Abstract
Glucosides of trans-zeatin occur widely in plant tissues, formed either by O-glucosylation of the hydroxylated side chain or N-glucosylation of the purine ring structure. O-Glucosylation is stereo-specific: the O-glucosyltransferase encoded by the Phaseolus lunatus ZOG1 gene has high affinity for trans-zeatin as the substrate, whereas the enzyme encoded by the maize (Zea mays) cisZOG1 gene prefers cis-zeatin. Here we show that hydroxylated derivatives of benzyladenine (topolins) are also substrates of ZOG1 and cisZOG1. The m-OH and o-OH derivatives are the preferred substrate of ZOG1 and cisZOG1, respectively. Among the hydroxylated derivatives of thidiazuron tested, the only enzyme/substrate combination resulting in conversion was cisZOG1/(o-OH) thidiazuron. The abilities of these cytokinins to serve as substrates to the glucosyltransferases were in a large part correlated with their biological activities in the P. lunatus callus bioassay, indicating that there may be similarities between cytokinin-binding sites on the enzymes and cytokinin receptors. Further support for this interpretation is provided by cytokinin recognition studies involving the Arabidopsis (Arabidopsis thaliana) CRE1/WOL/AHK4 and maize ZmHK1 receptors. The AHK4 receptor responded to trans-zeatin and m-topolin, while the ZmHK1 receptor responded also to cis-zeatin and o-topolin. Three-dimensional molecular models of the substrates were applied to explain the results.
Cytokinins are a group of plant growth regulators with important functions at all phases of plant development, from seed germination to senescence. The natural cytokinins are adenine derivatives, of which trans-zeatin (Fig. 1) is considered central due to its general occurrence and high activity in bioassays (Skoog and Armstrong, 1970). Also, cis-zeatin (Fig. 1) and its derivatives have been found in many plant species (discussed in Mok and Mok, 2001), often at relatively high levels; however, cis-zeatin has much lower cytokinin activity than trans-zeatin (Schmitz et al., 1972). Dihydrozeatin, the saturated counterpart of zeatin, also occurs in plant tissues, has high cytokinin activity in bioassays, and is more stable than trans-zeatin due to its resistance to cytokinin oxidases/dehydrogenases (Skoog and Armstrong, 1970; Armstrong, 1994).
Figure 1.
Chemical structures of cytokinins used in this study. The positions (o, m, and p) at which BAP and TDZ can be hydroxylated are indicated.
Although the isoprenoid cytokinins are the major components of endogenous cytokinins, aromatic cytokinins have been known to occur since their isolation from poplar (Horgan et al., 1973). Benzyladenine (BAP; Fig. 1) and its derivatives have now been identified in a number of plant species as minor components of the total cytokinins (Strnad, 1997; Sáenz et al., 2003). The hydroxylated derivatives of BAP were named topolins (Strnad, 1997). Of the topolins, only the meta form exhibits high cytokinin activity (Strnad, 1997; Holub et al., 1998).
Many cytokinin derivatives occur in plant tissues (for review, see Auer, 1997). The free bases may represent only a small portion of the total cytokinins, while the corresponding ribosides and especially nucleotides are frequently more abundant (Chen, 1997). In addition, most plant tissues analyzed contain cytokinin storage and deactivated forms, O- and N-glucosides (Auer, 1997; Vanková, 1999). Glucosides may accumulate to even higher levels than the free bases and ribosides under normal circumstances (Takagi et al., 1989; Frank et al., 2000; Veach et al., 2003) as well as stress conditions (Clarke et al., 1999). In a survey of endogenous cytokinins, some plant families were found to accumulate preferentially O-glucosides, others N-glucosides, and again others relatively high levels of both (Vanková, 1999).
A number of phenylurea derivatives exhibit cytokinin activity in bioassays but do not occur in nature (Shantz and Steward, 1955; Bruce and Zwar, 1966; Shudo, 1994). One of these is thidiazuron (TDZ; Fig. 1), a highly stable compound (Mok et al., 1982; Mok and Mok, 1985) now widely used for plant tissue cultures. TDZ can be glucosylated in plant tissues (Mok and Mok, 1985). These glucosides have not been characterized in depth, but evidence indicates occurrence of both N-glucosides and O-glucosides (after hydroxylation at the phenyl ring).
Enzymes mediating the conversions to O- and N-glucosides have been isolated. A bean (Phaseolus lunatus) enzyme from young seeds was found to mediate conversion of trans-zeatin to its O-glucoside (Dixon et al., 1989), while a related enzyme from Phaseolus vulgaris converted trans-zeatin to its O-xyloside (Turner et al., 1987). A radish enzyme could form N-glucosides of a range of cytokinins, with a preference for the N7 position (Entsch and Letham, 1979; Entsch et al., 1979). The gene encoding the P. lunatus O-glucosyltransferase (ZOG1) was cloned (Martin et al., 1999a) as well as the zeatin O-xylosyltransferase gene from P. vulgaris (ZOX1; Martin et al., 1999b). Two related genes (cisZOG1 and cisZOG2) were identified in maize (Zea mays), but the recombinant enzymes preferred cis-zeatin over trans-zeatin as substrate (Martin et al., 2001b; Veach et al., 2003).
The cytokinin glycosyltransferases are UDP-Glc- or UDP-Xyl-requiring and belong to family 1 of the 68 families of glycosyltransferases classified thus far (http://afmb.cnrs-mrs.fr/-cazy/CAZY/index.html). The substrates of some of these UDPG-glycosyltransferases (UGTs) have been identified, indicating recognition of a range of related substrates by each enzyme (Jackson et al., 2001; Lim et al., 2001; Fukuchi-Mizutani et al., 2003). Here we show that the recombinant enzymes ZOG1 and cisZOG1 recognize as substrates additional cytokinin-active compounds, topolins and hydroxylated phenylureas, and that they display specificity for a particular substituent position at the phenyl ring. We also show that the substrate specificities are, to a large extent, correlated with cytokinin structure-activity relationships in the P. lunatus bioassay and cytokinin recognition by the Arabidopsis (Arabidopsis thaliana) CRE1/WOL/AHK4 and maize ZmHK1 receptors (Inoue et al., 2001; Suzuki et al., 2001; Kakimoto, 2003; Yonekura-Sakakibara, 2004) and that the differences can be reconciled by molecular modeling of the compounds.
RESULTS
Substrate Recognition of ZOG1 and cisZOG1: Isoprenoid Cytokinins
Previously, we have described the isolation of the ZOG1 and cisZOG1 genes (GenBank accessions AF101972 and AF318075; Martin et al., 1999a, 2001b). The characterization of the ZOG1 enzyme was performed with recombinant protein with a tag of 7 His residues fused to the N terminus (Martin et al., 1999a). This enzyme had high affinity to trans-zeatin but formed negligible amounts of glucoside from cis-zeatin. However, even after purification on a nickel column the His-tagged recombinant protein was not pure; therefore, we have cloned ZOG1 into the pGex vector, forming a fusion protein with glutathione S-transferase that can be purified by cleavage on the affinity column. This yielded a virtually pure ZOG1 preparation as it did for other glucosyltransferases (Jackson et al., 2001). The purified ZOG1 enzyme converted cis-zeatin to its glucoside although at a much lower rate than trans-zeatin. The Km and Vmax were determined for both isomers (Table I). The Km for trans-zeatin was 54 μm, which is slightly higher than that found for the native enzyme (28 μm; Dixon et al., 1989). Affinity for cis-zeatin was significantly lower (Table I).
Table I.
Substrate specificity of the recombinant ZOG1 and cisZOG1 enzymes
| Protein | Substrate | Km | Vmax | kcat |
|---|---|---|---|---|
| mm | nmol mg−1 s−1 | s−1 | ||
| ZOG1 | trans-zeatin | 0.05 | 8.9 | 0.45 |
| ZOG1 | cis-zeatin | 0.40 | 0.7 | 0.04 |
| ZOG1 | m-topolin | 0.14 | 1.6 | 0.08 |
| cisZOG1 | cis-zeatin | 0.05 | 0.1 | 0.01 |
| cisZOG1 | o-topolin | 0.18 | 0.5 | 0.03 |
In contrast to ZOG1, cisZOG1 prefers cis-zeatin as substrate and mediates very low conversion of trans-zeatin to its glucoside (Table II). Although the Km of cisZOG1 for cis-zeatin is close to that of ZOG1 for trans-zeatin, the Vmax for the reaction is much lower (Table I).
Table II.
Relative activities of the recombinant ZOG1 and cisZOG1 enzymes with selected cytokinins
Recombinant ZOG1 and cisZOG1 were incubated with 500 μm cytokinin and 1 mm UDPG. The relative activity is presented as the percentage of zeatin (for ZOG1) or cis-zeatin (for cisZOG1).
| Substrate
|
Relative Activity (%)
|
|
|---|---|---|
| ZOG1 | cisZOG1 | |
| trans-Zeatin | 100 | 14 |
| cis-Zeatin | 3 | 100 |
| o-Topolin | 0.1 | 490 |
| m-Topolin | 23 | 0 |
| p-Topolin | 0.1 | 0 |
| TDZ | 0 | 0 |
| (o-OH)TDZ | 0 | 128 |
| (m-OH)TDZ | 0 | 0 |
| (p-OH)TDZ | 0 | 0 |
Substrate Recognition of ZOG1 and cisZOG1: Topolins and Hydroxylated Phenylureas
When leaves of transgenic tobacco (Nicotiana tabacum) harboring a 35S:ZOG1 construct were incubated with radiolabeled trans-zeatin, a large peak was formed coinciding with the elution of the O-glucoside of trans-zeatin, whereas the controls had only traces of this metabolite (Fig. 2, A and C). Interestingly, transgenic leaves incubated with radiolabeled m-topolin riboside (mTR) also yielded a larger peak eluting before the parent compound while control leaves did not (Fig. 2B). Although ZOG1 recognizes free bases and not the ribosides in in vitro assays, there is generally rapid interconversion between free bases and ribosides in plant tissues and therefore, feeding either free base or riboside provides substrate for glucosylation. Thus, we reasoned that the most likely candidate for the metabolite was the O-glucoside of m-topolin. Since no glucosylated standards for topolins are available, the product was further characterized by hydrolysis with β-glucosidase, which resulted in a shift to the m-topolin elution position (data not shown). The increase in this glucoside in transgenic leaves was accompanied by a corresponding decrease in m-topolin riboside (Fig. 2, B and D). Although only the 6-h data are shown in Figure 2, similar results were obtained after 24-h incubation, but with lower recovery of radiolabel in cytokinins and higher radiolabel in the fraction coeluting with adenine.
Figure 2.
Metabolism of 3H-labeled cytokinins in leaves of wild-type (control) and transformed (35S:ZOG1) tobacco. A, Chromatogram of leaf extracts after incubation with [3H]Z for 24 h, containing free bases, ribosides, and glucosides. B, Chromatogram of leaf extracts after incubation with m-[3H]TR for 24 h, containing free bases, ribosides, and glucosides. C, Relative amounts (% of total) of major products obtained from leaves incubated with [3H]Z for 6 and 24 h. D, Relative amounts (% of total) of major products obtained from leaves incubated with m-[3H]TR for 6 and 24 h. Cytokinins were extracted and purified according to the method of Dobrev and Kaminek (2002), resulting in two separate extracts from each sample: the fraction containing free bases, ribosides, and glucosides, and that containing the nucleotides. Radiolabeled metabolites were analyzed by HPLC coupled to a diode array detector and a flow-through radioactivity detector. Elution positions of purine and cytokinin standards in A and B are indicated by short horizontal lines. The data presented in C and D are the averages of two independent incubations and extractions. Z, trans-Zeatin; ZR, trans-zeatin riboside; Z7G, trans-zeatin-7-Glc; ZOG, trans-zeatin-O-glucoside; mT, m-topolin; and mTR, m-topolin riboside.
To confirm the observation with transgenic tissues that aromatic cytokinins are substrates for ZOG1 and to determine possible activities of the positional isomers, recombinant ZOG1 enzyme was incubated with o-, m-, and p-topolin in the presence of UDPG. Clearly, only the meta substituent yielded substantial amount of glucoside (Table II). The Km for m-topolin was 144 μm, higher than for cis-zeatin but lower than for trans-zeatin. The parent compound, BAP, did not give any product and neither did the riboside of m-topolin, which is consistent with the supposition that ZOG1 converts only free bases to O-glucosides. To compare the substrate recognition of ZOG1 with that of cisZOG1, the same substrates were incubated with cisZOG1 enzyme. Interestingly, o-topolin was found to be the best substrate, giving substantially higher conversion than cis-zeatin (Table II). In agreement with this, the Vmax of the reaction was also higher (Table I), but its affinity for the enzyme was lower (Km of 177 μm for o-topolin versus 46 μm for cis-zeatin).
When radiolabeled TDZ was supplied to P. lunatus callus cultures, the main metabolites formed were glucosides (Mok and Mok, 1985). To test whether ZOG1 could be responsible for this conversion, TDZ as well as the hydroxylated derivatives at the o-, m-, and p-position of the phenyl ring were incubated with recombinant enzyme. None of the positional isomers gave any conversion to glucosides (Table II). As expected, TDZ itself was not a substrate. However, (o-OH)TDZ was a substrate for cisZOG1, with slightly higher conversion rates than for cis-zeatin.
Several other compounds were tested, but none of them was converted to their glucosides by the two enzymes. They include cinnamic acid, β-indoleacetic acid, abscisic acid, kaempferol, and quercetin. The latter two are main flavonoids found in bean seed (Hempel and Bohm, 1996).
Cytokinin Activities in the P. lunatus Bioassay
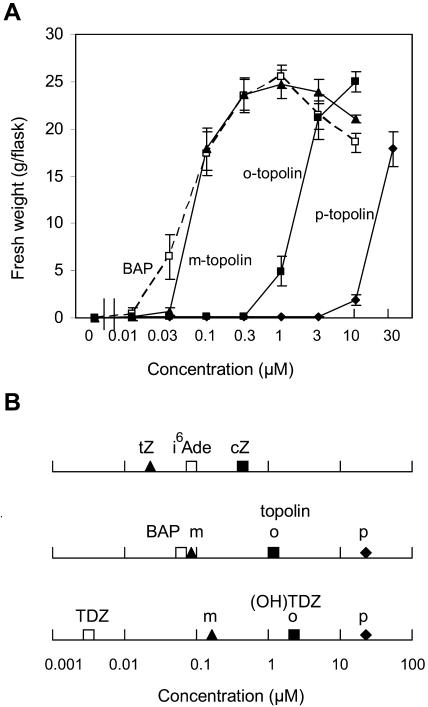
Cytokinin activities of the compounds used in the enzyme assays were compared in the P. lunatus callus bioassay to determine possible correlations of the relative conversions by ZOG1 and cisZOG1 with their abilities to promote growth in bioassays. Since the activities of the topolins in this bioassay have not been reported before, they are presented in detail in Figure 3A, while the activities of all three types of cytokinins (isoprenoid, aromatic, and TDZ derivatives) are summarized in Figure 3B as the midpoints of the response curves. The results show clearly that the relative activities and even the absolute activities of the corresponding positional isomers of the topolins and hydroxylated TDZ are very similar. Moreover, the relative activities of meta and ortho substituents are close to those of trans- and cis-zeatin. The main difference between the three groups resides in the activities of the parent compounds. While N6-(Δ2-isopentenyl)adenine (i6Ade) is a bit less active than trans-zeatin and BAP about equally active as m-topolin, TDZ has much higher cytokinin activity than its m-hydroxyl derivative (Fig. 3B). Although it is not known why this is the case, there seems to be a correlation with the stability of the compounds. Whereas i6Ade is highly labile and is broken down readily by cytokinin oxidases/dehydrogenases (Armstrong, 1994), BAP is more stable and TDZ is very stable (Mok and Mok, 1985).
Figure 3.
Cytokinin activities in the P. lunatus cv Kingston callus bioassay. A, Callus fresh weight in response to a range of concentrations (log scale) of BAP, m-, o-, and p-topolin. B, Summary of activities as indicated by the concentration at which one-half of the maximal growth is obtained of: (1) i6Ade and its hydroxylated derivatives, trans-zeatin and cis-zeatin; (2) BAP, m-topolin, o-topolin, and p-topolin; and (3) TDZ and its m-, o-, and p-OH derivatives. Three callus pieces of about 25 mg were placed in each Erlenmeyer with Murashige and Skoog medium (Murashige and Skoog, 1962) with a range of cytokinin concentrations (added dissolved in dimethyl sulfoxide after autoclaving). Cultures were grown in the dark at 27°C for 30 d. The values presented are means and se of three replicate experiments each with four flasks per treatment. tZ, trans-zeatin; cZ, cis-zeatin.
Recognition of Cytokinins by the AHK4 and ZmHK1 Receptors
To directly assess the responsiveness of receptors to the topolins and hydroxylated TDZs, AHK4 and ZmHK1 were expressed in the ΔrcsC and cps∷lacZ mutant background of Escherichia coli as described before (Suzuki et al., 2001; Yonekura-Sakakibara et al., 2004). Those studies demonstrated that AHK4 and ZmHK1 can induce lacZ expression in the presence of cytokinins recognized by the receptors (Suzuki et al., 2001; Yamada et al., 2001; Yonekura-Sakakibara et al., 2004). In this expression system AHK4 was responsive to trans-zeatin but not cis-zeatin, whereas ZmHK1 was responsive to both trans- and cis-zeatin (Yonekura-Sakakibara et al., 2004). Our assay with the topolins showed that only the meta isomer interacted with the AHK4 receptor (Fig. 4A), which is in agreement with a recent study (Spíchal et al., 2004). The response to m-topolin was stronger than to BAP, as was the response of trans-zeatin to its non-hydroxylated counterpart, i6Ade. The assays with ZmHK1 (Fig. 4B) showed that this receptor is slightly more responsive to m-topolin than o-topolin, comparable to the relationship between trans-zeatin and cis-zeatin. In the case of ZmHK1, responses to i6Ade and BAP were stronger than to their hydroxylated derivatives.
Figure 4.
Effects of cytokinins on expression of lacZ in E. coli (ΔrcsC. cps∷lacZ) harboring AHK4 (A) or ZmHK1 (B). The E. coli strains were cultured in the absence (C, control) or presence of 1 μm of the indicated cytokinins. cZ, cis-zeatin; tZ, trans-zeatin. The culture period was 8 h. The cells were harvested and the β-galactosidase activity was measured with o-nitrophenyl-β-d-galactopyranoside as a substrate. The values presented are means of triplicate samples with sd.
Similar assays with TDZ and derivatives showed that TDZ was recognized by AHK4, but none of the hydroxylated compounds were (Fig. 4A). Neither TDZ nor its hydroxylated derivatives were recognized by ZmHK1 (Fig. 4B).
Although only the 8-h data are shown here, results of the 2-h experiment were exactly the same with regard to relative responses but the absolute values were lower. A similar experiment with the control E. coli strain harboring the empty pIN-III vector gave only background β-gal activities in the presence of any of the cytokinins (data not shown), similar to the dimethyl sulfoxide controls presented in Figure 4.
Synopsis of Results and Molecular Modeling of Cytokinins
The relative conversions in the enzyme assays, activities in the bioassay, and recognition by the receptors are generally consistent for the adenine-type cytokinins. For instance, zeatin and m-topolin were preferred substrates of ZOG1, exhibited high activity in the bean bioassay, and were recognized by the AHK4 receptor. Likewise, cis-zeatin and o-topolin were good substrates of cisZOG1, showed much weaker activity in the bean bioassay, and were recognized by ZmHK1. Furthermore, p-topolin was not a substrate for the enzymes, did not exhibit much cytokinin activity, and did not induce detectable activity in the receptor assay.
Based on the results with the topolins, which also have a phenyl ring, one would expect (m-OH)TDZ to show the same enzyme and receptor recognition as m-topolin and (o-OH)TDZ as o-topolin. However, this was clearly not the case. Although (o-OH)TDZ was a substrate of cisZOG1, (m-OH)TDZ was neither a substrate of ZOG1 nor a cytokininin recognized by AHK4.
To further explain the results, we compared the molecular structure models of the cytokinins. One strategy to decipher the biochemical results is to compare the three-dimensional structures (rather than the flat chemical schematics in Fig. 1) of the various substrates (Fig. 5). For these comparisons, we first constructed geometry optimized molecular models of each compound, then superimposed the structures according to one or more of the corresponding ring systems.
Figure 5.
Comparison of molecular models of substrates. A and B, Overlay of trans-zeatin and m-topolin. The structure of trans-zeatin is shown with carbons in green and m-topolin with carbons in black. The structures are overlaid according to the atoms of the purine base to show the correspondence of the hydroxyl groups of the two model structures as a head-on view into the purine ring (A) and slightly canted (B). C and D, Overlay of cis-zeatin (green carbons) and o-topolin (black carbons). The hydroxyl group of the cis-configuration of zeatin is shown to correspond best with the hydroxyl group at the ortho position of topolin. Again, the overlays are shown as views into the purine ring (C) and slightly rotated (D). E to H, Overlay of BAP (carbons in black) and TDZ (carbons in green). In E, the five-membered ring of TDZ is overlaid with the five membered ring of the adenine of BAP, showing the correspondence of the ortho position, but not the meta or para positions of the phenyl ring. In F, the structures are overlaid according to the phenyl rings, while in G, the superposition is defined by the conformation of the linker between the benzyl and purine/thiadiazol rings of the two compounds. H is similar to F, except that the low energy form of a rotation isomer of BAP is shown (rotation about the link to the benzyl ring of BAP).
The molecular models show that zeatin and the topolins have obvious structural similarities (Fig. 5, A–D). Moreover, when the molecular models for zeatin and various topolins are superimposed, it becomes clear that m-topolin is very close to trans-zeatin (Fig. 5, A and B), which is in agreement with the results obtained with ZOG1, the bean bioassay, and the receptor assays. Similarly, the hydroxyl group of o-topolin (but not of m- and p-topolin) corresponds closely to that of cis-zeatin (Fig. 5, C and D), explaining the results with the cisZOG1 enzyme and ZmHK1.
There are several possible ways to overlay the structural models of BAP and TDZ (Fig. 5, E–H), with alignment of either the five-membered ring, the phenyl ring, or the linker between the rings. In overlays of the thiodiazol ring with the five-membered purine ring (Fig. 5E), there is close correspondence between the ortho but not the meta positions of the phenyl rings of BAP and TDZ, which is consistent with the results of the enzyme assays. The P. lunatus bioassay results would suggest recognition of the phenyl ring as the determinant, which is represented by overlays of the phenyl rings. Although at first glance the results with AHK4 may seem inconsistent with this interpretation, it should be noted that the activity of TDZ in the bioassay was 50 times higher than that of (m-OH)TDZ. If this differential also would exist for the receptor, (m-OH)TDZ activity would not be detectable in the AHK4 assay. The extremely high activity of TDZ-type compounds in tissue culture system is most likely related to their high stability (Mok and Mok, 1985). However, the ZmHK1 receptor does not seem to recognize TDZ and its derivatives, indicating a different conformation, such as represented by the overlay in Figure 5G.
Analyses of Endogenous Topolins
Topolins have been found in several genera (Strnad, 1997; Sáenz et al., 2003; Tarkowská et al., 2003). Their levels, however, are generally much lower than those of isoprenoid cytokinins. The presence of topolins in several plant species, combined with the fact that selected topolins are substrates of the ZOG1 and cisZOG1 enzymes, raises the question whether these compounds might be present in beans and maize. We have searched for these compounds in maize and bean leaves and roots, but did not detect any activity above our detection limits (about 1 pmol g−1 fresh weight). Thus, although topolins may be present in these species, their levels must be quite low, at least in roots and leaves.
DISCUSSION
Each of the two cytokinin UGTs, ZOG1 and cisZOG1, has a defined range of substrates. ZOG1 has the highest activity with trans-zeatin and also recognizes m-topolin as substrate but has much lower activity with cis-zeatin. cisZOG1 recognizes cis-zeatin, o-topolin, and (o-OH)TDZ as substrates while having lower catalytic activity with trans-zeatin. The compounds recognized as substrates by these enzymes are functionally related: all substrates have cytokinin activity. However, it is not known whether other, functionally unrelated metabolites are also substrates to the enzymes. We have tested a number of compounds, including kaempferol and quercetin, two flavonoids found in beans, but none of these were found to be substrates of ZOG1.
The various cytokinin O-glucosyltransferases differ widely in reaction velocities. The bean ZOG1 and ZOX1 enzymes have considerably higher reaction velocities than the maize cisZOG1 enzyme and also the Arabidopsis ZOG enzymes (R.C. Martin, D.W.S. Mok, and M.C. Mok, unpublished data). However, the reaction rate of the most active enzyme, ZOG1, was still lower than of the cytokinin oxidase/dehydrogenase of maize (Bilyeu et al., 2001), which is consistent with the generally rapid degradation of trans-zeatin in vivo.
The cytokinin-binding site on the enzyme is likely to have some features in common with other cytokinin-binding proteins such as receptors. Indeed, there is a close correlation between the cytokinin recognition of ZOG1 and AHK4. Both recognize trans-zeatin and to a lesser extent m-topolin, while they have very low or no recognition of cis-zeatin, o-, and p-topolin, or any of the hydroxylated TDZs. ZmHK1 has features in common with both enzymes, recognizing trans-zeatin and m-topolin (like ZOG1) as well as cis-zeatin and o-topolin (like cisZOG1). In all our assays, the activity of trans-zeatin was correlated with that of m-topolin, while activity of cis-zeatin was correlated with that of o-topolin. This relationship also applied to responses in the PARR∷GUS reporter assay in Arabidopsis (Spíchal et al., 2004). These findings are in agreement with predictions from molecular modeling, showing the similarities of trans-zeatin to m-topolin and cis-zeatin to o-topolin.
Comparisons of the molecular models of BAP and TDZ (Fig. 5, E–H) show that the two substrates can be superimposed using either the benzyl ring system or overlaying the thiadiazol ring of TDZ with either the five- or six-membered rings of the adenine moiety of BAP. In all cases, there is correspondence of one ring system, but not the other. This suggests that the various proteins (O-glucosyltransferases and receptors) could be recognizing different functional groups or different conformations of the molecules. This would explain the recognition of (o-OH)TDZ by cisZOG1 but lack of recognition of (m-OH)TDZ by ZOG1 (Fig. 5E) as well as the m>o>p relationship in the P. lunatus bioassay (Fig. 5F). Even though the bioassay and AHK4 receptor assay concern two different species, the lack of recognition of any of the hydroxylated TDZ derivatives by AHK4 is consistent with the bioassay results. TDZ was 80-fold more active than the most active hydroxylated derivative, (m-OH)TDZ, in the bioassay, while a similar relationship between TDZ and (m-OH)TDZ would lead to below detectable recognition of (m-OH)TDZ in the AHK4 assay.
UGTs may function in facilitating biosynthesis, inactivation, storage, and stability, as well as intra- and intercellular transport of a large array of natural compounds (Jones and Vogt, 2001; Paquette et al., 2003). In addition, some UGTs are involved in detoxification of xenobiotics (Sandermann et al., 1991; Leah et al., 1992; Loutre et al., 2003). Generally, glycosylation of hydrophobic compounds leads to increased hydrophilicity and decreased reactivity. Glycosylation of zeatin can lead to increased stability since zeatin is readily broken down by cytokinin oxidases/dehydrogenases, while zeatin O-glucoside is resistant to such enzymes. Although a function of cytokinin glycosides in transport has not been demonstrated, glycosides have been found in xylem sap (Letham, 1994; Kato et al., 2002). In tobacco leaf discs containing a ZOG1 transgene controlled by an inducible promoter, the increase in zeatin O-glucosylation led to a decrease of active cytokinin (Martin et al., 2001a). However, in intact plants containing constitutively expressed ZOG1, increased cytokinin glycosylation did not lead to significant decreases in the level of the free base or riboside (Martin et al., 2001a), indicating that plants precisely control active cytokinin levels. It seems that natural cytokinin glycosylation is one of the mechanisms whereby the plant can fine-tune its active cytokinins to levels appropriate for each tissue and stage of development. For instance, levels of glucosides increase during leaf maturation and senescence (Van Staden et al., 1988; Noodén and Letham, 1993).
Hydroxylation of natural compounds often precedes glucosylation (Paquette et al., 2003). This also seems to be the case for cytokinins. The mononucleotide of isopentenyl adenine was shown to be converted to that of zeatin and an enzyme mediating this conversion, with characteristics of a P450 hydroxylase, was partially purified from cauliflower microsomes (Chen and Leisner, 1984). Recently, two Arabidopsis genes encoding such hydroxylases have been identified (Takei et al., 2004). However, on the basis of results from deuterium-label dilution analysis, an alternative pathway for the formation of zeatin-type cytokinins, independent of i6AMP, was postulated (Åstot et al., 2000), which may possibly involve direct transfer of the hydroxylated side chain (Wolff et al., 2002) as shown for the Agrobacterium Tzs protein (Krall et al., 2002). Although formation of trans-zeatin can be accommodated by this pathway, hydroxylation can lead to formation of cis-zeatin and trans-zeatin as well as the topolins. Based on the results with the glucosyltransferases, we speculate that some hydroxylases may mediate conversion to trans-zeatin and m-topolin, while others may lead to formation of cis-zeatin and o-topolin. The occurrence of different hydroxylases for formation of cis- and trans-zeatin is supported by the finding that trans-zeatin is formed in plastids and cis-zeatin predominantly in the cytoplasm (Kasahara et al., 2004).
In conclusion, the cytokinin O-glucosyltransferases have a range of related substrates including natural and synthetic cytokinins but are defined by the position of the hydroxyl group. This range conforms to expectations based on molecular models. Moreover, the recognition of naturally occurring topolins by the enzymes corresponds with cytokinin activities displayed in the bean bioassay as well as activities in AHK4 and ZmHK1 receptor assays. The situation with the synthetic TDZ derivatives is more complex but can be explained to a great extent by our molecular models. Elucidation of the crystal structure of the ZOG1 enzyme and receptor proteins should provide further insight in the conformation of these proteins and their cytokinin binding sites and will serve as a critical test of our current interpretation.
MATERIALS AND METHODS
Cloning ZOG1 and cisZOG1 into the pGex-6P-1 Vector
The open reading frame of ZOG1 was obtained by PCR from genomic DNA of bean (Phaseolus lunatus) cv Kingston using primers with BamHI sites incorporated into each primer. The open reading frame of cisZOG1 was obtained by PCR from genomic DNA of maize (Zea mays) inbred B73 using primers with a BamHI site on the forward primer and a XhoI site on the reverse primer. The primers were as follows:
ZOG1F: CCAAATGGATCCATGGCTTTGAATGACAAAAGC
ZOG1B: GCTAGGATCCCAGGCTAAATGGTATGACTATTTAG
cisZOG1F: GATTGGATCCATGGCGGTTGACAC
cisZOG1B: GTCGACTCGAGATACTTTCACCTTGTGAT
PCR was performed using Cloned Pfu DNA Polymerase (Stratagene, La Jolla, CA). The PCR products were cloned using the Zero Blunt TOPO PCR Cloning kit (Invitrogen Life Technologies, Carlsbad, CA) and checked for sequence errors. Clones with perfect sequences were digested with BamHI (ZOG1) or BamHI and XhoI (cisZOG1) and ligated into pGex-6P-1 digested with appropriate enzymes. The plasmids were introduced into Escherichia coli strain XL1-Blue Competent cells (Stratagene).
Induction and Protein Purification
E. coli XL-1 Blue cells containing the recombinant plasmids were grown overnight at 37°C in 2× YT media containing 100 μg mL−1 carbenicillin. The next morning the cells were diluted 1:100 in 2× YT media with carbenicillin and grown for an additional 3.5 h. The cultures were grown for another hour at about 26°C and then isopropylthio-β-galactoside was added to a final concentration of 1 mm. The cultures were induced overnight and the cells were harvested by centrifugation at 7,000g. Cell pellets were stored at −80°C until purified. Cells were thawed in cold water and resuspended in phosphate buffered saline, pH 7.4, with 0.25 mg mL−1 lysozyme (Sigma, St. Louis), 0.1% Protease Inhibitor cocktail set III (Calbiochem, San Diego), 0.2 mm phenylmethylsulfonylfluoride (Sigma), 2 mm dithiotreitol (DTT), and 1 μL/mL Benzonase Nuclease (Novagen, Madison, WI)) and treated according to the manufacturer's recommendations (GST Gene Fusion System, Amersham Biosciences; Piscataway, NJ). Proteins were purified using Glutathione Sepharose 4 Fast Flow and cleaved on the column using PreScission Protease according to manufacturer's instructions (Amersham Biosciences).
Biochemical Characterization of Recombinant Enzyme
To test for enzyme activity with a particular substrate, recombinant enzyme (0.1–10 μg) was incubated with 500 μm of the substrate, 1 mm UDP-Glc (including 0.5 μCi UDP-[6-3H]Glc), and 5 mm DTT in 0.17 m Tris, pH7.5, for 30 min at 30°C. For determination of Km and Vmax, purified recombinant ZOG1 (0.1–1 μg) was incubated with 10 to 200 μm trans-zeatin (including 0.5 μCi trans-[2-3H]zeatin) or 20 to 800 μm cis-zeatin (including 0.5 μCi cis-[8-3H]zeatin), 4 mm UDP-Glc, and 5 mm DTT in 150 μL 0.17 m Tris, pH 7.5, for 30 min at 30°C. The reaction was terminated by addition of 15 μL cold trichloroacetic acid. Products were separated by HPLC and radioactivity in each fraction was determined as described in Dixon et al. (1989). The data presented in Tables I and II are the averages of three experiments.
Metabolism of 3H-Labeled Cytokinins in Tobacco Leaves
Apical portions of young but fully developed leaves of tobacco (Nicotiana tabacum) L. cv W38 transformed with the cauliflower 35S promoter∷ZOG1 and controls (1 g fresh weight) were incubated for 6 or 24 h in water with 1 μCi tritiated cytokinin (m-[2-3H]topolin riboside (specific activity 48 mCi/μmol) or trans-[2-3H]zeatin (specific activity 54 mCi/μmol) at room temperature and continuous light in the laminar flow hood.
Cytokinins were extracted and purified according to the method of Dobrev and Kaminek (2002). Leaf material was frozen in liquid nitrogen and extracted overnight with 10 mL methanol/water/formic acid (15:4:1, v/v/v, pH 2.5) at −20°C. The extract was passed through 2-mL Si-C18 columns (SepPak Plus, Waters, Milford, MA) to remove interfering lipophilic substances. After organic solvent evaporation in vacuo, the aqueous residue was applied to an Oasis MCX mixed mode (cation exchange and reverse-phase) column (150 mg, Waters). Adsorbed cytokinin nucleotides were eluted with 5 mL of 0.35 m ammonium in water; cytokinin bases, ribosides, and glucosides with 5 mL 0.35 m ammonium in 60% methanol. The eluates were evaporated in vacuo.
Radiolabeled metabolites were analyzed by HPLC. Dried samples were resuspended in 200 μL 20% (v/v) acetonitrile. The system contained a Series 200 Quaternary HPLC pump (Perkin Elmer, Boston) and Luna C18(2), 150 mm/4.6 mm/3 μm column (Phenomenex, Torrance, CA) coupled to a 235C diode array detector (Perkin Elmer), and RAMONA 2000 flow-through radioactivity detector (Raytest, Straubenhardt, Germany). The sample (10 μL) was eluted at a flow rate of 0.6 mL/min at 30°C. The mobile phase consisted of A, 40 mm CH3COOH adjusted to pH 4.1 with NH4OH; and B, CH3OH/CH3CN =1/1 (v/v). A linear gradient of 10% to 20% B over 2 min, 20% to 45% B over 17 min, and 45% to 100% B over 2 min was used. The radioactive metabolites were identified based on retention times of authentic standards. Since no standards for O-glucosides of m-topolin and its riboside are available, the main product of m-topolin riboside formed in ZOG1-containing leaves was characterized by treatment with β-glucosidase (Sigma). Extract containing cytokinin bases, ribosides, and glucosides was diluted with 1 mL 40 mm ammonium acetate buffer, pH 5.0, and split in two. One part was dried, the other treated with β-glucosidase (3.5 units) for 1 h at 37°C, applied to Si-C18 columns (SepPak Plus, Waters), washed with 5% MeOH, eluted with 80% MeOH, and dried. The two samples were then analyzed by HPLC as described above.
P. lunatus Cytokinin Bioassay
Cytokinin activity was determined in the P. lunatus cv Kingston callus bioassay as described earlier (Mok et al., 1978). The medium consisted of Murashige and Skoog inorganics (Murashige and Skoog, 1962), (L−1) 30 g Suc, 100 mg myo-inositol, 1 mg thiamine, 5 mg nicotinic acid, 0.5 mg pyridoxine, 2.5 μm picloram, and 8 g Difco Bacto agar. The pH of the medium was adjusted to 5.7 before autoclaving. Cytokinins, dissolved in dimethylsulfoxide, were added to autoclaved flasks (25 μL/50 mL medium). Three pieces of callus weighing about 25 mg each were planted per 125-mL Erlenmeyer flask containing 50 mL medium. Callus yield was determined after 30 d of growth at 27°C in the dark. Data were obtained from three experiments with four replicate flasks per treatment.
Expression of AHK4 and ZmHK1 in E. coli
A heterologous expression assay with the AHK4 and ZmHK1 cytokinin receptors in E. coli containing the ΔrscC and cps∷lacZ genetic background was used to determine the receptor recognition of cytokinins, as described previously (Suzuki et al., 2001; Takeda et al., 2001; Yonekura-Sakakibara et al., 2004). Briefly, transformants were grown overnight in Luria-Bertani medium supplemented with 50 mm potassium phosphate, pH 7, 40 mm Glc, and 50 μg mL−1 ampicillin. The overnight culture (1 mL) was inoculated in 100 mL fresh culture medium containing 1 μm cytokinin and then grown for 2 h or 8 h at 25°C with vigorous shaking. The cells were harvested and suspended in an appropriate volume of Z buffer (100 mm sodium phosphate, 10 mm KCl, and 1 mm MgSO4, pH 7.0). Cells were disrupted by sonication, and supernatant was recovered by centrifugation and assayed for β-galactosidase activity with o-nitrophenyl β-d-galactopyranoside as a substrate.
Models of Molecular Structures
Molecular models of the trans and cis forms of zeatin, and the isomers of topolin, BAP, and TDZ were constructed, and geometries optimized using the Builder function in the Insight II (Accelrys/MSI, San Diego). All ring and urea functional groups were defined as delocalized partial double-bond or as aromatic ring systems. Freely rotating single bonds were modeled in all possible staggered conformations prior to geometry optimization. The lowest energy structures for each compound are represented in Figure 5.
Analysis of Endogenous Cytokinins
Roots and leaves were obtained from 6-week-old maize (Z. mays) inbred B73 and 5-week-old P. lunatus cv Kingston grown in a hydroponic Hoagland solution under a 16-h light at 25°C. Tissues were lyophilized and cytokinins extracted as described earlier (Veach et al., 2003). Isoprenoid and aromatic cytokinins were analyzed in separate runs. The isoprenoid cytokinins were analyzed as in Veach et al. (2003), while an HPLC column AQUA, 250 mm/2 mm/5 μm (Phenomenex) was used to separate aromatic cytokinins, with a gradient of 15% to 60% acetonitrile in water and a constant level of acetic acid (0.05%, v/v) at a flow rate of 0.2 mL/min. Detection and quantification were carried out by mass spectrometry (MS) with a Finnigan LCQ operated in the positive ion, full-scan MS/MS mode. Aromatic cytokinins were identified by retention times and spectra of standards of BAP, m-topolin, o-topolin, and the corresponding ribosides. Since no deuterated internal standard of BAP and topolins were available, recovery rates were calculated based on those of N6-(Δ2-isopentenyl)adenine, its riboside and 9-glucoside, and trans-zeatin riboside. The electrospray ionization probe was installed with a sheath and auxiliary gasses at 96 and 6 units, respectively. The heated metal capillary temperature was maintained at 250°C and capillary voltage at 2.5 V. Two replicate samples were analyzed for each type of tissue.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AF101972 and AF318075.
Acknowledgments
We thank Dr. T. Mizuno, Nagoya University, for providing us with E. coli strains with the ΔrcsC and cps∷lacZ genetic background and pIN-III-AHK4.
This work was supported by the National Science Foundation (grant nos. IBN–9981974 and IBN–0086731), by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (grant no. 01–02015), by the National Institutes of Health (grant no. R1GM62957A), by the Grant Agency of the Czech Republic (project no. 522/04/0549), and by the Czech Ministry of Education, Youth and Sports (grant no. Kontakt ME 406).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.057174.
References
- Armstrong DJ (1994) Cytokinin oxidase and the regulation of cytokinin degradation. In DWS Mok, MC Mok, eds, Cytokinins:Chemistry, Activity, and Function. CRC Press, Boca Raton, FL, pp 139–154
- Åstot C, Dolezal K, Nordström A, Wang Q, Kunkel T, Moritz T, Chua N-H, Sandberg G (2000) An alternative cytokinin biosynthetic pathway. Proc Natl Acad Sci USA 97: 14778–14783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer CA (1997) Cytokinin conjugation: recent advances and patterns in plant evolution. Plant Growth Regul 23: 17–32 [Google Scholar]
- Bilyeu KD, Cole JL, Laskey JG, Riekhof WR, Esparza TJ, Kramer MD, Morris RO (2001) Molecular and biochemical characterization of a cytokinin oxidase from maize. Plant Physiol 125: 378–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce MI, Zwar JA (1966) Cytokinin activity of some substituted ureas and thioureas. Proc R Soc Lond B Biol Sci 165: 245–265 [DOI] [PubMed] [Google Scholar]
- Chen C-M (1997) Cytokinin biosynthesis and interconversions. Physiol Plant 101: 665–673 [Google Scholar]
- Chen C-M, Leisner SM (1984) Modification of cytokinins by cauliflower microsomal enzymes. Plant Physiol 75: 442–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SF, McKenzie MJ, Burritt DJ, Guy PL, Jameson PE (1999) Influence of white clover mosaic potexvirus infection on the endogenous cytokinin content of bean. Plant Physiol 120: 547–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SC, Martin RC, Mok MC, Shaw G, Mok DWS (1989) Zeatin glycosylation enzymes in Phaseolus: isolation of O-glucosyltransferase from P. lunatus and comparison to O-xylosyltransferase from P. vulgaris. Plant Physiol 90: 1316–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrev PI, Kaminek M (2002) Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogr 950: 21–29 [DOI] [PubMed] [Google Scholar]
- Entsch B, Letham DS (1979) Enzymic glycosylation of the cytokinin, 6-benzylaminopurine. Plant Sci Lett 14: 205–212 [Google Scholar]
- Entsch B, Parker CW, Letham DS, Summons RE (1979) Preparation and characterization using high-performance liquid chromatography of an enzyme forming glucosides of cytokinins. Biochim Biophys Acta 570: 124–139 [DOI] [PubMed] [Google Scholar]
- Frank M, Rupp H-M, Prinsen E, Motyka V, Van Onckelen H, Schmülling T (2000) Hormone autotrophic growth and differentiation identifies mutant lines of Arabidopsis with altered cytokinin and auxin content or signaling. Plant Physiol 122: 721–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi-Mizutani M, Okuhara H, Fukui Y, Nakao M, Katsumoto Y, Yonekura-Sakakibara K, Kusumi T, Hase T, Tanaka Y (2003) Biochemical and molecular characterization of a novel UDP-glucose:anthocyanin 3′-O-glucosyltransferase, a key enzyme for blue anthocyanin biosynthesis, from gentian. Plant Physiol 132: 1652–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel J, Bohm H (1996) Quality and quantity of prevailing flavonoid glycosides of yellow and green French beans (Phaseolus vulgaris L.). J Agric Food Chem 44: 2114–2116 [Google Scholar]
- Holub J, Hanuš J, Hanke DE, Strnad M (1998) Biological activity of cytokinins derived from ortho- and meta-hydroxybenzyladenine. Plant Growth Regul 26: 109–115 [Google Scholar]
- Horgan R, Hewett EW, Purse JG, Wareing PF (1973) A new cytokinin from Populus x robusta. Tetrahedron Lett 30: 2827–2828 [Google Scholar]
- Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, Tabata S, Shinozaki K, Kakimoto T (2001) Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409: 1060–1063 [DOI] [PubMed] [Google Scholar]
- Jackson RG, Lim E-K, Li Y, Kowalczyk M, Sandberg G, Hoggett J, Ashford DA, Bowles DJ (2001) Identification and biochemical characterization of an Arabidopsis indole-3-acetic acid glucosyltransferase. J Biol Chem 276: 4350–4356 [DOI] [PubMed] [Google Scholar]
- Jones P, Vogt T (2001) Glycosyltransferases in secondary plant metabolism: tranquilizers and stimulant controllers. Planta 213: 164–174 [DOI] [PubMed] [Google Scholar]
- Kakimoto T (2003) Perception and signal transduction of cytokinins. Annu Rev Plant Biol 54: 605–627 [DOI] [PubMed] [Google Scholar]
- Kasahara H, Takei K, Ueda N, Hishiyama S, Yamaya T, Kamiya Y, Yamaguchi S, Sakakibara H (2004) Distinct isoprenoid origins of cis- and trans-zeatin biosyntheses in Arabidopsis. J Biol Chem 279: 14049–14054 [DOI] [PubMed] [Google Scholar]
- Kato C, Kato H, Asami T, Yoshida S, Noda H, Kamada H, Satoh S (2002) Involvement of xylem sap zeatin-O-glucoside in cucumber shoot greening. Plant Physiol Biochem 40: 949–954 [Google Scholar]
- Krall L, Raschke M, Zenk MH, Baron C (2002) The Tzs protein from Agrobacterium tumefaciens C58 produces zeatin riboside 5′-phosphate from 4-hydroxy-3-methyl-2-(E)-butenyl diphosphate and AMP. FEBS Lett 527: 315–318 [DOI] [PubMed] [Google Scholar]
- Leah JM, Worrall TL, Cobb AH (1992) Isolation and characterization of two glucosyltransferases from Glycine max associated with bentazone metabolism. Pestic Sci 34: 81–87 [Google Scholar]
- Letham DS (1994) Cytokinins as phytohormones: sites of biosynthesis, translocation, and function of translocated cytokinin. In DWS Mok, MC Mok, eds, Cytokinins: Chemistry, Activity, and Function. CRC Press, Boca Raton, FL, pp 57–80
- Lim E-K, Li Y, Parr A, Jackson R, Ashford DA, Bowles DJ (2001) Identification of glucosyltransferase genes involved in sinapate metabolism and lignin synthesis in Arabidopsis. J Biol Chem 276: 4344–4349 [DOI] [PubMed] [Google Scholar]
- Loutre C, Dixon DP, Brazier M, Slater M, Cole DJ, Edwards R (2003) Isolation of a glucosyltransferase from Arabidopsis thaliana active in the metabolism of the persistent pollutant 3,4-dichloroaniline. Plant J 34: 485–493 [DOI] [PubMed] [Google Scholar]
- Martin RC, Mok DWS, Mok MC (2001. a) Development of transgenic tobacco harboring a zeatin O-glucosyltransferase gene from Phaseolus. In Vitro Cell Devel Biol – Plant 37: 354–360 [Google Scholar]
- Martin RC, Mok MC, Habben JE, Mok DWS (2001. b) A cytokinin gene from maize encoding an O-glucosyltransferase specific to cis-zeatin. Proc Natl Acad Sci USA 98: 5922–5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC, Mok MC, Mok DWS (1999. a) Isolation of a cytokinin gene, ZOG1, encoding zeatin O-glucosyltransferase of Phaseolus lunatus. Proc Natl Acad Sci USA 96: 284–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC, Mok MC, Mok DWS (1999. b) A gene encoding the cytokinin enzyme zeatin O-xylosyltransferase of Phaseolus vulgaris. Plant Physiol 120: 553–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok DWS, Mok MC (2001) Cytokinin metabolism and action. Annu Rev Plant Physiol Plant Mol Biol 52: 89–118 [DOI] [PubMed] [Google Scholar]
- Mok MC, Mok DWS (1985) The metabolism of [14C]-thidiazuron in callus tissues of Phaseolus lunatus. Physiol Plant 65: 427–432 [Google Scholar]
- Mok MC, Mok DWS, Armstrong DJ (1978) Differential structure-activity relationships in Phaseolus. Plant Physiol 61: 72–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok MC, Mok DWS, Armstrong DJ, Shudo K, Isogai Y, Okamoto T (1982) Cytokinin activity of N-phenyl-N'-1,2,3-thiadiazol-5-ylurea (Thidiazuron). Phytochemistry 21: 1509–1511 [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Noodén LD, Letham DS (1993) Cytokinin metabolism and signaling in the soybean plant. Aust J Plant Physiol 20: 639–653 [Google Scholar]
- Paquette S, Møller BL, Bak S (2003) On the origin of family 1 plant glucosyltransferases. Phytochemistry 62: 399–413 [DOI] [PubMed] [Google Scholar]
- Sáenz L, Jones LH, Oropeza C, Vláčil D, Strnad M (2003) Endogenous isoprenoid and aromatic cytokinins in different plant parts of Cocos nucifera (L.). Plant Growth Regul 39: 205–215 [Google Scholar]
- Sandermann H, Schmitt R, Eckey H, Bauknecht T (1991) Plant biochemistry of xenobiotics: isolation and properties of soybean O- and N-glucosyl and O- and N-malonyltransferases for chlorinated phenols and anilines. Arch Biochem Biophys 287: 341–350 [DOI] [PubMed] [Google Scholar]
- Schmitz RY, Skoog F, Playtis AJ, Leonard NJ (1972) Cytokinins: synthesis and biological activity of geometric and position isomers of zeatin. Plant Physiol 50: 702–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shantz EM, Steward FC (1955) The identification of compound A from coconut milk as 1,3-diphenylurea. J Am Chem Soc 77: 6351–6353 [Google Scholar]
- Shudo K (1994) Chemistry of phenylurea cytokinins. In DWS Mok, MC Mok, eds, Cytokinins: Chemistry, Activity, and Function. CRC Press, Boca Raton, FL, pp 35–42
- Skoog F, Armstrong DJ (1970) Cytokinins. Annu Rev Plant Physiol 21: 359–384 [Google Scholar]
- Spíchal L, Rakova NY, Riefler M, Mizuno T, Romanov GA, Strnad M, Schmulling T (2004) Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay. Plant Cell Physiol 45: 1299–1305 [DOI] [PubMed] [Google Scholar]
- Strnad M (1997) The aromatic cytokinins. Physiol Plant 101: 674–688 [Google Scholar]
- Suzuki T, Miwa K, Ishikawa K, Yamada H, Aiba H, Mizuno T (2001) The Arabidopsis sensor His-kinase, AHK4, can respond to cytokinins. Plant Cell Physiol 42: 107–113 [DOI] [PubMed] [Google Scholar]
- Takagi M, Yokota T, Murofushi N, Saka H, Takahashi N (1989) Quantitative changes of free-base, riboside, ribotide and glucoside cytokinins in developing rice grains. Plant Growth Regul 8: 349–364 [Google Scholar]
- Takeda S, Fujisawa Y, Matsubara M, Aiba H, Mizuno T (2001) A novel feature of the multistep phosphorelay in Escherichia coli: a revised model of the RcsC → YojN → RcsB signalling pathway implicated in capsular synthesis and swarming behavior. Mol Microbiol 40: 440–450 [DOI] [PubMed] [Google Scholar]
- Takei K, Yamaya T, Sakakibara H (2004) Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin. J Biol Chem 279: 41866–41872 [DOI] [PubMed] [Google Scholar]
- Tarkowská D, Doležal K, Tarkowski P, Åstot C, Holub J, Fuksová K, Schmülling T, Sandberg G, Strnad M (2003) Identification of new aromatic cytokinins in Arabidopsis thaliana and Populus x canadensis leaves by LC-(+)ESI-MS and capillary liquid chromatography/frit-fast atom bombardment mass spectrometry. Physiol Plant 117: 579–590 [DOI] [PubMed] [Google Scholar]
- Turner JE, Mok DWS, Mok MC, Shaw G (1987) Isolation and partial purification of an enzyme catalyzing the formation of O-xylosylzeatin in Phaseolus vulgaris embryos. Proc Natl Acad Sci USA 84: 3714–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Staden J, Cook EL, Noodén LD (1988) Cytokinins and senescence. In LD Noodén, AC Leopold, eds, Senescence and Aging in Plants. Academic Press, San Diego, pp 281–328
- Vanková R (1999) Cytokinin glycoconjugates: distribution, metabolism and function. In M Strnad, P Pec, E Beck, eds, Advances in Regulation of Plant Growth and Development. Peres Company, Prague, pp 67–78
- Veach YK, Martin RC, Mok DWS, Malbeck J, Vankova R, Mok MC (2003) O-Glucosylation of cis-zeatin in maize. Characterization of genes, enzymes, and endogenous cytokinins. Plant Physiol 131: 1374–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Suzuki T, Terada K, Takei K, Ishikawa K, Miwa K, Yamashino T, Mizuno T (2001) The Arabidopsis AHK4 histidine kinase is a cytokinin-binding receptor that transduces cytokinin signals across the membrane. Plant Cell Physiol 42: 1017–1023 [DOI] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K, Kojima M, Yamaya T, Sakakibara H (2004) Molecular characterization of cytokinin-responsive histidine kinases in maize. Differential ligand preferences and response to cis-zeatin. Plant Physiol 134: 1654–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M, Seemann M, Grosdemange-Billiard C, Tritsch D, Campos N, Rodríguez-Concepción M, Boronat A, Rohmer M (2002) Isoprenoid biosynthesis via the methylerythritol phosphate pathway. (E)-4-hydroxy-3-methylbut-2-enyl diphosphate: chemical synthesis and formation from methylerithrytol cyclodiphosphate by a cell-free system from Escherichia coli. Tetrahedron Lett 43: 2555–2559 [Google Scholar]