dynamics. For this, we first needed to firmly establish that that actomyosin contractility does not affect the curvature of perinuclear MTs in RFL-6 cells, the cell type that we are using in this study. RFL-6 cells were either mock-treated or treated with increasing concentrations (0.5–5 nM) of the actomyosin activator calyculin A (29). After 10 min of calyculin exposure, cell lysates were subjected to Western blot analysis. As shown in Fig. S3A, calyculin A treatment (1 and 5 nM) increased the levels of pS19 MLC as compared to the control, while it did not affect the total levels of MLC. Thus, both these concentrations activate actomyosin contractility RFL-6 cells. Next, we determined where activation of actomyosin contractility in the calyculin A exposed RFL-6 cells takes place. As shown in Fig. S3, B and C, immunofluorescence staining of phosphorylated MLC (phospho-Ser19-MLC) in control and calyculin A treated cells revealed a distribution of phosphorylated MLC in both perinuclear and peripheral areas of the cells with what appears to be a predominant staining in the peripheral areas. This is consistent with what is found in human fibroblast (NIH 3T3) cells which, following calyculin A treatment, revealed higher concentrations of phospho-MLC at the cell’s periphery (30). Having established that calyculin A treatment activates actomyosin contractility in RFL-6, we determined whether this results in changes in perinuclear MT curvature. RFL-6 cells were either mock-treated (control) or treated with 1 nM of calyculin A for 10 min, after which they were fixed, stained with anti-alpha-tubulin to visualize MTs (Fig. S3D), and subjected to immunofluorescence microscopy. Per each condition (control and calyculin A) MT curvature was evaluated as for the experiments presented in Figures 2 and 3. We found that calyculin A treatment does not affect perinuclear MT curvature (Fig. S3E). All measurements were taken in MTs with similar fluorescent intensity (Fig. S3F). We then performed a complementary experiment where perinuclear MT curvature was evaluated in RFL-6 cells treated with the myosin II inhibitor blebbistatin. Here, RFL-6 cells were mock-treated (control) or treated with 25 μM of blebbistatin for 1 h, fixed and stained with anti-alpha-tubulin to visualize MTs (Fig. S3G) and subjected to immunofluorescence microscopy. Per each condition (control and blebbistatin), MT curvature was evaluated as for the experiments presented in Figures 2 and 3 and described above. We found that blebbistatin treatment does not affect perinuclear MT curvature (Fig. S3H). All measurements were taken in MTs with similar fluorescent intensity (Fig. S3I).
Figure 3.
Overexpression of UNC-45A increases MT curvature in the perinuclear area of cells.A, top two panels, representative images of perinuclear MTs visualized via anti-alpha-tubulin staining in fixed GFP and UNC-45A-GFP–overexpressing RFL-6 cells. The white dotted lines indicate cell edges and black lines indicate nuclear membrane. Images were taken with the same exposure time. Three representative regions of interest (ROI) are shown. Middle two panels, close-up of one of representative areas shown in the top panels. Bottom two panels, representative magnified images region of single MT shown in middle panels where five curvature values were obtained. B, left, cumulative distribution of MT curvature calculated using the five curvature points per condition. The mean curvature values and SDs of GFP and UNC-45A-GFP were 0.510 rad/m 0.339 and 0.664 rad/m 0.508, respectively. Right, histogram of MT curvature distribution shown in B, left. C, left, quantification of MT fluorescence intensity (arbitrary fluorescence units, AFUs) along the length of the measured MTs. n represents number of total MTs evaluated per condition. Right, quantification of GFP mass (AFUs) in GFP and UNC-45A-GFP–overexpressing cells. D, top two panels, representative images of MTs visualized with Deep Red in live GFP and UNC-45A-GFP–overexpressing RFL-6 cells. The white dotted lines indicate cell edges and black lines indicate nuclear membrane. Images were taken using the same exposure time. Two representative regions of interest (ROI) are shown. Middle two panels, close-up of one of representative areas shown in the top panels. Bottom two panels, representative magnified images region of single MT shown in middle panels where five curvature values were obtained. For each MT, dots were placed every 0.5 μm for 2.5 μm long to record x-y coordinates. Yellow numbers indicate calculated curvature values at middle point using two adjacent points along MT. E, left, cumulative distribution of MT curvature calculated using the five curvature points per condition. The mean curvature values and SDs of GFP and UNC-45A-GFP were 0.336 rad/m 0.267 and 0.451 rad/m 0.341, respectively. Right, histogram of MT curvature distribution shown in E, left. F, left, quantification of MT fluorescence intensity (AFUs) along the length of the measured MTs. n represents number of total MTs evaluated per condition. Right, quantification of GFP mass (AFUs) in GFP and UNC-45A-GFP–overexpressing cells. All statistical significances of difference were assessed with an unpaired two-tailed Student's t test. MT, microtubule; UNC-45A, uncoordinated protein-45A.
After confirming that actomyosin contractility has no impact on perinuclear MT curvature in RFL-6 cells, our investigation proceeded to examine the influence of UNC-45A overexpression on these perinuclear MTs. This was done in both fixed and live cells looking at MTs within 10 μm from the nuclear membrane as identified via 4′,6-diamidino-2-phenylindole staining. In fixed cells (Fig. 3A), we found that UNC-45A overexpression led to increase in perinuclear MT curvature (Fig. 3B). All measurements were taken in MTs with similar fluorescent intensity (Fig. 3C, left) and in cells expressing equal amounts of GFP (Figs. 3C, right and S2A). We also looked at the effect of UNC-45A overexpression on perinuclear MTs curvature in live cells. We found that UNC-45A-GFP–overexpressing cells (Fig. 3D) had perinuclear MTs that were significantly more curved than the ones in control cells (Fig. 3E). All measurements were taken in MTs with similar fluorescent intensity (Fig. 3F, left) and in cells expressing equal amounts of GFP (Figs. 3F, right and S2E).
To confirm the effect of UNC-45A on MT curvature, we performed a complementary experiment where perinuclear MT curvature was evaluated in under conditions of UNC-45A loss. To this end, UNC-45A was knocked down via lentiviral-mediated delivery of scramble of UNC-45A shRNAs as we have previously performed (19). Because the lentiviral vector used expresses GFP, it is possible to monitor for efficiency of lentiviral infection (13). Western blot analysis revealed the efficiency of the KD with >60% reduction in UNC-45A expression levels (Fig. 4A). Next, scramble and UNC-45A KD RFL-6 cells were fixed, stained with anti-alpha-tubulin to visualize MTs (Fig. 4B) and subjected to immunofluorescence microscopy. Per each condition (scramble and UNC-45A KD cell), curvature was evaluated on perinuclear MTs (MTs that were within 10 μm from the nuclear membrane) as described above. We found that MTs in UNC-45A KD cells were less curved than scramble cells (Fig. 4C). All measurements were taken in MTs with similar fluorescent intensity (Fig. 4D, left) and in cells expressing equal amounts of GFP (Figs. 4D, right and S4). Taken together, this suggests UNC-45A may act on MT curvature independent of actomyosin forces.
Figure 4.
Loss of UNC-45A leads to a decrease in MT curvature.A, Western blot analysis of the levels of UNC-45A in scramble (Scr.) and UNC-45A knock down (KD) RFL-6 cells. Amido black was used as a loading control. Numbers represent the ratio between UNC-45A and amido black. B, top two panels, representative images of perinuclear MTs visualized via anti-alpha-tubulin staining in fixed scramble and UNC-45A knockdown (KD) RFL-6 cells. The white dotted lines indicate cell edges and black lines indicate nuclear membrane. Images were taken using the same exposure time. Rectangles indicate the representative areas shown in the bottom panels. Middle panels, close-up of one representative area shown in the top panels. Bottom two panels, magnified representative region of single MT where five curvature values were obtained. C, left, cumulative distribution of MT curvature calculated using the five curvature points per condition. The average curvature values and SDs of scramble and UNC-45A KD were 0.493 rad/m 0.347 and 0.444 rad/m 0.309, respectively. Right, histogram of MT curvature distribution shown in C, left. D, left, quantification of MT fluorescence intensity (arbitrary fluorescence units, AFUs) along the length of the measured MTs. n represents number of total MTs evaluated per condition. Right, quantification of GFP mass (AFUs) in scramble and UNC-45A KD cells. n represents number of cells evaluated per condition. All statistical significances of difference were assessed with an unpaired two-tailed Student's t test. MT, microtubule; UNC-45A, uncoordinated protein-45A.
UNC-45A counteracts the MT-straightening effects of Paclitaxel
The effect of Taxol treatment on MT curvature in vitro has been examined in some detail. Taxol-stabilized GTP MTs are less stiff and more wavy as compared to other in vitro MTs (1). However, these Taxol-treated MTs also display straighter individual tubulin protofilaments given that Taxol appears to decelerate their transition from a straight to curved conformation, contributing to this unique observation (31). Axonemal derived MTs treated with Taxol exhibited increased flexibility compared to the untreated ones. This enhanced flexibility led to the MTs becoming straightened when exposed to a flow (25). Addition of MT-stabilizing proteins such MAP2 and Tau to these MTs increases the rigidity of these MTs. The effect of Paclitaxel on MT curvature in cells, where MT are exposed to cellular environment including the presence of other cytoskeletal and organelle components and of cellular fluid has never been reported.
To investigate this, RFL-6 cells were either mock-treated (control) or treated with 500 nM of Paclitaxel for 30 min and subsequently fixed and stained with anti-alpha-tubulin antibody to visualize MTs (Fig. 5A). We found that Paclitaxel treatment decreased the perinuclear MT curvature compared to the control (Fig. 5, B and C), indicating that Paclitaxel straightens MTs in cells. All measurements were taken in MTs with similar fluorescent intensity (Fig. 5D). We have previously shown that UNC-45A is overexpressed in Paclitaxel-resistant cancer cells, where it counteracts the MT-stabilizing effects of the drug leading to cancer cells’ survival (19). In light of this and of the above-presented findings that UNC-45A increases the MT curvature, we looked at the effect of Paclitaxel on MT curvature under the condition of UNC-45A overexpression. RFL-6 cells were lentivirally infected with either GFP or UNC-45A-GFP and 12 h post infection cells were exposed or not (control) to 500 nM of Paclitaxel for 30 min, prior fixation and immunofluorescent visualization of MT stained with anti-alpha-tubulin antibody. Per each condition (GFP minus or plus Paclitaxel- Figure 6A left panels, and UNC-45A-GFP minus or plus Paclitaxel- Fig. 6A right panels) MT curvature was evaluated as we described above. We found that Paclitaxel treatment straightened MT curvature in GFP-expressing cells (Fig. 6B, top panel and 6C, left panel). This is consistent with our findings (Fig. 5) that Paclitaxel straightens MTs in uninfected RFL-6 cells. We also found that UNC-45A-GFP–expressing cells Paclitaxel treatment was still able to straighten MTs (Fig. 6B, bottom panel and Fig. 6C, right panel). Specifically, we found that Taxol-treated MTs curved in the presence of UNC-45A-GFP but not in its absence (Fig. 6D). All measurements were taken in MTs with similar fluorescent intensity (Fig. 6E) and in cells expressing equal amounts of GFP (Figs. 6F and S5). Taken together, this suggests that UNC-45A counteracts the MT-straightening effects of Paclitaxel in RFL-6 cells.
Figure 5.
Paclitaxel straightens perinuclear MTs in cells.A, top two panels, representative images of perinuclear MTs visualized via anti-alpha-tubulin staining in fixed RFL-6 cells treated or not (control) with Paclitaxel (500 nM for 30 min). The white dotted lines indicate cell edges and black lines indicate nuclear membrane. Images were taken with the same exposure time. Three representative regions of interest (ROIs) are shown. Middle two panels, close-up of one of representative areas shown in the top panels. Bottom two panels, representative magnified images region of single MT shown in middle panels where five curvature values were obtained. B, cumulative distribution of MT curvature calculated using the five curvature points per condition. The mean curvature values and SDs of control and Paclitaxel treatment were 0.533 rad/m 0.371 and 0.455 rad/m 0.343, respectively. C, histogram of MT curvature distribution shown in B. D, quantification of MT fluorescence intensity (AFUs) along the length of the measured MTs. n represents number of total MTs evaluated per condition. All statistical significances of difference were assessed with an unpaired two-tailed Student's t test. MT, microtubule; UNC-45A, uncoordinated protein-45A.
Figure 6.
UNC-45A counteracts the MT-straightening effects of Paclitaxel.A, top four panels, representative images of perinuclear MTs visualized via anti-alpha-tubulin staining in GFP and UNC-45A-GFP–overexpressing RFL-6 cells treated or not (control) with Paclitaxel (500 nM for 30 min) after fixation. The white dotted lines indicate cell edges and black lines indicate nuclear membrane. Images were taken with the same exposure time. Three representative regions of interest (ROI) areas are shown. Middle four panels, close-up of one of representative areas shown in the top panels. Bottom four panels, representative magnified image region of single MT shown in middle panels where five curvature values were obtained. B, top, cumulative distribution of MT curvature calculated using the five curvature points in GFP-expressing cells per condition. The mean curvature values and SDs of GFP minus and plus Paclitaxel were 0.546 rad/m 0.396 and 0.440 rad/m 0.328, respectively. Bottom, cumulative distribution of MT curvature calculated using the five curvature points in UNC-45A-GFP–expressing cells per condition. The mean curvature values and SDs of UNC-45A-GFP minus and plus Paclitaxel were 0.636 rad/m 0.436 and 0.559 rad/m 0.403, respectively. C, left and right, histograms of MT curvature distribution shown in B, top and bottom, respectively. D, left, cumulative distribution, right, histogram of Paclitaxel-treated MT curvature distribution minus and plus GFP-UNC-45A. E, quantification of MT fluorescence intensity (arbitrary fluorescence units, AFUs) along the length of the measured MTs. n represents number of total MTs evaluated per condition. F, quantification of GFP mass (AFUs) in GFP and UNC-45A-GFP–expressing cells. n represents number of cells evaluated per condition. All statistical significances of difference were assessed with an unpaired two-tailed Student's t test. MT, microtubule; UNC-45A, uncoordinated protein-45A.
Discussion
The curvature of MTs plays important roles both in normal physiological processes and in pathological conditions. Therefore, understanding the key regulators involved in MT curvature holds significance in both basic cell biology and its potential translational applications. In this study, we demonstrate that the MT-severing protein UNC-45A exhibits a preferential binding to curved MTs and actively contributes to the curvature of peripheral and perinuclear MTs in cells. This binding and contribution occur independently of actomyosin contractility, as it has little to no effect on perinuclear MTs. Furthermore, our research indicates that the presence of Paclitaxel, one of the most commonly used chemotherapy agents for human cancer treatment, results in the straightening of MTs. Despite this, UNC-45A retains its ability to induce curvature in Paclitaxel-exposed MTs. This finding suggests that UNC-45A counteracts the MT-straightening effects of Paclitaxel, presenting one possible mechanism through which UNC-45A overexpression in cancer cells is associated with chemoresistance.
MTs are the stiffest of the biological polymers (1, 2), yet, curved (also referred to as bent or buckled) MTs are often found in cells in both normal and pathological (4, 32, 33, 34, 35, 36). Three factors regulate MT curvature: mechanical interactions, interactions with MAPs, and exposure to MT-targeting agents. Mechanical interactions MTs have with the cellular matrix surrounding them, like for instance, interaction with actin filaments at the cell cortex (3, 4, 26, 37, 38) or interaction with other cellular organelles in crowded areas of the cell, increase MT curvature (39). MT curvature can also be influenced by their interaction with MAPs that acts on their protofilament, lattice, and/or ends, favoring or stabilizing curved or straight conformations. This is the case of kinesin-13 which depolymerizes MTs via inducing tubulin curvature and destabilization (7), of kinesin-1, which bend-locks MTs (8), or of Tau, which confers MTs’ protofilament a straight conformation (9). Lastly, MT-targeting agents can influence MT curvature. This is the case of Paclitaxel, which stabilizes straight MTs’ protofilament conformation while simultaneously increases MT flexibility in vitro (6, 31), or of vinblastine and colchicine, which favor and stabilize tubulin assembly and conformation, respectively (40, 41). MT bending has physiological and pathological significance. Curved MTs are usually found in mitotic spindles (32) and crowded areas of cells (39). Pathologically, bent and wavy MTs are often found in the axonal swelling associated with neurodegenerative diseases, aging, injuries, and axonopathies in general (35).
UNC-45A is a newly characterized MAP with ATP-independent MT-severing properties (13, 20, 42). UNC-45A-mediated MT severing is preceded by the presence of kinks and bent regions in the MTs (13). In cells, MTs often bend and break (3, 4). Thus, we sought to determine the contribution of UNC-45A to MT curvature in cells, if any. We started by looking at the distribution of UNC-45A on areas of MTs with different curvatures. We found that the UNC-45A signal increases in the curved region of MTs, suggesting that it may act on their curvature. This is interesting because the MT-stabilizing protein Tau preferentially binds to curved MTs (43) and prevents the access of katanin (44), the most well-known of MT-severing protein, to its severing site. Thus, hinting at the fact that MTs curvature may be an area of particular importance as far as MT stability regulation by severing proteins. We also found that in cells, UNC-45A actively contributes to increasing MT curvature of both peripheral and perinuclear MTs. UNC-45A has a dual nonmutually exclusive role in regulating NMII activity and MT stability (13). Thus, we cannot exclude that the contribution of UNC-45A to peripheral MT curvature could be partly due to its effect on actomyosin contractility. However, this does not seem to be the case for perinuclear MTs since this cell area is less prone to actomyosin forces (28) and given our results that neither increasing nor decreasing actomyosin contractility affects perinuclear MT curvature in RFL-6 cells. Furthermore, because UNC-45A overexpression results in loss of overall cellular MT mass over time (13), the effect of UNC-45A overexpression on MT curvature is not due to increased MT crowdedness and instead happens despite the opposite.
In vitro, Paclitaxel enhances MT lattice heterogeneity by changing lateral contacts between protofilaments (5, 6) and causes structural damage (nanodamages) in the lattice (45, 46). Both phenomena are consistent with decreased flexural rigidity of these Paclitaxel-treated MTs and increased curvature (6, 31, 47). Our in vitro experiments confirm that Paclitaxel-treated MTs are curvier than the untreated ones. In cells, we found that Paclitaxel straightens MTs. There are several possible explanations for what appears to be an inconsistency between in vitro and in vivo Paclitaxel-treated MTs with respect to their flexibility and curvature. First, Paclitaxel-treated MTs derived from axoneme are more flexible than untreated ones but straighter when exposed to a fluid flow (25). Thus, it is plausible to propose that while Paclitaxel-treated MTs may display reduced resistance to bending in RFL-6 cells, they straighten out when they align with the directional intracellular flow (48). Second, while Paclitaxel-nanodamaged MTs in vitro have a softer behavior if these damages are not repaired, they become stiffer if they are repaired via free tubulin availability in the in vitro system (47). Because a pool of free tubulin is present in cells, it is conceivable that Paclitaxel-treated MTs are repaired, which can account for their straightening. Lastly and most apparent, MTs in a cell-free system are not exposed to cellular MAPs, and this also likely contributes to a difference between MT behavior in the presence of Paclitaxel between in vitro and cellular MTs.
We also found that UNC-45A can still curve MTs in Paclitaxel-treated cells. This seemingly contradicts what we propose to be a preference of UNC-45A for curved regions of the MTs. However, as mentioned above, exposure to Paclitaxel, along with exposure to oxidative agents like hydrogen peroxide, causes nanodamages in MTs in vitro (45, 46, 49). Therefore, it is possible that UNC-45A preferentially binds and acts on MT defects that are present in both curved and Paclitaxel-nanodamaged areas of MTs. Notably, UNC-45A is the only MT-severing protein we know of that does not and ATPase domain and several human diseases like cancer and neurodegenerative diseases are characterized by both a reduction of ATP levels and a highly oxidative environment (50, 51). In this scenario, UNC-45A may have a functional advantage over other MT destabilizing proteins and have a significant role in human diseases from cancer to neurodegeneration. Overall, our study sheds light on the pivotal role of UNC-45A in regulating MT curvature and provides valuable insights into the implications of this process in both normal and pathological human contexts. These findings may pave the way for novel therapeutic strategies targeting MT dynamics to treat human diseases.
Experimental procedures
Preparation of MTs
Porcine brain tubulin (T240) and rhodamine-labeled tubulin (TL590M) were purchased from Cytoskeleton. GMPCPP was purchased from Jena Bioscience (NU-405S). Taxol was purchased from Cytoskeleton (TXD01). GMPCPP MTs were prepared as previously described with (13, 52) some modifications. Briefly, 1.5 mg/ml of tubulin (5:1 mixture of unlabeled and rhodamine-labeled tubulin, respectively), 1 mM GMPCPP, and 1 mM MgCl2 were mixed in BRB80 (80 mM 1,4-piperazinediethanesulfonic acid, 1 mM MgCl2, 1 mM EGTA, pH 6.9—pH was adjusted with KOH) and kept on ice for 5 min, followed by incubation at 37 °C for 1 to 2 h or until they were within 5 to 30 μm length range. Postincubation, the seeds were diluted 10× in warm BRB80 (with 10 μM Taxol when used). Taxol-stabilized GTP MTs were prepared as previously described (53) in BRB80. Briefly, 5 mg/ml of tubulin (5:1 mixture of unlabeled and rhodamine-labeled tubulin) was polymerized in the presence of 1 mM GTP for 20 min at 37 °C then 50 μM of Taxol was added and incubated for additional 20 min to equilibrate the Taxol. MTs were centrifuged at 14,000g for 10 min at 25 °C and resuspended in BRB80 containing 50 μM of Taxol. The volume of BRB80 was adjusted to have similar MT density as GMPCPP MTs.
Recombinant protein
GFP-tagged UNC-45A full length (UNC-45A WT; 1–944 aa) was cloned, expressed, and affinity purified as previously described with some modifications to optimally preserve protein activity (13, 54). The following detailed protocol refers to a 250 ml bacterial culture and can be scaled up or down as needed. GST-GFP-UNC-45A recombinant protein is expressed in Escherichia coli and isolated by lysing the bacterial cell pellet using 5 ml of a PBS-based solution supplemented with 10 mM magnesium chloride (MgCl2), 1 mM PMSF, protease inhibitor cocktail, 1% of Triton X-100, and 10% of glycerol. The cell lysis is achieved using an Avestin Emulsiflex Homogenizer C3, and the lysate is treated with 0.02 mg/ml of DNase and 400 mM of NaCl, with each treatment performed at 4 °C for a duration of 15 min. To remove cellular debris, the lysate is subjected to centrifugation at 12,000×g for 1 h at 4 °C. The supernatant is supplemented with 5 mM DTT and added to an affinity purification column containing 1.5 ml of prewashed Glutathione Sepharose 4B beads for 45 min at 4 °C. At the end of the incubation period, the column is washed with five column volumes of wash buffer (20 mM Tris–HCl pH7.5, 500 mM NaCl, 10 mM of MgCl2, 5 mM DTT, 1% of Triton X-100, and 10% glycerol), and the protein is eluted in fractions (6×) with half column volume of elution buffer (50 mM Tris–HCl, 300 mM NaCl, 10 mM of MgCl2, 5 mM DTT, 20 mM reduced glutathione, 1% Triton X-100, and 10% glycerol, pH = 8.8). Pooled fraction–containing protein (as verified by SDS-PAGE followed by Coomassie Blue staining) are subjected to thrombin digestion to remove the GST-tag at 16 °C for 4.5 h under gentile rocking. The thrombin-to-protein ratio exhibits a variability ranging from 1:10 to 1:40 μg of protein, contingent upon the specific batches of thrombin utilized. Thrombin and GST-tag are subsequently removed from the mixture by incubation with Glutathione Sepharose 4B beads and p-aminobenzodiazepine agarose at 4 °C for 45 min under gentle rocking. Following centrifugation, an aliquot of the supernatant-containing GFP-UNC-45A recombinant protein is subjected to SDS-PAGE and Coomassie Blue staining to determine protein purity. This protocol entails a 12-h process, during which the protein is kept in the above describe buffer at a temperature of 4 °C and protected from light. Following this, the protein undergoes centrifugation at 16,000×g at 4 °C for 10 min on the subsequent day to eliminate potential aggregates. The resulting supernatant is then subjected to buffer exchange (BRB80 buffer) using a spin-column with a 50 kDa cut-off, and the protein concentration is quantified using absorbance at 280 nm (A280). It is important to note that all experimental procedures are conducted within 48 h of the buffer exchange. Within this time frame, the recombinant GFP-UNC-45A protein remains soluble and does not undergo precipitation when stored in BRB-80 buffer at 4 °C.
Glycerol gradient sedimentation
Glycerol gradient sedimentation was performed as previously described with modifications (22, 55). Briefly, glycerol gradients were prepared by layering equal steps of 10%, 16.3%, 22.5%, 28.8%, and 35% glycerol in BRB80 buffer. Gradients were allowed to form overnight at 4 °C. GFP-UNC45A (200 μl containing 30 μg of protein) were applied to 5 ml of gradients. Gradients were spun in a SW55 Ti rotor at 45,000 rpm for 12 h at 4 °C. Sedimentation coefficient was determined relative to standards including bovine serum albumin (4.6S) and catalase (11.2S) as previously described (56).
Construction and preparation of flow chambers for TIRF imaging
TIRF chamber was prepared and MTs were immobilized as we previously described (13, 19). Each channel of the TIRF chamber was rinsed with BRB80 containing 1 mM DTT prior to the addition GFP-UNC-45A in imaging solution (50 mM KCl, 0.5% Pluronic F127, 0.2 mg/ml casein, 1.5% glycerol, 0.1% methylcellulose 4000 cP, 20 mM glucose, 110 μg/ml glucose oxidase, and 20 μg/ml catalase, 20 mM DTT in BRB80) as previously described (57). Images were taken 10 min after addition of GFP-UNC45A. For Taxol-stabilized MTs, the imaging buffer contained 1 μM of Taxol. Images were taken 10 min after addition of the imaging solution containing GFP-UNC-45A.
TIRF imaging
Time-lapse fluorescent images of MTs and GFP-UNC-45A were obtained with 561 nm and 488 nm lasers generated from TIRF mode on a Zeiss Axio observer Z1 inverted microscope using 100 × /1.46 NA objective lens. An oxygen scavenging system of glucose oxidase and catalase was employed to minimize photobleaching and photodamage during illumination with the laser. The standard exposure time was 20 ms for both 561 nm and 488 nm lasers.
Cell culture
Rat RFL-6 fibroblasts were purchased from the American Type Culture Collection and cultured in Ham's F-12K medium (Thermo Fisher Scientific) supplemented with 20% fetal bovine serum and 1% pen-strep. Cells were authenticated in December 2021, routinely tested negative for mycoplasma and were used between passage 6 and 8.
Modulation of UNC-45A expression levels in cells
For UNC-45A overexpression and knockdown, lentiviral supernatant containing either GFP empty vector control, UNC45A-GFP (in pRRL-3′GFP vector) or scramble and UNC-45A shRNAs (CCACCTCAAGCTGGAAGATTA plus microRNA-30 oligonucleotides cassette inserted into pRRL-PPTsin-3′GFP vector) were prepared and used to infect RFL-6 cells as previously described (19).
Antibody, chemicals, and tubulin
Mouse monoclonal anti-UNC-45A (Enzo, ADI-SRA-1800-D, 1:1000), phospho-myosin light chain 2 (Ser 19) antibody (Cell signaling, #3671, 1:1000), and monoclonal anti-myosin clone MY-21 (Sigma, M4401, 1:4000) were used for Western blot to detect recombinant UNC-45A, p-S19 MLC, and total MLC, respectively. Mouse monoclonal anti-alpha-tubulin (Sigma, T6074, 1:2000) and rabbit monoclonal anti-GFP (Thermo Fisher Scientific, G10362, 1:100) were used for immunofluorescence staining. Secondary antibodies used were peroxidase-linked anti-mouse IgG and peroxidase-linked anti-rabbit IgG (both Cytiva, formerly known as GE Healthcare Bio-Sciences, NA931 and NA934; 1:5000), Alexa Fluor 594–conjugated donkey anti-mouse IgG (1:250) and FITC-conjugated goat anti-rabbit IgG (1:200) (both Jackson ImmunoResearch Laboratories, 715-585-150 and 111-095-003, respectively). The specificity of UNC-45A and alpha-tubulin antibodies and the secondary antibodies were validated with RFL-6 cell lysates and in RFL-6 cells in our previous publication (42). The specificity of phospho-myosin light chain 2 and MLC antibodies were validated with RFL-6 cell lysates (5 μg, 10 μg, 20 μg of proteins). Paclitaxel was purchased from Cytoskeleton. Blebbistatin and calyculin A were purchased from Sigma-Aldrich (203,390 and C5552, respectively). Tubulin Tracker Deep Red was purchased from Thermo Fisher Scientific (T34077). Porcine brain tubulin (T240) and rhodamine-labeled tubulin (TL590M) were purchased from Cytoskeleton. GMPCPP was purchased from Jena Bioscience (NU-405S). Glutathione Sepharose 4B beads (17075605) were purchased from Cytiva. DNase I (10104159001) and PMSF (10837091001) were purchased from Millipore Sigma.
Live-cell imaging of MTs in RFL-6 cells
To determine the effects of UNC-45A overexpression on MT curvature in live cells, UNC45A-GFP–overexpressing RFL-6 cells (16 h postinfection) were treated with Tubulin Tracker Deep Red (Thermo Fisher Scientific T34077) and imaged as we have previously described (13). Briefly, GFP-control and UNC45A-GFP–overexpressing RFL-6 cells were treated with Tubulin Tracker Deep Red according to the manufacturer’s recommendation. Tubulin Tracker Deep Red was diluted 1:2000. This dilution corresponds to 500 nM Paclitaxel (personal communication from the Thermo Fisher Scientific technical support team). Cells infected with GFP control and UNC-45A-GFP–overexpressing lentivirus were treated with Tubulin Tracker Deep Red sequentially so that imaging was performed under the same conditions. Time-lapse fluorescent images were collected with a Zeiss Axio observer Z1 inverted microscope using a 100 × /1.46 NA objective lens. Digital images were collected at 8 s intervals over a period of 8 min using a Cy5 filter cube. Laser power and exposure time were minimized to avoid photobleaching and photodamage, and all images for experimental and control groups were taken under the same conditions.
Fixed cell imaging of MTs in RFL-6 cells
For studies on MT curvature in fixed cells, fixation and staining was performed as we previously described (13). Briefly, GFP-control and UNC45A-GFP–expressing RFL-6 cells or drug- (blebbistatin and Paclitaxel) treated RFL-6 cells were extracted in an MT-preserving buffer (BRB80 and 4 mM EGTA) with 0.5% Triton X-100 for 30 s to remove free tubulin and then fixed with 0.5% glutaraldehyde for 10 min. After fixation, cells were quenched for 7 min with 0.1% NaBH4 and then rehydrated in PBS before blocking in AbDil (2% Tris-buffered saline (TBS) and Tween 20 with 2% bovine serum albumin and 0.1% sodium azide) for 30 min. After blocking, cultures were stained with anti-α-tubulin and anti-GFP primary antibody, followed by Alexa Fluor 594- and FITC-conjugated secondary antibodies. Cells were then rinsed in TBS and Tween 20 and mounted in a Prolong Gold with 4′,6-diamidino-2-phenylindole. For p-S19 MLC staining, calyculin A–treated RFL-6 cells were fixed in 3.7% formaldehyde in PBS and permeabilized in 0.5% Triton X-100 in TBS before staining as described (30). Images were obtained on an Axiovert 200 microscope (Zeiss) equipped with a high-resolution charge-coupled device camera. All images were obtained using identical camera, microscope, and imaging criteria, such as gain, brightness, contrast, and exposure time. Digital gray values of image pixels representing arbitrary fluorescence units (AFUs) were obtained using Fiji software (https://imagej.net/software/fiji/downloads).
Western blot analysis
Total cellular protein (10 μg) from each sample was separated by SDS-PAGE, transferred to polyvinylidene fluoride membranes and subjected to Western blot analysis using the specified antibodies. Amido Black staining was performed to confirm equal protein loading. For detection of p-S19 MLC, RFL-6 cells were lysed in lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 1 × protease inhibitor mixture, 1 × phosphatase inhibitor mixture). For detection of UNC-45A, RFL-6 cells were lysed in 1% SDS lysis buffer. After secondary antibody incubation, the polyvinylidene fluoride membrane was developed with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, 34095) by following the manufacturer protocol.
Drug studies
To activate actomyosin contractility, RFL-6 cells were treated with calyculin A and used concentrations varying between 0.5 nM and 5 nM for 10 min to determine the optimum concentration, and RFL-6 cells were treated with 1 nM calyculin A for 10 min for p-S19 MLC and alpha-tubulin staining. To inhibit actomyosin contractility, RFL-6 cells were treated with 25 μM of blebbistatin for 1 h as previously described (13). For Paclitaxel treatment, RFL-6 cells were treated with either 500 nM Paclitaxel or dimethyl sulfoxide for 30 min.
Analysis of MT curvature in cells
All data were recorded as 16 bit images using Zen software (blue edition, Zeiss) (https://search.yahoo.com/yhs/search?hspart=airfind&hsimp=yhs-searchtool&p=ZEN+software). Single, clearly visible MTs were selected, and their segments (5–10 μm length) were used to extract x-y coordinates. MT regions that were crossing and bundling with other MTs were avoided.
Semiautomated MT tracking
In fixed cells, three representative regions of interest (ROIs) were selected within each cell and seven representative individual MTs per ROI were selected. The x-y coordinates of these MTs were extracted by semiautomated MT tacking algorism implemented in MATLAB as we have previously described (3, 26, 27). Briefly, the contour of single MT segments was estimated within user-defined rectangular regions, and MT backbone coordinates were estimated by fitting a Gaussian curve to each vertical line scan of the region. Approximately 0.5 μm interval of x-y coordinates were used to calculate MT curvature. The average Gaussian curve amplitude of vertical line scan at five highest curvature was expressed as MT AFU.
Manual MT tracking
To trace MT shapes in live cells and in vitro, we used protocols previously described in our laboratories (3, 4). Briefly, in live cells, two representative ROIs were selected and three representative single, clearly visible MTs were selected per ROI. Using ImageJ software (Fiji) (https://imagej.net/ij/download.html), x-y coordinate data from fluorescent images every 0.5 ± 0.05 μm along 5 to 10 μm length of single clearly visible MTs was collected in RFL-6 cells and in vitro. For MT AFU, the fluorescent intensity of MT at each coordinate and adjacent background were recorded with circular selection, which covers the entire width of the MT. The background intensity was subtracted from MT intensity at each coordinate and the average of MT fluorescence intensity per single MT was expressed as MT AFU.
MT curvature estimation
Once x-y coordinates of MT contour were extracted semiautomatically or manually, the curvature (k) was calculated locally at each coordinate by taking three adjacent points and computing the change in the angle (k) over the average arc length of the two adjacent segments ( and ) as we have previously described (3, 26)
For the curvature distribution with five highest curvature, five highest curvature values from each MT were plotted.
Fluorescence and chemiluminescence intensity analysis
The protocol to measure fluorescence intensity of GFP-UNC-45A on MT lattice was adapted from previously published work (58). In order to measure GFP-UNC-45A signal at the same coordinate where MT curvature was measured, circular ROI covering the entire width of MT and the integrated density from the ROI was measured on the GFP-UNC-45A channel. The background intensity of GFP-UNC-45A channel nearby MT curvature measurement was subtracted from ROI intensity to remove the background noise signal. This background intensity values were also reported to ensure that the GFP-UNC-45A channel had homogeneous background. In cells, GFP mass AFU and p-S19 MLC mass AFU in RFL-6 cells were quantified as previously described (13). For Western blotting and Coomassie stained gels, the band intensities were quantified by subtracting the integrated density of background near each band from the integrated density of each band. The fixed sized rectangular ROI was drawn by ImageJ to measure the integrated density of bands and background.
Statistical analysis
Results are reported as mean ± SD of three or more independent experiments. Unless otherwise indicated, statistical significance of difference was assessed with an unpaired two-tailed Student's t test using Prism (V.8 GraphPad) (https://www.graphpad.com/) and Excel (Microsoft) (https://www.microsoft.com/en-us/microsoft-365/excel). The level of significance was set at p < 0.05.
Data availability
Data are available from the corresponding authors upon reasonable request.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We thank Dr Guillermo Marques (University of Minnesota Imaging Center) for assistance with image analysis.
Author contributions
A. H. and M. B. conceptualization; A. H., B. C, D. O., and M. B. methodology; A. H., B. C., and M. B. software; A. H., V. C., M. S., and M. B., validation; A. H., V. C., M. S., and M. B. formal analysis; A. H., V. C., M. S., and M. B., investigation; M. B. resources; A. H. and M. B. data curation; A. H. and M. B. writing-original draft; A.H. visualization; M. B. supervision; M. B. project administration; D. O and M. B. funding acquisition.
Funding and additional information
This work was supported by the U.S. Department of Defense Ovarian Cancer Research Program (OC160377), the Minnesota Ovarian Cancer Alliance, the Randy Shaver Cancer Research, the Alzheimer’s Association (AARG-NTF-21-848680), Community Fund and the National Institute of General Medical Sciences (R01-GM130800 to M. B. and D. O.). The funders had no role in study design, data collection, and analysis, decision to publish or preparation of the manuscript. Deposited in PMC for release after 12 months.
Reviewed by members of the JBC Editorial Board. Edited by Enrique De La Cruz
Supporting information
Figure S1.
Figure S2.
Figure S3.
Figure S4.

Figure S5.

References
- 1.Mickey B., Howard J. Rigidity of microtubules is increased by stabilizing agents. J. Cell Biol. 1995;130:909–917. doi: 10.1083/jcb.130.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felgner H., Frank R., Schliwa M. Flexural rigidity of microtubules measured with the use of optical tweezers. J. Cell Sci. 1996;109:509–516. doi: 10.1242/jcs.109.2.509. [DOI] [PubMed] [Google Scholar]
- 3.Bicek A.D., Tüzel E., Kroll D.M., Odde D.J. Analysis of microtubule curvature. Methods Cell Biol. 2007;83:237–268. doi: 10.1016/S0091-679X(07)83010-X. [DOI] [PubMed] [Google Scholar]
- 4.Odde D.J., Ma L., Briggs A.H., DeMarco A., Kirschner M.W. Microtubule bending and breaking in living fibroblast cells. J. Cell Sci. 1999;112:3283–3288. doi: 10.1242/jcs.112.19.3283. [DOI] [PubMed] [Google Scholar]
- 5.Alushin G.M., Lander G.C., Kellogg E.H., Zhang R., Baker D., Nogales E. High-resolution microtubule structures reveal the structural transitions in alphabeta-tubulin upon GTP hydrolysis. Cell. 2014;157:1117–1129. doi: 10.1016/j.cell.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kellogg E.H., Hejab N.M.A., Howes S., Northcote P., Miller J.H., Díaz J.F., et al. Insights into the distinct mechanisms of action of taxane and non-taxane microtubule stabilizers from cryo-EM structures. J. Mol. Biol. 2017;429:633–646. doi: 10.1016/j.jmb.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W., Cantos-Fernandes S., Lv Y., Kuerban H., Ahmad S., Wang C., Gigant B. Insight into microtubule disassembly by kinesin-13s from the structure of Kif2C bound to tubulin. Nat. Commun. 2017;8:70. doi: 10.1038/s41467-017-00091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peet D.R., Burroughs N.J., Cross R.A. Kinesin expands and stabilizes the GDP-microtubule lattice. Nat. Nanotechnol. 2018;13:386–391. doi: 10.1038/s41565-018-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi M.C., Raviv U., Miller H.P., Gaylord M.R., Kiris E., Ventimiglia D., et al. Human microtubule-associated-protein tau regulates the number of protofilaments in microtubules: a synchrotron x-ray scattering study. Biophys. J. 2009;97:519–527. doi: 10.1016/j.bpj.2009.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni W., Odunuga O.O. UCS proteins: chaperones for myosin and co-chaperones for Hsp90. Subcell. Biochem. 2015;78:133–152. doi: 10.1007/978-3-319-11731-7_7. [DOI] [PubMed] [Google Scholar]
- 11.Lee C.F., Melkani G.C., Bernstein S.I. The UNC-45 myosin chaperone: from worms to flies to vertebrates. Int. Rev. Cell Mol. Biol. 2014;313:103–144. doi: 10.1016/B978-0-12-800177-6.00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price M.G., Landsverk M.L., Barral J.M., Epstein H.F. Two mammalian UNC-45 isoforms are related to distinct cytoskeletal and muscle-specific functions. J. Cell Sci. 2002;115:4013–4023. doi: 10.1242/jcs.00108. [DOI] [PubMed] [Google Scholar]
- 13.Habicht J., Mooneyham A., Hoshino A., Shetty M., Zhang X., Emmings E., et al. UNC-45A breaks the microtubule lattice independently of its effects on non-muscle myosin II. J. Cell Sci. 2021;134 doi: 10.1242/jcs.248815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazda L., Pokrzywa W., Hellerschmied D., Löwe T., Forné I., Mueller-Planitz F., et al. The myosin chaperone UNC-45 is organized in tandem modules to support myofilament formation in C. elegans. Cell. 2013;152:183–195. doi: 10.1016/j.cell.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venolia L., Waterston R.H. The unc-45 gene of Caenorhabditis elegans is an essential muscle-affecting gene with maternal expression. Genetics. 1990;126:345–353. doi: 10.1093/genetics/126.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venolia L., Ao W., Kim S., Kim C., Pilgrim D. unc-45 gene of Caenorhabditis elegans encodes a muscle-specific tetratricopeptide repeat-containing protein. Cell Motil. Cytoskeleton. 1999;42:163–177. doi: 10.1002/(SICI)1097-0169(1999)42:3<163::AID-CM1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 17.Jilani Y., Lu S., Lei H., Karnitz L.M., Chadli A. UNC45A localizes to centrosomes and regulates cancer cell proliferation through ChK1 activation. Cancer Lett. 2015;357:114–120. doi: 10.1016/j.canlet.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisa N.H., Jilani Y., Kainth K., Redd P., Lu S., Bougrine O., et al. The co-chaperone UNC45A is essential for the expression of mitotic kinase NEK7 and tumorigenesis. J. Biol. Chem. 2019;294:5246–5260. doi: 10.1074/jbc.RA118.006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mooneyham A., Iizuka Y., Yang Q., Coombes C., McClellan M., Shridhar V., et al. UNC-45A is a novel microtubule-associated protein and regulator of paclitaxel sensitivity in ovarian cancer cells. Mol. Cancer Res. 2019;17:370–383. doi: 10.1158/1541-7786.MCR-18-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clemente V., Hoshino A., Meints J., Shetty M., Starr T., Lee M., Bazzaro M. UNC-45A is highly expressed in the proliferative cells of the mouse genital tract and in the microtubule-rich areas of the mouse nervous system. Cells. 2021;10:1604. doi: 10.3390/cells10071604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartman J.J., Mahr J., McNally K., Okawa K., Iwamatsu A., Thomas S., et al. Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell. 1998;93:277–287. doi: 10.1016/s0092-8674(00)81578-0. [DOI] [PubMed] [Google Scholar]
- 22.Hartman J.J., Vale R.D. Microtubule disassembly by ATP-dependent oligomerization of the AAA enzyme katanin. Science. 1999;286:782–785. doi: 10.1126/science.286.5440.782. [DOI] [PubMed] [Google Scholar]
- 23.Sharp D.J., Ross J.L. Microtubule-severing enzymes at the cutting edge. J. Cell Sci. 2012;125:2561–2569. doi: 10.1242/jcs.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johjima A., Noi K., Nishikori S., Ogi H., Esaki M., Ogura T. Microtubule severing by katanin p60 AAA+ ATPase requires the C-terminal acidic tails of both alpha- and beta-tubulins and basic amino acid residues in the AAA+ ring pore. J. Biol. Chem. 2015;290:11762–11770. doi: 10.1074/jbc.M114.614768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dye R.B., Fink S.P., Williams R.C., Jr. Taxol-induced flexibility of microtubules and its reversal by MAP-2 and Tau. J. Biol. Chem. 1993;268:6847–6850. [PubMed] [Google Scholar]
- 26.Bicek A.D., Tüzel E., Demtchouk A., Uppalapati M., Hancock W.O., Kroll D.M., Odde D.J. Anterograde microtubule transport drives microtubule bending in LLC-PK1 epithelial cells. Mol. Biol. Cell. 2009;20:2943–2953. doi: 10.1091/mbc.E08-09-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prahl L.S., Castle B.T., Gardner M.K., Odde D.J. Quantitative analysis of microtubule self-assembly kinetics and tip structure. Methods Enzymol. 2014;540:35–52. doi: 10.1016/B978-0-12-397924-7.00003-0. [DOI] [PubMed] [Google Scholar]
- 28.Rey-Suarez I., Rogers N., Kerr S., Shroff H., Upadhyaya A. Actomyosin dynamics modulate microtubule deformation and growth during T-cell activation. Mol. Biol. Cell. 2021;32:1641–1653. doi: 10.1091/mbc.E20-10-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishihara H., Martin B.L., Brautigan D.L., Karaki H., Ozaki H., Kato Y., et al. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem. Biophys. Res. Commun. 1989;159:871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- 30.Peterson L.J., Rajfur Z., Maddox A.S., Freel C.D., Chen Y., Edlund M., et al. Simultaneous stretching and contraction of stress fibers in vivo. Mol. Biol. Cell. 2004;15:3497–3508. doi: 10.1091/mbc.E03-09-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elie-Caille C., Severin F., Helenius J., Howard J., Muller D.J., Hyman A.A. Straight GDP-tubulin protofilaments form in the presence of taxol. Curr. Biol. 2007;17:1765–1770. doi: 10.1016/j.cub.2007.08.063. [DOI] [PubMed] [Google Scholar]
- 32.Ivec A., Trupinić M., Tolić I.M., Pavin N. Oblique circle method for measuring the curvature and twist of mitotic spindle microtubule bundles. Biophys. J. 2021;120:3641–3648. doi: 10.1016/j.bpj.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mani N., Wijeratne S.S., Subramanian R. Micron-scale geometrical features of microtubules as regulators of microtubule organization. Elife. 2021;10 doi: 10.7554/eLife.63880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazarus C., Soheilypour M., Mofrad M.R. Torsional behavior of axonal microtubule bundles. Biophys. J. 2015;109:231–239. doi: 10.1016/j.bpj.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hahn I., Voelzmann A., Liew Y.T., Costa-Gomes B., Prokop A. The model of local axon homeostasis - explaining the role and regulation of microtubule bundles in axon maintenance and pathology. Neural Dev. 2019;14:11. doi: 10.1186/s13064-019-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brangwynne C.P., MacKintosh F.C., Kumar S., Geisse N.A., Talbot J., Mahadevan L., et al. Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J. Cell Biol. 2006;173:733–741. doi: 10.1083/jcb.200601060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prahl L.S., Bangasser P.F., Stopfer L.E., Hemmat M., White F.M., Rosenfeld S.S., Odde D.J. Microtubule-based control of motor-clutch system mechanics in glioma cell migration. Cell Rep. 2018;25:2591–2604.e8. doi: 10.1016/j.celrep.2018.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kent I.A., Rane P.S., Dickinson R.B., Ladd A.J.C., Lele T.P. Transient pinning and pulling: a mechanism for bending microtubules. PLoS One. 2016;11:e0151322. doi: 10.1371/journal.pone.0151322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakraborty S., Mahamid J., Baumeister W. Cryoelectron tomography reveals nanoscale organization of the cytoskeleton and its relation to microtubule curvature inside cells. Structure. 2020;28:991–1003.e4. doi: 10.1016/j.str.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Gigant B., Wang C., Ravelli R.B.G., Roussi F., Steinmetz M.O., Curmi P.A., et al. Structural basis for the regulation of tubulin by vinblastine. Nature. 2005;435:519–522. doi: 10.1038/nature03566. [DOI] [PubMed] [Google Scholar]
- 41.Cormier A., Marchand M., Ravelli R.B.G., Knossow M., Gigant B. Structural insight into the inhibition of tubulin by vinca domain peptide ligands. EMBO Rep. 2008;9:1101–1106. doi: 10.1038/embor.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Habicht J., Mooneyham A., Shetty M., Zhang X., Shridhar V., Winterhoff B., et al. UNC-45A is preferentially expressed in epithelial cells and binds to and co-localizes with interphase MTs. Cancer Biol. Ther. 2019;20:1304–1313. doi: 10.1080/15384047.2019.1632637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samsonov A., Yu J.Z., Rasenick M., Popov S.V. Tau interaction with microtubules in vivo. J. Cell Sci. 2004;117:6129–6141. doi: 10.1242/jcs.01531. [DOI] [PubMed] [Google Scholar]
- 44.Tan R., Lam A.J., Tan T., Han J., Nowakowski D.W., Vershinin M., et al. Microtubules gate tau condensation to spatially regulate microtubule functions. Nat. Cell Biol. 2019;21:1078–1085. doi: 10.1038/s41556-019-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rai A., Liu T., Glauser S., Katrukha E.A., Estévez-Gallego J., Rodríguez-García R., et al. Taxanes convert regions of perturbed microtubule growth into rescue sites. Nat. Mater. 2020;19:355–365. doi: 10.1038/s41563-019-0546-6. [DOI] [PubMed] [Google Scholar]
- 46.Rai A., Liu T., Katrukha E.A., Estévez-Gallego J., Manka S.W., Paterson I., et al. Lattice defects induced by microtubule-stabilizing agents exert a long-range effect on microtubule growth by promoting catastrophes. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2112261118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaedel L., John K., Gaillard J., Nachury M.V., Blanchoin L., Théry M. Microtubules self-repair in response to mechanical stress. Nat. Mater. 2015;14:1156–1163. doi: 10.1038/nmat4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldstein R.E., van de Meent J.W. A physical perspective on cytoplasmic streaming. Interf. Focus. 2015;5 doi: 10.1098/rsfs.2015.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldblum R.R., McClellan M., White K., Gonzalez S.J., Thompson B.R., Vang H.X., et al. Oxidative stress pathogenically remodels the cardiac myocyte cytoskeleton via structural alterations to the microtubule lattice. Dev. Cell. 2021;56:2252–2266.e6. doi: 10.1016/j.devcel.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheung E.C., Vousden K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer. 2022;22:280–297. doi: 10.1038/s41568-021-00435-0. [DOI] [PubMed] [Google Scholar]
- 51.Hou Y., Dan X., Babbar M., Wei Y., Hasselbalch S.G., Croteau D.L., Bohr V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 52.Coombes C., Yamamoto A., McClellan M., Reid T.A., Plooster M., Luxton G.W.G., et al. Mechanism of microtubule lumen entry for the alpha-tubulin acetyltransferase enzyme alphaTAT1. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E7176–E7184. doi: 10.1073/pnas.1605397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawkins T.L., Sept D., Mogessie B., Straube A., Ross J.L. Mechanical properties of doubly stabilized microtubule filaments. Biophys. J. 2013;104:1517–1528. doi: 10.1016/j.bpj.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diaz-Valencia J.D., Bailey M., Ross J.L. Purification and biophysical analysis of microtubule-severing enzymes in vitro. Methods Cell Biol. 2013;115:191–213. doi: 10.1016/B978-0-12-407757-7.00013-X. [DOI] [PubMed] [Google Scholar]
- 55.Hanson P.I., Roth R., Morisaki H., Jahn R., Heuser J.E. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- 56.Erickson H.P. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online. 2009;11:32–51. doi: 10.1007/s12575-009-9008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vemu A., Szczesna E., Roll-Mecak A. In vitro reconstitution assays of microtubule amplification and lattice repair by the microtubule-severing enzymes katanin and spastin. Methods Mol. Biol. 2020;2101:27–38. doi: 10.1007/978-1-0716-0219-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monroy B.Y., Sawyer D.L., Ackermann B.E., Borden M.M., Tan T.C., Ori-McKenney K.M. Competition between microtubule-associated proteins directs motor transport. Nat. Commun. 2018;9:1487. doi: 10.1038/s41467-018-03909-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding authors upon reasonable request.