Abstract
Lignocellulose is a plentiful and intricate biomass substance made up of cellulose, hemicellulose, and lignin. Cellulose and hemicellulose are polysaccharides characterized by different compositions and degrees of polymerization. As renewable resources, their applications are eco-friendly and can help reduce reliance on petrochemical resources. This review aims to illustrate cellulose, hemicellulose, and their structures and hydrolytic enzymes. To obtain desirable enzyme sources for the high hydrolysis of lignocellulose, highly stable, efficient and thermophilic enzyme sources, and new technologies, such as rational design and machine learning, have been introduced in detail. Generally, the efficient biodegradation of abundant natural biomass into fermentable sugars or other intermediates has great potential in practical applications.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-023-03819-1.
Keywords: Cellulose, Cellulase, Hemicellulose, Hemicellulase, Machine learning
Introduction
Lignocellulose refers to plant dry matter and mainly originates from plant cell walls (Capetti et al. 2021). Lignocellulose primarily consists of cellulose, hemicellulose, and lignin, which are common natural polymers and plant resources (Shi et al. 2021). These three components are the most prevalent in nature, and their contents vary due to differences in their sources (Table S1).
While the primary role of lignocellulose is structural support in plants, other functions have also been identified for a long time (Gao et al. 2023). For example, wood has been utilized as a raw material for the pulp and paper industry for many centeries, and the recognition of cellulose and hemicellulose as dietary fiber has been acknowledged for many years. In the context of carbon neutralization, using lignocellulose as a renewable biological resource to decrease reliance on petrochemical resources presents clear advantages (Dias et al. 2022). With the depletion of fossil resources, the efficient development and utilization of lignocellulosic biomass resources based on forest resources will be crucial as humanity enters the postoil era. Consequently, the rational exploitation and utilization of cellulose and hemicellulose have become a hotspot in the field of biomass energy and chemicals worldwide (Masek and Kosmalska 2022; Yang et al. 2020). In addition, lignocellulosic materials have significant potential for various industrial applications, including but not limited to biofuels, pharmaceuticals, cosmeceuticals, bioplastics, multifunctional carbon materials, and other eco-friendly specialty products. These materials offer unique advantages, such as their abundance, renewability, and sustainability, making them attractive for application in a broad range of industrial processes (Contreras et al. 2020; Maroldi et al. 2018; Okolie et al. 2021).
Lignin is a phenolic polymer that is a noncarbohydrate, unlike cellulose and hemicellulose, which are carbohydrate polymers. The formation of liginin involves the oxidative coupling of three types of phenylpropane building blocks or monoligonols (Abdel-Hamid et al. 2013). As a result, the linkages in lignin are either C–O ether or C–C linkages, which make lignin chemically recalcitrant to degradation and commonly cleaved by some oxidoreductases. These properties hinder lignin from producing highly valuable materials, and currently, most lignin is burned as fuel for generating energy. Thus, this review focuses on cellulose, hemicellulose and their hydrolases.
The native dense structures of cellulose and hemicellulose molecules and the protective layer derived from xylogen cannot be easily converted to biomass directly by microbes. Biodegradation will be an essential direction for the conversion of lignocellulosic raw materials in the future due to mild reaction conditions, reduced capital investment/equipment requirements, and environmental pollution (Deralia et al. 2021). There are two primary methods of bioconversion: (1) direct fermentation by microorganisms, leading to the degradation of cellulose and hemicellulose (Prieto et al. 2021); (2) degradation of raw lignocellulosic material by using cellulase and hemicellulose to low poly oligosaccharides or complete degradation to glucose and xylose (Yuan et al. 2022), which are then chemically and biologically fermented to generate important chemicals (Sahoo et al. 2020; Maroldi et al. 2018).
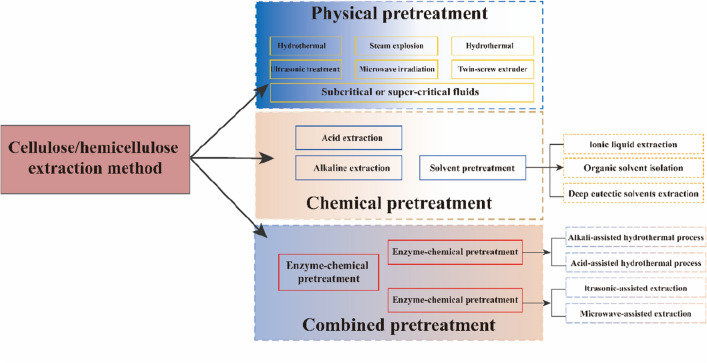
Traditional techniques for extracting cellulose and other compounds often involve acid and alkali hydrolysis (Ait Benhamou et al. 2022; Tavker et al. 2021). The products are obtained via alkali and bleaching treatment, followed by acid hydrolysis (Khan et al. 2020; Sun et al. 2021). Chemical methods can remove most hemicellulose and lignin but lead to significant loss of cellulose during extraction and substantial environmental pollution. In biological methods, various hydrolases are used to cleave and hydrolyze cellulose and other substances, allowing them to be degraded into reducing sugars for further use (Fig. 1). This approach is eco-friendly, efficient, and flexible, and it yields a higher quality of extracted products. As a result, biological methods are gradually replacing traditional chemical methods and gaining widespread applications.
Fig. 1.
Pretreatment of cellulose and hemicellulose via physical and/or chemical methods
This review begins by introducing cellulose and hemicellulose in detail, followed by a discussion of the associated enzymes, their structures and catalytic mechanisms. Furthermore, it outlines relevant and advanced research methods for studying these hydrolytic enzymes.
Chemical composition of cellulose and cellulase
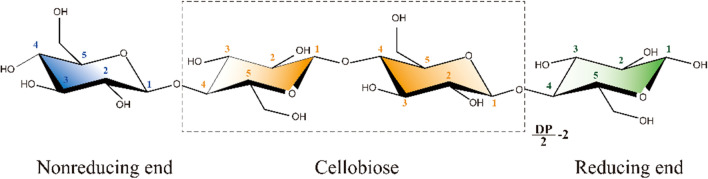
Cellulose is a linear polymer composed of repeated units of d-glucose connected by β-1,4 glycosidic bonds, which is a major component of renewable organic carbon in nature (Wang et al. 2022). The glucose molecules in cellulose are oriented in such a way that leads to the formation of cellobiose, a glucose dimer, as shown in Fig. 2. The cellulose chain has an asymmetrical structure, with one end linking a reducing end, such as a hemiacetal unit, and the other end lying on a side hydroxyl group, consisting of a nonreducing end. The degree of polymerization in the cell wall of different sources may vary, and plant cellulose molecules possess a relatively higher degree of polymerization than other components (Long et al. 2022).
Fig. 2.
Linear structure of cellulose with a reducing end and a nonreducing end
A group of hydrolytic enzymes called cellulases work in collaboration to break down cellulose by hydrolyzing its β-1,4 glycosidic bonds, resulting in the formation of cellobiose and glucose. Cellulose degradation involves a complex multicomponent enzyme system that works synergistically to break down polysaccharides into smaller glucose units (Payne et al. 2011; Horn et al. 2012). There are three types of cellulases classified according to their functions. The first type is endoglucanase (endo-1,4-β-d-glucanase, EC 3.2.1.4), which cleaves the glycosidic bond randomly inside the polysaccharides (Beckham et al. 2014; Chundawat et al. 2021). Endoglucanase can be either a nonsustained catalytic enzyme or a continuous catalytic enzyme that makes several consecutive cuts of polysaccharides via the active center (Vaaje-Kolstad et al. 2013; Taylor et al. 2013). The second type is exoglucanase (exo-1,4-β-d-glucanase, EC 3.2.1.74), which attacks the reducing or nonreducing end of polysaccharides. The continuous catalyst version of exoglucanase is called cellobiohydrolase (β-1,4-cellobiosidase, EC 3.2.1.91) and is dominant in both natural and commercial enzymes. The third type is β-glucosidase (EC 3.2.1.21), which converts the cellobiose formed by endoglucanase and cellobiohydrolase cleavage into the final product glucose.
Chemical composition of hemicellulose and hemicellulase
The structure of hemicellulose is complex, making it difficult to isolate specific components from the raw material. Hemicellulose has a much lower degree of polymerization than cellulose (Long et al. 2017). It can be primarily classified into three categories based on chemical composition: xylan, mannan, and arabinan.
Xylan and its hydrolase
Xylan is the primary type of hemicellulose, comprising approximately one-third of the total amount of renewable organic carbon in nature (Knapik et al. 2019; Dutta et al. 2010). It is a complex heteropolysaccharide with numerous branches. Xylan has a polypentose structure linked by β-d-1,4 xylosidic bonds, with various substituents, such as arabinose, acetyl, and glucuronoic acid (Cao et al. 2021; Alokika and Singh 2019). Various sources of xylan contain different side chain groups, especially that in hardwoods, the predominant form is O-acetyl-4-O-methylglucuronoxylan, while arabino-4-O-methylglucuronoxylan is predominant in softwoods (Bajaj and Mahajan 2019; Singh et al. 2003). Arabinoxylans are the main form of xylan in annual plants and herbs (Alcobaça et al. 2022; Kuramochi et al. 2016). Pinus sylvestris and tobacco xylans do not contain side chain groups, while some marine algae xylans exist in the form of xylopyranoside linked by β-D-1,3 xylosidic bonds and β-d-1,4 xylosidic bonds (Liab et al. 2000). The degree of polymerization varies significantly based on its origin, with hardwood xylan having a degree of polymerization ranging from 150 to 200, while softwood xylan ranges from 130 to 170.
The complete biodegradation of xylan consisting of a complex and heterogeneous polysaccharide requires a full complement of hydrolases (Dhiman et al. 2013; Jagtap et al. 2013). The main enzymes involved in this process are xylanase (EC 3.2.1.8) and β-xylosidase (EC 3.2.1.37), which act to cut xylan molecules into smaller xylooligosaccharides, which are then further converted into xylose or xylobiose (Ohta et al. 2010; Bankeeree et al. 2018). Other xylan degrading enzymes, such as α-arabinofuranosidase (EC 3.2.1.55), acetylxylanase (EC 3.1.1.72) and α-glucosidase (EC 3.2.1.139), are other important enzymes capable of removing side chain residues of xylan (Subramaniyan and Prema 2002). The complete decomposition of xylan requires the synergistic action of these enzymes (Fig. 3).
Fig. 3.
The basic structure and components of xylan and hydrolases are responsible for their degradation. These hydrolases mainly consist of xylanase and xylodisdase
Mannan and its hydrolase
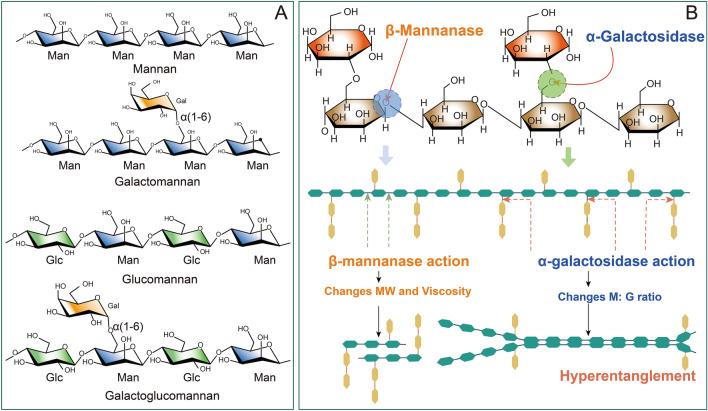
The hemicellulose component of angiosperms is mainly composed of xylan, while in gymnosperms, it is mainly expressed as mannan (Fig. 4) (Moreira and Filho 2008; Singh et al. 2018). Based on chemical composition, mannan can be categorized into linear mannan, galactomannan, glucomannan and galactoglucomannan. Linear mannan has a galactose content of less than 5%, while glucomannan is a type of polysaccharide that is made up of d-mannose and d-glucose units connected together through β-1,4 glycosidic bonds. In comparison, linear mannan and galactomannan also have backbones linked by β-1,4 glycosidic linkages of d-mannose residues but do not contain d-glucose residues. Galactomannans typically have galactose side chain residues, with 20% to 100% of the side chains being replaced by galactose. Various enzymes are needed for the complete hydrolysis of mannan, primarily including endo-β-1,4-mannanase (EC 3.2.1.78) and α-galactosidase (EC 3.2.1.22).
Fig. 4.
Structure and components of mannan and hydrolases responsible for their degradation. These hydrolases are primarily composed of mannanase and α-galactosidase
Arabinan and its hydrolase
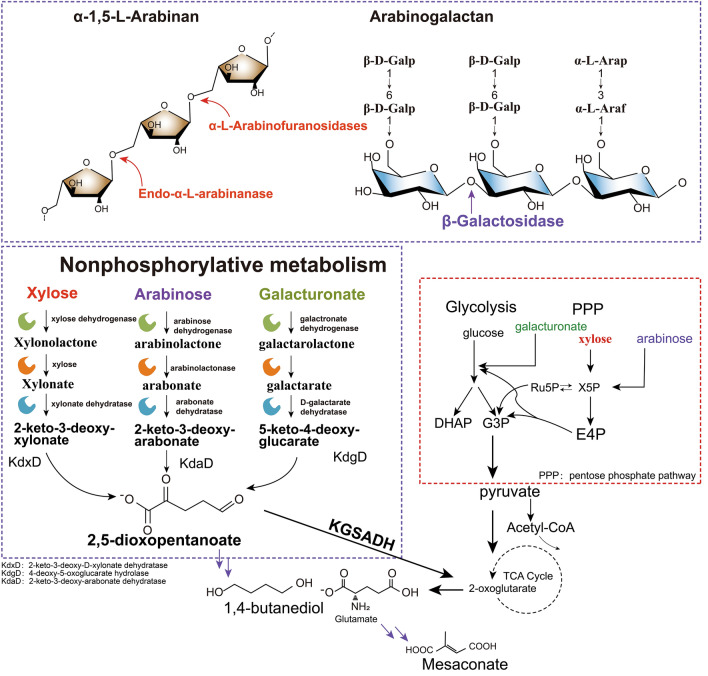
Arabinan can be extracted from various fruits and seeds, as well as from the bark of pine, geranium root, aspen, and willow. Initially, it was challenging to determine whether arabinan was hemicellulose or a mixture of unstable polysaccharide-lignin compounds, as it was primarily isolated from degraded pectin fragments under mild reaction conditions (Yamaguchi et al. 2018; Shi et al. 2014). Arabinan is a mixture of various polysaccharides made up of arabinose units. These units are connected to the main chain through α-1,5 glycosidic bonds, and additional linkages can also occur via α-1,2 and/or α-1,3 glycosidic bonds (Hong et al. 2009; Zhang et al. 2020). To fully hydrolyze arabinan, the enzymatic reactions of endo-arabinanase (EC 3.2.1.99) and arabinosidase need to work together (Fig. 5).
Fig. 5.
Structure and components of arabinan and hydrolases responsible for their degradation
Classification of cellulase and hemicellulase
Source and classification of cellulase
The bioconversion of cellulose raw materials has been shown to be an effective application technology with great potential in the development of new production processes and products (Ejaz et al. 2021; Sharma et al. 2016). Microbial cellulase is the most important biocatalyst in bioconversion. Cellulase is a widely distributed inducible enzyme found in fungi, bacteria (including some actinomycetes, as shown in Table S2), and some archaea (Cai et al. 2020). The cellulase-producing bacteria can be categorized into four types: aerobic, anaerobic, normal temperature and high-temperature resistant. Although Table S2 shows only a small fraction of the cellulase-producing bacteria, it is evident that these bacteria are highly diverse. The cellulases that have been extensively researched are derived from Cellulomonas, Clostridium, Trichoderma, Thermomonospora and Thermotoga.
Cellulase consists of domains, which are structurally and functionally autonomous modules. The glycosyl hydrolase (GH) families classify cellulases from different sources based on the amino acid's primary structure, the domain's composition and spatial structure, and the substrate of action. Endoglucanase is distributed in 12 GH families, exoglucanase in 4 GH families, and β-glucosidase in 6 GH families, as indicated in Table 1. As the database continues to accumulate more microbial genome sequences (https://www.ncbi.nlm.nih.gov), there will be ongoing updates to the cellulase GH family and its members.
Table 1.
Classification of cellulases in the glycoside hydrolase families
| Cellulase | Substrate | EC | Glycoside hydrolase family | Protein structure and PDB accession number |
|---|---|---|---|---|
| Endoglucanase | Cellulose | 3.2.1.4 | 5, 6, 7, 8, 9, 10, 12, 44, 45, 48, 51, 74, 124, 148 | 2ENG, 3AMG, 3WY6, etc |
| Exoglucanase (1,4-β-cellobiosidase) | Cello-oligosaccharide | 3.2.1.74 (3.2.1.91) | 1, 3, 5(5), (6), 7, (9), 39, (51) | 5YJ6, 5XYH, 7YHF, etc |
| β-Glucosidase |
Cello-oligosaccharide Cellobiose |
3.2.1.21 | 1, 2, 3, 5, 16, 30, 39, 116, 131, 175, 180 | 5OGZ, 1UYQ, 2CER, etc |
The three enzymes present in cellulase differ in source, species, and protein structure, mainly in the tertiary and quaternary structure of the protein. The structure determines the reaction conditions and catalytic properties of cellulose hydrolase from different sources. The Protein Data Bank (PDB, https://www.rcsb.org) is the largest database against protein structure, which contains almost all known protein three-dimensional structure models and related research dynamics, enabling us to study the structure of proteins. The PDB database shows that (1) eleven GH families of endoglucanases (e.g., GH 5, 6, and 7) have published three-dimensional protein structure models, and the structures are significantly different; (2) exoglucanases or 1,4-β-cellobiosidase belong to the GH 1, 3, 5, 6, 7, 9, 39 and 51 families, with only structural reports available in the PDB database; and (3) β-glucosidase belongs to the 1, 2, 3, 5, 30, 39, 116, 131, 175 and 180 GH families. In summary, cellulase is found in 25 GH familiesn (Table 1).
Source and classification of hemicellulase
Hemicellulase is another inducible enzyme that is widely distributed in fungi, actinomycetes, and bacteria (Echeverría and Eyzaguirre 2019; Brito-Cunha et al. 2013). However, since the structures of hemicelluloses are much more complex than those of cellulose, the complete degradation of hemicellulose requires a larger number of hydrolases (Table S3). A comparison of Tables S2 and S3 shows that many microorganisms that produce cellulase also produce hemicellulase. Furthermore, when querying the NCBI (https://www.ncbi.nlm.nih.gov) and CAZy (https://www.rcsb.org) databases, it was found that these cellulase- and hemicellulase-producing strains also contain many other GHs that are not included in the scope of this paper. Therefore, they will not be discussed further.
Hemicellulase is widely distributed in the GHs, contains a large amount of acetyl or ferulic acid lipase in the 9 GHs and is classified in a similar manner to cellulases (Tsai et al. 2017). Specifically, xylanase is found in GH 5, 6, 8, 10, 11,26, 30, 43, 51, 98 and 141 families; β-xylosidase in GH 1, 2, 3, 5, 8, 10, 30, 39, 43, 51, 52, 52, 54, 116 and 120 families; α-L-arabinofuranosidase in GH 2, 3, 5, 10, 43, 51, 54 62 and 159 families; α-glucuronidase in GH 67 and 115 families; arabinase in GH 43 family; mannanase in GH 5 and 26 families; β-mannosidase in GH 1, 2, and 5 families; α-galactosidase in GH 4, 27, 31, 36, 57, 97 and 110 families; galactanase in GH 53 and 147 families. Table 2 shows the substrate, classification, and structural model of the PDB database. According to statistics, the PDB data contain a large number of three-dimensional structural models of hemicellulase.
Table 2.
Classification of hemicellulases in the glycoside hydrolase families
| Hemicellulase | Substrates | EC | Glycoside hydrolase family | Protein structure and PDB accession number |
|---|---|---|---|---|
| Xylanase | β-xylan | 3.2.1.8 | 5, 6, 8, 10, 11, 26, 30, 43, 51, 98, 141 | 3HD8, 1NOF, 2B42, etc |
| β-Xylosidase | Xylooligosaccharides/xylobiose | 3.2.1.37 | 1, 2, 3, 5, 8, 10, 30, 39, 43, 51, 52, 54, 116, 120 | 3VSU, 7VC7, 4C1O, etc |
| Mannase | β-Mannan | 3.2.1.78 | 5, 26, 113,134 | 1RH9 |
| Endo-α-1,5-arabinanase | α-1,5-Arabinan | 3.2.1.99 | 43 | 2X8T, 1UV4, 6F1G, etc |
| β-mannosidase | Mannan-oligosaccharide, mannobiose | 3.2.1.25 | 1, 2, 5, 113, 164 | 6DDT, 4LYQ, 4NRS, etc |
| α-galactosidase | α-1,6-Galactosyl-mannan-oligosaccharide | 3.2.1.22 | 4, 27, 31, 36,57, 97, 110 | 1T0O, 3MI6, 4FNR, etc |
| Galactanase | β-Galactan | 3.2.1.89 | 53, 147 | 6Q3R, 7OSK, 4BF7, etc |
| β-glucosidase | β-1,4-glucomannan | 3.2.1.21 | 1, 2, 3, 5, 16, 30, 39, 116, 131, 175, 180 | 3VIK, 815P, 8I5P, etc |
| α-l-Arabinofuranosidase | Arabinoxylo-(α-1,2 or α-1,3)-oligosaccharides, α-1,5-arabinan | 3.2.1.55 | 2, 3, 5, 10, 43, 51, 54, 62, 159 | 1WD3, 1QW9, 2C8N, etc |
| α-Glucuronidase | 4-O-methyl-α-glucuronyl-xylan | 3.2.1.139 | 67, 115 | 1K9E, 1MQR, 4C9O, etc |
Catalytic mechanism of cellulase and hemicellulase
Cellulase and hemicellulase both belong to GH and share similar catalytic domains and mechanisms, especially in the same families. The primary and three-dimensional structures of cellulase and hemicellulase are crucial for understanding the active amino acid residues and substrate binding sites of these hydrolases. Enzymatic hydrolysis of glycosidic bonds typically involves the participation of two acidic residues, one acting as a proton donor to catalyze the hydrolysis of glycosidic bonds and the other as a nucleophilic group to stabilize the charge (Du et al. 2020; Yakovlieva and Walvoort 2020). These two active residues support one or two SN2 displacement reactions catalyzed by GHs (Akram et al. 2021), corresponding to the two catalytic mechanisms for those glycosidases: inverting and retaining mechanisms. Both mechanisms involve breaking down polysaccharides or oligosaccharides by hydrolysis while maintaining or reversing the anomeric atom configuration. The proton donor site is common to both mechanisms, wherein the oxygen atom of the internal glycoside forms a hydrogen bond at the same distance. However, these mechanisms vary in terms of the distance between the substrate’s anomeric carbon and the nucleophilic catalytic base. In the retaining mechanism, the nucleophilic catalytic base is located near the substrate’s anomeric carbon. However, in the converting mechanism, the distance between them is relatively larger. The average distance of the two catalytic amino acids in the relevant hydrolase in the retention mechanism is 5.5 Å, while the average distance of the two catalytic amino acids in the flipping mechanism is 10 Å (Shen et al. 2019) (Fig. 6). Lysozyme was the first GH to have a three-dimensional structure reported to have two catalytic amino acids (aspartic acid and glutamic acid) (Yang and Leśnierowski 2020; Shi et al. 2020). For most GHs, including cellulase and hemicellulase, the catalytically active amino acids are also aspartic acid and/or glutamic acid. In addition to these two amino acids, studies have shown that tyrosine has similar catalytic functions in viral neuraminidase and bacterial sialidase (Crennell et al. 1993).
Fig. 6.

Two major mechanisms of hydrolysis by cellulase and hemicellulase. a Retaining mechanism and b Inverting mechanism
Research methods of cellulase and hemicellulase
The benefits of thermophilic enzymes
The discovery of thermophilic microorganisms has made them a valuable source of industrial enzyme preparations, enriching the library of enzyme preparations. Heat-resistant enzymes have demonstrated outstanding application value in many industries that require highly thermally stable biocatalysts, such as food, feed processing, paper, and energy (Li et al. 2021; Vaaje-Kolstad et al. 2013). Thermophilic enzymes present several advantages in biotechnology compared to enzymes that function at normal temperatures. These advantages include (1) exceptional stability and resistance to deactivation at high temperatures; (2) reduced vulnerability to denaturing agents; (3) an accelerated reaction rate that can shorten reaction cycles and enhance economic efficiency; and (4) decreased susceptibility to contamination during the catalytic reaction process.
Thermophilic enzymes are derived from thermophilic microorganisms (60–80 °C) and extreme thermophilic microorganisms (≥ 80 °C), including archaea, bacteria, and some fungi (Esteves et al. 2019; Morgado and Vicente 2019). Since the discovery of thermophilic and thermostable enzymes originating from Thermus aquaticus (Pereira et al. 2010), research on thermotolerant and thermostable enzymes has become a hot topic (Li et al. 2021; Basen and Müller 2017; Okanishi et al. 2017). Heat-resistant enzymes, especially extremely heat-resistant enzymes, may solve the problem of efficiently degrading lignocellulosic raw materials due to their high thermal stability and excellent catalytic properties (Guo et al. 2011; Carvalho et al. 2018; Wang et al. 2021). With the determination of thermophilic and extreme thermophilic genomic DNA, research on cellulases and hemicellulases derived from thermophilic bacteria has become increasingly intense (Muderspach et al. 2021; Lee et al. 2018; Li et al. 2017). By using gene cloning technology, a large number of lignocellulosic hydrolases can be cloned and expressed from the genomes of extreme thermophiles (Míguez Amil et al. 2020; Yang and Han 2018), such as Pyrococcus, Sulfolobus, Ignisphaera, Thermotoga and Pseudothermotoga (Esteves et al. 2019; Shi et al. 2019).
Although some progress has been made in the study of high-temperature hydrolysis of lignocelluloses, the enzymes currently identified are mainly derived from extreme thermophilic bacteria. The enzyme activity and catalytic properties of archaeal high-temperature enzymes may still be far from ready for industrialization. The key to addressing this issue lies in the lack of effective basic research on the molecular mechanism and orientation modification of these thermophilic enzymes. Thus, future research should prioritize the molecular analysis and modification of exceedingly heat-resistant cellulases and hemicellulases. This approach will enable the acquisition of crucial enzymes capable of effectively and durably degrading lignocellulosic materials. At present, methods for studying thermophilic enzymes mainly include homologous sequence alignment, phylogenetic reconstruction, homology modeling and structural analysis, and theoretically designed site-directed mutagenesis.
Homologous sequence alignment
Comparing the primary structure of homologous proteins facilitates the identification of differences in amino acid composition between heat-resistant and normal-temperature proteins. The amino acid composition of a thermostable enzyme is distinct from that of a normal-temperature enzyme, which contributes to its high thermal stability (Yang et al. 2017a). For example, most extremely thermostable enzymes, such as endoglucanase and xylanase derived from Thermotoga maritima, and many thermostable enzymes, such as xylanase, xylosidase, and arabinase derived from Thermotoga thermarum, have low cysteine and histidine contents, reducing the likelihood of the enzyme forming a thermolabile disulfide bond and promoting thermal stability (Dumorné et al. 2017). The stability of microorganisms and their biomolecules under harsh environmental conditions is largely determined by temperature, which is considered one of the most critical parameters. To leverage their commercial and industrial value, it is necessary to fully utilize thermostable enzyme biotechnology during the reaction process. Thermostable and heat-resistant enzymes are key to efficiently and economically converting substrates into commercially applicable products (Rekadwad and Gonzalez 2019). Thermostable enzymes are known to exhibit higher levels of proline, alanine, threonine, and arginine than enzymes that function at normal temperatures. In addition, thermostable enzymes also have increased hydrophobicity and a greater number of charged amino acid residues (Murray et al. 2003).
Several software tools are available for amino acid sequence alignment, including Clustal and MEGA, which enable investigation of the amino acid composition and heat resistance of thermostable enzymes (Murray et al. 2004). Such alignment can provide a useful theoretical reference for further study on the heat resistance characteristics of these enzymes.
Phylogenetic reconstruction
Genetic mutation is the primary cause of evolution. When a mutation occurs, such as base substitution, insertion, or deletion, it can lead to changes in the amino acid or nucleotide sequence of a homologous protein from different species. These changes can then be diffused by natural selection at the population level and eventually fixed in the species. There are certain linkages in the evolution of homologous amino acid sequences of heat-resistant enzymes from different sources (Schiano-di-Cola et al. 2020). Therefore, phylogenetic reconstruction can be used to analyze the evolutionary relationships between thermostable and normal temperature enzymes and distinguish the classification status of thermostable enzymes from different sources (Zhu et al. 2020).
The significance of the phylogenetic reconstruction of thermostable enzymes is to explore their characteristics from an evolutionary perspective, providing an important reference for systematic analysis of thermostable enzymes from different sources (Shi et al. 2013a). Bioinformatics software such as MEGA, PAUP, and Bayes are frequently utilized in phylogenetic reconstruction, while analysis techniques, including neighbor-joining, maximum parsimony, and maximum likelihood, are commonly employed. For example, researchers built a continuous tree using MEGA 7 software. The analysis revealed that the endoglucanase obtained from the thermophilic Fervidobacterium nodosum and the endoglucanase from Thermotoga maritima grouped together. The related functional amino acids of the endoglucanase were estimated based on the sequence (Shi et al. 2013b). The xylanase and xylosidase of Thermotoga thermarum were systematically reconstructed, and the results showed that the xylanase in the taxonomic branch, T. thermarum and other similar enzymes of the genus Thermotoga were grouped together, showcasing slight yet statistically significant variations in their enzymatic characteristics (Boyce and Walsh 2018; Yang et al. 2017b).
Protein structure analysis and homology modeling
The structure of a protein plays a critical role in determining its nature and function, including heat resistance (Pucci et al. 2020). In the past 50 years, approximately 25% of the Nobel Prizes in Chemistry have been granted for research focused on the structure of biological macromolecules. This research has contributed to establishing a strong theoretical basis for protein redesign (Ioannidis et al. 2020). Technological advancements, such as X-ray crystallography, nuclear magnetic resonance, and homology modeling, have facilitated obtaining enzyme molecular structures and comprehending their catalytic and heat-resistant mechanisms in a sequential manner. The commonly used method for reporting protein crystals is X-ray crystal diffraction technology, which involves protein purification, crystal cultivation, diffraction, structural analysis, and model establishment (McPherson 2017).
Homology modeling is a computational method used to establish a three-dimensional structural model of a protein based on its amino acid sequence (target sequence) (Bertoni et al. 2017). To build a successful model, it is necessary to have a three-dimensional protein structure (known as a template) that has been resolved structurally, and the amino acid sequence of the template should exhibit noticeable similarity to the target sequence. SWISS-MODEL (https://swissmodel.expasy.org) was used to model the target protein's amino acid sequence based on the template structure. The specific steps include selecting an appropriate template, aligning the target sequence with the template, establishing the model, and modifying and evaluating it. The best protein structure model is then obtained, which depicts the three-dimensional structure of the exo-arabinase modeled using homology (Velasco et al. 2020).
Mutation based on theoretical design
Site-directed mutagenesis is a widely used technique in genetic research involving the introduction of a desired nucleotide into a DNA fragment, usually a plasmid containing a gene of interest, by reverse PCR (Yonemoto and Weyman 2017). Genetic transformation makes this technique valuable for modifying traits. Among enzyme molecular modification techniques, site-directed mutagenesis is the most commonly used and mature technology. Mutations can change the structure of proteins, thereby affecting their catalytic properties and functions. This technique has become an essential tool in the study of thermal stability mechanisms and enzyme modification of heat-resistant enzymes (Yonemoto and Weyman 2017; Dong et al. 2020).
Protein three-dimensional structure, homologous sequence alignment, and phylogenetic reconstruction can provide valuable information for the functional study of proteins. By designing experiments more efficiently and transforming proteins to meet the needs of protein engineering, researchers can improve protein thermostability, pH, and specific activity. Studies have shown that substitutions of certain amino acids, such as Lys → Arg, Asp → Glu, and Gly → Ala, can significantly enhance protein thermostability (Mylemans et al. 2020). The secondary structure of thermostable enzymes is generally conserved and related to their thermal stability. Functional amino acids are typically located in the linking regions of conserved structures such as loops, turns, coils, alpha-helices, and beta-sheets (Torktaz et al. 2018). Site-directed mutagenesis can not only improve the thermostability of enzymes but also increase their specific activity and even change their pH. For instance, substitutions of Leu → Asn and Gln → Glu in Clostridium cellulovorans endoglucanase have been shown to improve enzyme activity (Torktaz et al. 2018; Lee et al. 2010).
Advanced technologies in enzyme design
Enzyme engineering is a scientific process aimed at generating enhanced biocatalysts through modifications in their amino acid sequences. Traditionally, two main methodologies have been dominant in this discipline: rational design (Musil et al. 2018; Romero-Rivera et al. 2017) and directed evolution (Currin et al. 2015; Arnold 2015). However, a third paradigm, driven by machine learning (ML), has been progressively surfacing in recent years (Ge et al. 2023).
Unlike rational design, which is predicated on a model-centric approach, ML utilizes a data-centric methodology. It discerns patterns within extant data and leverages these identified patterns to extrapolate the properties of analogous but unobserved instances (Mazurenko et al. 2019). In contrast to directed evolution, which involves repeated selections from pre-existing mutations, an ML-guided strategy can engender novel and potentially beneficial variants that were previously unobserved, informed by the discerned data patterns.
The application of ML to enzyme design has yielded noteworthy advancements in the conception and optimization of novel designs, finding utility in diverse sectors, including medicine, chemistry, and agriculture (Mater and Coote 2019). With the growing recognition of the utility of ML in enzyme design, there has been particular interest in utilizing deep learning tools. These tools have the potential to assist protein engineers in uncovering optimal sequences conducive to desired enzymatic properties (Domingos 2012). Additionally, Priyakumar et al. introduced "SCONES", a self-consistent neural network purposed for predicting protein stability subsequent to mutation (Park and Kellis 2015). The degradation process of lignocellulosic biomass relies heavily on the involvement of hydrolytic enzymes. The development of these enzymes has been greatly influenced by recent advancements in machine learning (ML) in several ways. (1) Artificial neural networks (ANNs) have enabled predictive modeling of the optimal conditions for enzymatic hydrolysis. For example, Gama and his colleagues utilized an ANN effectively to model the enzymatic hydrolysis of apple pomace, considering different input variables. Their model, exhibiting an R2 value of 0.99 and a negligible mean square error (MSE), successfully predicted optimal conditions for apple pomace hydrolysis (Gama et al. 2017). (2) The design of artificial enzymes has been revolutionized by deep learning networks. For instance, the trRosetta structure prediction network was utilized to generate starting residue-residue distance maps from random amino acid sequences. Then, the amino acid sequence space was optimized by conducting Monte Carlo sampling. The contrast (Kullback–Leibler divergence) between predicted interresidue distance distributions and averaged background distributions was evaluated using this method across all proteins. Deep learning networks accurately determine the three-dimensional structures of these newly designed proteins (Anishchenko et al. 2021). (3) ML has been employed in the identification of enzyme inhibitors. For instance, support vector classifier-based ML models were used to predict inhibitors of the acetylcholinesterase (AChE) enzyme, which is implicated in the hydrolytic degradation of acetylcholine (Ach) (Ganeshpurkar et al. 2022). Although it is worth noting that ML cannot fully capture all aspects of computational protein engineering and enzyme design, it cannot be denied that these computational methodologies have become essential in the protein design process. As such, to obtain desirable enzymes, ML is poised to contribute significantly to future advancements in biotechnology.
Conclusions
In summary, cellulose and hemicellulose are two of the most abundant biomolecules in nature, with cellulose being the most abundant and hemicellulose being the second most abundant. Cellulose is composed of repeating units of d-glucose, forming a linear polysaccharide. However, the structure of hemicellulose is relatively complex, which makes it challenging to isolate specific components directly from raw materials. Biodegradation involving various enzymes is receiving increasing attention because it is eco-friendly, efficient, and flexible. Cellulases and hemicellulases are mainly derived from microorganisms, such as Cellulomonas, Clostridium, Trichoderma, Thermomonospora, and other thermophilic bacteria. The discovery of thermophilic microorganisms has significantly enriched the enzyme preparation library and made them an important source of industrial enzyme preparations. Thermostable enzymes, especially those derived from thermophilic microorganisms, have shown significant potential in various industries that require biocatalysts with high thermal stability, including food, feed processing, paper making, and energy. In our previous work, several thermophilic hemicellulases were cloned, expressed and characterized, and they showed great potential in the biodegradation of lignocellulose.
The mechanisms behind the thermostability of thermostable enzymes have been investigated by homologous sequence alignment, leading to an understanding of the amino acid composition and thermostability of these enzymes. These findings provide a theoretical reference for further research into the thermostable characteristics of enzymes. Moreover, the characteristics of thermostable enzymes can be explored through phylogenetic reconstruction, which is based on the fundamental principle of evolution, i.e., gene mutation. With powerful machine learning, these enzymes may be optimized and achieve higher catalytic activity and thermostability.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
H. L, M. X and X. N drafted the manuscript, H. L, Z. T and X. Y revised the manuscript, H. S, X. L and T. W administered the work, drafted the manuscript and critically revised the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation (No. 21878160) and Key R&D Plan Cultivation Project of Huaiyin Institute of Technology (No. 22HGZ001).
Data availability
Not applicable.
Declarations
Conflict of interests
All authors declare no competing interests.
Informed consent
All authors consent to publish this work.
Research involving Human Participants and/or Animals
Not applicable.
Contributor Information
Hao Shi, Email: ilyshihao@163.com, Email: ilyshihao@hyit.edu.cn.
Xun Li, Email: xunli@njfu.edu.cn.
Tao Wang, Email: wangtao@uga.edu.
References
- Abdel-Hamid AM, Solbiati JO, Cann IK. Insights into lignin degradation and its potential industrial applications. Adv Appl Microbiol. 2013;82:1–28. doi: 10.1016/B978-0-12-407679-2.00001-6. [DOI] [PubMed] [Google Scholar]
- Ait Benhamou A, Kassab Z, Boussetta A, Salim MH, Ablouh EH, Nadifiyine M, Qaiss AEK, Moubarik A, El Achaby M. Beneficiation of cactus fruit waste seeds for the production of cellulose nanostructures: extraction and properties. Int J Biol Macromol. 2022;203:302–311. doi: 10.1016/j.ijbiomac.2022.01.163. [DOI] [PubMed] [Google Scholar]
- Akram F, ul Haq I, Aqeel A, Ahmed Z, Shah FI. Thermostable cellulases: structure, catalytic mechanisms, directed evolution and industrial implementations. Renew Sust Energ Rev. 2021;151:111597. doi: 10.1016/j.rser.2021.111597. [DOI] [Google Scholar]
- Alcobaça OSA, Campanini EB, Ciancaglini I, Rocha SV, Malavazi I, Freire CCM, Nunes FMF, Fuentes ASC, Cunha AF. Identification of a new endo-β-1,4-xylanase prospected from the microbiota of the termite Heterotermes tenuis. Microorganisms. 2022;10(5):906. doi: 10.3390/microorganisms10050906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alokika SB. Production, characteristics, and biotechnological applications of microbial xylanases. Appl Microbiol Biot. 2019;103(21–22):8763–8784. doi: 10.1007/s00253-019-10108-6. [DOI] [PubMed] [Google Scholar]
- Anishchenko I, Pellock SJ, Chidyausiku TM, Ramelot TA, Ovchinnikov S, Hao J, Bafna K, Norn C, Kang A, Bera AK. De novo protein design by deep network hallucination. Nature. 2021;600(7889):547–552. doi: 10.1038/s41586-021-04184-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold FH. The nature of chemical innovation: new enzymes by evolution. Q Rev Biophys. 2015;48(4):404–410. doi: 10.1017/S003358351500013X. [DOI] [PubMed] [Google Scholar]
- Bajaj P, Mahajan R. Cellulase and xylanase synergism in industrial biotechnology. Appl Microbiol Biot. 2019;103(21–22):8711–8724. doi: 10.1007/s00253-019-10146-0. [DOI] [PubMed] [Google Scholar]
- Bankeeree W, Akada R, Lotrakul P, Punnapayak H, Prasongsuk S. Enzymatic hydrolysis of black liquor xylan by a novel xylose-tolerant, thermostable β-xylosidase from a tropical strain of Aureobasidium pullulans CBS 135684. Appl Biochem Biotech. 2018;184(3):919–934. doi: 10.1007/s12010-017-2598-x. [DOI] [PubMed] [Google Scholar]
- Basen M, Müller V. "Hot" acetogenesis. Extremophiles. 2017;21(1):15–26. doi: 10.1007/s00792-016-0873-3. [DOI] [PubMed] [Google Scholar]
- Beckham GT, Ståhlberg J, Knott BC, Himmel ME, Crowley MF, Sandgren M, Sørlie M, Payne CM. Towards a molecular-level theory of carbohydrate processivity in glycoside hydrolases. Curr Opin Biotech. 2014;27:96–106. doi: 10.1016/j.copbio.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Bertoni M, Kiefer F, Biasini M, Bordoli L, Schwede T. Modeling protein quaternary structure of homo- and hetero-oligomers beyond binary interactions by homology. Sci Rep. 2017;7(1):10480. doi: 10.1038/s41598-017-09654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce A, Walsh G. Purification and characterisation of a thermostable β-Xylosidase from Aspergillus niger van tieghem of potential application in lignocellulosic bioethanol production. Appl Biochem Biotech. 2018;186(3):712–730. doi: 10.1007/s12010-018-2761-z. [DOI] [PubMed] [Google Scholar]
- Brito-Cunha CC, de Campos IT, de Faria FP, Bataus LA. Screening and xylanase production by Streptomyces sp. grown on lignocellulosic wastes. Appl Biochem Biotech. 2013;170(3):598–608. doi: 10.1007/s12010-013-0193-3. [DOI] [PubMed] [Google Scholar]
- Cai M, Liu Y, Yin X, Zhou Z, Friedrich MW, Richter-Heitmann T, Nimzyk R, Kulkarni A, Wang X, Li W, Pan J, Yang Y, Gu JD, Li M. Diverse Asgard archaea including the novel phylum Gerdarchaeota participate in organic matter degradation. Sci China Life Sci. 2020;63(6):886–897. doi: 10.1007/s11427-020-1679-1. [DOI] [PubMed] [Google Scholar]
- Cao L, Zhang R, Zhou J, Huang Z. Biotechnological aspects of salt-tolerant xylanases: a review. J Agric Food Chem. 2021;69(31):8610–8624. doi: 10.1021/acs.jafc.1c03192. [DOI] [PubMed] [Google Scholar]
- Capetti CCM, Vacilotto MM, Dabul ANG, Sepulchro AGV, Pellegrini VOA, Polikarpov I. Recent advances in the enzymatic production and applications of xylooligosaccharides. World J Microb Biot. 2021;37(10):169. doi: 10.1007/s11274-021-03139-7. [DOI] [PubMed] [Google Scholar]
- Carvalho DR, Carli S, Meleiro LP, Rosa JC, Oliveira AHC, Jorge JA, Furriel RPM. A halotolerant bifunctional β-xylosidase/α-l-arabinofuranosidase from Colletotrichum graminicola: purification and biochemical characterization. Int J Biol Macromol. 2018;114:741–750. doi: 10.1016/j.ijbiomac.2018.03.111. [DOI] [PubMed] [Google Scholar]
- Chundawat SPS, Nemmaru B, Hackl M, Brady SK, Hilton MA, Johnson MM, Chang S, Lang MJ, Huh H, Lee SH, Yarbrough JM, López CA, Gnanakaran S. Molecular origins of reduced activity and binding commitment of processive cellulases and associated carbohydrate-binding proteins to cellulose III. J Biol Chem. 2021;296:100431. doi: 10.1016/j.jbc.2021.100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras F, Pramanik S, Rozhkova AM, Zorov IN, Korotkova O, Sinitsyn AP, Schwaneberg U, Davari MD. Engineering robust cellulases for tailored lignocellulosic degradation cocktails. Int J Mol Sci. 2020;21(5):1589. doi: 10.3390/ijms21051589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crennell SJ, Garman EF, Laver WG, Vimr ER, Taylor GL. Crystal structure of a bacterial sialidase (from Salmonella typhimurium LT2) shows the same fold as an influenza virus neuraminidase. P Natl Acad Sci USA. 1993;90(21):9852–8856. doi: 10.1073/pnas.90.21.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currin A, Swainston N, Day PJ, Kell DB. Synthetic biology for the directed evolution of protein biocatalysts: navigating sequence space intelligently. Chem Soc Rev. 2015;44(5):1172–1239. doi: 10.1039/c4cs00351a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deralia PK, Jensen A, Felby C, Thygesen LG. Chemistry of lignin and hemicellulose structures interacts with hydrothermal pretreatment severity and affects cellulose conversion. Biotechnol Progr. 2021;37(5):e3189. doi: 10.1002/btpr.3189. [DOI] [PubMed] [Google Scholar]
- Dhiman SS, Kalyani D, Jagtap SS, Haw JR, Kang YC, Lee JK. Characterization of a novel xylanase from Armillaria gemina and its immobilization onto SiO2 nanoparticles. Appl Microbiol Biot. 2013;97(3):1081–1091. doi: 10.1007/s00253-012-4381-9. [DOI] [PubMed] [Google Scholar]
- Dias MC, Zidanes UL, Martins CCN, de Oliveira ALM, Damásio RAP, de Resende JV, Vilas Boas EVB, Belgacem MN, Tonoli GHD, Ferreira SR. Influence of hemicellulose content and cellulose crystal change on cellulose nanofibers properties. Int J Biol Macromol. 2022;213:780–790. doi: 10.1016/j.ijbiomac.2022.06.012. [DOI] [PubMed] [Google Scholar]
- Domingos P. A few useful things to know about machine learning. Commun ACM. 2012;55(10):78–87. doi: 10.1145/2347736.2347755. [DOI] [Google Scholar]
- Dong M, Wang F, Li Q, Han R, Li A, Zhai C, Ma L. A single digestion, single-stranded oligonucleotide mediated PCR-independent site-directed mutagenesis method. Appl Microbiol Biot. 2020;104(9):3993–4003. doi: 10.1007/s00253-020-10477-3. [DOI] [PubMed] [Google Scholar]
- Du JJ, Klontz EH, Guerin ME, Trastoy B, Sundberg EJ. Structural insights into the mechanisms and specificities of IgG-active endoglycosidases. Glycobiology. 2020;30(4):268–279. doi: 10.1093/glycob/cwz042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumorné K, Córdova DC, Astorga-Eló M, Renganathan P. Extremozymes: a potential source for industrial applications. J Microbiol Biotechn. 2017;27(4):649–659. doi: 10.4014/jmb.1611.11006. [DOI] [PubMed] [Google Scholar]
- Dutta T, Bhattacharjee A, Majumdar U, Ray SS, Sahoo R, Ghosh S. In vitro renaturation of alkaline family G/11 xylanase via a folding intermediate: alpha-crystallin facilitates refolding in an ATP-independent manner. Appl Biochem Biotech. 2010;162(5):1238–1248. doi: 10.1007/s12010-009-8854-y. [DOI] [PubMed] [Google Scholar]
- Echeverría V, Eyzaguirre J. Penicillium purpurogenum produces a set of endoxylanases: identification, heterologous expression, and characterization of a fourth xylanase, xynD, a novel enzyme belonging to glycoside hydrolase family 10. Appl Biochem Biotech. 2019;187(1):298–309. doi: 10.1007/s12010-018-2782-7. [DOI] [PubMed] [Google Scholar]
- Ejaz U, Sohail M, Ghanemi A. Cellulases: from bioactivity to a variety of industrial applications. Biomimetics. 2021;6(3):44. doi: 10.3390/biomimetics6030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves AM, Graça G, Peyriga L, Torcato IM, Borges N, Portais JC, Santos H. Combined transcriptomics-metabolomics profiling of the heat shock response in the hyperthermophilic archaeon Pyrococcus furiosus. Extremophiles. 2019;23(1):101–118. doi: 10.1007/s00792-018-1065-0. [DOI] [PubMed] [Google Scholar]
- Gama R, Van Dyk JS, Burton MH, Pletschke BI. Using an artificial neural network to predict the optimal conditions for enzymatic hydrolysis of apple pomace. 3 Biotech. 2017;7(2):138. doi: 10.1007/s13205-017-0754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshpurkar A, Singh R, Singh RB, Kumar D, Kumar A, Singh SK. Identification of potential AChE inhibitors through combined machine-learning and structure-based design approaches. Indian J Biochem Bio. 2022;59(6):619–631. doi: 10.56042/ijbb.v59i6.61569. [DOI] [Google Scholar]
- Gao Y, Guo M, Wang D, Zhao D, Wang M. Advances in extraction, purification, structural characteristics and biological activities of hemicelluloses: a review. Int J Biol Macromol. 2023;225:467–483. doi: 10.1016/j.ijbiomac.2022.11.099. [DOI] [PubMed] [Google Scholar]
- Ge F, Chen G, Qian M, Xu C, Liu J, Cao J, Li X, Hu D, Xu Y, Wang D, Zhou J, Shi H, Tan Z. Artificial intelligence aided lipase production and engineering for enzymatic performance improvement. J Agric Food Chem. 2023 doi: 10.1021/acs.jafc.3c05029. [DOI] [PubMed] [Google Scholar]
- Guo L, Brügger K, Liu C, Shah SA, Zheng H, Zhu Y, Wang S, Lillestøl RK, Chen L, Frank J, Prangishvili D, Paulin L, She Q, Huang L, Garrett RA. Genome analyses of icelandic strains of Sulfolobus islandicus, model organisms for genetic and virus-host interaction studies. J Bacteriol. 2011;193(7):1672–1680. doi: 10.1128/jb.01487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong MR, Park CS, Oh DK. Characterization of a thermostable endo-1,5-alpha-l-arabinanase from Caldicellulorsiruptor saccharolyticus. Biotechnol Lett. 2009;31(9):1439–1443. doi: 10.1007/s10529-009-0019-0. [DOI] [PubMed] [Google Scholar]
- Horn SJ, Sørlie M, Vårum KM, Väljamäe P, Eijsink VG. Measuring processivity. Method Enzymol. 2012;510:69–95. doi: 10.1016/b978-0-12-415931-0.00005-7. [DOI] [PubMed] [Google Scholar]
- Ioannidis JPA, Cristea IA, Boyack KW. Work honored by Nobel prizes clusters heavily in a few scientific fields. PLoS ONE. 2020;15(7):e0234612. doi: 10.1371/journal.pone.0234612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagtap SS, Dhiman SS, Kim TS, Li J, Lee JK, Kang YC. Enzymatic hydrolysis of aspen biomass into fermentable sugars by using lignocellulases from Armillaria gemina. Bioresource Technol. 2013;133:307–314. doi: 10.1016/j.biortech.2013.01.118. [DOI] [PubMed] [Google Scholar]
- Khan MN, Rehman N, Sharif A, Ahmed E, Farooqi ZH, Din MI. Environmentally benign extraction of cellulose from dunchi fiber for nanocellulose fabrication. Int J Biol Macromol. 2020;153:72–78. doi: 10.1016/j.ijbiomac.2020.02.333. [DOI] [PubMed] [Google Scholar]
- Knapik K, Becerra M, González-Siso MI. Microbial diversity analysis and screening for novel xylanase enzymes from the sediment of the lobios hot spring in Spain. Sci Rep-UK. 2019;9(1):11195. doi: 10.1038/s41598-019-47637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramochi K, Uchimura K, Kurata A, Kobayashi T, Hirose Y, Miura T, Kishimoto N, Usami R, Horikoshi K. A high-molecular-weight, alkaline, and thermostable β-1,4-xylanase of a subseafloor Microcella alkaliphila. Extremophiles. 2016;20(4):471–478. doi: 10.1007/s00792-016-0837-7. [DOI] [PubMed] [Google Scholar]
- Lee CY, Yu KO, Kim SW, Han SO. Enhancement of the thermostability and activity of mesophilic Clostridium cellulovorans engD by in vitro DNA recombination with Clostridium thermocellum CelE. J Biosci Bioeng. 2010;109(4):331–336. doi: 10.1016/j.jbiosc.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Lee JP, Shin ES, Cho MY, Lee KD, Kim H. Roles of carbohydrate-binding module (CBM) of an endo-β-1,4-glucanase (Cel5L) from Bacillus sp. KD1014 in thermostability and small-substrate hydrolyzing activity. J Microbiol Biotechn. 2018;28(12):2036–2045. doi: 10.4014/jmb.1810.10001. [DOI] [PubMed] [Google Scholar]
- Li J, Xu X, Shi P, Liu B, Zhang Y, Zhang W. Overexpression and characterization of a novel endo-β-1,3(4)-glucanase from thermophilic fungus Humicola insolens Y1. Protein Expres Purif. 2017;138:63–68. doi: 10.1016/j.pep.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Li G, Zhou X, Li Z, Liu Y, Liu D, Miao Y, Wan Q, Zhang R. Significantly improving the thermostability of a hyperthermophilic GH10 family xylanase XynAF1 by semi-rational design. Appl Microbiol Biot. 2021;105(11):4561–4576. doi: 10.1007/s00253-021-11340-9. [DOI] [PubMed] [Google Scholar]
- Liab K, Azadi P, Collins R, Tolan J, Kim JS, Eriksson KL. Relationships between activities of xylanases and xylan structures. Enzyme Microb Tech. 2000;27(1–2):89–94. doi: 10.1016/s0141-0229(00)00190-3. [DOI] [PubMed] [Google Scholar]
- Long L, Tian D, Hu J, Wang F, Saddler J. A xylanase-aided enzymatic pretreatment facilitates cellulose nanofibrillation. Bioresource Technol. 2017;243:898–904. doi: 10.1016/j.biortech.2017.07.037. [DOI] [PubMed] [Google Scholar]
- Long L, Hu Y, Sun F, Gao W, Hao Z, Yin H. Advances in lytic polysaccharide monooxygenases with the cellulose-degrading auxiliary activity family 9 to facilitate cellulose degradation for biorefinery. Int J Biol Macromol. 2022;219:68–83. doi: 10.1016/j.ijbiomac.2022.07.240. [DOI] [PubMed] [Google Scholar]
- Maroldi MMC, Vasconcellos VM, Lacava PT, Farinas CS. Potential of mangrove-associated endophytic fungi for production of carbohydrolases with high saccharification efficiency. Appl Biochem Biotech. 2018;184(3):806–820. doi: 10.1007/s12010-017-2590-5. [DOI] [PubMed] [Google Scholar]
- Masek A, Kosmalska A. Technological limitations in obtaining and using cellulose biocomposites. Front Bioeng Biotech. 2022;10:912052. doi: 10.3389/fbioe.2022.912052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mater AC, Coote ML. Deep learning in chemistry. J Chem Inf Model. 2019;59(6):2545–2559. doi: 10.1021/acs.jcim.9b00266. [DOI] [PubMed] [Google Scholar]
- Mazurenko S, Prokop Z, Damborsky J. Machine learning in enzyme engineering. ACS Catal. 2019;10(2):1210–1223. doi: 10.1021/acscatal.9b04321. [DOI] [Google Scholar]
- McPherson A. Protein crystallization. Methods Mol Biol. 2017;1607:17–50. doi: 10.1007/978-1-4939-7000-1_2. [DOI] [PubMed] [Google Scholar]
- Míguez Amil S, Jiménez-Ortega E, Ramírez-Escudero M, Talens-Perales D, Marín-Navarro J, Polaina J, Sanz-Aparicio J, Fernandez-Leiro R. The cryo-EM structure of Thermotoga maritima β-galactosidase: quaternary structure guides protein engineering. ACS Chem Biol. 2020;15(1):179–188. doi: 10.1021/acschembio.9b00752. [DOI] [PubMed] [Google Scholar]
- Moreira LR, Filho EX. An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biot. 2008;79(2):165–178. doi: 10.1007/s00253-008-1423-4. [DOI] [PubMed] [Google Scholar]
- Morgado SM, Vicente ACP. Exploring tRNA gene cluster in archaea. Mem I Oswaldo Cruz. 2019;114:e180348. doi: 10.1590/0074-02760180348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muderspach SJ, Fredslund F, Volf V, Poulsen JN, Blicher TH, Clausen MH, Rasmussen KK, Krogh K, Jensen K, Lo Leggio L. Engineering the substrate binding site of the hyperthermostable archaeal endo-β-1,4-galactanase from Ignisphaera aggregans. Biotechnol Biofuels. 2021;14(1):183. doi: 10.1186/s13068-021-02025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PG, Collins CM, Grassick A, Tuohy MG. Molecular cloning, transcriptional, and expression analysis of the first cellulase gene (cbh2), encoding cellobiohydrolase II, from the moderately thermophilic fungus Talaromyces emersonii and structure prediction of the gene product. Biochem Bioph Res Co. 2003;301(2):280–286. doi: 10.1016/s0006-291x(02)03025-5. [DOI] [PubMed] [Google Scholar]
- Murray P, Aro N, Collins C, Grassick A, Penttilä M, Saloheimo M, Tuohy M. Expression in trichoderma reesei and characterisation of a thermostable family 3 beta-glucosidase from the moderately thermophilic fungus Talaromyces emersonii. Protein Expres Purif. 2004;38(2):248–257. doi: 10.1016/j.pep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Musil M, Konegger H, Hon J, Bednar D, Damborsky J. Computational design of stable and soluble biocatalysts. Acs Catal. 2018;9(2):1033–1054. doi: 10.1021/acscatal.8b03613. [DOI] [Google Scholar]
- Mylemans B, Noguchi H, Deridder E, Lescrinier E, Tame JRH, Voet ARD. Influence of circular permutations on the structure and stability of a six-fold circular symmetric designer protein. Protein Sci. 2020;29(12):2375–2386. doi: 10.1002/pro.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Fujimoto H, Fujii S, Wakiyama M. Cell-associated beta-xylosidase from Aureobasidium pullulans ATCC 20524: purification, properties, and characterization of the encoding gene. J Biosci Bioeng. 2010;110(2):152–157. doi: 10.1016/j.jbiosc.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Okanishi H, Kim K, Masui R, Kuramitsu S. Proteome-wide identification of iysine propionylation in thermophilic and mesophilic bacteria: Geobacillus kaustophilus, Thermus thermophilus, Echerichia coli, Bacillus subtilis, and Rhodothermus marinus. Extremophiles. 2017;21(2):283–296. doi: 10.1007/s00792-016-0901-3. [DOI] [PubMed] [Google Scholar]
- Okolie JA, Nanda S, Dalai AK, Kozinski JA. Chemistry and specialty industrial applications of lignocellulosic biomass. Waste Biomass Valori. 2021;12(5):2145–2169. doi: 10.1007/s12649-020-01123-0. [DOI] [Google Scholar]
- Park Y, Kellis M. Deep learning for regulatory genomics. Nat Biotechnol. 2015;33(8):825–826. doi: 10.1038/nbt.3313. [DOI] [PubMed] [Google Scholar]
- Payne CM, Bomble YJ, Taylor CB, McCabe C, Himmel ME, Crowley MF, Beckham GT. Multiple functions of aromatic-carbohydrate interactions in a processive cellulase examined with molecular simulation. J Biol Chem. 2011;286(47):41028–41035. doi: 10.1074/jbc.M111.297713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JH, Chen Z, McAndrew RP, Sapra R, Chhabra SR, Sale KL, Simmons BA, Adams PD. Biochemical characterization and crystal structure of endoglucanase Cel5A from the hyperthermophilic Thermotoga maritima. J Struct Biol. 2010;172(3):372–379. doi: 10.1016/j.jsb.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Prieto A, de Eugenio L, Méndez-Líter JA, Nieto-Domínguez M, Murgiondo C, Barriuso J, Bejarano-Muñoz L, Martínez MJ. Fungal glycosyl hydrolases for sustainable plant biomass valorization: talaromyces amestolkiae as a model fungus. Int Microbiol. 2021;24(4):545–558. doi: 10.1007/s10123-021-00202-z. [DOI] [PubMed] [Google Scholar]
- Pucci F, Kwasigroch JM, Rooman M. Protein thermal stability engineering using HoTMuSiC. Methods Mol Biol. 2020;2112:59–73. doi: 10.1007/978-1-0716-0270-6_5. [DOI] [PubMed] [Google Scholar]
- Rekadwad B, Gonzalez JM. Multidisciplinary involvement and potential of thermophiles. Folia Microbiol. 2019;64(3):389–406. doi: 10.1007/s12223-018-0662-8. [DOI] [PubMed] [Google Scholar]
- Romero-Rivera A, Garcia-Borràs M, Osuna S. Computational tools for the evaluation of laboratory-engineered biocatalysts. Chem Commun. 2017;53(2):284–297. doi: 10.1039/c6cc06055b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo K, Sahoo RK, Gaur M, Subudhi E. Cellulolytic thermophilic microorganisms in white biotechnology: a review. Folia Microbiol. 2020;65(1):25–43. doi: 10.1007/s12223-019-00710-6. [DOI] [PubMed] [Google Scholar]
- Schiano-di-Cola C, Kołaczkowski B, Sørensen TH, Christensen SJ, Cavaleiro AM, Windahl MS, Borch K, Morth JP, Westh P. Structural and biochemical characterization of a family 7 highly thermostable endoglucanase from the fungus rasamsonia emersonii. FEBS J. 2020;287(12):2577–2596. doi: 10.1111/febs.15151. [DOI] [PubMed] [Google Scholar]
- Sharma A, Tewari R, Rana SS, Soni R, Soni SK. Cellulases: classification, methods of determination and industrial applications. Appl Biochem Biotech. 2016;179(8):1346–1380. doi: 10.1007/s12010-016-2070-3. [DOI] [PubMed] [Google Scholar]
- Shen Y, Wang M, Chen Y, Xu L, Lu Y, Zhou Y, Tam JP, Han F, Yang H, Jia X. Convenient preparation of sagittatoside B, a rare bioactive secondary flavonol glycoside, by recyclable and integrated biphase enzymatic hydrolysis. Enzyme Microb Tech. 2019;121:51–58. doi: 10.1016/j.enzmictec.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Shi H, Li X, Gu H, Zhang Y, Huang Y, Wang L, Wang F. Biochemical properties of a novel thermostable and highly xylose-tolerant β-xylosidase/α-arabinosidase from Thermotoga thermarum. Biotechnol Biofuels. 2013;6(1):27. doi: 10.1186/1754-6834-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Zhang Y, Li X, Huang Y, Wang L, Wang Y, Ding H, Wang F. A novel highly thermostable xylanase stimulated by Ca2+ from Thermotoga thermarum: cloning, expression and characterization. Biotechnol Biofuels. 2013;6(1):26. doi: 10.1186/1754-6834-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Ding H, Huang Y, Wang L, Zhang Y, Li X, Wang F. Expression and characterization of a GH43 endo-arabinanase from Thermotoga thermarum. BMC Biotechnol. 2014;14:35. doi: 10.1186/1472-6750-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Liu Y, Guo J, Cao Y, Zhu X, Zhou J, Luo C, Wang P, Wang T, Li X. Thermostable manganese (II) dependent α-glycosidase from Pseudothermotoga thermarum. BioResources. 2019;14(3):7266–7274. doi: 10.15376/biores.14.3.7266-7274. [DOI] [Google Scholar]
- Shi K, Kurniawan F, Banerjee S, Moeller NH, Aihara H. Crystal structure of bacteriophage T4 spackle as determined by native SAD phasing. Acta Crystallogr D. 2020;76(Pt 9):899–904. doi: 10.1107/s2059798320010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Guo J, Yan X, Cui G, Tan Z, Zhu X, Zhou J, He S, Wang T, Li X. Characterization of a xyloglucananse in biodegradation of woody plant xyloglucan from caldicellulosiruptor kronotskyensis. BioResources. 2021;17(1):673–681. doi: 10.15376/biores.17.1.673-681. [DOI] [Google Scholar]
- Singh S, Madlala AM, Prior BA. Thermomyces lanuginosus: properties of strains and their hemicellulases. FEMS Microbiol Rev. 2003;27(1):3–16. doi: 10.1016/s0168-6445(03)00018-4. [DOI] [PubMed] [Google Scholar]
- Singh S, Singh G, Arya SK. Mannans: an overview of properties and application in food products. Int J Biol Macromol. 2018;119:79–95. doi: 10.1016/j.ijbiomac.2018.07.130. [DOI] [PubMed] [Google Scholar]
- Subramaniyan S, Prema P. Biotechnology of microbial xylanases: enzymology, molecular biology, and application. Crit Rev Biotechnol. 2002;22(1):33–64. doi: 10.1080/07388550290789450. [DOI] [PubMed] [Google Scholar]
- Sun SF, Yang J, Wang DW, Yang HY, Sun SN, Shi ZJ. Enzymatic response of ryegrass cellulose and hemicellulose valorization introduced by sequential alkaline extractions. Biotechnol Biofuels. 2021;14(1):72. doi: 10.1186/s13068-021-01921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavker N, Yadav VK, Yadav KK, Cabral-Pinto MM, Alam J, Shukla AK, Ali FAA, Alhoshan M. Removal of cadmium and chromium by mixture of silver nanoparticles and nano-fibrillated cellulose isolated from waste peels of citrus sinensis. Polymers. 2021;13(2):234. doi: 10.3390/polym13020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CB, Payne CM, Himmel ME, Crowley MF, McCabe C, Beckham GT. Binding site dynamics and aromatic-carbohydrate interactions in processive and non-processive family 7 glycoside hydrolases. J Phys Chem B. 2013;117(17):4924–4933. doi: 10.1021/jp401410h. [DOI] [PubMed] [Google Scholar]
- Torktaz I, Karkhane AA, Hemmat J. Rational engineering of Cel5E from clostridium Thermocellum to improve its thermal stability and catalytic activity. Appl Microbiol Biot. 2018;102(19):8389–8402. doi: 10.1007/s00253-018-9204-1. [DOI] [PubMed] [Google Scholar]
- Tsai AY, Chan K, Ho CY, Canam T, Capron R, Master ER, Bräutigam K. Transgenic expression of fungal accessory hemicellulases in Arabidopsis thaliana triggers transcriptional patterns related to biotic stress and defense response. PLoS ONE. 2017;12(3):e0173094. doi: 10.1371/journal.pone.0173094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaaje-Kolstad G, Horn SJ, Sørlie M, Eijsink VG. The chitinolytic machinery of Serratia marcescens—a model system for enzymatic degradation of recalcitrant polysaccharides. FEBS J. 2013;280(13):3028–3049. doi: 10.1111/febs.12181. [DOI] [PubMed] [Google Scholar]
- Velasco J, Oliva B, Gonçalves AL, Lima AS, Ferreira G, França BA, Mulinari EJ, Gonçalves TA, Squina FM, Kadowaki MAS, Maiorano A, Polikarpov I, Oliveira LC, Segato F. Functional characterization of a novel thermophilic exo-arabinanase from Thermothielavioides terrestris. Appl Microbiol Biot. 2020;104(19):8309–8326. doi: 10.1007/s00253-020-10806-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Cao X, Chen W, Xu J, Wu B. Identification and characterization of a thermostable GH36 α-galactosidase from Anoxybacillus vitaminiphilus WMF1 and its application in synthesizing isofloridoside by reverse hydrolysis. Int J Mol Sci. 2021;22(19):10778. doi: 10.3390/ijms221910778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Gao S, Lai C, Xie Y, Sun Y, Wang J, Wang C, Yong Q, Chu F, Zhang D. Upgrading wood biorefinery: an integration strategy for sugar production and reactive lignin preparation. Ind Crop Prod. 2022;187:115366. doi: 10.1016/j.indcrop.2022.115366. [DOI] [Google Scholar]
- Yakovlieva L, Walvoort MTC. Processivity in bacterial glycosyltransferases. ACS Chem Biol. 2020;15(1):3–16. doi: 10.1021/acschembio.9b00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Sogabe Y, Fukuoka S, Sakai T, Tada T. Structures of endo-1,5-α-L-arabinanase mutants from Bacillus thermodenitrificans TS-3 in complex with arabino-oligosaccharides. Acta Crystallogr F. 2018;74(Pt 12):774–780. doi: 10.1107/s2053230x18015947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Han Z. Understanding the positional binding and substrate interaction of a highly thermostable GH10 xylanase from Thermotoga maritima by molecular docking. Biomolecules. 2018;8(3):64. doi: 10.3390/biom8030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Leśnierowski G. Thermal modification of hen egg white lysozyme using microwave treatment. Acta Sci Polon-Techn. 2020;19(2):149–157. doi: 10.17306/j.Afs.0773. [DOI] [PubMed] [Google Scholar]
- Yang H, Shi P, Liu Y, Xia W, Wang X, Cao H, Ma R, Luo H, Bai Y, Yao B. Loop 3 of fungal endoglucanases of glycoside hydrolase family 12 modulates catalytic efficiency. Appl Environ Microb. 2017;83(6):e03123–e3116. doi: 10.1128/aem.03123-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Yang Y, Zhang L, Xu H, Guo X, Yang X, Dong B, Cao Y. Improved thermostability of an acidic xylanase from Aspergillus sulphureus by combined disulphide bridge introduction and proline residue substitution. Sci Rep. 2017;7(1):1587. doi: 10.1038/s41598-017-01758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, An X, Liu L, Tang S, Cao H, Xu Q, Liu H. Cellulose, hemicellulose, lignin, and their derivatives as multi-components of bio-based feedstocks for 3D printing. Carbohyd Polym. 2020;250:116881. doi: 10.1016/j.carbpol.2020.116881. [DOI] [PubMed] [Google Scholar]
- Yonemoto IT, Weyman PD. Facile site-directed mutagenesis of large constructs using gibson isothermal DNA assembly. Methods Mol Biol. 2017;1498:359–366. doi: 10.1007/978-1-4939-6472-7_24. [DOI] [PubMed] [Google Scholar]
- Yuan X, Zhao J, Wu X, Yao W, Guo H, Ji D, Yu Q, Luo L, Li X, Zhang L. Extraction of corn bract cellulose by the ammonia-coordinated bio-enzymatic method. Polymers. 2022;15(1):206. doi: 10.3390/polym15010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wright T, Wang X, Savary BJ, Xu J. Production of thermostable endo-1,5-α-l-arabinanase in Pichia pastoris for enzymatically releasing functional oligosaccharides from sugar beet pulp. Appl Microbiol Biot. 2020;104(4):1595–1607. doi: 10.1007/s00253-019-10238-x. [DOI] [PubMed] [Google Scholar]
- Zhu N, Jin H, Kong X, Zhu Y, Ye X, Xi Y, Du J, Li B, Lou M, Shah GM. Improving the fermentable sugar yields of wheat straw by high-temperature pre-hydrolysis with thermophilic enzymes of Malbranchea cinnamomea. Microb Cell Fact. 2020;19(1):149. doi: 10.1186/s12934-020-01408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.