Abstract
Introduction
Knee osteoarthritis (OA) is a common painful disorder. Intra-articular (IA) corticosteroid injections are frequently prescribed to treat knee pain. Lorecivivint (LOR), a novel IA cdc2-Like Kinase (CLK)/Dual-Specificity Tyrosine Phosphorylation-Regulated Kinase (DYRK) inhibitor thought to modulate Wnt and inflammatory pathways, has appeared safe and demonstrated improved patient-reported outcomes compared with placebo. While LOR is proposed for stand-alone use, in clinical practice, providers might administer LOR in close time proximity to IA corticosteroid. This open-label, parallel-arm, healthy volunteer study assessed potential short-term safety, tolerability and pharmacokinetic (PK) interactions between IA LOR and triamcinolone acetonide (TCA) administered 7 days apart.
Methods
Healthy volunteers were randomized to Treatment Sequence 1 (IA 40 mg TCA followed by IA 0.07 mg LOR) or Treatment Sequence 2 (IA 0.07 mg LOR followed by IA 40 mg TCA). Treatment-emergent adverse events (TEAEs) were categorized by “epoch”, with epoch 1 spanning from first until second injection, and epoch 2 spanning from second injection until end of study. Plasma PK was assessed pre injection and out to 22 days after to assess PK treatment interaction.
Results
A total of 18 TEAEs were reported by 11 (27.5%) of 40 enrolled participants, and there were no serious adverse events. Thirteen TEAEs were reported in Treatment Sequence 1 and five in Treatment Sequence 2, similarly distributed between epochs 1 and 2. In all participants and at all time points, plasma LOR concentrations were below the limit of quantification (0.100 ng/mL). Geometric mean concentrations and PK parameters for TCA were similar between treatment sequences.
Conclusion
No safety signals were observed. There were no quantifiable plasma concentrations of LOR in either Treatment Sequence. The PK of TCA was unaffected by previous LOR injection. These results suggest that IA administration of LOR and TCA in close time proximity is unlikely to pose a safety concern.
Trial Registration
ClinicalTrials.gov identifier, NCT04598542.
Supplementary Information
The online version supplementary material available at 10.1007/s40744-023-00604-7.
Keywords: Lorecivivint, Triamcinolone, Knee osteoarthritis, Knee pain, Intra-articular, Safety
Plain Language Summary
Knee osteoarthritis (OA) is a common disorder characterized by pain and loss of function. This clinical trial tested if two different treatments for OA injected into the same knee 1 week apart would impact the safety or exposure of either treatment. The treatments evaluated were an injection of a corticosteroid, triamcinolone acetonide, and a potential OA treatment in development, lorecivivint, a novel small molecule thought to inhibit inflammation and a biological pathway called the Wnt pathway. The amount of either treatment found in circulation was not different when injected before or after the other treatment. The order of injection did not change the safety profile for either agent, suggesting injection of the two agents 1 week apart is unlikely to pose a safety concern.
Supplementary Information
The online version supplementary material available at 10.1007/s40744-023-00604-7.
Key Summary Points
| Knee osteoarthritis (OA) is a common painful joint condition with high unmet need, often treated with intra-articular (IA) agents. |
| Lorecivivint (LOR) is an IA small molecule cdc2-Like Kinase (CLK)/Dual-Specificity Tyrosine Phosphorylation-Regulated Kinase (DYRK) inhibitor in development as a potential OA treatment. |
| This study evaluated if injection of LOR in close time proximity prior to or following IA corticosteroid (triamcinolone) would impact safety or pharmacokinetics of either agent in healthy volunteers. |
| These results suggest that IA administration of LOR and triamcinolone in close proximity (7 days apart) is unlikely to pose a safety concern. |
Introduction
Knee osteoarthritis (OA) is a common (US adult prevalence > 10%) painful joint disease that impairs function and reduces quality of life [1]. Limitations of available pharmacological OA treatments are evident by the differing recommendations from professional associations such as the Osteoarthritis Research Society International (OARSI), American College of Rheumatology (ACR), and American Academy of Orthopedic Surgeons (AAOS), with mixed recommendations for intra-articular (IA) hyaluronic acid and oral agents such as acetaminophen; IA corticosteroid injections are also recommended, and while usage is controversial because of concerns that repeat injections may be associated with greater OA progression, these injections remain a major therapeutic tool in patients with established OA [2]. The continued need for alternative safe and efficacious OA treatments remains high.
Lorecivivint (LOR) is a novel small-molecule inhibitor of cdc2-Like Kinases (CLKs) and Dual-Specificity Tyrosine Phosphorylation-Regulated Kinases (DYRKs), intra-nuclear kinases that regulate cellular gene expression, and are thought to modulate Wnt and inflammatory pathways. LOR is currently in phase 3 development as a potential IA disease-modifying therapy for knee OA. In a completed 24-week phase 2b knee OA clinical trial (NCT03122860), LOR appeared safe, and produced significant improvements from baseline in pain and function patient-reported outcomes compared with vehicle placebo (PBO) [3]. In a phase 1 study, IA LOR was undetectable by standard assay in plasma at doses up to 0.23 mg suggesting minimal systemic exposure following a single injection. Non-clinical studies of radiolabeled LOR suggest that LOR was detectable within Sprague–Dawley rat joints for up to 6 months following a single injection [4].
IA corticosteroid injections are frequently used to treat the pain of knee OA in clinical practice and have been widely studied. Although LOR is being developed as an IA monotherapy, if approved for clinical use, it is reasonable to assume that near-contemporaneous IA injections of LOR and corticosteroids would be a possibility in normal practice. While OA is rarely treated with two IA treatments at the same time (co-injected), some patients receive sequential treatments over the course of a month or two if additional pain relief is needed. One of the most frequently used corticosteroids is triamcinolone acetonide (TCA). Since both TCA and LOR could be present in the joint for extended periods of time after injection, it was deemed appropriate to study the safety and pharmacokinetic effects of both LOR followed by TCA and TCA followed by LOR. A 1-week timeframe between doses was chosen for this drug–drug interaction (DDI) study as a very conservative and clinically relevant dosing regimen to explore. To determine the potential effects on patient safety, this study analyzed safety and pharmacokinetic (PK) data in healthy volunteers who received same-knee IA injections of LOR and TCA 7 days apart.
Methods
Study Design
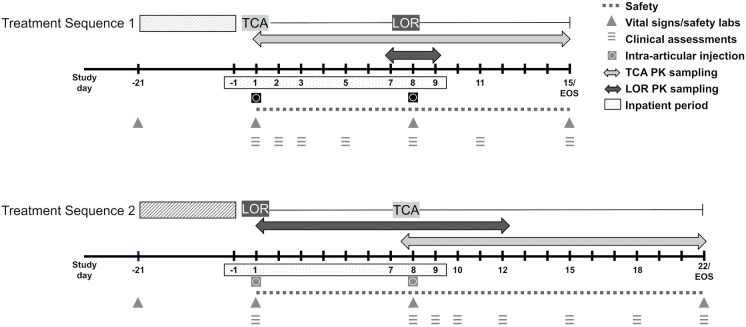
This was a single-site, randomized, open-label, parallel-arm study conducted from October 2020 to December 2020 (NCT04598542). Randomization occurred according to a permuted block design with block size of 4. Enrolled participants were healthy volunteers who were randomized to Treatment Sequence (TS) 1, a single IA injection into the knee of 40 mg TCA followed 7 days later by one IA 0.07 mg LOR injection in the same knee; or TS 2, a single IA injection of 0.07 mg LOR into the knee followed 7 days later by one IA 40 mg TCA injection (Fig. 1). The primary objectives of the study were to determine the safety and tolerability of an IA LOR injection when given in temporal proximity to TCA; the secondary objectives were to determine the PK of IA LOR after injection and to assess potential PK interactions between IA TCA and LOR. All studies were conducted in accordance with the Declaration of Helsinki and the International Conference for Harmonisation Good Clinical Practice Guidelines E6 [5]. Institutional review board approval was provided by Advarra (Columbia, MD) (approval date 7 October 2020, approval reference MOD00817729). Participants consented to the collection of de-identified data in support of this study, as well as the publication of any findings from the study to medical conferences and journals. All participants provided written informed consent prior to participating in any study-related procedures. Adverse event severity was classified using Clinical Data Interchange Standards Consortium (CDISC) with preferred terms assigned according to the Medical Dictionary for Regulatory Activities (MedDRA).
Fig. 1.
Overview of study design and assessments by treatment sequence. TCA triamcinolone acetonide, LOR lorecivivint, PK pharmacokinetics
Participants
Eligible participants were adults aged 18–55 years in generally good health with body mass index (BMI) ≤ 32 kg/m2. Exclusion criteria included any chronic medical condition, history of knee inflammatory disease, IA injections, or medical procedures; pregnancy, breastfeeding, and a refusal to use birth control if sexually active and of reproductive potential; use of recreational drugs or any medication besides occasional acetaminophen within 30 days of study day 1; history of psychiatric disorders; and active infection or a chronic infection that may compromise immune or liver function.
Study Protocol
All injections were performed on the right knee and were conducted according to the practitioner’s standard practice, which could include but did not require ultrasound guidance. Eligible participants checked into the clinic the evening before Day 1 (Day − 1) and remained in the clinic until Day 15 if in TS 1 or Day 22 if in TS 2. All participants had an end of study phone visit 14 days after leaving the clinic. The study was conducted at an inpatient phase 1 facility (Quotient Sciences, Miami, FL) (Fig. 1).
In TS 1, participants received IA TCA (40 mg) on Day 1 followed by IA LOR (0.07 mg) on Day 8 (Fig. 1). Additional study procedures and assessments were performed on Days 11 and 15, and the total study duration (including the screening period) for a given participant was up to 53 days. Blood was drawn for measurement of plasma TCA concentrations on Day 1 (before TCA dosing and 1, 2, 4, 6, 8, 10, and 12 h after TCA dosing), Day 2 (24 h after TCA dosing), Days 3, 5, and 8 (before LOR dosing and 1, 2, 4, 6, and 8 h after LOR dosing), Day 11, and Day 15. Blood was drawn for measurement of plasma LOR concentrations on Day 8 (before LOR dosing and 0.25, 0.5, 1, 2, 4, 6, and 8 h after LOR dosing).
In TS 2, participants received IA LOR (0.07 mg) on Day 1 followed by IA TCA (40 mg) on Day 8. Additional study procedures and assessments were performed on Days 10, 12, 15, 18, and 22, and total study duration for a given participant was up to 60 days. Blood was drawn for measurement of plasma LOR concentrations on Day 1 (before LOR dosing and 0.25, 0.5, 1, 2, 4, 6, and 8 h after LOR dosing), Day 8 (1, 2, 4, and 8 h after TCA dosing), Day 9 (24 h after TCA dosing), day 10, and Day 12. Blood was drawn for measurement of plasma TCA concentrations on Day 8 (before TCA dosing and 1, 2, 4, 6, 8, 10, and 12 h after TCA dosing), Day 9 (24 h after TCA dosing), and Days 10, 12, 15, 18, and 22.
Analyses
All participants underwent general medical evaluations including physical examinations, knee examinations, recording of vital signs, and clinical laboratory evaluations. Recording of adverse events (AEs) started following informed consent and continued at all subsequent visits until the participant completed the end of study phone visit. For each treatment sequence, treatment-emergent AEs (TEAEs) were categorized by “epoch”, with epoch 1 spanning the time from the first injection until the second injection, and epoch 2 spanning from the second injection until the end of the study. Relatedness of AEs was only assessed by investigators for relationship to LOR or LOR injection.
PK Assays
PK properties were analyzed by determining plasma LOR and TCA concentrations at the described time points (see “Study Protocol” section). Plasma concentrations for TCA were analyzed using validated methods at Covance, Inc, with a lower limit of quantification of 20.0 pg/mL. Plasma concentrations for LOR were analyzed using validated methods at Charles River Laboratories, with a lower limit of quantification of 0.100 ng/mL.
Statistics
All treated participants were included in all analyses. Baseline (prior to the first study injection) characteristics included age, sex, ethnicity, race, height, weight, and BMI (Table 1). When PK statistics were calculated for TCA, values below the lower limit of quantification (LLOQ) of 20.0 pg/mL were set to 1/2 LLOQ. For TCA, median value for Tmax (time taken to reach the maximum concentration) and geometric mean values for half-life, Cmax (maximum serum concentration), and area under the concentration curve (AUC) from baseline to 168 h post injection (AUC0–168) and AUC from baseline to infinity (AUC0–inf) were determined. In order to establish equivalence and no interaction between TCA and LOR on drug availability, two one-sided t tests (TOSTs) were used to compare the ratios of the geometric means for both AUC and Cmax between the treatment sequences. The results of the PK TOSTs were reported as ratios and combined 90% confidence intervals.
Table 1.
Participant characteristics
| Treatment sequence | |||
|---|---|---|---|
| TCA + LOR | LOR + TCA | Total | |
| N | 20 | 20 | 40 |
| Age (years)* | 41.8 (6.9) | 40.8 (7.7) | 41.3 (7.2) |
| Race [n (%)] | |||
| Black/African American | 4 (20%) | 4 (20%) | 8 (20%) |
| White | 16 (80%) | 16 (80%) | 32 (80%) |
| Sex [n (%)] | |||
| Female | 9 (45%) | 7 (35%) | 16 (40%) |
| Male | 11 (55%) | 13 (65%) | 24 (60%) |
| Body mass index (kg/m2)* | 27.36 (2.99) | 28.25 (2.97) | 27.80 (2.98) |
*Mean (SD) presented
TCA triamcinolone acetonide, LOR lorecivivint
Results
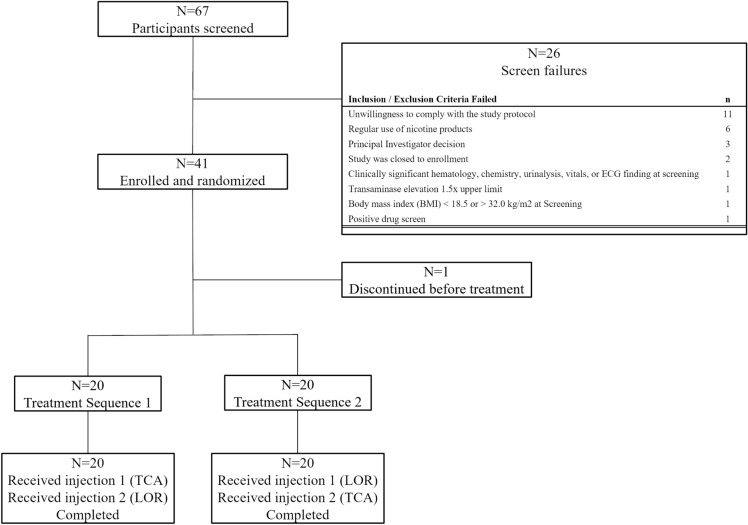
There were 67 participants screened, of whom 41 were randomized to TS 1 or TS 2 (n = 20 per arm; 1 randomized participant discontinued before treatment; Fig. 2). All dosed participants completed all PK measurements. Participant characteristics were similar between treatment sequences (Table 2).
Fig. 2.
Participant disposition. TCA triamcinolone acetonide, LOR lorecivivint
Table 2.
Treatment-emergent adverse events (TEAEs) by preferred term in the safety analysis set
| Subdivision of study timeline (epoch) | Treatment sequence 1 (N = 20) | Treatment sequence 2 (N = 20) | ||
|---|---|---|---|---|
| Epoch 1 (TCA) | Epoch 2 (TCA + LOR) | Epoch 1 (LOR) | Epoch 2 (LOR + TCA) | |
| Injection site bruising | 4/4 (20%) | 0/0 | 1/1 (5%) | 2/2 (10%) |
| Injection site pain | 0/0 | 2/1 (5%) | 0/0 | 0/0 |
| Back pain | 1/1 (5%) | 0/0 | 0/0 | 0/0 |
| Flank pain | 0/0 | 1/1 (5%) | 0/0 | 0/0 |
| Musculoskeletal discomfort | 0/0 | 1/1 (5%) | 0/0 | 0/0 |
| Extremity pain | 0/0 | 1/1 (5%) | 0/0 | 0/0 |
| Headache | 0/0 | 1/1 (5%) | 1/1 (5%) | 0/0 |
| Hypersensitivity | 0/0 | 0/0 | 0/0 | 1/1 (5%) |
| Skin abrasion | 0/0 | 1/1 (5%) | 0/0 | 0/0 |
| Adnexa uteri pain | 0/0 | 1/1 (5%) | 0/0 | 0/0 |
| Total TEAEs [events/n (%)] | 5/4 (20%) | 8/3 (15%) | 2/2 (10%) | 3/3 (15%) |
TCA triamcinolone acetonide, LOR lorecivivint, TEAEs treatment-emergent adverse events
Safety
A total of 18 TEAEs were reported by 11 (27.5%) participants out of 40 during the study; 7 (35.0%) participants in TS 1 and 4 (20.0%) participants in TS2, summarized in Table 2. The incidence of related TEAEs and related TEAEs at the injected knee were similar between treatment sequences. No severe TEAEs, serious TEAEs, or TEAEs leading to death occurred during the study. All TEAEs were mild in severity, except one moderate TEAE of hypersensitivity (environmental allergies) reported by one participant in TS 2 epoch 2 on Day 15 that resolved on Day 18. The event was not considered related to LOR. Injection site bruising was the most frequently reported TEAE overall, with four such TEAEs reported by 4 (20.0%) participants in TS 1 (all occurred in epoch 1, after the TCA injection) and three such TEAEs reported by 3 (15.0%) participants in TS 2 (one event in epoch 1 after the LOR injection and two events in epoch 2 after the TCA injection). All TEAEs resolved within a few days and with minimal or no medical intervention or supportive care measures.
Relatedness was only assessed by investigators for relationship to LOR or LOR injection. No relatedness assessments were made relative to TCA injections. A total of five TEAEs reported by 4 (10.0%) participants were assessed as related to LOR injection by the investigator; three TEAEs in TS 1 (TCA + LOR) and two TEAEs in TS 2 (LOR + TCA). Three of the five related TEAEs occurred at the injected knee and two were generalized mild headache. The knee-related events considered related to LOR included two TEAEs of injection site pain reported during epoch 2 by one participant in TS 1 and one TEAE of injection site bruising reported during epoch 1 by a participant in TS 2. There were no clinically significant changes in clinical laboratory evaluations (hematology, clinical chemistry, or urinalysis), vital signs, or physical and knee examinations.
When LOR administered after TCA was compared with LOR administered to TCA-naïve participants, there were more TEAEs reported by participants in which LOR was administered after TCA (eight TEAEs in TS 1 epoch 2 versus two TEAEs in TS 2 epoch 1) although those differences did not appear to suggest a specific tolerability or safety interaction.
PK
There were no quantifiable plasma concentrations of LOR detected in either treatment sequence following IA injection. Plasma LOR concentrations were below the limit of quantification (0.100 ng/mL) for all participants in both treatment sequences at all time points.
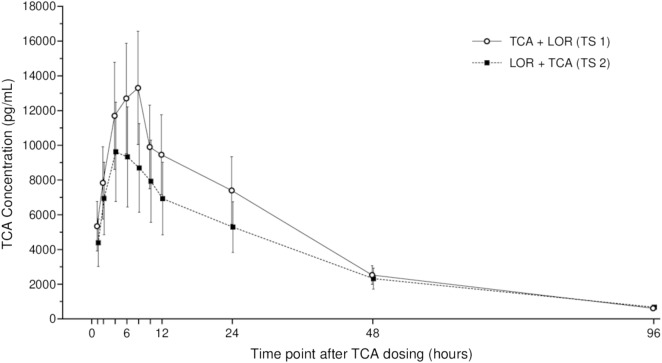
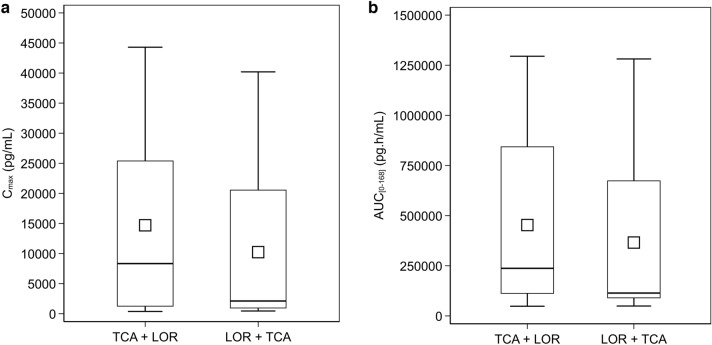
The plasma TCA concentration profiles (logarithmic scale) for both treatment sequences are presented in Fig. 3 with summary PK parameters in Table 3. The two treatment sequences had similar PK profiles in terms of concentration/shape, and both demonstrated high inter-subject variability consistent with previous reports of IA TCA [6]. The 90% CIs of the ratios of Cmax and AUC0–168 both contain 100% indicating no statistical differences between TCA administered 7 days after LOR (TS 2) compared to when TCA was administered without prior LOR exposure (Fig. 4, Table 4).
Fig. 3.
Plasma concentrations of TCA. All subjects received both study injections, TCA and LOR, in a randomized manner in either TS 1 (TCA then LOR) or TS 2 (LOR then TCA). Data are geometric mean ± geometric standard deviation (SD). Summary statistics were not calculated at any pre-dose time point. For all other summary statistics, values reported as < 20.0 were set to 1/2 × LLOQ (LLOQ = 20.0 pg/mL). TCA triamcinolone acetonide, LOR lorecivivint, LLOQ lower limit of quantification, TS treatment sequence
Table 3.
Plasma pharmacokinetics (PK) parameters of triamcinolone acetonide (TCA) (PKAS)
| Parameter | Statistic | Treatment sequence | |
|---|---|---|---|
| TCA + LOR (N = 20) | LOR + TCA (N = 20) | ||
| Tmax (h) | Median | 8.00 | 8.00 |
| Range | 1.00–336.00 | 1.00–48.00 | |
| Tlast (h) | Median | 336 | 336 |
| Range | 176–336 | 168–336 | |
| Cmax (pg/mL) | Geo mean (Geo CV%) | 6250 (318.3) | 3510 (333.7) |
| Range | 374–44,300 | 454–40,200 | |
| AUC0–168 (pg × h/mL) | Geo mean (Geo CV%) | 266,000 (155.6) | 201,000 (154.3) |
| Range | 48,100–1,290,000 | 49,200–1,280,000 | |
| AUC0–last (pg × h/mL) | Geo mean (Geo CV%) | 353,000 (108.0) | 281,000 (107.7) |
| Range | 104,000–1,300,000 | 89,500–1,330,000 | |
| AUC0–inf (pg × h/mL) | n | 7 | 5 |
| Geo mean (Geo CV%) | 915,000 (46.0) | 960,000 (28.4) | |
| AUCextrap (%) | n | 7 | 5 |
| Geo mean (Geo CV%) | 0.51 (780.2) | 0.19 (68.1) | |
| t1/2 (h) | n | 7 | 7 |
| Geo mean (Geo CV%) | 48.54 (122.3) | 53.99 (157.7) | |
LOR lorecivivint
Fig. 4.
Boxplots of individual treatment values by treatment sequence. a Cmax (PKAS). b AUC0–168 (PKAS). All subjects received both study injections, TCA and LOR, in a randomized manner in either Treatment Sequence 1 (TCA then LOR) or Treatment Sequence 2 (LOR then TCA). The boxplots display the minimum, 25th percentile, mean, median (50th percentile), 75th percentile, and maximum observations. The mean is represented by the square and the median is represented by the horizontal line within the box. TCA triamcinolone acetonide, LOR lorecivivint
Table 4.
Plasma pharmacokinetic (PK) parameters of triamcinolone acetonide (TCA): assessment of drug interaction (PKAS)
| Parameter | TS 2 LOR + TCA |
TS 1 TCA + LOR |
Ratio (%)b | 90% CI (%)c | P valued | ||
|---|---|---|---|---|---|---|---|
| n | Adj geo meana | n | Adj geo meana | ||||
| Cmax (pg/mL) | 20 | 3510 | 20 | 6250 | 56.12 | (24.35, 129.36) | 0.76 |
| AUC0–168 (pg × h/mL) | 20 | 201,000 | 20 | 266,000 | 75.49 | (41.85, 136.15) | 0.57 |
Results obtained from a mixed effect model of natural log transformed PK parameters including a term for sequence fitted as a fixed effect
TA triamcinolone acetonide, LOR lorecivivint, CI confidence interval, TS treatment sequence
aAdj geo mean = adjusted geometric mean from model
bRatio of adjusted geometric means with comparison presented as Treatment Sequence 2/Treatment Sequence 1
cCI for ratio of adjusted geometric means
dP value from two one-sided test [null hypothesis of nonequivalence] with the largest P value from the two one-sided tests presented
Discussion
This open-label, randomized parallel-arm study demonstrated that IA LOR administration appeared safe and well tolerated in generally healthy individuals when occurring as close as 1 week before or after an IA injection of TCA in the same knee. Treatment sequence was randomized to reduce allocation bias. An open-label design was chosen, in favor over a double-blinded two-period crossover design, which would have extended the in-patient duration for subjects and required additional injections. As all subjects received both treatments and PK is an objective endpoint, an open-label design was considered adequate to provide the optimal balance between unbiased outcomes and patient burden.
The safety profiles of both treatment sequences were comparable, there were no TEAEs indicative of a DDI, and there were no AEs reported in the time post enrollment and pre- first study injection. The incidence of related TEAEs was similar between treatment sequences. While there were more TEAEs when comparing LOR administered after TCA with LOR administered to TCA-naïve participants, many of these had alternative etiologies, such as poor mattress/bed quality, small cut while shaving, and ovarian cyst/history of adnexa uteri pain.
AEs related to the injection procedure were the most common TEAEs, with injection site bruising as the most reported TEAE overall. This study did not characterize injection events as related to TCA, although these would have been captured in a blinded trial. In aggregate, the injection-related AEs add to the body of knowledge regarding the tolerability of IA injections, independent of the injected agent.
LOR is in development as a stand-alone treatment for knee OA, with expected analgesic effects to last between 6 and 12 months based upon previous clinical data [3]. However, as IA corticosteroids are a commonly used treatment in clinical practice, it is possible that LOR and IA corticosteroids may be administered in close temporal proximity to each other. Radiolabeled animal studies indicate that LOR has a long residence time in the joint (up to 6 months) [7] and thus could potentially affect the safety and/or PK of subsequent or prior IA injections. Similar to previous results, LOR was not detectable systemically at any time point, and the PK of TCA was unchanged in relationship to LOR injection. As the PK and the safety profile of LOR and TCA were unchanged when both injections occurred within 1 week, these data support a margin of safety for use of these agents in clinical practice and are not indicative of DDIs (Tables 2, 3; Fig. 3).
To our knowledge, this is the first paper to investigate the potential of knee IA DDIs. Injections in close time proximity are frequently trial exclusion criteria and thus have not been studied in controlled trials despite the likelihood that these injections may occur in clinical practice. As safety concerns restrict the frequent use of most injectable OA therapeutic agents (e.g., corticosteroids), it is important to understand the safety of using different IA treatments in close temporal proximity.
There remains a tremendous need for additional OA therapeutics. IA corticosteroids are commonly used in clinical practice despite providing only moderate acute pain relief for a chronic condition. The risk–benefit of IA corticosteroids must be carefully evaluated, as concerns ranging from local effects such as accelerating OA structural progression, subchondral insufficiency fractures, and osteonecrosis have been reported [8], while systemic effects such as elevated circulating glucose levels are also well known [9]. The LOR clinical trial safety profile thus far has appeared similar to placebo arms, supporting its potential value in addressing the need for safe OA therapeutics [3, 4, 10].
This phase 1 study has several limitations. First, this study was conducted in healthy volunteers, and it is possible the PK and safety profile might be altered in an osteoarthritic knee joint based upon changes in inflammation-driven permeability, coupled with tissue and structural degeneration. Progression of these OA changes potentially complicates drug pharmacokinetics by affecting whole joint drug retention and targeting of individual joint tissues. In the healthy joint, molecules are generally cleared via capillaries and lymphatics. Both of these networks underlie the joint’s surrounding synovium within bone. However, in progressive OA, the increased presence of immune, fibrotic, and inflammatory cells, in and around the synovium, with accompanying destructive structural cartilage changes has potential to alter these usual mechanisms [11, 12]. To date research into the extent of OA pathology affecting drug clearance and targeting mechanisms remains sparse. Further, there may be differences in injection procedures between healthy and osteoarthritic joints that potentially affect injection tolerability. This study did not require ultrasound guidance for injections and thus it is possible that some injections were not delivered into the IA space. It is also possible that assessment of synovial fluid would provide additional or differential information regarding an interaction between LOR and TCA. Finally, the design of this study (injections 1 week apart vs co-injection) may be considered unconventional to examine DDIs, but this was done with clinical practice algorithms in mind. Co-injecting two separate agents for the treatment of OA is never recommended given constraints/differences in formulation pH, volume, and time–action profiles. Rather, treatment typically consists of trying one agent and then following with another several weeks to months later if symptoms persist. Our study was conducted to provide support for the safety of such an approach with LOR and provides a roadmap for how other IA DDI studies may be conducted.
Conclusion
Data from this study data indicated that plasma concentrations of LOR remained below the threshold of assay detection following IA injection, regardless of injection sequence. LOR did not appear to alter the peak exposure, total exposure, or PK variability of TCA when administered 7 days before TCA (TS 2) compared to TCA administered without prior LOR exposure (TS 1). The observed safety profiles of TS 1 and TS 2 were comparable regardless of the sequence of TCA and LOR injections, suggesting no knee-related or systemic adverse interactions between TCA and LOR administered in the joint 7 days apart.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge and thank the study participants, investigators, and associated research professionals who contributed their time and efforts to this trial. Additionally, the authors thank Amy Halseth, PhD (former employee of Biosplice Therapeutics Inc.), and Emily Solomon BSN, RN (employee of Biosplice Therapeutics Inc.) for their critical insights into the conduct of this trial.
Medical Writing/Editorial Assistance
Humza Ullah, Pharmacy Student, University of Arizona, provided editorial assistance (internship, no funding).
Author Contributions
Study design and methodology: Mark Fineman, PhD, Christopher J. Swearingen, PhD, Ismail Simsek, MD, Jeyanesh R. S. Tambiah, MD, Yusuf Yazici, MD; analysis design and implementation: Christopher J. Swearingen, PhD, Victor A. Lopez, MS; writing, original draft preparation: Sarah Kennedy, PhD; writing, review and editing: Mark Fineman, PhD, Timothy McAlindon, MD, Christian Lattermann, MD, Christopher J. Swearingen, PhD, Sarah Kennedy, PhD, Victor A. Lopez, MS, Ismail Simsek, MD, Jeyanesh R. S. Tambiah, MD, Yusuf Yazici, MD.
Funding
The trial described in this report, the preparation of this manuscript, and the journal’s Rapid Service Fee, were funded by Biosplice Therapeutics, Inc.
Data Availability
All data generated or analyzed during this study are included in this published article; subject-level PK data are included as a supplementary information file.
Declarations
Conflict of Interest
Mark Fineman, PhD, Christopher J. Swearingen, PhD, Sarah Kennedy, PhD, Victor A. Lopez, MS, Ismail Simsek, MD, Jeyanesh R.S. Tambiah, MD, and Yusuf Yazici, MD are current or past employees and shareholders of Biosplice Therapeutics. Timothy McAlindon, MD, MPH, is a consultant and investigator for Biosplice Therapeutics, a consultant to Kolon TissueGene, Organogenesis, Remedium-Bio, Medipost, ChemoCentryx, and Xalud, with business interest in Ambulomics, Inc. Christian Lattermann, MD is a consultant for Biopslice Therapeutics and a consultant to Organogenesis, Aesculap and Vericel, Scientifc Board Member of On-Foundation.
Ethical Approval
The research within this clinical trial was conducted in accordance with the Declaration of Helsinki, the International Council for Harmonisation Good Clinical Practice Guidelines, and all applicable regulations. The trial was conducted under institutional review board approval by Advarra (Columbia, MD) (approval date 7 October 2020, approval reference MOD00817729). All participants provided written informed consent prior to engaging in any study-related procedures.
References
- 1.Wallace IJ, Worthington S, Felson DT, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci U S A. 2017;114:9332–9336. doi: 10.1073/pnas.1703856114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richard MJ, Driban JB, McAlindon TE. Pharmaceutical treatment of osteoarthritis. Osteoarthr Cartil. 2023;31:458–466. doi: 10.1016/j.joca.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Yazici Y, McAlindon TE, Gibofsky A, et al. A phase 2b randomized trial of lorecivivint, a novel intra-articular CLK2/DYRK1A inhibitor and Wnt pathway modulator for knee osteoarthritis. Osteoarthr Cartil. 2021;29:654–666. doi: 10.1016/j.joca.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Yazici Y, McAlindon TE, Fleischmann R, et al. A novel Wnt pathway inhibitor, SM04690, for the treatment of moderate to severe osteoarthritis of the knee: results of a 24-week, randomized, controlled, phase 1 study. Osteoarthr Cartil. 2017;25:1598–1606. doi: 10.1016/j.joca.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 5.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Integrated addendum to ICH E6(R1): guideline for good clinical practice E6(R2). Federal Register Vol. 83, No. 41, p. 8882–3; 2016. https://www.ich.org/. Accessed 15 June 2023.
- 6.Kraus VB, Conaghan PG, Aazami HA, et al. Synovial and systemic pharmacokinetics (PK) of triamcinolone acetonide (TA) following intra-articular (IA) injection of an extended-release microsphere-based formulation (FX006) or standard crystalline suspension in patients with knee osteoarthritis (OA) Osteoarthr Cartil. 2018;26:34–42. doi: 10.1016/j.joca.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Deshmukh V, Hu H, Barroga C, et al. A small-molecule inhibitor of the Wnt pathway (SM04690) as a potential disease modifying agent for the treatment of osteoarthritis of the knee. Osteoarthr Cartil. 2018;26:18–27. doi: 10.1016/j.joca.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Ayub S, Kaur J, Hui M, et al. Efficacy and safety of multiple intra-articular corticosteroid injections for osteoarthritis—a systematic review and meta-analysis of randomized controlled trials and observational studies. Rheumatology (Bulgaria) 2021;60:1629–1639. doi: 10.1093/rheumatology/keaa808. [DOI] [PubMed] [Google Scholar]
- 9.Patel J, Schneider BJ, Smith CC. Fact finders for patient safety: intra-articular corticosteroid injections and hyperglycemia. Pain Med. 2018;19:1091–1092. doi: 10.1093/pm/pnx303. [DOI] [PubMed] [Google Scholar]
- 10.Yazici Y, McAlindon TE, Gibofsky A, et al. Lorecivivint, a novel intraarticular CDC-like kinase 2 and dual-specificity tyrosine phosphorylation-regulated kinase 1A inhibitor and Wnt pathway modulator for the treatment of knee osteoarthritis: a phase II randomized trial. Arthritis Rheumatol. 2020;72:1694–1706. doi: 10.1002/art.41315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao Y, Ma Y, Tao Y, Lin W, Wang P. Intra-articular drug delivery for osteoarthritis treatment. Pharmaceutics. 2021;13:2166. doi: 10.3390/pharmaceutics13122166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown S, Kumar S, Sharma B. Intra-articular targeting of nanomaterials for the treatment of osteoarthritis. Acta Biomater. 2019;93:239–257. doi: 10.1016/j.actbio.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article; subject-level PK data are included as a supplementary information file.