Abstract
Several processes during sexual reproduction in higher plants involve the movement of water between cells or tissues. Before flower anthesis, anther and pollen dehydration takes place before the release of mature pollen at dehiscence. Aquaporins represent a class of proteins that mediates the movement of water over cellular membranes. Aquaporins of the plasmamembrane PIP2 family are expressed in tobacco (Nicotiana tabacum) anthers and may therefore be involved in the movement of water in this organ. To gain more insight into the role these proteins may play in this process, we have analyzed their localization using immunolocalizations and generated plants displaying RNA interference of PIP2 aquaporins. Our results indicate that PIP2 protein expression is modulated during anther development. Furthermore, in tobacco PIP2 RNA interference plants, anther dehydration was slower, and dehiscence occurred later when compared with control plants. Together, our results suggest that aquaporins of the PIP2 class are required for efficient anther dehydration prior to dehiscence.
In plant sexual reproduction, control of water movement plays an important role. During development, for example, young anthers must first take up water for growth, but at later stages anthers and pollen dehydrate before dehiscence. In tobacco (Nicotiana tabacum) and many other species, pollen dehydration starts after the degeneration of the tapetum, and pollen grains are partially dehydrated at dehiscence (Goldberg, 1988; Franchi et al., 2002). The significance of correct pollen dehydration for the functions of the pollen grain is illustrated by the Arabidopsis (Arabidopsis thaliana) mutant raring-to-go (Johnson and McCormick, 2001). In these plants, pollen grains do not dehydrate as wild-type pollen and germinate within the anther when it is exposed to high humidity. However, besides this example, not much is known about the detailed mechanism of pollen dehydration.
Correct timing of anther dehiscence is important, as the time of pollen release is crucial for successful fertilization. It was shown that plant hormones are implicated in the dehiscence process. In Arabidopsis, mutations in several genes involved in jasmonic acid biosynthesis all result in delayed anther dehiscence, suggesting the involvement of jasmonic acid signaling in its regulation (Sanders et al., 2000; Ishiguro et al., 2001; Park et al., 2002). In tobacco, however, ethylene has been shown to control the timing of anther dehiscence (Rieu et al., 2003). Other processes associated with dehiscence involve the formation of secondary wall thickenings, the consecutive degeneration of various anther tissues, changes in carbohydrate metabolism, and the movement of water out of the anther (Keijzer, 1987; Bonner and Dickinson, 1990; Clement and Audran, 1995; Beals and Goldberg, 1997; Dawson et al., 1999; Steiner-Lange et al., 2003; Scott et al., 2004; see Table I for an overview of tobacco anther development). It has been suggested that the formation of secondary wall thickenings, in combination with dehydration of anther tissues, results in an outward-bending force that opens the anther when the stomium degenerates. Experimental evidence has shown that both the presence of the secondary wall thickenings and the correctly timed degeneration of the circular cell cluster and stomium are indispensable for dehiscence to occur (Beals and Goldberg, 1997; Dawson et al., 1999; Steiner-Lange et al., 2003). However, the significance of the changes in water movement or changes in carbohydrate metabolism remains to be resolved.
Table I.
Events during tobacco anther development
| Stagesa | Events in Anther Development |
|---|---|
| −7 to 1 | Tissue differentiation, meiosis, microspores in tetrads |
| 1–3 | Microspores released from tetrads, secondary wall thickenings formed |
| 4–6 | Degradation of CCC and tapetum, pollen wall formation |
| 7–9 | Degradation of connective tissue, anther bilocular, dehydration of anther tissues commences |
| 10–12 | Degradation of connective tissue continues, dehydration of anther and pollen completed, dehiscence |
Reference: Goldberg (1988).
Aquaporins are membrane proteins that mediate the movement of water according to osmotic and hydrostatic pressure gradients over cellular membranes (Maurel et al., 2002). Analysis of the Arabidopsis genome and maize (Zea mays) expressed sequence tag databases revealed the presence of 35 and 31 aquaporins, respectively, belonging to four different subfamilies: plasmamembrane intrinsic proteins (PIPs), tonoplast intrinsic proteins, NOD26-like intrinsic proteins, and small and basic intrinsic proteins (Chaumont et al., 2001; Johanson et al., 2001). All aquaporins have a molecular mass of about 30 kD and are present in the membrane as tetramers. Expression in Xenopus laevis oocytes revealed that members of all aquaporin families produce pores that allow water and/or glycerol to pass to a certain degree. However, some indications exist that aquaporins may also be permeable to a variety of other compounds, for example, ions or even gasses such as CO2 (Tyerman et al., 2002; Uehlein et al., 2003). The PIP subfamily, which is thought to be plasmamembrane localized, can be further divided into two classes and has been suggested to function in a number of cellular processes, such as elongation or osmoregulation (Tyerman et al., 2002). So far, the analysis of plants impaired in the expression of PIP1 or PIP2 proteins has shown their function in root water uptake and stress recovery (Kaldenhoff et al., 1998; Siefritz et al., 2002; Javot et al., 2003).
Previously, we isolated tobacco cDNA fragments representing both classes of the PIP subfamily of aquaporins. Expression studies on RNA and protein levels demonstrated that the genes corresponding to these cDNAs are expressed during anther development (Bots et al., 2005). In anthers, PIP1 proteins are strongly expressed early in development, but expression declines toward maturity. PIP2 transcripts accumulate in a complex temporal pattern during anther development, and a PIP2 antibody detects two proteins with distinct accumulation patterns. To investigate the role that PIP2 proteins may play in the regulation of water movement during anther development and dehiscence, we have analyzed their accumulation in anthers using immunolocalizations. Furthermore, we have generated tobacco plants displaying RNA interference (RNAi) of PIP2 genes. We found that anthers from these plants dehisce later than wild type and that this was caused by slower dehydration prior to dehiscence. Together, these results suggest that proteins of the PIP2 class are required for efficient anther dehydration and thereby contribute to the regulation of anther dehiscence.
RESULTS
Immunolocalization of PIP2 Proteins during Tobacco Anther Development
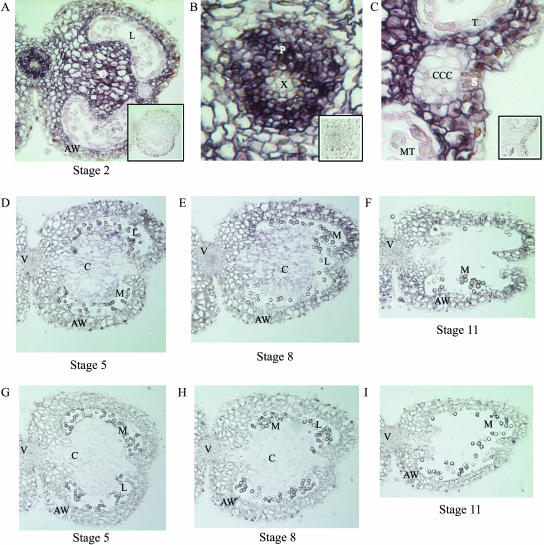
To analyze the localization of PIP2 aquaporins during anther development, we performed immunolocalizations using a PIP2 antibody on tobacco anther sections of stages 2, 5, 8, and 11 (flower stages according to Goldberg, 1988). Figure 1 shows that proteins of the PIP2 class were detected at all anther stages analyzed. In anthers at stage 2, all different tissues were present and intact. PIP2 proteins were detected in the connective tissue, anther wall, and phloem. No proteins were detected in the xylem, epidermis, or in the circular cell cluster (CCC), tapetum, and microspore tetrads, but some signal was observed in the cells of the stomium (Fig. 1, A–C). Sections of stage 5 anthers showed that the CCC had degenerated and that the microspores were released from the tetrads, but that the cells of the tapetum were still intact. At this stage, PIP2 protein expression was lower when compared with stage 2 anthers but still detectable, mainly in the cells of the connective tissue and anther wall (Fig. 1D). In anthers of stage 8, the tapetum had degenerated and the anther had become bilocular. PIP2 proteins were detected in the connective tissue and in the anther wall. Also, some signal could be detected in the vascular bundle (Fig. 1E). Finally, at stage 11, the connective had almost completely degenerated, and the anther only consisted of the upper and lower anther wall held together by the cells of the stomium. Here, PIP2 proteins were detected in the anther wall and vascular bundle, but not in the remains of the connective (Fig. 1F). No PIP2 proteins were detected in the cells of the epidermis and of the tapetum at any of the stages analyzed.
Figure 1.
Immunolocalization of PIP2 proteins in the tobacco anther at stages 2, 5, 8, and 11. PIP2 proteins can be detected at all stages analyzed. A to C, Stage 2; insets show preimmune controls. D to F, Stages 5, 8, and 11, respectively. G to I, Preimmune controls of the same stages. AW, Anther wall; C, connective tissue; CCC, circular cell cluster; L, locule; M, microspores; MT, microspore tetrads; P, phloem; S, stomium; T, tapetum; V, vascular bundle; X, xylem.
The localization results presented here, together with our previous results (Bots et al., 2005), indicate a role for aquaporins of the PIP2 class in anther dehydration.
Generation of Transgenic Plants Exhibiting RNAi of PIP2 Aquaporins
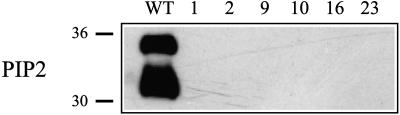
To obtain information about the function of PIP2 proteins in vivo, we suppressed their accumulation by RNAi. To this end, we used a 450-bp region of NtPIP2;1, chosen to silence aquaporins of the PIP2 family only, and placed it as a hairpin under control of the cauliflower mosaic virus 35S promoter. In six tobacco lines that were transformed with the PIP2 RNAi construct, no PIP2 proteins were detected in the microsomal protein fractions from leaves (data not shown) or anthers (Fig. 2). This indicated that silencing was very efficient.
Figure 2.
Western-blot analysis of PIP2 proteins in microsomal protein fractions of wild-type and transgenic tobacco anthers. The PIP2 antibody detects two proteins in the wild type (WT; Bots et al., 2005). No proteins are detected in the anthers from transgenic plants (1, 2, 9, 10, 16, and 23). Sizes of the molecular mass markers are indicated at left.
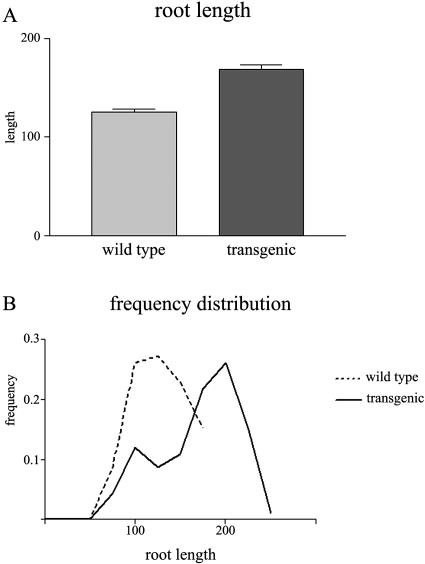
From these primary transformants, we arbitrarily chose the lines 2, 10, and 16 for further analyses. During development of their T2 offspring, we observed the presence of seedlings with a primary root longer than that of concurrently grown wild-type seedlings. To exclude possible deleterious effects of kanamycin or differences in microenvironment, wild-type and transgenic seedlings were grown side by side on nonselective plates and root lengths were measured. Figure 3 shows that the primary roots of these T2 seedlings were significantly longer than those of wild-type seedlings. This observation is in agreement with previous reports indicating the importance of PIP aquaporins in root water uptake and root development (Kaldenhoff et al., 1998; Siefritz et al., 2002; Javot et al., 2003) and shows that successful silencing of PIP2 in roots produces a similar phenotype as that described for PIP1.
Figure 3.
Analysis of root lengths of primary roots of wild-type seedlings and seedlings of the T2 PIP2 RNAi lines. A, Root length; B, frequency distribution. Seedlings were grown side by side on nonselective plates. Root lengths of the PIP2 RNAi lines 2, 10, and 16 were pooled. Error bars indicate se (n = 40).
PIP2-Silenced Tobacco Plants Show Delayed Anther Dehiscence
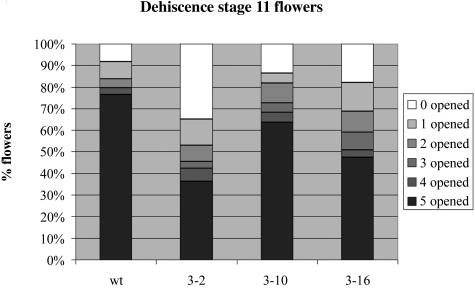
Anthers of transgenic plants morphologically appeared as wild-type anthers. However, the accumulation patterns of PIP2 aquaporins in tobacco anther development suggest that these proteins could play a role in anther dehydration and dehiscence (Fig. 1; Bots et al., 2005). Therefore, we performed a detailed analysis of the dehiscence process in the T2 generation of the PIP2 RNAi lines 2, 10, and 16. To this end, we counted the number of dehisced anthers in wild-type and transgenic flowers at developmental stage 11 (Goldberg, 1988). Figure 4 shows that in 76% of the wild-type tobacco flowers, all five anthers were dehisced, whereas only in 8% of the same flowers all anthers were nondehisced. By contrast, in the 3 transgenic lines, the percentage of flowers containing 5 dehisced anthers was always lower (36% in line 2, 63% in line 10, and 47% in line 16), whereas the percentage of flowers with 5 nondehisced anthers was invariably higher (35% in line 2, 14% in line 10, and 23% in line 16) as compared with the wild type. Although some variability existed between the lines, with line 2 showing the biggest delay in anther dehiscence, these results indicated that on average PIP2 RNAi anthers dehisced later than wild-type anthers.
Figure 4.
Characterization of delayed dehiscence of plants displaying RNAi of PIP2 genes. Bar graph displaying percentage of flowers at stage 11 containing 0, 1, 2, 3, 4, or 5 dehisced anthers in wild-type or T2 plants from transgenic lines 2, 10, and 16 (n > 75) is shown.
To test the possibility that the observed delay in anther dehiscence was indirectly caused by changes in flower development, we measured bud length and observed corolla coloration in developing wild-type and transgenic flowers. We found that bud growth and corolla coloration proceeded indistinguishably in wild-type and transgenic flowers. In addition, sections of wild-type and transgenic anthers observed by light microscopy revealed no anatomical differences during development (data not shown). Together, these results indicate that the delayed dehiscence was not caused by changes in flower development but was probably due to a specific physiological defect in the dehiscence program caused by the absence of aquaporins of the PIP2 class in anthers.
Transgenic Anthers Dehydrate More Slowly Than Wild-Type Anthers
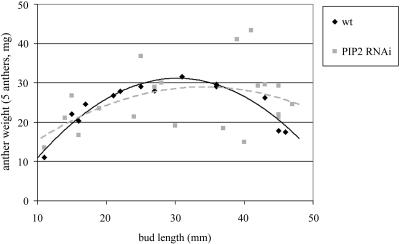
The process of anther dehiscence is preceded by dehydration of the anther and pollen (Keijzer, 1987). Because it may be expected that the absence of aquaporins in the anther has an effect on dehydration and thus on dehiscence, we followed the dehydration of wild-type and PIP2 RNAi anthers. We determined the fresh weight of nondehisced anthers from flowers at various stages, assuming that changes in weight are due to changes in water content, as was shown previously for tomato (Lycopersicon esculentum) anthers (Bonner and Dickinson, 1990). The results of these measurements are presented in Figure 5 and indicate that anthers of the PIP2 RNAi lines have larger weight variability than wild-type anthers. However, at the end of development (bud length 40–50 mm), wild-type anthers are lighter than transgenic anthers, suggesting that they dehydrate faster.
Figure 5.
Dehydration of wild-type and PIP2 RNAi anthers. Flower buds were collected at various developmental stages and the five anthers of each flower weighed.
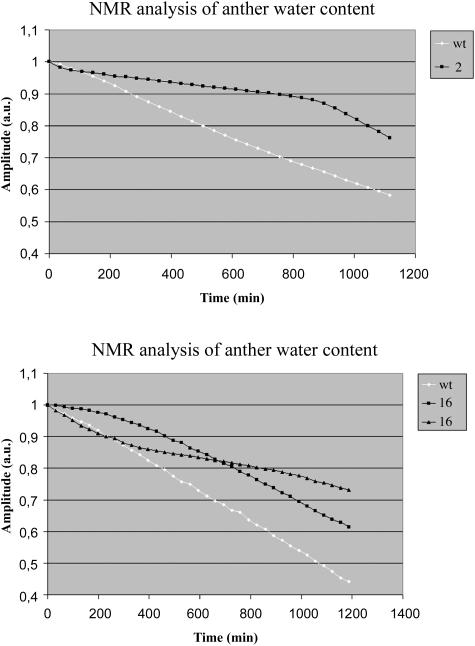
Therefore, we followed the changes in water content of wild-type and transgenic anthers in vivo using NMR relaxometry. This method has a number of advantages: (1) individual anthers can be analyzed; (2) water status can be measured in living tissue, over a prolonged period of time and in much more detail; and (3) it allows the discrimination of water in different compartments of the anther. To be able to follow water movement in anthers shortly before dehiscence, an anther of a flower at stage 9 with its filament attached was placed in the NMR system and analyzed for up to 20 h. Because the NMR system could only measure one anther at a time, wild-type and transgenic anthers were analyzed on consecutive days. T2 relaxation decays were measured and found to be best described by a sum of four exponentials with different decay times and amplitudes in a multiexponential analysis. The differences in decay times are explained by differences in the environment of water in various compartments; the amplitudes represent the amount of water in the different fractions. The T2 decay times of these 4 components were 200 ms, 80 ms, 17 ms, and 4 ms. It was found that these were similar for wild-type and transgenic anthers and did not change in time (data not shown), indicating that water is present in similar environments in transgenic and wild-type anthers. By contrast, the signal amplitudes of wild-type anthers decreased faster in time as compared with the amplitude of transgenic anthers. In particular, the amplitude of the fractions with decay times of 80 ms and 17 ms showed strong time dependence. In a typical wild-type anther, the amplitudes of the 80-ms component fell from 2.5E + 05 to 1.75E + 05, whereas the 17-ms component dropped from 1.75E + 05 to 1.0E + 05. For a transgenic of line 2, these changes were from 2.0E + 05 to 1.25E + 05 and from 1.50E + 05 to 1.25E + 05 for the 80-ms and 17-ms components, respectively. The signal amplitudes of the 200-ms and 4-ms components were approximately 5.0E + 04 for both wild type and transgenic and did not change during the experiment. Figure 6 shows the decrease in the sum of signal amplitudes in time. From the results, it appears that this difference is largest between the wild type and line 2, the same line that also showed strongest delay in dehiscence (Fig. 4). As the signal amplitude is correlated to the amount of intracellular water of a tissue (Van der Toorn et al., 2000), these results indicate that water moves more slowly out of anthers that do not contain aquaporins of the PIP2 class as compared with wild-type anthers.
Figure 6.
NMR analysis of anther water content. Flower stage 9 anthers of wild-type plants and the PIP2 RNAi lines 2 and 16 were analyzed using NMR relaxometry for up to 20 h. The sum of the signal amplitudes is shown. Initial amplitude was normalized to 1.
DISCUSSION
In plant sexual reproduction, the anther is the floral organ that produces the male gametophytes. Its growth during development is likely to be facilitated by water uptake, whereas dehiscence is preceded by dehydration. In some plant species, it has been shown that aquaporins are expressed in the anther, suggesting that they could function in the regulation of water movement in this organ (Ruiter et al., 1997; Marin-Olivier et al., 2000; O'Brien et al., 2002). Using PIP2-silenced tobacco plants, we showed that this class of aquaporins plays a significant role in anther dehiscence by allowing efficient water flow out of the anther.
The first occurrence of water movement in anther development is the influx of water to allow growth. Tobacco anthers enlarge until approximately stage 5 of anther development (Koltunow et al., 1990). Immunolocalizations revealed that in anthers at developmental stage 2, PIP2 aquaporins can be detected in most cell types (Fig. 1). Therefore, cell enlargement during anther development may be correlated with PIP2 aquaporin expression, and this observation would corroborate the idea that aquaporins are involved in regulating cell sizes and growth (Kirch et al., 2000; Maurel et al., 2002). Despite this, we did not observe a size difference between wild-type anther and PIP2 RNAi anthers (data not shown). However, because in situ hybridization experiments revealed that the spatial expression patterns of PIP1 and PIP2 genes in stage 2 anthers are very similar (Bots et al., 2005), it is possible that PIP1 and PIP2 aquaporins have overlapping roles in cell enlargement.
As the anther in its early stages of development requires a net influx of water, during the later stages the reverse is true. The anther then dehydrates before dehiscence, for example, in tobacco from stage 7 onward. At the same time, the pollen grains also dehydrate (Keijzer, 1987; Bonner and Dickinson, 1990; Koltunow et al., 1990; Ruiter et al., 1997). It has been suggested that in tomato, water moves out of the anther via the vascular bundle following an osmotic gradient (Bonner and Dickinson, 1990). In other species, evaporation may play a more significant role (Keijzer, 1987; Bonner and Dickinson, 1990). However, irrespective of the path the water might follow, it is likely that aquaporins could facilitate the process by increasing the hydraulic permeability of the anther cells. In agreement with this, western blots have shown that, in tobacco anthers at stage 8, PIP2 protein levels increase (Bots et al., 2005). Immunolocalization studies indicate that at the same stage these PIP2 proteins are present in those tissues that may permit water transport out of the anther via the vascular bundle (Fig. 1). However, we cannot exclude that dehydration occurs, at least partly, through evaporation.
Notably, no PIP2 signal was detected in the tapetum. In lily, the anther locule was found to be symplastically isolated from other parts of the anther (Clement and Audran, 1995), and in tomato water tracers have been used to demonstrate the symplastic isolation of the locules (Bonner and Dickinson, 1990). If the same is true for tobacco, our results could imply that hydraulic isolation of the locule might be achieved not only by the absence of plasmodesmata but also by the absence of plasmamembrane aquaporins.
Our immunolocalization results and previous literature allow the construction of a model that describes anther dehydration as part of the dehiscence program. Firstly, hydraulic continuity throughout the whole anther is established by the degeneration of the tapetum, which isolates the locule from the rest of the anther, and by increasing PIP2 accumulation in the connective and anther wall (Fig. 1). Secondly, an osmotic or hydrostatic pressure gradient must be formed to draw the water to the vascular bundle, as aquaporins only allow passive diffusion of water along such gradients. Indications exist that an osmotic gradient is formed in the anthers of a number of species (Bonner and Dickinson, 1990; Stadler et al., 1999; Pressman et al., 2002), suggesting that this may also occur in tobacco. Although the establishment of hydraulic continuity and the formation of the osmotic gradient are presented here as sequential events, they may in fact occur simultaneously. However, only when both processes are completed will efficient dehydration of the anther be possible.
This hypothesis predicts that if PIP2 expression in the anther is silenced, less efficient dehydration may occur because hydraulic continuity cannot be established fully throughout the anther. Indeed, our analysis of transgenic plants impaired in PIP2 expression shows that water moves more slowly out of transgenic anthers, as compared with the wild type (Figs. 4 and 5). Slower anther dehydration has no apparent consequences for pollen function, as fertility was normal in transgenic plants (data not shown). However, we found that slower anther dehydration causes delayed anther dehiscence (Fig. 4).
The current model describing anther dehiscence suggests that four processes should occur for efficient dehiscence. These processes are formation of secondary wall thickenings, degeneration of several anther tissues in a specific order, changes in carbohydrate metabolism, and dehydration of the anther. Previously, it has been shown that absence of secondary wall thickenings or inhibition of tissue degeneration results in indehiscent anthers (Beals and Goldberg, 1997; Dawson et al., 1999; Steiner-Lange et al., 2003), but the significance of anther dehydration or the changes in carbohydrate metabolism remained unknown. Here, we show that slower anther dehydration delays dehiscence. To our knowledge, this is the first direct evidence that a functional link between anther dehydration and dehiscence exists.
The delay in anther dehiscence is rather small, especially when compared with the dehiscence process described previously in various mutants (Beals and Goldberg, 1997; Dawson et al., 1999; Sanders et al., 2000; Ishiguro et al., 2001; Rieu et al., 2003; Steiner-Lange et al., 2003). This observation illustrates the complexity of the anther dehiscence process. In tobacco, dehiscence is regulated by the hormone ethylene, which coordinates anther dehydration and stomium degeneration (Rieu et al., 2003). In our RNAi lines, the absence of aquaporins of the PIP2 class in the anther does interfere with anther dehydration, but does not prevent stomium degeneration. In addition, some dehydration still takes place, indicating that other aquaporins or other dehydration mechanisms may also play a role during dehiscence.
Together, our results indicate that aquaporins of the PIP2 family may perform diverse functions in anther development. Expression and localization of PIP2 proteins in young anthers may suggest a function for these proteins in growth, possibly in cooperation with PIP1 proteins. Furthermore, the analysis of plants displaying RNAi of PIP2 indicates that they play a role in dehydration and dehiscence. However, because water movement across the plasmamembrane is driven by osmotic gradients, aquaporins have an accessory role in facilitating the water fluxes needed for growth or dehydration. This may be the main reason why growth is normal and dehiscence is only delayed.
MATERIALS AND METHODS
Plant Growth Conditions
Wild-type tobacco (Nicotiana tabacum cv Petit Havana SR1) plants were grown under standard greenhouse conditions. Transgenic seeds were sown on Murashige and Skoog (Duchefa, Amsterdam) medium containing 200 mg L−1 kanamycin to select for the presence of the transgene. After selection, resistant seedlings were grown in a growth chamber with 16 h light, 100 μmol m−2 s−1, at 22°C. Flower stages were determined according to Goldberg (1988).
Immunolocalization
Immunolocalization on sections of anthers was carried out according to standard procedures. Briefly, anthers were fixed in 3% paraformaldehyde and 0.5% glutaraldehyde in 0.05 m phosphate buffer, pH 7.2, for 2 h at room temperature. After rinsing in buffer, the anthers were embedded in paraplast (Sigma, St Louis). Sections of 7-μm thickness were attached to gelatin-coated microscope slides, deparafinized, and hydrated with successive xylene/ethanol and ethanol/water steps (Sternberger, 1979). All microscope slides contained a complete set of anther stages. Sections were incubated with 1:500 diluted antibody (anti-PIP2; Bots et al., 2005) in Tris-saline buffer, 0.05 m, pH 7.4, with 1% bovine serum albumin, and processed according to the peroxidase-anti-peroxidase procedure (Sternberger, 1979). After incubation for 2 h, sections were rinsed 3 times in Tris-saline buffer with bovine serum albumin and were incubated with 1:100 diluted horseradish peroxidase-coupled goat anti-rabbit (Pierce, Rockford, IL). Staining was carried out at room temperature for 10 min with 0.5 mg mL−1 3,3′-diaminobenzidine (Sigma) and 0.01% H2O2 in Tris-saline buffer as a substrate. Photographs were taken with a Leitz Orthoplan (Leica Microsystems GmbH, Wetzlar, Germany) microscope equipped with a Color Coolsnap digital camera (Roper Scientific, Tucson, AZ) and the MetaVue software (Universal Imaging, Westchester, PA). The PIP2 antibody used in the immunolocalizations was an affinity-purified antibody raised in rabbits against the peptide H2N-QQHGKDYVDPPPAPLC-CONH2 that represented the first 15 amino acids encoded by the tobacco cDNA NtPIP2;1 (Bots et al., 2005; GenBank accession no. AF440272).
Transgene Construction and Plant Transformation
The construct we used to induce RNAi of aquaporin genes was based on the vector pGSA1165 (Arabidopsis Biological Resource Center [ABRC] accession no. CD3-450), which in turn is derived from pCAMBIA 1200 (Cambia, Canberra, Australia. This vector allows easy cloning of a construct expressing an RNA hairpin driven by the 35S promoter. The aquaporin sequences used in making the constructs were amplified from the NtPIP2;1 cDNA, using primers NtPIP2ahps (5′-GGACTAGTGGCGCGCCCTGGTATCTCTGGAGGACATA-3′) and NtPIP2ahpas (5′-TAGGATCCATTTAAATGGGGTTGCTGCGGAAAGAAC-3′). These primers introduce the restriction sites SpeI and AscI on the 5′ end and SwaI and BamHI on the 3′ end of the PCR product. The PIP2 PCR product, digested with SpeI and BamHI, was cloned into the pGSA1165 SpeI and BamHI sites that are located upstream of the GUS spacer. This produced the sense arm of the hairpin. To increase the silencing efficiency (Smith et al., 2000), the GUS spacer was replaced by the chalcone synthase intron of vector pFGC5941 (ABRC accession no. CD3-447), using the restriction enzymes SwaI and BamHI that flank both fragments. Finally, the antisense arm of the hairpin was produced by cloning the AscI-SwaI-digested PIP2 PCR product downstream of the chalcone synthase intron. One microgram of the resulting vector was used to transform Agrobacterium tumefaciens LBA4404 cells via freeze-thaw transformation (Chen et al., 1994). Colonies resistant to the kanamycin selection were additionally tested by PCR for the presence of the RNAi construct. Tobacco leaf discs were transformed as described by Horsch et al. (1985).
Protein Isolation and Western-Blot Analysis
To extract proteins, the desired tissues were homogenized in homogenization buffer (330 mm Suc, 100 mm KCl, 1 mm EDTA, 50 mm Tris, 0.05% MES, 5 mm dithiothreitol, Complete proteinase inhibitor cocktail [Roche, Mannheim, Germany], pH 7.5). The homogenate was centrifuged at 1,000g for 15 min to collect cells and debris, and the supernatant was centrifuged at 10,000g for 15 min to collect the large cellular organelles. Finally, the microsomal fraction was pelleted by centrifugation at 20,000g for 75 min. The microsomal pellet was dissolved in membrane buffer (330 mm Suc, 200 mm dithiothreitol, 25 mm Tris, pH 8.5).
For the western blot, protein samples were prepared in the presence of 50 mm ethanedithiol. Fifteen micrograms of proteins was separated by 12% SDS-PAGE. The proteins were electroblotted onto a nitrocellulose membrane in 39 mm Gly, 48 mm Tris base, 0.037% SDS, 20% methanol, pH 8.3. For detection, the membranes were first incubated in blocking buffer (5% nonfat dried milk in PBS containing 0.1% Tween-20) for 2 h, after which they were incubated for 2 h with the PIP2 immune serum (1:500 in blocking buffer). After incubation with the primary antibody, the membranes were rinsed and incubated with horseradish peroxidase-conjugated goat anti-rabbit antibody (Pierce) 1:20,000 in blocking buffer for 1 h. All incubations were performed at room temperature. The membranes were developed using SuperSignal West Pico chemiluminescent substrate (Pierce) according to the manufacturer's protocol and exposed to Kodak X-omat AR scientific imaging films (Rochester, NY). Autoradiograms were digitized, and the contrast and brightness were adjusted with Adobe Photoshop (Adobe Systems, Mountain View, CA).
Root Length Determination
Transgenic and control seeds were sown on solid Murashige and Skoog medium (0.3% phytagel) without antibiotics in 12- × 12-cm petri dishes at an angle of 80°. After 2 weeks, an image of the bottom of each petri dish was obtained by scanning with a flat-bed scanner, and the root lengths were measured using Adobe Photoshop and analyzed using GraphPad Prism software.
Anther Dehiscence Analysis
Transgenic and control plants were grown in a growth chamber until they started to flower. Anther dehiscence was analyzed by picking all flowers of stages 11 and 12 from each plant and counting the number of dehisced and nondehisced anthers in these flowers. The flowers were picked daily at the same time, and the analysis continued at least 5 d or until more than 75 flowers were analyzed.
Anther weights were determined by collecting all five anthers of a single flower bud and weighing them.
NMR Spectroscopy
1H-NMR is a powerful technique to study the amount and physical state of water in living organisms (Callaghan, 1993). Water relations in biological materials are reflected in the magnetic resonance relaxation behavior of protons. Two types of relaxation processes can be distinguished experimentally: longitudinal or spin-lattice relaxation characterized by relaxation time T1, and transverse or spin-spin relaxation characterized by relaxation time T2. They are related to molecular mobilities in a system: for fast motions (pure water), T1 and T2 are equal. As the molecular mobility decreases, both T1 and T2 decrease. However, for slow motion T1 starts to increase again, whereas T2 continues to decrease as the motion becomes increasingly slower. These values are affected by the solutes present and their concentration. There also is a relation between T2 and compartment size (Brownstein and Tarr, 1979). This relation can be used to determine compartment sizes and water permeability of membranes between the compartments in living cells (Snaar and Van As, 1992, 1993; van der Weerd et al., 2001; van der Weerd et al., 2002). In NMR experiments, the relative abundance (amplitude) of each component, characterized by given values of T1 and T2, can be determined, which is directly related to the amount of protons present in that fraction. Since in plant tissue the proton signal is dominated by water protons, the NMR signal amplitude is proportional to the water content (Donker et al., 1997). Because NMR is a nondestructive technique, it allows obtaining of both structural and dynamical information about the water state in the same organism or population of organisms.
T2 relaxation times were measured using a CPMG sequence on a 0.7-T (31 MHz) pulse NMR spectrometer (MARAN Ultra; Resonance Instrument, Witney, UK). The decay due to relaxation was detected with a train of 2,048 echoes with an interecho time of 600 μs. Each echo was oversampled eight times, with 50 μs between the data points per echo resulting in a spectral width of 20 kHz. The decay was averaged 512 times, and the time between successive repeats was 3.8 s. One measurement took 32 min. Anthers were placed in a 5-mm NMR tube and measured during approximately 20 h. Four wild-type and eight PIP2 RNAi anthers were measured. All measurements were performed at 21°C. After phase correction, the real part of the data was analyzed by a multiexponential nonlinear least square fit as implemented SPLMOD (Provencher and Vogel, 1983).
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number AF440272.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.056408.
References
- Beals TP, Goldberg RB (1997) A novel cell ablation strategy blocks tobacco anther dehiscence. Plant Cell 9: 1527–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner LJ, Dickinson HG (1990) Anther dehiscence in Lycopersicon esculentum. New Phytol 115: 367–375 [DOI] [PubMed] [Google Scholar]
- Bots M, Feron R, Uehlein N, Weterings K, Kaldenhoff R, Mariani T (2005) PIP1 and PIP2 aquaporins are differentially expressed during tobacco anther and stigma development. J Exp Bot 56: 113–121 [DOI] [PubMed] [Google Scholar]
- Brownstein KR, Tarr CE (1979) Importance of classical diffusion in NMR studies of water in biological cells. Phys Rev A 19: 2446–2453 [Google Scholar]
- Callaghan PT (1993) Principles of Nuclear Magnetic Resonance Microscopy. Clarendon Press, Oxford
- Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R (2001) Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol 125: 1206–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Nelson RS, Sherwood JL (1994) Enhanced recovery of transformants of Agrobacterium tumefaciens after freeze-thaw transformation and drug selection. Biotechniques 16: 664–668, 670 [PubMed] [Google Scholar]
- Clement C, Audran JC (1995) Anther wall layers control pollen sugar nutrition in Lilium. Protoplasma 187: 172–181 [Google Scholar]
- Dawson J, Sozen E, Vizir I, Van Waeyenberge S, Wilson ZA, Mulligan BJ (1999) Characterization and genetic mapping of a mutation (ms35) which prevents anther dehiscence in Arabidopsis thaliana by affecting secondary wall thickening in the endothecium. New Phytol 144: 213–222 [Google Scholar]
- Donker HCW, Van As H, Snijder HJ, Edzes HT (1997) Quantitative 1H-NMR imaging of water in white button mushrooms (Agaricus bisporus). Magn Reson Imaging 15: 113–121 [DOI] [PubMed] [Google Scholar]
- Franchi GG, Nepi M, Dafni A, Pacini E (2002) Partially hydrated pollen: taxonomic distribution, ecological and evolutionary significance. Plant Syst Evol 234: 211–227 [Google Scholar]
- Goldberg RB (1988) Plants: novel developmental processes. Science 240: 1460–1467 [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry LE, Hoffman NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 27: 1229–1231 [DOI] [PubMed] [Google Scholar]
- Ishiguro S, Kawai Oda A, Ueda K, Nishida I, Okada K (2001) The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13: 2191–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javot H, Lauvergeat V, Santoni V, Martin Laurent F, Guclu J, Vinh J, Heyes J, Franck KI, Schaffner AR, Bouchez D, et al (2003) Role of a single aquaporin isoform in root water uptake. Plant Cell 15: 509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjovall S, Fraysse L, Weig AR, Kjellbom P (2001) The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol 126: 1358–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, McCormick S (2001) Pollen germinates precociously in the anthers of raring-to-go, an Arabidopsis gametophytic mutant. Plant Physiol 126: 685–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff R, Grote K, Zhu J-J, Zimmerman U (1998) Significance of plasmalemma aquaporins for water-transport in Arabidopsis thaliana. Plant J 14: 121–128 [DOI] [PubMed] [Google Scholar]
- Keijzer CJ (1987) The process of anther dehiscence and pollen dispersal. I. The opening mechanism of longitudinally dehiscing anthers. New Phytol 105: 487–498 [DOI] [PubMed] [Google Scholar]
- Kirch HH, Vera-Estrella R, Golldack D, Quigley F, Michalowski CB, Barkla BJ, Bohnert HJ (2000) Expression of water channel proteins in Mesembryanthemum crystallinum. Plant Physiol 123: 111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltunow AM, Truettner J, Cox KH, Wallroth M, Goldberg RB (1990) Different temporal and spatial gene expression patterns occur during anther development. Plant Cell 2: 1201–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Olivier M, Chevalier T, Fobis-Loisy I, Dumas C, Gaude T (2000) Aquaporin PIP genes are not expressed in the stigma papillae in Brassica oleracea. Plant J 24: 231–240 [DOI] [PubMed] [Google Scholar]
- Maurel C, Javot H, Lauvergeat V, Gerbeau P, Tournaire C, Santoni V, Heyes J (2002) Molecular physiology of aquaporins in plants. Int Rev Cytol 215: 105–148 [DOI] [PubMed] [Google Scholar]
- O'Brien M, Bertrand C, Matton DP (2002) Characterization of a fertilization-induced and developmentally regulated plasma-membrane aquaporin expressed in reproductive tissues, in the wild potato Solanum chacoense Bitt. Planta 215: 485–493 [DOI] [PubMed] [Google Scholar]
- Park JH, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J 31: 1–12 [DOI] [PubMed] [Google Scholar]
- Pressman E, Peet MM, Pharr DM (2002) The effect of heat stress on tomato pollen characteristics is associated with changes in carbohydrate concentration in the developing anthers. Ann Bot (Lond) 90: 631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW, Vogel RH (1983) Regularization techniques for inverse problems in molecular biology. In E Deufhard, E Hairer, eds, Numerical Treatment of Inverse Problems in Differential and Integral Equations. Birkhauser, Boston, pp 304–319
- Rieu I, Wolters Arts M, Derksen J, Mariani C, Weterings K (2003) Ethylene regulates the timing of anther dehiscence in tobacco. Planta 217: 131–137 [DOI] [PubMed] [Google Scholar]
- Ruiter RK, van Eldik GJ, van Herpen MMA, Schrauwen JAM, Wullems GJ (1997) Expression in anthers of two genes encoding Brassica oleracea transmembrane channel proteins. Plant Mol Biol 34: 163–168 [DOI] [PubMed] [Google Scholar]
- Sanders PM, Yun Lee P, Biesgen C, Boone JD, Beals TP, Weiler EW, Goldberg RB (2000) The Arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell 12: 1041–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RJ, Spielman M, Dickinson HG (2004) Stamen structure and function. Plant Cell (Suppl) 16: S46–S60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R (2002) PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. Plant Cell 14: 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NA, Singh SP, Wang MB, Stoutjesdijk PA, Green AG, Waterhouse PM (2000) Total silencing by intron-spliced hairpin RNAs. Nature 407: 319–320 [DOI] [PubMed] [Google Scholar]
- Snaar JEM, Van As H (1992) Probing water compartments and membrane permeability by proton NMR relaxation measurements. Biophys J 63: 1654–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaar JEM, Van As H (1993) NMR self-diffusion measurements in bounded systems with loss of magnetization at the wall. J Magn Reson A 102: 318–326 [Google Scholar]
- Stadler R, Truernit E, Gahrtz M, Sauer N (1999) The AtSUC1 sucrose carrier may represent the osmotic driving force for anther dehiscence and pollen tube growth in Arabidopsis. Plant J 19: 269–278 [DOI] [PubMed] [Google Scholar]
- Steiner-Lange S, Unte US, Eckstein L, Yang C, Wilson ZA, Schmelzer E, Dekker K, Saedler H (2003) Disruption of Arabidopsis thaliana MYB26 results in male sterility due to non-dehiscent anthers. Plant J 34: 519–528 [DOI] [PubMed] [Google Scholar]
- Sternberger LA (1979) Immunocytochemistry, Ed 2. John Wiley & Sons, New York
- Tyerman SD, Niemietz CM, Bramley H (2002) Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant Cell Environ 25: 173–194 [DOI] [PubMed] [Google Scholar]
- Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R (2003) The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425: 734–737 [DOI] [PubMed] [Google Scholar]
- Van der Toorn A, Zemah H, Van As H, Bendel P, Kamenetsky R (2000) Developmental changes and water status in tulip bulbs during storage: visualization by NMR imaging. J Exp Bot 51: 1277–1287 [DOI] [PubMed] [Google Scholar]
- van der Weerd L, Claessens M, Efde C, Van As H (2002) Nuclear magnetic resonance imaging of membrane permeability changes in plants during osmotic stress. Plant Cell Environ 25: 1539–1549 [Google Scholar]
- van der Weerd L, Claessens MM, Ruttink T, Vergeldt FJ, Schaafsma TJ, Van As H (2001) Quantitative NMR microscopy of osmotic stress responses in maize and pearl millet. J Exp Bot 52: 2333–2343 [DOI] [PubMed] [Google Scholar]