Abstract
Canola (Brassica napus L.) meal represents a prominent alternative plant-based source for protein isolation. This work aimed to investigate the combined effect of extraction and purification methods for the production of canola protein isolates (CPIs). CPIs were characterized in terms of process yield, protein recovery, basic composition, amino acid profile, in vitro protein digestibility, techno-functional properties, structural properties, and molecular features. The results showed that the Alk-Uf method enhanced yield (16.23 %) and protein recovery (34.88 %). Meanwhile, the Et-Alk-Uf method exhibited the highest crude protein (89.71 %) and free amino nitrogen (4.34 mg g protein−1) contents. Furthermore, protein digestibility (95.5 %) and protein digestibility corrected amino acid score (1.0) were improved using the Et-Alk-Ac method. Conversely, the amino acid composition, secondary structure, and electrophoretic profiles were generally similar for all CPIs. The Alk-Uf and Et-Alk-Uf methods produced isolates with the highest water solubility (∼39.18 %), water absorption capacity (∼3.86 g water g protein−1), oil absorption capacity (∼2.77 g oil g protein−1), and foaming capacity (∼505.26 %). Finally, the foaming stability (93.75 %) and foaming density (34.38 %) were increased when employing the Alk-Ac method. These findings suggest that, in general, the Alk-Uf and Et-Alk-Uf methods can be used to obtain CPIs with high added value for use in food formulations.
Keywords: Canola meal, Protein isolate, Alkaline extraction, Ultrafiltration, Food application
1. Introduction
Dietary protein is valuable in human nutrition. It provides the required amount of nitrogen and amino acids, which are essential to maintain the structural and biological functions of the body [1]. Inadequate dietary protein intake is considered a major public health concern in developing countries [2]. In this sense, the prevalence of malnutrition and the elevated cost of animal proteins have led scientists and technologists to explore plant-based alternative protein sources [3]. Among the most promising sources, agro-industrial by-products generated by the edible oil industry are a sustainable alternative for the recovery of proteins [4]. After processing raw seeds to extract oil, a large amount of defatted residues with an outstanding protein content is generated, which could be feasibly used for human consumption [3].
Under this context, canola (Brassica napus L.) is the third major cultivated oilseed crop in the world after palm and soybean [5]. Canola oil is one of the healthiest edible vegetable oils. It contains a low level of saturated fatty acids (SFAs, 6 %) and significant amounts of monounsaturated- (MUFAs, 62 %) and polyunsaturated-fatty acids (PUFAs, 32 %) [6]. After the oil extraction process, canola meal (CM) is the main by-product obtained and contains ca. 36–40 % of protein on a dry weight basis. This protein content is comprised of globular storage proteins cruciferin (12S globulin) and napin (1.7-2S albumin), and structural protein oleosin [7,8]. CM proteins can have several potential applications within the industry. For instance, they can be used to produce polymers, coatings, lubricants, detergents, and adhesives, among others [[9], [10], [11]]. Nevertheless, the direct food use of CM is scarce due to its color, flavor, and presence of antinutritional compounds, although its protein content has a well-balanced essential amino acid profile. For these reasons, the extraction and purification of proteins from CM can enhance the quality and functionality of this material, improving its potential to be used for several food applications [7,[12], [13], [14]].
Despite the large amount of defatted CM generated worldwide, and its properties, its use as a food ingredient is still limited due to the problem of separating the protein fraction from the non-protein part, which is comprised of fiber, phenols, and glucosinolates, among others [15]. The challenge is to design an efficient process that effectively removes non-protein compounds [16]. The most common process to purify canola proteins is alkaline and micellar extraction coupled with separation using isoelectric point and membrane technologies [13]. However, there are no studies of the extraction of canola proteins using ethanol pretreatment, which is known to remove part of non-protein polar components, including carbohydrates, soluble fiber, polyphenolic compounds, and pigments.
Based on the above, this work aimed to investigate the combined effect of extraction and purification methods to produce canola protein isolates (CPIs). CPIs were characterized in terms of process yield, protein recovery, basic composition, amino acid profile, in vitro protein digestibility, techno-functional properties, structural properties, and molecular features.
2. Materials and methods
2.1. Materials
Defatted CM was obtained from AGYDSA‒Aceites, Grasas y Derivados, S.A de C.V. (Zapopan, Jalisco, Mexico). Prior to each assay, a reduction in the particle size of defatted CM was carried out. For this purpose, the material was ground to powder using an electric grinder and passed through a 2 mm mesh sieve (Mill 300 AMA; Pulvex®, Ciudad de México, México). The particle size distribution (PSD) was determined with a laser light scattering in-situ particle size analyzer (Mastersizer 2000; Malvern Panalytical Ltd, UK). A commercial soy protein isolate (Fraca S.A de C.V., Tepatitlán de Morelos, Jalisco, México) was used as standard. All samples were kept in a freezer (−20 °C) until their use. All other reagents used were of analytical grade.
2.2. Extraction and purification of protein isolates
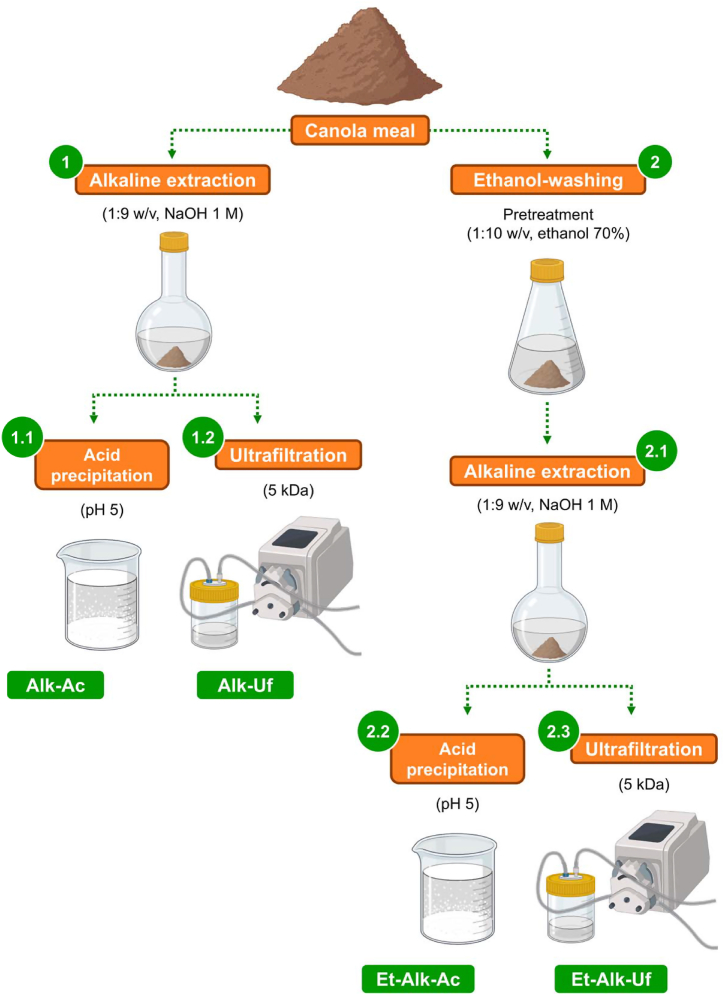
As depicted in the scheme in Fig. 1, four extraction and purification strategies, commonly used for the isolation of plant-based proteins [17], were evaluated for the production of CPIs. Alkaline extraction followed by acid precipitation, as well as pretreatment with organic solvent (ethanol) and subsequent membrane filtration (ultrafiltration), were selected based on previous studies reporting their simplicity and efficiency in preparing canola isolates [15,18]. The methods tested were i) alkaline protein extraction and acid precipitation (Alk-Ac, control method), ii) alkaline protein extraction and ultrafiltration (Alk-Uf), iii) pretreatment with ethanol, alkaline protein extraction, and acid precipitation (Et-Alk-Ac), or iv) pretreatment with ethanol, alkaline protein extraction, and ultrafiltration (Et-Alk-Uf). Details on how these treatments were performed are further described. Each treatment was replicated two times. After isolation, the protein suspension sample was lyophilized for 120 h (FreeZone 12L console; Labconco Corporation, Kansas City, MO, USA) and ground to powder (Cyclone mill 3010-014; Udy Corporation, Collins, CO, USA), which was stored at room temperature until analysis.
Fig. 1.
Scheme representation of methods employed for production of canola protein isolates (CPIs).
2.2.1. Alkaline extraction and acid precipitation (Alk-Ac)
The defatted CM was mixed with distilled H2O for alkaline extraction in a 1:9 (w/v) ratio. Then, the mixture solution was adjusted to pH 12 by the addition of NaOH (1.0 M) and incubated at 300 rpm and 60 °C for 15 min (Incubator-shaker RF1575; VWR International, Cornelius, OR, USA). After this period, the mixture solution was centrifuged at 3300×g and 4 °C for 10 min (Centrifuge 5804-R; Eppendorf®, Hamburg, Germany) and the supernatant was collected. The acid precipitation was based on the procedure of Klockeman et al. [19]. Briefly, the supernatant was mixed with acetic acid (glacial) until a pH of 5 was reached. Then, the precipitated protein was separated via centrifugation (3300×g and 4 °C for 10 min).
2.2.2. Alkaline extraction and ultrafiltration (Alk-Uf)
The alkaline extraction was performed as described in Section 2.2.1. Then, the collected supernatant was used directly for ultrafiltration. The ultrafiltration module consisted of a peristaltic pump (Sartoflow Tandem 1082; Sartorius AG, Göttingen, Germany) coupled with a polyethersulfone (PES) membrane with a molecular weight cut-off (MWCO) of 5 kDa. The system was operated in batch mode at room temperature, under a pressure range of 0.7–0.9 bar, and with recirculation of the retentate. When the retentate was reduced to 50 % volume, it was re-suspended with ultra-distilled H2O. The procedure was carried out seven times.
2.2.3. Ethanol pretreatment, alkaline extraction, and acid precipitation (Et-Alk-Ac)
The defatted CM was mixed with ethanol-H2O solution (70 % v/v) in a 1:10 (w/v) ratio for ethanol pretreatment. Then, the mixture solution was incubated at 300 rpm and 40 °C for 30 min. After this period, the mixture solution was centrifuged (3300×g and 4 °C for 10 min), and the supernatant was discarded. The obtained pellet was used for subsequent alkaline extraction and acid precipitation, as described in Section 2.2.1.
2.2.4. Ethanol pretreatment, alkaline extraction, and ultrafiltration (Et-Alk-Uf)
The ethanol pretreatment, alkaline extraction, and ultrafiltration procedures were carried out as described in Sections 2, 2.2.2.2.3, as appropriate.
2.3. Process yield and protein recovery
The process yield was determined as the ratio between the total mass of the recovered protein isolate, and the initial mass of defatted CM used in the extraction process (Equation (1)). Similarly, the protein recovery was estimated by comparing the total protein content in recovered protein isolate and the initial protein content in defatted CM (Equation (2)).
| (Equation 1) |
| (Equation 2) |
2.4. Characterization of protein isolates
2.4.1. Determination of moisture, protein, and free amino nitrogen contents
Moisture and crude protein (micro Kjeldahl method, N × 6.25) contents were determined using Association of Official Analytical Chemists (AOAC) standard methods 925.10 and 978.02, respectively [20]. To determine free α-amino nitrogen (FAN) content, the protein isolate was mixed with distilled H2O and incubated at room temperature for 1 h and 200 rpm. After this period, the mixture solution was centrifuged (3300×g and 4 °C for 20 min), and the supernatant was collected. Finally, the absorbance of the supernatant was spectrophotometrically recorded after carrying out the ninhydrin reaction as suggested by the AOAC 999.13 standard method [20]. The results were expressed as FAN content in milligrams per gram of protein isolate (mg g protein isolate−1).
2.4.2. Amino acid composition and protein digestibility
The amino acid composition was determined using high-pressure liquid chromatography (HPLC) in accordance with the AOAC 982.30 method [20]. On the other hand, the in vitro protein digestibility (IPD) was performed according to the method suggested by Hsu et al. [21]. Firstly, a multi-enzyme solution (pH 8) was prepared with trypsin (1.6 mg mL−1), chymotrypsin (3.1 mg mL−1), and protease (1.3 mg mL−1). Then, a protein-water solution (6.25 mg mL−1) was prepared and mixed with 1.5 mL of multienzyme solution. The mixture solution was incubated at 37 °C for 10 min in a water bath, and the pH drop was recorded. Finally, the IPD and protein digestibility corrected amino acid scores (PDCAAS) [22] were calculated according to Equations (Equation 3), (Equation 4), (Equation 5), as appropriate.
| (Equation 3) |
where Xf is the pH value of the mixture solution after hydrolysis.
| (Equation 4) |
| (Equation 5) |
when the limiting amino acid score value was ≥1, a truncated value of 1 was used.
2.4.3. Techno-functional properties
Water solubility index (WSI) and water absorption capacity (WAC) were analyzed following the method reported by Cheftel et al. [23]. The oil absorption capacity (OAC) was estimated according to the procedure of Ahn et al. [24]. Briefly, 0.25 g of protein isolate was mixed with 7.5 mL of soybean oil. Then, the mixture solution was incubated at room temperature for 30 min and 300 rpm. After this period, the mixture solution was centrifuged (14000×g and 4 °C for 10 min), and the supernatant was discarded. The results were expressed as grams of oil absorbed per gram of protein isolate (g oil g protein−1).
The foaming capacity (FC), stability (FS), and density (FD) were measured according to Haque & Kito [25]. First, a protein-water solution (3 % w/v) was prepared and whipped (Oster blender FPSTHM2600-013; Newell Brands México Ltd., Mexico City, Mexico) at high speed for 15 min. Next, the FC was estimated by comparing the foam volume of the solution after whipping and the initial volume of the solution (Equation (6)). Similarly, the FS was calculated as the ratio between the foam volume recorded 60 min after whipping and the initial foam volume (Equation (7)). Finally, the FD was estimated according to Equation (8).
| (Equation 6) |
| (Equation 7) |
| (Equation 8) |
2.4.4. Attenuated total reflectance-Fourier transform infrared spectroscopy
Secondary structure composition was determined by Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR) analysis. The spectra were obtained with a Spectrum One spectrometer (PerkinElmer, MA, USA). Air in the empty crystal was used as background, and 20 scans were recorded at 4/cm resolution, within the frequency range of 4000–800 cm−1. Fourier self-deconvolution was carried out using the software Spectrum with K-factor of 1.5. Peak intensities were obtained from the deconvoluted spectra to calculate the percentages of α-helix (1654 cm−1), β-sheet (1618 cm−1; 1625 cm−1; 1679 and 1695 cm−1), and disordered structures (1639 cm−1) [26].
2.4.5. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), under non-reducing conditions, was used to qualitatively determine the protein profile of isolates according to the method of Laemmli [27]. Electrophoresis was conducted in a Mini-Protean II electrophoresis cell (Bio-Rad Laboratories, Hercules, CA, USA) at a constant voltage (100 V) for 1 h. Aliquots containing 15 μg mL−1 of protein were loaded onto the specified lane. After migration, the intensities of bands were analyzed using the GelAnalyzer software (version 19.1; freely available at http://www.gelanalyzer.com).
2.5. Statistical analysis
Experimental data represent three independent repetitions and are reported as mean ± standard deviation (SD). For statistical analysis, one-way ANOVA was carried out, followed by Tukey-Kramer's multiple comparison test to settle the significance of differences (p < 0.05) among mean values. All statistical tests were performed using the Minitab 16 Statistical Software (Minitab, Inc., State College, PA, USA).
3. Results and discussion
3.1. Particle size distribution (PSD) of defatted CM
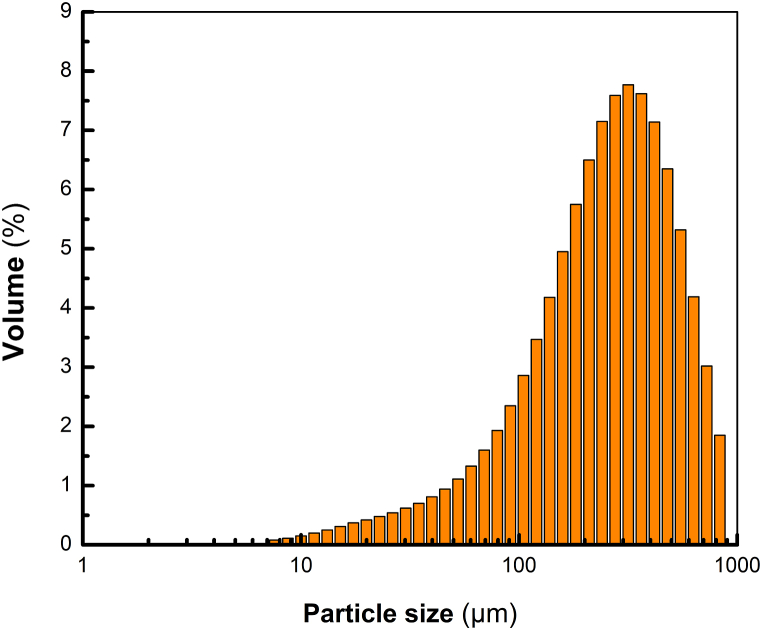
Fig. 2 presents the PSD of defatted CM. As can be observed, the material showed a unimodal distribution with a mean diameter (D50) value of 276.19 μm. Previous studies have suggested that the particle size of the material may influence protein recovery [28,29]. Indeed, particle size reduction has been reported to clearly enhance protein extraction by increasing surface contact between the solid and extraction solvent [30]. In this context, Rommi et al. [29] reduced the particle size (21 μm) of CM and evaluated the protein recovery after applying an alkaline extraction process (pH 12). The results showed that 74 % of the protein was recovered. Similarly, Das Purkayastha et al. [31] observed that reducing the particle size (250 μm) of CM improved the protein recovery by ca. 40–50 %. On the other hand, Vishwanathan et al. [32] achieved a maximum protein recovery of 93–97 % after reducing the particle size (<75 μm) of soybean and okara (Glycine max) flours and subjecting them to alkaline extraction. These results support the importance of reducing the particle size in raw material to impact protein recovery positively.
Fig. 2.
Particle size distribution of defatted canola meal (CM).
3.2. Process yield and protein recovery
Table 1 summarizes the process yield and protein recovery obtained by the different extraction and purification methods. Significant differences (p < 0.05) were detected among methods. The process yield ranged from 6.50 to 16.23 %, in ranking order of Alk-Ac < Et-Alk-Ac < Et-Alk-Uf < Alk-Uf. Meanwhile, the protein recovery reached values from 18.49 to 34.88 % with the ranking order of Et-Alk-Ac < Alk-Ac < Et-Alk-Uf < Alk-Uf. Overall, the results of both parameters agree with those previously reported to produce CM [31], yellow pea (Pisum sativum L.) [33], and lupin (Lupinus albus L.) [34] protein isolates.
Table 1.
Process yield, protein recovery, and basic composition of canola protein isolates (CPIs) obtained by different methods.
| Parameter | Soy protein | Alk-Ac* | Alk-Uf | Et-Alk-Ac | Et-Alk-Uf |
|---|---|---|---|---|---|
| Process yield (%) | – | 6.50 ± 0.52b | 16.23 ± 1.43a | 8.85 ± 0.59b | 9.65 ± 0.65b |
| Protein recovery (%) | – | 19.54 ± 0.37b | 34.88 ± 2.53a | 18.49 ± 0.53b | 20.61 ± 1.73b |
| Moisture (%)** | 9.56 ± 0.03a | 6.50 ± 0.39b | 6.15 ± 0.10b | 3.45 ± 0.32c | 3.99 ± 0.14bc |
| Crude protein (%)** | 68.45 ± 0.46c | 85.15 ± 2.66b | 88.1 ± 1.45ab | 88.17 ± 3.01ab | 89.71 ± 1.33a |
| FAN (mg g protein−1)** | 1.27 ± 0.07c | 1.76 ± 0.14bc | 4.27 ± 0.15a | 2.14 ± 0.21b | 4.34 ± 0.44a |
Different superscript letters within the same row indicate significant (p < 0.05) differences among treatments. ------, not determined. Alk-Ac, alkaline extraction and acid precipitation; Alk-Uf, alkaline extraction and ultrafiltration; Et-Alk-Ac, ethanol pretreatment, alkaline extraction, and acid precipitation; Et-Alk-Uf, ethanol pretreatment, alkaline extraction, and ultrafiltration; FAN, free amino nitrogen. *Control method. **Parameters expressed as dry basis.
The highest extraction and purification efficiency was obtained by using the Alk-Uf method. The effectiveness of alkaline extraction could be attributed to 1) the ability of NaOH to disrupt the fibers and extract more soluble matter from the raw material or 2) the neutralization of side amine groups of basic amino acids (e.g., lysine and arginine), thus increasing the negative surface charge and solubility of protein [33,35]. Meanwhile, ultrafiltration is one of the most efficient techniques to recover protein with high yield and purity due to its pore size and molecular cut-off [36]. Interestingly, the effect of ethanol pretreatment did not result in enhanced process yield and protein recovery, as the values of Et-Alk-Ac and Et-Alk-Uf were comparable to those of Alk-Ac. Peng et al. [37] have described that the low polarity of ethanol could inhibit cell wall disruption, limiting protein extractability. Based on this rationale, the Alk-Uf method can be successfully used for protein recovery and has the potential to be scaled up to a pilot plant model.
3.3. Moisture, protein, and FAN contents
The basic composition (moisture, crude protein, and FAN contents) of protein isolates obtained by the different extraction and purification methods are shown in Table 1. Significant differences (p < 0.05) were detected among methods. The moisture content ranged from 3.45 (Et-Alk-Ac) to 6.50 % (Alk-Ac). As noticed, the moisture was reduced when defatted CM was pretreated with ethanol (Table 1). This lower content could be due to the ability of ethanol to disrupt non-covalent interactions (e.g., hydrogen bonding or hydrophobic interactions), resulting in the release of interfacial water [38].
The crude protein content ranged from 85.15 (Alk-Ac) to 89.71 % (Et-Alk-Uf). It has been suggested that a protein mixture can be claimed as a protein isolate when its protein content is above 80 % [39]. Thus, the protein isolates obtained in the present study fulfill this criterion. On the other hand, the FAN content ranged from 1.76 (Alk-Ac) to 4.34 mg g protein−1 (Et-Alk-Uf). FAN is correlated with the hydrolysis degree of a protein. It determines α-amino acids, ammonia, and end-group amino nitrogen and is often linked to peptide size (molecular weight) [40]. The highest crude protein and FAN contents were obtained in ultrafiltered samples. This is directly attributed to their related protein recovery yields, where ultrafiltration exhibited the same trend (Table 1). Notably, other studies reported similar findings in which protein isolates from soybean [40], canola, hemp (Cannabis sativa), and flax (Linum usitatissimum) meals [41], and sunflower (Helianthus annuus) kernel [42] either pretreated with ethanol, obtained by alkaline extraction, or purified by ultrafiltration, resulted in the removal of moisture or enrichment of protein and FAN contents.
3.4. Amino acid composition and protein digestibility
The amino acid composition and IPD of protein isolates obtained by the different extraction and purification methods are presented in Table 2. In addition, the levels of essential amino acids were compared with those recommended by FAO/WHO/UNU for children (1–2 years old) and adults (>18 years old) [22]. Overall, the amino acid composition did not differ among methods; they share similar non-essential (NEAA) and essential amino acid (EAA) profiles with very few notable differences (Table 2). However, it is noteworthy that protein isolates contained a substantial concentration of all EAA, of which histidine, isoleucine, leucine, threonine, and methionine + cysteine were the dominant ones; meanwhile, the NEAA glutamic acid, glycine, and proline were the most abundant. These amino acid compositions were similar to commercial soy protein isolate (Table 2). Since all the protein isolates obtained in the present study exceeded the amino acid levels recommended by the FAO/WHO/UNU, we suggest they could be further valorized to produce nutritional supplements.
Table 2.
Amino acid composition, in vitro protein digestibility (IPD), and protein digestibility corrected amino acid score (PDCAAS) of canola protein isolates (CPIs) obtained by different methods.
| Amino acid (g 100 g protein−1) | Soy protein | Alk-Ac* | Alk-Uf | Et-Alk-Ac | Et-Alk-Uf | FAO/WHO/UNU suggested requirement (g 100 g protein−1) |
|
|---|---|---|---|---|---|---|---|
| Children | Adults | ||||||
| Non-essential amino acids | |||||||
| Alanine | 4.36 | 4.39 | 4.47 | 4.33 | 4.53 | NA | NA |
| Arginine | 7.46 | 6.91 | 6.78 | 6.91 | 6.70 | ||
| Aspartic Acid | 11.33 | 6.78 | 6.76 | 7.04 | 7.08 | ||
| Glutamic Acid | 18.96 | 21.80 | 22.71 | 21.99 | 22.78 | ||
| Glycine | 4.26 | 5.34 | 5.39 | 5.48 | 5.38 | ||
| Proline | 4.90 | 6.73 | 6.68 | 6.6 | 6.54 | ||
| Serine | 4.35 | 3.81 | 3.70 | 3.67 | 3.80 | ||
| Essential amino acids | |||||||
| Histidine | 2.62 | 3.15 | 3.16 | 3.09 | 3.10 | 1.8 | 1.5 |
| Isoleucine | 4.96 | 4.19 | 4.26 | 4.28 | 4.13 | 3.1 | 1.5 |
| Leucine | 7.87 | 7.56 | 7.54 | 7.56 | 7.52 | 6.3 | 2.1 |
| Lysine | 6.58 | 5.82 | 5.32 | 5.74 | 5.21 | 5.2 | 1.8 |
| Methionine + Cysteine | 2.76 | 5.20 | 4.92 | 5.12 | 4.80 | 2.6** | 2.0** |
| Phenylalanine + Tyrosine | 8.74 | 6.99 | 6.70 | 7.02 | 6.76 | 4.6*** | 2.1*** |
| Threonine | 3.82 | 3.85 | 3.95 | 3.72 | 3.97 | 2.7 | 1.1 |
| Tryptophan | 1.27 | 1.51 | 1.56 | 1.63 | 1.62 | 0.74 | 0.5 |
| Valine | 5.23 | 5.38 | 5.43 | 5.49 | 5.31 | 4.2 | 1.5 |
| Limiting amino acid score | 1 | 1 | 1 | 1 | 1 | NA | NA |
| IPD (%) | 91.4 ± 0.5ac | 92.7 ± 0.1b | 89.9 ± 0.8cd | 95.5 ± 1.0a | 88.1 ± 0.3d | ||
| PDCAAS | 0.97 | 1.00 | 0.92 | 1.00 | 0.88 | ||
For IPD values, different superscript letters indicate significant (p < 0.05) differences among treatments. The amino acid analysis represents the means of duplicate samples, with a coefficient of variation of less than 3 %. NA, not applicable. Alk-Ac, alkaline extraction, and acid precipitation; Alk-Uf, alkaline extraction, and ultrafiltration; Et-Alk-Ac, ethanol pretreatment, alkaline extraction, and acid precipitation; Et-Alk-Uf, ethanol pretreatment, alkaline extraction, and ultrafiltration. *Control method. **Suggested requirement for sulfur containing amino acids (e.g., Methionine + Cysteine). ***Suggested requirement for aromatic amino acids (e.g., Phenylalanine + Tyrosine).
On the other hand, significant differences (p < 0.05) in IPD were detected among methods. It ranged from 88.1 % to 95.5 %, in the ranking order of Et-Alk-Uf < Alk-Uf < Alk-Ac < Et-Alk-Ac. Concerning the PDCAAS, they reached values from 0.88 to 1.00 and exhibited the same trend as for IPD. When PDCAAS reaches the maximum score, i.e., 1.00, it indicates that one unit of the protein can supply all the essential amino acids after digestion [43]. It has been described that protein digestibility correlates with the nutritional value of protein and can be affected by processing methods. The highest IPD and PDCAAS values were obtained by using the Alk-Ac and Et-Alk-Ac methods. According to Sánchez-Reséndiz et al. [44], alkaline extraction can enhance the digestibility and quality of proteins by inducing protein structural changes or altering protein–protein interaction. It should be noted that these findings are comparable to or even higher than those reported by Sánchez-Reséndiz et al. [44], Khalesi & FitzGerald [45], and Onder et al. [46], for quinoa (Chenopodium petiolare), soybean, rice (Oryza sativa), and pea (Pisum sativum), and chickpea (Cicer arietinum) protein isolates, respectively.
3.5. Techno-functional properties
Table 3 summarizes the techno-functional properties (WSI, WAC, OAC, FC, FS, and FD) of protein isolates obtained using different extraction and purification methods. Significant differences (p < 0.05) were detected among methods. Regarding the WSI, it ranged from 16.70 (Alk-Ac) to 39.18 % (Et-Alk-Uf). Meanwhile, the WAC ranged from 2.50 (Et-Alk-Uf) to 3.86 g water g protein−1 (Alk-Uf). All CPIs produced showed lower WAC compared to soy protein isolate (control). Variation in WAC profiles among plant-based protein isolates has been attributed but not limited to differences in protein structure, conformation, and availability of polar amino acids, which mediate protein–water interactions [47]. As noted in Table 3, Alk-Ac and Et-Alk-Ac had a low WSI compared to Alk-Uf and Et-Alk-Uf, however, their WAC was high. The WSI and WAC of Alk-Uf were consistent, whereas the WSI of Et-Alk-Uf was high, but its WAC was low. This indicated that WAC might not have a direct relationship with WSI. Such trend has also been observed in soybean [48], whey, and chicken [49] protein isolates. Differences between WSI and WAC can be explained either by the presence/absence of polar amino acids and lower amounts of water-soluble protein or by forming larger protein aggregates, which are influenced by processing conditions [50,51].
Table 3.
Techno-functional properties of canola protein isolates (CPIs) obtained by different methods.
| Techno-functional property | Soy protein | Alk-Ac* | Alk-Uf | Et-Alk-Ac | Et-Alk-Uf |
|---|---|---|---|---|---|
| WSI (%) | 13.63 ± 0.19d | 16.70 ± 0.75cd | 32.09 ± 1.26b | 18.14 ± 0.82c | 39.18 ± 1.59a |
| WAC (g water g protein−1) | 6.38 ± 0.12a | 3.20 ± 0.07c | 3.86 ± 0.060b | 3.56 ± 0.12b | 2.50 ± 0.04d |
| OAC (g oil g protein−1) | 2.41 ± 0.03c | 2.33 ± 0.01d | 2.53 ± 0.01b | 2.31 ± 0.02d | 2.77 ± 0.0a |
| FC (%) | 610.42 ± 32.41a | 128.57 ± 0.0d | 233.33 ± 0.0c | 255.33 ± 19.66c | 505.26 ± 37.22b |
| FS (%) | 90.91 ± 0.0a | 93.75 ± 0.0a | 87.50 ± 0.0b | 83.51 ± 0.25c | 73.86 ± 1.61d |
| FD (%) | 14.09 ± 0.64d | 34.38 ± 0.0a | 30.0 ± 0.0b | 23.80 ± 1.25c | 16.55 ± 1.02d |
Different superscript letters within the same row indicate significant (p < 0.05) differences among treatments. Alk-Ac, alkaline extraction, and acid precipitation; Alk-Uf, alkaline extraction, and ultrafiltration; Et-Alk-Ac, ethanol pretreatment, alkaline extraction, and acid precipitation; Et-Alk-Uf, ethanol pretreatment, alkaline extraction, and ultrafiltration; WSI, water solubility index; WAC, water absorption capacity; OAC, oil absorption capacity; FC, foaming capacity; FS, foaming stability; FD, foaming density. *Control method.
As mentioned above, ethanol pretreatment promoted moisture evaporation by disrupting non-covalent interactions (Section 3.3). However, alteration of protein interactions can lead to partial protein denaturation, thus affecting protein functionalities such as decreased WAC [52]. Based on our results, we inferred that ethanol pretreatment did not alter protein structure and cause denaturation, as WAC profiles were comparable among all CPIs. High solubility and water absorption are preferable for further application, as they form stable dispersions and influence the texture and viscosity when incorporated into food systems [48].
On the other hand, the OAC ranged from 2.31 (Et-Alk-Ac) to 2.77 g oil g protein−1 (Et-Alk-Uf). Overall, the methods tested share similar OAC values with very few notable differences (Table 3). The obtained results are comparable to or even higher than those OAC values for sunflower meal (1.49 g oil g protein−1) [53], CM (3.26 g oil g protein−1) [54], chickpea (2.30 g oil g protein−1) [55] protein isolates. The OAC of protein represents its capacity to interact with lipids. It can be affected or influenced by factors including amino acid composition, protein conformation, presence of non-polar side chains (hydrophobicity), and type of oil employed [56]. High OAC is desirable for food formulation, as it correlates with flavor retention, shelf-life, and emulsifying properties [57].
Regarding foaming properties, the FC ranged from 128.57 (Alk-Ac) to 505.26 % (Et-Alk-Uf). Deng et al. [56] have stated that FC is closely related to protein solubility. As shown in Table 3, the obtained FC values correspond to the reported WSI values. In particular, the highest WSI and FC values were obtained when employing the Et-Alk-Uf method. Meanwhile, the FS ranged from 73.86 to 93.75 %, in ranking order of Et-Alk-Uf < Et-Alk-Ac < Alk-Uf < Alk-Ac. Concerning the FD, it ranged from 16.55 to 34.38 % and exhibited the same trend as for FS (Table 3). The highest FS and FD values were obtained by using the Alk-Ac and Alk-Uf methods. It has been described that FC depends on the ability of the protein to diffuse at the water-air interface due to the unfolding of its structure. In contrast, the FS and FD are determined by the interactions among proteins and their capacity to form cohesive interfacial layers, stabilizing the air bubbles [54,56]. Overall, the foaming properties obtained in this study are comparable to or greater than those reported previously for protein isolates from soybean-maize (FC = 388 %, FS = 85 %, and FD = 20 %) [40], soybean (FC = 10–60 %, FS = 75–95 %) [58], and CM (FC = 90 %, FS = 73 %) [59] either obtained by alkaline extraction or purified by ultrafiltration. Foaming properties are essential in many food formulations, as they contribute to the uniform distribution of air cells in the structure of the products, which improves the appearance and textural properties [60].
3.6. ATR-FTIR spectrometry analysis
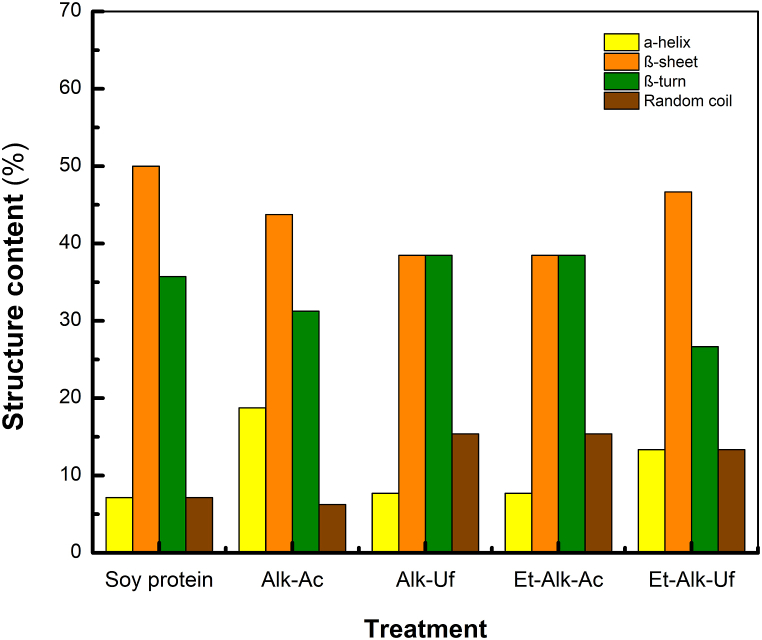
ATR-FTIR spectroscopy reflects the unique vibrations of secondary structural units of a protein and provides information on conformational changes after processing [61,62]. In the infrared region, proteins mainly contain three absorption bands: amide I, amide II, and amide III bands, which are correlated with α-helix, β-sheet, β-turn, and random coil configurations [63]. Fig. 3 shows the secondary structural features of protein isolates obtained by the different extraction and purification methods. Overall, no pronounced effect on secondary structural changes was observed among methods, indicating that they do not lead to critical modifications in the protein structure. It has been described that conformational changes in proteins can impact their techno-functional properties [64]. Interestingly, the β-sheet (ca. 38–46 %) and β-turn (ca. 26–38 %) secondary structures were dominant in all of the isolates (Fig. 3). The higher β-sheet and β-turn contents could be attributed to protein isolates containing substantial concentrations of tryptophan, threonine, valine, and phenylalanine + tyrosine, which prefer to adopt β-conformations [65]. These results are in accordance with the characterization of lentils (Lens culinaris) [61], quinoa [63], and lupin [66] protein isolates, where the secondary structure mainly consisted of β-sheet.
Fig. 3.
Secondary structure composition of canola protein isolates (CPIs) based on ATR-FTIR analysis (α-helix = 1654 cm−1; β-sheet = 1618 cm−1, 1625 cm−1, 1679 cm−1, and 1695 cm−1; and disordered = 1639 cm−1).
3.7. SDS-PAGE
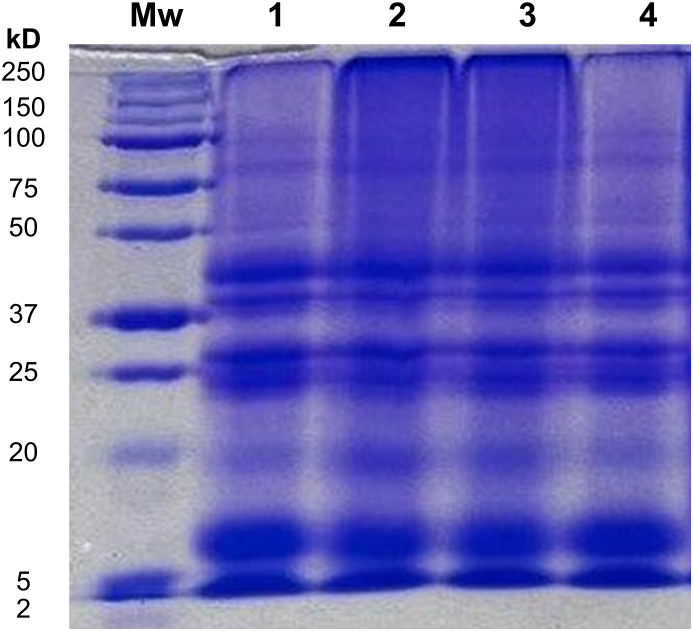
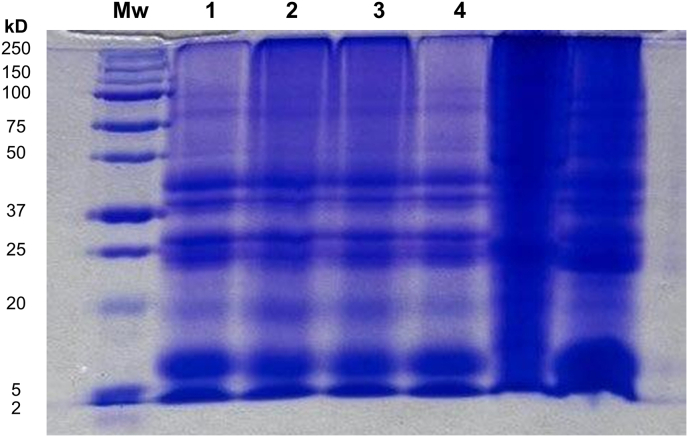
Fig. 4 depicts the electrophoretic profiles obtained by SDS-PAGE of protein isolates obtained by the different extraction and purification methods. The molecular weight distribution showed great similarities among all isolates and exhibited several bands ranging from ca. 20–45 kDa. Notably, none of the methods affected the protein profiles since the primary structure of isolates is not altered. Previous studies showed that hemp and flax [41] and CM [54] protein isolates possessed similar protein fractions.
Fig. 4.
SDS-PAGE of canola protein isolates (CPIs). Mw lane = molecular weight marker; lane 1 = Et-Alk-Uf, ethanol pretreatment, alkaline extraction, and ultrafiltration; lane 2 = Et-Alk-Ac, ethanol pretreatment, alkaline extraction, and acid precipitation; lane 3 = Alk-Uf, alkaline extraction and ultrafiltration; and lane 4 = Alk-Ac, alkaline extraction and acid precipitation (control method). Full SDS-PAGE is provided in Supplementary Figure S1.
Canola globular storage proteins are comprised of cruciferin (12S globulin), napin (2S albumin), and oleosin [67]. Other studies previously identified these proteins [9,68]. As shown in Fig. 4, the first predominant protein band had a molecular weight of ca. 7–11 kDa, which according to the densitometry analysis, represented ca. 25 %. Therefore, it was ascribed to the napin fraction, a basic protein with low molecular weight and a higher level of amidated amino acids [69]. The second and third predominant bands had molecular weights ranging from ca. 23–45 kDa and were ascribed to the globulin fractions. Indeed, they specifically corresponded to cruciferin, which is a neutral protein (200–310 kDa) comprised of a polypeptide chain (50-20 kDa) [69]. Finally, the fourth predominant band had a molecular weight of ca. 20 kDa and was ascribed to the oleosin fraction. This fraction is considered the major structural protein associated with the oil fraction [70].
4. Conclusion
This work has demonstrated the significant role of the extraction and purification method in defining the properties of protein isolates. In general, CPIs were successfully produced by all methods tested. However, the Alk-Uf and Et-Alk-Uf methods resulted in a higher process yield, protein recovery, basic composition, and an excellent essential amino acid profile and IPD. Considering the great quality and techno-functionality of protein isolates obtained by these methods, we suggested that they have the potential to be scaled up to a pilot plant model. Indeed, protein isolates had significant WSI, OAC, and foaming properties, which make them suitable for application in food formulations. Since defatted CM is the main by-product obtained after the oil extraction, it is noteworthy that this study demonstrated its potential to be revalorized and be a significant alternative protein source for the food industry. Despite these promising results, some study limitations must be addressed. To provide a more complete characterization and not create significant challenges for product developers, future research should focus on determining the sensory, rheological, and thermal properties of CPIs. On the other hand, during the extraction process, polyphenols present in CM can interact with proteins and affect the quality of isolates, thus studies are required to evaluate the effect of polyphenol removal on the properties of CPIs. Finally, comparison of different drying methods (e.g., freeze-, spray-, and vacuum-drying) used for the preparation of CPIs must be conducted to investigate their impact on the functionality of isolates.
Funding
This research was supported by the Centro de Investigación y Desarrollo de Proteínas (CIDPRO) and the Instituto para la Investigación en Obesidad from Tecnologico de Monterrey.
Data availability statement
Data associated with this study have not been deposited into a publicly available repository and will be made available on request.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Cristina Cháirez-Jiménez: Writing – original draft, Visualization, Investigation, Formal analysis, Data curation. Cecilia Castro-López: Writing – original draft, Visualization, Investigation, Formal analysis, Data curation. Sergio Serna-Saldívar: Writing – review & editing. Cristina Chuck-Hernández: Writing – review & editing, Supervision, Resources, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
C.C-J. express her sincere thanks to Mexico's National Council of Humanities, Sciences and Technologies (CONAHCyT) for the postgraduate scholarship granted to perform doctoral studies (CVU 442080). A special acknowledgment is due to the Centro del Agua from Tecnológico de Monterrey, as well as to Andrea Nucamendi and Jesús Abraham Díaz for their support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21938.
Contributor Information
Cristina Cháirez-Jiménez, Email: A01053142@tec.mx.
Cristina Chuck-Hernández, Email: cristina.chuck@tec.mx.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
figs1.
References
- 1.Bandyopadhyay S., Kashyap S., Calvez J., Devi S., Azzout-Marniche D., Tomé D., Kurpad A.V., Gaudichon C. Evaluation of protein quality in humans and insights on stable isotope approaches to measure digestibility – a review. Adv. Nutr. 2022;13(4):1131–1143. doi: 10.1093/advances/nmab134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalu R.E., Etim K.D. Factors associated with malnutrition among under five children in developing countries: a review. Global J. Pure Appl. Sci. 2018;24(1):69. doi: 10.4314/gjpas.v24i1.8. [DOI] [Google Scholar]
- 3.Almeida Sá A.G., Franco Moreno Y.M., Mattar Carciofi B.A. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci. Technol. 2020;97:170–184. doi: 10.1016/j.tifs.2020.01.011. [DOI] [Google Scholar]
- 4.Singh R., Langyan S., Sangwan S., Rohtagi B., Khandelwal A., Shrivastava M. Protein for human consumption from oilseed cakes: a review. Front. Sustain. Food Syst. 2022;6 doi: 10.3389/fsufs.2022.856401. [DOI] [Google Scholar]
- 5.Food and Agriculture Organization Corporate Statistical Database (FAOSTAT) 2023. Crops and Livestock Products.https://www.fao.org/faostat/en/#data/QCL [Google Scholar]
- 6.Lin L., Allemekinders H., Dansby A., Campbell L., Durance-Tod S., Berger A., Jones P.J. Evidence of health benefits of canola oil. Nutr. Rev. 2013;71(6):370–385. doi: 10.1111/nure.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia W., Curubeto N., Rodríguez-Alonso E., Keppler J.K., van der Goot A.J. Rapeseed protein concentrate as a potential ingredient for meat analogues. Innovat. Food Sci. Emerg. Technol. 2021;72 doi: 10.1016/j.ifset.2021.102758. [DOI] [Google Scholar]
- 8.Perera S.P., McIntosh T.C., Wanasundara J.P.D. Structural properties of cruciferin and napin of Brassica napus (canola) show distinct responses to changes in pH and temperature. Plants. 2016;5(3):64–74. doi: 10.3390/plants5030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandara N., Esparza Y., Wu J. Exfoliating nanomaterials in canola protein derived adhesive improves strength and water resistance. RSC Adv. 2017;7(11):6743–6752. doi: 10.1039/c6ra27470f. [DOI] [Google Scholar]
- 10.He R., He H.Y., Chao D., Ju X., Aluko R. Effects of high pressure and heat treatments on physicochemical and gelation properties of rapeseed protein isolate. Food Bioprocess Technol. 2014;7(5):1344–1353. doi: 10.1007/s11947-013-1139-z. [DOI] [Google Scholar]
- 11.Zhang Y., Liu Q., Rempel C. Processing and characteristics of canola protein-based biodegradable packaging: a review. Crit. Rev. Food Sci. Nutr. 2018;58(3):475–485. doi: 10.1080/10408398.2016.1193463. [DOI] [PubMed] [Google Scholar]
- 12.Gerzhova A., Mondor M., Benali M., Aider M. A comparative study between the electro-activation technique and conventional extraction method on the extractability, composition and physicochemical properties of canola protein concentrates and isolates. Food Biosci. 2015;11:56–71. doi: 10.1016/j.fbio.2015.04.005. [DOI] [Google Scholar]
- 13.Witczak M., Chmielewska A., Ziobro R., Korus J., Juszczak L. Rapeseed protein as a novel ingredient of gluten-free dough: rheological and thermal properties. Food Hydrocolloids. 2021;118 doi: 10.1016/j.foodhyd.2021.106813. [DOI] [Google Scholar]
- 14.Yang J., Faber I., Berton-Carabin C.C., Nikiforidis C.v., van der Linden E., Sagis L.M.C. Foams and air-water interfaces stabilised by mildly purified rapeseed proteins after defatting. Food Hydrocolloids. 2021;112 doi: 10.1016/j.foodhyd.2020.106270. [DOI] [Google Scholar]
- 15.Aider M., Barbana C. Canola proteins: composition, extraction, functional properties, bioactivity, applications as a food ingredient and allergenicity – a practical and critical review. Trends Food Sci. Technol. 2011;22(1):21–39. doi: 10.1016/j.tifs.2010.11.002. [DOI] [Google Scholar]
- 16.Wanasundara J.P.D., McIntosh T.C., Perera S.P., Withana-Gamage T.S., Mitra P. Canola/rapeseed protein-functionality and nutrition. OCL. 2016;23(4):D407. doi: 10.1051/ocl/2016028. [DOI] [Google Scholar]
- 17.Kumar M., Tomar M., Potkule J., Verma R., Punia S., Mahapatra A., et al. Advances in the plant protein extraction: mechanism and recommendations. Food Hydrocolloids. 2021;115 doi: 10.1016/j.foodhyd.2021.106595. [DOI] [Google Scholar]
- 18.Tan S.H., Mailer R.J., Blanchard C.L., Agboola S.O. Canola proteins for human consumption: extraction, profile, and functional properties. J. Food Sci. 2011;76(1):R16–R28. doi: 10.1111/j.1750-3841.2010.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klockeman D.M., Toledo R., Sims K.A. Isolation and characterization of defatted canola meal protein. J. Agric. Food Chem. 1997;45(10):3867–3870. doi: 10.1021/jf970026i. [DOI] [Google Scholar]
- 20.Association of Official Analytical Collaboration International (AOAC International) 1992. Official Methods of Analysis. Washington, DC, USA. [Google Scholar]
- 21.Hsu H.W., Vavak D.L., Satterlee L.D., Miller G.A. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977;42(5):1269–1273. doi: 10.1111/j.1365-2621.1977.tb14476.x. [DOI] [Google Scholar]
- 22.Food and Agriculture Organization/World Health Organization (FAO/WHO) Food and Agriculture Organization/World Health Organization; Geneva, Switzerland: 2007. Protein and Amino Acid Requirements in Human Nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation, WHO Technical Report Series 935.https://apps.who.int/iris/handle/10665/43411 [PubMed] [Google Scholar]
- 23.Cheftel J.C., Cuq J.L., Lorient D. In: Proteínas Alimentarias: Bioquímica. Propiedades Funcionales. Valor Nutritivo. Modificaciones Químicas. Cheftel J.C., Cuq J.L., Lorient D., editors. Acribia S.A.; Zaragoza, España: 1989. Propiedades funcionales de las proteínas; p. 346. [Google Scholar]
- 24.Ahn H.J., Kim J.H., Ng P.K.W. Functional and thermal properties of wheat, barley, and soy flours and their blends treated with a microbial transglutaminase. J. Food Sci. 2005;70(6):380–386. doi: 10.1111/j.1365-2621.2005.tb11433.x. [DOI] [Google Scholar]
- 25.Haque Z., Kito M. Lipophilization of alpha Sl-casein. 2. Conformational and functional effects. J. Agric. Food Chem. 1983;31(6):1231–1237. doi: 10.1021/JF00120A022. [DOI] [Google Scholar]
- 26.Withana-Gamage T.S., Wanasundara J.P., Pietrasik Z., Shand P.J. Physicochemical, thermal and functional characterisation of protein isolates from Kabuli and Desi chickpea (Cicer arietinum L.): a comparative study with soy (Glycine max) and pea (Pisum sativum L.) J. Sci. Food Agric. 2011;91(6):1022–1031. doi: 10.1002/jsfa.4277. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Drakos A., Malindretou K., Mandala I., Evageliou V. Protein isolation from jet milled rye flours differing in particle size. Food Bioprod. Process. 2017;104:13–18. doi: 10.1016/j.fbp.2017.04.004. [DOI] [Google Scholar]
- 29.Rommi K., Holopainen U., Pohjola S., Hakala T.K., Lantto R., Poutanen K., Nordlund E. Impact of particle size reduction and carbohydrate-hydrolyzing enzyme treatment on protein recovery from rapeseed (Brassica rapa L.) press cake. Food Bioprocess Technol. 2015;8(12):2392–2399. doi: 10.1007/s11947-015-1587-8. [DOI] [Google Scholar]
- 30.Russin T.A., Arcand Y., Boye J.I. Particle size effect on soy protein isolate extraction. J. Food Process. Preserv. 2007;31(3):308–319. doi: 10.1111/j.1745-4549.2007.00127.x. [DOI] [Google Scholar]
- 31.Das Purkayastha M., Dutta G., Barthakur A., Mahanta C.L. Tackling correlated responses during process optimisation of rapeseed meal protein extraction. Food Chem. 2015;170:62–73. doi: 10.1016/j.foodchem.2014.08.053. [DOI] [PubMed] [Google Scholar]
- 32.Vishwanathan K.H., Singh V., Subramanian R. Influence of particle size on protein extractability from soybean and okara. J. Food Eng. 2011;102(3):240–246. doi: 10.1016/j.jfoodeng.2010.08.026. [DOI] [Google Scholar]
- 33.Gao Z., Shen P., Lan Y., Cui L., Ohm J.-B., Chen B., Rao J. Effect of alkaline extraction pH on structure properties, solubility, and beany flavor of yellow pea protein isolate. Food Res. Int. 2020;131 doi: 10.1016/j.foodres.2020.10904. [DOI] [PubMed] [Google Scholar]
- 34.Albe-Slabi S., Mesieres O., Mathé C., Ndiaye M., Galet O., Kapel R. Combined effect of extraction and purification conditions on yield, composition and functional and structural properties of lupin proteins. Foods. 2022;11(11):1646. doi: 10.3390/foods11111646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerliani N., Hammami R., Aïder M. Extraction of protein and carbohydrates from soybean meal using acidic and alkaline solutions produced by electro-activation. Food Sci. Nutr. 2020;8(2):1125–1138. doi: 10.1002/fsn3.1399. [DOI] [Google Scholar]
- 36.Rastogi Y., Priya, Gogate P.R. Intensified recovery of whey proteins using combination of enzyme in free or immobilized form with ultrafiltration. Chem. Eng. Process. 2022;179 doi: 10.1016/j.cep.2022.109076. [DOI] [Google Scholar]
- 37.Peng Y., Kyriakopoulou K., Ndiaye M., Bianeis M., Keppler J.K., van der Goot A.J. Characteristics of soy protein prepared using an aqueous ethanol washing process. Foods. 2021;10(9):2222. doi: 10.3390/foods10092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng Y., Ma X., Kong B., Chen Q., Liu Q. Ethanol induced changes in structural, morphological, and functional properties of whey proteins isolates: influence of ethanol concentration. Food Hydrocolloids. 2021;111 doi: 10.1016/j.foodhyd.2020.106379. [DOI] [Google Scholar]
- 39.Ghribi A.M., Gafsi I.M., Blecker C., Danthine S., Attia H., Besbes S. Effect of drying methods on physico-chemical and functional properties of chickpea protein concentrates. J. Food Eng. 2015;165:179–188. doi: 10.1016/j.jfoodeng.2015.06.021. [DOI] [Google Scholar]
- 40.Soria-Hernández C.G., Serna-Saldívar S.O., Chuck-Hernández C. Comparison of physicochemical, functional and nutritional properties between proteins of soybean and a novel mixture of soybean-maize. Appl. Sci. 2020;10(19):6998. doi: 10.3390/app10196998. [DOI] [Google Scholar]
- 41.Teh S.S., Bekhit A.E.D., Carne A., Birch J. Effect of the defatting process, acid and alkali extraction on the physicochemical and functional properties of hemp, flax and canola seed cake protein isolates. J Food Meas Charact. 2014;8(2):92–104. doi: 10.1007/s11694-013-9168-x. [DOI] [Google Scholar]
- 42.Jia W., Sutanto I.R., Ndiaye M., Keppler J.K., van der Goot A.J. Effect of aqueous ethanol washing on functional properties of sunflower materials for meat analogue application. Food Struct. 2022;33 doi: 10.1016/j.foostr.2022.100274. [DOI] [Google Scholar]
- 43.Millward D.J. Amino acid scoring patterns for protein quality assessment. Br. J. Nutr. 2012;108(S2):S31–S43. doi: 10.1017/S0007114512002462. [DOI] [PubMed] [Google Scholar]
- 44.Sánchez-Reséndiz A.I., Escalante-Aburto A., Andía-Ayme V., Chuck-Hernández C. Structural properties, functional evaluation, and in vitro protein digestibility of black and yellow quinoa (Chenopodium petiolare) protein isolates. CYTA J Food. 2019;17(1):864–872. doi: 10.1080/19476337.2019.1669714. [DOI] [Google Scholar]
- 45.Khalesi M., FitzGerald R.J. In vitro digestibility and antioxidant activity of plant protein isolate and milk protein concentrate blends. Catalysts. 2021;11(7):787. doi: 10.3390/catal11070787. [DOI] [Google Scholar]
- 46.Onder S., Karaca A.C., Ozcelik B., Alamri A.S., Ibrahim S.A., Galanakis C.M. Exploring the amino-acid composition, secondary structure, and physicochemical and functional properties of chickpea protein isolates. ACS Omega. 2023;8(1):1486–1495. doi: 10.1021/acsomega.2c06912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bocarando-Guzmán M.D., Luna-Suárez S., Hernández-Cázares A.S., Herrera-Corredor J.A., Hidalgo-Contreras J.V., Ríos-Corripio M.A. Comparison of the physicochemical and functional properties of flour and protein isolate from moringa (Moringa oleifera Lam.) leaves. Int. J. Food Prop. 2022;25(1):733–747. doi: 10.1080/10942912.2022.2058533. [DOI] [Google Scholar]
- 48.Hima J., Shekh M.M., Saroj K.G., Lalan K.S. Rheological properties and particle size distribution of soy protein isolate as affected by drying methods. Nutri Food Sci Int J. 2018;7(5) doi: 10.19080/NFSIJ.2018.07.555721. [DOI] [Google Scholar]
- 49.Chen X., Li Y., Zhou R., Liu D., Xu X., Zhou G. Water-soluble myofibrillar proteins prepared by high-pressure homogenisation: a comparison study on the composition and functionality. Int. J. Food Sci. Technol. 2017;52(11):2334–2342. doi: 10.1111/ijfs.13515. [DOI] [Google Scholar]
- 50.Waghmare A.G., Salve M.K., LeBlanc J.G., Arya S.S. Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Bioresour Bioprocess. 2016;3:16. doi: 10.1186/s40643-016-0094-8. [DOI] [Google Scholar]
- 51.Yousefi N., Abbasi S. Food proteins: solubility & thermal stability improvement techniques. Food Chemistry Advances. 2022;1 doi: 10.1016/j.focha.2022.100090. [DOI] [Google Scholar]
- 52.Xin Tan J., Tan C.-C., Dharmawan J., Jan Leong S.S. Effects of ethanol washing on off-flavours removal and protein functionalities of pea protein concentrate. Food Bioprod. Process. 2023;141:73–80. doi: 10.1016/j.fbp.2023.07.004. [DOI] [Google Scholar]
- 53.Ivanova P., Ivanova Chalova V., Koleva L. Functional properties of proteins isolated from industrially produced sunflower meal. Int J Food Stud. 2014;3:203–212. doi: 10.7455/ijfs/3.2.2014.a6. [DOI] [Google Scholar]
- 54.Flores-Jiménez N.T., Ulloa J.A., Silvas J.E.U., Ramírez J.C.R., Ulloa P.R., et al. Effect of high-intensity ultrasound on the compositional, physicochemical, biochemical, functional and structural properties of canola (Brassica napus L.) protein isolate. Food Res. Int. 2019;121:947–956. doi: 10.1016/j.foodres.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 55.El Sohaimy S.A., Brennan M.A., Darwish A.M.G., Brennan C.S. Chickpea protein isolation, characterization and application in muffin enrichment. Int J Food Stud. 2021;10:SI57–SI71. doi: 10.7455/ijfs/10.SI.2021.a5. [DOI] [Google Scholar]
- 56.Deng Y., Huang L., Zhang C., Xie P., Cheng J., Wang X., Li S. Physicochemical and functional properties of Chinese quince seed protein isolate. Food Chem. 2019;283:539–548. doi: 10.1016/j.foodchem.2019.01.08. [DOI] [PubMed] [Google Scholar]
- 57.Ngo N.T.T., Shahidi F. Functional properties of protein isolates from camelina (Camelina sativa (L.) Crantz) and flixweed (sophia, Descurainis sophia L.) seed meals. Food Prod Process and Nutr. 2021;3:31. doi: 10.1186/s43014-021-00076-8. [DOI] [Google Scholar]
- 58.Kim H.J., Kim B.K. Comparison of soy protein concentrates produced using membrane ultrafiltration and acid precipitation. Food Sci. Biotechnol. 2015;24(1):67–73. doi: 10.1007/s10068-015-0011-5. [DOI] [Google Scholar]
- 59.Li X., Shi J., Scanlon M., Xue S.J., Lu J. Effects of pretreatments on physicochemical and structural properties of proteins isolated from canola seeds after oil extraction by supercritical-CO2 process. LWT. 2021;137 doi: 10.1016/j.lwt.2020.110415. [DOI] [Google Scholar]
- 60.Amagliani L., Silva J.V.C., Saffon M., Dombrowski J. On the foaming properties of plant proteins: current status and future opportunities. Trends Food Sci. Technol. 2021;118(Part A):261–272. doi: 10.1016/j.tifs.2021.10.001. [DOI] [Google Scholar]
- 61.Gunes Z.S., Can Karaca A. Examining the amino acid composition, secondary structure, and physicochemical and functional properties of proteins isolated from local lentil landraces of Anatolia. Cereal Chem. 2022;99(1):78–89. doi: 10.1002/cche.10446. [DOI] [Google Scholar]
- 62.Kong J., Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007;39(8):549–559. doi: 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 63.Wang X., Zhao R., Yuan W. Composition and secondary structure of proteins isolated from six different quinoa varieties from China. J. Cereal. Sci. 2020;95 doi: 10.1016/j.jcs.2020.103036. [DOI] [Google Scholar]
- 64.Rahman M.S., Go G.-W., Seo J.K., Gul K., Choi S.G., Yang H.S. Thiol concentration, structural characteristics and gelling properties of bovine heart protein concentrates. LWT. 2019;111:175–181. doi: 10.1016/j.lwt.2019.05.030. [DOI] [Google Scholar]
- 65.Nham Tran T.L., Miranda A.F., Mouradov A., Adhikari B. Physicochemical characteristics of protein isolated from thraustochytrid oilcake. Foods. 2020;9(6):779. doi: 10.3390/foods9060779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Devkota L., Kyriakopoulou K., Bergia R., Dhital S. Structural and thermal characterization of protein isolates from Australian lupin varieties as affected by processing conditions. Foods. 2023;12(5):908. doi: 10.3390/foods12050908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu J., Muir A.D. Comparative structural, emulsifying, and biological properties of 2 major canola proteins, cruciferin and napin. J. Food Sci. 2008;73(3):C210–C216. doi: 10.1111/j.1750-3841.2008.00675.x. [DOI] [PubMed] [Google Scholar]
- 68.Manamperi W.A.R., Wiesenborn D.P., Chang S.K.C., Pryor S.W. Effects of protein separation conditions on the functional and thermal properties of canola protein isolates. J. Food Sci. 2011;76(3):266–273. doi: 10.1111/j.1750-3841.2011.02087.x. [DOI] [PubMed] [Google Scholar]
- 69.Aider M., Barbana C. Canola proteins: composition, extraction, functional properties, bioactivity, applications as a food ingredient and allergenicity - a practical and critical review. Trends Food Sci. Technol. 2011;22(1):21–39. doi: 10.1016/j.tifs.2010.11.002. [DOI] [Google Scholar]
- 70.Wanasundara J.P.D. Proteins of brassicaceae oilseeds and their potential as a plant protein source. Crit. Rev. Food Sci. Nutr. 2011;51(7):635–677. doi: 10.1080/10408391003749942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study have not been deposited into a publicly available repository and will be made available on request.