Abstract
Herbivore feeding elicits defense responses in infested plants, including the emission of volatile organic compounds that can serve as indirect defense signals. Until now, the contribution of plant tissue wounding during the feeding process in the elicitation of defense responses has not been clear. For example, in lima bean (Phaseolus lunatus), the composition of the volatiles induced by both the insect caterpillar Spodoptera littoralis and the snail Cepaea hortensis is very similar. Thus, a mechanical caterpillar, MecWorm, has been designed and used in this study, which very closely resembles the herbivore-caused tissue damage in terms of similar physical appearance and long-lasting wounding period on defined leaf areas. This mode of treatment was sufficient to induce the emission of a volatile organic compound blend qualitatively similar to that as known from real herbivore feeding, although there were significant quantitative differences for a number of compounds. Moreover, both the duration and the area that has been mechanically damaged contribute to the induction of the whole volatile response. Based on those two parameters, time and area, which can replace each other to some extent, a damage level can be defined. That damage level exhibits a close linear relationship with the accumulation of fatty acid-derived volatiles and monoterpenes, while other terpenoid volatiles and methyl salicylate respond in a nonlinear manner. The results strongly suggest that the impact of mechanical wounding on the induction of defense responses during herbivore feeding was until now underestimated. Controlled and reproducible mechanical damage that strongly resembles the insect's feeding process represents a valuable tool for analyzing the role of the various signals involved in the induction of plant defense reactions against herbivory.
Herbivorous arthropods, mainly insect larvae, represent a major challenge for plants in their natural environment. For defense against herbivorous insects, plants are endowed with both constitutive and inducible mechanisms. Constitutive defense mechanisms are represented by physical barriers, such as cuticles, thorns, and trichomes, or preexisting secondary metabolites that are harmful or even toxic to the insect. Inducible defense involves biosynthetic processes and/or the up-regulation of gene expression. Moreover, defense mechanisms can be divided into direct and indirect responses (for review, see Gatehouse, 2002; Kessler and Baldwin, 2002; Van Poecke and Dicke, 2004). A direct defense element, such as a proteinase inhibitor, affects by itself the feeding insect. In contrast, an indirect defense mechanism eventually results in attraction of predators and parasitoids of the particular herbivores feeding on the infested plant (Kessler and Baldwin, 2002). An intensively studied example of an inducible, indirect defense response is the synthesis and emission of volatile organic compounds (VOCs), which are employed in the attraction of carnivorous insects searching for their prey (Walling, 2000; Gatehouse, 2002; Kessler and Baldwin, 2002). The insect feeding-induced emission of VOCs has been demonstrated for several plant species (for overview, see Van Poecke and Dicke, 2004), including maize (Zea mays; Turlings et al., 1990), cotton (Gossypium hirsutum; Röse et al., 1996), lima bean (Phaseolus lunatus; Dicke et al., 1990; Ozawa et al., 2000), and tobacco (Nicotiana attenuata; Kessler and Baldwin, 2001).
Recently, from oral secretions of feeding insects, herbivore-specific compounds have been isolated with elicitor capabilities, e.g. certain enzymes (Glc oxidase, Felton and Eichenseer, 1999; β-glucosidase, Mattiacci et al., 1995; and alkaline phosphatase, Funk, 2001) or fatty acid-amino acid conjugates such as volicitin (Alborn et al., 1997). Some of these elicitors exhibited high VOC-inducing activities when added to certain mechanically wounded plants (Mattiacci et al., 1995; Alborn et al., 1997; Landolt et al., 1999; Halitschke et al., 2001; Schmelz et al., 2001, 2003). The general structures of the fatty acid-amino acid conjugate-signaling compounds have been determined as N-acyl-Glns, where the fatty acid moiety is represented mainly by linolenic acid (C18:3), linoleic acid (18:2), and their derivatives (Alborn et al., 1997; Pohnert et al., 1999; Spiteller and Boland, 2003). However, these insect-derived elicitors are not generally active, as in lima bean and cotton, and no induction of VOCs could be demonstrated (Spiteller et al., 2001). Nevertheless, a detergent-like effect of exogenously added N-acyl-Glns on the cytosolic calcium signature in soybean cell cultures could be shown (Maffei et al., 2004). Thus, the relative contribution of both the insect elicitors and the feeding event to the induction of the VOC emission response is not that clear.
Almost all herbivores, in particular chewing insects, cause substantial injury to the site of their attack. Such mechanical wounding of plant tissues is an inevitable consequence of herbivory, although the intensity and extent of damage is different and may vary with the mode of feeding, e.g. sucking or chewing. Of course, in all studies analyzing the effects of insect feeding or insect elicitors on volatile responses, control experiments with wounded plants have been performed using either a razor blade for scratching the leaf (Turlings et al., 1990; Schmelz et al., 2001; Spiteller et al., 2001), a forceps for crushing the leaf (Reymond et al., 2000), or a pattern wheel for puncturing the leaf (Halitschke et al., 2001). However, it is questionable whether or not these treatments indeed resemble insect feeding for at least two reasons: (1) the extent of damaged tissue and (2) the different time of treatment. The plant might recognize and discriminate a continuously sustained damage by feeding insects from mechanical wounding that was set only once.
To address this question, we first compared the blend of VOCs emitted from lima bean leaves due to treatments by unrelated herbivores, caterpillars and snails, which feed on the plant in completely different ways. Moreover, we introduce a mechanical caterpillar (MecWorm) that was engineered to execute permanent damage over a long time period, mimicking real insect feeding. Using this mechanical caterpillar, we have been able to analyze the impact of various parameters, such as time or size of wounding, with respect to the induction of VOCs.
RESULTS
Induction of Volatiles by Snails and Insect Larvae
Volatile synthesis and emission has been investigated mainly in response to feeding insects or spider mites (Walling, 2000). To analyze whether VOC formation in plants represents either a general reaction upon herbivore feeding or a unique reaction arising only upon insect infestation, we compared the VOC blend of lima bean leaves challenged by different herbivores. On the one hand, chewing Spodoptera littoralis larvae have been used; on the other hand, Cepaea hortensis snails have been used, rasping the plant tissue with their radula. As shown in Figure 1, in both cases VOC emission was induced and, moreover, the qualitative composition of the volatiles was nearly identical to each other and to that observed after spider mite damage (Dicke et al., 1990). Only indole was not detected in the headspace of C. hortensis-treated lima bean leaves. Although relative amounts of the particular compounds varied to some extent between various experiments, the similarity of the volatile blends in terms of quality suggested induction by similar underlying mechanisms. On a quantitative basis, only the fatty acid derivative octen-3ol, the monoterpene C10H14, and the sesquiterpenes β-caryophyllene and nerolidol were observed in significantly lower amounts upon snail treatment than upon S. littoralis feeding (Table I).
Figure 1.
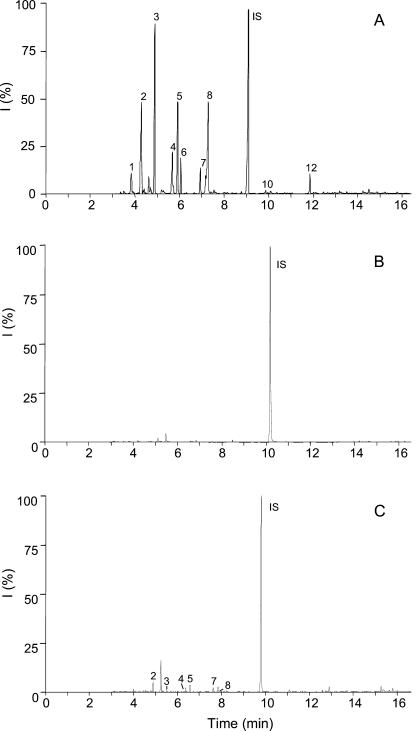
GC profiles of induced VOCs emitted from lima bean leaves upon feeding of different herbivores. Headspace volatiles were collected for 24 h during the whole treatment. A, Feeding by S. littoralis larvae. B, Control without any feeding. C, Feeding by C. hortensis. Identification of compounds was performed by MS: (1) octen-3-ol; (2) Hex-Ac; (3) β-ocimene; (4) linalool; (5) DMNT; (6) C10H14; (7) MeSA; (8) C10H16O; (9) indole; (10) β-caryophyllene; (11) nerolidol; (12) TMTT. IS, Internal standard (1-bromodecane, 0.9 mm). All experiments have been conducted at least six times with similar results.
Table I.
VOCs emitted from lima bean leaves upon feeding by S. littoralis, C. hortensis, and the mechanical larva MecWorm
The relative amount of the volatiles was determined by the ratio of the peak area of the particular compound (AVOC) to the peak area of the internal standard (AIS). Medians over 6 to 10 replications with range of values (minimum to maximum) shown in parentheses are given; treatments and number of compounds according to Figures 1, A and C, and 3A.
| Compound (No.)
|
S. littoralis
|
C. hortensis
|
MecWorm
|
|---|---|---|---|
| Relative Amount of VOCs (AVOC/AIS) | |||
| Octen-3-ol (1) | 0.09 (0.06–0.19)a | 0.03 (0.01–0.05)b | 0.37 (0.17–0.69)c |
| Hex-Ac (2) | 0.47 (0.14–1.37) | 0.16 (0.07–0.41)b | 0.43 (0.34–0.59) |
| β-Ocimene (3) | 0.65 (0.16–1.42) | 0.49 (0.01–0.67) | 0.28 (0.09–1.21) |
| Linalool (4) | 0.37 (0.19–0.63) | 0.37 (0.10–0.78) | 0.19 (0.11–0.47)c |
| DMNT(5) | 0.51 (0.21–1.29) | 0.36 (0.14–0.88) | 0.31 (0.07–0.47)c |
| C10H14 (6) | 0.27 (0.08–0.48)a | 0.05 (0.01–0.07) | 0.08 (0.03–0.26) |
| MeSA (7) | 0.01 (0.01–0.03) | 0.13 (0.07–0.59) | 0.03 (0.01–0.09) |
| C10H16O (8) | 0.89 (0.28–1.92) | 0.26 (0.05–0.32) | 0.27 (0.07–0.88)c |
| Indole (9) | 0.00 (n.d.–0.28) | n.d.d | n.d.d |
| β-Caryophyllene (10) | 0.07 (0.03–0.17)a | tre | 0.02 (n.d.–0.06)c |
| Nerolidol (11) | 0.03 (0.01–0.07)a | tre | n.d.d |
| TMTT (12) | 0.12 (0.05–0.21) | 0.26 (0.02–0.67) | 0.03 (0.01–0.07)c |
The Mann-Whitney U test was used to determine the significance of differences in VOC amounts between S. littoralis and S. hortensis. Statistical evaluations were done with SPSS (version 11.5).
The Mann-Whitney U test was used to determine the significance of differences in VOC amounts between S. hortensis and MecWorm. Statistical evaluations were done with SPSS (version 11.5).
The Mann-Whitney U test was used to determine the significance of differences in VOC amounts between S. littoralis and MecWorm. These comparisons yielded a P value < 0.05 and were considered to be statistically significant.
n.d., Compound not detectable.
tr, Traces (AVOC/AIS ≤ 0.005).
Induction of Volatiles by Continuous Mechanical Wounding
The finding that feeding of spider mites (Dicke et al., 1990), S. littoralis larvae, and snails induced similar patterns of VOCs in lima bean leaves tempted us to speculate about the common ground in the entirely different feeding processes of the three herbivores. To investigate the impact of leaf tissue damage, we developed and used the computer-controlled unit MecWorm (Fig. 2) that can mimic the mechanical treatment of herbivores in terms of long-lasting and continuous wounding. A damage program was applied that punched the leaf every 5 s over a period of about 17 h, resulting in a damaged area of 733 mm2, which corresponded to about 20% to 25% of the total leaf surface. The headspace of this plant contained nearly all VOCs that could be detected in the headspace of herbivore-challenged lima bean leaves, except for the minor compounds indole and nerolidol (Fig. 3A; Table I). However, there were some significant quantitative differences among the treatments. Continuous mechanical damage gave less linalool, 4,8-dimethylnona-1,3,7-triene (DMNT), 4,8,12-trimethyltrideca-1,3,7,11-tetrane (TMTT), and more octen-3-ol than S. littoralis feeding. On the other hand, mechanical damage gave significantly more (Z)-3-hexenyl acetate (Hex-Ac) and octen-3-ol than C. hortensis feeding. Mechanical wounding using a pattern wheel did not induce any substantial volatile emission (Fig. 3B), confirming the results obtained with razor blade wounding (Spiteller et al., 2001).
Figure 2.
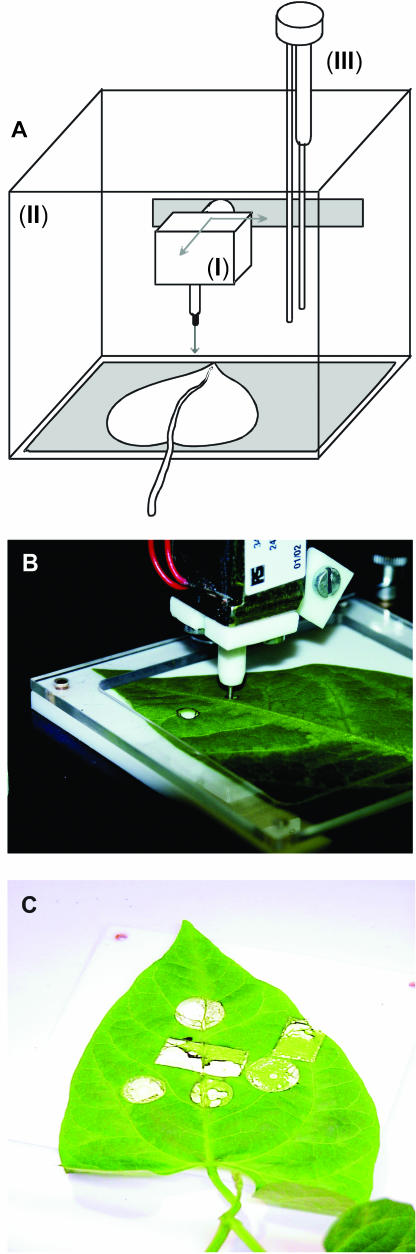
MecWorm, the mechanical caterpillar. A, Design of MecWorm. I, Punching unit; II, plexiglass cabinet; III, volatile collection unit. B, Section of the punching unit of MecWorm, including the aglet treating a lima bean leaf. C, Lima bean leaf damaged by MecWorm.
Figure 3.
GC profile of induced VOCs emitted from lima bean leaves upon mechanical damage. A, Treatment with MecWorm. Headspace volatiles were collected for 24 h during the whole treatment (time = 17 h, 7 min; area = 733 mm2). B, Treatment with a pattern wheel. C, Collection of volatiles emitted from the nontreated opposite leaf. Identification of compounds was performed by MS (see Fig. 1). Experiments have been repeated nine (A), four (B), and eight times, respectively, with similar results.
Herbivory induces a systemic volatile response in the infested plants (Turlings and Tumlinson, 1992; Dicke et al., 1993; Röse et al., 1996). To investigate whether or not the mechanical wounding also induced a systemic induction of VOCs, both the treated and the nontreated lima bean leaves were analyzed separately. Strikingly, Hex-Ac, three monoterpenes, DMNT, and the phenolic compound methyl salicylate (MeSA) could be detected in the headspace of the opposite nonwounded leaf, although in low amounts (Fig. 3C). In these experiments, the extent of punching of the corresponding damaged leaf has been carried out to ensure a full VOC response, as shown in Figure 2 (time = 17 h, 7 min; area = 733 mm2). This result strongly indicates that continuous wounding is indeed already sufficient to induce a systemic volatile response in the plant.
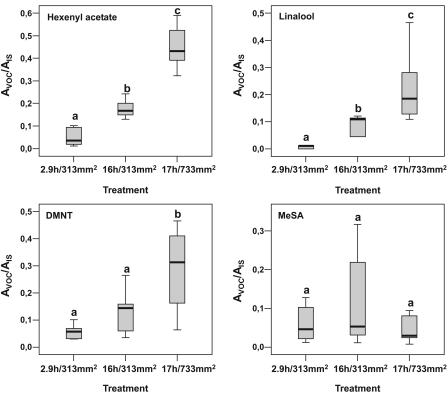
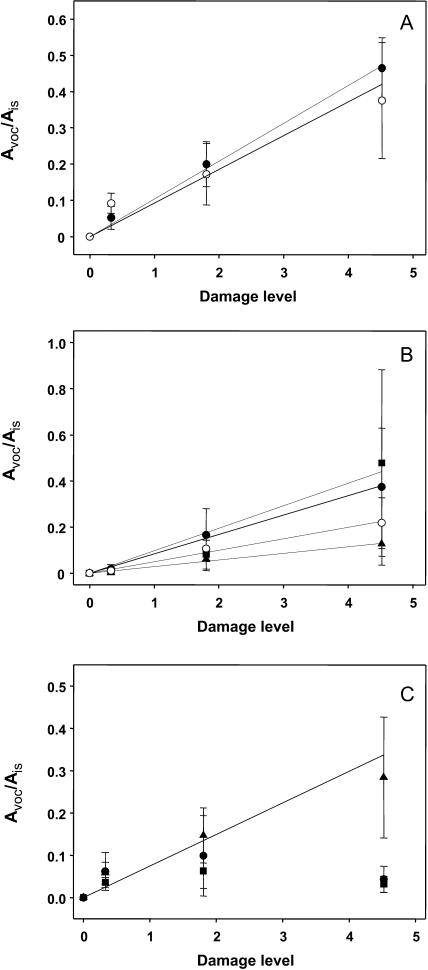
A shorter time of treatment leading to a smaller damage area resulted in less VOC formation. This observation raised the question as to what is the most important parameter responsible for the volatile induction: the duration of treatment, the size of the treated area, or both? To address this issue, the accumulation of certain VOCs representing typical compounds of different biosynthetic pathways have been analyzed under various conditions in more detail: the fatty acid derivative Hex-Ac, the phenolic compound MeSA, the monoterpene linalool, and the homoterpene DMNT. A comparison of the relative amounts of these volatiles emitted upon mechanical damage of a defined damage area (313 mm2) in either 2.9 or 16 h (representing a punching event taking place every 2 and 11 s, respectively) showed a significant increase in the concentration of Hex-Ac and linalool associated with the longer treatment, but not for DMNT and MeSA (Fig. 4). Keeping the time of treatment nearly constant and varying the damage area (16 h, 313 mm2 and 17 h, 733 mm2) this approach again resulted in a significant increase of emitted Hex-Ac and linalool, as well as DMNT, due to the larger area that was treated. However, the amount of MeSA remained largely unaffected (Fig. 4). These results suggest an impact of both the time and the area wounded. Thus, the integration of these two parameters based on multiplying time by area was defined as damage level. For quantitative considerations, the relative amount of VOCs was determined by calculating the ratio of the peak area of the particular compound to the peak area of the fixed amount of the internal standard. This calculation has been performed individually for all main compounds found in the headspace of stimulated lima bean leaves. To investigate whether the length of time and the treated area might replace each other in their contribution to a particular damage level, an additional treatment (733 mm2, 6.8 h) that had a damage level identical to the one obtained by treatment of 313 mm2 for 16 h was included in further analyses. As shown in Figure 5, there was a linear relationship between the damage level and amount of the two fatty acid-derived compounds octen-3-ol and Hex-Ac, the monoterpenes ocimene (C10H16O), and linalool (C10H14), and the sesquiterpene DMNT, respectively. In contrast, for the accumulation of MeSA and the homoterpene TMTT, no obvious correlation was found (Fig. 5).
Figure 4.
Relative amounts of various emitted VOCs in relation to time and extent of mechanical treatments in lima bean leaves. Volatiles have been induced by either mechanical damage of a fixed area at various or similar lengths of time combined with various areas. The relative increase or decrease in the amount of volatiles was determined by the ratio of the peak area of the particular compound (AVOC) to the peak area of the internal standard (AIS). Statistical evaluations were done with SPSS (version 11.5). Different letters indicate significant differences between treatments (P < 0.05) according to one-way ANOVA on rank-transformed data. The Student-Newman-Keuls test was used to determine post hoc differences. Sample sizes are n = 5 (2.9 h, 313 mm2), n = 6 (16 h, 313 mm2), and n = 10 (17 h, 733 mm2).
Figure 5.
Correlation of the relative amounts of volatiles with the damage levels of mechanical wounding in lima bean leaves. The relative amount of the volatiles was determined by the ratio of the peak area of the particular compound (AVOC) to the peak area of the internal standard (AIS). The damage levels have been determined with 0 (no treatment), 0.33 (2.9 h, 313 mm2), 1.80 (6.85 h, 733 mm2; 16 h, 313 mm2), and 4.52 (17 h, 733 mm2), respectively. A, Octen-3-ol (○) and Hex-Ac (•). B, Ocimene (▪), C10H16O (•), linalool (○), and C10H14 (▴). C, DMNT (▴), MeSA (•), and TMTT (▪). MeSA and TMTT did not show linear correlations. The results are the mean ± sd (n = 5–10).
Comparison of Herbivore and Mechanical Leaf Damage
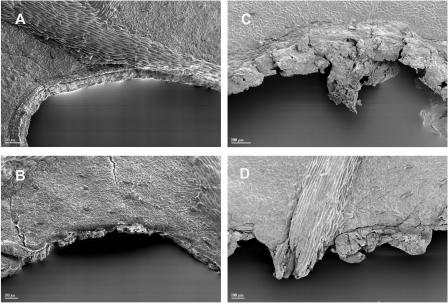
To visually compare the mode and extent of leaf wounding caused by S. littoralis caterpillars and the mechanical device, respectively, scanning electron microscopy analysis was performed directly at the site of feeding and punching. As shown in Figure 6, the feeding zone of S. littoralis larvae is characterized by a straight border that was formed by the biting activities of the insect (Fig. 6, A and B). The mechanical wounding generated to some extent a small, frayed zone at the borderline (Fig. 6, C and D). This latter zone was formed due to the conical shape of the aglet that crushed the leaf tissue at its very outer edge. In all cases, about 200 μm behind the border, the leaf tissue cracked open, showing flaws parallel to the zone of treatment.
Figure 6.
Scanning electron micrographs of lima bean leaf damage zones after feeding of S. littoralis larvae (A and B) and after treatment with the mechanical caterpillar (C and D).
DISCUSSION
Induction of defense is accompanied by additional metabolic and fitness costs (for review, see Heil and Baldwin, 2002; Kessler and Baldwin, 2002). Thus, a simple touch or a single wounding event (e.g. by falling twigs) should not initiate the whole machinery of inducible defense responses directed against herbivores. However, in most experiments designed to discriminate the response of herbivore-affected and simply injured tissues, controls have been performed only by a single wounding event. This approach does not take into account that the plant might specifically recognize and discriminate between continuous damage and a single wounding event. Moreover, to analyze the particular contribution of wounding trauma and herbivore-derived elicitors (Alborn et al., 1997; Halitschke et al., 2001) on the induction of certain defense responses, it is necessary to imitate the damage during a feeding process as accurately as possible. Surprisingly, snail-infested lima bean leaves qualitatively released a very similar bouquet of VOCs as insect-damaged leaves (Fig. 1), a result that emphasizes the putative impact of wounding. However, we cannot exclude a contribution of yet-unknown snail-derived elicitors in that process.
Based on these considerations, we developed an experimental concept that included all aspects for a highly comparable and reproducible mode to treat plant tissue with respect to naturally occurring herbivory. Consequently, a computer-controlled mechanical caterpillar was designed. The main advantages of this apparatus are its ability to carry out a damage process continuously, for a long defined time, and step by step, only on a small area that is treated by each punching event. Moreover, the time between single punches can be determined as well as the areas to be wounded. To mimic herbivory, this mode of action is clearly superior to other methods that approach the herbivore-induced wounding process, such as razor blades for scratching the leaves (maize, Turlings et al., 1990; Schmelz et al., 2001; P. lunatus, Spiteller et al., 2001), a pattern wheel for puncturing leaves (N. attenuata; Halitschke et al., 2001; Fig. 3B), or even simply cutting the leaves (Fig. 1B). In none of these cases was a volatile profile obtained that resembled herbivory. In contrast, using continuous damage via the mechanical caterpillar on lima bean leaves, we obtained virtually the same blend of volatiles as from actual herbivore damage, although there were some quantitative differences. Strikingly, emission of some VOCs that are typically induced by larval feeding was detected not only localized at the damaged leaf but also systemically upon persisting mechanical treatment. This result contradicts the common view that mechanical wounding alone is not sufficient for the induction of herbivory-like VOC blend (e.g. Alborn et al., 1997; Pare and Tumlinson, 1999; Schmelz et al., 2001).
For some plants, it has been described that different, but closely related, herbivores can induce different volatile compositions in terms of both quality and quantity and thereby attract the parasitoids selectively (De Moraes et al., 1998). In one case, even the same species, the lepidopteran herbivore Heliothis subflexa was differently vulnerable to its parasitoid when feeding on different organs of the host plant Physalis angulata. Infested leaves emitted a volatile blend containing linalool and (E)-β-ocimene, whereas infested fruits did not. This was due to the presence (leaves) or absence (fruits) of linolenic acid, a biosynthetic precursor of both the phytohormone jasmonic acid and herbivore elicitors, N-acyl Gln conjugates (De Moraes and Mescher, 2004). In contrast, Van Poecke et al. (2003) showed that Cotesia parasitoids do not discriminate between volatile blends induced by Pieris rapae, their natural host, and by the nonhost, Spodoptera exigua, when feeding on Arabidopsis (Arabidopsis thaliana) leaves. Moreover, both herbivores induced highly similar gene expression profiles in Arabidopsis, suggesting that the plant response is more general than specific (Reymond et al., 2004). Furthermore, some predators have a polyphagous prey, as in the case of the predatory mite Phytoseiulus persimilis and the herbivorous spider mite Tetranychus urticae (Helle and Sabelis, 1985). Thus, P. persimilis faces a large variability in the composition of emitted volatiles. Nevertheless, the presence of certain key compounds, such as MeSA, is sometimes sufficient to attract the predators, as shown for P. persimilis and T. urtica, which were feeding on lima bean (De Boer and Dicke, 2004). Consequently, it is tempting to speculate that the differences between MecWorm damage and that of the two herbivores may affect the attraction of carnivores or parasitoids. However, whether or not the MecWorm-induced volatile blend can attract any lima bean herbivore-related parasitoids remains to be investigated.
Another aspect of the mechanical caterpillar, and even an advantage over real herbivore feeding, is the fact that it enables us to define and control both time and extent of wounding. That means that a reference is available for the kinetics of volatile synthesis and the amount of emitted compounds. This reference can be the area of destroyed tissue or the duration of wounding or both. Thus, by integrating time and area to a defined damage level, it was obvious that the formation of the fatty acid-derived compounds, monoterpenes, and DMNT is exclusively induced by the wounding process in lima bean, because a linear correlation between the damage level and the amount of these volatiles in the headspace of the plant has been detected (Fig. 5). On the other hand, the volatile sesquiterpene TMTT, as well as MeSA, displayed a diverse accumulation pattern, suggesting different dynamics or regulation during their induction (Fig. 5C), a finding that needs further investigation.
In lima bean, the role of N-acyl-Gln conjugates seems to be different compared to maize (Alborn et al., 1997) and tobacco (Halitschke et al., 2001), as these insect-derived elicitors are unable to induce VOCs when exogenously applied to wounds (Spiteller et al., 2001). Instead, these compounds elicit ion fluxes (Maffei et al., 2004) and the up-regulation of salicylate (T. Koch and W. Boland unpublished data), which in the lima bean even acts antagonistically to the jasmonic acid-induced volatile biosynthesis (Engelberth et al., 2001). This raises the question of whether, in lima bean, other yet-unidentified compounds might have an elicitor function or whether such chemical signals are not necessary at all. It is tempting to speculate that, in N-acyl-Gln-responsive plants (i.e. volatile production), these and other signal compounds, e.g. Glc oxidase (Felton and Eichenseer, 1999) and other enzymes (Mattiacci et al., 1995; Funk, 2001), somehow accelerate responses that would develop by continuous wounding as well. It is also conceivable that N-acyl-Glns sensitize the whole system to respond to a much lower extent of wounding than required for simple mechanical damage alone. However, various mechanisms of VOC induction might exist in different plants or plant-herbivore interactions. Genuine traits and response mechanisms to identify continuous mechanical damage of high impact (feeding) should be of outstanding importance for the survival of plants. Without such mechanisms, insects could easily circumvent the plant's defense responses by simply developing feeding habits that avoid the introduction of salivary secretions into the attacked leaf by first cutting and completely removing leaf segments prior to the admixture of secretions. Single wounding of a continuous sequence (insect feeding) may lead to an accumulation of damage-related products (reactive oxygen species, oxylipins, protein phosphorylation, and other effects) until a certain threshold is reached. Above the limit, a set of defense programs is activated typically involved in defense against insect herbivores. In such a scenario, even herbivores that are completely devoid of salivary secretions would finally trigger defense responses in plants.
The overall results of this study reveal that the mechanical caterpillar is a valuable tool to dissect the role and impact of wounding and signaling compounds in plant herbivore interactions. It mimics very realistically the feeding process as indicated by the induction of a complete blend of herbivory-related volatiles in lima bean without any addition of signaling compounds. The attractiveness of this blend to various parasitoids of lima bean herbivores will be addressed next to learn more about its biological relevance. A more detailed analysis of the plant responses, including changes in phytohormone levels, reactive oxygen species, and oxylipin accumulation and herbivory-related gene activations, as well as kinetic analyses of inducible compounds upon treatment with the mechanical caterpillar in combination with the application of chemical elicitors, will also be performed in the near future. Furthermore, other plants, such as maize, cotton, and tobacco, are currently being investigated to find out whether or not continuous mechanical damage without simultaneous entrainment of chemical elicitors is generally sufficient to elicit plant defense responses.
MATERIALS AND METHODS
Plant Material
All experiments have been carried out with 12- to 16-d-old freshly detached plantlets of lima bean (Phaseolus lunatus) L. cv Ferry Morse var Jackson Wonder Bush, showing two fully developed primary leaves. Individual plants were grown from seed in a plastic pot using sterilized potting soil. Growing conditions were 23°C, 60% humidity, and 270 μE m−2 s−1 during a 16-h photoperiod.
Animal Material
Larvae of Spodoptera littoralis Boisd. (Lepidoptera, Noctuidae) were grown at 22°C to 24°C and a 14- to 16-h photoperiod in petri dishes or plastic boxes and fed as described previously (Bergomaz and Boppré, 1986). For feeding experiments, third-instar larvae were used. Adult snails, Cepaea hortensis (O.F. Müll.; Stylommatophora, Helicidae), were collected at local meadows near Jena, Thuringia. They were kept in plastic boxes at 22°C to 24°C and a 14- to 16-h photoperiod and fed with lima bean leaves.
Mechanical Caterpillar
The mechanical caterpillar MecWorm (Fig. 2) was engineered to user specification for the imitation of wounding that emerges upon caterpillar feeding. In principle, a kind of hole-punching unit, with a metal punch (φ = 0.5 mm), punches out small parts of the treated tissue (Fig. 2, A and B). The overall time of treatment, as well as the time slice between single punching events, can be defined individually by the user for each experiment. Moreover, the shape (circle, rectangle), area, and exact position of punching can be determined as well by a two-dimensional (x, y) moving punching unit. For example, in a typical experiment, a lima bean leaf has been treated at 6 different sectors (in summary, about 733 mm2) for 17 h, 6 min, and 50 s (time slice = 5 s) according to 12,322 single punching events (Fig. 2C).
Electron Microscopy
Pieces of leaves were fixed with 2.5% glutaraldehyde in 75 mm cacodylate buffer, pH 7.0, postfixed with osmium tetroxide, dehydrated in a graded series of acetone solutions, and critical-point dried from liquid CO2, mounted on stubs, and coated with 3 to 5 nm platinum with a magnetron sputter coater. The specimens were examined with a Hitachi (Tokyo) S-4100 field emission scanning electron microscope operated at 5 kV.
Induction Experiments and Analysis of Headspace Volatiles
Plantlets of lima beans were cut with a razor blade and transferred immediately into vials containing tap water. Control experiments indicated no differences concerning volatile response in detached and nondetached leaves, respectively, in contrast to results obtained in maize (Zea mays; Schmelz et al., 2001). For headspace analyses, the vials containing plantlets were enclosed in desiccators (750 mL) and either six Spodoptera littoralis caterpillars or two Cepaea hortensis snails were allowed to feed on the leaves. Plantlets used for continuous mechanical damage were enclosed in a Plexiglas cabinet (approximately 500 mL) and punched therein for the time indicated. In some cases, only the treated leaf was placed in the cabinet, whereas the opposite leaf was analyzed separately for systemic responses. In the latter case, the opposite leaf was enclosed in a polyester bag (Toppitz, Minden, Germany). Headspace volatiles emitted by lima bean leaves treated with either caterpillars, snails, or the mechanical caterpillar were continuously collected for 24 h on charcoal traps (1.5 mg of charcoal) using air circulation as described; efficient trapping also avoids accumulation of volatiles in the headspace (Koch et al., 1999) and effects associated with some of the emitted volatiles on plant physiology (Farag and Paré, 2002). All experiments were started at noon. Setups were kept at 22°C to 24°C with a light/dark rhythm of 9 h light, 10 h dark, 5 h light. For all samples, after volatile collection, absorbed compounds were eluted with dichloromethane (2 × 15 μL, supplemented with 1-bromodecan as internal standard [0.9 mm final concentration] and adjusted to a final volume of 40 μL) and directly analyzed by gas chromatography (GC)-mass spectrometry (MS) using fused-silica capillary tubes.
To normalize the data, relative increase or decrease of the amount of VOCs was determined by calculating the ratio of the peak area of the particular compound and the peak area of the internal standard present in all samples analyzed by GC-MS. The damage levels (mm2·s, × 107) were determined with 0 (no treatment), 0.33 (2.9 h, 313 mm2), 1.80 (6.85 h, 733 mm2; 16 h, 313 mm2), and 4.52 (17 h, 733 mm2), based on the multiplication of time (s) and area (mm2).
Acknowledgments
We thank A. Lehr, S. Dobler, and A. Biedermann for excellent technical assistance, C. Kost and H. Maischak for help with the statistical analysis, M. Leitner for drawing Figure 2A, the Plant Protection Centre of Bayer AG (Monheim, Germany) for providing S. littoralis egg clutches, A. Berg for culturing caterpillars, and M. Mithöfer and G. Paysan for collecting snails. We also thank D. Scholz, T. Bahrmann, S. Eiseleit, P. Hause, and R. Bark from the mechanical workshop of the Department of Physics, Friedrich Schiller University, Jena, Germany, for construction and development of MecWorm.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.054460.
References
- Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH (1997) An elicitor of plant volatiles from beet armyworm oral secretion. Science 276: 945–949 [Google Scholar]
- Bergomaz R, Boppré M (1986) A simple insect diet for rearing Arctiidae and other moths. J Lepid Soc 40: 131–137 [Google Scholar]
- De Boer JG, Dicke M (2004) The role of methyl salicylate in prey searching behavior of the predatory mite Phytoseiulus persimilis. J Chem Ecol 30: 255–271 [DOI] [PubMed] [Google Scholar]
- De Moraes CM, Lewis WJ, Paré WB, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393: 570–574 [Google Scholar]
- De Moraes CM, Mescher MC (2004) Biochemical crypsis in the avoidance of natural enemies by an insect herbivore. Proc Natl Acad Sci USA 101: 8993–8997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicke M, van Baarlen P, Wessels R, Dijkman H (1993) Herbivory induces systemic production of plant volatiles that attract predators of the herbivore—extraction of endogenous elicitor. J Chem Ecol 19: 581–599 [DOI] [PubMed] [Google Scholar]
- Dicke M, van Beek TA, Posthumus MA, Ben Dom N, van Bokhoven H, de Groot AE (1990) Isolation and identification of volatile kairomone that affects acarine predator-prey interactions. Involvement of host plants in its production. J Chem Ecol 16: 381–396 [DOI] [PubMed] [Google Scholar]
- Engelberth J, Koch T, Schüler G, Bachmann N, Rechtenbach J, Boland W (2001) Ion channel-forming alamethicin is a potent elicitor of volatile biosynthesis and tendril coiling. Cross talk between jasmonate and salicylate signaling in lima bean. Plant Physiol 125: 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag MA, Paré PW (2002) C6-green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry 61: 545–554 [DOI] [PubMed] [Google Scholar]
- Felton GW, Eichenseer H (1999) Herbivore saliva and its effects on plant defense against herbivores and pathogens. In AA Agrawal, S Tuzun, E Bent, eds, Induced Plant Defenses against Pathogens and Herbivores: Ecology and Agriculture. American Phytopathology Society Press, St. Paul, pp 19–36
- Funk CJ (2001) Alkaline phosphatase activity in whitefly salivary glands and saliva. Arch Insect Biochem Physiol 46: 165–174 [DOI] [PubMed] [Google Scholar]
- Gatehouse JA (2002) Plant resistance towards insect herbivores: a dynamic interaction. New Phytol 156: 145–169 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol 125: 711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Baldwin IT (2002) Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends Plant Sci 7: 61–67 [DOI] [PubMed] [Google Scholar]
- Helle W, Sabelis MW (1985) World Crop Pest: Spider Mites. Their Biology, Natural Enemies and Control, Vol 1B. Elsevier, Amsterdam
- Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141–2144 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53: 299–328 [DOI] [PubMed] [Google Scholar]
- Koch T, Krumm T, Jung V, Engelberth J, Boland W (1999) Differential induction of plant volatile biosynthesis in the lima bean by early and late intermediates of the octadecanoid-signaling pathway. Plant Physiol 121: 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt PJ, Tumlinson JH, Alborn DH (1999) Attraction of Colorado potato beetle (Coleoptera: Chrysomelidae) to damaged and chemically induced potato plants. Environ Entomol 28: 973–978 [Google Scholar]
- Maffei M, Bossi S, Spiteller D, Mithöfer A, Boland W (2004) Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol 134: 1752–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiacci L, Dicke M, Posthumus MA (1995) Beta-glucosidase—an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Natl Acad Sci U S A 92: 2036–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa R, Arimura G, Takabayashi J, Shimoda T, Nishioka T (2000) Involvement of jasmonate- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol 41: 391–398 [DOI] [PubMed] [Google Scholar]
- Pare PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121: 325–331 [PMC free article] [PubMed] [Google Scholar]
- Pohnert G, Jung V, Haukioja E, Lempa K, Boland W (1999) New fatty acid amides from regurgitant of lepidopteran (Noctuidae, Geometridae) caterpillars. Tetrahedron 55: 11275–11280 [Google Scholar]
- Reymond P, Bodenhausen N, Van Poecke RMP, Krishnamurthy V, Dicke M, Farmer EE (2004) A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell 16: 3132–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röse USR, Manukian A, Heath RR, Tumlinson JH (1996) Volatile semiochemicals released from undamaged cotton leaves. Plant Physiol 111: 487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Tumlinson JH (2001) The influence of intact-plant and excised-leaf bioassay designs on volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays. Planta 214: 171–179 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Tumlinson JH (2003) Synergistic interactions between volicitin, jasmonic acid and ethylene mediate insect-induced volatile emission in Zea mays. Physiol Plant 117: 403–412 [DOI] [PubMed] [Google Scholar]
- Spiteller D, Boland W (2003) N-(15,16-Dihydroxylinoleoyl)-glutamine and N-(15,16-epoxylinoleoyl)-glutamine isolated from oral secretions of lepidopteran larvae. Tetrahedron 59: 135–139 [Google Scholar]
- Spiteller D, Pohnert G, Boland W (2001) Absolute configuration of volicitin, an elicitor of plant volatile biosynthesis from lepidopteran larvae. Tetrahedron Lett 42: 1483–1485 [Google Scholar]
- Turlings TC, Tumlinson JH (1992) Systemic release of chemical signals by herbivore-injured corn. Proc Natl Acad Sci USA 89: 8399–8402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlings TC, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250: 1251–1253 [DOI] [PubMed] [Google Scholar]
- Van Poecke RMP, Dicke M (2004) Indirect defence of plants against herbivores: using Arabidopsis thaliana as a model plant. Plant Biol 6: 387–401 [DOI] [PubMed] [Google Scholar]
- Van Poecke RMP, Roosjen M, Pumarino L, Dicke M (2003) Attraction of the specialist parasitoid Cotesia rubecula to Arabidopsis thaliana infested by host or non-host herbivore species. Entomol Exp Appl 107: 229–236 [Google Scholar]
- Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19: 195–216 [DOI] [PubMed] [Google Scholar]