Abstract
Background
No study has examined the association between constipation and atopic dermatitis (AD) in infants and toddlers. We aimed to explore that association in toddlers using the data from a nationwide birth cohort study.
Methods
From the Japan Environment and Children’s Study, a nationwide prospective birth cohort study that began in 2011, children born in a singleton live birth were analyzed. Participants completed questionnaires containing questions related to bowel movements and AD, during 1.5 to 3 years after birth. Constipation at 1 year of age was defined as having ≤2 bowel movements per week. AD was defined based on participant’s responses to the modified ISAAC questionnaire and/or self-reported physician’s diagnosis. Outcome was defined as the cumulative number of AD cases that occurred until 3 years of age. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for development of AD were calculated by a multivariable logistic regression.
Results
From a total of 62,777 participants who met the study inclusion criteria, 14,188 children (22.6%) were affected by AD between the ages of 1.5 and 3 years. The adjusted OR of developing AD for the presence versus absence of constipation at 1 year of age was 1.18 (95% CI, 1.01–1.38).
Conclusion
Constipation at 1 year of age was associated with a slightly higher risk of AD until 3 years of age.
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1265/ehpm.23-00103.
Keywords: Atopic dermatitis, Child, Cohort study, Constipation, Dysbiosis, Eczema, Gut microbiota
Introduction
Atopic dermatitis/eczema (AD) is a skin disease commonly seen around the world, affecting approximately 10% to 20% of children in Japan [1–3]. Although the pathogenesis of AD has not been fully elucidated, multiple genetic and environmental factors are thought to influence the onset and progress of the disease [1, 4, 5]. With the recent improvements in genetic analysis technology, comprehensive genetic studies of the gut microbiota revealed that the diversity of the gut microbiota was reduced in children with AD, called as dysbiosis [6–8].
As for functional constipation defined as constipation with no underlying organic cause, studies of stools in children with constipation have shown that dysbiosis affects constipation through a variety of mechanisms including, (1) changes in the composition of the intestinal microflora, such as an increase in the ratio of Clostridium spp. and Bifidobacterium spp., (2) changes in peristaltic movement due to the action of gases and organic substances produced by the metabolism of the intestinal microflora, (3) changes in peristalsis due to neuroendocrine factors such as gastrin, serotonin, and motilin [9–11]. Previously two epidemiological studies of adolescents and adults examined the association between constipation and AD [12, 13]; both of which reported that constipation was a risk factor for the development of AD. However, no such study has examined this issue in children, especially in infants and toddlers.
In the present study, we aimed to investigate whether functional constipation at 1 year of age is a risk factor for the development of AD.
Methods
Study design
The present study analyzed data from the Japan Environment and Children’s Study (JECS), a government-funded, prospective birth cohort study that began in January 2011 [14, 15]. For the JECS, 15 Regional Centres were selected: Hokkaido, Miyagi, Fukushima, Chiba, Kanagawa, Koshin, Toyama, Aichi, Kyoto, Osaka, Hyogo, Tottori, Kochi, Fukuoka and Southern Kyushu/Okinawa. Expectant women who were living in the Study Area at the time of enrollment; who were expected to continue to live there for the foreseeable future; whose due date of delivery was between January 2011 and March 2014; and who were able to participate in the study without difficulty (that is, had adequate Japanese language comprehension to completely respond to self-administered questionnaire), were included in the JECS study population. Details of the JECS protocol have been described elsewhere [14, 15]. We obtained outcomes, exposures, and covariates data through self-administered questionnaires, and medical record transcriptions. In JECS, the questionnaires were distributed at enrollment, during the second or third trimesters of pregnancy, one month after childbirth, and every six months after childbirth until 3 years of age. In addition, medical records, at enrollment, delivery, and one month after birth, were transcribed by physicians, midwives/nurses, and/or Research Co-ordinators.
Study population
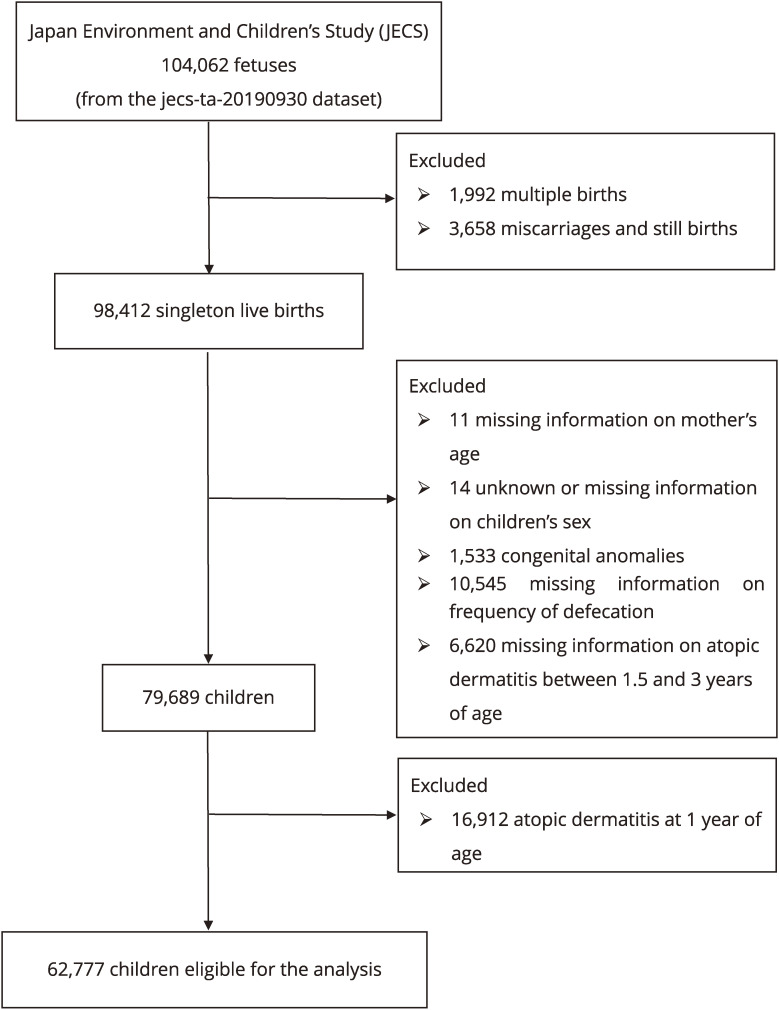
We used the jecs-ta-20190930 data set, which was released in October 2019 and revised in June 2021. Among 104,062 fetal records, 98,412 singleton live births were included. Following cases were excluded: missing information on mother’s age (n = 11), unknown or missing information on children’s sex (n = 14), congenital anomalies which potentially cause constipation (n = 1,533), missing information on frequency of defecation (n = 10,545), and missing information on AD (n = 6,620). The missing information on AD was defined as no response to the question on AD at 1.5, 2, and 3 years of age. Also, children with the presence of AD at 1 year of age were excluded (n = 16,912). The final population of this study comprised of 62,777 participants (Fig. 1).
Fig. 1.
Flow chart of the participant selection
Exposure variables
From the questionnaire, we obtained data regarding constipation at 1 year of age. Constipation in young children is mostly functional constipation [16], which is often diagnosed by the ROME criteria when, two or more of the six questions in the questionnaire are satisfactorily answered [17, 18]. In the JECS questionnaire, there are only two sequential questions on constipation for the age of 1 year of age, “does your child poop almost every day?”, and “if not, how many times a week does your child poop?”. Based on the response, we defined “constipated” as having ≤2 bowel movements per week, and “normal” as defecation almost every day, which we used as a reference group.
Outcome measures
We obtained the data regarding AD from the questionnaires at 1.5, 2, and 3 years of age. Outcome was defined as the cumulative number of AD cases that occurred between 1.5 and 3 years of age, and the number of cases were counted if AD occurred at least once in the questionnaire at 1.5, 2, or 3 years of age. The definition of AD was based on the answer of “yes” to the question, “Has your child ever had a recurring itchy rash for at least two months? (in the questionnaire at 1.5 years of age)”, and “Has your child ever had an itchy recurring rash for at least 6 months? (in the questionnaires at 2 and 3 years of age)”. These questions are partly adapted from Japanese version of the ISAAC questionnaire for ages 6–7, which were translation validated in Japanese [19–21]. Self-reported physician’s diagnosis was also included as the definition of AD.
Covariates
The following items were analyzed as potential confounding factors during pregnancy: mother’s age at delivery (<25, 25–29, 30–34, ≥35 years), maternal body mass index (BMI) before pregnancy (<18.5, 18.5–19.9, 20–22.9, 23–24.9, ≥25 kg/m2), maternal smoking during pregnancy (yes, no), maternal educational status (<13, 13–15, ≥16 years), maternal allergic history including AD, asthma, hay fever, food allergy, and allergic conjunctivitis (yes, no). Also, the following items were analyzed as potential confounding factors during childhood: child’s sex (male, female), birth weight (<2500, 2500–2999, 3000–3499, ≥3500 g), mode of delivery (vaginal, cesarean section), nutrition until six months of age (exclusive breastfeeding, partial breastfeeding, formula feeding), day care attendance at six months of age (yes, no), and keeping pets indoors (yes, no). We obtained the data regarding maternal BMI (height and weight) from medical records transcription at enrollment; maternal history of allergic diseases and smoking from the questionnaire during the first trimester; maternal educational status from the questionnaire during the second or third trimester; maternal age, child’s sex, child’s birthweight from medical records transcription at delivery; regarding child’s feeding style, day nursery attendance from the questionnaire at six months after birth; and pet ownership from the questionnaire at 1.5 years after birth.
Statistical analysis
The association between constipation at 1 year of age, and cumulative incidence of AD until 1.5, 2, and 3 years of age was assessed. We used multivariable logistic regression analyses to estimate the crude, and adjusted odds ratios (ORs and aORs, respectively) of the AD with 95% confidence intervals (CI), using the “normal” group as a reference. We also performed the regression analyses when AD was defined solely by the responses to the questionnaire and when AD was defined solely by the self-reported physician’s diagnosis. For multivariate analysis, mother’s age at delivery, maternal BMI before pregnancy, smoking during pregnancy, educational status, allergic history (AD, asthma, hay fever, food allergy, and allergic conjunctivitis), child’s sex, birth weight, mode of delivery, feeding pattern, day care attendance, and keeping pets indoors were adjusted.
We conducted all statistical analyses using SAS statistical software version.9.4 (SAS Institute Inc., Cary, NC, USA). p < .05 was considered statistically significant.
Results
There were 104,062 fetal records in the JECS 2019 dataset, and 62,777 participants met the study inclusion criteria (Fig. 1). Table 1 summarizes the sociodemographic and medical characteristics of the participants. Overall, 14,188 children (22.6%) were suffered from AD until 3 years of age. Table 2 presents ORs and aORs with 95% CI of the association between constipation at 1 year of age, and cumulative incidence of AD until 3 years of age. The cumulative incidence of AD was significantly higher among constipated children who had had ≤2 bowel movements per week at 1 year of age, with aOR of 1.18 (95% CI, 1.01–1.38). Such an association was similarly observed for the cumulative incidence of AD until 2 years of age, and at 1.5 years of age, with aOR of 1.18 (95% CI, 0.99–1.40), and 1.15 (95% CI, 0.92–1.43), respectively.
Table 1.
Characteristics of the participants
|
Total (%)
(N = 62,777) |
Cases (%)
(n = 14,188) |
|||
| Frequency of bowel movements at 1 year of age (per week) | ||||
| Almost everyday | 54,672 | (87.1) | 12,328 | (86.9) |
| 5–6 | 2,624 | (4.2) | 561 | (4.2) |
| 3–4 | 4,631 | (7.4) | 989 | (7.4) |
| ≤2 times per week | 850 | (1.4) | 207 | (1.5) |
| (Maternal and family characteristics) | ||||
| Maternal age at delivery (year) | ||||
| <25 | 5,205 | (8.3) | 1,235 | (8.7) |
| 25–29 | 17,090 | (27.2) | 4,013 | (28.3) |
| 30–34 | 22,835 | (36.4) | 5,112 | (36.0) |
| ≥35 | 17,647 | (28.1) | 3,828 | (27.0) |
| Maternal history of atopic dermatitis | ||||
| No | 53,943 | (85.9) | 11,441 | (80.6) |
| Yes | 8,834 | (14.1) | 2,747 | (19.4) |
| Maternal history of asthma | ||||
| No | 56,577 | (90.1) | 12,419 | (87.5) |
| Yes | 6,200 | (9.9) | 1,769 | (12.5) |
| Maternal history of hay fever | ||||
| No | 40,780 | (65.0) | 8,688 | (61.2) |
| Yes | 21,997 | (35.0) | 5,500 | (38.8) |
| Maternal history of food allergy | ||||
| No | 60,052 | (95.7) | 13,409 | (94.5) |
| Yes | 2,725 | (4.3) | 779 | (5.5) |
| Maternal history of allergic conjunctivitis | ||||
| No | 56,836 | (90.5) | 12,522 | (88.3) |
| Yes | 5,941 | (9.5) | 1,666 | (11.7) |
| Maternal BMI before pregnancy (kg/m2) | ||||
| <18.5 | 10,097 | (16.1) | 2,306 | (16.3) |
| 18.5–19.9 | 15,648 | (24.9) | 3,472 | (24.5) |
| 20–22.9 | 24,035 | (38.3) | 5,396 | (38.0) |
| 23–24.9 | 6,646 | (10.6) | 1,569 | (11.1) |
| ≥25 | 6,314 | (10.1) | 1,437 | (10.1) |
| Missing | 37 | (0.1) | 8 | (0.1) |
| Maternal smoking during pregnancy | ||||
| No | 59,718 | (95.1) | 13,384 | (94.3) |
| Yes | 2,352 | (3.8) | 636 | (4.5) |
| Missing | 707 | (1.1) | 168 | (1.2) |
| Maternal educational status (year) | ||||
| ≤12 | 21,136 | (33.7) | 4,772 | (33.6) |
| 13–15 | 26,822 | (42.7) | 6,185 | (43.6) |
| ≥16 | 14,175 | (22.6) | 3,071 | (21.7) |
| Missing | 644 | (1.0) | 160 | (1.1) |
| Child’s sex | ||||
| Male | 31,234 | (49.8) | 7,112 | (50.1) |
| Female | 31,543 | (50.3) | 7,076 | (49.9) |
| Birth weight (g) | ||||
| <2,500 | 5,003 | (8.0) | 1,104 | (7.8) |
| 2,500–2,999 | 24,532 | (39.1) | 5,526 | (39.0) |
| 3,000–3,499 | 26,342 | (42.0) | 5,966 | (42.1) |
| ≥3,500 | 6,745 | (10.7) | 1,558 | (11.0) |
| Missing | 155 | (0.3) | 34 | (0.2) |
| Mode of delivery | ||||
| Vaginal delivery | 50,935 | (81.1) | 11,541 | (81.3) |
| Caesarean section | 11,571 | (18.4) | 2,584 | (18.1) |
| Missing | 271 | (0.4) | 63 | (0.4) |
| Nutirtion until the age of 6 months | ||||
| Exclusive breastfeeding | 24,700 | (39.4) | 5,683 | (40.1) |
| Partial breastfeeding | 24,891 | (39.7) | 5,579 | (39.3) |
| Formula feeding | 12,720 | (20.3) | 2,826 | (19.9) |
| Missing | 466 | (0.7) | 100 | (0.7) |
| Day care attendance at 6 months of age | ||||
| No | 58,039 | (92.5) | 12,903 | (90.9) |
| Yes | 4,136 | (6.6) | 1,140 | (8.0) |
| Missing | 602 | (1.0) | 145 | (1.1) |
| Pet ownership at 1.5 years of age | ||||
| No | 51,390 | (81.9) | 11,609 | (81.8) |
| Yes | 10,143 | (16.2) | 2,312 | (16.3) |
| Missing | 1,244 | (2.0) | 267 | (1.9) |
BMI: body mass index
Table 2.
Association between frequency of bowel movements and cumulative incidence of atopic dermatitis
| Frequency of bowel movements | ||||
|
| ||||
| Alomost everyday | 5–6 times/wk | 3–4 times/wk | ≤2 times/wk | |
| Cumulative incidence until 3 years of age | ||||
| No. at risk | 54,672 | 2,624 | 4,631 | 850 |
| No. of cases (%) | 12,328 (22.6) | 598 (22.8) | 1043 (22.5) | 219 (25.8) |
| Crude OR (95% CI) | ref | 1.01 (0.92–1.11) | 1.00 (0.93–1.07) | 1.19 (1.02–1.39) |
| Multivariable OR (95% CI)a | ref | 1.02 (0.93–1.12) | 1.00 (0.93–1.07) | 1.18 (1.01–1.38) |
| Cumulative incidence until 2 years of age | ||||
| No. at risk | 56,579 | 2,717 | 4,805 | 882 |
| No. of cases (%) | 9,253 (16.4) | 468 (17.2) | 778 (16.2) | 165 (18.7) |
| Crude OR (95% CI) | ref | 1.06 (0.96–1.18) | 0.99 (0.91–1.07) | 1.18 (0.99–1.40) |
| Multivariable OR (95% CI)a | ref | 1.07 (0.97–1.19) | 0.99 (0.91–1.07) | 1.18 (0.99–1.40) |
| Cumulative incidence until 1.5 years of age | ||||
| No. at risk | 57,820 | 2,782 | 4,885 | 905 |
| No. of cases (%) | 5,175 (9.0) | 263 (9.5) | 437 (9.0) | 92 (10.2) |
| Crude OR (95% CI) | ref | 1.06 (0.93–1.21) | 1.00 (0.90–1.11) | 1.15 (0.93–1.43) |
| Multivariable OR (95% CI)a | ref | 1.07 (0.94–1.22) | 1.00 (0.90–1.11) | 1.15 (0.92–1.43) |
CI: confidence interval; OR: odds ratio
aAdjusted by maternal characteristics such as mother’s age at delivery, maternal body mass index before pregnancy, maternal smoking, maternal educational status, maternal allergic history including atopic dermatitis, asthma, hay fever, food allergy, and allergic conjunctivitis. Also adjusted by child’s characteristics such as child’s sex, birth weight, mode of delivery, exclusive breastfeeding for 6 months or more, day care attendance, and pet ownership.
The above associations were similarly observed when AD was defined solely by the responses to the questionnaire (Supplementary Table 1). However, when AD was defined solely by the physician’s diagnosis, no associations were observed.
Discussion
To the best of our knowledge, this is the first study to investigate the association between constipation and AD using large cohort data of children. In this study, constipation (the frequency of defecation ≤2 times per week) at 1 year of age was associated with a higher risk of AD until 3 years of age, compared to no constipation. No excess risk of AD was found for the frequency of defecation of 5–6 times per week, or 3–4 times per week.
These association did not change materially when AD was defined solely by the responses of “yes” to the questionnaire. However, no associations were observed when AD was defined solely by the physician’s diagnosis. Many physicians were general pediatricians but not an allergist or a dermatologist so that the diagnosis of AD varied largely which led to the dilution of the associations.
Two previous reports examined the association between constipation, and the development of AD. Tokunaga et al. conducted a cross-sectional study of approximately 20,000 Japanese high school students aged 15 years and older [12], and showed that constipation, based on self-administered questionnaire, increased the prevalence of AD (diagnosed by an allergist, or a dermatologist), by approximately 17%, compared with the normal group. Huang YC et al. conducted a retrospective cohort study using the Taiwan National Insurance database [13], and reported that patients with constipation (ICD-9-CM codes = 564.0: with at least three outpatient visits or one hospitalization) had a higher risk of developing AD (ICD-9-CM codes = 691) than non-constipated patients, with adjusted hazard ratios of 1.89 (95% CI: 1.58–2.27) for ages <6 years, and of 2.31 (95% CI: 2.17–2.46) for all ages including ≥65 years.
Although the pathophysiological mechanisms between constipation and AD have not been fully elucidated, Lee SY et al. proposed that the gut microbiota under no constipation may influence the pathogenesis of host AD through three pathways [22]. The first is the probiotic-mediated immunological pathway, which can lead pro-inflammatory or anti-inflammatory status through the production of various kinds of cytokines, depending on the probiotic strains. The second is the metabolic pathway, in which dietary fiber ingested by the host is metabolized by the gut microbiome to increase the amount of short-chain fatty acids in the intestinal tract, which have anti-inflammatory effects. Moreover, Kynurenic acid produced by the ingestion of certain lactic acid bacteria reduces itching sensation. The third is a neuroendocrine pathway in which tryptophan produced by the gut microbiota aggravates itching sensation, while gamma aminobutyric acid produced by some lactic acid bacteria reduces itching sensation.
There are several limitations of this study. The JECS questionnaire differed from the ROME criteria [17, 18], which is used globally. The prevalence of childhood constipation was reported to be 9.5% in the meta-analysis by Koppen et al. [23] in which constipation was defined by ROME criteria; the present study showed that the proportion of constipated children who had ≤2 bowel movements per week at 1 year of age was only 1.35%, indicating that constipation may have been underestimated. The second is, since the dataset used in this study is mostly Japanese, it is not clear whether the findings of this study can be generalized to other ethnic groups. Finally, residual confounding could have occurred from unmeasured confounding variables, such as history of post-natal antimicrobial use.
Our study provides a new insight for the role of constipation on the development of AD in early childhood albeit the magnitude of excess risk was small and clinical significance may be limited. It is possible to detect AD at an early stage by recognizing the signs of constipation using the frequency of defecation, and the early treatment of constipation could prevent the risk or worsening of AD, which will need to be verified by further investigation.
Conclusion
Functional constipation at 1 year of age was associated with a slightly higher risk of AD until 3 years of age. Further studies are necessary to examine the association after 3 years of age because AD may develop at later ages.
Abbreviations
- AD
atopic dermatitis
- aOR
adjusted odds ratio
- BMI
body mass index
- CI
confidence intervals
- ICD-9-CM
International Classification of Diseases 9th Revision Clinical Modification
- ISAAC
International Study of Asthma and Allergies in Childhood
- JECS
Japan Environment and Children’s Study
- OR
odds ratio
Supplementary information
Additional file 1: Supplemental Table 1 Association between frequency of bowel movements and cumulative incidence of atopic dermatitis.
Declarations
Ethics approval and consent to participate
The JECS was conducted in accordance with the Declaration of Helsinki and local regulations; the JECS protocol was reviewed and approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies and the Ethics Committees of all participating institutions. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Availability of data and material
Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restricts the open sharing of the epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.
Competing interests
The authors declare that they have no conflicts of interest.
Funding
The study was funded by the Ministry of the Environment, Japan. The findings in this article are exclusively the responsibility of the authors and are not the official position of the above-mentioned government.
Author’s contributions
YT contributed to the study conception, the formal analysis and wrote the first version of the manuscript. YA contributed to the formal analysis and reviewed the manuscript and contributed to the discussion. SI, KT, SB, KO, and ST reviewed the manuscript and contributed to the discussion. HI reviewed the manuscript, contributed to the discussion, and supervised the study.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Acknowledgements
The authors thank all participants of the JECS and all staff members involved in this study.
The members of JECS group as of the year 2023 are as follows: Members of the JECS Group as of 2023: Michihiro Kamijima (principal investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Tomotaka Sobue (Osaka University, Suita, Japan), Masayuki Shima (Hyogo Medical University, Nishinomiya, Japan), Hiroshige Nakamura (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
Author’s information
ORCID ID: Yoshihiko Takano https://orcid.org/0000-0002-7105-8328
References
- 1.Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy Eur J Allergy Clin Immunol. 2014;69(1):3–16. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg JI, Barbarot S, Gadkari A, Simpson EL, Weidinger S, Mina-Osorio P, et al. Atopic dermatitis in the pediatric population: A cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126(4):417–428.e2. doi: 10.1016/j.anai.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Deckers IAG, McLean S, Linssen S, Mommers M, van Schayck CP, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: A systematic review of epidemiological studies. PLoS One. 2012;7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katoh N, Ohya Y, Ikeda M, Ebihara T, Katayama I, Saeki H, et al. Japanese guidelines for atopic dermatitis 2020. Allergol Int. 2020;69(3):356–69. doi: 10.1016/j.alit.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–22. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- 6.Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129(2). [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Kim H. Microbiome of the Skin and Gut in Atopic Dermatitis (AD): Understanding the Pathophysiology and Finding Novel Management Strategies. J Clin Med. 2019;8(4):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddel S, Del Chierico F, Quagliariello A, Giancristoforo S, Vernocchi P, Russo A, et al. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci Rep. 2019;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohkusa T, Koido S, Nishikawa Y, Sato N. Gut microbiota and chronic constipation: A review and update. Front Med. 2019;6(FEB):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avelar Rodriguez D, Popov J, Ratcliffe EM, Toro Monjaraz EM. Functional Constipation and the Gut Microbiome in Children: Preclinical and Clinical Evidence. Front Pediatr. 2021;8(January):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Yu YB. Intestinal microbiota and chronic constipation. Springerplus. 2016;5(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokunaga T, Ninomiya T, Osawa Y, Imoto Y, Ito Y, Takabayashi T, et al. Factors associated with the development and remission of allergic diseases in an epidemiological survey of high school students in Japan. Am J Rhinol Allergy. 2015;29(2):94–9. [DOI] [PubMed] [Google Scholar]
- 13.Huang YC, Wu MC, Wang YH, Wei JCC. Influence of constipation on atopic dermatitis: A nationwide population-based cohort study in Taiwan. Int J Clin Pract. 2021;75(3):1–9. [DOI] [PubMed] [Google Scholar]
- 14.Kawamoto T, Nitta H, Murata K, Toda E, Tsukamoto N, Hasegawa M, et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health. 2014;14(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michikawa T, Nitta H, Nakayama SF, Yamazaki S, Isobe T, Tamura K, et al. Baseline profile of participants in the Japan environment and children’s study (JECS). J Epidemiol. 2018;28(2):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauvat F. Diagnosis of constipation in children. Ann Nestle. 2007;65(2):63–71. [Google Scholar]
- 17.Hyman PE, Milla PJ, Benninga MA, Davidson GP, Fleisher DF, Taminiau J. Childhood Functional Gastrointestinal Disorders: Neonate/Toddler. Gastroenterology. 2006;130(5):1519–26. [DOI] [PubMed] [Google Scholar]
- 18.Fukudo S, Hongo M MK. ROME III: the functional gastrointestinal disorders. 3rd ed. Tokyo: Kyowa Kikaku, Tokyo; 2008. [Google Scholar]
- 19.Ellwood P, Asher MI, Beasley R, Clayton TO, Stewart aW. Phase Three Manual of the International Study of Asthma and Allergies in Childhood (ISAAC). Int J Tuberc Lung Dis. 2005;9(August 2004):10–6. [PubMed] [Google Scholar]
- 20.Weiland SK, Björkstén B, Brunekreef B, Cookson WOC, von Mutius E, Strachan DP, et al. Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC II): Rationale and methods. Eur Respir J. 2004;24(3):406–12. [DOI] [PubMed] [Google Scholar]
- 21.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International study of asthma and allergies in childhood (ISAAC): Rationale and methods. Eur Respir J. 1995;8(3):483–91. [DOI] [PubMed] [Google Scholar]
- 22.Lee SY, Lee E, Park YM, Hong SJ. Microbiome in the gut-skin axis in atopic dermatitis. Allergy Asthma Immunol Res. 2018;10(4):354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koppen IJN, Vriesman MH, Saps M, Rajindrajith S, Shi X, van Etten-Jamaludin FS, et al. Prevalence of Functional Defecation Disorders in Children: A Systematic Review and Meta-Analysis. J Pediatr. 2018;198:121–130.e6. doi: 10.1016/j.jpeds.2018.02.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table 1 Association between frequency of bowel movements and cumulative incidence of atopic dermatitis.
Data Availability Statement
Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restricts the open sharing of the epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.