Abstract
Objective
In this cohort study, we assessed perinatal factors known to be related to maternal and neonatal inflammation and hypothesized several would be associated with emotional, cognitive, and behavioral dysregulation in youth.
Method
The Environmental Influences on Child Health Outcomes is a research consortium of 69 pediatric longitudinal cohorts. We used a subset of 18 cohorts that had both Child Behavior Checklist (CBCL) data on children (6–18 years) and information on perinatal exposures including maternal prenatal infections. Children were classified as having the CBCL dysregulation profile (CBCL-DP) if the sum of their T-Scores for three CBCL subscales (attention, anxious/depressed, and aggression) was ≥ 180. Perinatal factors associated with maternal and/or neonatal inflammation were our primary exposures and we assessed associations between these and our outcome.
Results
Approximately 13.4 % of 4,595 youth met criteria for the CBCL-DP. Boys were affected more than girls (15.1% vs 11.5%). More youth with the CBCL-DP (35%) were born to mothers with prenatal infection(s), compared to 28% of youth without the CBCL-DP. Adjusted odds ratios indicated the following were significantly associated with dysregulation: having a first degree relative with a psychiatric disorder, being born to a mother with lower educational attainment, who was obese, had any prenatal infection and/or who smoked tobacco during pregnancy.
Conclusion
In this large study, a few modifiable maternal risk factors with established roles in inflammation (maternal lower education, obesity, prenatal infections, and smoking) were strongly associated with the CBCL-DP and could be targets for interventions to improve offspring’s behavioral outcomes.
Diversity & Inclusion Statement:
We worked to ensure race, ethnic, and/or other types of diversity in the recruitment of human participants. One or more of the authors of this paper self-identifies as a member of one or more historically underrepresented sexual and/or gender groups in science. We actively worked to promote sex and gender balance in our author group. The author list of this paper includes contributors from the location and/or community where the research was conducted who participated in the data collection, design, analysis, and/or interpretation of the work.
Keywords: dysregulation, CBCL, children, adolescents, inflammation
INTRODUCTION
Emotional, cognitive, and behavioral dysregulation in childhood is associated with impaired psychosocial functioning, poor school performance and with increased rates of psychiatric disorders, suicidality, and functional impairment in adulthood.1–4 To prevent a lifetime of participatory challenges for those affected, it is critical to understand the prenatal or early life antecedents associated with the development of dysregulation. Studying the risk factors for emotional, cognitive, and behavioral dysregulation will facilitate the identification of factors that are potentially modifiable through treatment and/or education to ensure a healthier developmental trajectory for children.
One common tool used in research and clinical settings to assess for emotional, cognitive, and behavioral concerns in youth is the Child Behavior Checklist.5,6 Among the outcomes that can be derived from the Child Behavior Checklist (CBCL) is the Dysregulation Profile (CBCL-DP). The criterion for dysregulation is a sum of the T-scores for three subscales of the CBCL (Aggressive Behavior, Anxious/Depressed, and Attention Problems) being ≥ 180.7–9 Several recent studies have used a latent class analysis (LCA) for defining the CBCL-DP to provide a robust and well delineated class for each data set and society, given that the 3-peak model for CBCL-DP can be relatively rare.7,10 Nonetheless, high scores on all three of CBCL-DP subscales correlate with the Total problems score on the CBCL and are associated with significant maladjustment.10,11 Therefore, using the three peak definition of the CBCL-DP in early childhood may represent a general measure of overall psychopathology that is predictive of significant functional impairment in daily life and multiple neuropsychiatric outcomes during adolescence and adulthood.1,2,10,12 Thus, epidemiologic studies that identify modifiable risk factors for the CBCL-DP would have tremendous potential benefits for public health.
In the Extremely Low Gestational Age Newborns (ELGAN) cohort, the CBCL-DP at two years of age (age adjusted for degree of prematurity) was associated with maternal passive smoke exposure during pregnancy, maternal education of high school or less, as well as with Mycoplasma cultured from the placenta.8 All these factors were associated with neonatal systemic inflammation in the ELGAN cohort13,14 suggesting an overarching hypothesis that neonatal systemic inflammation increases the risk of emotional, cognitive and behavioral concerns as measured by the CBCL-DP.
This is the first study to our knowledge to examine various factors associated with maternal and/or neonatal inflammation and the CBCL-DP across a number of pediatric cohorts, including term born and preterm cohorts. To pursue our line of inquiry, we leveraged the unique dataset from the National Institutes of Health (NIH) Environmental Influences on Child Health Outcomes (ECHO) research program to identify early life factors associated with CBCL-DP, with a focus on prenatal factors that conceptually could be associated with neonatal systemic inflammation in the fetus. This limited set of risk factors fulfills two criteria: (1) factors included in the ECHO-wide data platform of extant data and (2) factors that we or others have found to be associated with maternal immune activation as well as maternal and/or neonatal systemic inflammation as these are potentially modifiable risk factors.13,14 Therefore, based on prior research we hypothesized that factors known to be associated with inflammation including lower maternal education, lower household income, pre-pregnancy obesity, maternal diabetes,15 maternal thyroid disease,16 tobacco smoke exposure during pregnancy (active and/or passive), fetal growth restriction, and maternal genito-urinary tract infections as well as any other prenatal infection would be associated with emotional, cognitive, and behavioral dysregulation (the CBCL-DP) in childhood and adolescence. Our access to a large sample size allowed us to adequately assess the relationships between exposures and outcomes of interest. Finally, this is the first study to evaluate sex as an effect modifier on these relationships. We hypothesized that we would observe sex differences in these associations with male participants being more impacted.
METHOD
Participants
The ECHO Program is an NIH-funded 7 year research consortium project that began in the Fall of 2016, consisting of 69 existing pediatric longitudinal observational cohorts with approximately 50,000 children and their caregivers from 44 US states and Puerto Rico.17 The ECHO Program includes two broad activities: 1. Analyses of pooled extant data previously collected prospectively by the longitudinal pediatric cohorts and 2. Analyses of prospectively collected data and biospecimens from these same 69 cohorts. Therefore, once ECHO was launched, a number of variables and measures (including the exposure and outcome data used in this manuscript) that had previously been collected in a prospective manner in each individual cohort prior to the ECHO program were shared in the centralized data base and underwent data harmonization. The project also enrolled study participants for new data collection using a standardized data collection protocol.18 ECHO aims to investigate the effects of early life environmental exposures (e.g., biological, chemical, social) on child health by focusing on five key pediatric health outcome areas:19,20 (1) prenatal, perinatal, and postnatal outcomes (e.g., small for gestational age, prematurity), (2) obesity, (3) upper and lower airway health (e.g., asthma), (4) neurodevelopment (e.g., cognition, psychopathology), and (5) positive health/well-being. Cohort-specific and/or central ECHO institutional review boards approved the protocols, and participants provided informed consent. This analysis included all ECHO cohorts that had CBCL5 data at age 6 to 18 years, collected prenatal infection information, and contributed data for at least 10 children. Specifically, the current analysis included 4595 children from 18 ECHO cohorts with prenatal infection information and CBCL data collected from 2009 to August 31, 2021 (Figure 1). Nearly all data used in this analysis were collected prospectively and all data were collected prior to the ECHO collaborative. These extant data were provided to ECHO and then were harmonized. All CBCLs are parent reports collected prospectively. Please see Table S1, available online (details about each cohort).
Figure 1.
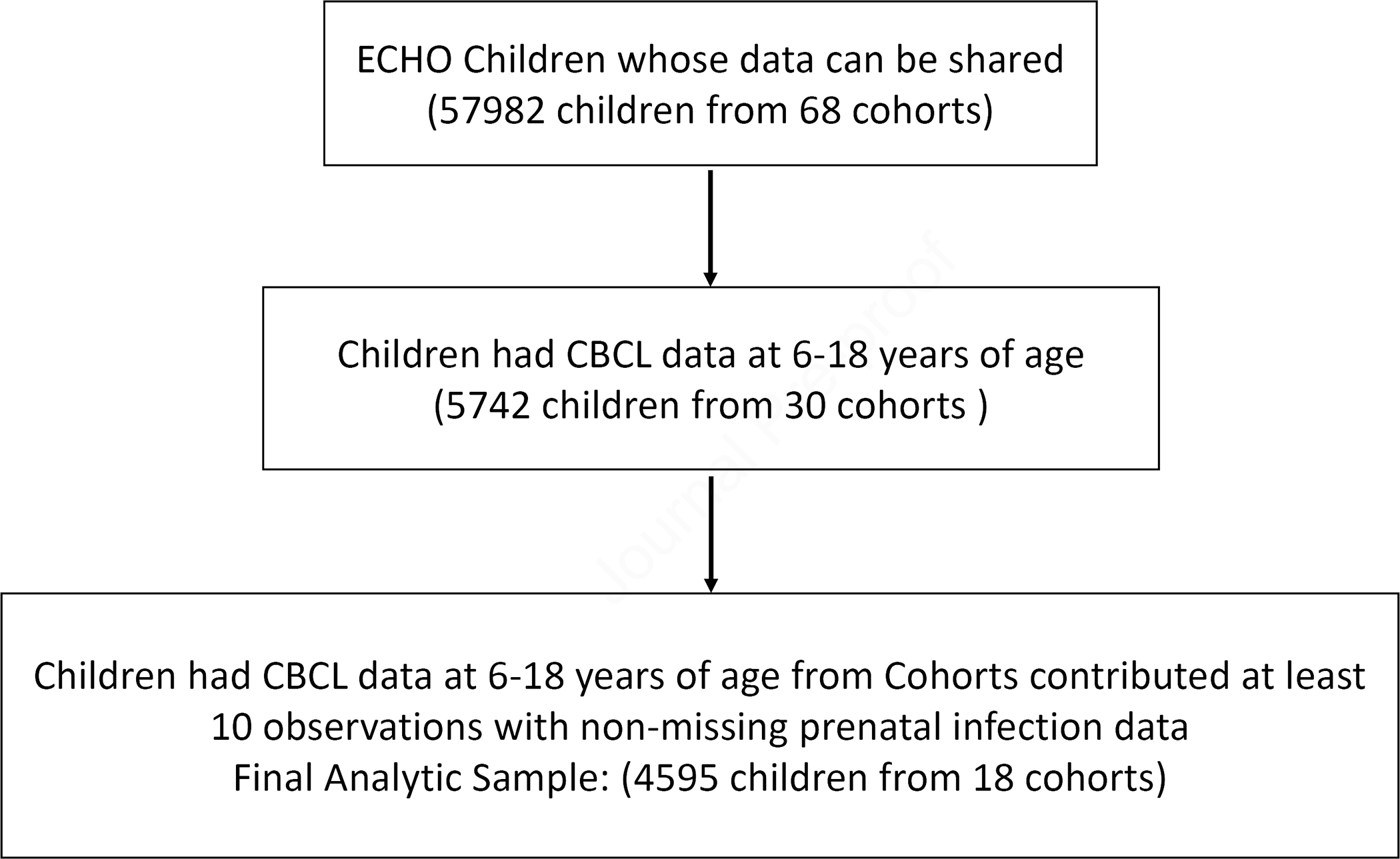
Flow Chart
Outcome
The outcome in this analysis was the CBCL-DP which is derived from the CBCL. The CBCL is a comprehensive parent report screener of behavioral problems and competencies among children and adolescents ages 6 to 18 years, consisting of 120 questions about the child/adolescent’s behavior.5 The criterion used for the CBCL-DP is a sum of T-Scores for three CBCL subscales (attention, anxious/depressed, and aggression) ≥ 180.7 For the three CBCL subscales used, we summed the corresponding item responses first, and then used the corresponding sex and age-standardized T-scores in analysis. When longitudinal data were available, we used the first CBCL assessment for each child between 6 and 18 years.
Exposures, Confounders, Effect Modifier, and Covariates
The exposures of interest included: (1) self-reported maternal tobacco smoke exposure during pregnancy (prenatal), (2) maternal pre-pregnancy overweight (BMI > 25 and < 30; compared to reference group with BMI ≤ 25) or obese (BMI ≥ 30), (3) fetal growth restriction indicated by small for gestational age (birth weight <10th percentile for gestational age), (4) socioeconomic disadvantage identified by lower maternal education (<high school, high school, > high school (reference group), (5) gestational diabetes, (6) prenatal maternal thyroid disease or thyroid medications, and (7) prenatal infections, which included fever, flu, sexually transmitted infections (including Genital Herpes, HIV, Trichomonas, positive lab results for Gonorrheal screening, chlamydia screening, HIV, HBsAg-positive, and HCV-positive), sexually transmitted infections known to impact brain development (determined by positive lab results on Zika or Syphilis (VDRL)), genitourinary tract infections (including urinary tract infection (bladder/kidney), infection in utero, vaginal infection, Group B Strep vaginal infection, other genitourinary tract infection, pelvic inflammatory disease), pneumonia, and skin infections (including Cellulitis).
We created a directed acyclic graph to guide the selection of potential confounders for adjustment in multivariable models (see Figure S1, available online). Potential confounders included gestational age (<28, 28–36, ≥37 weeks-reference group) and a first degree relative (biologic mother, father, sister, brother) with a psychiatric condition (including major depression, dysthymia, bipolar disorder, anxiety disorder NOS, generalized anxiety disorder, specific phobia, panic disorder, obsessive compulsive disorder, social anxiety, post-traumatic stress disorder, attention deficit hyperactivity disorder, eating disorder, schizophrenia, alcoholism or other substance abuse, and autism spectrum disorder). Other covariates included in multivariable regression were child age at CBCL assessment (6–8 years-reference group, 9–11, 12–14, and 15–18 years old), and child race/ethnicity.
All variables used in this analysis were generated by an ECHO-wide data harmonization process that used both new data collected by standardized protocols and cohort specific extant data to maximize the sample size and power for analysis. Adjusted ORs were obtained from multivariable logistic regression models.
Missing Data
We used multiple imputation to account for missing data for variables with ≤ 50 % missing data by fully conditional specification (FCS) with a discriminant function method 21 for categorical and binary variables, such as race/ethnicity, maternal education, small for gestational age, prenatal tobacco use, first degree relatives with psychiatric disorder, prenatal infection, gestational diabetes, and prenatal thyroid disorders. We used the FCS predictive mean matching method for imputing the continuous variables of gestational age and maternal pre-pregnancy BMI. We included all variables in the analysis model in the imputation model to impute the following variables for 25 imputations: child race/ethnicity (0.6% missing), maternal education (2.5%), gestational age (7.6%), maternal pre-pregnancy BMI (10.1%), gestational diabetes (12.5%), first degree relatives with psychiatric disorder (14.6%), prenatal infection (18.8%), prenatal tobacco use (25.3%), and prenatal thyroid disorder (39.3%). ECHO cohort site was also included in the imputation model as a classification variable (one categorical variable with 18 Cohort ID levels).
Statistical Analysis
Descriptive statistics were used to describe and compare characteristics between children with and without CBCL-DP based on raw data with missing values. We then conducted exploratory bivariate analyses of associations between our primary exposures and our outcome by using chi-square analysis or Fisher’s exact test or T-test.
In the process of statistical modeling, the 25 imputed complete data sets were each analyzed first, and their estimates were combined to calculate average effect estimates and standard errors incorporating the imputation variability.22 Specifically, univariable, and multivariable logistic regression models with random intercept for controlling cohort effect were fitted to quantify the association between possible predictors and CBCL-DP. To evaluate effect modification on CBCL-DP, we ran stratified analyses by child sex. To examine whether the effect of predictors on CBCL-DP differ by sex, we ran separate multivariable logistic regression models including an interaction term of sex and each of the predictors; the odds ratio for one variable was adjusted for all other variables in the table as well as for child age at time of CBCL, child sex, birth weight, race, and gestational age. In addition, we did a similar analysis for each subscale of the CBCL-DP (attention, anxious/depressed and aggression) and this is included in Tables S2A–C, available online.
We did a leave-one-out sensitivity analysis by excluding one cohort successively to examine the influence of individual cohort, which might be caused by unmeasured confounding or other cohort characteristics (e.g., one cohort had many subjects with autism spectrum disorder) (see Figure S2, available online). In other sensitivity analyses we modeled the outcome of CBCL-DP as a continuous variable.
For multiple comparisons we used the Benjamini and Hochberg (BH) adjustment method that controls for the false discovery rate to obtain adjusted p-values.23 All analyses were conducted in SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Characteristics of Study Population (See Table 1):
Table 1.
Characteristics of Study Population
| Characteristics | No CBCL-DP N (%) | Yes CBCL-DP N (%) |
|---|---|---|
|
| ||
| Number of Children | 3,979(86.6) | 616(13.4) |
| Age (years) at CBCL assessment, N (%) with data | 3,979(100) | 616(100) |
| 6–8 | 2,298(58) | 318(52) |
| 9–11 | 755(19) | 131(21) |
| 12–14 | 152(4) | 37(6) |
| 15–18 | 774(19) | 130(21) |
| Child Gender, N (%) with data | 3,979(100) | 616(100) |
| Female | 1,917(48) | 249(40) |
| Male | 2,062(52) | 367(60) |
| Child Race and Ethnicity, N(%) with data | 3,955(99.4) | 611(99.2) |
| Hispanic | 537(14) | 97(16) |
| Non-Hispanic White | 2,045(52) | 325(53) |
| Non-Hispanic Black | 972(25) | 127(21) |
| Non-Hispanic Asian | 77(2) | < 10 |
| Non-Hispanic Native Hawaiian or other Pacific Islander | <10 | 0(0) |
| Non-Hispanic American Indian or Alaska Native | <10 | <5 |
| Non-Hispanic Multiple Race | 306(8) | 53(9) |
| Non-Hispanic Other Race | <5 | 0(0) |
| CBCL Respondent, N(%) with data | 3,945(99.1) | 612(99.4) |
| Mother | 3,331(84) | 515(84) |
| Father | 106(3) | 11(2) |
| Other | 508(13) | 86(14) |
| Gestational Age at Birth, N(%) with data | 3,674(92.3) | 570(92.5) |
| >=37 weeks | 2,762(75) | 414(73) |
| 28–36 weeks | 289(8) | 57(10) |
| <28 weeks | 623(17) | 99(17) |
| Birth Weight(kg), N(%) with data | 3,825(96.1) | 586(95.1) |
| Mean(SD) | 2.9(1) | 2.9(1.1) |
| Highest Maternal Education, N(%) with data | 3,880(97.5) | 598(97.1) |
| < High School | 286(7) | 63(11) |
| High School | 597(15) | 98(16) |
| Some college and above | 2,997(77) | 437(73) |
| Prenatal Income, N(%) with data | 1,087(27.3) | 157(25.5) |
| <$30,000 | 616(57) | 99(63) |
| $30,000-$49,999 | 131(12) | 15(10) |
| $50,000-$74,999 | 225(21) | 32(20) |
| $75,000-$99,999 | 20(2) | <5 |
| $100,000 or more | 95(9) | <10 |
| Behavior Summary Scores | Mean (SD) | Mean (SD) |
| Anxious/Depressed Syndrome T-score | 52(3.9) | 63(8.8) |
| Attention Problems T-score | 54(4.6) | 67(8.7) |
| Aggressive Behavior T-score | 52(3.6) | 65(8.2) |
| Sum of above 3 T-score | 158(8.3) | 195(15.3) |
Note: CBCL-DP = Child Behavior Checklist- Dysregulation Profile
As compared to the entire ECHO cohort, the sample used in this CBCL-DP analysis was enriched in participants born preterm and participants with autism, and the average maternal education was higher, but the distribution of sex was similar (Table 1).
We included 4,595 children and adolescents across 18 cohorts from the ECHO program in this data analysis. These children were selected because they had both prenatal infection information and a CBCL. Among the respondents on the CBCL, 84% were mothers, 3% were fathers and 13% were other. Six hundred and sixteen (13.4%) of these 4,595 children and adolescents met criteria for the CBCL-DP. Of those with the CBCL-DP, 52% were between 6–8 years of age, 21% were between 9–11 years, 6% were between 12–14 years, and 21% were between 15–18 years. A greater percentage of those with the CBCL-DP (48%) were 9 years of age or older compared to their peers (42%). The sex breakdown of those who had the CBCL-DP was 1.5:1 (boys vs girls) compared to a sex ratio of closer to 1:1 in those youth without the profile (p<0.001). The frequency of the CBCL-DP was 11.5% and 15.1% for girls and boys respectively. Of those who met threshold criteria for the CBCL-DP, 16% were Hispanic, 9% were Non-Hispanic Multiple Race, 53% were Non-Hispanic White, 21% were Non-Hispanic Black, and 1% were Non-Hispanic Asian.
Twenty-five percent of the study sample was born preterm (before 37 weeks of gestation), and 17% was born extremely preterm (before 28 weeks of gestation). Eight percent were small for gestational age at birth. The distribution of maternal education was as follows: 8% had not completed high school, 15% had completed high school but did not attend college, and 77% had at least some college education.
Associations between Exposures and the CBCL-DP (See Table 2 and 3)
Table 2.
Associations between Exposures and CBCL-Dysregulation Profile
| No CBCL-DP | Yes CBCL-DP | P-Valuea | P-Adjustedb | |
|---|---|---|---|---|
| Characteristics | N (%) | N (%) | ||
|
| ||||
| Number of Children | 3979(86.6) | 616(13.4) | ||
| Small for Gestational Age (SGA) at Birth, N(%) with data | 3200(80.4) | 503(81.7) | 0.183 | 0.290 |
| Yes | 244(8) | 47(9) | ||
| First Degree Relatives had Psychiatric Disorder, N(%) with data | 3411(85.7) | 512(83.1) | <0.001 | <0.001 |
| Yes | 1704(50) | 346(68) | ||
| Maternal pre-pregnancy BMI, N(%) with data | 3574(89.8) | 556(90.3) | 0.004 | 0.012 |
| <=25 | 1911(53) | 264(47) | ||
| 25–29.9 | 830(23) | 128(23) | ||
| >30 | 833(23) | 164(29) | ||
| Prenatal Tobacco use, N(%) with data | 2982(74.9) | 452(73.4) | <0.001 | 0.001 |
| Yes | 418(14) | 94(21) | ||
| Any Prenatal Infection, N(%) with data | 3226(81.1) | 507(82.3) | 0.004 | 0.012 |
| Yes | 918(28) | 176(35) | ||
| Flu during Pregnancy, N(%) with data | 1335(33.6) | 259(42) | 0.064 | 0.119 |
| Yes | 125(9) | 34(13) | ||
| Fever during Pregnancy, N(%) with data | 773(19.4) | 171(27.8) | 0.897 | 0.897 |
| Yes | 84(11) | 18(11) | ||
| Sexually transmitted diseases (STD) during pregnancy, N(%) with data | 2327(58.5) | 384(62.3) | 0.272 | 0.346 |
| Yes | 180(8) | 36(9) | ||
| STD impacting brain development during pregnancy, N(%) with data | 1218(30.6) | 217(35.2) | 0.151 | 0.264 |
| Yes | 0(0) | <5 | ||
| Genitourinary tract infections during this pregnancy, N(%) with data | 3120(78.4) | 496(80.5) | 0.196 | 0.290 |
| Yes | 632(20) | 113(23) | ||
| Pneumonia during this pregnancy, N(%) with data | 803(20.2) | 130(21.1) | 0.254 | 0.339 |
| Yes | 5(1) | <5 | ||
| Skin infections during this pregnancy, N(%) with data | 241(6.1) | 35(5.7) | 0.421 | 0.464 |
| Yes | <5 | <5 | ||
| Gestational Diabetes, N(%) with data | 3472(87.3) | 548(89) | 0.008 | 0.020 |
| Yes | 176(5) | 43(8) | ||
| Prenatal thyroid disorder or on medications*, N(%) with data | 2411(60.6) | 376(61) | 0.031 | 0.064 |
| Yes | 43(2) | 13(3) | ||
| Prenatal thyroid disorder, N(%) with data | 2409(60.5) | 376(61) | 0.032 | 0.064 |
| Yes | 43(2) | 13(3) | ||
| Prenatal usage of thyroid disorder medicationsc, N(%) with data | 283(7.1) | 46(7.5) | 0.207 | 0.290 |
| Yes | 26(9) | 7(15) | ||
| Highest Maternal Education, N(%) with data | 3880(97.5) | 598(97.1) | 0.017 | 0.040 |
| < High School | 286(7) | 63(11) | ||
| High School | 597(15) | 98(16) | ||
| Some college and above | 2997(77) | 437(73) | ||
Note: CBCL-DP = Child Behavior Checklist- Dysregulation Profile
P-Value were obtained from Chi-square test, or Fisher Exact test, or T-test where appropriate
Adjusted P-Value using Benjamini and Hochberg correction method for multiple comparisons
Thyroid disorder medications included Levothyroxine, methimazole, and propylthiouracil
Family Psychiatric History and Child CBCL-DP
Sixty-eight percent of those with the CBCL-DP had a first degree relative with a psychiatric disorder as compared to 50% of children without the CBCL-DP (p<0.001). The adjusted OR for having the CBCL-DP if the youth had a first degree relative with a psychiatric disorder was 2.08 (CI:1.69,2.55).
Maternal Health and Child CBCL-DP
As compared to youth without the CBCL-DP, youth with the CBCL-DP were more likely to be born to mothers with pre-pregnancy BMIs in the overweight category or greater (52% vs 46%; p=0.012), more likely to be exposed to prenatal tobacco use (21% versus 14%; p=0.001), more likely to be exposed to any prenatal infection (35% versus 28%;p=0.012), more likely to be exposed to maternal gestational diabetes (8% versus 5%; (p=0.02), and more likely to be born to a mother with a thyroid disorder (3% versus 2%; (trend p=0.064).
After adjusting for all variables in Table 3 as well as for child age at time of CBCL, child sex, birth weight, race and gestational age, children with mothers whose pre-pregnancy BMI was in the obese range (≥30 BMI) had a greater odds of having the CBCL-DP [OR:1.39 (CI:1.11, 1.74)] compared to those youth with mothers whose BMI < 25. Other factors associated with CBCL-DP were exposure to prenatal tobacco [OR: 1.66 (CI: 1.24; 2.23) and prenatal infection [[OR: 1.35 (CI:1.1, 1.65)].
Table 3.
Adjusted Odds Ratios of Child Behavior Checklist (CBCL)-Dysregulation Profile
| Characteristics | Overall OR (95% CI) |
Boys
OR (95% CI) |
Girls
OR (95% CI) |
Sex
Difference P-Value |
|---|---|---|---|---|
|
| ||||
| Small for Gestational Age at Birth (ref = No) | 1.25 (0.89,1.76) | 1.24 (0.8,1.92) | 1.32 (0.77,2.29) | 0.957 |
| First Degree Relatives had Psychiatric Disorder (ref = No) | 2.08 (1.69,2.55) | 1.93 (1.48,2.52) | 2.33 (1.68,3.23) | 0.246 |
| Maternal pre-pregnancy BMI (ref = ≤ 25) | ||||
| Obese (BMI ≥30) | 1.39 (1.11,1.74) | 1.44 (1.08,1.91) | 1.4 (0.98,2) | 0.578 |
| Overweight (BMI 25–29.9) | 1.13 (0.89,1.44) | 1.03 (0.75,1.42) | 1.27 (0.89,1.82) | 0.491 |
| Prenatal Tobacco use (ref = No) | 1.66 (1.24,2.23) | 1.21 (0.8,1.82) | 2.2 (1.42,3.42) | 0.01 |
| Any Prenatal Infection (ref = No) | 1.35 (1.1,1.65) | 1.49 (1.14,1.95) | 1.17 (0.85,1.62) | 0.401 |
| Gestational Diabetes (ref = No) | 1.28 (0.9,1.8) | 1.41 (0.92,2.15) | 1.05 (0.59,1.87) | 0.347 |
| Prenatal thyroid disorder or on medications (ref = No) | 1.84 (0.96,3.52) | 1.15 (0.46,2.87) | 3.55 (1.42,8.89) | 0.099 |
| Highest Maternal Education (ref = Some college or above) | ||||
| < High School | 1.52 (1.1,2.11) | 1.25 (0.8,1.96) | 1.78 (1.11,2.86) | 0.142 |
| High School | (1.11 (0.86,1.43) | 1.03 (0.73,1.44) | 1.19 (0.8,1.76) | 0.42 |
Note: Adjusted Odd Ratios (ORs) were obtained from multivariable logistic regression models. An OR for one variable was adjusted for all other variables in Table 3 as well as for child age at time of CBCL, child sex, birth weight, race, and gestational age.
Sex Difference P-Value represents the interaction of each predictor and sex
Numbers in bold font have p-value <0.05
Maternal Education and Child CBCL-DP
Lower maternal education was significantly associated with a youth having the CBCL-DP (p=0.017). Youth with a mother with less than a high school education had higher odds of having the CBCL-DP compared to youth whose mother had some college or above [1.52(CI:1.1, 2.11)].
Sex Differences in Associations between Maternal Health and CBCL-DP (see Table 3).
In analyses of effect moderation, the only statistically significant finding was a stronger association with prenatal tobacco smoke exposure among girls with the CBCL-DP compared to girls without the CBCL-DP. Girls exposed to prenatal tobacco use had an OR of having the profile of 2.2 (CI:1.42,3.42) which was significantly different from boys (p=0.01). Although the interaction between sex and exposure to prenatal thyroid disorder or medication was not statistically significant (p = 0.1), the odds ratio for this exposure among girls was 3.55 (1.42, 8.89) as compared to an odds ratio of 1.15 (0.46, 2.87) in boys.
The sensitivity analysis in which we sequentially excluded the data from each cohort (including one comprised primarily of children with autism spectrum disorder) did not alter the key findings (see Supplementary Figure 2). In addition, the results of sensitivity analyses in which the outcome was treated as a continuous variable (the sum of T-scores for attention, anxious/depressed and aggression) are summarized in Figure S3, available online, which depicts the distribution of the continuous outcome as a function of exposures. Comparisons across exposure levels lead to conclusions similar to those from analyses using a clinical cut of poin
DISCUSSION
In this study which includes 18 longitudinal pediatric cohorts, we observed that approximately 13.4 % of study participants were classified as having the CBCL-DP, an indicator of emotional, cognitive, and behavioral dysregulation. This rate of the CBCL-DP is well within the range of prevalence rates (2–18%) identified using the latent class analysis approach for defining the CBCL-DP in 56,666 children ranging in age from 6–16 across 29 societies.11 We also found that boys were more often affected than girls consistent with the literature.24 This study is unique in its investigation of the associations between proinflammatory conditions such as maternal obesity, gestational diabetes, thyroid disease, prenatal infections, and prenatal maternal smoking during pregnancy and emotional, cognitive, and behavioral dysregulation in their offspring as assessed by the CBCL-DP. Overall, the most interesting finding of our study is that maternal prenatal infection, obesity, maternal education of less than high school and smoking were associated with significantly increased odds of having the CBCL-DP in offspring beyond the risk conferred by having a first degree relative with a psychiatric disorder and independent of the effects of each of these exposures.
Maternal and sociodemographic characteristics
In our study, youth with the CBCL-DP were more likely than their peers to be 9 years of age or older and male, to have a first degree relative with a psychiatric disorder), and to have a mother with less than a high school education. We found that the rates of CBCL-DP differed depending on the age range of the child. This not only may relate to the developmental stage of the child but also may reflect changes in the parent-child/peer-child relationships at different age ranges as well as ongoing developmental interventions. This also may reflect the increased contributions of anxiety and depression to the CBCL-DP in late adolescence which is often harder to detect at younger ages and might reflect that ADHD symptoms decline at early adolescence,28 lowering the attention subscale score.
Maternal Education
Maternal education was used as a socioeconomic surrogate in our analyses due to the large amount of missing data on family income. We found an association between low maternal education (our SES proxy) and dysregulation, consistent with other reports about health and cognitive outcomes in youth who are at risk.25–27 Several studies have indicated that the stressors experienced by mothers who are disadvantaged, and the early care and social interactions experienced by infants, have been associated with epigenetic changes that impact brain development and function conferring increased risk for emotional, cognitive, and behavioral dysregulation and mental health conditions.27,29
Family Psychiatric History
An elevated score on the CBCL-DP is highly heritable and genetic associations support its validity as a distinct phenotype.30–32 Also, in our analysis of adjusted odds ratios where one variable was adjusted for all other exposures of interest, having a first degree relative with a history of any psychiatric disorder was most strongly associated with having CBCL-DP with an adjusted OR of 2.08 (CI: 1.69,2.55). This may reflect that there is genetic vulnerability for developing the CBCL-DP which confers eventual risk of psychopathology.33,34
While the CBCL-DP is highly heritable it is also associated with environmental adversity such as impaired peer relationships and difficulties at home and at school.3,35 Kim and colleagues reported that parents of children with the CBCL-DP had more psychopathology and more significant marital strife than parents of children without the profile.3 Therefore, there might be an element of modeling of behavior36 within the home that influences the development of the CBCL-DP in youth, highlighting the complicated relationship between genetic and environmental factors that might set the stage for a youth to develop the dysregulation profile.
The CBCL-DP (using the three-peak definition) likely reflects both genetic and early life experiences and is a measure indicating general psychopathology. In fact, Deutz and colleagues found in a longitudinal study of 1, 073 children that the general factor of psychopathology (GP) on the CBCL and the CBCL-DP were quite similar in terms of stability, antecedents (including family factors (home environment and parenting) and maternal depression) and outcomes. They also pointed out that the using the CBCL-DP model might be “more parsimonious” than the GP as it required fewer items of the CBCL and likely would not require the same power as a study using the GP.12
Maternal Health Parameters
A variety of maternal characteristics were associated with youth who met the criteria for CBCL-DP. For example, mothers of children with the profile were more likely to have an elevated prenatal body mass index. Being overweight is associated with a heighted inflammatory state37 and this might result in an inflammatory milieu for the developing fetus38 which in turn could have epigenetic consequences with associated emotional, cognitive, and behavioral dysregulation.39,40
Maternal prenatal tobacco use was associated with the CBCL-DP in offspring, with this association being more common among girls with the CBCL-DP than boys. Tobacco exposure may have direct and indirect effects via epigenetic changes on child development41 and prenatal exposure also may implicate later conditions such as asthma and ADHD. Pregnant women typically under-report their tobacco use.42 For example, more than 20% of pregnant women who denied using tobacco had cotinine levels that were considered characteristic of smokers.43 Therefore, it is possible that if maternal cotinine levels were available that there would be an even greater association between this exposure and the CBCL-DP in youth. Smoking is known to be associated with increased inflammatory markers in the body44 and has an impact on brain development and function.45 In addition, smoking during pregnancy has been associated with infant irritability and behavioral dysregulation during childhood, including ADHD.46,47 Our findings of an association between maternal smoking and the CBCL-DP and maternal obesity and the CBCL-DP also are similar to the findings reported by Frazier and colleagues in an extremely preterm born cohort.8
Our finding that any maternal infection during pregnancy was associated with the CBCL-DP in offspring is consistent with prior research that suggests that prenatal maternal infections are associated with infections in the newborn48 as well as with psychiatric disorders, such as autism, schizophrenia, and attention deficit hyperactivity disorder, in offspring.49 Similarly, some of these findings have also been linked to perinatal inflammation in ELGANs.50 Moreover, a recent very large study from the UK found that maternal reported infections and not hospital recorded infections during pregnancy were associated with increased emotional problems during childhood in their offspring.49 In addition, there is evidence in the literature that early life inflammation in infants is associated with subsequent behavioral outcomes, including ADHD.51 Unfortunately, we did not have access to first month blood spots to directly evaluate this association in this study, but we were able to assess prenatal factors that have been associated with neonatal systemic inflammation in prior studies. As a result of the associations between exposures that trigger maternal immune activation and behavioral outcomes, infants exposed to prenatal infections may require enhanced monitoring allowing for early identification of and interventions for adverse emotional, cognitive, and behavioral outcomes.
We found that children with CBCL-DP were more likely to have mothers with gestational diabetes. Maternal obesity and gestational diabetes have been associated with chronic inflammation which changes the fetal and placenta milieu.15 Yet at least one large study indicated that maternal obesity but not gestational diabetes, was associated with impaired child neurodevelopment and behavioral difficulties in preschool children.54 Consistent with these findings, in our study maternal obesity but not gestational diabetes, after adjustment, remained significantly associated with having the CBCL-DP. We also noted a trend (after adjusting for multiple comparisons) for maternal thyroid disease and treatment that was associated with CBCL-DP in girls. This finding is consistent with a recent systematic review and a large study highlighting the association between maternal thyroid dysfunction, particularly hypothyroidism (a pro-inflammatory condition), during pregnancy and behavioral health problems in offspring, including ADHD and externalizing behaviors.52,53
The strengths of this study include that it used one of the largest sample sizes to study the CBCL and had availability of information about maternal infections. In addition, we were able to evaluate a variety of other factors associated with maternal immune activation and neonatal inflammation to identify possible modifiable risk factors. There are genetic, epigenetic, and environmental factors interacting in complex ways to result in emotional, cognitive, and behavioral dysregulation in youth.
With regard to the limitations of this study, we did not have nationally representative samples, a potential source of bias that could limit the generalizability of our findings. Our analysis included many disparate cohorts in relation to age, location, maturity at birth, as well as other variables which might be a potential source of bias. However, the leave-one-out sensitivity analysis showed the robustness of our results. In addition, data on important socioeconomic variables, such as household income, were missing from a large proportion of participants, creating the possibility of bias due to informative missingness. Consequently, we used maternal education as a socioeconomic surrogate in our analysis. Since maternal education is associated with offspring having the CBCL-DP, the prevalence of dysregulation in this CBCL subsample may underrepresent the extent of dysregulation in the overall ECHO sample. Lacking data on specific prenatal infections, and the timing of infections, we were not able to examine the effect of each specific infection type, and of timing of the infections on the likelihood of CBCL-DP. We also had relatively small, but meaningful, effect sizes so the possibility of modifying only one variable in isolation likely will have a small impact on the likelihood of developing the CBCL-DP. However, modifying several variables could possibly have an additive impact on developing the CBCL-DP. In addition, we relied on a single informant report of the CBCL and did not have access to a structured diagnostic psychiatric interview. Lastly, some forms used by the cohorts collected family history of psychiatric disorder on the child’s biologic mother, father, and siblings, but others collected information only on the mother, which might have resulted in misclassification when only mother’s information was available and used in defining the first-degree relatives having psychiatric disorder. Overall, however, ECHO cohorts represent geographic, economic, racial, and ethnic diversity, and the current study is the largest study evaluating the associations between maternal immune activation and the CBCL-DP in offspring.
The findings of this study lend support to the contribution of prenatal exposures to the later development of CBCL-DP. Social and educational interventions could possibly target the modifiable risk factors (e.g., maternal obesity, prenatal tobacco use, maternal education) and modify the developmental trajectories associated with the CBCL-DP, particularly if there are multiple modifiable risk factors seen in an individual child, as addressing the risk factors collectively could have an additive impact on the child’s outcome. Given the prevalence, the extent of impairment and the chronicity of the CBCL-DP, early screening in primary care practices of children who have antecedents that are associated with increased risk of emotional, cognitive, and behavioral problems would be beneficial. Early therapeutic approaches, including Parent Child Interaction Therapy (PCIT) and pharmacological intervention could be implemented to target the symptoms or diagnoses (e.g. ADHD) seen in these children to improve outcomes.55 In addition, youth who have the CBCL-DP often require integrated models of care to facilitate their development. Both the child and family require systems that more fully can address their needs. Contingency management and cognitive behavior therapy can help older children acquire more adaptive problem-solving abilities. Critically important to the overall management of these dysregulated youth is more fully understanding the drivers behind a child’s behavior and providing support and intervention in a sustained way.56
Future research would benefit from focusing on identifying the physiological mechanisms linking prenatal risks and the CBCL-DP and on whether various interventions can reverse or partially reverse these physiological mechanisms that contribute to the development of emotional, cognitive, and behavioral dysregulation.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of the Director, National Institutes of Health, under Award Numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 (PRO Core), UH3OD023248, UH3OD023282, UH3OD023365, UH3OD023271, UH3OD023389, UH3OD023342, UH3OD023348, UH3OD023285, UH3OD023290, UH3OD023249, UH3OD023305, UH3OD023337, UH3OD023318, UH3OD023328, UH3OD023332, U24OD023319, U24OD023382, and R03HD090430. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors wish to thank our ECHO colleagues; the medical, nursing, and program staff; and the children and families participating in the ECHO cohorts. The Environmental influences on Child Health Outcomes (ECHO) program: https://www.nih.gov/research-training/environmental-influences-child-health-outcomes-echo-program.
Footnotes
Disclosure: Dr. Frazier has received research support from Quadrant, Healx, and Tetra Pharmaceuticals. Dr. Posner has received research support from Takeda (formerly Shire) and Aevi Genomics and consultancy fees from Innovative Science. Drs. Li, Kong, Hooper, Joseph, Cochran, Kim, Fry, Brennan, Msall, Fichorova, Hertz-Picciotto, Daniels, Lai, Boles, Zvara, Jalnapurkar, Schweitzer, Singh, Bennett, Kuban, and O’Shea have reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jean A. Frazier, Eunice Kennedy Shriver Center, Worchester, Massachusetts, and the UMass Chan Medical School, Worchester, Massachusetts.
Xiuhong Li, John’s Hopkins University, Baltimore, Maryland.
Xiangrong Kong, John’s Hopkins University, Baltimore, Maryland.
Stephen R. Hooper, University of North Carolina at Chapel Hill.
Robert M. Joseph, Boston University School of Medicine, Boston, Massachusetts.
David M. Cochran, Eunice Kennedy Shriver Center, Worchester, Massachusetts, and the UMass Chan Medical School, Worchester, Massachusetts.
Sohye Kim, Eunice Kennedy Shriver Center, Worchester, Massachusetts, and the UMass Chan Medical School, Worchester, Massachusetts.
Rebecca C. Fry, University of North Carolina at Chapel Hill.
Patricia A. Brennan, Emory University, Atlanta, Georgia.
Michael E. Msall, University of Chicago Comer Children’s Hospital, Chicago, Illinois, and Kennedy Research Center on Intellectual and Neurodevelopmental Disabilities, Baltimore, Maryland.
Raina N. Fichorova, Brigham and Women’s Hospital, Boston, Massachusetts, and Harvard Medical School, Boston, Massachusetts.
Irva Hertz-Picciotto, University of California, Davis.
Julie L. Daniels, University of North Carolina at Chapel Hill.
Jin-Shei Lai, Northwestern University Feinberg School of Medicine, Chicago, Illinois.
Richard E. Boles, University of Colorado Anschutz Medical Campus, Aurora, Colorado.
Bharathi J. Zvara, University of North Carolina at Chapel Hill.
Isha Jalnapurkar, Eunice Kennedy Shriver Center, Worchester, Massachusetts, and the UMass Chan Medical School, Worchester, Massachusetts.
Julie B. Schweitzer, University of California, Davis.
Rachana Singh, Tufts Medical Center, Boston, Massachusetts..
Jonathan Posner, Duke University, Durham, North Carolina.
Deborah H. Bennett, University of California, Davis.
Karl C. K. Kuban, Boston University Medical Center, Boston, Massachusetts.
T. Michael O’Shea, University of North Carolina at Chapel Hill.
REFERENCES
- 1.Holtmann M, Buchmann AF, Esser G, Schmidt MH, Banaschewski T, Laucht M. The Child Behavior Checklist-Dysregulation Profile predicts substance use, suicidality, and functional impairment: A longitudinal analysis. Journal of Child Psychology and Psychiatry. 2011;52(2):139–147. doi: 10.1111/j.1469-7610.2010.02309.x [DOI] [PubMed] [Google Scholar]
- 2.Hyde R, O’Callaghan MJ, Bor W, Williams GM, Najman JM. Long-term outcomes of infant behavioral dysregulation. Pediatrics. 2012;130(5):e1243–e1251. DOI: 10.1542/peds.2010-3517 [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Carlson GA, Meyer SE, et al. Correlates of the CBCL-dysregulation profile in preschool-aged children. Journal of Child Psychology and Psychiatry. 2012;53(9):918–926. doi: 10.1111/j.1469-7610.2012.02546.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McQuillan ME, Kultur EC, Bates JE, et al. Dysregulation in children: Origins and implications from age 5 to age 28. Development and psychopathology. 2018;30(2):695–713. doi: 10.1017/S0954579417001572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatrics in review. 2000;21(8):265–271. DOI: 10.1542/pir.21-8-265 [DOI] [PubMed] [Google Scholar]
- 6.Mick E, McGough J, Loo S, et al. Genome-wide association study of the child behavior checklist dysregulation profile. J Am Acad Child Adolesc Psychiatry. 2011;50(8):807–817.e808. DOI: 10.1016/j.jaac.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Althoff RR. Dysregulated children reconsidered. 2010; 49 (4): 302–304. DOI: 10.1097/00004583-201004000-00004 [DOI] [PubMed] [Google Scholar]
- 8.Frazier JA, Wood ME, Ware J, et al. Antecedents of the child behavior checklist–dysregulation profile in children born extremely preterm. Journal of the American Academy of Child & Adolescent Psychiatry. 2015;54(10):816–823. DOI: 10.1016/j.jaac.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer SE, Carlson GA, Youngstrom E et al. Long-term outcomes of youth who manifested the CBCL-Pediatric Bipolar Disorder phenotype during childhood and/or adolescence. Journal of Affective Disorders. 2009;113(3):227–235. DOI: 10.1016/j.jad.2008.05.024 [DOI] [PubMed] [Google Scholar]
- 10.Rescorla LA, Jordan P, Zhang S, Baelen-King G, Althoff RR, Ivanova MY & International ASEBA Consortium. Latent Class Analysis of the CBCL Dysregulation Profile for 6- to 16-Year-Olds in 29 Societies, Journal of Clinical Child & Adolescent Psychology. 2021; 50(5): 551–564. DOI: 10.1080/15374416.2019.1697929 [DOI] [PubMed] [Google Scholar]
- 11.Jordan P, Rescorla LA, Althoff RR, & Achenbach TM.International comparisons of the Youth Self-Report dysregulation profile: Latent class analyses in 34 societies. Journal of the American Academy of Child & Adolescent Psychiatry. 2016; 55: 1046–1053. doi: 10.1016/j.jaac.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 12.Deutz MHF, Geeraerts SB, Belsky J, Deković M, van Baar AL, Prinzie P, Patalay General Psychopathology and Dysregulation Profile in a Longitudinal Community Sample: Stability, Antecedents and Outcomes. Child Psychiatry & Human Development.2020; 51:114–126. DOI: 10.1007/s10578-019-00916-2 [DOI] [PubMed] [Google Scholar]
- 13.Fichorova RN, Onderdonk AB, Yamamoto H, et al. Maternal microbe-specific modulation of inflammatory response in extremely low-gestational-age newborns. MBio. 2011;2(1):e00280–00210. DOI: 10.1128/mBio.00280-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leviton A, Allred EN, Dammann O, et al. Socioeconomic status and early blood concentrations of inflammation-related and neurotrophic proteins among extremely preterm newborns. PloS one. 2019;14(3):e0214154.DOI: 10.1371/journal.pone.0214154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pantham P, Aye IL, Powell TL. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta. 2015;36(7):709–715. DOI: 10.1016/j.placenta.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alemu A, Betelihem Terefe B,Abebe M and Belete Biadgo B.Thyroid hormone dysfunction during pregnancy: A review Int J Reprod Biomed. 2016; 14(11): 677–686. DOI: 10.29252/ijrm.14.11.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blaisdell CJ, Park C, Hanspal M, et al. The NIH ECHO Program: investigating how early environmental influences affect child health. Pediatric Research. 2022; 92(5):1215–1216. DOI: 10.1038/s41390-021-01574-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeWinn KZ, Caretta E, Davis A, Anderson AL, Oken E. SPR perspectives: Environmental influences on Child Health Outcomes (ECHO) Program: Overcoming challenges to generate engaged, multidisciplinary science. Pediatric Research. 2022;92(5):1262–1269. DOI: 10.1038/s41390-021-01598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paneth N, Monk C. The importance of cohort research starting early in life to understanding child health. Current opinion in pediatrics. 2018;30(2):292–296. DOI: 10.1097/MOP.0000000000000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romano ME, Buckley JP, Elliott AJ, Johnson CC, Paneth N. SPR Perspectives: scientific opportunities in the Environmental influences on Child Health Outcomes Program. Pediatric research. 2022;92(5):1255–1261. doi: 10.1038/s41390-021-01577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, De A. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. International journal of statistics in medical research. 2015;4(3):287–295. doi: 10.6000/1929-6029.2015.04.03.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin DB. Multiple imputation after 18+ years. Journal of the American statistical Association. 1996;91(434):473–489. DOI: 10.80/01621459.1996.10476908 [DOI] [Google Scholar]
- 23.Benjamini Y, and Hochberg Y Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B, 1995; 57: 289–300. http://www.jstor.org/stable/2346101. DOI: 10.1111/j.2517-6161.1995.tb02031. [DOI] [Google Scholar]
- 24.Althoff RR, Ayer LA, Crehan ET, Rettew DC, Baer JR, Hudziak JJ. Temperamental profiles of dysregulated children. Child Psychiatry Hum Dev. 2012;43(4):511–522. DOI: 10.1007/s10578-012-0280-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aizer A, Currie J. The intergenerational transmission of inequality: Maternal disadvantage and health at birth. science. 2014;344(6186):856–861. DOI: 10.1126/science.1251872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko G, Shah P, Lee SK, Asztalos E. Impact of maternal education on cognitive and language scores at 18 to months among extremely preterm neonates. American journal of perinatology. 2013;30(09):723–730. DOI: 10.1055/s-0032-1331034 [DOI] [PubMed] [Google Scholar]
- 27.Murgatroyd C, Spengler D. Epigenetics of early child development. Frontiers in psychiatry. 2011;2:16. doi: 10.3389/fpsyt.2011.00016.eCollection 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and Development of Psychiatric Disorders in Childhood and Adolescence. Arch Gen Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837 [DOI] [PubMed] [Google Scholar]
- 29.Johnson SB, Riley AW, Granger DA, Riis J. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics. 2013;131(2):319–327. DOI: 10.1542/peds.2012-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Althoff RR, Rettew DC, Faraone SV, Boomsma DI, Hudziak JJ. Latent class analysis shows strong heritability of the child behavior checklist–juvenile bipolar phenotype. Biological psychiatry. 2006;60(9):903–911. DOI: 10.1016/j.biopsych.2006.02.025 [DOI] [PubMed] [Google Scholar]
- 31.Hudziak JJ, Althoff RR, Derks EM, Faraone SV, Boomsma DI. Prevalence and genetic architecture of Child Behavior Checklist–juvenile bipolar disorder. Biological Psychiatry. 2005;58(7):562–568. DOI: 10.1016/j.biopsych.2005.03.024 [DOI] [PubMed] [Google Scholar]
- 32.Mick E, Monuteaux M, Wilens T, Wozniak J, Byrne D, Faraone S. A Genetic association study of emotional dysregulation indexed by the Child Behavior Checklist (CBCL) in Children with Attention-Deficit/Hyperactivity Disorder (ADHD). Paper presented at: American Academy of Child and Adolescent Psychiatry 57th Annual Meeting2010. [Google Scholar]
- 33.Kendler KS. Twin studies of psychiatric illness: an update. Archives of general psychiatry. 2001;58(11):1005–1014. DOI: 10.1001/archpsyc.58.11.1005 [DOI] [PubMed] [Google Scholar]
- 34.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of general psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 35.Jucksch V, Salbach-Andrae H, Lenz K, et al. Severe affective and behavioural dysregulation is associated with significant psychosocial adversity and impairment. Journal of Child Psychology and Psychiatry. 2011;52(6):686–695. DOI: 10.1111/j.1469-7610.2010.02322.x [DOI] [PubMed] [Google Scholar]
- 36.Burstein M, Ginsburg GS. The effect of parental modeling of anxious behaviors and cognitions in school-aged children: An experimental pilot study. Behaviour research and therapy. 2010;48(6):506–515. DOI: 10.1016/j.brat.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Archives of medical science. 2017;13(4):851–863. DOI: 10.5114/aoms.2016.58928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Burg JW, Allred EN, McElrath TF, et al. Is maternal obesity associated with sustained inflammation in extremely low gestational age newborns? Early human development. 2013;89(12):949–955. DOI: 10.1016/j.earlhumdev.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 39.Lester BM, Conradt E, Marsit CJ. Are epigenetic changes in the intrauterine environment related to newborn neurobehavior? Epigenomics. 2014;6(2):175–178. DOI: 10.2217/epi.14.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Chen Q, Tsai HJ, et al. Maternal preconception body mass index and offspring cord blood DNA methylation: exploration of early life origins of disease. Environmental and molecular mutagenesis. 2014;55(3):223–230. DOI: 10.1002/em.21827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knopik VS, Maccani MA, Francazio S, McGeary JE. The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Development and psychopathology. 2012;24(4):1377–1390. DOI: 10.1017/S0954579412000776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aurrekoetxea JJ, Murcia M, Rebagliato M, et al. Determinants of self-reported smoking and misclassification during pregnancy, and analysis of optimal cut-off points for urinary cotinine: a cross-sectional study. BMJ open. 2013;3(1):e002034. DOI: 10.1136/bmjopen-2012-002034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shipton D, Tappin DM, Vadiveloo T, Crossley JA, Aitken DA, Chalmers J. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. Bmj. 2009;339: b4347.doi: 10.1136/bmj.b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elisia I, Lam V, Cho B, et al. The effect of smoking on chronic inflammation, immune function and blood cell composition. Scientific reports. 2020;10(1):1–16. DOI: 10.1038/s41598-020-76556-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ekblad M, Korkeila J, Lehtonen L. Smoking during pregnancy affects foetal brain development. Acta paediatrica. 2015;104(1):12–18. DOI: 10.1111/apa.12791 [DOI] [PubMed] [Google Scholar]
- 46.Leech S, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicology and teratology. 1999;21(2):109–118. DOI: 10.1016/s0892-0362(98)00042-7 [DOI] [PubMed] [Google Scholar]
- 47.Stroud LR, Paster RL, Goodwin MS, et al. Maternal smoking during pregnancy and neonatal behavior: a large-scale community study. Pediatrics. 2009;123(5):e842–e848. DOI: 10.1542/peds.2008-2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fichorova RN, Beatty N, Sassi RR, et al. Systemic inflammation in the extremely low gestational age newborn following maternal genitourinary infections. American Journal of Reproductive Immunology. 2015;73(2):162–174. DOI: 10.1111/aji.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall HA, Speyer LG, Murray AL, Auyeung B. Prenatal maternal infections and children’s socioemotional development: findings from the UK Millennium Cohort Study. European child & adolescent psychiatry. 2021;30(10):1641–1650. DOI: 10.1007/s00787-020-01644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuban KC, O’Shea TM, Allred EN, et al. The breadth and type of systemic inflammation and the risk of adverse neurological outcomes in extremely low gestation newborns. Pediatric neurology. 2015;52(1):42–48. DOI: 10.1016/j.pediatrneurol.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Shea TM, Joseph RM, Kuban KC, et al. Elevated blood levels of inflammation-related proteins are associated with an attention problem at age 24 mo in extremely preterm infants. Pediatric research. 2014;75(6):781–787. DOI: 10.1038/pr.2014.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fetene DM, Betts KS, Alati R. Mechanisms in Endocrinology: Maternal thyroid dysfunction during pregnancy and behavioural and psychiatric disorders of children: A systematic review. European journal of endocrinology. 2017;177(5):R261–R273. DOI: 10.1530/EJE-16-0860 [DOI] [PubMed] [Google Scholar]
- 53.Young J, Savoy C, Colman I, Ferro M, Van Lieshout RJ. Psychiatric Disorders in the Adolescent Offspring of Mothers with Thyroid Problems During Pregnancy. Child Psychiatry & Human Development. 2020;51(3):461–470. DOI: 10.1007/s10578-020-00957-y [DOI] [PubMed] [Google Scholar]
- 54.Daraki V, Roumeliotaki T, Koutra K, et al. Effect of parental obesity and gestational diabetes on child neuropsychological and behavioral development at 4 years of age: the Rhea mother–child cohort, Crete, Greece. European child & adolescent psychiatry. 2017;26(6):703–714. DOI: 10.1007/s00787-016-0934-2 [DOI] [PubMed] [Google Scholar]
- 55.Thomas R, Abell B, Webb HJ, Avdagic E, Zimmer-Gembeck MJ. Parent-Child Interaction Therapy: A Meta-analysis. Pediatrics. 2017;140(3):e20170352.doi: 10.1542/peds.2017-0352.. [DOI] [PubMed] [Google Scholar]
- 56.Carlson GA, Singh MK, Amaya-Jackson L, et al. Narrative review: impairing emotional outbursts: what they are and what we should do about them. Journal of the American Academy of Child & Adolescent Psychiatry. 2023. Feb;62(2):135–150. doi: 10.1016/j.jaac.2022.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


