Abstract
Purpose
To assess the diagnostic efficacy of 99mTc-sulfur colloid lymphoscintigraphy in chylothorax and chylous ascites, and the utility of single-photon emission computed tomography-computed tomography (SPECT/CT) in localizing the sites of leaks.
Methods
Data from patients who underwent lymphoscintigraphy for clinical suspicion of chylothorax or chylous ascites were retrospectively analyzed. Biochemical fluid analysis was taken as the reference standard. Pleural fluid triglyceride level > 110 mg/dL (with pleural fluid/serum ratio > 1) and a cholesterol level < 200 mg/dL (with pleural fluid/serum ratio < 1) were considered confirmatory for chylothorax. Ascitic fluid triglyceride level > 200 mg/dL with a low cholesterol level (ascites fluid/serum ratio < 1) was considered confirmatory for chylous ascites.
Results
26 patients (15 males, 57.7%) aged 9 months to 68 years were enrolled in the study. Based on the reference standard, 17 had chylothorax or chylous ascites (9 with surgical history). Lymphoscintigraphy was positive in 16 (with 1 false positive) and negative in 10 (with 2 false negatives). The sensitivity, specificity, negative predictive value, positive predictive value, and accuracy of lymphoscintigraphy were 88.2% (63.6–98.5%), 88.9% (51.8–99.7%), 80.0% (51.6–93.8%), 93.8% (70.1–99.0%), and 88.5% (69.9–97.6%), respectively. SPECT/CT could localize sites of leaks in 61.5% (8/13) with a localization rate of 77.8% (7/9) and 25.0% (1/4) in patients with surgical and nonsurgical causes, respectively.
Conclusion
99mTc-sulfur colloid lymphoscintigraphy is a highly efficacious noninvasive modality to diagnose chylothorax or chylous ascites with a high positive predictive value. SPECT/CT could localize the sites of leaks more frequently in patients with surgical causes.
Keywords: Lymphoscintigraphy, SPECT/CT, Chylous effusion, Chyle leak, Chylothorax, Chylous ascites
Introduction
The occurrence of chylothorax or chylous ascites is a rare phenomenon commonly caused by post-surgical injuries, infections, and trauma [1]. Although the gold standard for diagnosing the condition is the detection of chylomicrons in effusion fluids by lipoprotein electrophoresis, the technology is costly, labor-intensive, and not widely available. Thus, the evaluation of fluids for triglyceride and cholesterol is routinely followed in clinical practice. A pleural fluid triglyceride level > 110 mg/dL and a cholesterol level < 200 mg/dL are diagnostic of chylothorax. The pleural fluid to serum triglyceride ratio should be > 1 and the pleural fluid to serum cholesterol ratio < 1 [2]. An ascitic fluid triglyceride level > 200 mg/dL with a low cholesterol level (ascites fluid/serum ratio < 1) is diagnostic of chylous ascites [3].
Conservative management in the form of percutaneous drainage, dietary modifications, or total parenteral nutrition is successful in most cases. Adjunctive pharmacological management includes the administration of octreotide or somatostatin which acts by reducing splanchnic blood flow and decreasing intestinal absorption of fats. Untreated extensive or recurrent cases or those refractory to conservative or pharmacological management have several deleterious effects including hypovolemia, malnutrition, electrolyte imbalances, immunosuppression, and vitamin deficiencies [2, 3]. Such patients may need surgical or procedural intervention, and hence, assessment of chylothorax and chylous ascites, and identifying the site of the leak by imaging is prudent for optimal patient management [4–7].
Although lymphangiography is considered the gold standard imaging modality in the evaluation of chylothorax or chylous ascites, it is an invasive procedure with several risks, some being serious. Infection, pain, allergic reactions, intra-alveolar hemorrhage, contrast extravasation into the deep tissues, and oil embolism to the lungs, brain, kidneys, and liver are known complications of the procedure [1]. The presence of fluid collection can be confirmed noninvasively by chest radiography, ultrasonography, or computed tomography (CT) of the abdomen or chest. However, these modalities cannot differentiate chylous fluid collection from other causes of fluid collection. CT scan of the chest or abdomen may additionally show the cause of chylothorax or chylous ascites like mass lesions or evidence of trauma [8]. However, it has limitations in localizing the exact site of the leak. Different magnetic resonance (MR) lymphangiography techniques have been developed for imaging the lymphatic system and assessing chylothorax and chylous ascites [9, 10]. Unenhanced MR lymphangiography cannot specifically distinguish lymphatic vessels from surrounding tissues with high T2 signals, especially in patients with large effusions [11]. Though dynamic contrast-enhanced MR lymphangiography can be helpful in delineating the anatomy, function, and pathologic changes in the lymphatic system, it is a time-consuming procedure, and it also requires intranodal injection of a gadolinium-based contrast agent [10]. Lymphoscintigraphy is a simple, technically non-demanding, and non-invasive modality that can confirm the presence of chylothorax and chylous ascites although ascertaining the exact site of leak is difficult on routine planar lymphoscintigraphy images. The advent of hybrid three-dimensional single-photon emission computed tomography-computed tomography (SPECT/CT) might redeem this limitation of planar lymphoscintigraphy in localizing the site of chyle leak.
The primary objective of the present study was to assess the diagnostic efficacy of 99mTc-sulfur colloid lymphoscintigraphy in chylothorax and chylous ascites. The secondary objective was to assess the utility of SPECT/CT in localizing the sites of chyle leaks.
Materials and Methods
The current study had an observational-analytic cross-sectional design. The Institute Ethics Committee approved the retrospective study (IEC-398/06.05.2022). The committee waived the requirement for informed written consent due to the retrospective nature of the study.
Patients
We retrospectively reviewed the data of patients who underwent lymphoscintigraphy for clinical suspicion of chylothorax or chylous ascites from January 2014 to January 2022. The study enrolled only those patients for whom biochemical fluid analysis for confirmation of the presence of chylothorax or chylous ascites was available.
Lymphoscintigraphy and SPECT/CT Acquisition
Under strict aseptic precautions, 0.25 mCi (9.25 MBq) of 99mTc-sulfur colloid in 0.1–0.2 ml volume was administered intradermally into each first and second webspace of the lower and/or upper limbs using a 26-gauge needle (tuberculin syringe). After the radiotracer administration, patients were allowed to lie supine with feet towards the gantry in a dual-head GE Discovery NM/CT 670 SPECT/CT system. Whole-body static images in anterior and posterior views were acquired using a low-energy high-resolution collimator, matrix size 1024 × 256, zoom 1, and 20% energy window centered on 140 keV photopeak. The whole-body acquisition was performed in continuous mode at a speed of 10–13 cm/min and exposure time per pixel of 180 s. Image acquisition was performed within 5 min following tracer administration and at 30 min, 1 h, 2 h, 4 h, and in some cases 24 h. Regional static imaging of the region of clinically suspected chyle leak and/or abnormal tracer concentration was acquired in anterior and posterior views using matrix size 256 × 256 and with the parameters mentioned above to acquire 700–1000 kcounts. SPECT of the region of clinically suspected chyle leak and/or abnormal tracer concentration on planar imaging was acquired in step-and-shoot mode with 40 s per view for 64 views using a matrix of 128 × 128. After the SPECT acquisition, CT was acquired on the 16‑slice spiral CT of the hybrid SPECT/CT system with a slice thickness of 5 mm, pitch of 1.5, and matrix of 512 × 512. Images were processed on a dedicated Xeleris 4 DR workstation. SPECT images were reconstructed by iterative method ordered subset expectation maximization (four iterations and ten subsets). CT data were used for attenuation correction and anatomical correlation. SPECT emission images were co-registered and fused with the transmission CT images using the object versus target matrix method. Fused emission and transmission images were visually inspected for the correctness of co-registration. Reconstructed images were displayed for review in axial, coronal, and sagittal planes, as well as in maximum intensity projections and three-dimensional cine mode.
Image Analysis
All the scans were analyzed by two experienced nuclear medicine physicians on a dedicated Xeleris 4 DR workstation. Visualization of localized extra-lymphatic concentration of radiotracer on planar whole-body and/or regional static images not explainable by the normal biodistribution pattern was considered positive for chylothorax or chylous ascites. On SPECT/CT, focal or localized extra-nodal radiotracer uptake, along the expected path of the thoracic duct or its tributaries, and not conforming to the normal biodistribution pattern was considered the site of chyle leak.
Reference Standard
Biochemical fluid analysis performed not more than 4 weeks from the date of lymphoscintigraphy was taken as the reference standard. Pleural fluid triglyceride level > 110 mg/dL (with pleural fluid/serum ratio > 1) and a cholesterol level < 200 mg/dL (with pleural fluid/serum ratio < 1) were considered confirmatory for chylothorax. Ascitic fluid triglyceride level > 200 mg/dL with a low cholesterol level (ascites fluid/serum ratio < 1) was considered confirmatory for chylous ascites.
Statistical Analysis
Descriptive statistics such as range and frequencies were used to describe the demographic and clinical profiles of the patients. Sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), and accuracy of lymphoscintigraphy with 95% confidence intervals were calculated using biochemical fluid analysis as the reference standard. The utility of SPECT/CT in localizing the site of chyle leak was expressed as frequency and percentage. The statistical package MedCalc® 20.009 (MedCalc Software, Mariakerke, Belgium) was used for the statistical analyses.
Results
Patient Characteristics
Data from a total of 46 patients who underwent lymphoscintigraphy from January 2014 to January 2022 for clinical suspicion of chylothorax or chylous ascites could be retrieved from the PACS (Picture Archiving and Communication System) and hospital records. Biochemical fluid analysis was available for 26 patients who were finally included in the study. The age of the enrolled patients ranged from 9 months to 68 years. There were 15 males and 11 females. Based on the reference standard, 17 patients had chylothorax or chylous ascites (14 chylothorax, 3 chylous ascites) out of which, 9 patients had a history of surgical intervention (8 chylothorax post-cardiothoracic surgery, 1 chylous ascites post-resection of neuroblastoma). 6/17 patients had tuberculosis (4 chylothorax with pulmonary tuberculosis, 1 chylothorax with disseminated tuberculosis, and 1 chylous ascites with abdominal tuberculosis). The remaining 2 patients had idiopathic chylous ascites and congenital chylothorax respectively. A tabulated summary and comprehensive representation of the patient characteristics and clinico-imaging details are depicted in Tables 1 and 2.
Table 1.
Summary characteristics of enrolled patients
| Characteristics | |
|---|---|
| Age (range) | 9 months–68 years |
| Sex | |
| Male | 15 (57.7%) |
| Female | 11 (42.3%) |
| Reference standard | |
| Chylothorax | 14 (53.9%) |
| Chylous ascites | 3 (11.5%) |
| None | 9 (34.6%) |
| Fluid analysis | |
| Fluid TG | 333.3 ± 301.8 mg/dL |
| Fluid Cho | 49.7 ± 22.2 mg/dL |
| Fluid-serum TG ratio | 2.8 ± 2.4 |
| Fluid-serum Cho ratio | 0.4 ± 0.2 |
| History of surgical intervention | |
| Present | 9 (34.6%) |
| Absent | 17 (65.4%) |
| Injection site | |
| LL | 19 (73.1%) |
| UL and LL | 7 (26.9%) |
| Lymphoscintigraphy | |
| Positive | 16 (61.5%) |
| Negative | 10 (38.5%) |
| SPECT/CT | |
| Performed | 15 (57.7%) |
| Not performed | 11 (42.3%) |
| SPECT/CT results | |
| Positive | 8 (53.3%) |
| Negative | 7 (46.7%) |
TG triglyceride, Cho cholesterol, LL lower limb, UL upper limb, SPECT/CT single-photon emission computed tomography-computed tomography
Table 2.
Patient characteristics and clinico-imaging details
| Sl No | Age | Sex | Clinical history | Injection site | Fluid TG (mg/dL) | Fluid Cho (mg/dL) | Fluid-serum TG ratio | Fluid-serum Cho ratio | Lymphoscintigraphy | SPECT/CT (localization of chyle leak) | Reference standard |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 years | M | TGA correction | LL | 621 | 17 | 3.7 | 0.1 | Positive | Not localized | Chylothorax |
| 2 | 9 months | F | Bidirectional Glenn shunt and atrial septectomy | LL | 614 | 49 | 5.6 | 0.5 | Positive | Localized; 2 foci of tracer uptake: 1. Left posterior mediastinum at D5 level adjacent to LPA, 2. Paraaortic region near surgical clip adjacent to aortic arch | Chylothorax |
| 3 | 7 years | M | TGA correction | LL | 249 | 51 | 2.9 | 0.5 | Positive | Localized to sub-precarinal region between SVC and RPA | Chylothorax |
| 4 | 16 years | F | Idiopathic chylous ascites | LL | 302 | 29 | 2.5 | 0.3 | Positive | Not localized; focal tracer uptake in the region of cisterna chyli (aortocaval/retrocaval region) at L1-2 level, considered as physiological uptake | Chylous ascites |
| 5 | 30 years | M | Suspected chylous ascites | LL | 163 | 52 | 1.2 | 0.3 | Negative | NA | No chylous ascites |
| 6 | 23 years | F | Pulmonary TB with suspected chylothorax | UL and LL | 733 | 63 | 4.8 | 0.4 | Negative | NA | Chylothorax |
| 7 | 48 years | M | Suspected chylous ascites | LL | 111 | 39 | 0.8 | 0.2 | Negative | NA | No chylous ascites |
| 8 | 31 years | F | Suspected chylothorax | LL | 57 | 23 | 0.7 | 0.1 | Negative | Not localized | No chylothorax |
| 9 | 6 years | M | Cardiac surgery for CHD and permanent pacemaker implantation | LL | 233 | 22 | 1.3 | 0.1 | Positive | Localized; 2 foci of tracer uptake: 1. Left anterior mediastinum anterolateral to aortic arch and anterior to esophagus at D4-5 level, 2. Left paravertebral region at D6-7 level | Chylothorax |
| 10 | 3 years | M | Resection of left suprarenal neuroblastoma | LL | 560 | 80 | 4.5 | 0.7 | Positive | Localized to left paravertebral region adjacent to surgical clip at L2 level | Chylous ascites |
| 11 | 1 year 9 months | M | Congenital chylothorax | LL | 465 | 38 | 4.6 | 0.4 | Positive | NA | Chylothorax |
| 12 | 9 years | M | Abdominal TB with chylous ascites | LL | 382 | 23 | 6.4 | 0.1 | Positive | NA | Chylous ascites |
| 13 | 9 years | M | Disseminated TB with left pleural effusion | UL and LL | 412 | 41 | 4.4 | 0.4 | Positive | NA | Chylothorax |
| 14 | 68 years | M | Right supraclavicular neck swelling with suspected chylous fluid; FNA: suppurative lymphadenitis | LL | 67 | 78 | 0.3 | 0.3 | Negative | NA | No chylothorax |
| 15 | 63 years | F | Filariasis with suspected chylous ascites | LL | 124 | 48 | 1.1 | 0.5 | Negative | NA | No chylous ascites |
| 16 | 1 year 9 months | M | Suspected chylothorax | LL | 72 | 35 | 0.3 | 0.1 | Negative | NA | No chylothorax |
| 17 | 55 years | F | Suspected chylous ascites | LL | 147 | 45 | 1.2 | 0.4 | Negative | NA | No chylous ascites |
| 18 | 19 years | F | Suspected chylous ascites; pseudomonas species in ascitic fluid | UL and LL | 192 | 24 | 1.6 | 0.2 | Negative | NA | No chylous ascites |
| 19 | 8 years | F | Excision of lymphangioma of anterior mediastinum with suspected chylothorax | LL | 181 | 59 | 2.4 | 0.5 | Positive | Localized to mediastinum around ascending aorta | Chylothorax |
| 20 | 13 years | F | Pulmonary TB with suspected chylothorax | LL | 218 | 85 | 1.7 | 0.8 | Positive | Not localized | Chylothorax |
| 21 | 32 years | M | Surgery for coarctation of aorta | LL | 325 | 76 | 2.5 | 0.7 | Positive | Localized to mediastinum near surgical clip between aortic arch and esophagus at D3-4 level | Chylothorax |
| 22 | 28 years | M | Cardiothoracic surgery | LL | 1494 | 105 | 10.8 | 0.5 | Positive | Localized to left superior mediastinum just above aortic arch at D2-3 level | Chylothorax |
| 23 | 48 years | M | Tubercular pleural effusion with suspected chylothorax | UL and LL | 71 | 46 | 0.5 | 0.2 | Positive | Not localized | No chylothorax |
| 24 | 54 years | F | Pulmonary TB with suspected chylothorax | UL and LL | 331 | 65 | 1.6 | 0.5 | Positive | Localized to paraesophageal region at D6-7 level | Chylothorax |
| 25 | 6 years | M | Bidirectional Glenn cardiac surgery for CHD | UL and LL | 331 | 40 | 2.9 | 0.4 | Positive | Not localized | Chylothorax |
| 26 | 31 years | F | Pulmonary TB with suspected chylothorax | UL and LL | 210 | 59 | 2.4 | 0.4 | Negative | Not localized | Chylothorax |
M male, F female, TGA transposition of great arteries, TB tuberculosis, CHD congenital heart disease, FNA fine needle aspiration, LL lower limb, UL upper limb, TG triglyceride, Cho cholesterol, SPECT/CT single-photon emission computed tomography-computed tomography, LPA left pulmonary artery, SVC superior vena cava, RPA right pulmonary artery, D dorsal vertebra level, L lumbar vertebra level, NA not applicable (SPECT/CT not done)
Performance of Lymphoscintigraphy
Lymphoscintigraphy was positive in 16 patients of whom 1 patient with tubercular pleural effusion was false positive (Fig. 1) based on the reference standard. Of the remaining 10 patients with negative lymphoscintigraphy findings, 2 were false negative and the remaining 8 were true negative (Fig. 2). Lymphoscintigraphy was positive in all the 9 chylothorax or chylous ascites patients (100%) who had a history of surgical intervention and in 66.7% (4/6) of chylothorax or chylous ascites patients secondary to tuberculosis. The remaining 2 patients with idiopathic chylous ascites and congenital chylothorax respectively were also positive on lymphoscintigraphy resulting in an overall positivity rate of 75.0% (6/8) in patients with no history of surgical intervention. The sensitivity, specificity, NPV, PPV, and accuracy of lymphoscintigraphy for detecting chylothorax or chylous ascites were 88.2 (63.6–98.5%), 88.9 (51.8–99.7%), 80.0% (51.6–93.8%), 93.8% (70.1–99.0%), and 88.5% (69.9–97.6%), respectively.
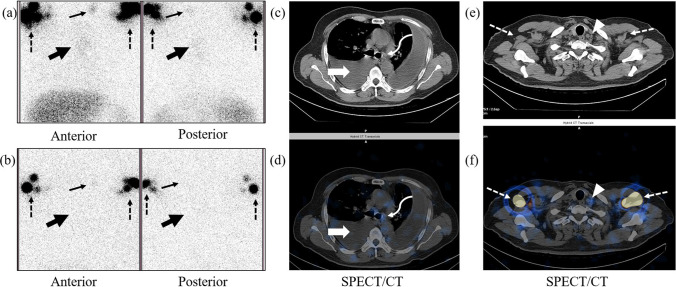
Fig. 1.
False-positive lymphoscintigraphy findings in a 48-year-old male with tubercular pleural effusion and no evidence of chylothorax on fluid analysis. Planar lymphoscintigraphy image at 3 h (a) reveals radiotracer concentration in the left paramedian thoracic region (solid thick black arrows) which disappears in the delayed image (b). SPECT/CT (c, d) reveals bilateral pleural effusion with no radiotracer concentration (solid thick white arrows). However, faint radiotracer concentration is seen in the mediastinal lymph nodes (curved arrows in c and d). Another focal area of radiotracer concentration is seen in the planar images (solid thin black arrows in a and b) which is localized to a small left supraclavicular lymph node on SPECT/CT (arrowheads in e and f). Dotted arrows in a, b, e, and f indicate radiotracer concentration in bilateral axillary lymph nodes
Fig. 2.
True-negative lymphoscintigraphy findings in a 31-year-old female with pleural effusion and no evidence of chylothorax on fluid analysis. Planar lymphoscintigraphy image at 3 h (a) reveals physiological distribution of radiotracer (solid black arrows, liver; dotted arrows, inguino-pelvic-abdominal lymph nodes). SPECT/CT (b, c) reveals right pleural effusion with no radiotracer concentration (solid white arrows). Faint radiotracer concentration is seen in the mediastinal lymph nodes (curved arrows in b and c)
Performance of SPECT/CT
SPECT/CT was performed in 13 patients with positive and 2 patients with negative lymphoscintigraphy findings respectively. SPECT/CT could localize sites of leaks in 61.5% (8/13) of biochemically confirmed chylothorax or chylous ascites patients with a localization rate of 66.7% (8/12) in those who had true positive lymphoscintigraphy findings. All the 9 chylothorax or chylous ascites patients with a history of surgical intervention underwent SPECT/CT and it could localize the sites of leaks in 77.8% (7/9) (6 chylothorax post-cardiothoracic surgery, 1 chylous ascites post-resection of neuroblastoma) (Figs. 3, 4, and 5). Four chylothorax or chylous ascites patients with no history of surgical intervention (3 chylothorax patients with pulmonary tuberculosis, 1 patient with idiopathic chylous ascites) underwent SPECT/CT. Out of these 4 patients, SPECT/CT could localize the site of leak in 1 chylothorax patient with pulmonary tuberculosis (25.0%; 1/4). One patient with idiopathic chylous ascites was positive on lymphoscintigraphy with SPECT/CT showing focal radiotracer concentration in the region of cisterna chyli which was considered physiological uptake. Out of the 8 chylothorax or chylous ascites patients in whom SPECT/CT could localize the sites of leaks, there were 2 patients with 2 sites of leaks each, and both patients had a history of cardiothoracic surgery.
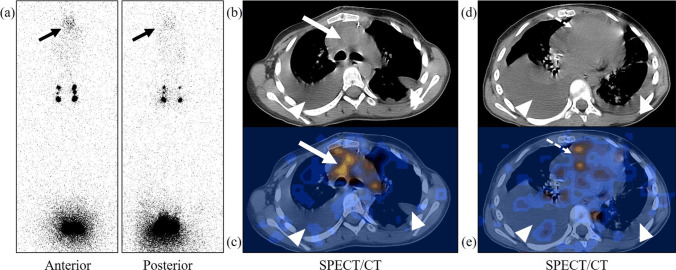
Fig. 3.
8-year-old female with a history of excision of anterior mediastinal lymphangioma and subsequent development of chylothorax confirmed by fluid analysis. Planar lymphoscintigraphy image (a) reveals abnormal radiotracer concentration in the thorax (solid black arrows). SPECT/CT (b–e) reveals fluid collection in the bilateral pleural spaces (arrowheads) and localization of the leak site to the mediastinum around ascending aorta (solid white arrows in b and c). Note is also made of pericardial effusion with radiotracer concentration (dotted arrows in d and e)
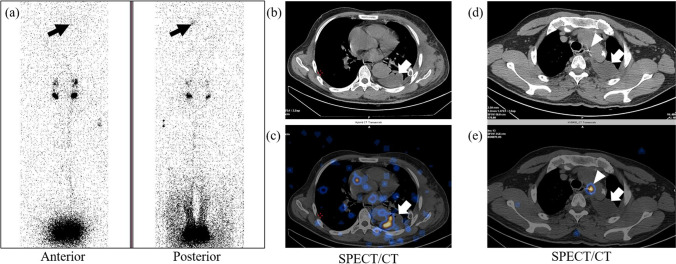
Fig. 4.
32-year-old male with a history of surgery for coarctation of the aorta with subsequent development of chylothorax confirmed by fluid analysis. Planar lymphoscintigraphy image (a) reveals abnormal radiotracer concentration in the left hemithorax (solid black arrows) that is better visualized in the posterior image. SPECT/CT (b–e) reveals fluid collection in the left pleural space with areas of radiotracer concentration (solid white arrows). SPECT/CT localizes the site of the leak to the mediastinum near the surgical clip between the aortic arch and esophagus at D3-4 level (arrowheads in d and e)
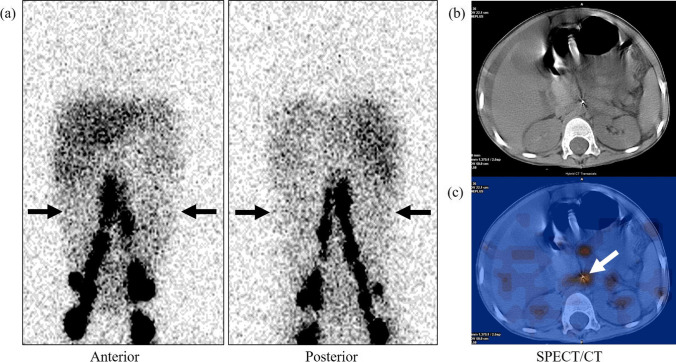
Fig. 5.
3-year-old male with a history of resection of left suprarenal neuroblastoma and subsequent development of chylous ascites confirmed by fluid analysis. Planar lymphoscintigraphy image (a) reveals a diffuse concentration of radiotracer in the abdominal cavity (black arrows). SPECT/CT (b, c) localizes the site of the leak to the paraaortic retroperitoneum at L2 level (white arrow)
Discussion
Imaging plays a crucial role in the evaluation of chylothorax and chylous ascites to assess the extent, and regions of involvement and also to identify the sites of leaks. Identification of the sites of leaks is critical in the management of extensive or recurrent cases or those refractory to conservative management, especially in patients with chylothorax or chylous ascites due to traumatic causes. These patients may require surgical or procedural interventions to prevent the development of detrimental effects of long-standing, recurrent, and/or extensive chylothorax or chylous ascites [4–7].
Our study is one of the very few original studies assessing the utility of lymphoscintigraphy in the evaluation of chylothorax and chylous ascites. Weiss et al. [12], in their study on 24 patients, showed that lymphoscintigraphy had a sensitivity of 88%, specificity of 100%, NPV of 80%, and PPV of 100% respectively for the detection of chylothorax or chylous ascites. This is in line with the results of our study except that there was one false positive in our study while Weiss et al. had none. The false-positive patient in our study had abnormal tracer concentration in the left paramedian thoracic region in the initial images which disappeared in the delayed images. However, faint radiotracer concentration was apparent in the mediastinal lymph nodes on SPECT/CT. The reason for the washout in the delayed images could not be explained. Turpin et al. [13] reported the utility of lymphoscintigraphy in their series of 21 patients with suspected chylous effusion and recommended injection in all four limbs. Also, the authors suggested the introduction of a high-fat diet and withholding octreotide prior to imaging as these maneuvers might reduce the possibility of false negative results by increasing intestinal chyle production and drainage. Their study population consisted of only pediatric patients while our study had both pediatric and adult populations. Also, unlike in our study, biochemical fluid analysis was not taken as the reference standard in their study. In our study, not all patients had four-limb injections, and the status of a high-fat diet or octreotide was not available. Further studies are suggested to assess if these maneuvers improve the detection rate of chylothorax or chylous ascites on lymphoscintigraphy.
The pitfall of planar lymphoscintigraphy is the inherent lack of detailed spatial and anatomical information which makes it difficult to precisely localize the sites of chyle leaks. SPECT/CT is a hybrid imaging modality that provides three-dimensional information on radiotracer distribution along with detailed anatomical information for the localization of any abnormal radiotracer accumulation. There are few studies, the majority being case reports, showing the utility of SPECT/CT in precisely localizing the sites of chyle leaks [12, 14–17]. In our study, SPECT/CT could localize the sites of chyle leaks in 61.5% (8/13) of patients with biochemically proven chylothorax or chylous ascites overall and in 66.7% (8/12) with positive findings on lymphoscintigraphy. In one chylothorax patient with negative lymphoscintigraphy findings, SPECT/CT could not localize the site of chyle leak. This is in line with the observations of Weiss et al. [12] who also did not find any incremental value of SPECT/CT in patients with negative lymphoscintigraphy findings. Hence, in patients with negative lymphoscintigraphy findings, SPECT/CT may be avoided thereby reducing the time of acquisition and radiation dose to the patients. The study, though, does not have enough power to generalize this statement; further studies with larger sample sizes are suggested in this respect.
Another interesting finding was that lymphoscintigraphy was positive in all the 9 chylothorax or chylous ascites patients (100%) who had a history of surgical intervention as compared to 75.0% (6/8) in those with no history of surgical intervention. SPECT/CT could localize the sites of leaks more frequently in patients with a history of surgical intervention (77.8%; 7/9) than in those without (25.0%; 1/4). This is also in agreement with that reported by Weiss et al. [12]. The reason for the higher lymphoscintigraphy positivity rate and leak localization rate in this subset of patients could be the higher flux of chyle leak due to traumatic or surgical causes as compared to non-traumatic or non-surgical causes. And hence, patients with traumatic chylothorax or chylous ascites who need surgical or procedural intervention might be benefitted from lymphoscintigraphy and SPECT/CT. Furthermore, larger studies are suggested to affirm this hypothesis.
The major limitation of the study is its retrospective nature. Hence, there could have been an unavoidable patient referral bias since selection bias is inherent in retrospective studies. This might have led to the inclusion of more patients with chylothorax than those with chylous ascites. Another limitation of the study was the small sample size. Also, sufficient follow-up data with respect to the subsequent management of the patients was not available due to the non-availability of a full-fledged electronic hospital information system. Hence, the significance of our findings on patient outcomes could not be analyzed. This is more specifically related to the performance of SPECT/CT in localizing the sites of chyle leaks, as surgical confirmation of the sites of leaks was not available. In addition, there was a lack of standardization of the injection sites; the majority of the patients had lower limbs injections, and only a few had four-limbs injections. Furthermore, records on pre-imaging patient preparations such as the administration of a high-fat diet or withholding of octreotide were not available.
Conclusion
99mTc-sulfur colloid lymphoscintigraphy is a highly efficacious noninvasive modality to diagnose chylothorax or chylous ascites with a high positive predictive value. SPECT/CT could localize the sites of chyle leaks more frequently in patients with surgical causes.
Author Contribution
JJ contributed to the study concept and design, data mining, scan review, and prepared the first draft manuscript. SN, ASB, and PG contributed to data mining, material preparation, and data entry. BCK designed the study concept, contributed to scan review, performed the data analysis, and prepared the final manuscript. CP and RK contributed to the study concept and design, proofreading, and editing of the draft manuscript. All authors read and approved the final manuscript.
Data availability
Original raw data is available on reasonable request to the corresponding author.
Declarations
Conflicts of Interest
Jasim Jaleel, Syeddharvesh Nasurudeen, Anushna Sunila Babu, Priyanka Gupta, Bangkim Chandra Khangembam, Chetan Patel, and Rakesh Kumar declare no conflict of interest.
Ethical Approval
All procedures involving human participants were in accordance with the Declaration of Helsinki 1964 as revised in 2013 and its later amendments or comparable ethical standards. The Institute Ethics Committee, All India Institute of Medical Sciences, New Delhi, approved the retrospective study (IEC-398/06.05.2022).
Informed Consent
The Institute Ethics Committee waived the requirement for informed written consent.
Consent for Participation
Not applicable.
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deso S, Ludwig B, Kabutey N-K, Kim D, Guermazi A. Lymphangiography in the diagnosis and localization of various chyle leaks. Cardiovasc Intervent Radiol. 2012;35:117–126. doi: 10.1007/s00270-010-0066-x. [DOI] [PubMed] [Google Scholar]
- 2.Braun CM, Ryu JH. Chylothorax and pseudochylothorax. Clin Chest Med. 2021;42:667–675. doi: 10.1016/j.ccm.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Bhardwaj R, Vaziri H, Gautam A, Ballesteros E, Karimeddini D, Wu GY. Chylous ascites: a review of pathogenesis, diagnosis and treatment. J Clin Transl Hepatol. 2018;6:105–113. doi: 10.14218/JCTH.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besselink MG, van Rijssen LB, Bassi C, Dervenis C, Montorsi M, Adham M, et al. Definition and classification of chyle leak after pancreatic operation: a consensus statement by the International Study Group on Pancreatic Surgery. Surgery. 2017;161:365–372. doi: 10.1016/j.surg.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 5.Tai E, Min A, Rajan DK. A single-center experience with percutaneous interventional management of refractory chylous ascites. Can Assoc Radiol J. 2021;72:871–875. doi: 10.1177/0846537120929429. [DOI] [PubMed] [Google Scholar]
- 6.Kamarajah SK, Siddaiah-Subramanya M, Parente A, Evans RPT, Adeyeye A, Ainsworth A, et al. Risk factors, diagnosis and management of chyle leak following esophagectomy for cancers: an international consensus statement. Ann Surg Open. 2022;3:e192. doi: 10.1097/AS9.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal A, Chaddha U, Kaul V, Desai A, Gillaspie E, Maldonado F. Multidisciplinary management of chylothorax. Chest. 2022;162:1402–1412. doi: 10.1016/j.chest.2022.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Sachs PB, Zelch MG, Rice TW, Geisinger MA, Risius B, Lammert GK. Diagnosis and localization of laceration of the thoracic duct: usefulness of lymphangiography and CT. Am J Roentgenol. 1991;157:703–705. doi: 10.2214/ajr.157.4.1892021. [DOI] [PubMed] [Google Scholar]
- 9.Dori Y. Novel lymphatic imaging techniques. Tech Vasc Interv Radiol. 2016;19:255–261. doi: 10.1053/j.tvir.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Krishnamurthy R, Hernandez A, Kavuk S, Annam A, Pimpalwar S. Imaging the central conducting lymphatics: initial experience with dynamic MR lymphangiography. Radiology. 2015;274:871–878. doi: 10.1148/radiol.14131399. [DOI] [PubMed] [Google Scholar]
- 11.Pieper CC, Feisst A, Schild HH. Contrast-enhanced interstitial transpedal MR lymphangiography for thoracic chylous effusions. Radiology. 2020;295:458–466. doi: 10.1148/radiol.2020191593. [DOI] [PubMed] [Google Scholar]
- 12.Weiss M, Schwarz F, Wallmichrath J, Baumeister R, Frick A, Bartenstein P, et al. Chylothorax and chylous ascites. Clinical utility of planar scintigraphy and tomographic imaging with SPECT/CT. Nuklearmedizin. 2015;54:231–40. doi: 10.3413/Nukmed-0723-15-02. [DOI] [PubMed] [Google Scholar]
- 13.Turpin S, Lambert R. Lymphoscintigraphy of Chylous Anomalies: Chylothorax, chyloperitoneum, chyluria, and lymphangiomatosis – 15-year experience in a pediatric setting and review of the literature. J Nucl Med Technol. 2018;46:123–128. doi: 10.2967/jnmt.117.203281. [DOI] [PubMed] [Google Scholar]
- 14.Prevot N, Tiffet O, Avet J, Quak E, Decousus M, Dubois F. Lymphoscintigraphy and SPECT/CT using 99mTc filtered sulphur colloid in chylothorax. Eur J Nucl Med Mol Imaging. 2011;38:1746. doi: 10.1007/s00259-011-1793-1. [DOI] [PubMed] [Google Scholar]
- 15.Kotani K, Kawabe J, Higashiyama S, Shiomi S. Lymphoscintigraphy with single-photon emission computed tomography/computed tomography is useful for determining the site of chyle leakage after esophagectomy. Indian J Nucl Med. 2012;27:208–209. doi: 10.4103/0972-3919.112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Codreanu I, Zhuang H. Minimal lymphatic leakage in an infant with chylothorax detected by lymphoscintigraphy SPECT/CT. Pediatrics. 2014;134:e606–e610. doi: 10.1542/peds.2013-2689. [DOI] [PubMed] [Google Scholar]
- 17.Das J, Thambudorai R, Ray S. Lymphoscintigraphy combined with single-photon emission computed tomography-computed tomography (SPECT-CT): A very effective imaging approach for identification of the site of leak in postoperative chylothorax. Indian J Nucl Med. 2015;30:177–179. doi: 10.4103/0972-3919.152988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original raw data is available on reasonable request to the corresponding author.