Abstract
The landscape for managing type 1 diabetes during pregnancy has been transformed by increasing use of continuous glucose monitoring (CGM). Women are aiming for pregnancy-specific glucose targets or 70% time in range for pregnancy (TIRp; 63–140 mg/dL) as soon as possible, knowing that every extra 5% TIRp has benefits for reducing the risks of complications in their babies. Ongoing monitoring of maternal A1C (at pregnancy confirmation and at 20, 28, and 36 weeks’ gestation) remains useful. Intensification of glycemic management and instruction in using CGM (if not already used) is recommended for individuals with an A1C >6.0% after 20 weeks. A better understanding of CGM-documented glycemic changes throughout pregnancy is needed to inform future management of gestational diabetes and pregnancy in people with type 2 diabetes. Research regarding overcoming barriers to CGM use and optimal TIRp targets for pregnant individuals with type 2 diabetes from diverse racial/ethnic groups is urgently needed.
Continuous glucose monitoring (CGM) empowers individuals to manage their daily glucose levels, alerting them if their glucose is too high or too low and providing unprecedented options for data-sharing with partners, parents, and clinicians. This article provides an overview and roadmap of the effective use of CGM in pregnancy (Figure 1).
Figure 1.
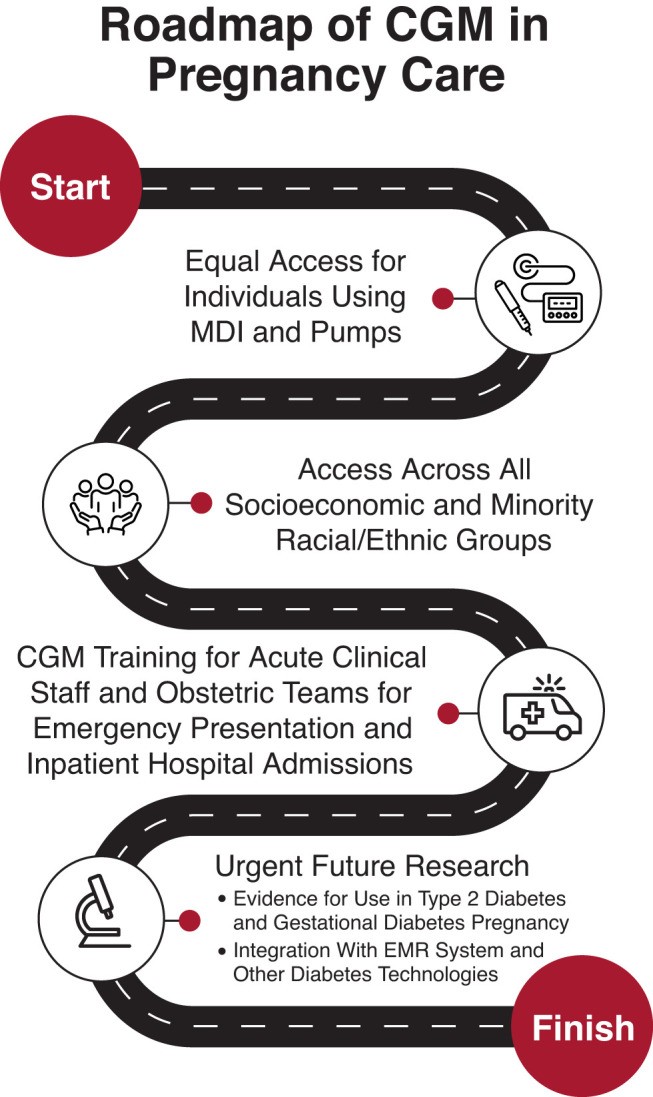
A roadmap to the effective use of CGM in pregnancy. EMR, electronic medical record; MDI, multiple daily injection insulin therapy.
The landscape for managing type 1 diabetes outside of pregnancy has been transformed by increasing use of CGM in the past 5 years. Likewise, use of CGM in type 1 diabetes pregnancy is now widespread based on randomized controlled trial data showing that CGM use improves maternal glucose levels and reduces the frequency and duration of neonatal care unit admissions, meaning that it is both clinically and cost-effective during pregnancy in people with type 1 diabetes (1–3). Based on data from the CONCEPTT (Continuous Glucose Monitoring in Pregnant Women With Type 1 Diabetes) trial (1) and changes in remote care during the coronavirus disease 2019 pandemic (4), CGM use is widely established as a standard of care in type 1 diabetes pregnancy. Listening to women’s voices has become increasingly pertinent, in society at large and especially in maternity health care settings (5).
CGM gives women more information, empowering them to make informed diabetes treatment decisions. Unlike laboratory A1C measurements, which assess average glucose over the preceding 8–12 weeks and are announced to patients by clinicians, patients hold their own daily CGM glucose information (e.g., mean glucose and time spent in, above, and below the target glycemic range) on their smartphones. Patients find CGM time in range (TIR) information engaging because it provides immediate feedback on changes they make to their dietary intake, physical activity, and diabetes treatment. The patient-centeredness of CGM TIR data are strongly endorsed by people with diabetes, who ranked TIR as the factor that, only after their food choices, has the biggest impact on their daily lives (6). This sentiment was summarized by a pregnant study participant with type 1 diabetes, who said, “I really feel it’s a game-changer in helping me understand where I am. It determines if I’m on track, and, when I’m not, I know things need to be done” (7).
Pregnant women are uniquely motivated to achieve tight glucose targets for their babies to have the best possible health outcomes. They are aiming for glucose levels between 63 and 140 mg/dL for at least 16 hours, 48 minutes, per day or 70% time in pregnancy range (TIRp) throughout the type 1 diabetes pregnancy (8). The challenge of achieving and maintaining 70% TIRp in the face of early pregnancy nausea, changing eating patterns, and gestational changes in insulin sensitivity should not be underestimated. Achieving 70% TIRp is broadly similar to achieving 90% standard TIR (70–180 mg/dL) outside of pregnancy. Furthermore, pregnant women have to balance the consequences of above-target glucose increasing their babies’ risk of preterm birth, large birth weight, and neonatal hypoglycemia with their own immediate risk of hypoglycemic events (9). Pregnant CGM users often share their glucose alerts (particularly low glucose alerts) with a partner or CGM follower. Participants in type 1 diabetes pregnancy trials reiterate that being able to share CGM alerts when glucose levels are dangerously low provides crucial reassurance, especially during early pregnancy, when the risk of severe hypoglycemia is particularly pertinent. As one said, “I had an overwhelming fear that I would go to bed and not wake up, so having someone like [partner] check in was so important. I always had a follower, so that I didn’t die” (7).
Studies of type 1 diabetes pregnancy have demonstrated that very small changes in maternal glucose levels are associated with large effects on neonatal health outcomes (10). Knowing that every extra 5% TIRp has benefits for reducing their babies’ risks of complications is crucially important information for pregnant women who are struggling to achieve the recommended 70% TIRp (11).
Although the target of 70% TIRp was based on consensus opinion, there are increasing data relating CGM TIRp metrics to A1C and clinical outcomes for mothers and babies in type 1 diabetes pregnancy (1,12). We used data from the CONCEPTT trial to compare how useful A1C and key CGM metrics (TIRp and time above range for pregnancy [TARp]) were at 12–13, 24–25, and 34–35 weeks’ gestation for predicting common complications of type 1 diabetes pregnancy (i.e., preeclampsia, preterm delivery, large for gestational age [LGA], neonatal hypoglycemia, and admission to the neonatal intensive care unit). Even though CGM metrics were only available for 1 week, they were still important predictors of obstetric and neonatal complications (13).
Most CGM metrics (e.g., mean sensor glucose, TIRp, and TARp) are closely correlated with A1C. In clinical practice, just a few key CGM metrics (i.e., mean sensor glucose, TIRp, TARp, and time below range for pregnancy [TBRp]) are used routinely to assess maternal glycemia and guide treatment decisions. Although its associations with pregnancy outcomes are unclear, maintaining a TBRp (time <63 mg/dL) ≤4% (1 hour/day) is crucially important for maternal safety. This is even lower than the standard TBR (time <70 mg/dL) outside of pregnancy. Modern glucose sensors may allow women to achieve lower TBRp targets.
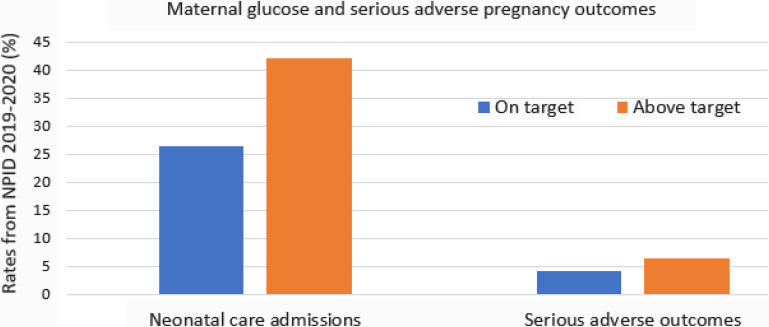
Ongoing monitoring of maternal A1C (at pregnancy confirmation and at approximately 20, 28, and 34–36 weeks’ gestation) remains useful because of the established association of A1C with pregnancy outcomes and discrepancies between A1C and the glucose management indicator (a CGM-derived estimate of A1C). Based on data from the National Pregnancy in Diabetes (NPID) audit (Figure 2), an urgent action plan and multidisciplinary team review advising CGM use is implemented for individuals with an A1C >6.5% after 20–24 weeks’ gestation. Those with an A1C between 6.0 and 6.5% are reviewed for intensification of glycemic management and CGM use (if not already initiated), and those with an A1C <6.0% are supported to continue their current management plan (9).
Figure 2.
Maternal glucose and serious adverse pregnancy outcomes in individuals with early-onset type 2 diabetes.
Underserved Population: Individuals With Early-Onset Type 2 Diabetes
There are stark differences in the characteristics of pregnant women in the United Kingdom. Those with type 1 diabetes are predominantly of White European race/ethnicity, have lower BMIs, and are less socioeconomically disadvantaged, whereas those with early-onset type 2 diabetes (defined as onset at <39 years of age) are from more diverse racial/ethnic backgrounds, have higher BMIs, and live in more socioeconomically deprived communities. Therefore, we cannot necessarily extrapolate the benefits of CGM use in type 1 diabetes pregnancy to type 2 diabetes pregnancy (9). Furthermore, women with type 1 diabetes receive extensive diabetes support and specialist multidisciplinary team care from the time of diagnosis. By contrast, those with early-onset type 2 diabetes are usually managed in primary care settings and get very little specialist diabetes support. The U.K. NPID audit data showed that only 18% of pregnant women with early-onset type 2 diabetes were treated with insulin and routinely monitored their capillary glucose levels before pregnancy (9). As one such patient noted, “I was told to take pills or lose weight. I know one or two [high glucose levels] might not have been the end of the world, but people really don’t see how important it is to get it right for the safety of the babies and indeed the mother” (personal communication). This disparity in care in part stems from the fact that health care services for individuals with type 2 diabetes traditionally have been targeted to older age-groups and also from the considerable stigma and negative emotions associated with maternal overweight, obesity, and early-onset type 2 diabetes; fewer than 5% of research participants with type 2 diabetes are 18–39 years of age, with women who are pregnant or planning pregnancy often excluded from type 2 diabetes trials (14). Furthermore, anxiety and depression are particularly common in this patient population, with a recent Danish study suggesting that 36% experienced anxiety and 14% had depressive symptoms during pregnancy (15).
Data regarding CGM use in type 2 diabetes pregnancy are extremely limited (16). A 2019 systematic review (17) found only three trials that included small numbers of pregnant women with type 2 diabetes (n = 25 from the United Kingdom, n = 31 from Denmark, and n = 82 from the Netherlands). All three of these studies were focused primarily on pregnant women with type 1 diabetes and used older-generation, less user-friendly CGM systems intermittently rather than continuously (18–20).
Recent data from the U.K. NPID audit suggest that, for optimal obstetric and neonatal outcomes, pregnant women with type 2 diabetes may need even tighter pregnancy glucose targets than pregnant women with type 1 diabetes (9). However, there are no consensus or evidence-based CGM targets to guide glycemic management in type 2 diabetes pregnancy. This information is urgently needed, as pregnancies in women with early-onset type 2 diabetes are rapidly increasing. In the United Kingdom, pregnancies in those with early-onset type 2 diabetes have doubled in the past 2 decades (9).
In the United States, the TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) study highlighted alarming pregnancy outcomes for mothers and babies in youth-onset type 2 diabetes pregnancy (21). Only 15% of young sexually active women and girls used any form of contraception, so most of these pregnancies were unplanned. This patient group entered pregnancy with above-target glycemia (mean A1C 8.7%) and consequently had high rates of major congenital anomaly (10%). Congenital anomaly rates were <5% in those with an early pregnancy A1C of <8% and almost 20% in those who entered pregnancy with an A1C >8%, suggesting that many congenital anomalies could have been prevented by supporting women and girls to use contraception until safe A1C levels are achieved. Individuals in this group also experienced high rates of pregnancy loss and pregnancy complications, including hypertension and preeclampsia. Only 43% of pregnancies resulted in a live term birth, with approximately one-fourth of babies delivered before 37 weeks. Similar to the TODAY participants, individuals with early-onset type 2 diabetes who took part in our research said they didn’t just want to hear about all the pregnancy risks; they also wanted, as one put it, “more focus on the positivity of managing glucose levels and the results for my pregnancy/birth, for example, being able to deliver naturally—proper support to manage diabetes without compromising my mental health and unborn baby” (H.R.M., personal communication with focus group participant). Research regarding the role of CGM use, overcoming barriers, and optimal TIRp targets for pregnant individuals with early-onset type 2 diabetes is urgently needed.
Role of CGM in Gestational Diabetes
Glucose levels are dynamic, with glucose tolerance and insulin sensitivity varying across the 24-hour day with a circadian rhythm. Insulin sensitivity also varies across pregnancy, with insulin resistance increasing with advancing gestation. Because the oral glucose tolerance test (OGTT), which is the traditional screening method for detecting gestational diabetes mellitus (GDM), relies on just two glucose readings taken 2 hours apart on 1 day, it cannot detect all of the nuances of daily glycemic variations or changes across pregnancy.
CGM provides the most objective method of assessing fetal exposure to maternal glucose in daily life. Although there have been small, short-term studies of glucose metabolism in healthy pregnant women and those with risk factors for hyperglycemia, comprehensive, longitudinal description of gestational changes in CGM profiles in both healthy and GDM-complicated pregnancy is lacking (22,23). It is also unknown how CGM metrics relate to traditional screening for GDM by OGTT and whether CGM metrics (e.g., mean sensor glucose, TIRp, and TARp) are correlated with fetal growth parameters and neonatal health outcomes in GDM pregnancy. CGM could potentially also be used to detect glucose dysregulation earlier in pregnancy, allowing earlier initiation of dietary changes and pharmacotherapy; however, data from adequately powered, high-quality randomized trials examining the use of CGM as a diagnostic or therapeutic tool in GDM pregnancy are lacking. A small study comparing CGM compared with capillary glucose monitoring found no differences, but this study was not powered to detect differences in TIRp or pregnancy outcomes (24).
The optimal timing for diagnosing GDM (in the first trimester vs. the traditional 24–28 weeks’ gestation) is also unclear. The TOBOGM (Treatment of Gestational Diabetes Mellitus Diagnosed Early in Pregnancy) trial detected minimal differences in maternal and neonatal outcomes between those with GDM detected during a first trimester and those with GDM diagnosed with the traditional OGTT at 24–28 weeks (25). However, we have previously shown that excess fetal growth assessed by ultrasound scan is detectable from 20 weeks’ gestation, pre-dating biochemical diagnosis of GDM (26). We also know that performing a conventional OGTT at 24–28 weeks’ gestation is often too late to prevent abnormal fetal growth, particularly in individuals with a higher BMI, but there are no validated screening and/or diagnostic criteria for earlier diagnosis of GDM.
Indeed, despite the use of the OGTT, the majority of LGA babies are born to mothers without a GDM diagnosis. This fact suggests that pregnant women who could benefit from earlier diagnosis and earlier treatment of GDM potentially are not being identified correctly. The OGTT is an outdated test for diagnosis of type 2 diabetes and is no longer widely used outside of pregnancy. It is poorly reproducible during pregnancy; 40% of pregnant women who had a second OGTT immediately after an abnormal OGTT had normal results (27). OGTT reproducibility was pertinent in the TOBOGM trial, with discrepancies between early and late OGTTs among one-third of TOBOGM trial participants (25). Among the milder glycemic cohort, discrepancies between the early and late OGTTs were noted in 50% of TOBOGM participants (25). Whether this finding relates to gestational variations in maternal glycemia or to poor reproducibility of OGTT results remains unclear.
Gaining a better understanding of glycemic changes by using CGM throughout pregnancy is urgently needed to inform both the diagnostic criteria for and management of GDM. Data from the United Kingdom suggest that women from higher-risk racial/ethnic groups, with higher BMIs, and from more resource-challenged communities are least likely to attend visits for an OGTT. Thus, broadening inclusion in future research is imperative (28).
Directions for Future Research
In clinical practice, women with type 1 diabetes are increasingly entering pregnancy using a range of commercially available hybrid closed-loop automated insulin delivery systems, so more information regarding the safety and efficacy of these systems throughout pregnancy is needed. Future research should also evaluate whether the use of new technology is associated with more positive pregnancy experiences. It is imperative that patients, clinicians, and researchers acknowledge the crucial role of maternal glucose management in reducing adverse pregnancy outcomes in individuals with early-onset type 2 diabetes as well as type 1 diabetes. A better understanding of CGM-documented glycemic changes throughout pregnancy is needed to inform future GDM and type 2 diabetes pregnancy management. Prioritizing these patient groups is essential to address health care inequalities in research and access to technology for pregnant women with diabetes.
Article Information
Duality of Interest
H.R.M. serves on U.K. and European Medtronic scientific advisory boards; has received research support (devices at reduced cost) from Abbott Diabetes Care, Dexcom, Johnson & Johnson, and Medtronic; and has received speaking honoraria from Dexcom, Medtronic, Novo Nordisk, Roche, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contribution
As the sole author of this article, H.R.M. researched the data and wrote and revised the manuscript and is the guarantor of this work.
References
- 1. Feig DS, Donovan LE, Corcoy R, et al.; CONCEPTT Collaborative Group . Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 2017;390:2347–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmed RJ, Gafni A, Hutton EK, et al.; CONCEPTT Collaborative Group . The cost implications of continuous glucose monitoring in pregnant women with type 1 diabetes in 3 Canadian provinces: a posthoc cost analysis of the CONCEPTT trial. CMAJ Open 2021;9:E627–E634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murphy HR, Feig DS, Sanchez JJ, de Portu S, Sale A; CONCEPTT Collaborative Group . Modelling potential cost savings from use of real-time continuous glucose monitoring in pregnant women with type 1 diabetes. Diabet Med 2019;36:1652–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murphy HR. Managing diabetes in pregnancy before, during, and after COVID-19. Diabetes Technol Ther 2020;22:454–461 [DOI] [PubMed] [Google Scholar]
- 5. Sibley M. Ockenden report: the refusal of our healthcare service to take patient experience seriously. BMJ 2022;377:o875. [DOI] [PubMed] [Google Scholar]
- 6. Runge AS, Kennedy L, Brown AS, et al. Does time-in-range matter? Perspectives from people with diabetes on the success of current therapies and the drivers of improved outcomes. Clin Diabetes 2018;36:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee TTM, Collett C, Man MS, et al.; AiDAPT Collaborative Group . AiDAPT: automated insulin delivery amongst pregnant women with type 1 diabetes: a multicentre randomized controlled trial - study protocol. BMC Pregnancy Childbirth 2022;22:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murphy HR, Howgate C, O’Keefe J, et al.; National Pregnancy in Diabetes (NPID) Advisory Group . Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: a 5-year national population-based cohort study. Lancet Diabetes Endocrinol 2021;9:153–164 [DOI] [PubMed] [Google Scholar]
- 10. Scott EM, Murphy HR, Kristensen KH, et al. Continuous glucose monitoring metrics and birth weight: informing management of type 1 diabetes throughout pregnancy. Diabetes Care 2022;45:1724–1734 [DOI] [PubMed] [Google Scholar]
- 11. Murphy HR. Continuous glucose monitoring targets in type 1 diabetes pregnancy: every 5% time in range matters. Diabetologia 2019;62:1123–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kristensen K, Ögge LE, Sengpiel V, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia 2019;62:1143–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meek CL, Tundidor D, Feig DS, et al.; CONCEPTT Collaborative Group . Novel biochemical markers of glycemia to predict pregnancy outcomes in women with type 1 diabetes. Diabetes Care 2021;44:681–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sargeant JA, Brady EM, Zaccardi F, et al. Adults with early-onset type 2 diabetes (aged 18–39 years) are severely underrepresented in diabetes clinical research trials. Diabetologia 2020;63:1516–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ásbjörnsdóttir B, Vestgaard M, Do NC, et al. Prevalence of anxiety and depression symptoms in pregnant women with type 2 diabetes and the impact on glycaemic control. Diabet Med 2021;38:e14506. [DOI] [PubMed] [Google Scholar]
- 16. Murphy HR, Rayman G, Duffield K, et al. Changes in the glycemic profiles of women with type 1 and type 2 diabetes during pregnancy. Diabetes Care 2007;30:2785–2791 [DOI] [PubMed] [Google Scholar]
- 17. Jones LV, Ray A, Moy FM, Buckley BS. Techniques of monitoring blood glucose during pregnancy for women with pre-existing diabetes. Cochrane Database Syst Rev 2019;5:CD009613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Secher AL, Ringholm L, Andersen HU, Damm P, Mathiesen ER. The effect of real-time continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care 2013;36:1877–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Voormolen DN, DeVries JH, Sanson RME, et al. Continuous glucose monitoring during diabetic pregnancy (GlucoMOMS): a multicentre randomized controlled trial. Diabetes Obes Metab 2018;20:1894–1902 [DOI] [PubMed] [Google Scholar]
- 20. Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ 2008;337:a1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murphy HR, Moses RG. Pregnancy outcomes of young women with type 2 diabetes: poor care and inadequate attention to glycemia. Diabetes Care 2022;45:1046–1048 [DOI] [PubMed] [Google Scholar]
- 22. Harmon KA, Gerard L, Jensen DR, et al. Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: metabolic determinants of fetal growth. Diabetes Care 2011;34:2198–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hernandez TL, Friedman JE, Van Pelt RE, Barbour LA. Patterns of glycemia in normal pregnancy: should the current therapeutic targets be challenged? Diabetes Care 2011;34:1660–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lai M, Weng J, Yang J, et al. Effect of continuous glucose monitoring compared with self-monitoring of blood glucose in gestational diabetes patients with HbA1c<6%: a randomized controlled trial. Front Endocrinol (Lausanne) 2023;14:1174239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simmons D, Immanuel J, Hague WM, et al.; TOBOGM Research Group . Treatment of gestational diabetes mellitus diagnosed early in pregnancy. N Engl J Med 2023;388:2132–2144 [DOI] [PubMed] [Google Scholar]
- 26. Sovio U, Murphy HR, Smith GC. Accelerated fetal growth prior to diagnosis of gestational diabetes mellitus: a prospective cohort study of nulliparous women. Diabetes Care 2016;39:982–987 [DOI] [PubMed] [Google Scholar]
- 27. Neiger R, Coustan DR. The role of repeat glucose tolerance tests in the diagnosis of gestational diabetes. Am J Obstet Gynecol 1991;165:787–790 [DOI] [PubMed] [Google Scholar]
- 28. Meek CL, Lindsay RS, Scott EM, et al. Approaches to screening for hyperglycaemia in pregnant women during and after the COVID-19 pandemic. Diabet Med 2021;38:e14380. [DOI] [PMC free article] [PubMed] [Google Scholar]