Abstract
Objectives
Accurate and reproducible measurements of the pediatric airway are critical for diagnostic evaluation and management of subglottic stenosis. Endoluminal functional lumen imaging probe (EndoFLIP) is a catheter-based imaging probe which utilizes impedance planimetry to calculate luminal parameters, including cross-sectional area and compliance. Herein, we demonstrate the feasibility of this system for multidimensional evaluation of the pediatric airway.
Methods
3D-printed pediatric laryngotracheal models were created based on computed tomography scans, then artificially deformed to simulate both circumferential and posterior subglottic stenosis. Two observers made six measurements of the minimum cross-sectional area (MCSA) and length of stenosis of each model with EndoFLIP. Agreement between observer measurements and model dimensions was evaluated using Lin’s concordance correlation coefficient; inter-observer reliability was assessed using intraclass correlation.
Results
Four models were created: two without pathology (MCSA: 132.4, 44.3 mm2) and two with subglottic stenosis (MCSA: 28.7, 59.7 mm2, stenotic length 27.8, 24.4 mm). Observer measurements of MCSA and length of stenosis demonstrated high concordance with the models (r = 0.99, 0.95, p<0.001) with a mean error of 4.5% and 18.2% respectively. There was a low coefficient of variation (0.6–2.8%) for measurements, indicating high precision. Interrater reliability was high for both MCSA and stenotic length (ICC: 0.99, 0.98).
Conclusions
The EndoFLIP system allows for accurate and reproducible measurements of cross-sectional area and stenotic length in pediatric airway models. This method may provide further advantages in the evaluation of airway distensibility, as well as measurements of asymmetric airway pathology.
Keywords: airway evaluation, subglottic stenosis, multidimensional measurement, EndoFLIP
Introduction
Subglottic and tracheal stenosis is a challenging disorder involving the narrowing of the upper airway anywhere between the vocal folds and carina. The documented prevalence of pediatric subglottic stenosis ranges from 2.7–4.2%, with a recent prospective study reporting rates as high as 11.4%[1, 2]. Evaluation may involve transnasal fiberoptic laryngoscopy and radiographic imaging, however, the gold standard remains intraoperative microlaryngoscopy and bronchoscopy[3]. Understandably, quantification of the dimensions of the narrowed segment in this setting is key for treatment selection and longitudinal assessment of intervention efficacy.
The standard method of measuring airway stenosis intraoperatively was first proposed by Cotton and Myer and utilizes endotracheal tubes and age-matched norms to determine the percentage of obstruction [4]. Although this method allows for determination of the narrowest airway diameter, it is limited in its description of other facets of pathologic stenosis, including the length and stiffness of the narrowed segment. There is also a ceiling to its precision, as endotracheal tubes are manufactured with diameters at increments of 0.5 mm, with a variation of ±0.15 mm of the stated size. These difficulties are compounded when evaluating more distal lesions in tracheal stenosis. Other proposed methods include quantitative endoscopy and ultrasonography, which may be difficult to achieve intraoperatively.
The endoluminal functional lumen imaging probe (EndoFLIP) is a device used in gastroenterology to assess the caliber and compressibility of the upper digestive tract. It provides important luminal data in real time including diameter, cross-sectional area (CSA), pressure, compliance, and distensibility index[5]. Beyond the esophagus, the tool has been used to assess pyloric sphincter function and even to measure of distensibility in the cervix [6, 7].
Currently, there is a need for a reliable and user-friendly technique for multidimensional airway measurements intraoperatively. We propose that EndoFLIP technology can provide a more comprehensive and precise method of describing airway pathology compared to existing tools. In this proof-of-concept study, we demonstrate that this device provides accurate and reproducible measurements of pediatric tracheal dimensions.
Material and Methods
This is a pilot study investigating the feasibility of utilizing the EndoFLIP system in the measurement of pediatric airways. Approval for this study was obtained from the Johns Hopkins School of Medicine Institutional Review Board (IRB00310635)
3D printing of the trachea
Deidentified chest CT imaging was obtained from the Johns Hopkins Department of Otolaryngology-Head and Neck Surgery under IRB approval. Anatomical segmentation was performed using the open-source medical image viewer 3D Slicer. First, the lumen of the trachea was segmented using a standard thresholding method for air. The wall of the trachea was then delineated by generating a 3 mm margin around the lumen, reflecting the average thickness of the trachea[8]. The superior and inferior aspects of the segmented trachea were cropped to expose the lumen. 3D meshes of the segmented trachea were then exported and edited in Autodesk Meshmixer to model tracheal stenosis. Using the 3D surface brush, the 3D mesh of the trachea was deformed either circumferentially or posteriorly to simulate an approximate 50% reduction of lumen size.
EndoFLIP Impedance Planimetry System
The primary measuring device is comprised of a 1 meter long probe with a 12 cm cylindrical polyurethane bag at the tip. There are a series of 17 impedance sensors placed 5–10 mm apart along the tip of the probe, as well as excitation electrodes at each end of the catheter (Figure 1A). During use, the bag is slowly infused with saline via an external pump to an appropriate pressure (Figure 1B), and the excitation electrodes are activated. The voltage difference recorded between sensors is a function of the conductivity of the saline and the cross-sectional area. CSA and diameter can then be determined given adequate pressures within the probe; these estimates are given at a resolution of 0.1 mm.
Figure 1.
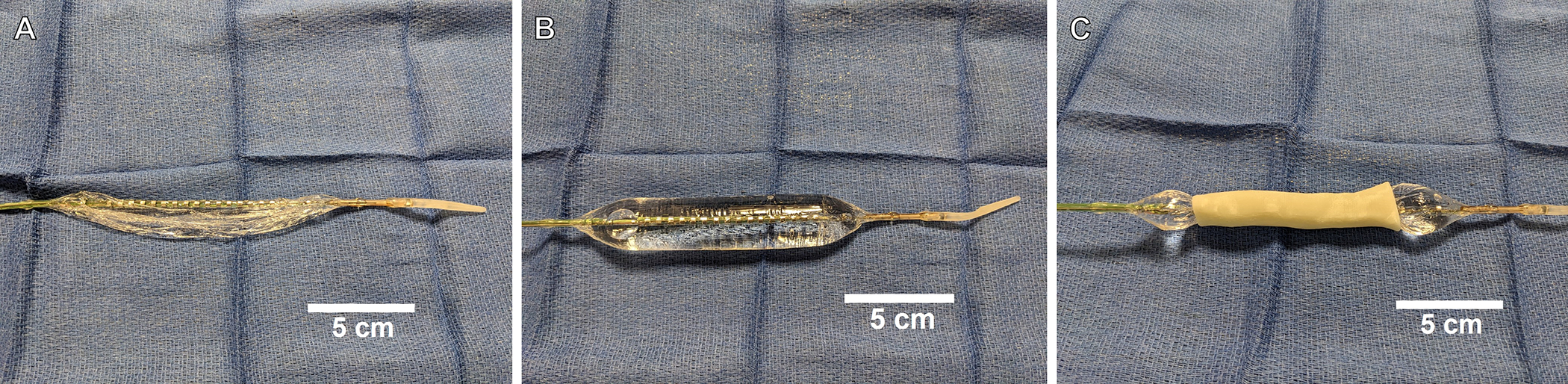
A) Deflated EndoFLIP catheter B) EndoFLIP catheter inflated with 20 cc of normal saline C) EndoFLIP measurement technique using a 3D-printed pediatric tracheal model
Measurement technique
The 3D-printed trachea was placed horizontally on the surgical field. The catheter was inserted through the proximal opening and advanced until the distal balloon edge approximated the carina. The balloon was slowly infused with normal saline at 2 milliliters per second to a volume of 20 – 25 cc (Figure 1C). This volume was selected to achieve a pressure of 20–60 mmHg depending on the model. Measurements, including estimated diameter at each increment, estimated minimum cross-sectional area, and balloon pressure were recorded.
Statistical analysis
Two observers made 6 independent measurements of the minimum cross-sectional area (MCSA) and length of stenosis of each model with the EndoFLIP system. Agreement between observer measurements and model dimensions was evaluated using Lin’s concordance correlation coefficient, which measures how well a set of bivariate data compares to a gold standard. Inter-observer reliability was assessed using intraclass correlation. Statistical analysis was performed on R Studio version 2022.12.0 (Vienna, Austria) and a significance level of p<0.05 was use for all analyses.
Results
De-identified patient scans were imported and the laryngotracheal complex was isolated. For subglottic stenotic models, the airway was artificially deformed to create both circumferential and posterior narrowing (Figure 2)
Figure 2.
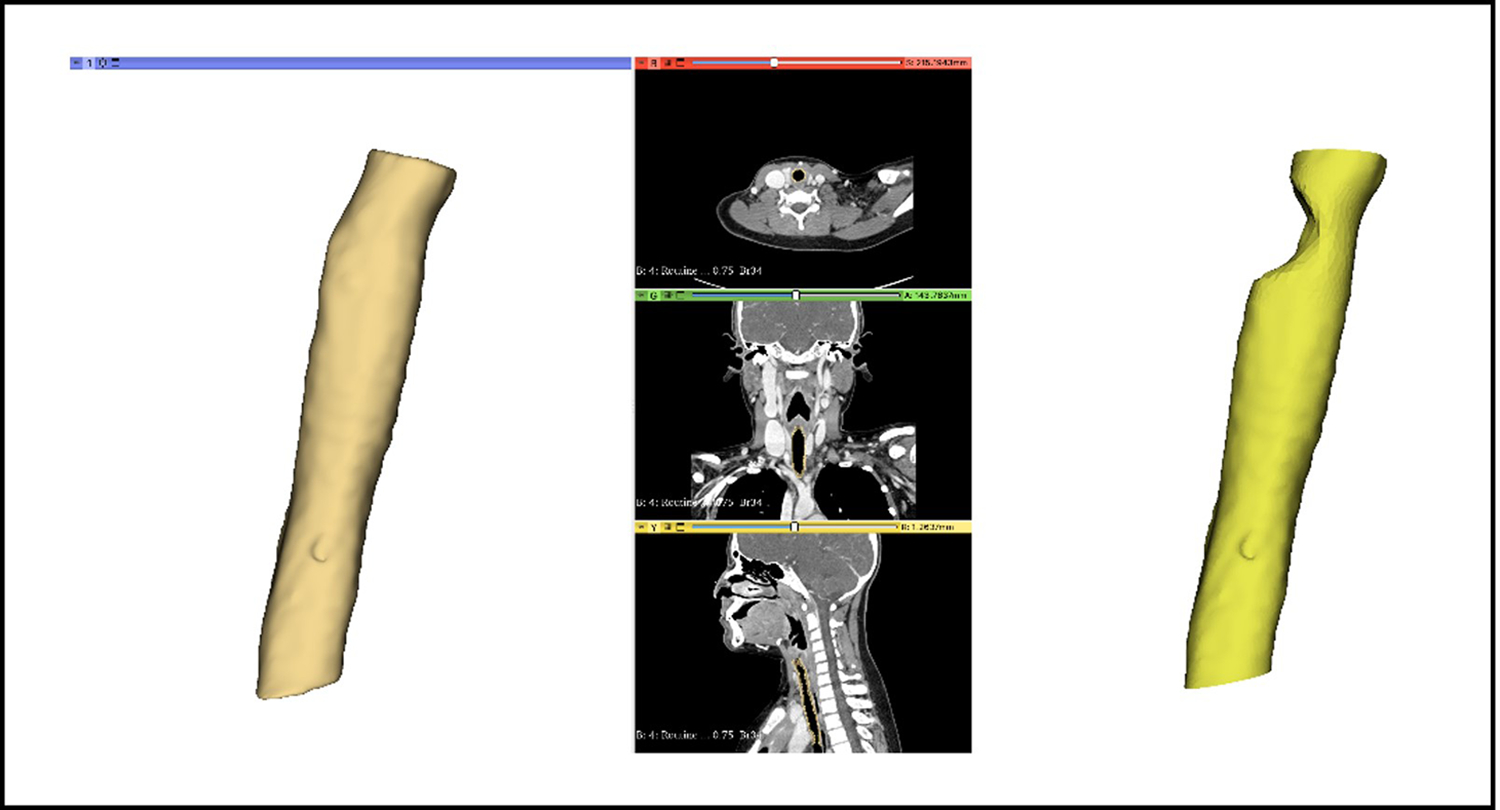
Creation of normal and pathologic pediatric tracheal models based on de-identifed CT scans using Slicer.
Measurements were taken as described in the methods section. Representative EndoFLIP outputs are shown in Figure 3.
Figure 3.
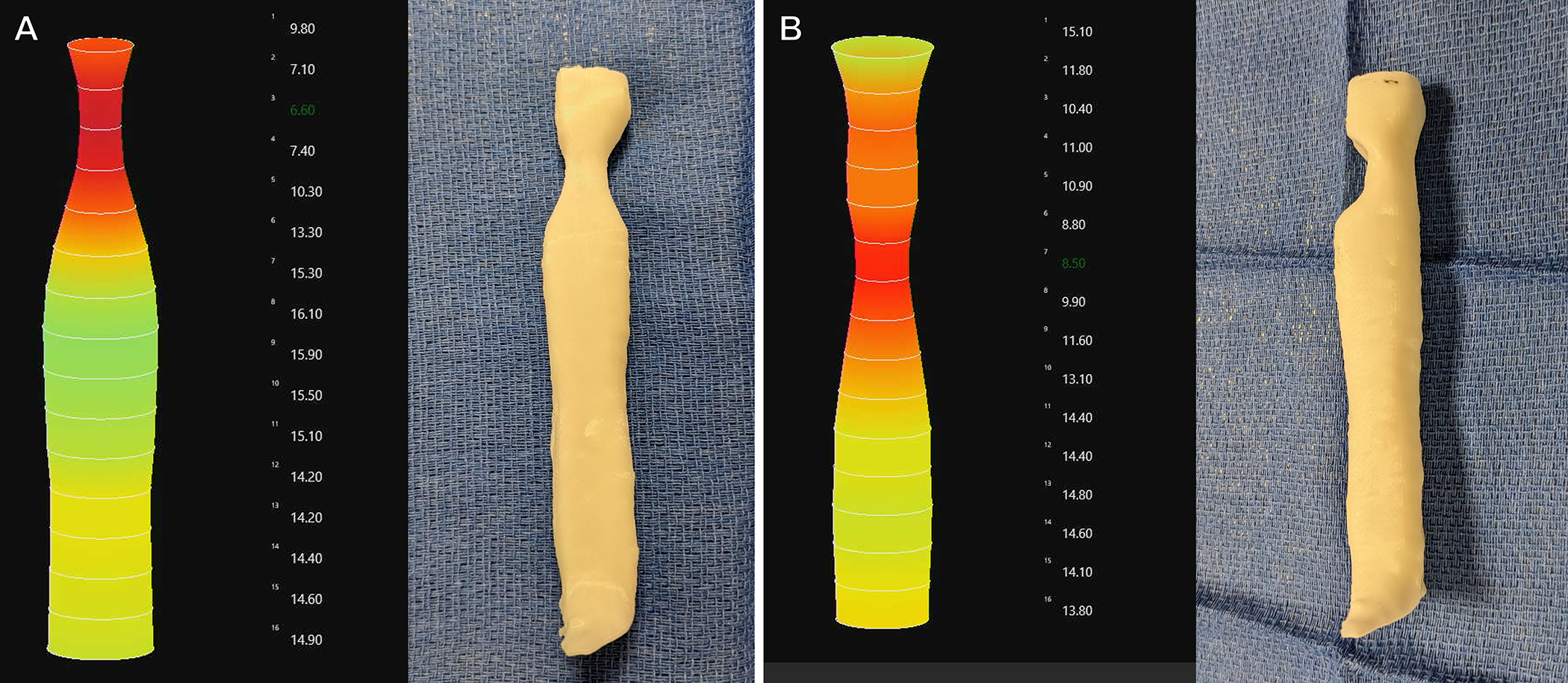
Representative measurements of the 3D tracheal models using the EndoFLIP. Numbers to the right of the EndoFLIP figure are estimated diameter in millimeters. A) Circumferential stenosis model B) Posterior stenosis model
The exact agreement between reference (CT scan, 3D model) and observed (EndoFLIP) was high for both MCSA (95.5%) and stenotic length (81.8%). This translates to a mean error of 1.4mm in measurements of diameter, and a 4mm error in stenotic length. There was a substantial level of concordance between the observer findings and model dimensions (MCSA: r = 0.99, stenotic length: r = 0.95). There was a low coefficient of variation (0.6–2.8%) for measurements, indicating high precision. Intra-observer reliability was high for measurements of both MCSA (r = 0.99, 0.995, p<0.01) and length of stenosis (r = 0.98, 0.99, p<0.01) for Observer #1 and Observer #2. Finally, there was substantial interobserver agreement for minimum cross-sectional area (r=0.99, p<0.01) and stenotic length (r=0.98, p<0.01)
Discussion
Quantitative assessment of subglottic and tracheal stenosis remains a challenge in otolaryngology. Patients with these findings may require clinical intervention and subsequent follow-up to evaluate treatment efficacy. Treatment decision often requires in-depth knowledge of the type of stenosis including length, thickness, and compliance. Appropriate longitudinal care of these patients is predicated on accurate and repeatable measurements of the upper airway. The current study demonstrated that a catheter-based, impedance planimetry system can be used for quantitative and reproducible assessments of a 3D model of the subglottic airway.
Within our study, we found that there is significant concordance between measurements obtained through the EndoFLIP system and the dimensions of our CT-scan based 3-D printed airway models. Using our method, the diameter and the length of the stenosis was estimated to within 1.4mm and 3.9mm, respectively. These ranges are consistent to slightly improved compared to previously described alternate methods of measurement, including both intraoperative measuring sticks and ultrasonography.[9, 10]. Furthermore, there was significant inter-rater and intra-rater reliability (>0.99, >0.99), indicating that this measurement system could be reproduced with high fidelity between different providers.
Real-time, intraoperative measurements of the airway can provide critical information for the otolaryngologist in identifying appropriate treatment strategies and assessing pre- and post-intervention changes. Currently, the standard method of evaluating subglottic stenosis described by Myer et.al [4] is limited by its single dimensionality; it does not provide other important information, such as the location or length of stenosis. Non-invasive methods that utilize radiographic studies, including computed tomography and magnetic resonance imaging, have been demonstrated accuracy in detection and quantification of subglottic stenosis [11–13]. 3D airway reconstructions of the airway based on endoscopic video evaluation have been shown to be reliable strategies in both phantom airway modeling, as well as ex-vivo animal experiments [14–16]. However, these processes can be time consuming, may subject patients to ionizing radiation, and do not provide intraoperative feedback.
The EndoFLIP system allows for rapid, multi-dimensional analysis of the length of the trachea. Its operation is similar to that of balloon dilation, a standard tool in the otolaryngologist’s arsenal, and one that is already a key component in the endoscopic management of laryngotracheal stenosis [17]. A primary difference between the two is in the type of balloon utilized. Current pediatric airway balloons are non-compliant and are generally inflated to 7–12 atmospheric pressures, compared to the 1–1.5 ATM filling pressures utilized in the EndoFLIP system, which allows the balloon to mold to the airway lumen Despite the significantly lower pressures in these compliant balloons, both types have been shown to create significant expansile force[18]. As currently designed, the catheter measures cross-sectional area with 0.1 mm resolution at 5 mm increments along the laryngotracheal lumen, which would capture stenotic length and location, even in the instances of multi-segment pathology. Furthermore, changes in the filling pressures within the balloon allow for quantification of lumen compressibility, which may provide critical information on the rigidity of the stenotic segment and associated scar thickness. Although certain imaging modalities, such as optical coherence tomography, have the potential to quantify scar thickness [19], EndoFLIP would be able to provide novel insight into the stiffness of pathologically narrow segments.
There are limitations to this approach for pediatric airway measurement. Firstly, the 6 mm overall diameter of the probe and catheter precludes utilization within the neonatal population. Secondly, the sensor distribution on the catheter limits the detection to 5 mm increments. Although this resolution should be adequate in the majority of clinical scenarios, it may omit particularly short stenotic segments. We postulate that multiple measurements with slight changes in catheter insertion depth may help overcome this limitation. Finally, this measurement method entails temporary occlusion of the airway lasting approximately 30 seconds. We anticipate that the risk profile of EndoFLIP deployment should be no higher than endoscopic high-pressure balloon dilation, which is routinely used to address pediatric subglottic stenosis [20]. Notably, filling and deflating times of the EndoFLIP are longer than standard airway dilation devices. As such, appropriate surgical precautions and careful patient selection, should be taken account to further mitigate these risks.
Conclusion
Intraoperative measurements of subglottic stenosis provide valuable assessments of treatment efficacy and help guide management decisions. In this pilot study, we demonstrate that the EndoFLIP system allows for multidimensional quantification of 3D-printed pediatric tracheal models in a single, reproducible, measurement. Further investigation is needed to assess the system’s applicability using a non-rigid model within an intraoperative setting.
Footnotes
Funding and Conflicts of Interest: None
Meeting:
This work was accepted for a podium presentation at COSM 2023: Boston, MA. May 3 – 7, 2023
Level of Evidence: N/A
References
- 1.Schweiger C, et al. , Incidence of post-intubation subglottic stenosis in children: prospective study. The Journal of Laryngology & Otology, 2013. 127(4): p. 399–403. [DOI] [PubMed] [Google Scholar]
- 2.Parkin JL, Stevens MH, and Jung AL, Acquired and congenital subglottic stenosis in the infant. Ann Otol Rhinol Laryngol, 1976. 85(5 Pt.1): p. 573–81. [DOI] [PubMed] [Google Scholar]
- 3.Marston AP and White DR, Subglottic Stenosis. Clin Perinatol, 2018. 45(4): p. 787–804. [DOI] [PubMed] [Google Scholar]
- 4.Myer CM 3rd, O’Connor DM, and Cotton RT, Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol, 1994. 103(4 Pt 1): p. 319–23. [DOI] [PubMed] [Google Scholar]
- 5.Hirano I, Pandolfino JE, and Boeckxstaens GE, Functional Lumen Imaging Probe for the Management of Esophageal Disorders: Expert Review From the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol, 2017. 15(3): p. 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hee L, et al. , Cervical stiffness evaluated in vivo by endoflip in pregnant women. PLoS One, 2014. 9(3): p. e91121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng K, et al. , Utility of functional lumen imaging probe in esophageal measurements and dilations: a single pediatric center experience. Surg Endosc, 2020. 34(3): p. 1294–1299. [DOI] [PubMed] [Google Scholar]
- 8.Furlow PW and Mathisen DJ, Surgical anatomy of the trachea. Ann Cardiothorac Surg, 2018. 7(2): p. 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma GK, Foulad A, and Verma SP, A novel device for measurement of subglottic stenosis in 3 dimensions during suspension laryngoscopy. JAMA Otolaryngol Head Neck Surg, 2015. 141(4): p. 377–81. [DOI] [PubMed] [Google Scholar]
- 10.Lambert EM, Tran HD, and Ongkasuwan J, Comparison of Endoscopic and Ultrasonographic Measurements of the Subglottic Airway in Children. Otolaryngol Head Neck Surg, 2020. 163(6): p. 1264–1269. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi DB, et al. , Quantitative Evaluation of Subglottic Stenosis Using Ultrashort Echo Time MRI in a Rabbit Model. Laryngoscope, 2021. 131(6): p. E1971–e1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourdillon AT, et al. , Correlations of Radiographic and Endoscopic Observations in Subglottic Stenosis. Ann Otol Rhinol Laryngol, 2022. 131(7): p. 724–729. [DOI] [PubMed] [Google Scholar]
- 13.Sirisopana M, et al. , Novel measurements of the length of the subglottic airway in infants and young children. Anesth Analg, 2013. 117(2): p. 462–70. [DOI] [PubMed] [Google Scholar]
- 14.Meisner EM, et al. , Anatomical reconstructions of pediatric airways from endoscopic images: a pilot study of the accuracy of quantitative endoscopy. Laryngoscope, 2013. 123(11): p. 2880–7. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, et al. , Anatomical reconstruction from endoscopic images: toward quantitative endoscopy. Am J Rhinol, 2008. 22(1): p. 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francom CR, et al. , Clinical validation and reproducibility of endoscopic airway measurement in pediatric aerodigestive evaluation. International Journal of Pediatric Otorhinolaryngology, 2019. 116: p. 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavrysen E, et al. , Endoscopic Treatment of Idiopathic Subglottic Stenosis: A Systematic Review. Front Surg, 2019. 6: p. 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson CM, et al. , Mechanical Modeling of the Human Cricoid Cartilage Using Computer-Aided Design: Applications in Airway Balloon Dilation Research. Ann Otol Rhinol Laryngol, 2016. 125(1): p. 69–76. [DOI] [PubMed] [Google Scholar]
- 19.Ridgway JM, et al. , Optical coherence tomography of the newborn airway. Ann Otol Rhinol Laryngol, 2008. 117(5): p. 327–34. [PMC free article] [PubMed] [Google Scholar]
- 20.Whigham AS, et al. , Outcomes of balloon dilation in pediatric subglottic stenosis. Ann Otol Rhinol Laryngol, 2012. 121(7): p. 442–8. [DOI] [PubMed] [Google Scholar]


