Unstructured abstract
Direct cortical stimulation has been applied in epilepsy for nearly one century and has experienced a renaissance given unprecedented opportunities to probe, excite and inhibit the human brain. Evidence suggests stimulation can increase diagnostic and therapeutic utility in patients with drug-resistant epilepsies. However, choosing appropriate stimulation parameters is not a trivial issue, which is further complicated by the fact that epilepsy is characterized by complex brain state dynamics. In this article derived from discussions at the ICTALS 2022 conference, we succinctly review the literature on cortical stimulation applied acutely and chronically to the epileptic brain for localization, monitoring, and therapeutic purposes. In particular, we discuss how stimulation is used to probe brain excitability, discuss evidence on usefulness of stimulation to trigger and stop seizures, review therapeutic applications of stimulation, and finally discuss how stimulation parameters are impacted by brain dynamics. Although research has advanced considerably over the past decade, there are still significant hurdles to optimize use of this technique. For example, it remains unclear to what extent short timescale diagnostic biomarkers can predict long-term outcomes and to what extent these biomarkers add information to already existing biomarkers from passive EEG recordings. Further questions include the extent to which closed loop stimulation offers advantages over open loop stimulation, what the optimal closed loop timescales may be, and whether biomarker-informed stimulation can lead to seizure freedom. The ultimate goal of bioelectronic medicine remains not just to stop seizures but rather to cure epilepsy and its comorbidities.
Keywords: electrical stimulation, epilepsy, diagnosis, treatment, deep brain stimulation
Introduction
The discovery of the nervous system as an excitable tissue dates back to Luigi Galvani and his studies on so-called “animal electricity” (Piccolino, 1997). Applying electrical pulses to neural tissue can generate responses that have a life of their own. Neural responses outlive and propagate beyond the site of stimulation, sometimes leading to dramatic amplifications. Seizures exemplify this principle of excitability, so why stimulate the epileptic brain? How might induced excitation on “hyperexcitable” tissue yield positive outcomes? Despite the counter-intuition, stimulating the epileptic brain can improve seizure control, although a full understanding of the optimal parameters to achieve this is not yet within reach. In this article, we succinctly review the literature on cortical stimulation applied acutely and chronically to the epileptic brain for localization, monitoring, and therapeutic purposes. We emphasize current opinions of experts who participated in the ICTALS 2022 conference on when and how the epileptic brain may be stimulated to probe, trigger or stop seizures.
Using stimulation to probe excitability
Electrical stimulation can be used to activate cortical tissue, and recordings of the brain’s response to electrical perturbation can give insight into both cortical excitability and the pathological connectivity of the epileptic brain network. Specifically, single-pulse electrical stimulation (SPES) is a procedure in which brief pulses of current are delivered at specific sites across the brain. The responses, often termed cortico-cortical evoked potentials (CCEPs), are recorded to define effective connections between the stimulus and response regions (Matsumoto et al., 2004; 2017). The canonical CCEP waveform includes the N1 potential, which is a sharp negative potential with a peak at 10–50 ms following stimulation, and the N2 potential, which is a later slow-wave like potential with a peak between 50 and 300 ms (Matsumoto et al., 2004). However, the CCEP waveform may be variable with a larger inter-electrode distance being believed to be correlated with smaller amplitude CCEPs. Moreover, the CCEP waveform may change polarity according to the exact location of the stimulating or recording electrodes. The issue of polarity might be overcome by the measurement of the spectral power (Sonoda et al., 2021) or the line length (Lepeu et al., BioRxiv 2021) of the CCEPs. Although CCEPs were initially investigated to define physiological effective connectivity in highly functionalized circuits (Matsumoto et al., 2004; 2007; 2017), the technique rapidly translated to epilepsy in an attempt to identify regions of high cortical excitability and define epileptogenic network hubs (for a comprehensive review, see Matsumoto et al., 2017).
Specific CCEP properties have been investigated for their potential to reflect cortical excitability. In complementary paradigms, a site’s excitability can be explored by the morphology of the evoked activity in response to stimulation elsewhere or by the morphology and distribution of the responses elicited by stimulation to the single site. For example, the N1 amplitude is generally larger (Iwasaki et al., 2010; Enatsu et al., 2012; Hays et al., 2022) with accompanying stronger high-frequency activity (van’t Klooster et al., 2011; 2017; Mouthaan et al., 2016) in response sites associated with epileptogenicity, suggesting a greater sensitivity to input stimuli in pathological brain areas (Figure 1 A–C). Further, SPES can implicate response regions as pathological through the presence of evoked waveforms such as delayed responses, which resemble interictal epileptiform discharges that occur 100ms-1s after the stimulus (Valentín et al., 2002; 2005). Removal of areas that consistently elicited delayed responses have corresponded to favorable surgical outcome (Valentín et al., 2005), although this finding has not been replicated in larger cohorts of patients. On the other hand, stimulation sites have been classified as pathological based on the network properties they reveal, such as stronger interconnections between epileptogenic zone (EZ) nodes (Guo et al., 2020) or the influence of specific nodes in a dynamical network (Smith et al., 2022). Finally, the amplitude and waveform of CCEPs may be affected by specific dynamics in given cortices/pathways. This variable might need to be considered when developing a model for localizing the epileptogenic zone using electrical stimulation, as it could confound the results. For instance, certain studies have observed larger CCEPs following stimulation of the primary motor or sensory cortex, as well as the language cortex, even when controlling for inter-electrode distance (Matsuzaki et al., 2013; Guo et al., 2020; Sonoda et al., 2021).
Figure 1.
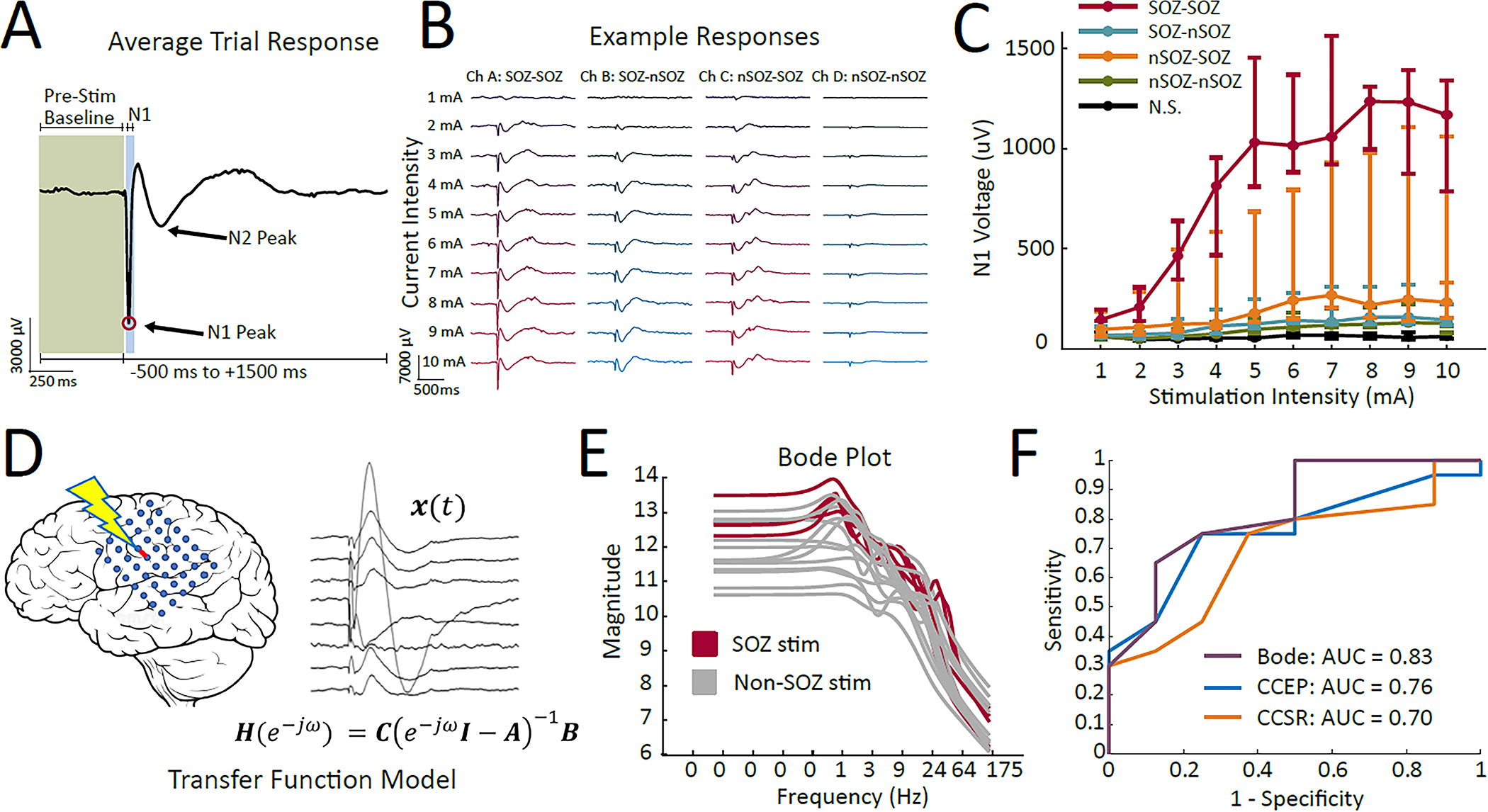
Probing the epileptic brain with single-pulse electrical stimulation. A) The CCEP waveform includes a sharp N1 peak and a slower N2 peak, but alternative waveforms have been described for different functional areas and tissue types. Blocks of 40–50 trials are often used to compute the average trial response. B) Increasing amplitude nonlinearly amplifies the N1 and N2 peaks of the waveform, and C) these response amplitudes are generally most excitable in response regions associated with the SOZ (red and yellow lines). In contrast, stimulation sites have been categorized as epileptogenic by D) using CCEP responses to build transfer function models for specific stimulation sites. E) The transfer function model for each stimulation site produces a Bode plot, which reflects the magnitude of the output of the system when driven at a specific frequency (x-axis). F) Bode plot features classified SOZ regions and reflected surgical outcome with slightly higher performance than the traditional N1 amplitude analysis (CCEP) or spectral responses to stimulation (CCSR). Figure adapted from (Hays et al. 2022) and (Smith et al. 2022).
Though rather simple analyses of stimulation and response pairings with CCEPs have historically been quite powerful, the hierarchical and complex network structures in the epileptic brain have necessitated the development of new analysis techniques. In recent years, algorithms employing deep learning (Johnson et al., 2022) and graph theory (Zhao et al., 2019; Hays et al., 2021) have been applied to stimulation-evoked potentials to uncover epileptogenic regions. Dynamical network and transfer function models utilizing CCEPs have been particularly useful in localizing seizure onset regions because they mathematically describe the complex relationships between input stimuli and the remote output responses (Kamali et al., 2020a; 2020b; Smith et al., 2020). The models have been used to identify “resonant” regions in the epileptogenic network that corresponded to focal epileptogenic areas, and then were used to predict triggering of native seizures using periodic stimulation at the resonant frequency (Smith et al., 2022) (Figure 1, D–F). This trend of increased crossover of CCEPs with novel techniques in computational neuroscience will continue to unveil how stimulation-evoked potentials can enable epileptogenic network discrimination and cortical hyperexcitability measurement in epilepsy.
The stimulation parameters used to probe cortical excitability with CCEPs, such as stimulation current or voltage, frequency, pulse width, and polarity, can vary widely from center to center. Typically, 30–50 trials of stimulation pulses are delivered at 0.5–1 Hz, and stimulation occurs at a large percentage of grey matter electrodes, though white matter stimulation is increasingly common (Hays et al., 2022; Paulk et al., 2022). The effect of stimulation current on the CCEP has been most studied; there exists a nonlinear positive relationship between current and the N1 peak amplitude, with an eventual plateau of the CCEP amplitude at high stimulation currents (Kundu et al, 2020; Hays et al., 2021). Using a biphasic pulse with 0.15 ms/phase width, reliable CCEP responses emerge with a stimulation current of 2–3 mA and plateau in response amplitude at 5.5–7 mA (Hays et al., 2021; 2022; Paulk et al., 2022). Slightly higher stimulation currents (or voltages) can be used in ECoG electrodes due to the increase in electrode surface area, however the overall charge density should not exceed approximately 57 μC/cm2per phase (Kuncel and Grill 2004; Prime et al. 2018). For example, using SEEG electrodes with a surface area of around 0.06–0.08 cm2 (Kuncel and Grill 2004; Hays et al. 2021) and 0.15 ms/phase width, a stimulation current up to 10 mA remains under 25 uC/cm2/phase (Hays et al. 2021). Though overall charge per phase has been thought to drive CCEP morphology (Donos et al., 2016), further exploration of the effects of stimulation parameters on specific features of the CCEP waveform is warranted. Efforts to differentiate pathologically excitable tissues from physiological levels are currently hindered by the lack of understanding of how CCEPs are modulated by factors such as stimulation location in grey versus white matter or specific brain areas (Basu et al., 2019; Paulk et al., 2022), varying stimulation parameters (Donos et al., 2016; Kundo et al., 2020; Hays et al., 2022), montage choice (Mitsuhashi et al., 2020), lesion presence, and electrode type (Grande et al., 2020). Large, multi-center studies (Trebaul et al., 2018) that can parse the effects of these factors on CCEPs and their reflection of cortical excitability are extremely important in this regard.
Clinical evidence for triggering patient-typical electro-clinical seizures
Electrical cortical stimulation is used for both functional mapping and delineation of the epileptogenic zone (Rosenow & Luders, 2001) as approximated by the seizure-onset zone (SOZ). In contrast to its wide use for functional mapping, its use for identifying the SOZ is less well established despite having been first described in the early twentieth century (Cushing, 1909; Foerster & Penfield, 1930) and despite its longstanding tradition in the French school of stereo-electroencephalography (Bancaud & Talairach, 1965; Isnard et al., 2018). A review from 2016 suggested that evidence remained inconclusive regarding the usefulness of triggering patient-typical electro-clinical seizures for postsurgical outcome prediction (Kovac et al., 2016). More recently, two large multicenter studies shed light on this question (Cuello Oderiz et al., 2019; Trébuchon et al., 2020); both studies demonstrated that stimulation of patient-typical electro-clinical seizures during stereo-electroencephalography can be useful to identify the SOZ and to predict postsurgical outcome.
Cuello-Oderiz et al. (2019) investigated a total of 103 patients who underwent SEEG with at least one electrical stimulation session and subsequent open resective surgery. Patient-typical electro-clinical seizures were evoked in 59 (57%) patients. The percentage of patients with cortical stimulation–induced patient-typical electroclinical seizures was higher in the good (Engel I) than in the poor outcome group (Engel II-IV). Also, the median percentage of the resected contacts encompassing the cortical stimulation induced SOZ was higher in the good versus the poor surgical outcome group (Figure 2). Importantly, this result did not differ from that observed for spontaneous seizures suggesting that stimulation-induced patient-typical seizures and spontaneous seizures can provide similar information about the SOZ. Trébuchon et al. (2020) investigated 346 patients and were able to stimulate seizures in 75.3% of patients. They further found that the occurrence of patient-typical ictal events after low-frequency 1-Hz stimulation predicts a favorable epilepsy outcome, with only 44% chance of disabling seizure recurrence at last follow-up. In a multivariate analysis, stimulation-induced patient-typical seizures provided information independent of classical post-surgical predictors such as informative MRI, type II focal cortical dysplasia, and tumor (Trébuchon et al., 2020).
Figure 2.
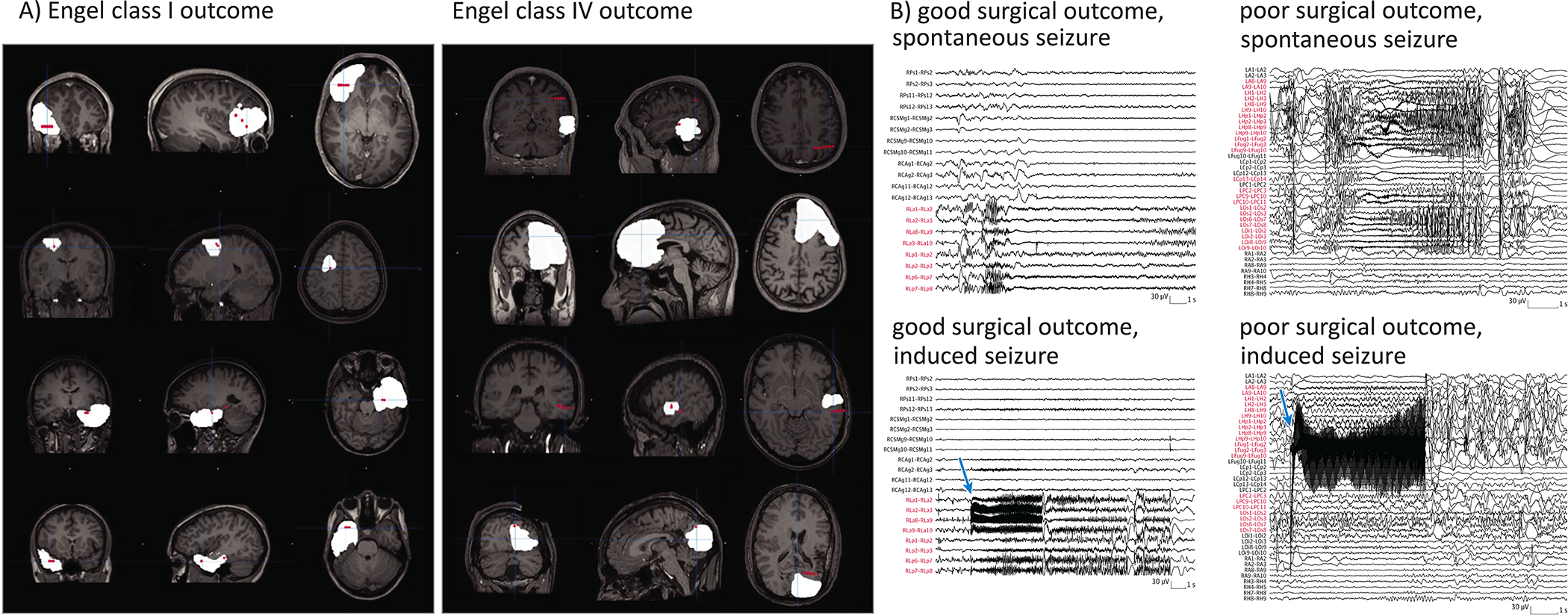
Panel A. Proportion of Resected Contacts in Patients With Good or Poor Surgical Outcome. Left side: Examples of Engel class I or good surgical outcome. Right side: Examples of Engel class IV or poor surgical outcome. The contacts of the seizure-onset zone informed by cortical stimulation are reconstructed as red dots, and the resection cavity is superimposed in white. The examples illustrate that in patients with good surgical outcome, a higher proportion of the contacts were removed compared with patients with poor outcome. Panel B. Comparison in Outcomes Between Spontaneous Seizure and Cortical Stimulation–Induced Seizure in 2 Patient Examples, Blue arrows point to the beginning of the cortical stimulation (CS). Contacts RLa 4–6 in patient 217 and contacts LFug 4–6 in patient 260 were stimulated (using 50 Hz). Even though the seizure-onset patterns show differences, the seizure-inducing channels (labeled in red) were similar. Cuello-Oderiz et al., JAMA Neurol 2019, with permission of the American Medical Association.
Early work showed that seizures are induced more readily with 50 Hz stimulation than 1 Hz stimulation, with seizure onsets usually only identifiable after the stimulation artifact in the case of 50 Hz stimulation (Munari et al., 1993). Cuello-Oderiz et al. (2019) found that among all investigated factors, only 50 Hz vs. 1 Hz stimulation (54.9% vs. 18.2%) and longer time (> 24 hrs) since the last seizure were associated with a higher likelihood of induced patient-typical electro-clinical seizures. The same conclusion was drawn by Trébuchon et al. (2020) (6.6% only by low-frequency stimulation, 40.8% only by high frequency stimulation, 27.9% by both). In addition, they found that if patient-typical seizures were evoked by 1 Hz stimulation, this was a significant positive predictive factor for a good seizure outcome after epilepsy surgery. One immediate clinical advantage of triggering patient-typical seizures is that the sequence of symptoms and signs, as well as the electro-clinical correlation can be observed in a controlled manner. Highlighting the importance of studying the patient-typical seizure, inducing the aura or the complete clinical seizure but not a subsequent part of the seizure, such as the seizure without preceding aura, was associated with a good outcome (Trebuchon et al., 2020).
Regarding differences in the underlying pathology, the literature suggests that stimulation-induced seizures are more likely in certain pathologies such as focal cortical dysplasia type 2 (Chassoux et al., 2000).
Similar to probing excitability, stimulation parameters for inducing patient-typical electro-clinical seizures vary across the different centers, with sites using either high-frequency, low-frequency, or high- and low-frequency stimulation (Trébuchon & Chauvel, 2016). The initial intensity is selected depending on the type of stimulation (high-frequency vs. low-frequency), pulse duration and electrode type (Prime et al., 2018), the anatomical structure or pathology studied (lower intensities are needed for the sensorimotor cortices, the mesio-temporal lobe as well as dysplastic tissue), current antiseizure medication drug dosage, history of generalization, and time after last seizure. To avoid tissue damage, a maximum charge density of ~57 μC/cm2 (Gordon et al., 1990) should not be exceeded. As charge density is not only dependent on output current and pulse width, but also electrode surface area, typical stimulation intensities may differ between sEEG and subdural contacts, and even between manufacturers. As suggested in a recent review, the following stimulation parameters are reasonable for high-frequency stimulation: pulse width of 300 μs, current spanning 1–6 mA in depth electrodes and 1–11 mA in subdural contacts, stimulation time of 5 s maximum, and at least 10 s break in between the stimulations (Suller-Marti et al., 2022). An increase in intensity is recommended only for 50 Hz stimulation until after discharges can be induced or maximum charge density has been reached. In contrast, an increase in intensity is not recommended for 1 Hz stimulation (many sites use an intensity of 3 or 5 mA), where increasing intensities result mainly in slowing of the background activity due to exhaustion of the neuronal pool. Re-initiation of anti-seizure medication or administration of an anti-seizure medication loading dosage should be considered prior to stimulation to avoid non-habitual seizures and to minimize the risk of focal to bilateral tonic-clonic seizures (Arya et al., 2018).
If well explained to the patient and performed according to accepted protocols, side effects of electrical stimulation for triggering patient-typical electro-clinical seizures are well tolerated by the majority of patients. False positive stimulation results, i.e., the stimulation of non-habitual electro-clinical seizures, are low with SEEG reported as ~8% for high-frequency and 1.5% for low-frequency stimulation (Cuello-Oderiz et al., 2019). Despite its demonstrated utility, further study is still needed to establish the added value of stimulation of patient-typical electro-clinical seizures in our arsenal of markers of postsurgical outcome.
Clinical evidence of neurostimulation to stop seizures and decrease interictal epileptic activity
A significant amount of stimulation research has focused on reducing seizure burden in patients (Ryvlin et al., 2021; Simpson et al, 2022). Well-designed controlled clinical trials as well as registry data have demonstrated the long-term clinical efficacy of stimulation approaches (Fisher et al., 2010; Morrell et al., 2011; Englot et al., 2016; Nair et al., 2020, Salanova et al., 2021). Unlike medications, there are many stimulation parameters to consider and precise arrangements of electrical contacts relative to underlying brain structures are important (Figure 3). Despite widely varying parameters for different approaches (Table 1) and different stimulation targets, overall long-term efficacies are similar across multiple brain stimulation approaches, often with an approximate 50–70% median seizure reduction after 3–5 years. In addition, data suggest improving efficacy over months to years, suggesting that at least some mechanisms of action work slowly. At least in some cases, interictal epileptiform activity (Arcot Desai et al., 2019) and brain connectivity (Khambhati et al., 2021) show apparent changes to stimulation over a period of months to years although more rapid decreases have also been reported (Lundstrom et al., 2019). In general, the primary outcome has been reduction of seizure burden, rather than acutely aborting seizures.
Figure 3.
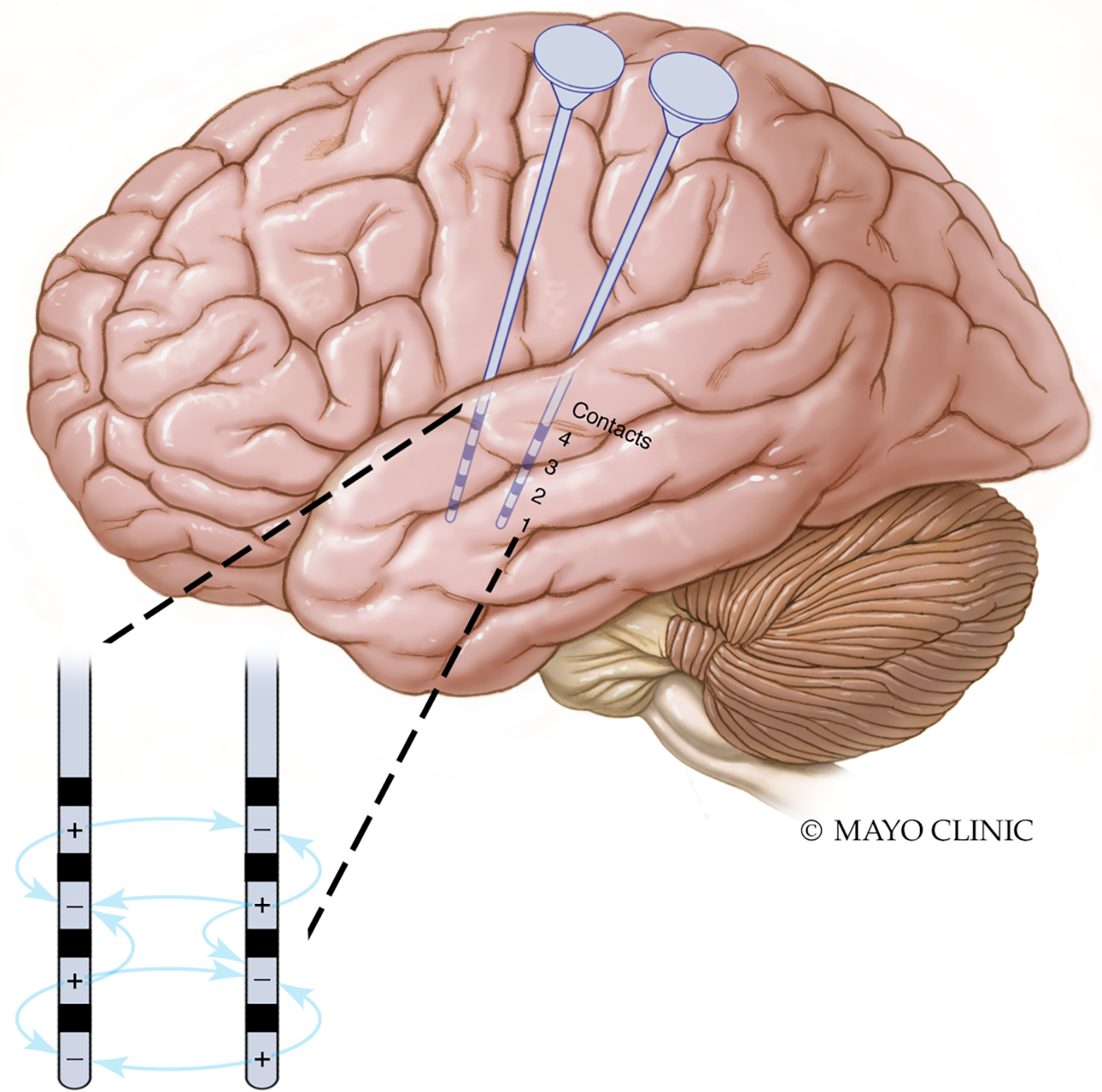
Stimulation parameters include not only amplitude, frequency, and pulse width, but also the geometrical arrangement of cathodes (negatively charged contacts) and anodes (positively charged contacts) that determine the electrical fields.
Table 1. Typical stimulation settings for VNS, DBS, and RNS.
| Current, voltage, or charge density | Pulse width | Frequency | Duty cycle %, ON/OFF (min) | |||||
|---|---|---|---|---|---|---|---|---|
| Initial | Increment | Target | Initial | Target | ||||
| VNS | Normal mode | 0.125 – 0.25 mA | 0.125 – 0.25 mA | 1.5–2 mA | 250 μs | 20–30 Hz | 10%, 0.5 ON / 5 OFF | 16–58% |
| Autostim | 0.25 – 0.375 mA | 0.125 – 0.25 mA | 1.5–2 mA | 250 μs | 20–30 Hz | ON 1 | ON 1 | |
| Magnet mode | 0.5 mA | 0.25 mA | 2 mA | 500 μs | 20–30 Hz | ON 1 | ON 1 | |
| DBS (ANT) | SANTE protocol | 2–3 mA/V per cathode | 1–2 mA/V | 5–6 mA/V | 90 μs | 145 Hz | 17%, 1 ON / 5 OFF | |
| RNS | Initial | 0.5 μC/cm2 | 0.5 μC/cm2 | 2–6 μC/cm2 | 160 μs | 200 Hz | ||
The evidence for acutely aborting seizures with electrical stimulation in human focal epilepsy is limited, and not likely an optimal long-term therapeutic strategy. There is definitive evidence that high frequency stimulation (HF) can abort focal seizures and after-discharges. The seminal work of Lesser et al. (1999) used the unique opportunity of stimulation during human brain mapping to investigate the ability of electrical stimulation to stop induced seizures and after-discharges (Lesser et al., 1999) and observed that electrical stimulation induced after-discharges could be suppressed with HF electrical stimulation (300 microsecond pulse width, charge balanced square waves, 50 Hz). A subsequent multicenter trial sponsored by NeuroPace, Inc., using a custom bedside external responsive neurostimulation (eRNS) system further explored this exciting finding and later developed a fully implantable RNS system (personal communication with MJ Morrell) that received FDA approval in 2013.
However, stopping a patient’s habitual, spontaneous seizure after onset, in contrast to seizures provoked by very focal electrical stimulation during brain mapping, has proven difficult in ambulatory patients. With the NeuroPace RNS device, epileptologists infrequently see seizures directly aborted by stimulation, and nonetheless commonly see improved clinical outcomes and seizure reductions with hundreds to thousands of stimulations per day (Nair et al., 2020). While there are certainly electrographic examples of aborting spontaneous human seizures with RNS, this seems to be the exception. The reason is likely the complex organization of the focal epileptogenic brain (Stead et al., 2010). By the time seizures are visible on macroelectrodes, they have recruited more extended networks that are very difficult to control with focal electrical brain stimulation. When acute cessation does occur, HF stimulation may create a functional lesion by a depolarization block that suppresses local neuronal activity. Generally, the topic of how seizures terminate naturally remains an important question in epileptology (Timofeev et al., 2004; Lado et al., 2008; Kramer et al, 2012; Jiruska et al., 2013). Because no study directly compared open-loop ongoing cortical stimulation to closed-loop responsive cortical stimulation, the different and potentially complementary mechanisms by which they act on cortical physiology remain unaddressed.
Finally, seizure networks may respond differently to stimulation during an ongoing seizure. Indeed, a study found that single-pulse induced CCEPs were absent during mesiotemporal seizures, but maintained during neocortical seizures (Russo et al., 2023). Thus, characteristics of stimulation targets and underlying dynamics related to seizure initiation, propagation and termination are likely important for aborting seizures with electrical stimulation or, more broadly, chronically reducing seizure burden.
Modulation of nodes and networks with deep brain stimulation
Deep brain stimulation (DBS) is an emerging therapy for patients with drug-resistant epilepsy. It involves the implantation of electrodes in subcortical structures with the aim of modulating brain networks involved in seizure generation and propagation by acting locally and remotely in connected areas. Relatively little is known about the optimal targets and parameters of DBS in epilepsy, which is a current limitation of therapeutic development (Kaufmann et al., 2020). The choice of the target is a crucial aspect of neuromodulation as it determines how DBS impacts epileptogenic networks. These networks are indeed characterized by their anatomical specificity with important relationships between cortical and subcortical regions (Bartolomei et al., 2017). Increased synchronization and functional connectivity have been found in epileptogenic networks (epileptogenic zone and propagation regions) (Lagarde et al, 2022). One proposed mechanism for neurostimulation is a desynchronization of these networks to approximate the normal state (Yu et al 2018; Deutschová et al, 2021, Piper et al, 2022).
The thalamus is the most common target, and the anterior nucleus of the thalamus (ANT) is the only approved target for epilepsy stimulation (Ryvlin et al., 2021). ANT stimulation leads to a significant reduction in focal seizure frequency compared to controls (Fisher et al., 2010), and its effect seems to increase with time. Indeed, a long-term open-label extension study reported a progressive decrease in seizure frequency and a response rate of 50% that reached 74% after 7 years of follow-up in those who continued treatment (Salanova et al., 2021). The ANT is a key component of the limbic system for episodic memory. The main input to the ANT, via the mammillothalamic tract, originates from the hippocampus and the mammillary body. Other cortical afferents originate from the cingulate cortex, orbito-medial prefrontal cortex, retrosplenial cortex, and inferior parietal lobule (Child & Benarroch, 2013). Within the ANT the location of stimulation also appears to be important with an anterior rather than posterior stimulation location associated with improved effectiveness (Lehtimäki et al., 2016). However, the connectivity of the ANT is relatively restricted and may suggest that other targets are preferred in cases where the epileptogenic networks are extra-limbic.
The centromedian nucleus (CM) of the thalamus has widespread projections to the cortex but particularly to the motor cortices and the basal ganglia (Smith et al., 2004, Velasco et al., 2021). The CM has been the subject of several open-label studies in Lennox-Gastaut syndrome reporting a high rate of responders (up to 90%) (Valentín et al., 2013; Cukiert et al., 2020; Velasco et al., 2021). The recent ESTEL study, a blinded controlled study, did not show a significant difference between treatment and control arms at 3 months (responder rate of 50% versus 22%, p=0.25) but did show a significant reduction of electrographic seizures (Dalic et al., 2022). Recently the same group reported that efficacious CM‐DBS for Lennox-Gataut syndrome was linked to stimulation of the parvocellular part of the CM and the adjacent ventral lateral nucleus (Warren et al., 2022). Efficacy was also associated with connectivity to areas of a priori EEG-fMRI activation, including premotor and prefrontal cortex, putamen, and pontine brainstem (Warren et al., 2022).
Another promising thalamic target is the medial pulvinar (PuM; Figure 4). PuM is associated with directed attention, executive functions and working memory and has many bidirectional connections including the inferior temporal, posterior parietal, prefrontal cortical areas as well as the amygdala and the superior colliculus (Homman-Ludiye & Bourne, 2019). Several concordant studies highlighted its role in focal seizures in humans (Guye et al., 2006; Rosenberg et al., 2006; Capecchi et al., 2020; Pizzo et al., 2021). SEEG recordings of the pulvinar demonstrated involvement during mesial temporal seizures as well as other seizure types (Pizzo et al., 2021). Furthermore, SEEG studies have shown that the PuM is implicated in increased functional synchrony observed during propagation of temporal lobe seizures (Guye et al., 2006) and potentially in their termination (Evangelista et al., 2015). The role of this structure in the alteration of awareness has also been shown, as the PuM is a hub region for the pathological synchronization of fronto-parietal regions involved in normal conscious processing (Arthuis et al., 2009). Acute PuM stimulation is well-tolerated and can be effective in reducing the duration and severity of seizures (Filipescu et al., 2019). This effect could be linked to a desynchronizing impact in responders (Deutschová et al., 2021). A current open study is ongoing to test DBS PuM stimulation in patients with refractory focal epilepsies (PULSE protocol, NCT04692701). Another recent off-label development relates to the incorporation of the PuM into RNS procedures, which may be beneficial for cases with regional drug-resistant epilepsy of the posterior cortex (Burdette et al., 2020).
Figure 4.
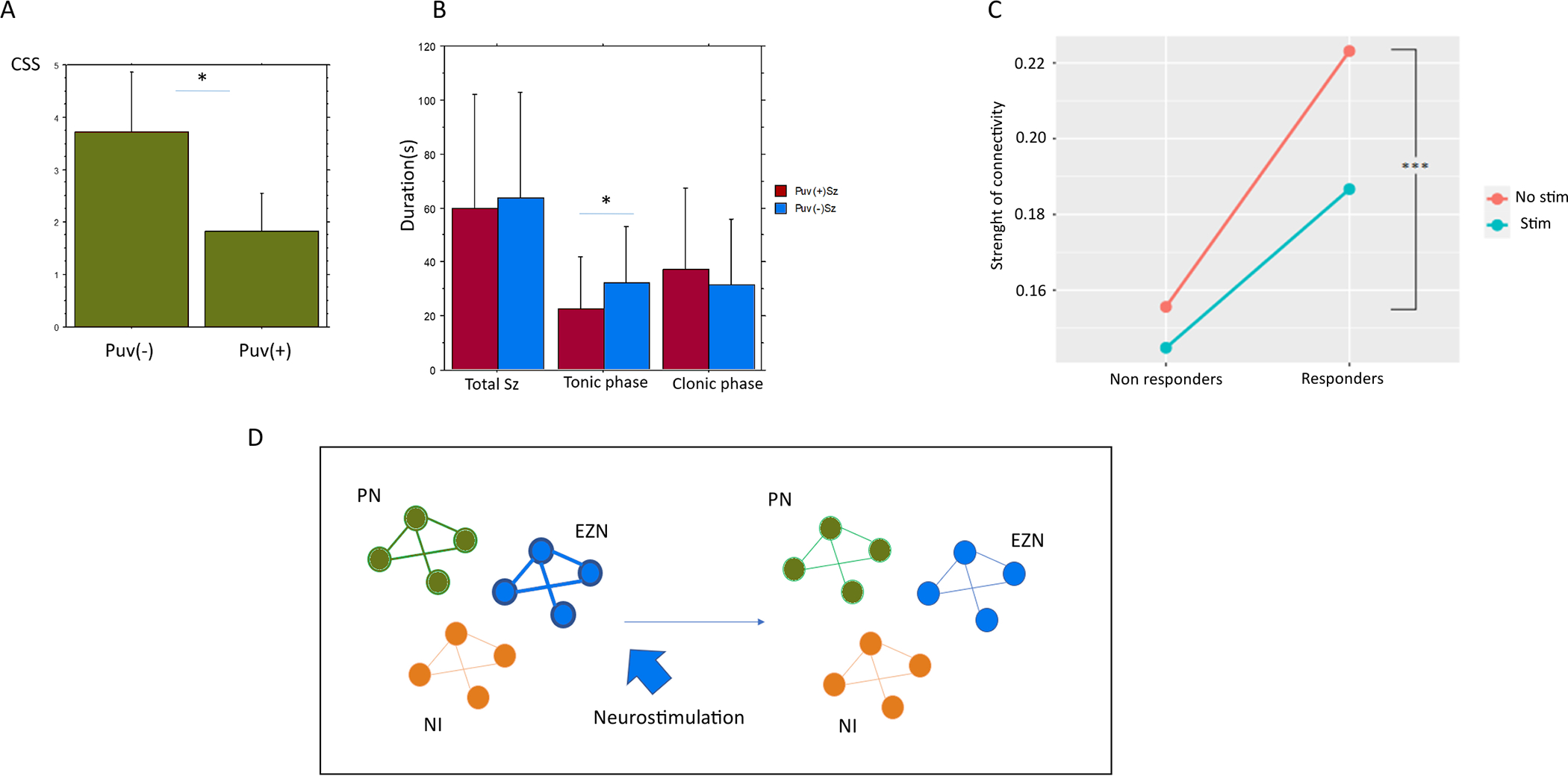
Figure showing the effect of medial pulvinar stimulation on temporal lobe seizures. (A) A decrease in the alteration of consciousness is observed. (B) A shortening of the duration of the tonic phase of the seizure is observed (from Filipescu et al, 2019). (C) A desynchronizing effect is observed in responders as opposed to non-responders (from Deutschová et al, 2021). (D) Potential effect of deep brain stimulation of the thalamus on the alteration of interictal connectivity. On the left, the interictal period is characterized by a relative increase of the epileptogenic zone networks (EZN) and propagation networks (PZ) compared to the non-involved regions (NI) (Lagarde et al, 2022). On the right, the effect of stimulation is thought to decrease the increased functional connectivity (FC) in the epileptogenic networks. CSS, consciousness seizure scale; Puv, pulvinar; stim, stimulation.
The example of DBS in these three thalamic nuclei demonstrates the complexities of targeting and highlights the importance of even localization at the subnuclei level. Stimulation-related interactions between subcortical and cortical structures remain incompletely understood. Although it is tempting to make broad generalizations from apparent anatomic connectivity to treatment efficacy, there remains much unknown about mechanisms underlying stimulation efficacy and the complexities of epilepsy network connectivity.
Adjustment of stimulation parameters to dynamotypes in a dynamical systems framework
It may be helpful to recall that neurons, the foundational computational unit of the brain, communicate via electricity and that electrical oscillations form a cornerstone of neural communication. Thus, aspects of electrical brain function may best be understood within the framework of dynamical systems (Strogatz, 1994).
In epilepsy, relevant brain states can broadly be categorized as including interictal and ictal states. The brain can exist in these states with varying degrees of stability, and the brain may transition to the ictal state when near a tipping point (Maturana et al., 2020). Electrical impulses can either increase or decrease stability for these states. For example, the goal of external electrical stimulation is to therapeutically increase stability for the interictal state. Evidence suggests that endogenous electrical impulses such as interictal epileptiform discharges can either increase the chance of transition to a seizure state or can stabilize the interictal state (Chvojka et al., 2021). In other words, for a given neural subnetwork interictal discharges may act as an external perturbation that shifts the system to a different stable region, such as a seizure state, when it is near a tipping point. The brain as a dynamical system undergoing interictal to ictal transitions via a phase transition is an attractive phenomenological model but critical slowing down, one of the hallmarks of such systems, remains controversial (Wilkat et al., 2019).
The dynamics of seizures themselves are complex. Classified according to EEG amplitudes and time intervals between epileptiform discharges at seizure onset and offset, 16 distinct types of dynamics, or dynamotypes, have been described (Saggio et al., 2020). Even within single patients, dynamics may differ from seizure to seizure. Further, seizure propagation patterns and seizure durations vary independently (Schroeder et al., 2022). As might be expected given these differing dynamics, EEG biomarkers of epileptogenicity also vary in accuracy as brain dynamics vary (Smith & Stacey, 2021). Despite these differences, similarities still exist such that large numbers of cross-patient invasive EEG seizure records can be effectively categorized (Arcot et al., 2022).
Given the complexity of brain dynamics, it should not be surprising that a given set of stimulation parameters may have a different effect depending on the patient and brain state. For example, RNS with 200 Hz burst stimulation was associated with improved effectiveness when the brain was in a high-risk state for seizures, while 100 Hz stimulation was associated with improved effectiveness when the brain was in a low-risk state (Chiang et al., 2021). In general, stimulation frequency is often considered a key stimulation parameter, and, for example, stimulation at 100 Hz or greater is typical for RNS. Nonetheless, 7 Hz low frequency RNS can lead to notable improvements compared to high frequency (100–200 Hz) stimulation in selected patients (Alcala-Zermeno et al., 2022b). In a chronic rat limbic epilepsy model, stimulation to stop seizures was more effective when stimulation frequency matched the natural frequency measured during seizure termination, where frequencies varied from 7 to 300 Hz (Sobayo et al., 2016). These data are supported by computational work showing that stimulation frequency required to abort seizure-related activity depended on underlying dynamics; slower seizure dynamics required slower stimulation frequencies to be effective (Köksal Ersöz et al., 2020).
Therapeutic stimulation also differs by the degree of feedback used to inform stimulation. RNS systems provide stimulation based on short timescale feedback from physician-programmed detectors, whereas other approaches provide continuous stimulation. In one example, stimulation of the medial septum in a rat hippocampal kindling model showed that responsive closed loop stimulation at the level of individual stimulation pulses (which is not the case with RNS) was effective whereas open loop stimulation was not (Takeuchi et al., 2021). Despite these differences, it is interesting that outcomes are generally similar across a wide range of stimulation approaches (Alcala-Zermeno et al., 2022a). Certainly, stimulation parameters and details of brain state dynamics can affect outcomes, and yet sometimes even basic, standardized approaches are similarly effective.
Outlook and open questions
Electrical stimulation can be used for the diagnosis and treatment of epilepsy, but requires clinicians to have a familiarity with the physics of electricity and consider numerous factors when optimizing therapy for individual patients (Simpson et al., 2022). It can be easy to overlook the dynamical complexity of the brain (Nunez et al., 2006). Electrical stimulation is not as simple as choosing an antiepileptic medication and its target dosage.
At present, evidence suggests the need for continued openness regarding how electrical stimulation works and might best be implemented. Umbrella statements might best be avoided. Electrical stimulation can induce seizures and can inhibit seizures. Data do not yet support clear differences in long-term effectiveness for open vs. closed loop stimulation or high vs. low frequency stimulation, although it is often assumed that closed loop stimulation and high frequency stimulation may be more effective. In contrast, evidence suggests low frequency stimulation may be more effective for probing epileptic cortex. Although seizures are more readily induced with high-frequency stimulation, prognostication of surgical outcome appears to be improved with low-frequency stimulation (Cuello Oderiz et al., 2019, Trebuchon et al., 2020). Brain states are always in flux, and electrical stimulation will likely have multiple effects over space and time. Questions remain regarding: 1) to what extent short timescale diagnostic biomarkers can predict long-term outcomes, and if these biomarkers are adding information to already existing biomarkers from passive EEG recording, 2) under what circumstances and over what timescales closed loop stimulation offers advantages over open loop stimulation, and 3) whether biomarker-informed stimulation can lead to seizure freedom.
Bioelectronic medicine holds the promise of using electricity, which is in some sense the language of the brain, to dramatically improve diagnosis and treatment of epilepsy. The goal remains not just to stop seizures but rather to cure epilepsy.
Key points:
Properties of cortico-cortical evoked potentials can reflect pathological excitability in both stimulation and response areas.
Stimulation to induce patient-typical seizures is useful to identify the seizure-onset zone and to predict postsurgical outcome.
Evidence for consistent acute cessation of individual seizures with electrical stimulation is limited, while evidence for long-term seizure reduction is strong.
Carefully selected implantation targets and stimulation parameters are crucial for achieving an optimal response of brain stimulation in intractable epilepsy.
Given the complexity of brain dynamics, a given set of stimulation parameters may have different effects depending on the patient and brain state.
Acknowledgments
We acknowledge the generous sponsorship through unrestricted educational donations for the ICTALS 2022 conference in Bern including the University of Bern, the Inselspital, University Hospital Bern, the Alliance for Epilepsy Research, the Swiss National Science Foundation, UCB, FHC, the Wyss Center for bio- and neuro-engineering, the American Epilepsy Society (AES), the CURE epilepsy Foundation, Ripple neuro, Sintetica, DIXI medical, UNEEG medical and NeuroPace. BF’s research was supported by the Canadian Institutes of Health Research (PJT-175056), the Hewitt Foundation, and a salary award (“Chercheur-boursier clinicien Senior”) from the Fonds de Recherche du Québec – Santé (2021–2025). BNL was supported by NIH NINDS K23NS112339.
Footnotes
Disclosure of Conflicts of Interest
MOB declares shares with Epios SA, a medical device company. BNL declares intellectual property licensed to Cadence Neuroscience Inc (contractual rights waived; all funds to Mayo Clinic) and Seer Medical Inc (contractual rights waived; all funds to Mayo Clinic), site investigator (Medtronic EPAS, NeuroPace RESPONSE, Neuroelectrics tDCS for Epilepsy), and industry consultant (Epiminder, Medtronic, Neuropace, Philips Neuro; all funds to Mayo Clinic). GAW declares intellectual property licensed to Cadence Neuroscience Inc. and NeuroOne Inc, site investigator (Medtronic Plc. and NeuroPace Inc. studies), and has sponsored research from Cadence Inc. and Medtronic Plc. The remaining authors have no conflict of interest.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Alcala-Zermeno JL, Gregg NM, Starnes K, Mandrekar JN, Van Gompel JJ, Miller K, et al. Invasive neuromodulation for epilepsy: Comparison of multiple approaches from a single center. Epilepsy Behav EB. 2022. Oct 27;137(Pt A):108951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcala-Zermeno JL, Starnes K, Gregg NM, Worrell G, Lundstrom BN. Responsive Neurostimulation with Low Frequency Stimulation. Epilepsia. 2022. Nov: doi: 10.1111/epi.17467. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcot Desai SA, Tcheng TK, Morrell MJ. Quantitative electrocorticographic biomarkers of clinical outcomes in mesial temporal lobe epileptic patients treated with the RNS system. Clin Neurophysiol 2019. Aug;130(8):1364–1374. [DOI] [PubMed] [Google Scholar]
- Arya R, Aungaroon G, Zea Vera A, Horn PS, Byars AW, Greiner HM et al. , Fosphenytoin pre-medication for pediatric extra-operative electrical stimulation brain mapping. Epilepsy Res 2018. Feb;140:171–6. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Lagarde S, Wendling F, McGonical A, Jirsa V, Guye M, Benar C. Defining epileptogenic networks: Contribution of SEEG and signal analysis. Epilepsia. 2017. Jul;58(7):1131–47. [DOI] [PubMed] [Google Scholar]
- Basu I, Robertson MM, Crocker B, Peled N, Farnes K, Vallejo-Lopez DI, et al. Consistent linear and non-linear responses to invasive electrical brain stimulation across individuals and primate species with implanted electrodes. Brain Stimulat. 2019;12(4):877–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette D, Mirro EA, Lawrence M, Patra SE. Brain-responsive corticothalamic stimulation in the pulvinar nucleus. Epilepsia Open 2021. Sep;6(3):611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi F, Mothersill I, Imbach LL. The medial pulvinar as a subcortical relay in temporal lobe status epilepticus. Seizure. 2020. Oct;81:276–9. [DOI] [PubMed] [Google Scholar]
- Chassoux F, Devaux B, Landré E, Turak B, Nataf F, Varlet P, et al. Stereoelectroencephalography in focal cortical dysplasia: A 3D approach to delineating the dysplastic cortex. Brain. 2000. Aug 1;123(8):1733–51. [DOI] [PubMed] [Google Scholar]
- Chiang S, Khambhati AN, Wang ET, Vannucci M, Chang EF, Rao VR. Evidence of state-dependence in the effectiveness of responsive neurostimulation for seizure modulation. Brain Stimulat. 2021. Apr;14(2):366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child ND, Benarroch EE. Anterior nucleus of the thalamus: functional organization and clinical implications. Neurology. 2013. Nov 19;81(21):1869–76. [DOI] [PubMed] [Google Scholar]
- Chvojka J, Kudlacek J, Chang WC, Novak O, Tomaska F, Otahal J, et al. The role of interictal discharges in ictogenesis - A dynamical perspective. Epilepsy Behav EB. 2021. Aug;121(Pt B):106591. [DOI] [PubMed] [Google Scholar]
- Cuello Oderiz C, von Ellenrieder N, Dubeau F, Eisenberg A, Gotman J, Hall J, et al. Association of Cortical Stimulation-Induced Seizure With Surgical Outcome in Patients With Focal Drug-Resistant Epilepsy. JAMA Neurol. 2019. Sep 1;76(9):1070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukiert A, Cukiert CM, Burattini JA, Mariani PP. Seizure outcome during bilateral, continuous, thalamic centromedian nuclei deep brain stimulation in patients with generalized epilepsy: a prospective, open-label study. Seizure. 2020. Oct;81:304–9. [DOI] [PubMed] [Google Scholar]
- Cushing H A Note upon the Faradic Stimulation of the Postcentral Gyrus in Conscious Patients. Brain J Neurol. 1909;32:44–53. [Google Scholar]
- Dalic LJ, Warren AEL, Bulluss KJ, Thevathasan W, Roten A, Churilov L, et al. DBS of Thalamic Centromedian Nucleus for Lennox-Gastaut Syndrome (ESTEL Trial). Ann Neurol. 2022. Feb;91(2):253–67. [DOI] [PubMed] [Google Scholar]
- Deutschová B, Pizzo F, Giusiano B, Villalon SM, Carron R, Bénar C, et al. Ictal connectivity changes induced by pulvinar stimulation correlate with improvement of awareness. Brain Stimulat. 2021;14(2):344–6. [DOI] [PubMed] [Google Scholar]
- Donos C, Mîndruţă I, Ciurea J, Mălîia MD, Barborica A. A comparative study of the effects of pulse parameters for intracranial direct electrical stimulation in epilepsy. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2016. Jan;127(1):91–101. [DOI] [PubMed] [Google Scholar]
- Enatsu R, Piao Z, O’Connor T, Horning K, Mosher J, Burgess R, et al. Cortical excitability varies upon ictal onset patterns in neocortical epilepsy: A cortico-cortical evoked potential study. Clin Neurophysiol. 2012. Feb 1;123(2):252–60. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Rolston JD, Wright CW, Hassnain KH, Chang EF. Rates and predictors of seizure freedom with vagus nerve stimulation for intractable epilepsy. Neurosurgery 2016. Sep;79(3):345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista E, Bénar C, Bonini F, Carron R, Colombet B, Régis J, et al. Does the Thalamo-Cortical Synchrony Play a Role in Seizure Termination? Front Neurol. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipescu C, Lagarde S, Lambert I, Pizzo F, Trébuchon A, McGonigal A, et al. The effect of medial pulvinar stimulation on temporal lobe seizures. Epilepsia. 2019. Apr;60(4):e25–30. [DOI] [PubMed] [Google Scholar]
- Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010. May;51(5):899–908. [DOI] [PubMed] [Google Scholar]
- Foerster O, Penfield W. The structural basis of traumatic epilepsy and results of radical operation. Brain 1930;53:99–119. [Google Scholar]
- Grande KM, Ihnen SKZ, Arya R. Electrical Stimulation Mapping of Brain Function: A Comparison of Subdural Electrodes and Stereo-EEG. Front Hum Neurosci. 2020. Dec;14:611291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZH, Zhao BT, Toprani S, Hu WH, Zhang C, Wang X, et al. Epileptogenic network of focal epilepsies mapped with cortico-cortical evoked potentials. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2020. Nov;131(11):2657–66. [DOI] [PubMed] [Google Scholar]
- Guye M, Régis J, Tamura M, Wendling F, McGonigal A, Chauvel P, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain J Neurol. 2006. Jul;129(Pt 7):1917–28. [DOI] [PubMed] [Google Scholar]
- Hays MA, Coogan C, Crone NE, Kang JY. Graph theoretical analysis of evoked potentials shows network influence of epileptogenic mesial temporal region. Hum Brain Mapp. 2021;42(13):4173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays MA, Smith RJ, Wang Y, Coogan C, Sarma SV, Crone NE, et al. Cortico-cortical evoked potentials in response to varying stimulation intensity improves seizure localization. Clin Neurophysiol [Internet]. 2022. Sep; 42;4173–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homman-Ludiye J, Bourne JA. The medial pulvinar: function, origin and association with neurodevelopmental disorders. J Anat. 2019. Sep;235(3):507–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon B, Lesser RP, Rance NE, Hart H Hr, Webber R, Uematsu S, Fisher RS. Parameters for direct cortical electrical stimulation in the human: histopathological confirmation. Electroencephalogr Clin Neurophysiol. 1990. May;75(5) :371–7. [DOI] [PubMed] [Google Scholar]
- Isnard J, Taussig D, Bartolomei F, Bourdillon P, Catenoix H, Chassoux F, et al. French guidelines on stereoelectroencephalography (SEEG). Neurophysiol Clin Clin Neurophysiol. 2018. Feb;48(1):5–13. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Enatsu R, Matsumoto R, Novak E, Thankappen B, Piao Z, et al. Accentuated cortico-cortical evoked potentials in neocortical epilepsy in areas of ictal onset. Epileptic Disord. 2010. Dec 1;12(4):292–302. [DOI] [PubMed] [Google Scholar]
- Jiruska P, de Curtis M, Jefferys JGR, Schevon CA, Schiff SJ, Schindler K. Synchronization and desynchronization in epilepsy: controversies and hypotheses. J Physiol 2013. Feb 15;591(4):787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GW, Cai LY, Doss DJ, Jiang JW, Negi AS, Narasimhan S, et al. Localizing seizure onset zones in surgical epilepsy with neurostimulation deep learning. J Neurosurg. 2022. Sep 23;1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali G, Smith RJ, Hays M, Coogan C, Crone NE, Kang JY, et al. Transfer Function Models for the Localization of Seizure Onset Zone From Cortico-Cortical Evoked Potentials. Front Neurol. 2020;11:579961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali G, Smith RJ, Hays M, Coogan C, Crone NE, Sarma SV, et al. Localizing the seizure onset zone from single pulse electrical stimulation responses using transfer function models. Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Int Conf. 2020. Jul;2020:2524–7. [DOI] [PubMed] [Google Scholar]
- Kaufmann E, Bartolomei F, Boon P, Chabardes S, Colon AJ, Eross L, et al. European Expert Opinion on ANT-DBS therapy for patients with drug-resistant epilepsy (a Delphi consensus). Seizure. 2020. Oct;81:201–9. [DOI] [PubMed] [Google Scholar]
- Khambhati AN, Shafi A, Raoi VR, Chang EF. Long-term brain network reorganization predicts responsive neurostimulation outcomes for focal epilepsy. Sci Transl Med 2021. Aug 25;13 (608):eabf6588. [DOI] [PubMed] [Google Scholar]
- Köksal Ersöz E, Modolo J, Bartolomei F, Wendling F. Neural mass modeling of slow-fast dynamics of seizure initiation and abortion. PLoS Comput Biol. 2020. Nov;16(11):e1008430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovac S, Kahane P, Diehl B. Seizures induced by direct electrical cortical stimulation--Mechanisms and clinical considerations. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2016. Jan;127(1):31–9. [DOI] [PubMed] [Google Scholar]
- Kramer MA, Truccolo W, Eden UT, Lepage KQ, Hochberg LR, Eskandar EN, et al. Human seizures self-terminate across spatial scales via a critical transition. PNAS 2012. Dec;109 (51):21116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuncel Alexis M., and Grill Warren M.. 2004. “Selection of Stimulus Parameters for Deep Brain Stimulation.” Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology 115 (11): 2431–41. [DOI] [PubMed] [Google Scholar]
- Kundu B, Davis TS, Philip B, Smith EH, Arain A, Peters A, et al. A systematic exploration of parameters affecting evoked intracranial potentials in patients with epilepsy. Brain Stimulat. 2020;13(5):1232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lado FA, Moshé SL. How do seizures stop? Epilepsia. 2008;49(10):1651–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde S, Bénar CG, Wendling F, Bartolomei F. Interictal Functional Connectivity in Focal Refractory Epilepsies Investigated by Intracranial EEG. Brain Connect. 2022;12(10):850–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtimäki K, Möttönen T, Järventausta K, Katisko J, Tähtinen T, Haapasalo J, et al. Outcome based definition of the anterior thalamic deep brain stimulation target in refractory epilepsy. Brain Stimulat. 2016;9(2):268–75. [DOI] [PubMed] [Google Scholar]
- Lepeu G, Van Maren E, Slabeva K, Fuchs M, Anso J, Z/Graggen WJ, et al. Probing crotical excitability under GABAergic modulation. BioRxiv 2021. doi: 10.1101/2021.02.18.431873. [DOI] [Google Scholar]
- Lesser RP, Kim SH, Beyderman L, Miglioretti DL, Webber WR, Bare M, et al. Brief bursts of pulse stimulation terminate afterdischarges caused by cortical stimulation. Neurology. 1999. Dec 10;53(9):2073–81. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Van Gompel J, Khadjevand F, Worrell G, Stead M. Chronic subthreshold cortical stimulation and stimulation-related EEG biomarkers for focal epilepsy. Brain Commun 2019;1(1):fcz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto R, Kunieda T, Nair D. Single pulse electrical stimulation to probe functional and pathological connectivity in epilepsy. Seizure. 2017. Jan;44:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Bingaman W, Shibasaki H, Lüders HO. Functional connectivity in human cortical motor system: a cortico-cortical evoked potential study. Brain J Neurol. 2007. Jan;130(Pt 1):181–97. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, et al. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain J Neurol. 2004. Oct;127(Pt 10):2316–30. [DOI] [PubMed] [Google Scholar]
- Matsuzaki N, Juhasz C, Asano E. Cortico-cortical evoked potentials and stimulation-elicited gamma activity preferentially propagate from lower- to higher-order visual areas. Clin Neurophysiol 2013. Jul;124(7):1290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturana MI, Meisel C, Dell K, Karoly PJ, D’Souza W, Grayden DB, et al. Critical slowing down as a biomarker for seizure susceptibility. Nat Commun. 2020. May 1;11(1):2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi T, Sonoda M, Iwaki H, Luat AF, Sood S, Asano E. Effects of depth electrode montage and single-pulse electrical stimulation sites on neuronal responses and effective connectivity. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2020. Dec;131(12):2781–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell MJ RNS System in Epilepsy Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011. Sep 27;77(13):1295–304. [DOI] [PubMed] [Google Scholar]
- Mouthaan BE, van ‘t Klooster MA, Keizer D, Hebbink GJ, Leijten FSS, Ferrier CH, et al. Single Pulse Electrical Stimulation to identify epileptogenic cortex: Clinical information obtained from early evoked responses. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2016. Feb;127(2):1088–98. [DOI] [PubMed] [Google Scholar]
- Munari C, Kahane P, Tassi L, Francione S, Hoffmann D, Russo GL, et al. Intracerebral Low Frequency Electrical Stimulation: a New Tool for the Definition of the “Epileptogenic Area”? In: Meyerson BA, Broggi G, Martin-Rodriguez J, Ostertag C, Sindou M, editors. Advances in Stereotactic and Functional Neurosurgery 10. Vienna: Springer; 1993. p. 181–5. [DOI] [PubMed] [Google Scholar]
- Nair DR, Laxer KD, Weber PB, Murro AM, Park YD, Barkley GL, et al. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology. 2020. Sep 1;95(9):e1244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain : the neurophysics of EEG [Internet]. 2nd ed. Oxford ; New York: Oxford University Press; 2006. xvi, 611 p. p. Available from: http://www.loc.gov/catdir/enhancements/fy0916/2005040874-t.html http://www.loc.gov/catdir/enhancements/fy0916/2005040874-d.html [Google Scholar]
- Paulk AC, Zelmann R, Crocker B, Widge AS, Dougherty DD, Eskandar EN, et al. Local and distant cortical responses to single pulse intracranial stimulation in the human brain are differentially modulated by specific stimulation parameters. Brain Stimulat. 2022;15(2):491–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M Luigi Galvani and animal electricity: two centuries after the foundation of electrophysiology. Trends Neurosci. 1997. Oct;20(10):443–8. [DOI] [PubMed] [Google Scholar]
- Piper RJ, Richardson RM, Worrell G, Carmichael DW, Baldeweg T, Litt B, Denison T, Tisdall MM. Towards network-guided neuromodulation for epilepsy. Brain. 2022;145(10):3347–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo F, Roehri N, Giusiano B, Lagarde S, Carron R, Scavarda D, et al. The Ictal Signature of Thalamus and Basal Ganglia in Focal Epilepsy: A SEEG Study. Neurology. 2021. Jan 12;96(2):e280–93. [DOI] [PubMed] [Google Scholar]
- Prime D, Rowlands D, O’Keefe S, Dionisio S. Considerations in performing and analyzing the responses of cortico-cortical evoked potentials in stereo-EEG. Epilepsia. 2018. Jan;59(1):16–26. [DOI] [PubMed] [Google Scholar]
- Ryvlin P, Rheims S, Hirsch LJ, Sokolov A, Jehi L. Neuromodulation in epilepsy: state-of-the-art approved therapies. Lancet Neurol 2021;20:1038–47. [DOI] [PubMed] [Google Scholar]
- Rosenow F, Lueders H. Presurgical evaluation of epilepsy. Brain. 2001. Sep;124 (Pt9):1683–700. [DOI] [PubMed] [Google Scholar]
- Rosenberg DS, Mauguière F, Demarquay G, Ryvlin P, Isnard J, Fischer C, et al. Involvement of medial pulvinar thalamic nucleus in human temporal lobe seizures. Epilepsia. 2006. Jan;47(1):98–107. [DOI] [PubMed] [Google Scholar]
- Ryvlin P, Rheims S, Hirsch LJ, Sokolov A, Jehi L. Neuromodulation in epilepsy: state-of-the-art approved therapies. Lancet Neurol. 2021. Dec;20(12):1038–47. [DOI] [PubMed] [Google Scholar]
- Russo S, Mikulan E, Zauli FM, Sartori I, Solbiati M, Furregoni G, Porro M, et al. Neocortical and medial temporal seizures have distinct impacts on brain responsiveness. Epilepsia 2023. Mar; doi: 10.1111/epi.17580. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Saggio ML, Crisp D, Scott JM, Karoly P, Kuhlmann L, Nakatani M, et al. A taxonomy of seizure dynamotypes. eLife. 2020. Jul 21;9:e55632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanova V, Sperling MR, Gross RE, Irwin CP, Vollhaber JA, Giftakis JE, et al. The SANTÉ study at 10 years of follow-up: Effectiveness, safety, and sudden unexpected death in epilepsy. Epilepsia. 2021. Jun;62(6):1306–17. [DOI] [PubMed] [Google Scholar]
- Schroeder GM, Chowdhury FA, Cook MJ, Diehl B, Duncan JS, Karoly PJ, et al. Multiple mechanisms shape the relationship between pathway and duration of focal seizures. Brain Commun. 2022;4(4):fcac173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson HD, Schulze-Bonhage A, Cascino GD, Fisher RS, Jobst BC, Sperling MR, et al. Practical considerations in epilepsy neurostimulation. Epilepsia. 2022. Jun 14;63(10):2445–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G, Stacey WC. The accuracy of quantitative EEG biomarker algorithms depends upon seizure onset dynamics. Epilepsy Res. 2021. Oct;176:106702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Hays MA, Kamali G, Coogan C, Crone NE, Kang JY, et al. Stimulating native seizures with neural resonance: a new approach to localize the seizure onset zone. Brain. 2022. Nov 1;145(11):3886–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Kamali G, Hays M, Coogan CG, Crone NE, Sarma SV, et al. State-space models of evoked potentials to localize the seizure onset zone. In: 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). 2020. p. 2528–31. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004. Sep;27(9):520–7. [DOI] [PubMed] [Google Scholar]
- Sobayo T, Mogul DJ. Should stimulation parameters be individualized to stop seizures: Evidence in support of this approach. Epilepsia. 2016. Jan;57(1):131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda M, Silverstein BH, Jeong JW, Sugiura A, Nakai Y, Mitsuhashi T, et al. , Six-dimensional dynamic tractography atlas of language connectivity in the development brain. Brain 2021. Dec; 144(11):3340–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead M, Bower M, Brinkmann BH, Lee K, Marsh WR, Meyer FB, et al. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain. 2010. Sep;133(9):2789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strogatz SH. Nonlinear dynamics and Chaos: with applications to physics, biology, chemistry, and engineering. Reading, Mass.: Addison-Wesley Pub.; 1994. xi, 498 p. p. (Studies in nonlinearity). [Google Scholar]
- Suller Marti A, Mirsattari SM, Steven DA, McLachlan RS, Parrent AG, Hayman-Abello S, et al. Extraoperative electrical stimulation mapping in epilepsy presurgical evaluation: a proposal and review of the literature. Clin Neurol Neurosurg. 2022. Mar;214:107170. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Harangozó M, Pedraza L, Földi T, Kozák G, Li Q, et al. Closed-loop stimulation of the medial septum terminates epileptic seizures. Brain J Neurol. 2021. Apr 12;144(3):885–908. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004. Jan 1;123(2):299–336. [DOI] [PubMed] [Google Scholar]
- Trebaul L, Deman P, Tuyisenge V, Jedynak M, Hugues E, Rudrauf D, et al. Probabilistic functional tractography of the human cortex revisited. NeuroImage. 2018. Nov 1;181:414–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trébuchon A, Chauvel P. Electrical Stimulation for Seizure Induction and Functional Mapping in Stereoelectroencephalography. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2016. Dec;33(6):511–21. [DOI] [PubMed] [Google Scholar]
- Trébuchon A, Racila R, Cardinale F, Lagarde S, McGonigal A, Lo Russo G, et al. Electrical stimulation for seizure induction during SEEG exploration: a useful predictor of postoperative seizure recurrence? J Neurol Neurosurg Psychiatry. 2021. Jan;92(1):22–6. [DOI] [PubMed] [Google Scholar]
- Valentín A, Alarcón G, Honavar M, García Seoane JJ, Selway RP, Polkey CE, et al. Single pulse electrical stimulation for identification of structural abnormalities and prediction of seizure outcome after epilepsy surgery: a prospective study. Lancet Neurol. 2005. Nov;4(11):718–26. [DOI] [PubMed] [Google Scholar]
- Valentín A, Anderson M, Alarcón G, Seoane JJG, Selway R, Binnie CD, et al. Responses to single pulse electrical stimulation identify epileptogenesis in the human brain in vivo. Brain J Neurol. 2002. Aug;125(Pt 8):1709–18. [DOI] [PubMed] [Google Scholar]
- Valentín A, García Navarrete E, Chelvarajah R, Torres C, Navas M, Vico L, et al. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia. 2013. Oct;54(10):1823–33. [DOI] [PubMed] [Google Scholar]
- van ‘t Klooster MA, van Klink NEC, van Blooijs D, Ferrier CH, Braun KPJ, Leijten FSS, et al. Evoked versus spontaneous high frequency oscillations in the chronic electrocorticogram in focal epilepsy. Clin Neurophysiol. 2017. May 1;128(5):858–66. [DOI] [PubMed] [Google Scholar]
- van ‘t Klooster MA, Zijlmans M, Leijten FSS, Ferrier CH, van Putten MJAM, Huiskamp GJM. Time-frequency analysis of single pulse electrical stimulation to assist delineation of epileptogenic cortex. Brain J Neurol. 2011. Oct;134(Pt 10):2855–66. [DOI] [PubMed] [Google Scholar]
- Velasco F, Saucedo-Alvarado PE, Reichrath A, Valdés-Quiroz H, Aguado-Carrillo G, Velasco AL. Centromedian Nucleus and Epilepsy. J Clin Neurophysiol 2021. Nov 1;38(6):485–93. [DOI] [PubMed] [Google Scholar]
- Warren AEL, Dalic LJ, Bulluss KJ, BAppSci AR, Thevathasan W, Archer JS. The Optimal Target and Connectivity for Deep Brain Stimulation in Lennox-Gastaut Syndrome. Ann Neurol. 2022. Jul;92(1):61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkat T, Rings T, Lehnertz K. No evidence for critical slowing down prior to human epileptic seizures. Chaos 2019;29:091104. [DOI] [PubMed] [Google Scholar]
- Yu T, Wang X, Li Y, et al. High-frequency stimulation of anterior nucleus of thalamus desynchronizes epileptic network in humans. Brain. 2018;141:2631–2643. [DOI] [PubMed] [Google Scholar]
- Zhao C, Liang Y, Li C, Gao R, Wei J, Zuo R, et al. Localization of Epileptogenic Zone Based on Cortico-Cortical Evoked Potential (CCEP): A Feature Extraction and Graph Theory Approach. Front Neuroinformatics [Internet]. 2019. [cited 2022 Nov 25];13. Available from: 10.3389/fninf.2019.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]


