Abstract
Objective
SAPHO (Synovitis, Acne, Pustulosis, Hyperostosis and Osteitis) syndrome is a heterogeneous disease that clinically manifests as chronic inflammatory osteoarticular and dermatological lesions. Few reports have described familial clustering of SAPHO syndrome cases. This research aimed to illustrate the family aggregation of SAPHO syndrome and investigate the prevalence of autoimmune disorders among SAPHO syndrome patients and first-degree relatives in a large cohort.
Methods
We retrospectively reviewed the medical records of 233 SAPHO patients diagnosed at Peking Union Medical College Hospital. Direct phone calls were made to each first-degree relatives. All relatives of the patients who reported SAPHO syndrome were asked for a detailed outpatient evaluation.
Results
A total of 233 patients and 1227 first-degree relatives were recruited. Six (2.6 %) patients had positive SAPHO family history, including four mother-daughter pairs and two sister pairs. Twenty-one (9.0 %) patients presented at least one kind of autoimmune disease, including 12 rheumatoid arthritis and 4 ulcerative colitis cases. Fifty-eight (24.9 %) SAPHO syndrome patients had 68 (5.5 %) first-degree relatives with at least one autoimmune disorder. The palmoplantar pustulosis, psoriasis vulgaris, and rheumatoid arthritis prevalence in our subjects were each higher than reference rates.
Conclusion
This is the first evaluation of familial aggregation for SAPHO syndrome in a large cohort. SAPHO syndrome has a weak familial aggregation. There is a relatively high prevalence of coexisting autoimmune disease among patients with SAPHO syndrome and their first-degree relatives. These results would prompt physicians to screen SAPHO syndrome patients and their family members for concomitant autoimmune diseases.
Keypoints
This study suggesting a potential genetic component in the pathogenesis of SAPHO syndrome. This study is the first to evaluate the family aggregation of SAPHO syndrome in a large cohort.
Keywords: SAPHO syndrome, Familial aggregation, Autoimmune disease, Rheumatoid arthritis
1. Introduction
SAPHO syndrome (Synovitis, Acne, Pustulosis, Hyperostosis and Osteitis) is a rare and underestimated disease characterized by chronic inflammatory osteoarticular and dermatological lesions. The clinical manifestations of SAPHO syndrome are heterogeneous [1]. The most commonly affected skeletal sites are the anterior chest wall, spine (for adults), and long bone (for children). Palmoplantar pustulosis (PPP), psoriasis vulgaris (PV), severe acne (SA), or other neutrophilic dermatosis are the main skin abnormalities in patients with SAPHO syndrome [2]. Chronic recurrent multifocal osteomyelitis (CRMO) is considered a subtype of SAPHO syndrome; the symptoms of CRMO are also included in the SAPHO syndrome diagnostic criteria [3].
The pathogenesis of SAPHO syndrome is likely multifactorial and probably involves a combination of genetic, infectious, and immunological aspects with the participation of NK cells, Th17, and Treg cells [4,5]. Most of the published English works of literature regarding SAPHO syndrome are based on sporadic cases. Reports of familial clustering of SAPHO syndrome cases are limited, but such clustering has been described a monozygotic twin pair [[6], [7], [8]], a sibling pair [9,10], a parent-child pair [6], and a grandparent-child pair [11]. Unfortunately, because most of these cases were described in case reports or cohort studies with relatively small sample sizes, the clinical characteristics of some patients were not clearly illustrated. Notably, the proportion of the familial inheritance of SAPHO syndrome remains unknown and has not been systematically evaluated in large cohorts.
There is growing evidence suggesting that autoimmune diseases tend to coexist both within individuals and within families [12]. The high prevalence of autoimmune diseases co-occurring within patients and families may indicate an autoimmune diathesis. Moreover, the co-aggregation of SAPHO syndrome and certain other autoimmune diseases may imply overlapping pathogenesis that deserves further elucidation [13]. Some studies on SAPHO syndrome have also reported that a small percentage of patients had a family history of autoimmune disease or chronic inflammation disorders [14,15]. However, the prevalence of each autoimmune disease among SAPHO patients and their first-degree relatives is yet to be determined.
In this study, we reviewed the medical records of 233 patients with SAPHO syndrome diagnosed in our medical center. This research was conducted on a large cohort to illustrate the family aggregation of SAPHO syndrome and understand the coexisting autoimmune disorders within SAPHO syndrome patients and their families.
2. Methods
2.1. Participants and ethics
From January 2015 to February 2017, patients with SAPHO syndrome were continuously recruited for the present study from our former cohort [16]. All patients met Kahn's 2003 diagnostic criteria [17]. Written consent was obtained from each patient, and verbal consent was obtained from each relative. This study was approved by the Institutional Review Board of Peking Union Medical College Hospital (PUMCH) (approval number: ZS-944).
2.2. Data collection
Detailed demographic and clinical data, including age, cutaneous manifestations, bone manifestations, and all autoimmune disorder comorbidities, were collected during each patient's first visit at the PUMCH outpatient clinic. At this time, the patients were also questioned regarding a family history of SAPHO syndrome and provided contact information of each of their first-degree relatives (i.e., parents, all siblings, and all children). Direct telephone surveys lasting at least 15 min with each relative were then conducted to reveal their medical history of SAPHO syndrome or other autoimmune disorders (233 relatives from a queue of 354 patients completed this survey). For some influent telephone users, such as some elderly and little children, the medical history was reported by other relatives who were familiar with their condition. The telephone survey was conducted by two physicians (LC, SYM) of this study with a question list in supplement material 1. The questions were mostly the same for each relative, except some specific questions regarding different clinical symptoms, laboratory tests, medications, and therapeutic effects of different diseases. All subjects agreed to participate in a telephone survey and completed all the questions by telephone.
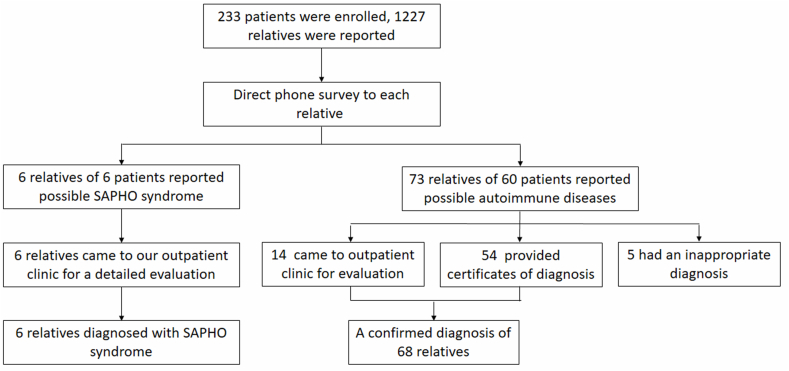
After the telephone survey, all of the relevant first-degree relatives who reported a possible diagnosis of SAPHO syndrome were asked to visit our clinic, where they received a comprehensive evaluation, including clinical symptom assessment, laboratory workup, and imaging. All relatives received detailed examinations of computerized tomography scan, magnetic resonance imaging and 99mTc-MDP bone scintigraphy for the osteoarticular evaluation. All patients and relatives with SAPHO syndrome were followed up regularly at least twice a year. For those relatives only reporting other concurrent autoimmune diseases, those who lived in Beijing were also asked to visit our outpatient clinic for a detailed evaluation. Providing an electronic version certificate of diagnosis from a local qualified tertiary hospital was also accepted for those relatives living in other cities. Otherwise, the autoimmune disease reported would not be counted (Fig. 1).
Fig. 1.
Schematic of patient enrollment.
This study did not restrict the investigated autoimmune diseases to a specific list; instead, the patients and their relatives were encouraged to report any related or unrelated diseases they suffered. Since PPP and PV are cutaneous presentations of SAPHO syndrome, these two diseases were not regarded as comorbidities of SAPHO syndrome patients. PPP and PV were only considered as distinct chronic inflammatory diseases for those relatives who were not diagnosed with SAPHO syndrome.
To compare the disease prevalence in our study to that of the general Chinese population, we searched the available epidemiologic data on Chinese and East-Asian people. Because these groups are ethnically and socioeconomically comparable, the data from East-Asian populations served as an acceptable representation for the Chinese people. However, some diseases lacked reliable prevalence data from Chinese or Asian populations; for these, the data of Caucasian populations were referenced.
2.3. Statistical analysis
Descriptive data are expressed as the proportion (%) for categorical variables and the mean (SD) for continuous variables. A Student's t-test and chi-square test (or Fisher's exact test) were used for continuous and categorical variables, respectively. Comparisons with the general population were made using the one-sample z-test. Relative risk (RR) was calculated as the prevalence of each autoimmune disease among SAPHO syndrome patients or their relatives, divided by the prevalence of each autoimmune disease in the general population; 95 % confidence intervals (CI) were also calculated. All tests were two-sided, and a p-value of less than 0.05 was considered to indicate a statistically significant difference. All recorded data were analyzed using SPSS statistics software version 22.
3. Results
3.1. Demographic characteristics
A total of 233 SAPHO syndrome patients and 1227 first-degree relatives of these patients, including 466 parents, 512 siblings, and 249 children, were included in the analysis. All subjects were Chinese. The mean age of these patients was 43.2 years old (SD: 12.0 years, range: 10–70 years), and the female/male ratio of the patients was 2.1:1 (Table 1).
Table 1.
Autoimmune diseases in SAPHO patients and their first-degree relatives.
| Characteristics | Patient |
Relatives |
Prevalence, % | |||||
|---|---|---|---|---|---|---|---|---|
| Number | RR (95 % CI) | Parent | Sibling | Child | Total | RR (95 % CI) | ||
| Total N | 233 | / | 466 | 512 | 249 | 1227 | / | |
| Female sex | 68.2 % | / | 50 % | 48.8 % | 49.3 % | 49.2 % | / | |
| Disease, n (%) | ||||||||
| SAPHO | 233 (100) | NA | 1 (0.21) | 2 (0.39) | 3 (1.20) | 6 (0.49) | NA | NA |
| PPP | 185 (79.40) | / | 7 (1.50) | 5 (0.98) | 2 (0.80) | 14 (1.14) | 9.81 (5.83–16.51) | 0.12 [24] |
| SA | 33 (14.16) | / | 0 (0.00) | 0 (0.00) | 1 (0.40) | 1 (0.08) | 0.18 (0.03–1.30) | 0.45 [25] |
| Psoriasis | 29 (12.44) | / | 10 (2.15) | 11 (2.15) | 0 (0.00) | 21 (1.71) | 6.46 (3.68–11.34) | 0.27 [18] |
| RA | 12 (5.15) | 18.14 (9.41–34.98) | 11 (2.36) | 2 (0.39) | 1 (0.40) | 14 (1.14) | 4.02 (2.14–7.56) | 0.28 [18] |
| SS | 2 (0.86) | 19.96 (4.86–81.92) | 1 (0.21) | 2 (0.39) | 0 (0.00) | 3 (0.24) | 5.69 (1.77–18.30) | 0.043 [20] |
| Vitiligo | 2 (0.86) | 1.54 (0.38–6.19) | 5 (1.07) | 0 (0.00) | 0 (0.00) | 5 (0.41) | 0.73 (0.30–1.79) | 0.56 [21] |
| SLE | 1 (0.43) | 15.10 (1.58–144.64) | 0 (0.00) | 1 (0.20) | 0 (0.00) | 1 (0.08) | 2.87 (0.30–27.55) | 0.03 [18] |
| AIT | 4 (1.72) | 0.36 (0.14–0.95) | 0 (0.00) | 1 (0.20) | 0 (0.00) | 1 (0.08) | 0.02 (0.00–0.12) | 4.80 [22] |
| Scleroderma | 1 (0.43) | 45.29 (2.84–721.91) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | NA | 0.01 [18] |
| UC | 4 (1.72) | 343.35 (92.78–1270.58) | 2 (0.43) | 0 (0.00) | 0 (0.00) | 2 (0.16) | 32.60 (6.33–167.87) | 0.005 [19] |
| MCTD | 0 (0.00) | NA | 2 (0.43) | 0 (0.00) | 0 (0.00) | 2 (0.16) | NA | NA |
| DM | 0 (0.00) | NA | 2 (0.43) | 0 (0.00) | 1 (0.40) | 3 (0.24) | 11.11 (3.33–37.08) | 0.022 [18] |
| AS | 0 (0.00) | NA | 1 (0.21) | 1 (0.20) | 0 (0.00) | 2 (0.16) | 0.98 (0.24–3.94) | 0.167 [27] |
| ReA | 0 (0.00) | NA | 1 (0.21) | 2 (0.39) | 0 (0.00) | 3 (0.24) | NA | NA |
| NMO | 0 (0.00) | NA | 1 (0.21) | 0 (0.00) | 0 (0.00) | 1 (0.08) | 9.70 (1.21–77.48) | 0.008 [26] |
| AIHA | 0 (0.00) | NA | 1 (0.21) | 0 (0.00) | 0 (0.00) | 1 (0.08) | NA | NA |
| Any, except SAPHOa | 21 (9.01)b | NA | 43 (9.23) | 20 (3.91) | 5 (2.01) | 68 (5.52) | NA | NA |
PPP: Palmoplantar pustulosis; SA: Severe acne; RA: Rheumatoid arthritis; SS: Sjögren's syndrome; SLE: Systemic lupus erythematosus; AIT: Autoimmune thyroiditis; UC: ulcerative colitis; MTCD: Mixed connective tissue disease; DM: Dermatomyositis; AS: Ankylosing spondylitis; ReA: Reactive arthritis; NMO: Neuromyelitis optical; AIHA: Autoimmune hemolytic anemia; NA: Not acquired.
Patients with two or more autoimmune diseases (except SAPHO) are not counted repeatedly, each person is only counted once.
PPP, SA, and PV are considered cutaneous manifestations of SAPHO rather than concomitant diseases for patients with SAPHO syndrome, so patients who only suffer from one or more diseases in PPP, SA, PV are not included.
3.2. Familial aggregation of SAPHO syndrome
Six (2.6 %) patients had a positive SAPHO syndrome family history. All 12 patients in these families were female; there were four mother-daughter pairs and two sister pairs. This phenomenon indicates a possible familial aggregation among female patients (Fig. 2A–F).
Fig. 2.
Pedigree of six SAPHO patients. Solid symbols denote affected individuals, and arrows point to the probands in each pedigree. Gray symbols denote individuals with other autoimmune disorders.
The clinical characteristics of the SAPHO syndrome patients with a positive family history illustrate the heterogeneity of this disease. Five families had PPP as a cutaneous manifestation, whereas two patients in pedigree E had no skin lesions. The skin manifestations showed a relatively stable inheritance pattern (Table 2).
Table 2.
Detailed clinical characteristics of SAPHO patients with a positive family history.
| Patienta | Kinship | Gender | Onset age | Cutaneous manifestation | Bone disease locations | Complication of autoimmune diseases |
|---|---|---|---|---|---|---|
| A-II-4 | Proband | Female | 46 | PPP | ACW + Spine | NA |
| A-I-2 | Mother | Female | 69 | PPP | ACW | NA |
| B-II-5 | Proband | Female | 30 | PPP | ACW + Spine + Mandible | NA |
| B-III-4 | Daughter | Female | 10 | PPP | ACW | NA |
| C–I-2 | Proband | Female | 57 | PPP | ACW + Spine | NA |
| C-II-2 | Daughter | Female | 35 | PPP | ACW | NA |
| D-II-3 | Proband | Female | 63 | PPP | ACW + Spine | NA |
| D-III-2 | Daughter | Female | 35 | PPP | ACW + Spine | NA |
| E-II-6 | Proband | Female | 54 | NA | ACW + Periphral bone | Vitiligo |
| E-II-4 | Sister | Female | 55 | NA | Spine | NA |
| F-II-6 | Proband | Female | 56 | PPP | ACW | NA |
| F-II-7 | Sister | Female | 50 | PPP | ACW + Spine + Periphral bone | NA |
ACW: anterior chest wall; PPP: Palmoplantar pustulosis; NA: Not acquired.
Number of patients referred to in the pedigree shown in Fig. 2.
In contrast, the bone lesion sites varied even among afflicted subjects within the same pedigree. Except for pedigree D, all the pedigrees had different bone lesion sites for the proband compared with their affected first-degree relative. Among these 12 patients, the anterior chest wall (including the sternum, clavicle, and rib) was still the most commonly affected site, except for subject E–II–4. Seven patients showed lesions in the spine (including the vertebrae and sacroiliac joint). Two patients, subjects E–II–6 and F-II-7, had peripheral joints involved (i.e., shoulder joint and metatarsophalangeal joint). Subject B-II-5 reported involvement of the mandible (Table 2).
3.3. Concomitant autoimmune diseases among SAPHO patients
The cutaneous symptoms of the 233 SAPHO syndrome patients included PPP (n = 185, 79.40 %), SA (n = 33, 14.16 %), and PV (n = 29, 12.44 %). No other neutrophilic dermatosis was reported, including hidradenitis suppurative, pyoderma gangrenosum, and Sweet's syndrome. (Table 1).
A total of 21 (9.0 %) patients presented at least one kind of autoimmune disease; there were 12 with rheumatoid arthritis (RA), 2 with Sjögren's syndrome (SS), 2 with vitiligo, 4 with autoimmune thyroiditis (AIT), 1 with systemic lupus erythematosus (SLE), 1 with scleroderma, and 4 with ulcerative colitis (UC). Five patients had more than one diagnosed autoimmune disorder: two had concomitant RA and SS, two had concurrent RA and AIT, and one had SLE and AIT. The prevalence of most of these diseases was higher than that of the general population, particularly that of RA (5.15 % vs. 0.28 %, p = 0.001; RR: 18.14, 95%CI: 9.41–34.98) and UC (1.72 % vs. 0.005 %, p = 0.046; RR: 343.35, 95%CI: 92.78–1270.58) [18,19]. The prevalence of SS, vitiligo, SLE, and scleroderma each trended slightly higher than that of the general population but was not significantly different: 0.86 % vs. 0.043 % (p = 0.180), 0.86 % vs. 0.56 % (p = 0.623), 0.43 % vs. 0.03 % (p = 0.353), and 0.43 % vs. 0.01 % (p = 0.330), respectively. AIT was relatively rare among SAPHO patients compared with the general population (1.72 % vs. 4.80 %) [18,[20], [21], [22]] (Table 1).
Among patients with a positive family history of SAPHO syndrome, subject E–II–6 reported coexisting vitiligo. An unaffected family member in the same pedigree, E-I-2, reported a history of RA (Table 2).
Even though most autoimmune diseases predominantly affect females [23], the prevalence of autoimmune diseases did not significantly differ between the men and women in our cohort (9.3 % of male subjects vs. 8.9 % of female subjects, p = 0.871).
3.4. Other autoimmune diseases among first-degree relatives of SAPHO patients
A total of 58 (24.9 %) SAPHO syndrome patients had 68 (5.5 %) first-degree relatives who suffered from at least one autoimmune disorder, including 43 (9.2 %) parents, 20 (3.9 %) siblings, and 5 (2.0 %) children (Table 1).
For the first-degree relatives not diagnosed as SAPHO syndrome patients, PPP and PV were classified as independent autoimmune disorders rather than as manifestations of SAPHO. Fourteen (1.14 %) and 21 (1.71 %) subjects had PPP and PV, respectively. Another 14 (1.14 %) relatives reported a history of RA. These rates are all significantly higher than those of the general population (p = 0.001, RR = 9.81, 95%CI: 5.83–16.51 for PPP; p < 0.001, RR = 6.46, 95%CI: 3.68–11.34 for PV; p = 0.005, RR = 4.02, 95%CI: 2.14–7.56 for RA) [18,24]. Only one boy reported SA [25]. Other autoimmune disorders were also reported in these subjects; UC, SS, vitiligo, and SLE occurred among two, three, five, and one family, respectively. There were no cases of scleroderma reported in these subjects, and only one relative reported AIT. Sporadic cases of several new autoimmune diseases, including mixed connective tissue disease, dermatomyositis, ankylosing spondylitis, reactive arthritis, neuromyelitis optical, and autoimmune hemolytic anemia, were also reported, and most had a slightly higher prevalence than the reference value for the general population [18,26,27] (Table 1).
Because the risk of an autoimmune disease is increased in relatives of patients with some autoimmune diseases [28], we also examined this potential connection in our study. The results show that whether a first-degree relative had an autoimmune disease was unrelated to sex (p = 0.387), but it was linked to whether the SAPHO syndrome patient had an autoimmune disease (p = 0.021).
4. Discussion
Our analysis of the clinical data of 233 SAPHO syndrome patients and 1227 of their first-degree relatives revealed that 2.6 % of SAPHO syndrome patients had a positive family history, suggesting a potential genetic component in the pathogenesis of SAPHO syndrome. This study also uncovered a higher prevalence of autoimmune disorders among 9.0 % of SAPHO patients and 5.5 % of their first-degree relatives. This study is the first to evaluate the family aggregation of SAPHO syndrome in a large cohort.
Autoimmune diseases can occur when the immune response is unbalanced. Genetic factors are known to modify the immune response. Multiple environmental factors have also been suggested to involve with the pathogenesis of autoimmune diseases. Few extensive cohort studies have focused on the family inheritance of SAPHO. In one cohort of 89 Caucasian non-bacterial osteitis (NBO) patients, at least two family members were affected with NBO in 6 % of the families, and autoimmune diseases were reported in 40 % of the families, potentially indicating a genetic basis for NBO [14]. These two rates are higher than the related ones from the present work, possibly due to the small sample size (only 39 SAPHO (CRMO) patients were included) and different inclusion criteria of their study. Furthermore, even though NBO and SAPHO syndrome shares similar clinical features, the previous study may not represent the full genetic picture of SAPHO syndrome. Additionally, because the diagnosis of NBO mainly focuses on bone lesions, patients with skin manifestations, such as PPP and PV, were regarded as having independent autoimmune diseases in the NBO study and were counted in the 40 %; this may have led to a higher positive rate for coexisting autoimmune disorders. Another study of 45 CRMO patients also reported a higher prevalence of chronic inflammatory diseases among first- and second-degree relatives [15].
Together with the results of previous work, our findings point to a potential familial aggregation of SAPHO syndrome. However, our data analysis suggests that the inheritance pattern is not simply monogenic dominant or recessive. The present Chinese cohort study observed an apparent familial aggregation among female patients; the most commonly observed family relationships were mother-daughter pairs and sister pairs. Notably, male patients have also been reported to be involved in the inheritance of SAPHO syndrome, although some cases lacked sufficient osteoarticular evaluation [7,9,10]. Thus, a recruitment bias may exist in this study. Both in this study and previous reports, the clinical presentations, especially regarding bone lesions, showed heterogeneity even within the same pedigree, [8,10,11]. Additionally, PPP was the cutaneous manifestation of 5 families in this cohort, while SA and PV have also been reported in other published cases [[7], [8], [9]]. The variety of presenting factors, including patients of either sex, different family relationships within clusters, and diverse bone and skin manifestations, indicates that SAPHO syndrome is heterogeneous and its genetic influences are likely complex. Moreover, the co-aggregation of SAPHO syndrome and certain other autoimmune diseases suggests the potential for overlapping pathogenesis that deserves further elucidation. The high prevalence of autoimmune diseases among SAPHO syndrome patients and their families could indicate a genetic background of a disordered immune system in these individuals; if so, this may also indicate that immunological aspects contribute to the pathogenesis of SAPHO syndrome [4].
Marzano et al.'s study showed that comprehensive hidradenitis suppurativa is a polygenic autoimmune inflammatory disease, and the association between different variants and different types and clinical manifestations was determined through the whole-exome sequencing method. This provides important clues for diagnosis and research [29]. Similarly, the genetic associations of SAPHO syndrome have also been studied at the molecular level. In mouse models with nonsynonymous homozygous mutations in the PSTPIP2 gene, some mice developed characteristics similar to those of CRMO [30]. However, mutations of PSTPIP2 may not be specific, as they were not found more frequently in SAPHO syndrome patients when compared with healthy controls [10,14]. Several single nucleotide polymorphisms (SNPs) were found to be associated with the susceptibility of SAPHO syndrome patients. Three SNP sites, including rs10889677 and rs2201841 of interleukin (IL)-23 R and rs2243248 of IL-4, showed a significant correlation with the occurrence of SAPHO syndrome in a Chinese population [31]. Significant increases in the frequencies of Mdm2 SNP T309G and p53 SNP G72C were found in patients with SAPHO syndrome, but not in individuals with psoriasis or psoriatic arthritis, which indicates that SAPHO syndrome may be linked to an imbalance between MDM2 and p53 regulation [32]. Another study on Chinese patients concluded that people who carry the risk allele T of rs6908425 in CDKAL1 might be more prone to developing SAPHO [33]. Moreover, SAPHO syndrome shares some similar presentations with Majeed syndrome, a disease proven to be an autosomal-recessive disorder caused by an LPIN2 mutation. However, no clear association between LPIN2 and SAPHO syndrome has been detected [10,34]. Future studies may use genome-wide association data from a large cohort to detect additional predisposition genes, which may shed light on the pathogenesis of SAPHO. Moreover, different autoimmune diseases may share overlapping genetic susceptibility loci and a large proportion of their genetic background. Some loci mentioned above (i.e., rs10889677 and rs2201841 of IL-23 R, rs2243248 of IL-4, and of CDKAL1) were also reported to be associated with other autoimmune disorders, such as IBD, AS, RA, PV, gout, alopecia areata, and multiple sclerosis [[35], [36], [37], [38], [39], [40], [41], [42]]. The presence of these loci may also imply overlapping pathogenesis between SAPHO syndrome and other autoimmune diseases. It also supports the optimistic hypothesis that ‘one cure for many diseases’ may be possible [43].
The two major cutaneous manifestations of SAPHO syndrome, PPP and PV, occurred at a greater rate than expected in the first-degree relatives of SAPHO patients. PPP is the most common skin lesion of SAPHO syndrome, accounting for 60 % of all dermatologic manifestations [2]. PV represents up to one-third of all skin involvement, and it is frequently seen in combination with other lesions [3]. Since PPP and PV are in the spectrum of SAPHO syndrome, some relatives may be in a kind of forms with incomplete penetration of SAPHO. However, for some patients with no osteoarticular lesions, PPP or PV alone could not lead to a confirmed diagnosis of SAPHO syndrome. Thus, PPP and PV were considered as distinct diseases. Familial aggregation for PV was previously reported, and approximately 40 % of individuals with psoriasis or psoriatic arthritis had a family history of the disease [44]. Variations in the IL19, IL20, IL24, and CARD14 genes may influence the risk for both PPP and PV [45,46]. However, mutations of these loci were not detected in SAPHO syndrome patients.
The presenting coexisting autoimmune diseases were heterogeneous. Notably, RA appeared more prevalent among our subjects, and all these patients met the 2010 ACR/EULAR classification criteria for RA. There was also a previous report on the coexistence of RA and SAPHO syndrome [47]. Specific neutrophilic dermatosis, such as rheumatoid neutrophilic dermatitis (RND) and palisading neutrophilic granulomatous dermatitis (PNGD), are known skin manifestations of RA [48]. However, neutrophilic dermatosis is also a common skin manifestation of SAPHO syndrome. On the other hand, since SAPHO syndrome and RA share similar clinical presentations, some SAPHO syndrome patients, especially those with lesions in the peripheral joints, may be misdiagnosed as RA. Importantly, the positive rates of rheumatoid factor (RF) and anti-citrullinated protein antibody (ACPA) are relatively low in SAPHO syndrome patients, so these markers may be a useful clue for differential diagnosis [49,50].
Our study had some limitations. First, the study was a retrospective study, and data collection was based on patients’ self-reports, which can cause information bias. However, we applied various methods to gather and verify information to minimize the recall and data-missing bias. Second, since not all the first-degree relatives were asked to our clinic, their autoimmune conditions could not be fully confirmed. We addressed this by conducting a detailed telephone survey, followed by direct outpatient clinic or providing certificate of diagnosis from a local qualified tertiary hospital to verify the diagnosis. Third, even though this study was already the largest cohort examining familial aggregations and coexisting autoimmune diseases for SAPHO syndrome, recruitment bias may still exist. Future studies with more cases enrolled are still needed. Last, some of the referenced prevalence rates of autoimmune diseases may not represent the true prevalence in Chinese population due to variations in age, races, and other confounding factors.
5. Conclusions
This study is the first evaluation of a family aggregation of SAPHO syndrome in a large cohort. It shows that 2.6 % of all SAPHO patients have a positive family history, highlighting a potential familial aggregation for this disease. The prevalence rates of coexisting autoimmune diseases among SAPHO syndrome patients and their first-degree relatives were high, especially for RA, PPP, and PV. These results could help alert physicians to the potential of additional inflammatory diseases in SAPHO syndrome patients and inform the potential screening of family members.
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 82074246.
Data availability statement
Data relevant to this study are not stored in publicly available repositories. Data will be made available on request.
Ethics approval and consent to participate
Written consent was obtained from each patient. This study was approved by the Institutional Review Board of Peking Union Medical College Hospital (approval number: ZS-944).
Consent to participate
Informed consent to participate in the study was obtained from all participants.
Consent to publication
Informed consent to publication was obtained from relevant participants.
CRediT authorship contribution statement
Chen Li: Project administration. Hesong Wang: Writing – original draft. Haixu Jiang: Writing – review & editing. Yuming Shao: Writing – review & editing. Guangrui Huang: Data curation. Kai Yuan: Conceptualization. Shufeng Wei: Resources.
Declaration of competing interest
The authors declare no conflict of interest. CL, HSW, HXJ and YMS contributed equally to this work.
Acknowledgments
The authors apologize to all colleagues whose works have not been separately cited or discussed here due to space or knowledge limitations.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21541.
Contributor Information
Chen Li, Email: casio1981@163.com.
Kai Yuan, Email: yuankai@bucm.edu.cn.
Shufeng Wei, Email: Weishufeng2021@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Li C., Cao Y., Zhang W. Clinical heterogeneity of SAPHO syndrome: challenge of diagnosis. Mod. Rheumatol. 2018;28:432–434. doi: 10.1080/14397595.2017.1416733. [DOI] [PubMed] [Google Scholar]
- 2.Firinu D., Garcia-Larsen V., Manconi P.E., et al. SAPHO syndrome: current developments and approaches to clinical treatment. Curr. Rheumatol. Rep. 2016;18:35. doi: 10.1007/s11926-016-0583-y. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen M.T., Borchers A., Selmi C., et al. The SAPHO syndrome. Semin. Arthritis Rheum. 2012;42:254–265. doi: 10.1016/j.semarthrit.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Rukavina I. SAPHO syndrome: a review. J Child Orthop. 2015;9:19–27. doi: 10.1007/s11832-014-0627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu D., Liu X., Lu C., et al. Reduction of peripheral natural killer cells in patients with SAPHO syndrome. Clin. Exp. Rheumatol. 2019;37:12–18. [PubMed] [Google Scholar]
- 6.Golla A., Jansson A., Ramser J., et al. Chronic recurrent multifocal osteomyelitis (CRMO): evidence for a susceptibility gene located on chromosome 18q21.3-18q22. Eur. J. Hum. Genet. 2002;10:217–221. doi: 10.1038/sj.ejhg.5200789. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez T., Gantes M., Bustabad S., et al. Acne fulminans associated with arthritis in monozygotic twins. J. Rheumatol. 1985;12:389–391. [PubMed] [Google Scholar]
- 8.Darley C.R., Currey H.L., Baker H. Acne fulminans with arthritis in identical twins treated with isotretinoin. J. R. Soc. Med. 1984;77:328–330. doi: 10.1177/014107688407700415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumolard A., Gaudin P., Juvin R., et al. SAPHO syndrome or psoriatic arthritis? A familial case study. Rheumatology. 1999;38:463–467. doi: 10.1093/rheumatology/38.5.463. [DOI] [PubMed] [Google Scholar]
- 10.Hurtado-Nedelec M., Chollet-Martin S., Chapeton D., et al. Genetic susceptibility factors in a cohort of 38 patients with SAPHO syndrome: a study of PSTPIP2, NOD2, and LPIN2 genes. J. Rheumatol. 2010;37:401–409. doi: 10.3899/jrheum.090456. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson P.J., Lokuta M.A., El-Shanti H.I., et al. Neutrophil dysfunction in a family with a SAPHO syndrome-like phenotype. Arthritis Rheum. 2008;58:3264–3269. doi: 10.1002/art.23942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somers E.C., Thomas S.L., Smeeth L., et al. Autoimmune diseases co-occurring within individuals and within families: a systematic review. Epidemiology. 2006;17:202–217. doi: 10.1097/01.ede.0000193605.93416.df. [DOI] [PubMed] [Google Scholar]
- 13.Kuo C.F., Grainge M.J., Valdes A.M., et al. Familial risk of sjogren's syndrome and Co-aggregation of autoimmune diseases in affected families: a nationwide population study. Arthritis Rheumatol. 2015;67:1904–1912. doi: 10.1002/art.39127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansson A., Renner E.D., Ramser J., et al. Classification of non-bacterial osteitis: retrospective study of clinical, immunological and genetic aspects in 89 patients. Rheumatology. 2007;46:154–160. doi: 10.1093/rheumatology/kel190. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson P.J., El-Shanti H.I. Autoinflammatory bone disorders. Curr. Opin. Rheumatol. 2007;19:492–498. doi: 10.1097/BOR.0b013e32825f5492. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y., Li C., Xu W., et al. Spinal and sacroiliac involvement in SAPHO syndrome: a single center study of a cohort of 354 patients. Semin. Arthritis Rheum. 2019;48:990–996. doi: 10.1016/j.semarthrit.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Kahn M. Paper Presented at: American College of Rheumatology 67th Annual Scientific Meeting. 2003. Proposed classification criteria of SAPHO syndrome. [Google Scholar]
- 18.Li R., Sun J., Ren L.M., et al. Epidemiology of eight common rheumatic diseases in China: a large-scale cross-sectional survey in Beijing. Rheumatology. 2012;51:721–729. doi: 10.1093/rheumatology/ker370. [DOI] [PubMed] [Google Scholar]
- 19.Chuang C.H., Lin S.H., Chen C.Y., et al. Increasing incidence and lifetime risk of inflammatory bowel disease in Taiwan: a nationwide study in a low-endemic area 1998-2010. Inflamm. Bowel Dis. 2013;19:2815–2819. doi: 10.1097/01.MIB.0000435436.99612.27. [DOI] [PubMed] [Google Scholar]
- 20.Qin B., Wang J., Yang Z., et al. Epidemiology of primary Sjogren's syndrome: a systematic review and meta-analysis. Ann. Rheum. Dis. 2015;74:1983–1989. doi: 10.1136/annrheumdis-2014-205375. [DOI] [PubMed] [Google Scholar]
- 21.Wang X., Du J., Wang T., et al. Prevalence and clinical profile of vitiligo in China: a community-based study in six cities. Acta Derm. Venereol. 2013;93:62–65. doi: 10.2340/00015555-1397. [DOI] [PubMed] [Google Scholar]
- 22.Jozkow P., Lwow F., Slowinska-Lisowska M., et al. Trends in the prevalence of autoimmune thyroiditis in the leading private health-care provider in Poland. Adv. Clin. Exp. Med. 2017;26:497–503. doi: 10.17219/acem/60862. [DOI] [PubMed] [Google Scholar]
- 23.Rubtsova K., Marrack P., Rubtsov A.V. Sexual dimorphism in autoimmunity. J. Clin. Invest. 2015;125:2187–2193. doi: 10.1172/JCI78082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubota K., Kamijima Y., Sato T., et al. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen Y., Wang T., Zhou C., et al. Prevalence of acne vulgaris in Chinese adolescents and adults: a community-based study of 17,345 subjects in six cities. Acta Derm. Venereol. 2012;92:40–44. doi: 10.2340/00015555-1164. [DOI] [PubMed] [Google Scholar]
- 26.Asgari N., Lillevang S.T., Skejoe H.P., et al. A population-based study of neuromyelitis optica in Caucasians. Neurology. 2011;76:1589–1595. doi: 10.1212/WNL.0b013e3182190f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dean L.E., Jones G.T., MacDonald A.G., et al. Global prevalence of ankylosing spondylitis. Rheumatology. 2014;53(4):650–657. doi: 10.1093/rheumatology/ket387. [DOI] [PubMed] [Google Scholar]
- 28.Priori R., Medda E., Conti F., et al. Risk factors for Sjogren's syndrome: a case-control study. Clin. Exp. Rheumatol. 2007;25:378–384. [PubMed] [Google Scholar]
- 29.Marzano A.V., Genovese G., Moltrasio C., et al. Whole-exome sequencing in 10 unrelated patients with syndromic hidradenitis suppurativa: a preliminary step for a genotype-phenotype correlation. Dermatology. 2022;238(5):860–869. doi: 10.1159/000521263. [DOI] [PubMed] [Google Scholar]
- 30.Liao H.J., Chyuan I.T., Wu C.S., et al. Increased neutrophil infiltration, IL-1 production and a SAPHO syndrome-like phenotype in PSTPIP2-deficient mice. Rheumatology. 2015;54:1317–1326. doi: 10.1093/rheumatology/keu481. [DOI] [PubMed] [Google Scholar]
- 31.Guo C., Li C., Han F., Gao J., et al. Association analysis of interleukin-23 receptor SNPs and SAPHO syndrome in Chinese people. Int. J. Rheum. Dis. 2019;22:2178–2184. doi: 10.1111/1756-185X.13741. [DOI] [PubMed] [Google Scholar]
- 32.Assmann G., Wagner A.D., Monika M., et al. Single-nucleotide polymorphisms p53 G72C and Mdm2 T309G in patients with psoriasis, psoriatic arthritis, and SAPHO syndrome. Rheumatol. Int. 2010;30:1273–1276. doi: 10.1007/s00296-009-1136-8. [DOI] [PubMed] [Google Scholar]
- 33.Li N., Ma J., Li K., et al. Different contributions of CDKAL1, KIF21B, and LRRK2/MUC19 polymorphisms to SAPHO syndrome, rheumatoid arthritis, ankylosing spondylitis, and seronegative spondyloarthropathy. Genet. Test. Mol. Biomarkers. 2017;21:122–126. doi: 10.1089/gtmb.2016.0112. [DOI] [PubMed] [Google Scholar]
- 34.Al-Mosawi Z.S., Al-Saad K.K., Ijadi-Maghsoodi R., et al. A splice site mutation confirms the role of LPIN2 in Majeed syndrome. Arthritis Rheum. 2007;56:960–964. doi: 10.1002/art.22431. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Y., Jiang H., Chen Z., et al. Genetic association between IL23R rs11209026 and rs10889677 polymorphisms and risk of Crohn's disease and ulcerative colitis: evidence from 41 studies. Inflamm. Res. 2020;69:87–103. doi: 10.1007/s00011-019-01296-y. [DOI] [PubMed] [Google Scholar]
- 36.Tabatabaei-Panah P.S., Moravvej H., Delpasand S., et al. IL12B and IL23R polymorphisms are associated with alopecia areata. Gene Immun. 2020;21:203–210. doi: 10.1038/s41435-020-0100-1. [DOI] [PubMed] [Google Scholar]
- 37.Han R., Xia Q., Xu S., et al. Interleukin-23 receptor polymorphism (rs10889677 A/C) in ankylosing spondylitis: meta-analysis in Caucasian and Asian populations. Clin. Chim. Acta. 2018;477:53–59. doi: 10.1016/j.cca.2017.11.038. [DOI] [PubMed] [Google Scholar]
- 38.Liu S., Zhou Z., Wang C., et al. Associations between interleukin and interleukin receptor gene polymorphisms and risk of gout. Sci. Rep. 2015;5 doi: 10.1038/srep13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou Q., Zhao Y., Wang Y., et al. Associations between IL-23R gene polymorphisms and the susceptibility of rheumatoid arthritis: a meta-analysis. Artif. Cells, Nanomed. Biotechnol. 2019;47:951–956. doi: 10.1080/21691401.2019.1579731. [DOI] [PubMed] [Google Scholar]
- 40.Banerjee A.A., Mahale S.D. Extracellular loop 3 substitutions K589N and A590S in FSH receptor increase FSH-induced receptor internalization and along with S588T substitution exhibit impaired ERK1/2 phosphorylation. Arch. Biochem. Biophys. 2018;659:57–65. doi: 10.1016/j.abb.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Al-Naseri M.A., Salman E.D., Ad'hiah A.H. Association between interleukin-4 and interleukin-10 single nucleotide polymorphisms and multiple sclerosis among Iraqi patients. Neurol. Sci. 2019;40:2383–2389. doi: 10.1007/s10072-019-04000-4. [DOI] [PubMed] [Google Scholar]
- 42.Coto-Segura P., Batalla A., Gonzalez-Fernandez D., et al. CDKAL1 gene variants affect the anti-TNF response among Psoriasis patients. Int. Immunopharm. 2015;29:947–949. doi: 10.1016/j.intimp.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Zhernakova A., Withoff S., Wijmenga C. Clinical implications of shared genetics and pathogenesis in autoimmune diseases. Nat. Rev. Endocrinol. 2013;9:646–659. doi: 10.1038/nrendo.2013.161. [DOI] [PubMed] [Google Scholar]
- 44.Gladman D.D., Anhorn K.A., Schachter R.K., et al. HLA antigens in psoriatic arthritis. J. Rheumatol. 1986;13:586–592. [PubMed] [Google Scholar]
- 45.Kingo K., Mossner R., Koks S., et al. Association analysis of IL19, IL20 and IL24 genes in palmoplantar pustulosis. Br. J. Dermatol. 2007;156:646–652. doi: 10.1111/j.1365-2133.2006.07731.x. [DOI] [PubMed] [Google Scholar]
- 46.Mossner R., Frambach Y., Wilsmann-Theis D., et al. Palmoplantar pustular psoriasis is associated with missense variants in CARD14, but not with loss-of-function mutations in IL36RN in European patients. J. Invest. Dermatol. 2015;135:2538–2541. doi: 10.1038/jid.2015.186. [DOI] [PubMed] [Google Scholar]
- 47.Nissen M.J., Syrogiannopoulou A., Gabay C. Coexistence of seropositive rheumatoid arthritis and SAPHO syndrome. Joint Bone Spine. 2013;80:674–676. doi: 10.1016/j.jbspin.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 48.Ergun T., Inanc N., Tuney D., et al. Skin manifestations of rheumatoid arthritis: a study of 215 Turkish patients. Int. J. Dermatol. 2008;47:894–902. doi: 10.1111/j.1365-4632.2008.03708.x. [DOI] [PubMed] [Google Scholar]
- 49.Li C., Zuo Y., Wu N., et al. Synovitis, acne, pustulosis, hyperostosis and osteitis syndrome: a single centre study of a cohort of 164 patients. Rheumatology. 2016;55:1023–1030. doi: 10.1093/rheumatology/kew015. [DOI] [PubMed] [Google Scholar]
- 50.Grosjean C., Hurtado-Nedelec M., Nicaise-Roland P., et al. Prevalence of autoantibodies in SAPHO syndrome: a single-center study of 90 patients. J. Rheumatol. 2010;37:639–643. doi: 10.3899/jrheum.090863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data relevant to this study are not stored in publicly available repositories. Data will be made available on request.