Abstract
Introduction
Baricitinib, an orally available small-molecule inhibitor of Janus kinase (JAK)1 and JAK2, is indicated to treat active moderate-to-severe rheumatoid arthritis (RA).
Objective
This systematic review described the real-world clinical characteristics of baricitinib-treated patients with RA, prescription patterns, effectiveness, drug persistence, patient-reported outcomes (PROs; physical function, pain, health-related quality of life [HRQoL]), patient global assessment (PGA), and safety of baricitinib.
Methods
A PRISMA systematic review of real-world studies was conducted to identify relevant literature published between January 2016 and September 2022 using MEDLINE®, EMBASE®, and evidence-based medicine review databases. Websites or online repositories of the American College of Rheumatology and the European Alliance of Associations for Rheumatology were searched manually to include relevant abstracts from conferences held between January 2016 and November 2022.
Results
A total of 11,472 records were identified by searching online databases. Seventy studies were included in the study, of which 40 were abstracts. Most patients were older (51–71 years), female, and with mean RA duration of 4–19 years. Baricitinib was mostly used after the failure of one or more bDMARDs, and 4 mg dosing was prevalent in patients with RA (range 22–100%). Clinical effectiveness of baricitinib was reported in real-world settings regardless of prior biologic/targeted synthetic disease-modifying antirheumatic drug (DMARD) use and concomitant conventional synthetic DMARD use. Achievement of Clinical Disease Activity Index (CDAI) remission was reported in 8.7–60% of patients at week 12 and CDAI low disease activity (LDA) in 20.2–81.6% at week 24. The proportion of patients attaining Simple Disease Activity Index (SDAI) remission was reported in 12% at week 4 to 45.4% at 24 weeks. Drug persistence was high, similar, or equal to anti-tumor necrosis factor drugs. No new safety signals were identified.
Conclusion
Baricitinib demonstrated effectiveness in the real-world setting with a consistent safety profile observed in clinical studies. Better persistence rates for baricitinib compared to bDMARDs with improvement in PROs were reported, although baricitinib-treated patients had RA with poor prognostic characteristics.
Keywords: Baricitinib, Real-world evidence, Janus kinase inhibitors, Rheumatoid arthritis
Key Summary Points
| Baricitinib received European Medicines Agency and Food and Drug Administration approval in February 2017 and in May 2018, respectively, for the treatment of rheumatoid arthritis (RA). |
| Comprehensive data on baricitinib survival, safety, effectiveness, and patient-reported outcomes (PROs) in patients with RA are lacking. |
| This systematic review of literature provides real-world evidence in baricitinib-treated patients with moderate-to-severe active RA, specifically on patient characteristics, treatment patterns, clinical effectiveness, drug survival, PROs, and safety related to baricitinib therapy in RA. |
| Baricitinib demonstrated effectiveness in the real-world setting with a consistent safety profile observed in clinical studies. |
| Discontinuation rates of baricitinib were lower than for biologic disease-modifying antirheumatic drugs (DMARDs) and similar between patients on baricitinib monotherapy and in combination with conventional synthetic DMARDs. |
Introduction
Janus kinase (JAK) inhibitors are the targeted synthetic disease-modifying antirheumatic drugs (tsDMARDs) that expanded the therapeutic landscape of rheumatoid arthritis (RA) [1]. JAK inhibitors enter cellular cytoplasm and reversibly block JAKs and signal transduction for several proinflammatory cytokines and growth factors [2]. JAK inhibitors offer a targeted oral treatment option with quick onset of action and higher efficacy than the gold standard of care—tumor necrosis factor (TNF) inhibitors [3–5]. JAK inhibitors are recommended by the American College of Rheumatology (ACR) 2021 and the European Alliance of Associations for Rheumatology (EULAR) 2022 in the treatment algorithm for patients with inadequate response or intolerant to methotrexate monotherapy (MTX-IR) [6, 7].
Baricitinib, an oral selective JAK1 and JAK2 inhibitor with less affinity for JAK3 and tyrosine kinase 2, received European Medicines Agency (EMA) approval in February 2017 and Food and Drug Administration (FDA) approval in May 2018 for the treatment of RA [8, 9]. Clinical trials have evaluated the efficacy and safety of baricitinib in monotherapy and in combination with methotrexate (MTX) in patients with active RA [10, 11]. In addition, in a 52-week, phase 3, double-blind, placebo- and active-controlled study including MTX-IR patients with active RA on the background of MTX, baricitinib was associated with significant clinical improvements compared with placebo (ACR50 response at week 12: 45% vs. 17%; ACR70: 19% vs. 5%; P ≤ 0.001) and adalimumab (ACR50 response at week 12: 45% vs. 35%, P ≤ 0.01; ACR70: 19% vs. 13%; P ≤ 0.05). In addition, baricitinib was superior to adalimumab according to the mean change in 28-joint count Disease Activity Score (DAS28) using C-reactive protein (CRP) at week 12 (− 2.24 for baricitinib vs. − 1.95 for adalimumab; P < 0.001) [4]. The importance of real-world evidence to complement such clinical study data is recognized for informing routine clinical practice.
Therefore, we conducted a systematic literature review (SLR) that focuses on real-world studies of baricitinib in patients with moderate-to-severe active RA. The aim of this review was to describe the clinical characteristics of the patients, treatment pattern, effectiveness, drug survival and persistence, and safety of baricitinib as monotherapy and combination therapy in a real-world setting. Data on patient-reported outcomes (PROs; physical function, pain, health-related quality of life [HRQoL]), patient global assessment (PGA), treatment satisfaction, x-ray, ultrasound, and magnetic resonance imaging (MRI) outcomes were also reported.
Methods
Data Sources and Search Strategy
This SLR was performed according to guidance issued by the Centre for Reviews and Dissemination and the Cochrane Collaboration [12, 13]. An English language search of the MEDLINE®, EMBASE®, and evidence-based medicine reviews databases was conducted to identify relevant literature (full-text and abstracts) published between January 2016 and September 2021. The literature search was updated following the same strategy (September 2021–2022) to ensure that new evidence is incorporated into the findings. The OVID® platform was used to perform the searches using a combination of free text and controlled vocabulary terms for the disease and study designs. Validated search filters of the Scottish Intercollegiate Guidelines Network [14] were modified and adapted for the searches. In addition, websites or online repositories of the ACR and the EULAR were searched manually to include relevant abstracts from conferences held between January 2016 and November 2022.
Eligibility Criteria
The eligibility of studies was based on the patients, interventions, comparators, outcomes, and study design criteria. Full-text observational (prospective, retrospective, case-control, and cross-sectional) and pragmatic studies reporting outcomes using baricitinib in patients aged ≥ 18 years with moderate-to-severe active RA were included. Studies with mixed populations were included if the proportion of adult patients was ≥ 80%.
Duplicate and non-English articles were excluded. Furthermore, all clinical studies, including pragmatic, practical, or naturalistic studies; case studies or case series; protocols; commentaries; editorials; and letters were excluded. Studies were excluded where the authors aimed to validate the translation of an instrument or developed a new instrument and tested it.
Study Selection and Data Extraction
Citations, titles, and abstracts were exported into DistillerSR for screening. The studies identified for potential inclusion were screened by a single reviewer (SG), and 10% of the studies at each selection stage were referred to a second reviewer (MK) for quality check. Any discrepancies were resolved via mutual consensus. Data extraction was performed by a single reviewer (SG) using Microsoft® Excel. The extracted data were cross-checked by the second reviewer (MK), and any disputes were discussed and resolved. Data extractors were not blinded to any study information.
Major Outcomes
Outcome of interest included clinical effectiveness, safety, medication adherence, persistence, discontinuation, and switching. In addition, PROs including functional disability, pain, PGA, HRQoL, and treatment satisfaction were also of interest.
The specific efficacy outcomes of interest were ACR/EULAR remission. Index-based ACR/EULAR remission was defined as Clinical Disease Activity Index [CDAI] score ≤ 2.8; Simple Disease Activity Index [SDAI] score ≤ 3.3; and DAS28 < 2.6. Boolean-based ACR/EULAR remission was defined as tender joint count [TJC] ≤ 1, swollen joint count [SJC] ≤ 1, CRP ≤ 1 mg/dl, and PGA ≤ 1 [on a 0–10 scale] at any point) [15].
Additionally, outcomes measuring morning joint stiffness and/or joint pain (severity and duration), tiredness or fatigue, and radiographic measures of improvement in joint inflammation were also considered. Image measures included synovitis, bone erosion, joint effusion, and tenosynovitis detected by plain radiography, ultrasonography, and MRI.
Risk of Bias Assessment
The risk of bias in this SLR was assessed using a modified version of the Newcastle-Ottawa Scale [16], as per the recommendations of the Cochrane Collaboration [12]. This instrument appraises the quality of non-randomized studies based on eight domains of assessment.
For both single-arm and comparator studies, bias assessment was conducted based on the representativeness of the exposed cohort, ascertainment of the exposure, assessment of the outcome, and follow-up. The follow-up period was assessed based on whether the length of follow-up was adequate for outcomes to occur and if the follow-up of cohorts was adequate. In addition to these criteria, the comparability of cohorts was assessed based on the design or analysis, wherever applicable.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new data with human participants or animals performed by any of the authors.
Results
Search Results
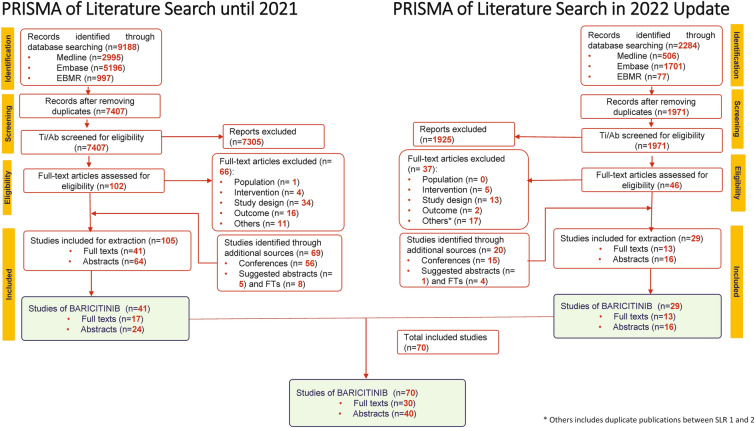
Initial literature search was conducted between January 2016 and September 22, 2021. A total of 9188 records were identified by searching online databases. After removing duplicates, 7407 potentially relevant records were identified and screened based on titles and abstracts. Of 7407 studies, 102 full-text articles were selected. Of these, 36 were included based on the inclusion criteria. In addition, 69 studies from additional sources (56 abstracts from ACR and the EULAR, published between 2016 and 2021, and 13 studies suggested by peer reviewers) were included. Of the final 105 studies (41 full-text articles and 64 conference abstracts), 41 studies (17 full-text articles and 24 abstracts) on baricitinib were included in the study (Fig. 1).
Fig. 1.
PRISMA flow diagram. AB/FT abstract/full text, EBMR evidence-based medicine, EULAR European Alliance of Associations for Rheumatology, Embase Excerpta Medica database, FT full-text article, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-analyses, Ti/Ab title/abstract
The updated search resulted in 2284 records. Of these, 13 full-text articles published between September 2021 and September 2022 and 16 abstracts published between September 2021 and November 2022 were included. Overall, this SLR inluded 70 articles on baricitinib, of which 30 were available as full texts and 40 as abstracts (Fig. 1).
Characteristics of Population and Studies
The main characteristics of 70 included studies are summarized in Table 1. A total of 40 studies included both biologic disease-modifying antirheumatic drug (bDMARD)-naïve and -experienced patients. Fifteen studies included bDMARD-experienced patients. Twelve studies did not report treatment status of the patients (bDMARD-naïve or experienced).
Table 1.
Patient and study characteristics
| Author (year) | Number of patients receiving baricitinib | Age (mean ± SD or range), years | Female sex, % | Disease duration (months/years) | bDMARDs exp (1/2/3/4/ ≥ 5), n (%)a | Dose | Concomitant medication, n (%) | Prior tsDMARDs, n (%)a | |
|---|---|---|---|---|---|---|---|---|---|
| MTX | Other csDMARDs or steroids | ||||||||
| Naïve, n (%)a | |||||||||
| Full-text articles | |||||||||
| Miyazaki et al. (2021) [17] | 141 | 58.2 (13.3) | 81.6 | 122.3 (120.0) months | bDMARDs exp: 38/24/20/9/10 | 2/4 mg QD | NR | NR | NR |
| Naïve: 40 (28.4) | |||||||||
| Kim et al. (2021) [18] | 20 | 53.5 (47.5–61.3) | 75 | NR | bDMARDs exp: 75 | 4 mg QD | NR | NR | NR |
| Naïve: 25 | |||||||||
| Iwamoto et al. (2021) [19] | 81 | 66 (56–74) | 84 | NR | 2.00 [1.00–3.00] | 2 mg/4 mg QD | 37 (45.7) | Oral GCC: 38 (46.9) | 2.00 [1.00–3.00] |
| Naïve: 18 (22.2) | |||||||||
| Asai et al. (2021) [20] | 48 | 61 (14) | 79 | 9 (8) years | 60 | NR | 63 | GCC: 52 | NR |
| Guidelli et al. (2021) [21] | 446 | Naïve: 56 (49–66) | 81 | NR | Naïve: 150 (34) | 4 mg QD | NR | Oral GCC: 109 (72.7) | NR |
| exp: 60 (53–68) | bDMARDs exp: 296 (66) | Oral GCC: 218 (73.6) | NR | ||||||
| Ebina et al. (2021) [22] | 166 | 60.2 (13.5) | 86.7 | 12.6 (10.6) years |
2/ ≥ 3 bDMARDs exp: 23.5/54.2 TNFi: 57.8 Anti-IL-6R: 36.1 Abatacept: 31.9 Naïve: 22.3 |
NR | 64.5 |
PSL: 42.8 SASP: 11.4 |
Prior JAKi: 20.5 2nd/ ≥ 3rd JAKi: 23.5, 54.2 |
| Retuerto et al. (2021) [23] | 15 | 62 (51–67) | 80.6 | NR |
bDMARDs exp: 87 Anti-TNF: 77.4 Non-anti-TNF: 70.9 |
2 mg/4 mg QD | 54.9 |
PSL: 84% Leflunomide: 16.1 |
NR |
| Spinelli et al. (2020) [24] | 59 | NR | NR | NR | bDMARDs exp 1/2/3/ ≥ 4: 12 (20.3)/16 (27.1)/3 (5.1)/19 (32.1) | 4 mg QD | 26 (83.9%) |
PSL: 54.2 HCQ: 19.4 Leflunomide: 6.5 SSZ: 9.7 |
NR |
| Naïve: 9 (15.3) | |||||||||
| Gonzalez-Freire et al. (2021) [25] | 20 | 61 (41–79) | 70 | NR | bDMARDs exp: 15 (75) | 4 mg BID | NR | NR | NR |
| Sagdeo et al. (2020) [26] | 37 | < 40–80 median (IQR) | 81 | Median IQR: 5 to > 10 years | All other patients had been previously exposed to one or more csDMARDs or bDMARDs | 4 mg QD | NR | NR | NR |
| Tesei et al. (2021) [27] | 43 | 56.09 (11.15) | 86.05 | 150.91 (120.17) Months | bDMARDs exp 1/2/3 ≥ 4: 12 (27.91), 6 (13.95), 10 (23.26), 15 (34.88) | 4 mg QD |
csDMARDs: 30 (69.77) Steroid treatment: 32 (74.42) |
NR | |
| Naïve: 12 (27.91) | |||||||||
| Deprez et al. (2020) [28] | TOFA and BARI: 55 | 58 | 81.80 | 11 | NR | 5 mg BID and 4 mg QD | 30 (54.5%) | Corticosteroids: 23 (41.8%) | NR |
| Gonzalez Mazarío et al. (2021) [29] | 32 | 54.1 (13.2) | 88.78 | NR | bDMARDs exp 1/2/ > 3: 24.49, 13.27, 28.57 | NR | NR | GCC: 71.43 | NR |
| Naïve: 28.57 | |||||||||
| Cronin et al. (2021) [30] | JAKi: 28 | Median: 69 (62.3–75) | 64.3 | NR |
Rituximab: 12 (42.9%) TNF-α inhibitor: 15 (53.6%) Tocilizumab: 6 (21.4%) Abatacept: 6 (21.4%) |
4 mg QD | 4 (14.3%) |
PSL: 13 (46.4%) AZA: 2 (7.1%) Leflunomide: 2 (7.1%) HCQ: 6 (21.4%) SSZ: 3 (10.7%) |
NR |
| Naïve: 8 (28.6%) | |||||||||
| Redeker et al. (2021) [31] | 13,991 (JAKi = 713) | 57.7 | 75.2 | Median IQR: 7.0 (3.0–13.0) | NR | NR | 263 (36.9) | GCC: 312 (43.9) | NR |
| Perrone et al. (2020) [32] | 41,290 (BARI = 149) | 57.6 | 73 | 103 (46) | bDMARDs exp: 93 (4.3%) | NR | NR | NR | NR |
| Naïve: 56 (2.7%) | |||||||||
| Perrone et al. (2021) [33] | 445 | 59.2 (12) | 63.59 | NR | NR | NR | NR | NR | NR |
| Amstad et al. (2022) [34] | 73 | NR | 79.3 | NR | NR | NR | NR | NR | NR |
| Barbulescu et al. (2022) [35] | 1,420 | 61 (52–71) | 81.6 | NR | NR | 2 mg/4 mg QD | NR | NR | NR |
| Choi et al. (2022) [36] | 416 | 60.2 (11.8) | 93.9 | NR | bDMARDs exp: 22 (66.7) | 4 mg QD | NR | NR | NR |
| Ebina et al. (2022) [37] | 166 | 60.2 ± 13.5 | 86.7 | NR |
2nd/ ≥ 3rd bDMARDs exp: 23.5, 54.2 TNFi: 57.8 Anti-IL-6: 36.1 Abatacept: 31.9 bDMARDs or JAKi naïve: 22.3 |
NR | NR | NR | JAKi: 20.5 |
| Egeberg et al. (2022) [38] | 275 | 58.77 (12.40) | 84 | NR | bDMARDs exp 1/2/ ≥ 3: 29 (10.55)/51 (18.55)/186 (67.64) | NR | NR | NR | NR |
| Naïve: 9 (3.27) | |||||||||
| Fitton (2021) [39] | 69 | 55.8 (14.3) | 78.2 | NR | On average patients had received 3 previous bDMARDs | 2 mg | NR | NR | NR |
| Naïve: 11 (9.6%) | |||||||||
| Mazarío et al. (2021) [29] | 32 | 53.2 (13.1) | 96.68 | NR | Naïve: 8 (25) | NR | NR | NR | NR |
| Song et al. (2022) [40] | 63 | 55.2 (± 13.5) | 86.9 | < 10 years | NR | NR | NR | NR | NR |
| Song et al. (2022) [40] | 980 | 61.5 (12.7) | 78.6 | NR | NR | NR | NR | NR | NR |
| Gouverneur et al. (2022) [41] | 61 | Median IQR: 65.7 [56.1–75.8] | 65.2 | NR |
bDMARDs (excluding TNFi): 29.4 TNFi: 31.5 |
2 mg | NR | NR | – |
| Hoisnard et al. (2022) [42] | N = 8,481 (exposed group—initiated JAKi) | Median [IQR] for exposed group = 440 [203–846] | Exposed = 78.3 | NR | Bio-naïve in the exposed group: 33 | 2 mg QD = 1,034; 4 mg QD = 4,016 | NR | NR | – |
| Salinas et al. (2022) [43] | 9,013 |
HealthVerity PS20: BARI = 55 (± 11); ARTIS: BARI = 59 (± 14) |
HealthVerity PS20: BARI = 86; ARTIS: BARI = 82 |
NR |
Both; bio experienced: HealthVerity PS20: 36% ARTIS: 54% SNDS: 56% |
2 or 4 mg QD | NR | NR | NR |
| Hernández-Cruz et al. (2022) [44] | 182 | 62.2 (± 12.3) | 83.5 | 13.2 (10.8) years | bDMARDs exp: 78 | 2 or 4 mg QD | NR | NR | NR |
| Abstracts | |||||||||
| Burmester et al. (2021) [45] | 509 | 59.1 (13.2) | 76.6 | 10.0 (9.1) | bDMARDs exp 1/2/ > 2: 67 (13. 2), 110 (21. 6), 8 7 (17.1) | 2 mg/4 mg QD | NR |
csDMARD: 250 (49.1) Oral GCC: 218 (42.8) |
NR |
| Naïve ts/bDMARDs: 48.1 | |||||||||
| Yamane et al. (2020) [46] | 7 | 56.4 | NR | 9.2 Mean Years | bDMARDs exp, median (range): 2.3 (1–4) | NR | 42.85 | PSL: 57.14 | TOFA (IR): 3.4 (1–10) |
| Rosas et al. (2019) [47] | 40 | 58.95 (10.8) | 77 | 9.6 (8.8) years | bDMARDs exp 1/2/3/4/5: 24/2/5/6/3 | NR | NR | csDMARDs: 94 | NR |
| Spinelli et al. (2020) [24] | 51 | 59 (12) | NR | 163 (101) | bDMARDs exp: 2 (1–4) | NR | 52.9 | PSL | NR |
| Torikai et al. (2020) [48] | Discontinuation group: 23 | 66.9 (8.6) | 73.9 | 7.6 (10.3) | bDMARDs exp 1/2/ > 3: 2/0/0 | 4 or mg QD | MTX | SPL | NR |
| Naïve: 21 | |||||||||
| Continuation group: 28 | 67.9 (12.7) | 85.7 | 8.3 (9.9) | bDMARDs exp 1/2/ > 3: 6/4/1 | 4 or 2 mg QD | MTX | SPL | NR | |
| Naïve: 17 | |||||||||
| Gilbert et al. (2021) [49] | 273 | 59 (14) | 78 | 13 (10) years | bDMARDs exp 2/3/4 or later: 20/19/44/78 | NR | NR | csDMARD 41 | 33 |
| Naïve: 20 | |||||||||
| Torikai et al. (2019) [50] | 19 | 66.4 (9.0) | 84.21 | 3.81 (5.80) | Naïve: 19 | 4 or 2 mg QD | NR | PSL | NR |
| 13 | 69.8 (11.2) | 69.2 | 4.57 (3.13) | bDMARDs exp: 13 | 4 or 2 mg QD | NR | PSL | NR | |
| Littlejohn et al. (2020) [51] | JAKi: 14,501 | NR | NR | NR | NR | NR | NR | NR | NR |
| Ponce et al. (2021) [52] | TOFA and BARI: 21 | 58.6 (26.4–84.7) | 92.5 | 14.4 (0.18–37.51) years | bDMARDs exp: 1 (0–7) | NR | NR |
PSL: 46% NSAIDs: 23.8% csDMARDs: 46% |
NR |
| Philippoteaux et al. (2021) [53] | After propensity score matching: 116 | 58.7 ± 15.3 | 70.8 | 12 (6–20) years | bDMARDs exp 1/2/ 3 or more: 38.8, 50.6 | NR | NR | csDMARD: 43 (37.1) | NR |
| Naïve: 12 (10.7) | |||||||||
| Fitton et al. (2019) [39] | TOFA and BARI: 77 | 55.9 (12.52) | 80 | 13.8 (5.34) years | bDMARDs exp: 4 (0–9) | NR | NR | NR | NR |
| Naïve: 5 | |||||||||
| Atsumi et al. (2020) [54] | 1,992 | Mean = 64, median = 66 | 80 | Mean = 11, median = 9 years | bDMARDs exp: 75 | 2 mg/4 mg QD | 55% | GCC: 43% | 21 |
| Yamasaki (2021) [55] | 154 | NR | 83.77 | 11.4 (7.8) years | bDMARDs exp: 48.7 | 2 mg/4 mg QD | NR | NR | NR |
| Naïve: 51.3 | |||||||||
| Morena de la et al. (2019) [56] | TOFA and BARI: 28 | 58.39 | 42–58 | 230.11 months | bDMARDs exp: 1/2/3: 35.7/10.7/17.8 | 10 mg QD and 4 mg QD | NR | NR | NR |
| Naïve: 35.71 | |||||||||
| Kanayama et al. (2021) [57] | 16 | 55.9 | NR | 10.2 years | NR | NR | 75% | NR | NR |
| Page (2019) [58] | 374 | NR | NR | NR | Experienced 1/ > 2—63 (14%)/211 (48%) | NR | TOFA + BARI: 185 (43%) | TOFA + BARI: Oral steroids, 133 (31%) | NR |
| Naive: 112 (25%) | |||||||||
| Kellerhals et al. (2021) [59] | 12 | 61 | 91.67 | NR | bDMARDs exp: 1–75 | 4 mg QD | NA | NR | NR |
| Cometi et al. (2021) [60] | 90 | 57 (12) | NR | 131 (100) | NR | BARI: 4 mg QD | NR | PSL: 5.5 (5.3) | NR |
| Favalli et al. (2021) [61] | JAKi: 1,027 | 56.9 (13.5) | 79.8 | NR | NR | NR | NR | NR | NR |
| Baldi et al. (2021) [62] | 30 | NR | NR | NR | All patients had failed at least one anti-TNFi | BARI: 4 mg QD | NR | PSL | NR |
| Delcoigne et al. (2021) [63] | 2,4083b | NR | 75 | NR | NR | NR | NR | NR | NR |
| Vega et al. (2021) [64] | JAKi: 257 | NR | 84.4 | NR | NR | NR | 71.1% |
Leflunomide: 21.2 Other csDMARDs: 7.7 |
NR |
| Rodriguez et al. (2021) [65] | TOFA and BARI: 40 | 54 (9) | 86 | 11 (7) | bDMARDs exp: 2 (0–4) | TOFA and BARI: 5 mg BID, 4/2 mg QD | 53 | Leflunomide: 8 | NR |
| Guillen et al. (2021) [66] | TOFA and BARI: 42 | 56 (29–78) | 90.5 | NR | bDMARDs exp: 78.6 | NR | NR | NR | 21.4 |
| Frisell et al. (2022) [67] | BARI and bDMARDs: 1,665 | NR | NR | NR | NR | NR | NR | NR | NR |
| Rosas et al. (2022) [68] | 63 | 63 (6) | 89 | NR | NR | 2 mg, 4 mg | NR | NR | NR |
| Alten et al. (2022) [69] | 510 | Cohort A: 59.1 (13.2) | NR | 10.0 (9.1) | NR | 2 mg, 4 mg | NR | NR | NR |
| Bayat et al. (2022) [70] | 93 | 58.4 (12.8) | 98 | 9.7 years | NR | NR | NR | NR | NR |
| Aymon et al. (2022) [71] | 273 | NR | NR | NR | NR | NR | NR | NR | NR |
| Tsuda et al. (2022) [72] | NR | NR | NR | NR | NR | NR | NR | NR | |
| Yoshi et al. (2022) [73] | 31 | NR | NR | ≥ 10 years | NR | 4 mg QD | NR | NR | NR |
| Kemenes et al. (2022) [74] | 30 | 53.4 (12.6) | 24 | NR | bDMARD exp: 14 | 4 mg QD | NR | NR | NR |
| Naïve: 16 | |||||||||
| Codes-Mendez et al. (2022) [75] | 44 | 63 (± 13) | 86.7 | NR | All patients had previously received a median (range) of 3 (0–8) bDMARDs | NR | NR | NR | NR |
| Vassallo et al. (2022) [76] | BARI, TOFA, UPADA: 26 | NR | NR | NR | bDMARD exp: 23 (88) | NR | NR | NR | NR |
| Scheepers et al. (2022) [77] | BARI, TOFA, UPADA: 5,455 | NR | NR | NR | NR | NR | NR | NR | NR |
| Valero Jaimes et al. (2022) [78] | 17 | 71 (63–68) | 4 | NR | NR | 4 mg/2 mg QD | NR | NR | NR |
| Cometi et al. (2022) [60] | 49 | 57 (± 12) | NR | 131 ± 100 months | NR | NR | NR | NR | NR |
| Edwards et al. (2022) [79] | 409 | NR | 76 | NR | bDMARD exp: 63 | 2 or 4 mg QD | NR | GCC: 30% | NR |
| Ciciriello (2022) [80] | 1,875 | NR | NR | NR | NR | NR | NR | NR | NR |
| Alten et al. (2022) [69] | 509 | NR | NR | NR |
Naïve: cohort A, 19.6 Cohort B, 39.4 |
2 or 4 mg QD | NR | NR | NR |
| Gilbert et al. (2022) [81] | BARI (n = 164/273); BARI + csDMARD (n = 109/273) |
BARI monotherapy: 60 (± 15) BARI + csDMARD: 57 (± 11) |
BARI monotherapy = 82 and BARI + csDMARD = 73 | NR | Naïve: 17% (monotherapy), 18% (combination therapy) | NR | NR | NR | NR |
ARTIS antirheumatic therapy in Sweden, AZA azacitidine, BARI baricitinib, BID twice daily, bDMARD biologic DMARD, csDMARD conventional synthetic DMARD, DMARD disease-modifying antirheumatic drug, exp experienced, GCC glucocorticoid, HCQ hydroxychloroquine, IQR interquartile range, JAKi Janus kinase inhibitors, MTX methotrexate, n number of subjects, NR not reported, NSAID nonsteroidal anti-inflammatory drugs, PSL prednisolone, QD once daily, SD standard deviation, SSZ sulfasalazine, TNF tumor necrosis factor, TNFi TNF inhibitors, TOFA tofacitinib, tsMARD targeted synthetic DMARD, UPADA upadacitinib
aNumber of patients were reported in case of percentage data not reported
bThe study included 24,083 patients during treatment of rheumatoid arthritis with etanercept, adalimumab, infliximab, certolizumab pegol, golimumab, rituximab), abatacept, tocilizumab, BARI, or TOFA
Most of the patients’ characteristics were suggestive of having difficult-to-treat RA. Most patients were older (mean age range: 51–71 years), females (range: 42–97%), with long disease duration (mean range: 4–19 years), and bDMARDs-experienced. In addition, most of the patients received baricitinib 4 mg/day and in combination with MTX or other conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and glucocorticoids (GCCs). Overall, in the real-world setting, the baricitinib-treated patients had poor prognostic characteristics indicating more aggressive course of RA.
Real-World Treatment Patterns of Baricitinib
Lines of Baricitinib Therapy
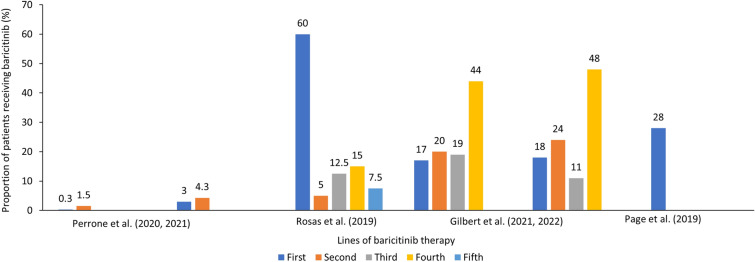
Baricitinib was used in all treatment lines, but differences in frequency were observed depending on the geography [41–51]. In Italy, baricitinib was used very rarely in the first (0.3–2.7%; N = 47,711–41,290) [32] and in the second line of treatment (1.5–4.3%) [33]. In the OPAL dataset from Australia (N = 45,317), higher use of baricitinib was reported in the second to the sixth line of treatment [51]. In Sweden, baricitinib (n = 1420) was more frequently used as second- or third-line treatment, while tofacitinib was used as in later lines of therapy [35]. Similarly, in nationwide registries DANBIO and DERMBIO (n = 5104), only 3.3% of baricitinib patients were bio-naïve [38]. Slightly higher percentage of patients (17%) were bio-naïve in Switzerland (2017–2020; n = 273) [49, 58]. In European patients treated with baricitinib in the multinational RA-BE-REAL cohort (n = 509; 51% on monotherapy), 48.1% were bio-naïve [45]. Also, higher percentage was observed in BSRBR-RA registry from UK (n = 443), wherein 28% of bio-naïve patients received baricitinib. Spanish ORBIT-RA study also showed that 22% of patients were bio-naïve. The number of patients who had previously been treated with one, two, or three or more bDMARDs was 24.2, 17.0, and 36.8%, respectively. Interestingly, in another study from Spain (n = 40), 24 (60%) patients received baricitinib before bDMARDs, and in the remaining 16 (40%) patients, baricitinib was primarily used as the fourth-line treatment (17%). However, the small sample and single-center data source may influence final results [47].
In summary, baricitinib was mostly used in patients with RA with failure to csDMARDs and bDMARDs across the countries (Table 1 and Fig. 2).
Fig. 2.
Proportion of patients reporting the line of therapy in the included studies
Baricitinib Dosing
Of the 70 studies, only 13 reported data on the proportion of patients receiving 2 or 4 mg/day dosing. Eighteen studies mentioned baricitinib dosing (2 or 4 mg/day) but not the proportion of patients. Most of the studies (39) did not report baricitinib dosing (Table 2).
Table 2.
Studies reporting proportion of patients receiving 2 or 4 mg baricitinib dosing
| Study | Country | 2 mg, n (%) | 4 mg, n (%) |
|---|---|---|---|
| Rosas et al. [68] | NR | 23 (36) | 40 (64) |
| Valero Jaimes et al. [78] | Spain | 5 (30) | 12 (70) |
| Alten et al. [69, 82, 83] and Burmester et al. [45] | Germany, France, UK, Spain, and Italy | 11.6% | 88.4% |
| Barbulescu et al. [35] | Sweden | 76.30% | 22.20% |
| Gouverneur et al. [41] | France | 14 (23) | 47 (77) |
| Hoisnard et al. [42] | France | 1034 (20.5) | 4016 (79.5) |
| Hernández-Cruz et al. [44] | Spain | 18 (9.9) | 164 (90.1) |
| Edwards et al. [79] | UK | 16% | 84% |
| Atsumi et al. [54] | Japan |
2 mg: 27% 4–2 mg: 5% |
4 mg: 62% 2–4 mg: 4% |
| Morena et al. [56] | Spain | None | 28 (100) |
| Rodriguez et al. [65] | NR | NR | 50% |
| Miyazaki et al. [17] | Japan |
Before IPTW: 16 (11.6) After IPTW: 19 (13.5) |
Before IPTW: 122 (88.4%) After IPTW: 122 (86.5) |
| Kim et al. [18] | South Korea | None | 20 (100) |
| Spinelli et al. [84] | Italy | None | 59 (100) |
| González-Freire et al. [25] | Spain | 0 (0) | 20 (45.5) |
IPTW inverse probability treatment weighting; NR, not reported
In a small 4-year retrospective study (n = 63), 64% of the patients were prescribed 4 mg/day dose; however, the use of the 2 mg/day dose predominated in patients older than 65 years. Interestingly, survival with baricitinib 2 mg/day was significantly higher in the first 24 months (P = 0.003) and decreased by 30 months [68]. On the other hand, as of December 2019, data from 1992 patients in an all-case post-marketing study of baricitinib in Japan reported that although the population had a mean age of 64 years and RA duration of 11 years, 4 mg/day dosing was prevalent in 62% of patients [54]. By February 2021 (N = 4731, mean ± standard deviation [SD] age 63.9 ± 13.1 years; 1059 [22.38%] were aged ≥ 75 years), 64.6% (n = 3058) of patients received 4 mg/day baricitinib dose compared to 35.1% (n = 1661) of patients on 2 mg/day baricitinib dose [85].
In addition, in the Spanish ORBIT-RA study (N = 182) funded by Lilly, most patients started treatment with baricitinib at 4 mg/day (90.1%), and 43.4% received baricitinib as monotherapy. Eighteen patients (9.9%) changed the starting dose during follow-up. Of 20 changes in starting dose, 14 (70.0%) were dose reductions to 2 mg/day due to remission (64.3%) and adverse events (AEs; 14.3%). The mean (standard deviation, SD) time until change in dosing was 272 (200) days and 326 (195) days until decreasing the dose from 4 to 2 mg daily. Six (30.0%) dose increases from 2 to 4 mg/day were observed. The mean time until increasing the dose from 2 to 4 mg/day was 92 (40) days [44]. In another Spanish study, no dose-related adverse drug reactions were observed in patients with median age of 61 years who received baricitinib 4 mg/day. In another retrospective study of 37 patients who received baricitinib between January 2017 and December 2019, in 9 (24.3%) patients, the dose was reduced to either 2 mg/day every day or 2 and 4 mg/day on alternate days leading to fewer infections while still maintaining moderate improvement in their RA. One of the patients experienced worsening chronic kidney disease and another developed neutropenia on the 4 mg/day dose but remained stable on a 2 mg/day dose. Notably, 12 (32.4%) patients were aged 70–80 years in this study [26]. In the RA-BE-REAL study, 88.2% of patients were treated with baricitinib 4 mg/day. At the time of enrollment, patients treated with baricitinib were more likely to commence treatment as a monotherapy compared to patients treated with tsDMARDs or bDMARD who are more likely to commence treatment in combination with csDMARDs (P < 0.001). Patients treated with baricitinib were more likely to be older (mean age: 59.2 vs. 57.0 years; P = 0.009) [82].
Overall, baricitinib dosing varied by geography and patient population. A greater proportion of patients were reported to have received 4 mg/day compared to 2 mg/day dose of baricitinib. Cases of down-titration of baricitinib 4–2 mg/day were reported, which were mainly due to differences in patient characteristics. Patients receiving baricitinib 2-mg/day were of older age, with greater functional disability, previous bDMARD therapy, and AEs.
Monotherapy Versus Combination Therapy
Of 70 studies, 19 reported baricitinib monotherapy, 13 reported both monotherapy and in combination with csDMARDs, 22 reported only combination with csDMARDs, and 26 did not report monotherapy or combination status (Fig. 3). Most of the patients received baricitinib in combination with csDMARDs, mainly MTX (in 11–84% of cases; Table 1).
Fig. 3.
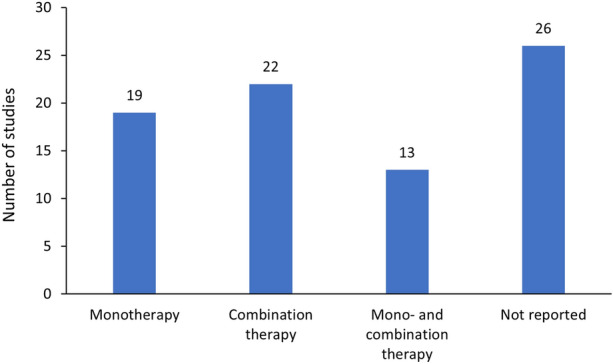
Number of included publications by types of treatment
GCC-Sparing Potential of Baricitinib
Concomitant use of GCCs was reported in several studies in the range of 17% [17] to 95% [18] of the patients initiating treatment with baricitinib. Multiple studies demonstrated steroid-sparing potential of baricitinib. In a multicentric observational study from Italy (N = 446), > 70% of bDMARD-naïve patients were on GCCs at baseline, which reduced to 46.7% after 3 months and 21% at 1 year. Significantly more bDMARD-naïve patients were able to withdraw GCCs at 12 months (bDMARD-naïve vs. bDMARD-experienced: 21% vs. 42%; P = 0.0093) [21]. In the Spanish ORBIT-RA study (bDMARD-experienced: 78%; on monotherapy: 43.4%), 54.9% of patients received GCCs (n = 112) and 23.2% (n = 26) of them changed the dose after baricitinib initiation, 96.2% of which reduced or stopped GCCs [44]. In a single-center study of patients with moderate-to-severe active RA from Italy (N = 59; 47.5% on monotherapy), the proportion of patients receiving GCCs reduced from 78% (5 mg/day) at baseline to 44.7% at 24 weeks (P < 0.0001) and 34.8% at 48 weeks [84]. In another single-center study of patients from Italy (N = 43; 30% on baricitinib monotherapy, > 70% bio-experienced), the mean dose of oral prednisone significantly decreased from 6.25 mg (± 5.06) at baseline to 4.36 mg (± 4.16) at 1 month (P < 0.05), 2.75 mg (± 2.98) at 3 months (P < 0.0001), and 1.86 mg (± 2.85) at 6 months (P < 0.0001) [27]. In a small study, 49 patients received baricitinib and 41 bDMARDs (17 abatacept, 12 TNF inhibitors, 11 tocilizumab, and 1 rituximab) with no statistically significant difference in age, sex, disease duration, disease activity, pain visual analogue scale (VAS), prior tsDMARD/bDMARD use, concomitant csDMARDs, and prednisone dose. Baricitinib showed a significantly higher reduction of mean prednisone dosage at 3 months (− 3.2 ± 5.1 vs. − 1.7 ± 3.7 mg) and 6 months (− 4.1 ± 5.3 vs. − 1.9 ± 4.6 mg), which was not significant after adjusting for baseline prednisone dose [60].
Overall, studies reported steroid-sparing potential of baricitinib, especially in bDMARD-naïve patients.
Clinical Effectiveness
Achieving Remission or LDA
Table 3 shows the studies reporting proportion of patients achieving remission or low disease activity (LDA). Most studies reported a follow-up period of 24 weeks/6 months. Achievement of CDAI remission was reported in 8.7% (in intolerant to bDMARD [bDMARD-IR] patients at 3 months) [21] to 60% of patients at week 12 [48] and CDAI LDA in 20.2% [48] to 81.6% of patients at week 24 [17]. The proportion of patients attaining SDAI remission was reported in the range of 12% at week 4 [27] to 45.4% at 24 weeks [17]. The proportion of patients attaining SDAI LDA was reported from 41% [44] to 81.5% at 24 weeks [17].
Table 3.
Studies reporting proportion of patients achieving remission or LDA
| CDAI | SDAI | DAS28 | DAS28 ESR | DAS28 CRP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author | Country | BARI sample size, n | Time points | LDA (n [%]) | Remission (n [%]) | LDA (n [%]) | Remission (n [%]) | LDA (n [%]) | Remission (n [%]) | LDA (n [%]) | Remission (n [%]) | LDA (n [%]) | Remission (n [%]) |
| Alten et al. [82] | France, Germany, Italy, Spain, and UK | 1073 | 12 months | BARI 2 or 4 mg cohort A: 39.4% | 24.1% (BARI 2 or 4 mg -cohort A) | NR | NR | NR | NR | NR | NR | NR | NR |
| Bayat et al. [70] | NR | 139 | 4, 12, 24, 36, 48, 60, 72, 84, and 96 weeks | NR | NR | NR | NR | NR | NR | NR |
BARI monotherapy: 96 weeks: 3.01% (remission) BARI with MTX: 96 weeks: 2.86% (remission) |
NR | NR |
| Yoshi et al. [73] | NR | 82 | 1, 2, 3, 6, 9, and 12 months | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Kemenes et al. [74] | NR | 30 | Week 24, 48 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Vassallo et al. [76] | NR | 26 | 3 months | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Barbulescu et al. [35] | Sweden | 1420 | 3 months and 1 year | NR | Differences between proportions of CDAI remissions at 1 year: 15 (11.7–18.3) | NR | NR | NR | NR | NR | NR | NR | NR |
| Fitton et al. [39] | UK | 69 | Baseline, 3 months, 6 months | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Mazarío et al. [29] | Spain | 32 | Baseline and at months 1, 3, 6, 12, 18 and 24 | NR | NR | NR | NR | NR | NR | NR | NR |
1 month: 16% After 3 months: 13% At 6 months- 64% |
1 month- 9% After 3 months- 30% At 6 months- 64% |
| Hernández-Cruz et al. [44] | Spain | 182 | 6, 12, 18 months |
6 months: 43.38 12 months: 45.69 |
6 months: 23.5 12 months: 32.45 |
6 months: 41.01 12 months: 45.34 |
6 months: 21.22 12 months: 29.66 |
NR | NR |
6 months: 16.67 12 months: 8.17 |
6 months: 56.2 12 months: 65.68 |
NR | NR |
| Edwards et al. [79] | UK | 409 | 6 months | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Alten et al. [82] | Europe | 509 | 12 months |
Total no. of patients achieving LDA: n = 382; 39.4 BARI mono: 33.8 BARI combo: 44.3 |
Total no. of patients achieving remission: n = 24.1 BARI mono: 21.8 BARI combo: 26.2 |
NR | NR | NR | NR | NR | NR | NR | NR |
| Burmester et al. [45] | EU 4 and UK | 509 | 3, 6, and 24 months | At 6 months: 36.8% | At 6 months: 25.6% | NR | NR | NR | NR | NR | NR | NR | NR |
| Yamane et al. [46]a | Japan | 7 | 2, 4, 8, and 12 weeks | Week 12; n = 5 | NR | Week 12; n = 5 | NR | NR | NR | NR | NR | Week 12; n = 2 | Week 12; n = 1 |
| Rosas et al. [47]b | Spain | 40 | Last visit | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Spinelli et al. [84] | Italy | 67 | 12 and 24 weeks | NR | NR | NR | NR | NR | NR | NR | NR |
Week 4- 51.8% Week 12- 60.7% Week 24- 70.3% |
Week 4- 27% Week 12- 32.1% Week 24- 42.2% |
| Torikai et al. [48]c | Japan | 51 | 24 weeks |
Week 24: 20.2% (Group D) Week 24: 36.6% (Group C) Week 24: 49.9% (re-initiation group) |
Week 24: 27.7% (Group D) Week 24: 56.3% (Group C) Week 24: 50.1% (re-initiation group) |
NR | NR | NR | NR | NR | NR | NR | NR |
| Gilbert et al. [49] | Switzerland | 273 | 12 months | 65.40% | 19.80% | NR | NR | NR | NR | NR | NR | NR | NR |
| Torikai et al. [50] | Japan | 32 | 3 months | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Ponce et al. [52] | Spain | JAKi: 21 (TOFA, BARI) | After treatment | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Fitton et al. [39] | UK | 39 | 3 months | NR | NR | NR | NR | NR | n = 4; 10.25% | NR | NR | NR | NR |
| Yamasaki et al. [55]d | Japan | 154 | 12 weeks |
45 (59.5%): b/tsDMARDs naïve at 12 weeks 35(46.7%): b/tsDMARDs IR group at 12 weeks |
18 (22.8%): b/tsDMARDs naïve at 12 weeks 11(14.7%): b/tsDMARDs IR group at 12 weeks |
NR | NR | NR | NR | NR | NR | NR | NR |
| Kellerhals et al. [59]e | Switzerland | 12 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Cometi L[60]f | Italy | 100 | BL-3 M and BL-6 M | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Baldi et al. [62]g | Italy | NR | Evaluated at baseline (T0) and then after 1 month (T1), 3 months (T2), and 6 months (T3) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Rodriguez et al. [65]h | NR | 36 (BARI and TOFA) | 6 and 12 months | NR | NR | NR | NR | NR | NR | NR |
At 6 months- 22% At 12 months- 0% |
NR | NR |
| Miyazaki et al. [17] | Japan |
Before IPTW- BARI, n = 138 After IPTW- BARI, n = 141 |
24 weeks | 115/141 = 81.6%; P = 0.02 | 57/141 = 40.4%; P = 0.04 | 115/141 = 81.5%; P = 0.02 | 64/141 = 45.4%; P = 0.04 | NR | NR | NR | NR | NR | NR |
| Kim et al. [18] | South Korea | 20 | 12 and 24 weeks | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Iwamoto et al. [19] | Japan | 81 | 24 weeks | 60.5 | 22.2 | 61.7 | 27.2 | NR | NR |
Overall, BARI: 45.7 BARI with MTX: 27.3 BARI without MTX: 26.3 |
BARI: 24.7 BARI with MTX: 26.3 BARI without MTX: 22.3 |
NR | NR |
| Asai et al. [20] | Japan | 284 and 113 patients treated with tocilizumab and BARI (before propensity score matching), BARI (n = 48) [after propensity score matching] | 24 weeks | NR | NR | NR | NR | NR | NR | NR | NR | 12.2 | 45.2 |
| Guidelli et al. [21] | Italy | 446 | 3 and 6 months |
All patients: (%) 3 months: 39.8 6 months: 44.4 12 months:42.9 bDMARD-naïve: (%) 3 months: 48.3 6 months: 45.5 12 months: 38.1 bDMARD-IR: (%) 3 months: 34.9 6 months: 43.7 12 months: 47.6 |
All patients: (%) 3 months: 12 6 months: 25 12 months: 38.9 bDMARD-naïve: (%) 3 months: 17.5 6 months: 38.2 12 months: 50.8 bDMARD-IR: (%) 3 months: 8.7 6 months: 15.8 12 months: 27 |
NR | NR | NR | NR | NR | NR |
All patients: (%) 3 months: 19.8 6 months: 15.9 12 months: 16.7 bDMARD-naïve:(%) 3 months: 21.9 6 months: 14.6 12 months: 14.3 bDMARD-IR: (%) 3 months: 18.5 6 months: 16.8 12 months: 18.7 |
All patients: (%) 3 months: 36.3 6 months: 51.6 12 months: 64.3 bDMARD-naïve: (%) 3 months: 48.3 6 months: 70 12 months: 77.8 bDMARD-IR: (%) 3 months: 29.5 6 months: 40.2 12 months: 50.8 |
| Retuerto et al. [23] | Spain | 15 | Post-treatment | NR | NR | NR | NR |
Disease activity during the first year of follow-up after start of the second JAKi: (%) 3 months: 17.3 6 months: 7.54 9 months: 18.8 12 months: 18.3 |
Disease activity during the first year of follow-up after start of the second jakinib: (%) 3 months: 29.7 6 months: 46.4 9 months: 44.1 12 months: 73.2 |
NR | NR | NR | NR |
| Spinelli et al. [84] | Italy | 59 | 4, 12, 24, and 48 weeks |
Week 4–48% Week 12–66% Week 24–66% Week 48–60% |
Week 4–12% Week 12–18% Week 24–20% Week 48–22% |
Week 4–47% Week 12–64% Week 24–63% Week 48–60% |
Week 4–12% Week 12–20% Week 24–16% Week 48–26% |
NR | NR | NR | NR |
Week 4–47% Week 12–64% Week 24–71% Week 48–56% |
Week 4–29% Week 12–40% Week 24–42% Week 48–39% |
| González-Freire et al. [25] | Spain | 20 | 6 and 12 months | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sagdeo et al. [26] | UK | 37 | 6 months | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Tesei G[27] | Italy | 43 | 1, 3, and 6 months | NR | NR |
53.8% at 3 months 51.3% at 6 months |
5/39 (12.8%) at 3 months 8/36 (21.6%) at 6 months |
NR | NR | NR | NR | NR | NR |
| Deprez et al. [28] | France | 55 | 3, 6, and 12 months | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Gonzalez et al. [29] | Spain |
All JAKi patients n = 98 (100%) BARI group n = 32 (32.65%) TOFA group n = 66 (67.35%) |
1, 3, 6, 12, 18 and 24 months | NR | NR | NR | NR |
BARI: (%) 1 month: 14% 3 months: 23% 6 months: 29% 12 months: 18% 18 months: 49% 24 months: 50% JAKi: 1 month: 16% 3 months: 13% 6 months: 28% 12 months: 23% 18 months: 50% 24 months: 33% JAKi Monotherapy: 1 month: 9% 3 months: 10% 6 months: 32% 12 months: 42% 18 months: 64% JAKi combination therapy: 1 month: 19% 3 months: 14% 6 months: 24% 12 months: 12% 18 months: 39% 24 months: 39% |
BARI: (%) 1 month: 15% 3 months: 33% 6 months: 29% 12 months: 38% 18 months: 16% 24 months: 16% JAKi: 1 month: 9% 3 months: 30% 6 months: 36% 12 months: 40% 18 months: 25% 24 months: 33% JAKi Monotherapy: 1 month: 17% 3 months: 26% 6 months: 25% 12 months: 13% 18 months: 32% JAKi combination therapy: 1 month: 10% 3 months: 35% 6 months: 45% 12 months: 52% 18 months: 19% |
NR | NR | NR | NR |
BARI baricitinib, bDMARD, biologic DMARD, CDAI Clinical Disease Activity Index, CRP C-reactive protein, csDMARD conventional synthetic DMARD, DAS28 Disease Activity Score 28-joint count, DMARD disease-modifying antirheumatic drug, ESR erythrocyte sedimentation rate, EU4 Germany, France, Spain, and Italy, IPTW inverse probability treatment weighting, IR inadequate responders, JAKi Janus kinase inhibitors, LDA low disease activity, MTX methotrexate, NR not reported, RA rheumatoid arthritis, SDAI Simple Disease Activity Index, TNF tumor necrosis factor, TOFA tofacitinib, tsMARD targeted synthetic DMARD
aPatients on TOFA-IR and TOFA were administered the drug for 3.4 months
bIn this study, 94% of patients continued concomitant treatment with some csDMARD
cPatients divided into two groups: a discontinuation group (D group; n = 23) and a continuation group (C group; n = 28)
dIn this study, 79 (51.3%) b/tsDMARDs naïve and 75 (48.7%) b/tsDMARDs-IR patients were enrolled
eIn this study, patients were treated with 4 mg BARI/day after TOFA was discontinued
fIn this study, 90 of 100 RA patients were evaluated; 49 received BARI
gAll patients had failed at least one anti-TNF
hIn this study, 4 patients received both treatments (BARI + TOFA)
In the RA-BE-REAL study (N = 1073), more baricitinib-treated patients were in CDAI remission (24.1% vs. 16.6%) compared to patients treated with b/tsDMARDs. Proportion of patients with LDA was 39.4% vs. 43.4% [69]. In a multicenter study (N = 242) from Japan, where most patients on baricitinib were bDMARD-IR (80%) and on monotherapy (53%), tofacitinib and baricitinib had comparable CDAI remission (18% vs. 22%) and LDA rates (65.8% vs. 60.5%) at 24 weeks. Remission and LDA rates for baricitinib were similar in combination with csDMARDs but 10% higher than for tofacitinib in monotherapy (31% vs. 41%) [19]. Similarly, in the ORBIT-RA study, remission or LDA was reported in 71.6% and 76.3% of patients at 6 and 12 months, respectively, at any index: DAS28-erythrocyte sedimentation rate (ESR) (73.1 and 73.5%), SDAI (62.4 and 75.0%), and CDAI (66.7 and 78.1%). Good and moderate EULAR response was noted in 80.0% and 78.2% of patients, respectively [44].
In a single-center study of patients treated with baricitinib (n = 32), a significant reduction in the DAS28 disease activity was obtained within 1 month of treatment initiation. Within the treatment groups (vs. tofacitinib), no statistically significant difference in response was observed between the patients regarding their therapeutic status (i.e., bDMARD-naïve vs. prior bDMARD experience and monotherapy vs. combination therapy) [29]. In a Japanese study, both DAS28 and SDAI scores were consistently lower in the baricitinib-treated patients than in the tofacitinib-treated patients from 1 month after baseline [73]. In BAREBONE, a prospective, interventional, open-label, monocentric single-center study (N = 30), DAS28 score decreased from 4.8 (4.5–5.1) at baseline to 2.9 (2.5–3.3) at week 48 [74]. However, two single-center studies showed that baricitinib and tofacitinib were both comparable in terms of effectiveness in real-world conditions [39, 86].
Overall, improvements in disease activity were observed after treatment initiation with baricitinib, irrespective of the composite disease activity measure used.
Baricitinib Survival and Persistence
A total of 52 studies reported data on discontinuation and persistence rates of baricitinib. In a prospective observational study (N = 139), baricitinib persistence was high over time, attributed to 66.5% of the patients at 1 year and 56.4% of the patients at 2 years irrespective of its use as a monotherapy or combination with csDMARDs (69% vs. 67%, respectively, at 1 year; 62% vs. 56%, respectively, at 2 years) [70].
In the RA-BE-REAL study (baricitinib, n = 509), at 12 months, patients naïve to b/tsDMARD were least likely to discontinue baricitinib compared to those who had more than two previous b/tsDMARD treatments. A similar percentage of patients discontinued baricitinib whether as monotherapy (24.7%) or combination therapy with any csDMARD (28.8%) [83]. In a multicentric observational study from Italy (N = 446; 49% on baricitinib monotherapy), baricitinib discontinuation rates at 3, 6, and 12 months were 4%, 10%, and 24%, respectively. Seropositivity (P < 0.022) and bDMARD-naïve status (P < 0.043) were identified as protective factors for baricitinib discontinuation due to inefficacy. At the same time, bDMARD-IR status and older age were risk factors for discontinuation due to AE for each additional year that they continued baricitinib (P = 0.008) [21].
Several other studies also documented lower discontinuation rate of baricitinib compared to tofacitinib and other bDMARDs. These studies used persistence and adherence rates to show the drug survival [73, 75, 77]. The continuation rate at 12 months after the start of administration was highest for baricitinib (89.3%), followed by tofacitinib (86.4%) and golimumab (69.0%) in a study of small sample size (n = 31) [73]. Another small study reported adherence (medication possession ratio [MPR]) and persistence of tofacitinib and baricitinib in RA patients in a real-life setting. Between 2017 and 2021, 40 (29.4%) and 38 (27.9%) patients treated with tofacitinib and baricitinib discontinued the treatment. Mean treatment persistence was 363 days (95% confidence interval [CI]: 2–1.282) in the tofacitinib group and 406 days (95% CI 8–1.300) in the baricitinib group. There were no statistical differences in treatment survival (hazard ratio [HR] = 1.01 [95% CI 0.59–1.71]; P = 0.97) [75]. In a retrospective observational study (N = 5455) conducted using the Australian Medicare Database (from January 2006 till October 2021), persistence rates on first-line JAK inhibitors were 70% for baricitinib and 57% for tofacitinib; persistence rates dropped to 63% for baricitinib and 47% for tofacitinib in the second-line setting. Median treatment persistence was 27.1 months for baricitinib and 15.2 months for tofacitinib [77].
A few studies reported persistence of the baricitinib treatment differently. A nationwide cohort study from the DANBIO and DERMBIO registries (n = 5104) used confounder-adjusted models of drugs in the analysis. The highest drug survival was observed for rituximab followed by baricitinib, etanercept, and tocilizumab. Among the two JAK inhibitors, drug survival was higher for baricitinib compared to tofacitinib with an HR of 1.42 (95% CI: 1.06 to 1.89). A Swedish cohort study of national registers used crude drug retention rates. In this study, baricitinib showed higher treatment retention and overall equivalent or better treatment responses compared with bDMARDs. Treatment retention for tofacitinib was lower than that for baricitinib, but treatment responses were not significantly different from those of bDMARDs or baricitinib [35]. A Spanish retrospective study (2017–2021, n = 96) analyzed the survival of baricitinib or TNF inhibitors and reported baricitinib's superiority to TNF inhibitors during the first 4 years of treatment (HR: 0.47, 95% CI 0.24–0.91; P = 0.026) [68]. Treatment maintenance was observed in a single-center study of 55 patients from France (48 patients on baricitinib; 55% with concomitant MTX), and baricitinib maintenance was reported by 67.6% at 12 months. Baricitinib maintenance was independent of concomitant MTX use, prior use of interleukin-6 inhibitor therapy, or a bDMARD-naïve status. The factors associated with JAK inhibitor discontinuation after 1 year due to AEs were Charlson comorbidity index, age, and GCC use at the initiation [28].
In summary, most of the studies showed equal or better baricitinib survival compared to TNF inhibitors and other bDMARDs, although drug survival measures were different among studies.
Patient-Reported Outcomes
Pain Outcomes
Of 70 studies, 14 observed the effect of baricitinib on pain outcomes [21, 44, 45, 57–62, 69, 73, 84]. In the ORBIT-RA study (n = 182), pain assessment (VAS 10 cm) was 6.6 (2.0) at baseline, which reduced to 2.5 (− 3.0, − 2.0) and 3.0 (− 3.6, − 2.5) cm at 6 and 12 months, respectively (P < 0.0001) [44]. In the RA-BE-REAL study (n = 509), in patients who started treatment with baricitinib, the mean pain VAS score (0–100 mm) at 6 months was 34.5 (27.1) with mean change from baseline of − 22.4 (28.6). At 12 months, the mean pain (VAS) reduction from baseline was − 24.6 and − 19.3 in baricitinib- and b/tsDMARDs-treated patients, respectively [45, 69].
In a real-world multicenter study (n = 446), baricitinib induced significant improvement in pain scores as early as 3 months, which further improved with longer treatment duration. Improvements were similar between treatment groups of monotherapy and combination therapy. The improvement in pain (VAS scores) was observed to be better among bDMARD-naïve patients compared to bDMARD-IR patients at all time points, independent of concomitant MTX use [21]. In a monocentric real-life clinical setting (N = 102; baricitinib, n = 61), at baseline, 75.4% of patients showed tenosynovitis involving at least one tendon, with a median score of 2 (interquartile range [IQR] 3.5), significantly decreasing after 24 weeks (P = 0.02) [87]. Similar observations were reported in another monocentric study at weeks 4, 12, 24, and 48 (P < 0.001) [84]. In a study of bDMARD-experienced patients (N = 30), baricitinib (4 mg/day with concomitant MTX) simultaneously improved pain VAS and CRP over 6 months. By 1 month, significant changes were obtained from baseline. Baricitinib also demonstrated a significant parallel and fast improvement in VAS pain at as early as 1 month (baseline vs. 1 month; P < 0.0098) [62].
Overall, treatment with baricitinib was observed to rapidly improve pain in a way comparable to bDMARDs.
Patient Global Assessment
Nine studies reported data on PGA [20, 24, 28, 45, 46, 60–62, 69]. In the RA-BE-REAL study, 6 months after treatment initiation, European patients (N = 1074) achieved a mean − 2.3 reduction in PGA scores from baseline with baricitinib (n = 509; 51% on monotherapy). The mean change was not significantly different compared to patients receiving other b/tsDMARDs [69]. Deprez et al. (2021) reported improvement in PGA outcomes with baricitinib in French patients (n = 55; 45% monotherapy), regardless of previous therapeutic status (bDMARD-naïve vs. experienced) and concomitant MTX use. The baseline PGA VAS reduced from approximately 70 to 30 mm within 3 months of treatment, which was accompanied by improvements in pain VAS and other clinical parameters (number of tender joints, number of swollen joints, and morning stiffness) [28]. Treatment with baricitinib resulted in an early reduction in PGA VAS, as reported in Japanese patients (N = 32). Significantly reduced PGA scores (P < 0.001) from baseline to 1 month were observed in both bio-naïve (49.9 ± 18.6 to 21.2 ± 17.6) and bio-experienced patients (66.6 ± 25.2 to 25.4 ± 24.3), with lower mean values at 3 and 6 months in the bio-naïve patients [50]. These results were supported by another study in Japanese patients (N = 59; 47.5% monotherapy) with inadequate response or intolerance to ≥ 1 csDMARDs (47.5% monotherapy), wherein treatment with baricitinib significantly improved the PGA VAS and pain VAS within 4 weeks of treatment. This response was maintained throughout the follow-up period [84].
Overall, studies showed that PGA was significantly improved with baricitinib, regardless of the previous therapeutic status.
Improvement in Physical Function
Of 70 studies, 11 articles reported data on Health Assessment Questionnaire (HAQ) Disability Index (DI) or other functional disability measures [17, 28, 29, 35, 45, 50, 66–69, 73]. Improvements of ≥ 0.22 points in the HAQ scores was taken to be a minimum clinically important difference (MCID) [88–90]. Functional remission was defined as low impairments in physical function with HAQ-DI score ≤ 0.5 [91].
In a Japanese study (n = 32), early improvements with baricitinib in HAQ-DI scores were observed from baseline (0.43 ± 0.24) and sustained until 6 months (0.28 ± 0.17; P < 0.001 vs. baseline) in bio-naïve patients [50]. In another retrospective study (n = 49), significant reduction in HAQ scores was observed at 3 and 6 months in patients treated with baricitinib [60]. In the RA-BE-REAL study, improvements in HAQ-DI scores were reported with baricitinib with mean (SD) difference of 1.0 (0.8) from baseline to 6 months. The post-treatment HAQ-DI scores were similar between treatment groups receiving baricitinib versus ts/bDMARDs [45, 69]. In the Swedish patients with RA (n = 1420), baricitinib reduced physical function compared with baseline at 3 months and retained its relative advantage to other bDMARDs on the HAQ-DI scale. For baricitinib, results showed statistically significant gains in improvement compared with TNF inhibitors (0.06 units higher improvement [95% CI 0.02, 0.10]). Adjusted 1-year response proportions were consistently lower on TNF inhibitors compared with baricitinib with differences of − 9.9 (− 14.4 to − 5.4) for HAQ-DI improvement. Baricitinib initiators also achieved HAQ-DI improvement more frequently than any bDMARDs except rituximab [35]. In a Japanese study (n = 82), the HAQ-DI score at 6 months after baseline was significantly lower in the baricitinib than in the golimumab [73]. In a small multicenter observational study with baricitinib (n = 67), no statistically significant difference in HAQ-DI score at 24 weeks was observed between patients who discontinued treatment (n = 23) after achieving CDAI LDA and those who maintained (n = 28) the treatment, although the median HAQ-DI score changed from 0.28 to 0.45 for patients of the discontinued group. The study concluded that baricitinib could be discontinued without deterioration of HAQ-DI. Re-initiation of baricitinib, in case of disease flare, could result in reintroduction of CDAI LDA [48]. Additionally, in a Japanese study (n = 32), early improvements with baricitinib in HAQ-DI scores were observed in ≤ 1 month (0.76 ± 0.24) and sustained until 6 months (0.28 ± 0.17; P < 0.001 vs. baseline) [50]. In another retrospective study (n = 49), significant reduction in HAQ scores was observed at 3 and 6 months in patients treated with baricitinib [60].
In all real-world studies, treatment with baricitinib improved functional disability better than all other treatments options.
HRQoL and Treatment Satisfaction
Patient-reported measures of HRQoL and satisfaction among patients treated with baricitinib were reported in limited studies. Treatment of European patients with baricitinib in the multinational RA-BE-REAL cohort (n = 509; 51% on monotherapy) resulted in improved HRQoL at 6 months (mean change of 0.1 [± 0.2] on EuroQol 5 Dimension 5 Levels [EQ-5D-5L]) [45] and at 12 months [69]. The mean change in EQ-5D-5L was not significantly different from the cohort treated with bDMARDs (n = 565; 0.1 ± 0.3) [45]. Similar results were observed in an another single-center study (n = 51); rapid improvement (by week 4) in clinical endpoints and high patient satisfaction were observed in patients (82%) receiving baricitinib or tofacitinib, as measured using the Patient Acceptable Symptom State questionnaire after a median treatment duration of 10 days [24].
Clinical studies with baricitinib monotherapy or combination therapy (baricitinib + MTX) have reported greater improvement in SF-36 physical component score and EQ-5D-5L at weeks 24 and 52 compared with MTX (P ≤ 0.01) [92] and placebo at weeks 12 and 24 [93]. Observations from the above studies are consistent with the clinical study data.
Radiographic Progression and Ultrasound Evaluation of Treatment Effect
Eight studies reported radiographic outcomes on treatment with baricitinib [18, 27, 44, 52, 57, 62, 74, 84]. In BAREBONE, a prospective, open-label, interventional, single-center study (N = 30), in baricitinib (4 mg/day)-treated patients with RA, total RA MRI scores (RAMRIS) slightly decreased from 20.6 (95% CI 14.4–27.8) at baseline to 18.3 (11.5–26.5) at week 48 and mainly as a result of reduced MRI synovitis. A significant difference in RAMRIS synovitis change for biologic naïve − 3.8 (− 5.2 to − 2.6) vs. biologic failure − 1.0 (− 2.2 to 0.4) was observed at week 48 [74].
Ultrasonography is a more accessible assessment method, and more data were reported on it. In a study of patients with moderate-to-severely active RA and inadequate response or intolerance to ≥ 1 csDMARDs (N = 59; 47.5% on baricitinib monotherapy), improvements in joint inflammation (observed with ultrasonography) started as early as 4 weeks and were sustained at each time point of observation. At baseline, 31 patients (60.8%) showed positivity for power Doppler in at least one ultrasonography-assessed joint. This percentage decreased to 29.5% at 4 weeks, to 15.8% at 12 weeks, and to 22.6% at 24 weeks. Radiographic improvements were in parallel to a significant decrease in disease activity (DAS28-CRP, CDAI, and SDAI scores) and pain VAS [84].
Another study (n = 20) demonstrated a favorable effect of baricitinib treatment at 24 weeks. Compared to baseline, significant improvement of grayscale synovitis (2.00 [IQR 1.00–3.00] vs. 1.00 [IQR 0.00–2.00]; P = 0 0.002), power Doppler synovitis (PDS; 1.00 [IQR 0.00–2.00] vs. 0.00 [IQR 0.00–1.00]; P = 0.030), and joint effusion (3.00 [IQR 2.00–3.00] vs. 2.00 [IQR 1.0–3.0]; P = 0.002). Bone erosion scores were not different between baseline and 24 weeks (P = 0.317) [18]. In the other study involving patients with moderate-to-severe active RA and ≥ 1 csDMARD experience (N = 43), statistically significant improvements were observed in ultrasound imaging parameters (grayscale synovitis, grayscale tenosynovitis, and PDS) at 1 month of treatment with baricitinib (30.2% on monotherapy). Improvements were sustained at 3 and 6 months [27]. Study of patients treated with baricitinib or TNF inhibitors (N = 61; baricitinib, 16; TNF inhibitors, 45; concomitant MTX use 75% vs. 89%) showed improvements in grayscale and PDS starting at 4 weeks with both treatments. The improvement in PDS was significantly greater with baricitinib at weeks 12 and 24: Degree of improvement at week 12 (− 9.2 vs. − 3.8; P = 0.049) and week 24 (− 11.3 vs. − 5.7; P = 0.062). The difference in rate of PDS improvement at week 24 was − 77.1% versus − 50.1% (P = 0.048). Differences in grayscale were not statistically significant. The results suggested that baricitinib induced early improvements in inflammatory synovitis compared with TNF inhibitors [57]. In a monocentric, longitudinal study (N = 59, 31 on baricitinib), a significant reduction of ultrasonography score from 12 (IQR 12.5) at 4 weeks (P < 0.0001) to 8 (IQR 9) at 12 weeks (P < 0.0001), 8 (IQR 10) at 24 weeks (P < 0.0001), and 8 (IQR 9) at 48 weeks (P < 0.0001) was seen. The positivity for power Doppler in at least one ultrasonography-assessed joint decreased from 31 patients (60.8%) at baseline to 29.5% at 4 weeks, 15.8% at 12 weeks, and 22.6% at 24 weeks [84].
Overall, treatment with baricitinib improved early image parameters of disease progression in the patients from baseline. A favorable effect on ultrasound-detected abnormalities (including synovitis and bone erosion) was reported with baricitinib. These observations are an important addition to those reported in the 5-year RA-BEYOND study in which approximately 40%–72% of patients treated with baricitinib 2 or 4 mg combined with a csDMARD (or 4 mg monotherapy for disease-modifying antirheumatic drug disease-modifying antirheumatic drug [DMARD]-naïve patients) had no radiographic progression (threshold of van der Heijde modified total Sharp score ≤ 0) over 5 years [90].
Real-World Safety Profile of Baricitinib
Most of the studies included in this review reported data on the safety profile of baricitinib [17, 19–40, 46, 47–50, 51, 54, 86]. As of December 2019, in the ongoing Japanese, all-case, post-marketing study of baricitinib (N = 1992), 536 patients (27%) had AEs and 79 (4%) had serious AEs (SAEs) at 24 weeks. The major AEs were varicella zoster virus (VZV) infection (n = 58), serious infections (n = 29), low hemoglobin or anemia (n = 26), liver dysfunction (n = 68), hyperlipidemia (n = 27), major adverse cardiovascular events (MACE) (n = 15), interstitial pneumonia (n = 5), malignancy (n = 7), and venous thromboembolism (VTE) (n = 2). At this time, the median age of patients was 66 years with a median disease duration of 9 years. Also, 62% of patients received 4 mg compared to 27% of patients receiving 2 mg; > 50% of patients were > 65 years of age; 73% continued treatment for 24 weeks. Similar results were observed in the safety analyses to February 2021, which included 4731 patients (initial baricitinib dose: 4 mg/day, n = 3058; 2 mg/day, n = 1661; other, n = 12); 1059 (22.38%) were ≥ 75 years, and 3362 (71.06%) previously received biologic therapy [54, 85]. In the Italian multicenter study with baricitinib (N = 446; n = 217, baricitinib monotherapy), six cases of VZV reactivation were observed; however, the VZV reactivation was significantly associated with concomitant use of oral GCCs (83% vs. 25% in the other infections; P = 0.034 with Yates correction). Among the entire cohort, four thrombotic events were observed in patients younger than 65 years. Overall, 13% baricitinib withdrawal due to AEs was reported with a higher rate reported with older age and previous use of bDMARDs [21].
In data obtained from the RABBIT registry, the risk of herpes zoster virus (HZV) infection was significantly increased in patients treated with JAK inhibitors, with a significantly higher risk in older patients and in those with concomitant GCC therapy [31]. The rates of AEs were similar between baricitinib (N = 81) and tofacitinib (N = 161) treatment groups. During the 24-week follow-up period, 38 (23.6%) patients discontinued tofacitinib and 15 (18.5%) patients discontinued baricitinib. In the baricitinib group, lack of efficacy (n = 10) and an AE (pneumonia, HZV, breast cancer, headache, and elevation of creatine kinase; n = 5) were the reasons. However, this study had a small sample size and short observation period [19]. Remarkably, dose reduction of baricitinib (2 mg daily or 2 mg/4 mg on alternate days) was found to control the infections while sustaining the clinical response [26]. Despite EMA’s warning in 2019 regarding the elevated risk of VTE with tofacitinib 5 mg twice a day, a multicenter study from Europe observed that the patients initiating index treatment with a JAK inhibitor (N = 232; 155 on baricitinib) had elevated risk of VTE. Although not significant, the proportion of JAK inhibitor initiators with thromboembolic history was noted to be numerically lower after 2019 [53]. In the cohort study using nationwide Swedish register data (n = 1420), a difference in crude proportions of treatments stopped for safety reasons within the first year after initiation was observed between baricitinib (9.4%) and tofacitinib (14.6%) [35]. However, among patients recruited from the FIRST registry (tofacitinib, 156; baricitinib, 138), no difference was observed in the incidence of AEs leading to discontinuation of in the tofacitinib and baricitinib groups [17].
In a study that used the Japanese Adverse Drug Event Report (JADER) database (2014–2020), the proportion of patients with infections was more in patients treated with baricitinib (49.2%) than in those treated with tofacitinib (37%). In addition, solid tumors were more frequent in patients treated with abatacept or baricitinib, and more frequencies of MACE were noted in patients treated with baricitinib and golimumab. Cases of VTE were more frequent in patients treated with baricitinib and tofacitinib compared to those treated with other DMARDs [72]. The same observations were made in a single-center retrospective study (2017–2020) in which the incidence of infection and serious infection was numerically higher in the baricitinib group than in the tofacitinib group but with no statistical signification. Overall, 29 (29.59%) definitive discontinuations were observed in this study, of which 10 (10.2%) cases were in the baricitinib group and 19 (19.39%) in the tofacitinib group. No cases of thrombotic-related entities (deep-vein thrombosis, prostate artery embolization, or even MACE) or malignancy were detected. However, more serious infections were detected in the baricitinib group (16% against 3% in tofacitinib group), and most were pneumonia [86].
In a retrospective pharmacovigilance disproportionality analysis using data from the AE reports from the FDA, general AEs in terms of System Organ Classes were highest in tofacitinib (29.0%), and infections were highest in both baricitinib and upadacitinib (31.7 and 23.5%, respectively). Among the top ten potential important medical event terms (IMEs), thrombosis, pulmonary embolism, and deep-vein thrombosis were the main potential AEs occurring with tofacitinib or baricitinib. The incidence rates of overall malignancy were 0.85 and 0.80 per 100 patient-years for tofacitinib and baricitinib, respectively. In this study, breast cancer was also reported as an IME in patients taking baricitinib with odds ratio of 4.54 (95% CI 2.04–10.14). However, among the spontaneous AE reports, 86.2% of patients taking tofacitinib and 91.9% of patients taking upadacitinib were reported from North America, whereas only 59.7% of patients taking baricitinib were from North America and 30.7% from Europe [95].
A multi-database study, across 14 post-marketing data sources in Europe, the USA, and Japan, of patients in routine care using disease registries and claims databases suggested increased risk of VTE with baricitinib versus TNF inhibitors (incident rate ratio [IRR] = 1.51, 95% CI 1.10, 2.08). Risk of MACE was also numerically greater with baricitinib versus TNF inhibitors, although not statistically significant, during a mean overall exposure of 8 months (IRR = 1.54, 95% CI 0.93, 2.54). However, overall incidence rates were not estimated in this study, and comparative risk should be interpreted in terms of patient cohorts or populations rather than individual risk. In addition, comparisons were limited by the small number of patients with events. The mean age of patients with a VTE was higher (mean age in antirheumatic therapy in Sweden [ARTIS], 64 years; mean age in Système National des Données de Santé [SNDS], 68 years) than the mean age of patients included in VTE analyses (mean age in ARTIS, 59 years; mean age in SNDS, 58 years). In addition, in the ARTIS, SNDS, and Betriebskrankenkasse [BKK] data sources, almost all patients in the baricitinib cohort with a VTE during follow-up were male, unlike for TNF inhibitor cohorts [43]. On the other hand, the risk of MACEs and VTEs did not significantly differ between initiating a JAK inhibitor and adalimumab in a nationwide population-based cohort study (N = 15,835) of the French national health data system. This study assessed the risk of MACEs and VTEs among patients initiating tofacitinib or baricitinib (79.5% received 4 mg daily) versus adalimumab. Also, risk of MACEs (both myocardial infarction and stroke) and VTEs was not significant between baricitinib and tofacitinib groups [42].
Overall, HZ and respiratory infections, gastrointestinal issues, and elevated blood lipids were observed with the use of baricitinib in the real-world setting. Treatment with baricitinib is also associated with the risk of VTE and MACE; however, this treatment had a similar risk with TNF inhibitors. The infection rates were not different than those observed in the randomized clinical trials (RCTs), and no new safety signal was identified in any of the observational studies.
Discussion
A recent SLR has provided an update on the evolving evidence from RCTs from 2019 to January 2022 on efficacy of cs/b/tsDMARDs in RA [96]. This, to the best of our knowledge, is the first SLR to provide real-world data, specifically on patient characteristics, treatment patterns, clinical effectiveness, drug survival, PROs, and safety related to baricitinib therapy in RA.
This SLR reports that, in most of the studies, baricitinib was used in the older population, with long course of the disease, and in advance lines of treatment. In addition, the baseline level of disease activity also was high. The use of baricitinib at an earlier stage (first- or second-line use) was reported by a few studies, which could be due to the characteristics of the health system of different countries.
As recommended by the EMA summary of product characteristics [97], most of the patients were reported to be receiving 4 mg baricitinib for the treatment of RA. A few studies reported using 2 mg baricitinib dosing; however, the patients were generally at higher risk of VTE, MACE, and malignancy; aged ≥ 65 years; and had a history of chronic or recurrent infections. These observations were in congruence with the recommended dosing in patients with RA.
In all cases, baricitinib was reported to be effective even in patients with prior b/tsDMARD use. Baricitinib in combination with MTX was more effective in reducing signs and symptoms of RA and improving HRQoL when compared with placebo + MTX in MTX-IR patients from Brazil, Argentina, and China [98, 99]. In real-world settings, baricitinib may be more effective than TNF inhibitors [35]. Effectiveness of baricitinib observed in a real-world setting is broadly in line with evidence from RCTs. RA-BEAM [4] reported baricitinib to be superior to adalimumab, while ORAL-STANDARD [100] and ORAL-STRATEGY [101] found similar efficacy of tofacitinib vs. adalimumab.
Long-term drug performance including effectiveness, safety, and tolerability can be indirectly measured by assessing drug survival of a medicine [38]. Exploring drug survival in routine clinical practice may help patients and clinicians identify the best treatment option and avoiding or delaying treatment failure, especially in chronic disease such as RA [102]. Studies included in this SLR reported comparable drug survival of baricitinib monotherapy or combination therapy in a real-life setting and equal or better baricitinib survival compared to TNF inhibitors and other bDMARDs, although persistence measures were different between studies.
Chronic GCC treatment may have detrimental bone, metabolic, cardiovascular, and infective side effects [103]. Steroid-sparing effect was observed with baricitinib regardless of line of bDMARDs treatment but was much more evident in bio-naïve population. Together with higher rates of remission compared to TNF inhibitors, it may have an additional effect on lowering the number of associated comorbidities related to prolong GCC use such as atherosclerosis, metabolic diseases, serious infections, or osteoporosis.
A wide variation in the effectiveness of data was observed. This is probably due to the differences in study methodology including sample size and baseline patient characteristics. Monotherapy of baricitinib was consistently observed to have an efficacy comparable to combination therapy with MTX. The extent to which prior bDMARD use affects the response of patients to baricitinib was reported in several studies. While some studies reported it to be a significant factor, others did not. Nonetheless, improvements in disease activity and physical function were observed frequently before 24 weeks of baricitinib treatment. The changes from baseline were statistically significant, and the response was sustained at later time points.
Shared decision making is a basis for treat-to-target strategy, as it enables better communication between physicians and patients and allows alignment on common treatment goal [104]. Thanks to this approach, PROs became important in disease activity monitoring as a factor needed for better outcomes. Although common contributors to HRQoL (pain or fatigue or disease perceptions) were assessed in RCT and were significantly improved in patients receiving treatment with baricitinib, patient-reported measures of HRQoL in RWE were not commonly assessed. Treatment with baricitinib was observed to rapidly improve pain and the patient’s perception of the disease. Pain reduction with baricitinib was observed to be only partially attributable to the reduction of inflammatory markers. Improvements in PROs in real-world settings are similar to those observed in RCTs [92, 105]. Baricitinib 2 or 4 mg/day provided significant improvement versus placebo in PROs across different domains of RA, including physical function, morning joint stiffness, fatigue, pain, and HRQoL, suggesting that baricitinib can be a potentially valuable addition to the RA treatment options for patients struggling with this common and disabling condition [93]. Data from two phase 3 studies, RA-BUILD (NCT01721057; csDMARD-IR patients) and RA-BEACON (NCT01721044; bDMARD-IR patients), showed that baricitinib-treated patients with RA achieved MCID improvement in PROs (pain, physical function, fatigue, HRQoL, and PGA data) at weeks 4 and 12 and maintained those improvements over time. In addition, substantial PRO responses were achieved quickly [106].
Lack of efficacy and AEs were common reasons of treatment discontinuation in baricitinib. HZV and respiratory infections, gastrointestinal symptoms, and hyperlipidemia were observed with the use of baricitinib in the real-world setting. However, the infection rates were not different than those observed in RCTs. Despite the large number of patients who are older and have more comorbidities than those enrolled in RCTs, no new safety concerns were identified. Baricitinib maintained a safety profile similar to that previously reported, with incidence rates of safety events of special interest (including deaths, malignancies, MACE, and VTE) remaining stable [107–110].
From this SLR, several gaps in the availability of data were identified. None of the studies measured adherence or persistence specifically for baricitinib. Similarly, switching and dose changes during treatment were not reported frequently. Dose tapering of oral GCCs and the decrease in the proportion of patients receiving it concomitantly with baricitinib were mostly conducted in patients from Italy. Several high-quality studies included in this review were conducted with patients from Japan. Most of the data on baricitinib versus other JAK inhibitors regarding relative efficacy and tolerance were reported in these studies, limiting its interpretation for other populations. In addition, ORAL Surveillance (NCT02092467), an FDA-mandated, post-authorization, phase IIIb/IV randomized, open-label, non-inferiority study, evaluated the safety and efficacy of tofacitinib as compared with a TNF inhibitor in patients with RA who were 50 years of age or older and had at least one additional cardiovascular risk factor. Tofacitinib (combined dose 5 and 10 mg twice a day) did not achieve non-inferiority of the co-primary endpoints of MACE and malignancy [111]. As a result of this and post-hoc analyses [112], the Pharmacovigilance Risk Assessment Committee (PRAC) and EMA recommended to carry out a review to determine whether these risks are associated with all JAK inhibitors, including baricitinib, authorized in the European Union for the treatment of inflammatory disorders [113]. Recent EMA warning and precautions for AEs of special interest in populations at risk were not properly addressed in the current SLR, as this reports safety data from the studies published up to November 2022 before final PRAC recommendations. However, extended data from baricitinib clinical studies have been published looking at this population [114].
This systematic review has many potential limitations that should be appreciated by all readers. First, this study included only baricitinib and lacks studies including comparators in the treatment of RA. More real-world studies are needed to investigate the usefulness and differences of JAK inhibitors as a treatment option in clinical settings, especially with baricitinib. Second, the summary provided in a systematic review of the literature is only as reliable as the methods used to estimate the effect in each of the primary studies. Real-world evidence goes beyond the constraints of RCTs, however have inherent biases such as the presence of confounders and selection bias. The generalizability of these results can also be a limitation, and conclusions about the differences observed should be made with caution. Additionally, new evidence can and does emerge during the production and publication process, especially following the latest EMA warning and precautions for all JAKs. This review included the articles published until November 22, 2022, so recently published data on baricitinib could have been missed.
Conclusion
In conclusion, baricitinib is efficacious in the treatment of RA in a real-world setting. Discontinuation rates of baricitinib were lower than those in bDMARDs and similar between patients on baricitinib monotherapy and in combination with csDMARDs. Persistence rates for baricitinib were higher compared to bDMARDs. Real-world observations of AEs/SAEs were consistent with RCT findings.
Acknowledgements
Medical Writing, Editorial, and Other Assistance
Amit Kumar Koushik and Suchita Dubey, employees of Eli Lilly Services India Private Ltd., provided medical writing support. Sneha G (SG) and Madhuri K (MK), also employees of Eli Lilly Services India Private Ltd., reviewed included studies and the data extracted.
Author Contributions
Conceptualization: Tamas Treuer, Ewa Haladyj, Jens Gerwien. Data Curation: Chandreyee Dutta Gupta. Formal Analysis: Chandreyee Dutta Gupta. Funding Acquisition: Tamas Treuer. Investigation: Ewa Haladyj. Methodology: Tamas Treuer, Chandreyee Dutta Gupta, Blanca Hernández-Cruz, Fabrizio Conti. Project Administration: Tamas Treuer. Resources: Tamas Treuer. Software: Chandreyee Dutta Gupta. Supervision: Tamas Treuer. Visualization: Chandreyee Dutta Gupta. Writing—Original Draft Preparation: Tamas Treuer, Ewa Haladyj, Chandreyee Dutta Gupta, Blanca Hernández-Cruz, Fabrizio Conti. Writing—Review and Editing: Tamas Treuer, Ewa Haladyj, Chandreyee Dutta Gupta, Blanca Hernández-Cruz, Uta Kiltz, Jérôme Avouac, Fabrizio Conti.
Funding
This study and the journal’s Rapid Service Fee are funded by Eli Lilly and Company.
Data Availability
No additional data are available from the authors.
Declarations
Conflict of Interest
Tamas Treuer: Tamas Treuer is an employee and shareholder of Eli Lilly and Company. Ewa Haladyj: Ewa Haladyj is an employee and shareholder of Eli Lilly and Company. Jens Gerwien: Jens Gerwien is an employee and shareholder of Eli Lilly and Company. Chandreyee Dutta Gupta: Chandreyee Dutta Gupta is an employee and shareholder of Eli Lilly and Company. Blanca Hernández-Cruz: Grant and research support and consultancy fees in the last 3 years from AbbVie, Amgen, BMS, Eli Lilly, Fresenius, Grunnenta, STAD, and Gilead. Uta Kiltz: Grant and research support and consultancy fees from AbbVie, Amgen, Biocad, Biogen, BMS, Chugai, Eli Lilly, Fresenius, Gilead, Grünenthal, GSK, Hexal, Janssen, MSD, Novartis, onkowoessen.de, Pfizer, Roche, UCB, and Viatris.. Jérôme Avouac: Honoraria from Pfizer, Lilly, Bristol Myers Squibb, UCB, Roche, Nordic Pharma, Novartis, Sanofi, Boehringer, AbbVie, Chugai, Galapagos, Biogen, Fresenius Kabi, Sandoz, and AstraZeneca. Research grants: Pfizer, Bristol Myers Squibb, Fresenius Kabi, Novartis, Nordic Pharma, Galapagos. Fabrizio Conti: Speaker fee: Eli Lilly, Biogen, BMS, Galapagos, AbbVie, Pfizer, UCB.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Favoino E, Prete M, Catacchio G, Ruscitti P, Navarini L, Giacomelli R, et al. Working and safety profiles of JAK/STAT signaling inhibitors. Are these small molecules also smart? Autoimmun Rev. 2021;20(3):102750. doi: 10.1016/j.autrev.2021.102750. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka Y, Luo Y, O'Shea JJ, Nakayamada S. Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach. Nat Rev Rheumatol. 2022;18(3):133–145. doi: 10.1038/s41584-021-00726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung YK, Lee YH. Comparative effectiveness and safety of non-tumour necrosis factor biologics and Janus kinase inhibitors in patients with active rheumatoid arthritis showing insufficient response to tumour necrosis factor inhibitors: a Bayesian network meta-analysis of randomized controlled trials. J Clin Pharm Ther. 2021;46(4):984–992. doi: 10.1111/jcpt.13380. [DOI] [PubMed] [Google Scholar]
- 4.Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen ML, Reyes Gonzaga J, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–662. doi: 10.1056/NEJMoa1608345. [DOI] [PubMed] [Google Scholar]
- 5.Fleischmann R, Pangan AL, Song IH, Mysler E, Bessette L, Peterfy C, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788–1800. doi: 10.1002/art.41032. [DOI] [PubMed] [Google Scholar]
- 6.Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2021;73(7):924–939. doi: 10.1002/acr.24596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smolen JS, Landewé RBM, Bergstra SA, Kerschbaumer A, Sepriano A, Aletaha D, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82(1):3–18. doi: 10.1136/ard-2022-223356. [DOI] [PubMed] [Google Scholar]
- 8.Markham A. Baricitinib: first global approval. Drugs. 2017;77(6):697–704. doi: 10.1007/s40265-017-0723-3. [DOI] [PubMed] [Google Scholar]
- 9.Mayence A, Vanden Eynde JJ, Baricitinib: A, Novel FDA-approved small molecule inhibiting janus kinases. Pharmaceuticals (Basel) 2018 doi: 10.3390/ph12010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleischmann R, Takeuchi T, Schlichting DE, Macias WL, Rooney T, Gurbuz S, et al. Baricitinib, methotrexate, or baricitinib plus methotrexate in patients with early rheumatoid arthritis who had received limited or no treatment with disease-modifying anti-rheumatic drugs (DMARDs): phase 3 trial results. Arthritis & Rheumatology: Wiley-Blackwell 111 RIVER ST, HOBOKEN 07030–5774, NJ USA; 2015.
- 11.Durez P, Walker D, Geusens P, Van den Bosch F, Shaikh S, Roccatello D, et al. Baricitinib, methotrexate, or baricitinib plus methotrexate in patients with moderately-to-severely active rheumatoid arthritis who had received limited or no treatment with DMARDs: efficacy and safety results from the 52-week phase 3 ra-begin study. Rheumatology: Oxford Univ Press Great Clarendon St, Oxford Ox2 6dp, England; 2017. p. 134–5.
- 12.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. Updated February 2021 ed. Cochrane. 2021.
- 13.Centre for Reviews and Dissemination . CRD’s guidance for undertaking reviews in health care. York: Centre for Reviews and Dissemination, University of York; 2009. [Google Scholar]
- 14.Scottish Intercollegiate Guidelines Network: SIGN search filters for observational studies. https://www.sign.ac.uk/what-we-do/methodology/search-filters/ Accessed 13 Aug 2021.
- 15.Bykerk VP, Massarotti EM. The new ACR/EULAR remission criteria: rationale for developing new criteria for remission. Rheumatology (Oxford) 2012;51 Suppl 6:vi16–20. doi: 10.1093/rheumatology/kes281. [DOI] [PubMed] [Google Scholar]
- 16.Wells G, Shea B, O'Connell D, Peterson J, Welch, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014.
- 17.Miyazaki Y, Nakano K, Nakayamada S, Kubo S, Inoue Y, Fujino Y, et al. Efficacy and safety of tofacitinib versus baricitinib in patients with rheumatoid arthritis in real clinical practice: analyses with propensity score-based inverse probability of treatment weighting. Ann Rheum Dis. 2021;80(9):1130–1136. doi: 10.1136/annrheumdis-2020-219699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SK, Jung UH, Kim JW, Choe JY. The beneficial effect of baricitinib on ultrasound-detected synovial inflammation and bone damage in rheumatoid arthritis: Preliminarily data from single center-based observational study for 24 weeks. Medicine (Baltimore) 2021;100(30):e26739. doi: 10.1097/MD.0000000000026739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwamoto N, Sato S, Kurushima S, Michitsuji T, Nishihata S, Okamoto M, et al. Real-world comparative effectiveness and safety of tofacitinib and baricitinib in patients with rheumatoid arthritis. Arthritis Res Ther. 2021;23(1):197. doi: 10.1186/s13075-021-02582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asai S, Takahashi N, Kobayakawa T, Kaneko A, Watanabe T, Kato T, et al. Comparison of the effects of baricitinib and tocilizumab on disease activity in patients with rheumatoid arthritis: a propensity score matching analysis. Clin Rheumatol. 2021;40(8):3143–3151. doi: 10.1007/s10067-021-05815-3. [DOI] [PubMed] [Google Scholar]
- 21.Guidelli GM, Viapiana O, Luciano N, De Santis M, Boffini N, Quartuccio L, et al. Efficacy and safety of baricitinib in 446 patients with rheumatoid arthritis: a real-life multicentre study. Clin Exp Rheumatol. 2021;39(4):868–873. doi: 10.55563/clinexprheumatol/pudtpo. [DOI] [PubMed] [Google Scholar]
- 22.Ebina K, Hirano T, Maeda Y, Yamamoto W, Hashimoto M, Murata K, et al. Drug retention of sarilumab, baricitinib, and tofacitinib in patients with rheumatoid arthritis: the ANSWER cohort study. Clin Rheumatol. 2021;40(7):2673–2680. doi: 10.1007/s10067-021-05609-7. [DOI] [PubMed] [Google Scholar]
- 23.Retuerto M, Trujillo E, Valero C, Fernandez-Espartero C, Soleto CY, Garcia-Valle A, et al. Efficacy and safety of switching Jak inhibitors in rheumatoid arthritis: an observational study. Clin Exp Rheumatol. 2021;39(3):453–455. doi: 10.55563/clinexprheumatol/cbanza. [DOI] [PubMed] [Google Scholar]
- 24.Spinelli FR, Garufi C, Ceccarelli F, Mancuso S, Duca I, Alessandri C, et al. FRI0134 effect of JAK inhibitors on pain and quality of life in rheumatoid arthritis patients. Ann Rheum Dis. 2020;79(Suppl 1):649. doi: 10.1136/annrheumdis-2020-eular.4903. [DOI] [Google Scholar]
- 25.González-Freire L, Giménez-Candela RM, Castro-Luaces S, Veiga-Villaverde AB, Crespo-Diz C. Baricitinib and tofacitinib in patients with rheumatoid arthritis: results of regular clinical practice. Farm Hosp. 2021;45(4):165–169. doi: 10.7399/fh.11586. [DOI] [PubMed] [Google Scholar]
- 26.Sagdeo A, Askari A, Morrissey H, Ball PA. Baricitinib in rheumatoid arthritis—real world cross-sectional study. Open Rheumatol J. 2020;14:28–33. doi: 10.2174/1874312902014010028. [DOI] [Google Scholar]
- 27.Tesei G, Cometi L, Nacci F, Terenzi R, Tofani L, Capassoni M, et al. Baricitinib in the treatment of rheumatoid arthritis: clinical and ultrasound evaluation of a real-life single-centre experience. Ther Adv Musculoskelet Dis. 2021;13:1759720X211014019. doi: 10.1177/1759720X211014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deprez V, Le Monnier L, Sobhy-Danial J-M, Grados F, Henry-Desailly I, Salomon-Goëb S, et al. Therapeutic maintenance of baricitinib and tofacitinib in real life. J Clin Med. 2020;9(10):3319. doi: 10.3390/jcm9103319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.González Mazarío R, Fragío Gil JJ, Ivorra Cortés J, Grau García E, Cañada Martínez AJ, González Puig L, et al. Real-world effectiveness and safety of jak inhibitors in rheumatoid arthritis: a single-centre study. Reumatolog Clín. doi: 10.1016/j.reuma.2021.08.001. [DOI] [PubMed]
- 30.Cronin O, McKnight O, Keir L, Ralston SH, Hirani N, Harris H. A retrospective comparison of respiratory events with JAK inhibitors or rituximab for rheumatoid arthritis in patients with pulmonary disease. Rheumatol Int. 2021;41(5):921–928. doi: 10.1007/s00296-021-04835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redeker I, Albrecht K, Kekow J, Burmester GR, Braun J, Schäfer M, et al. Risk of herpes zoster (shingles) in patients with rheumatoid arthritis under biologic, targeted synthetic and conventional synthetic DMARD treatment: data from the German RABBIT register. Ann Rheum Dis. 2022;81(1):41–47. doi: 10.1136/annrheumdis-2021-220651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perrone V, Losi S, Rogai V, Antonelli S, Fakhouri W, Giovannitti M, et al. Real-world analysis of therapeutic patterns in patients affected by rheumatoid arthritis in italy: a focus on baricitinib. Rheumatol Ther. 2020;7(3):657–665. doi: 10.1007/s40744-020-00218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrone V, Losi S, Rogai V, Antonelli S, Fakhouri W, Giovannitti M, et al. Treatment patterns and pharmacoutilization in patients affected by rheumatoid arthritis in Italian settings. Int J Environ Res Public Health. 2021 doi: 10.3390/ijerph18115679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amstad A, Papagiannoulis E, Scherer A, Rubbert-Roth A, Finckh A, Mueller R, et al. Comparison of drug retention of TNF inhibitors, other biologics and JAK inhibitors in RA patients who discontinued JAK inhibitor therapy. Rheumatology (Oxford) 2022;62(1):89–97. doi: 10.1093/rheumatology/keac285. [DOI] [PubMed] [Google Scholar]
- 35.Barbulescu A, Askling J, Chatzidionysiou K, Forsblad-d'Elia H, Kastbom A, Lindström U, et al. Effectiveness of baricitinib and tofacitinib compared with bDMARDs in RA: results from a cohort study using nationwide Swedish register data. Rheumatology (Oxford) 2022;61(10):3952–3962. doi: 10.1093/rheumatology/keac068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi W, Ahn SM, Kim YG, Lee CK, Yoo B, Hong S. Safety of JAK inhibitor use in patients with rheumatoid arthritis who developed herpes zoster after receiving JAK inhibitors. Clin Rheumatol. 2022;41(6):1659–1663. doi: 10.1007/s10067-022-06096-0. [DOI] [PubMed] [Google Scholar]
- 37.Ebina K, Hirano T, Maeda Y, Yamamoto W, Hashimoto M, Murata K, et al. Factors affecting drug retention of Janus kinase inhibitors in patients with rheumatoid arthritis: the ANSWER cohort study. Sci Rep. 2022;12(1):134. doi: 10.1038/s41598-021-04075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egeberg A, Rosenø NAL, Aagaard D, Lørup EH, Nielsen ML, Nymand L, et al. Drug survival of biologics and novel immunomodulators for rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, and psoriasis—a nationwide cohort study from the DANBIO and DERMBIO registries. Semin Arthritis Rheum. 2022;53:151979. doi: 10.1016/j.semarthrit.2022.151979. [DOI] [PubMed] [Google Scholar]
- 39.Fitton J, Melville AR, Emery P, Nam JL, Buch MH. Real-world single centre use of JAK inhibitors across the rheumatoid arthritis pathway. Rheumatology (Oxford) 2021;60(9):4048–4054. doi: 10.1093/rheumatology/keaa858. [DOI] [PubMed] [Google Scholar]
- 40.Song YJ, Cho SK, Kim H, Kim HW, Nam E, Choi CB, et al. Risk factors for herpes zoster in Korean patients with rheumatoid arthritis treated with JAK inhibitor: a nested case-control study. RMD Open. 2022 doi: 10.1136/rmdopen-2021-001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gouverneur A, Avouac J, Prati C, Cracowski JL, Schaeverbeke T, Pariente A, et al. JAK inhibitors and risk of major cardiovascular events or venous thromboembolism: a self-controlled case series study. Eur J Clin Pharmacol. 2022;78(12):1981–1990. doi: 10.1007/s00228-022-03402-2. [DOI] [PubMed] [Google Scholar]
- 42.Hoisnard L, Pina Vegas L, Dray-Spira R, Weill A, Zureik M, Sbidian E. Risk of major adverse cardiovascular and venous thromboembolism events in patients with rheumatoid arthritis exposed to JAK inhibitors versus adalimumab: a nationwide cohort study. Ann Rheum Dis. 2022 doi: 10.1136/ard-2022-222824. [DOI] [PubMed] [Google Scholar]
- 43.Salinas CA, Louder A, Polinski J, Zhang TC, Bower H, Phillips S, et al. Evaluation of VTE, MACE, and serious infections among patients with RA treated with baricitinib compared to TNFi: a multi-database study of patients in routine care using disease registries and claims databases. Rheumatol Ther. 2022 doi: 10.1007/s40744-022-00505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernández-Cruz B, Rosas J, Díaz-Torné C, Belzunegui J, García-Vicuña R, Inciarte-Mundo J, et al. Correction to: real-world treatment patterns and clinical outcomes of baricitinib in rheumatoid arthritis patients in Spain: results of a multicenter, observational study in routine clinical practice (The ORBIT-RA Study) Rheumatol Ther. 2022;9(4):1245–1246. doi: 10.1007/s40744-022-00463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burmester G FB, Alten R, Matucci-Cerinic M, Salmon J, Holzkaemper T, de la Torre I, Lopez-Romero P, Fakhouri W, Gentzel-Jorczyk A. A Multinational, Prospective, observational study in patients with rheumatoid arthritis receiving baricitinib, targeted synthetic or biologic disease-modifying therapies: 6-month effectiveness and patient reported outcome data from the European Cohort [abstract]. Arthritis Rheumatol. 2021;73. https://acrabstracts.org/abstract/a-multinational-prospective-observationalstudy-in-patients-with-rheumatoid-arthritis-receiving-baricitinib-targeted-synthetic-or-biologic-disease-modifyingtherapies-6-month-effectiveness-and-pat/ [DOI] [PMC free article] [PubMed]
- 46.Yamane T, Hashiramoto A. AB0364 Efficacy of baricitinib (BARI) in patients with rheumatoid arthritis (RA) whose response was inadequate to tofacitinib (TOFA) Ann Rheum Dis. 2020;79(Suppl 1):1483. doi: 10.1136/annrheumdis-2020-eular.4349. [DOI] [Google Scholar]
- 47.Rosas J, Senabre-Gallego JM, Soler GS, Bernal-Vidal JA, Salas-Heredia E, Pons A, et al. AB0442 Response to baricitinib in patients with rheumatoid arthritiswith failure to conventional synthetic DMARD and/or biological DMARD: data from a local registry. Ann Rheum Dis. 2019;78(Suppl 2):1684. doi: 10.1136/annrheumdis-2019-eular.3820. [DOI] [Google Scholar]
- 48.Torikai E, Hirano Y, Suzuki D, Kanayama Y. FRI0137 Discontinuation of baricitinib after achieving low disease activity in patients with rheumatoid arthritis in clinical practice; a multicenter observational study. Ann Rheum Dis. 2020;79(Suppl 1):651. doi: 10.1136/annrheumdis-2020-eular.1546. [DOI] [Google Scholar]
- 49.Gilbert B, Courvoisier D, Mongin D, Lauper K, Perrier C, Muller R, et al. POS0668 Real world effectiveness of baricitinib in the Swiss Rheumatoid Arthritis Register (SCQM-RA) Ann Rheum Dis. 2021;80(Suppl 1):577–578. doi: 10.1136/annrheumdis-2021-eular.1781. [DOI] [Google Scholar]
- 50.Torikai E, Suzuki D. THU0196 Efficacy and safety up to 24 weeks of baricitinib for Japanese patients with rheumatoid arthritis in real world multicenter clinical data. Ann Rheum Dis. 2019;78(Suppl 2):375–376. doi: 10.1136/annrheumdis-2019-eular.4496. [DOI] [Google Scholar]
- 51.Littlejohn G, Smith T, Tymms K, Youssef P, Cooley H, Ciciriello S, et al. THU0209 Uptake of Janus kinase inhibitors for management of rheumatoid arthritis in Australia. Ann Rheum Dis. 2020;79(Suppl 1):329. doi: 10.1136/annrheumdis-2020-eular.5055. [DOI] [Google Scholar]
- 52.Ponce A F-SB, Julio R, Sapena N, Gumucio R, Ruiz-Esquide V, Morlà R, Bassas M, Cañete J, Gomez-Puerta J. Composite articular index including acute phase reactants should not be used in patients with rheumatoid arthritis treated with Il6 inhibitors but may be useful in those receiving Jak inhibitors: ultrasound evidence [abstract]. Arthritis Rheumatol. 2021;73. https://acrabstracts.org/abstract/time-to-discontinuation-and-effectivenesswith-baricitinib-in-rheumatoid-arthritis-12-month-european-data-from-a-multinational-prospective-observationalstudy/
- 53.Philippoteaux CDV, Letarouilly J, Cailliau E, Houvenagel E, Deprez X, Nottez A, Philippe P, Pascart T, Goeb V, Flipo R. Characteristics of RA patients treated with JAK inhibitors before versus after VTE warnings: results of a real-world multicentric study [abstract]. . Arthritis Rheumatol. 2021;73. https://acrabstracts.org/abstract/characteristics-of-ra-patients-treatedwith-jak-inhibitors-before-versus-after-vte-warnings-results-of-a-real-world-multicentric-study/
- 54.Atsumi T ON, Takahashi N, Tamura N, Nakajima A, Nakajima A, Fujii T, Matsuno H, Takahashi Y, Inui F, Tsujimoto N, Nishikawa A, Ishii T, Takeuchi T, Kuwana M, Takagi M. Safety of baricitinib in patients with rheumatoid arthritis: interim report from all-case post-marketing study in clinical use [abstract]. Arthritis Rheumatol. 2020;72. https://acrabstracts.org/abstract/safety-of-baricitinib-inpatients-with-rheumatoid-arthritis-interim-report-from-all-case-post%E2%80%91marketing-study-in-clinical-use/
- 55.Yamasaki M. Efficacy and safety of baricitinib in B/tsDMARDs naive and B/tsDMARDs-IR patients with rheumatoid arthritis [abstract]. Arthritis Rheumatol, p. 73
- 56.Morena I, Paz Solarte JA, Bedoya D, Larraz PT. AB0435 Real world data of a patient cohort with rheumatoid artritis treated with JAK/STAT inhibitors. Ann Rheum Dis. 2019;78(Suppl 2):1680. doi: 10.1136/annrheumdis-2019-eular.8254. [DOI] [Google Scholar]
- 57.Kanayama Y, Nagata A, Shimotake M, Miyachi F, Fujita K, Koyama M, et al. POS0635 comparing the ultrasonographic evaluation in patients with Japanese rheumatoid arthritis between baricitinib and tnf antagonist therapy. Ann Rheum Dis. 2021;80(Suppl 1):555. doi: 10.1136/annrheumdis-2021-eular.3483. [DOI] [Google Scholar]
- 58.Page J, Kearsley-Fleet L, Davies R, Watson K, Hyrich K, Lunt M. AB0434 Early experience with JAK inhibitor prescribing in the uk: results from the British Society For Rheumatology Biologics Register For Rheumatoid Arthritis (BSRBR-RA) Ann Rheum Dis. 2019;78(Suppl 2):1680. doi: 10.1136/annrheumdis-2019-eular.508. [DOI] [Google Scholar]
- 59.Kellerhals S, Amsler J, Schulze-Koops H, Hügle T, Nissen MJ, Paul H, et al. Effectiveness of a switch from tofacitinib to baricitinib in rheumatoid arthritis: a retrospective analysis of real-world data in Switzerland. Ann Rheum Dis. 2021;80(Suppl 1):1161. doi: 10.1136/annrheumdis-2021-eular.2872. [DOI] [Google Scholar]
- 60.Cometi L, Bruni C, Tofani L, Tesei G, Nacci F, Fiori G, et al. AB0256 Baricitinib (BARI) versus biologics impact on steroid tapering in rheumatoid arthritis (RA) Ann Rheum Dis. 2021;80(Suppl 1):1154–1155. doi: 10.1136/annrheumdis-2021-eular.1343. [DOI] [Google Scholar]
- 61.Favalli EG, Iannone F, Gremese E, Gorla R, Foti R, Conti F, et al. POS0675 The comparative 3-year retention rate of targeted-synthetic and biologic drugs for rheumatoid arthritis: real-life data from the Italian GISEA registry. Ann Rheum Dis. 2021;80(Suppl 1):582. doi: 10.1136/annrheumdis-2021-eular.3557. [DOI] [Google Scholar]
- 62.Baldi C, Falsetti P, Conticini E, Khayyat SG, Bardelli M, Gentileschi S, et al. POS0661 Rapid response to baricitinib in patients with rheumatoid arthritis and an inadequate response to methotrexate and at least one biologic DMARD: a clinical and power doppler ultrasound study. Ann Rheum Dis. 2021;80(Suppl 1):573. doi: 10.1136/annrheumdis-2021-eular.1007. [DOI] [Google Scholar]
- 63.Delcoigne B, Ljung L, Provan SA, Glintborg B, Lederballe Gron K, Hetland ML, et al. OP0114 Short- and longer-term risks for acute coronary syndrome in patients with rheumatoid arthritis starting treatment with disease-modifying anti-rheumatic drugs. A collaborative observational head-to-head study across five Nordic rheumatology registers. Ann Rheum Dis. 2021;80(Suppl 1):63–64. doi: 10.1136/annrheumdis-2021-eular.2626. [DOI] [Google Scholar]
- 64.Vega L, Calvo I, Ibarguengoitia O, Montero D, García C, Blanco JM, et al. 4CPS-327 Effectiveness, safety and adherence of baricitinib and tofacitinib in rheumatoid arthritis. Ann Rheum Dis. 2021;80(Suppl 1):1104. doi: 10.1136/annrheumdis-2021-eular.1141. [DOI] [Google Scholar]
- 65.Rodriguez Mauriz R, Seguí Solanes C, Almendros-Abad N, Sosa-Pons A, Rudi Sola N. 4CPS-327 Effectiveness, safety and adherence of baricitinib and tofacitinib in rheumatoid arthritis. Eur J Hosp Pharm. 2021;28(Suppl 1):A78-A. doi: 10.1136/ejhpharm-2021-eahpconf.159. [DOI] [Google Scholar]
- 66.Guillen E, et al. Observational real-world study of patients with rheumatoid arthritis treated with baricitinib and/or tofacitinib in a tertiary hospital, collected in the register of patients and treatments. Pharmacoepidemiol Drug Saf. 2021;30(Suppl.):410. doi: 10.1002/pds.5306. [DOI] [Google Scholar]
- 67.Frisell T, Bower H, Baecklund E, Di Giuseppe D, Delcoigne B, Feltelius N, et al. POS0637 SAfety of b/tsdmards for ra as used in clinical practice-results from the last decade of the ARTIS program. Ann Rheum Dis. 2022;81(Suppl 1):587–588. doi: 10.1136/annrheumdis-2022-eular.341. [DOI] [Google Scholar]
- 68.Rosas J, Pons A, Barber X, Senabre-Gallego JM, Santos Soler G, Soler-Giner E, et al. POS0657 Survival of baricitinib vs anti-tnf as the first biological drug in patients with rheumatoid arthritis, in clinical practice: results of a local registry. Ann Rheum Dis. 2022;81(Suppl 1):600–601. doi: 10.1136/annrheumdis-2022-eular.3949. [DOI] [Google Scholar]
- 69.Alten R, Burmester GR, Matucci Cerinic M, Ostor A, Zaremba-Pechmann L, Herrera M, et al. POS0666 A multinational, prospective, observational study in patients with rheumatoid arthritis receiving baricitinib, targeted synthetic or biologic disease-modifying therapies: 12 month time to discontinuation, effectiveness and patient reported outcome data from the European cohort. Ann Rheum Dis. 2022;81(Suppl 1):606–607. doi: 10.1136/annrheumdis-2022-eular.265. [DOI] [Google Scholar]
- 70.Bayat S, Tascilar K, Bohr D, Simon D, Krönke G, Hartmann F, et al. POS0699 Similar efficacy and drug survival rates of baricitinib monotherapy and baricitinib/methotrexate combination therapy in real-life treatment of rheumatoid arthritis—results from a prospective cohort of baricitinib-treated patients. Ann Rheum Dis. 2022;81(Suppl 1):629. doi: 10.1136/annrheumdis-2022-eular.3989. [DOI] [Google Scholar]
- 71.Aymon R, Gilbert B, Mongin D, Nham E, Laedermann C, Müller R, et al. POS1420 Doubly robust estimator for average treatment effect as sensitivity analysis for comparative effectiveness research. An example comparing drug maintenance between baricitinib and alternative biologic DMARDs. Ann Rheum Dis. 2022;81(Suppl 1):1053. doi: 10.1136/annrheumdis-2022-eular.1822. [DOI] [Google Scholar]
- 72.Tsuda N, Inokuma S, Noguchi H, Yamaji M, Harada T, Misaki M, et al. Comparison of adverse events (aes) related to major anti-rheumatic drugs, reported to the official Japanese Adverse Drug Event Report Database (JADER) Ann Rheum Dis. 2022;81(Suppl 1):1318. doi: 10.1136/annrheumdis-2022-eular.118. [DOI] [Google Scholar]
- 73.Yoshii I, Sawada N, Chijiwa T. AB0383 Comparison of efficacy and safety in jak inhibitor due to a difference of selectivity—tofacitinib vs. baricitinib. Ann Rheum Dis. 2022;81(Suppl 1):1318–1319. doi: 10.1136/annrheumdis-2022-eular.129. [DOI] [Google Scholar]
- 74.Kemenes S, Bayat S, Simon D, Krönke G, Bohr D, Valor L, et al. AB0385 Baricitinib leads to rapid and persistent resolution of synovitis as measured by hand mri in patients with active rheumatoid arthritis (RA) failing cs/bDMARD tharapy. Ann Rheum Dis. 2022;81(Suppl 1):1320–1321. doi: 10.1136/annrheumdis-2022-eular.476. [DOI] [Google Scholar]
- 75.Codes-Mendez H, Martinez-Molina C, Masip M, Riera P, Pagès Puigdemont N, Riera Magallón A, et al. AB0402 therapeutic adherence and persistence of tofacitinib and baricitinib in rheumatoid arthritis patients in daily clinical practice. Ann Rheum Dis. 2022;81(Suppl 1):1330. doi: 10.1136/annrheumdis-2022-eular.2116. [DOI] [Google Scholar]
- 76.Vassallo C, Sammut L. AB0412 Cycling of jak-inhibitors in patients with rheumatoid arthritis: a single-centre experience. Ann Rheum Dis. 2022;81(Suppl 1):1334–1335. doi: 10.1136/annrheumdis-2022-eular.4004. [DOI] [Google Scholar]
- 77.Scheepers L, Jones G. AB0414 drug persistence on janus kinase (JAK) inhibitors compared to biologic dmards in patients with rheumatoid arthritis: retrospective study in the australian population. Ann Rheum Dis. 2022;81(Suppl 1):1335. doi: 10.1136/annrheumdis-2022-eular.4149. [DOI] [Google Scholar]
- 78.Valero Jaimes JA, De Diego SA, Alcorta Lorenzo N, Egües Dubuc CA, Belzunegui Otano JM, Uriarte Isacelaya E, et al. AB0330 Experience in the current practice with baricitinib in patients with rheumatoid arthritis and intersticial lung disease of the Donostia University Hospital. Ann Rheum Dis. 2022;81(Suppl 1):1289. doi: 10.1136/annrheumdis-2022-eular.269. [DOI] [Google Scholar]
- 79.Edwards CJ, Mount J, Meeks A, Zaremba-Pechmann L, Mian A, Larsson E, et al. O33 Baricitinib in the BSRBR-RA registry: characteristics and status of patients at first follow-up. Rheumatology. 2021 doi: 10.1093/rheumatology/keab246.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ciciriello S LG, Treuer T, Gibson K, Youssef P, Bird P, OSullivan C, Smith T, Deakin C. Real-world utilisation and switching between janus kinase inhibitors in patients with rheumatoid arthritis in the Australian OPAL Dataset [abstract. Arthritis Rheumatol. 2022;74. https://acrabstracts.org/abstract/real-world-utilisation-and-switching-between-janus-kinase-inhibitors-in-patients-with-rheumatoid-arthritis-in-the-australian-opal-dataset/ [DOI] [PubMed]
- 81.Gilbert B, Mongin D, Nham E, Courvoisier D, Lauper K, Laedermann C, et al. POS0435 Impact of combination therapy with csdmards on the effectiveness of biologic or targeted synthetic dmards in a real-life setting: results from the Swiss Rheumatoid Arthritis Register (SCQM-RA) Ann Rheum Dis. 2022;81(Suppl 1):472–473. doi: 10.1136/annrheumdis-2022-eular.3770. [DOI] [Google Scholar]
- 82.Alten R, Burmester GR, Matucci-Cerinic M, Salmon JH, López-Romero P, Fakhouri W, et al. AB0261 A multinational, prospective, observational study in patients with rheumatoid arthritis receiving baricitinib, targeted synthetic or biologic disease-modifying therapies (RA-BE-REAL) – study design and baseline characteristics. Ann Rheum Dis. 2021;80(Suppl 1):1157. doi: 10.1136/annrheumdis-2021-eular.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alten R BG, Matucci-Cerinic M, Ostor A, Zaremba-Pechmann L, Treuer T, Ng K, Gerwien J, Gibson K, Fautrel B. Time to discontinuation and effectiveness with baricitinib in rheumatoid arthritis: 12-month European data from a multinational, prospective, observational study [abstract]. Arthritis Rheumatol. 2022;74. https://acrabstracts.org/abstract/time-to-discontinuation-and-effectivenesswith-baricitinib-in-rheumatoid-arthritis-12-month-european-data-from-a-multinational-prospective-observationalstudy/ [DOI] [PMC free article] [PubMed]
- 84.Spinelli FR, Ceccarelli F, Garufi C, Duca I, Mancuso S, Cipriano E, et al. Effectiveness and safety of baricitinib in rheumatoid arthritis: a monocentric, longitudinal, real-life experience. Clin Exp Rheumatol. 2021;39(3):525–531. doi: 10.55563/clinexprheumatol/lfg83z. [DOI] [PubMed] [Google Scholar]
- 85.Takagi M, Atsumi T, Matsuno H, Tamura N, Fujii T, Okamoto N, et al. Safety and effectiveness of baricitinib for rheumatoid arthritis in Japanese clinical practice: 24-week results of all-case post-marketing surveillance. Mod Rheumatol. 2022 doi: 10.1093/mr/roac089. [DOI] [PubMed] [Google Scholar]
- 86.González Mazarío R, Fragío Gil JJ, Ivorra Cortés J, Grau García E, Cañada Martínez AJ, González Puig L, et al. Real-world effectiveness and safety of JAK inhibitors in rheumatoid arthritis: a single-centre study. Reumatolog Clín (English Edition) 2022;18(9):523–530. doi: 10.1016/j.reumae.2021.08.004. [DOI] [PubMed] [Google Scholar]
- 87.Ceccarelli F, Spinelli FR, Garufi C, Mancuso S, Alessandri C, Di Franco M, et al. The role of musculoskeletal ultrasound in predicting the response to JAK inhibitors: results from a monocentric cohort. Clin Exp Rheumatol. 2022;40(5):921–927. doi: 10.55563/clinexprheumatol/totvyv. [DOI] [PubMed] [Google Scholar]
- 88.Kosinski M, Zhao SZ, Dedhiya S, Osterhaus JT, Ware JE., Jr Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis Rheum. 2000;43(7):1478–1487. doi: 10.1002/1529-0131(200007)43:7<1478::Aid-anr10>3.0.Co;2-m. [DOI] [PubMed] [Google Scholar]
- 89.Strand V, Tugwell P, Bombardier C, Maetzel A, Crawford B, Dorrier C, et al. Function and health-related quality of life: results from a randomized controlled trial of leflunomide versus methotrexate or placebo in patients with active rheumatoid arthritis. Leflunomide Rheumatoid Arthritis Investigators Group. Arthritis Rheum. 1999;42(9):1870–1878. doi: 10.1002/1529-0131(199909)42:9<1870::Aid-anr11>3.0.Co;2-d. [DOI] [PubMed] [Google Scholar]
- 90.van der Heijde D, Kartman CE, Xie L, Beattie S, Schlichting D, Mo D, et al. Radiographic progression of structural joint damage over 5 years of baricitinib treatment in patients with rheumatoid arthritis: results from RA-BEYOND. J Rheumatol. 2022;49(2):133–141. doi: 10.3899/jrheum.210346. [DOI] [PubMed] [Google Scholar]
- 91.Studenic P, Aletaha D, de Wit M, Stamm TA, Alasti F, Lacaille D, et al. American College of Rheumatology/EULAR remission criteria for rheumatoid arthritis: 2022 revision. Arthritis Rheumatol. 2023;75(1):15–22. doi: 10.1002/art.42347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schiff M, Takeuchi T, Fleischmann R, Gaich CL, DeLozier AM, Schlichting D, et al. Patient-reported outcomes of baricitinib in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Res Ther. 2017;19(1):208. doi: 10.1186/s13075-017-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Emery P, Blanco R, Maldonado Cocco J, Chen YC, Gaich CL, DeLozier AM, et al. Patient-reported outcomes from a phase III study of baricitinib in patients with conventional synthetic DMARD-refractory rheumatoid arthritis. RMD Open. 2017;3(1):e000410. doi: 10.1136/rmdopen-2016-000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fitton J, Melville A, Dass S, Emery P, Nam J, Buch M. THU0169 Janus kinase inhibitors demonstrate effectiveness in a real-world multi-biologic DMARD refractory rheumatoid arthritis population. Ann Rheum Dis. 2019;78(Suppl 2):358–359. doi: 10.1136/annrheumdis-2019-eular.7246. [DOI] [Google Scholar]
- 95.Song YK, Song J, Kim K, Kwon JW. Potential Adverse Events Reported With the Janus Kinase Inhibitors Approved for the Treatment of Rheumatoid Arthritis Using Spontaneous Reports and Online Patient Reviews. Front Pharmacol. 2021;12:792877. doi: 10.3389/fphar.2021.792877. [DOI] [PMC free article] [PubMed]
- 96.Kerschbaumer A, Sepriano A, Bergstra SA, Smolen JS, van der Heijde D, Caporali R, et al. Efficacy of synthetic and biological DMARDs: a systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2023;82(1):95–106. doi: 10.1136/ard-2022-223365. [DOI] [PubMed] [Google Scholar]
- 97.EMA. Summary of Product Charecteritics-Olumiant.
- 98.Li Z, Hu J, Bao C, Li X, Li X, Xu J, et al. Baricitinib in patients with rheumatoid arthritis with inadequate response to methotrexate: results from a phase 3 study. Clin Exp Rheumatol. 2020;38(4):732–741. [PubMed] [Google Scholar]
- 99.Yang Y, Xu J, Xu J, Li X, Hu J, Li X, et al. Patient-reported outcomes from a randomized, double-blind, placebo controlled, phase III study of baricitinib versus placebo in patients with moderately to severely active rheumatoid arthritis and an inadequate response to methotrexate therapy: results from the RA-BALANCE study. Ther Adv Musculoskelet Dis. 2021;13:1759720x211006964. doi: 10.1177/1759720x211006964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367(6):508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 101.Fleischmann R, Mysler E, Hall S, Kivitz AJ, Moots RJ, Luo Z, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. 2017;390(10093):457–468. doi: 10.1016/s0140-6736(17)31618-5. [DOI] [PubMed] [Google Scholar]
- 102.Di Sanzo LSR, Soriano ER, Citera G, Mysler E, Wei JC-C, Ríos MHC. Drug Survival: treatment of rheumatic diseases in the biologic era. Front Med. 2022;9:858817. doi: 10.3389/fmed.2022.858817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yasir M GA, Sonthalia S. Corticosteroid Adverse Effects. [Updated 2022 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. [PubMed]
- 104.Bartlett SJ, De Leon E, Orbai AM, Haque UJ, Manno RL, Ruffing V, et al. Patient-reported outcomes in RA care improve patient communication, decision-making, satisfaction and confidence: qualitative results. Rheumatology (Oxford) 2020;59(7):1662–1670. doi: 10.1093/rheumatology/kez506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smolen JS, Kremer JM, Gaich CL, DeLozier AM, Schlichting DE, Xie L, et al. Patient-reported outcomes from a randomised phase III study of baricitinib in patients with rheumatoid arthritis and an inadequate response to biological agents (RA-BEACON) Ann Rheum Dis. 2017;76(4):694–700. doi: 10.1136/annrheumdis-2016-209821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sholter D, Wu J, Jia B, Zhang H, Griffing K, Birt J, et al. Maintenance of patient-reported outcomes in baricitinib-treated patients with moderate-to-severe active rheumatoid arthritis: post hoc analyses from two phase 3 trials. Rheumatol Ther. 2022;9(2):541–553. doi: 10.1007/s40744-021-00415-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen YC, Yoo DH, Lee CK, Li KJ, Won JE, Wu WS, et al. Safety of baricitinib in East Asian patients with moderate-to-severe active rheumatoid arthritis: an integrated analysis from clinical trials. Int J Rheum Dis. 2020;23(1):65–73. doi: 10.1111/1756-185x.13748. [DOI] [PubMed] [Google Scholar]
- 108.Taylor PC, Takeuchi T, Burmester GR, Durez P, Smolen JS, Deberdt W, et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann Rheum Dis. 2022;81(3):335–343. doi: 10.1136/annrheumdis-2021-221276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Taylor PC, Weinblatt ME, Burmester GR, Rooney TP, Witt S, Walls CD, et al. Cardiovascular safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheumatol. 2019;71(7):1042–1055. doi: 10.1002/art.40841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Winthrop KL, Harigai M, Genovese MC, Lindsey S, Takeuchi T, Fleischmann R, et al. Infections in baricitinib clinical trials for patients with active rheumatoid arthritis. Ann Rheum Dis. 2020;79(10):1290–1297. doi: 10.1136/annrheumdis-2019-216852. [DOI] [PubMed] [Google Scholar]
- 111.Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 112.Charles-Schoeman C, Buch MH, Dougados M, Bhatt DL, Giles JT, Ytterberg SR, et al. Risk of major adverse cardiovascular events with tofacitinib versus tumour necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: a post hoc analysis from ORAL Surveillance. Ann Rheum Dis. 2023;82(1):119–129. doi: 10.1136/ard-2022-222259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.EMA. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC). EMA starts safety review of Janus kinase inhibitors for inflammatory disorders. 2022.
- 114.Taylor PC, Bieber T, Alten R, Witte T, Galloway J, Deberdt W, et al. Baricitinib safety for events of special interest in populations at risk: analysis from randomised trial data across rheumatologic and dermatologic indications. Adv Ther. 2023;40(4):1867–1883. doi: 10.1007/s12325-023-02445-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available from the authors.