Abstract
Introduction
Psoriasis (Pso) and psoriatic arthritis (PsA) can reduce the quality of life (QoL) and are known to be associated with depression. Within this study, we aimed to assess the burden of disease, functional capacity, quality of life, and depressive symptoms and identify factors predicting functional impairment and depression in patients with psoriatic disease.
Methods
A cross-sectional survey was conducted in a cohort of 300 patients with psoriatic disease including 150 patients from a university hospital dermatology outpatient clinic and 150 patients from a university hospital rheumatology outpatient clinic. Questionnaire-based assessment of signs of arthritis (Psoriasis Epidemiology Screening Tool; PEST), functional status (Functional Questionnaire Hannover; FFbH), quality of life (World Health Organization Quality of Life Brief Version; WHOQOL-BREF), and depressive symptoms (Patient health questionnaire 9; PHQ-9) and retrospective medical chart analysis were performed.
Results
Despite treatment, burden of disease was high. Joint pain was reported in multiple regions in patients with Pso (n = 111) and patients with PsA (n = 189), but with differences in frequency and distribution patterns of symptoms. Functional impairment in everyday life was independently associated with diagnosis of PsA (odds ratio [OR] 9.56, p = 0.005), depressive symptoms (OR 5.44, p < 0.001) and age (OR 1.04, p = 0.033). At least mild depressive symptoms were demonstrated in 54% and 69% of patients with Pso and PsA, respectively. In a logistic regression model, depressive symptoms were independently associated with functional impairment (OR 4.50, p = 0.003), axial complaints (OR 2.80, p = 0.030), diagnosis of psoriatic arthritis (OR 2.69, p = 0.046), and number of joint regions with complaints (OR 1.10, p = 0.032).
Conclusion
Functional impairment, QoL, and depressive symptoms are mutually interdependent. Early diagnosis of PsA and initiation of anti-inflammatory therapy are essential to avoid long-term damage, disability, and mental health complications. However, despite therapy many patients with PsA, and especially female patients, report a substantial residual disease burden due to their psoriatic disease which will need to be addressed by a more patient-centered approach.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-023-00602-9.
Keywords: Psoriasis, Psoriatic arthritis (PsA), Quality of life, Depression, Functional impairment
Key Summary Points
| Why carry out this study? |
| Psoriatic disease can impair the quality of life. Furthermore, both psoriasis and psoriatic arthritis are known to be associated with depression. |
| We aimed to assess the burden of disease as well as factors predicting functional impairment and depression in patients with psoriasis and patients with psoriatic arthritis. |
| What was learned from the study? |
| Functional impairment, quality of life and depressive symptoms are mutually interdependent. While functional impairment in everyday life is independently associated with depressive symptoms, diagnosis of psoriatic arthritis, and age, depressive symptoms are predicted by functional impairment, diagnosis of psoriatic arthritis, axial complaints, and the number of joint regions with complaints. |
| Early diagnosis and initiation of anti-inflammatory therapy are crucial to avoid long-term damage, disability, and mental health complications. Awareness and addressing of the identified risk factors may help to overcome unmet needs and improve quality of life for these patients. |
| Depression is underdiagnosed and undertreated in patients with psoriatic disease, which may contribute to impaired quality of life, increased disease activity, and reduced therapy adherence. Patients should thus be screened for depressive symptoms and access to mental health care should be facilitated. |
Introduction
Psoriasis constitutes a chronic immune-mediated skin condition affecting 2–3% of the population in Western countries [1]. Approximately 30% of patients with psoriasis develop psoriatic arthritis (PsA) in the course of the disease [2]. Up to 15% of patients with PsA remain undiagnosed as clinical manifestations may be heterogeneous [3], including peripheral arthritis, enthesitis, dactylitis, and axial disease manifestations. Predisposing factors for the development of PsA include psoriasis severity, scalp or flexural skin involvement, nail involvement, and certain HLA alleles [4, 5]. Untreated, PsA may lead to irreversible joint destruction associated with a high rate of disability [6]. Thus, timely diagnosis and initiation of disease-modifying therapy are essential.
Psoriasis and psoriatic arthritis may both have a major impact on quality of life (QoL). In particular, pain and functional impairment negatively influence health-related QoL [7]. Other studies described musculoskeletal manifestations, such as enthesitis, tender joints, and back pain, to have deleterious effects on QoL [8, 9]. Furthermore, psoriasis and PsA may lead to loss of productivity, work absenteeism, increased healthcare costs, and may impose a financial burden on patients and their family carers [10, 11]. In general, the impact of PsA on patients’ lives seems to be broad, covering all aspects of life including physical and functional aspects, social participation, emotional well-being, fatigue, and sleep [12].
Psoriasis and PsA may both significantly impact mental health and an association with depression is well established [13, 14]. Different aspects play a role: Skin psoriasis has been linked with a negative body image and reduced self-esteem as well as feelings of stigmatization in addition to physical discomfort and pain [15–17]. Discomfort, pain, and disability play an even bigger role regarding mental health in PsA [14]. Depression and anxiety thus constitute important comorbidities, with a prevalence of approximately 33% and 20%, respectively, in patients with PsA [18]. While patients with more severe disease may have an elevated risk of developing mental health comorbidities, depression was also shown to increase the perception of pain [18, 19].
Within this study, we assessed the burden of disease, functional status, QoL, and depressive symptoms in a cohort of 300 patients with psoriatic disease and identified factors associated with functional impairment and depression.
Methods
Patient Cohort and Data Collection
For this project, 300 patients with psoriatic disease from the dermatology outpatient clinic and the rheumatology outpatient clinic of the University Medical Center Freiburg, Freiburg, Germany were recruited and assessed by survey. Patients with PsA had to fulfil the Classification of Psoriatic Arthritis (CASPAR) criteria. A detailed description of the patient cohort can be found in the “Results” section.
The study was conducted under the ethics protocol 190/17 (ethics committee of the University of Freiburg, Germany). Patients gave their written consent according to International Conference on Harmonization Good Clinical Practice (ICH GCP) guidelines. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Patients were assessed with the following questionnaires: To measure functional capacity in everyday life/disability the Functional Questionnaire Hannover (Funktionsfragebogen Hannover; FFbH) was used. FFbH is a validated tool developed in Germany to assess functional capacity in patients with rheumatic diseases or other joint problems [20] and comprises 18 items, which assess whether the patient’s ability to complete 18 different tasks of everyday life either independently without difficulties (2 points), with effort (1 point), or unable/need help (0 points). To calculate a patient’s total score, points are multiplied by 100 and divided by 2 × the number of valid answers. Relevant functional impairment is defined as a functional capacity less than 60% on this scale.
The Psoriasis Epidemiology Screening Tool (PEST) questionnaire was developed as a tool to screen patients with psoriasis for PsA and comprises five questions as well as a manikin for patient markup to indicate regions with joint problems [21]. The PEST questionnaire was shown to have an overall sensitivity up to 92% and a specificity of 78% for PsA [21].
Quality of life was assessed by WHOQOL-BREF questionnaire, i.e., the short version of the World Health Organization Quality of Life questionnaire, for the domains subjective quality of life, physical and mental health-related QoL, social relationships, and environmental QoL, divided into 26 items [22]. The questionnaire assesses well-being within the previous 2 weeks, rated on a five-point Likert scale from very poor/very unsatisfactory to very good/very satisfactory. Domain scores are calculated from the average score of items within the domain. Domain scores are subsequently transformed onto a 0–100 scale, with higher scores indicating a better quality of life.
Depressive symptoms were assessed using the Patient health questionnaire 9 (PHQ-9), which constitutes a nine-item screening instrument for the diagnosis of depression [23].
Survey data were supplemented with demographic and clinical data from retrospective medical chart analysis, including age, height, body weight, body mass index (BMI), disease duration, and therapy.
Statistics
In order to summarize demographic and disease characteristics, descriptive statistics including mean, standard deviation, and percentages were calculated. To compare categorical variables between groups, chi-square test was employed, whereas for the comparison of continuous variables Student’s or Welch’s t tests were used; analysis of variance (ANOVA) was used for multiple groups.
To determine factors independently associated with depression, multivariate logistic regression was performed. Multivariate models were calculated employing stepwise backwards elimination. All statistical analyses were performed using jamovi version 2.3.21.0 (The jamovi project (2023). jamovi (Version 2.3) [Computer Software], retrieved from https://www.jamovi.org) [24] or GraphPad Prism (version 9.5 for Mac, GraphPad Software, San Diego, California USA, www.graphpad.com).
Results
Patient Cohort
A total of 300 patients with psoriatic disease were analyzed, including 150 patients from the dermatology department and 150 patients from the rheumatology outpatient clinic. In total, 111 patients had skin psoriasis (Pso) and 189 psoriatic arthritis (PsA); 54% of patients were male and 46% were female. Mean age at assessment was 54.3 years and mean disease duration was 15.4 years. Mean age was higher (56.2 vs. 51.0 years, p = 0.005) in patients with PsA than in patients with Pso, but mean disease duration was shorter (14.0 vs. 17.4 years, p = 0.035). Furthermore, patients with PsA had a higher mean serum C-reactive protein (CRP) concentration compared to patients with Pso (p = 0.016). Epidemiological data are summarized in Table 1. Smoking was more prevalent in the skin psoriasis cohort (57.3% vs. 22.0%, p < 0.001). Mean BMI of both groups was within the overweight spectrum (28.2 vs. 27.6 kg/m2).
Table 1.
Clinical description of patient cohort
| Total (n = 300) | Pso (n = 111) | PsA (n = 189) | p value | |
|---|---|---|---|---|
| Age, years, mean (SD) | 54.3 (14.5) | 51.0 (16.0) | 56.2 (13.3) | 0.005 |
| Male, n (%)/female, n (%) | 162 (54.0%)/138 (46.0%) | 55 (49.5%)/56 (50.5%) | 107 (56.6%)/82 (43.4%) | 0.236 |
| BMI, kg/m2, mean (SD) (n = 274) | 27.8 (5.65) | 28.2 (6.29) | 27.6 (5.29) | 0.413 |
| Smokers, n (%) (n = 243) | 80 (32.9%) | 43 (57.3%) | 37 (22.0%) | < 0.001 |
| Disease duration, years, mean (SD) (n = 266) | 15.4 (12.5) | 17.4 (14.1) | 14.0 (11.2) | 0.035 |
| DAS28, mean (SD) | NA | NA | 2.51 (1.01) | NA |
| CRP, mean (SD) | 6.18 (7.17) | 5.01 (3.68) | 6.82 (8.43) | 0.016 |
| Highest educational degree | ||||
| Postgraduate, n (%) | 5 (1.7) | 0 (0.0) | 5 (2.7) | 0.170 |
| University degree, n (%) | 18 (6.0) | 8 (7.3) | 10 (5.3) | |
| Polytechnic degree, n (%) | 19 (6.4) | 7 (6.4) | 12 (6.4) | |
| High school diploma (Abitur), n (%) | 15 (5.0) | 4 (3.6) | 11 (5.9) | |
| Polytechnic entrance qualification, n (%) | 20 (6.7) | 5 (4.5) | 15 (8.0) | |
| Intermediate school certificate, n (%) | 93 (31.2) | 34 (30.9) | 59 (31.4) | |
| Lower secondary education, n (%) | 120 (40.3) | 46 (41.8) | 74 (39.4) | |
| No degree, n (%) | 8 (2.7) | 6 (5.5) | 2 (1.1) | |
| Therapy | ||||
| Topical, n (%) | 38 (12.7) | 35 (31.5) | 3 (1.6) | < 0.001 |
| Systemic, n (%) | 232 (77.3) | 76 (68.5) | 156 (82.5) | 0.005 |
| csDMARD, n (%) | 87 (29.0) | 8 (7.2) | 79 (41.8) | < 0.001 |
| bDMARD, n (%) | 154 (53.3) | 46 (41.4) | 108 (60.7) | 0.001 |
| tsDMARD, n (%) | 23 (7.7) | 10 (9.0) | 13 (6.9) | 0.503 |
| Combination b/tsDMARD + csDMARD, n (%) | 48 (16.0) | 1 (0.9) | 47 (24.9) | < 0.001 |
| NSAID monotherapy, n (%) | 3 (1.0) | 0 (0.0) | 3 (1.6) | 0.182 |
For parameters for which data from individual patients were missing, the number (n) for the individual item is given in the first column
bDMARD biological disease-modifying antirheumatic drug, BMI body mass index, CRP C-reactive protein, csDMARD conventional synthetic disease-modifying antirheumatic drug, n number, NA not available, NSAID non-steroidal anti-inflammatory drugs, PsA psoriatic arthritis, Pso psoriasis, SD standard deviation, tsDMARD targeted synthetic disease-modifying antirheumatic drug
Regarding the educational level, 14.1% of patients had a university or polytechnic degree; 11.7% reported a high school diploma or polytechnic entrance qualification, whereas 71.5% reported lower secondary education or an intermediate school certificate as their highest educational degree; 2.7% of patients did not have a degree. There were no significant differences between patients with Pso and PsA regarding educational level.
At the time of assessment, 77.3% of patients had a systemic immunomodulatory therapy; 53.3% of patients were treated with a biological (b) disease-modifying antirheumatic drug (bDMARD), 7.7% with a targeted synthetic (ts) DMARD. A combination of b/tsDMARD with a conventional DMARD was used in 16% of patients. The most commonly used biologics in this cohort were interleukin (IL)-17 inhibitors (29.1%), followed by tumor necrosis factor (TNF) alpha inhibitors (18.3%) and IL-(12/)23 inhibitors (7.3%). Patients with PsA significantly more often had a biological therapy, a conventional synthetic (cs) DMARD therapy, and a combination therapy of b/tsDMARD + csDMARD (p = 0.001, p < 0.001, and p < 0.001, respectively). In the group of patients with Pso, 31.5% were treated with a topical therapy. Detailed numbers on therapies for both subgroups can be found in Table 1.
The queried patients with Pso reached on average 1.68 points in the PEST questionnaire compared to 3.72 points in the confirmed PsA cohort; 27.9% of patients with Pso scored ≥ 3 points, suspicious of psoriatic arthritis.
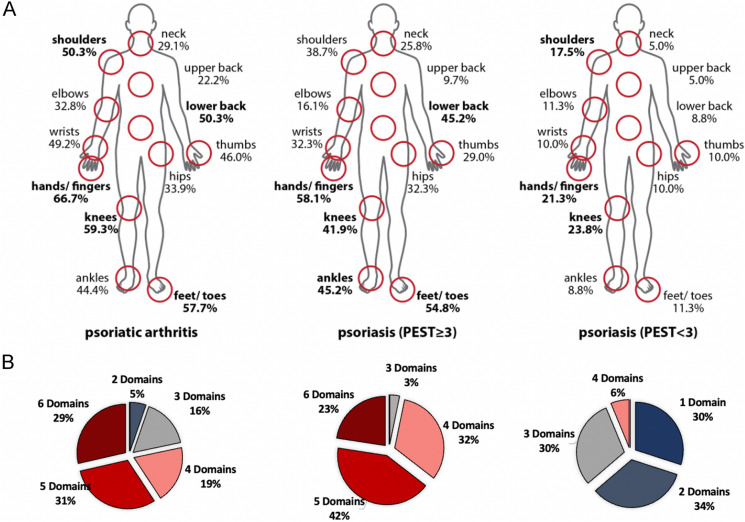
The great majority of patients in this cohort had multidomain disease: Only 21.6% of the included patients with Pso had only one single affected disease domain, whereas all included patients with PsA had at least two affected domains out of six possible disease domains (skin psoriasis, nail involvement, peripheral arthritis, axial disease, enthesitis, dactylitis). Patients with Pso had on average 2.88 affected domains (PEST ≥ 3, 4.84 domains; PEST < 3, 2.13 domains) compared to a mean of 4.61 affected domains in patients with PsA.
Functional Impairment and Burden of Disease
Of patients with Pso (PEST ≥ 3, 6.5%; PEST < 3, 0.0%), 1.8% had a clinically significant functional impairment in everyday life (FFbH < 60%) compared to 19.0% in the PsA cohort (p < 0.001). Of the queried patients with Pso, 73.9% reported peripheral and 25.2% reported axial joint pain with on average 3.39 of 21 joint regions affected. In the confirmed PsA cohort 96% of patients reported joint pain in on average of 8.39 joint regions (min 0, max 21), 94% had peripheral joint pain, and 60% complained of axial pain. The most commonly affected joint regions were the hands/fingers, followed by the knees, feet and toes, and lower back pain (Fig. 1). Patients with PsA scored significantly worse regarding their mobility and their ability to work than patients with Pso (p < 0.001, p = 0.013).
Fig. 1.
Joint distribution and affected disease domains. a Self-reported regions with joint pain or joint problems in psoriasis (n = 111) and psoriatic arthritis (n = 189). The most commonly affected regions were hands/fingers, knees, feet/toes, and lower back in patients with PsA; hands/fingers, feet/toes, lower back, and ankles in patients with Pso with a PEST score ≥ 3 (n = 31); knees, hands/fingers, and shoulders in patients with Pso with a PEST score < 3 (n = 80). b Number of affected disease domains (self-reported) out of six possible domains (skin psoriasis, nail involvement, peripheral arthritis, axial disease, enthesitis, dactylitis). PEST Psoriasis Epidemiology Screening Tool, PsA psoriatic arthritis, Pso psoriasis
In order to identify factors associated with relevant functional impairment, we performed logistic regression analysis. After adjustment for sex, disease duration, BMI, and smoking status, a diagnosis of PsA (odds ratio [OR] 9.56, p = 0.005), depressive symptoms (OR 5.44, p < 0.001), and age (OR 1.04, p = 0.033) were independently associated with relevant functional impairment in everyday life (Table 2).
Table 2.
Binomial logistic regression analysis of factors associated with relevant functional impairment (FFbH < 60%)
| Predictors | OR | 95% CI | B | p |
|---|---|---|---|---|
| Diagnosis of PsA | 9.56 | 1.96–46.73 | 2.26 | 0.005 |
| Depressive symptoms (PHQ-9 > 10) | 5.44 | 2.22–13.33 | 1.69 | < 0.001 |
| Age | 1.04 | 1.004–1.08 | 0.04 | 0.033 |
Multivariable logistic regression adjusted for sex, disease duration, smoking status, BMI
BMI body mass index, CI confidence interval, FFbH Functional Questionnaire Hannover, OR odds ratio, PHQ-9 Patient health questionnaire 9, PsA psoriatic arthritis
Quality of Life
Subjective overall quality of life did not differ significantly between patients with Pso and PsA. However, patients with PsA reached significantly lower scores in the physical health-related quality of life domain than patients with Pso (p < 0.001). In particular, patients with PsA reported more pain and malaise (p < 0.001) as well as more dependency on medication and support (p < 0.001). There were no significant differences between the two groups regarding overall mental health-related quality of life; however, patients with PsA scored worse than patients with Pso regarding energy and fatigue (p = 0.009) and more commonly reported sleeping problems and tiredness (both p = 0.001).
Patients with Pso who had a PEST score ≥ 3 rated their overall subjective quality of life significantly worse than patients with PEST score < 3 points (p = 0.003) and demonstrated a significantly reduced QoL in the health- and mental health-related domains as well as regarding their living environment (domains 1, 2, and 4; p < 0.001, p = 0.008, and p = 0.011, respectively) (see also Table 3 for comparison of PsA and Pso patients with PEST score ≥ 3 and PEST score < 3). In particular, Pso patients with a PEST score ≥ 3 reported more pain, fatigue, and sleeping problems (p < 0.001, p = 0.002, and p = 0.010, respectively).
Table 3.
Quality of life assessed by WHOQOL-BREF questionnaire
| Quality of life (WHOQOL-BREF) | Pso (PEST ≥ 3) (n = 31) | Pso (PEST < 3) (n = 80) | PsA (n = 189) | p value |
|---|---|---|---|---|
| Dimension 1: Physical health | 59.54 | 74.71 | 57.94 | < 0.0001 |
| Dimension 2: Mental health | 57.53 | 67.87 | 64.33 | 0.0028 |
| Dimension 3: Social relationships | 68.14 | 71.47 | 67.45 | 0.3189 |
| Dimension 4: Environment | 68.29 | 77.24 | 73.80 | 0.0027 |
| Subjective quality of life | 3.129 | 3.688 | 3.404 | 0.0038 |
| Satisfaction with health | 2.621 | 3.150 | 2.884 | 0.0280 |
Comparison of groups by ANOVA
n number, PEST Psoriasis Epidemiology Screening Tool, PsA psoriatic arthritis, Pso psoriasis, WHOQOL-BREF World Health Organization Quality of Life Brief Version
In general, patients less than 45 years of age reported a better subjective quality of life and a better health-related quality of life compared to patients aged over 45 years (p = 0.005, p < 0.001). Furthermore, subjective quality of life correlated significantly with educational level (p = 0.003).
In the PsA subgroup, female patients had a worse functional status than male patients as well as more joint regions with complaints (p = 0.019, p = 0.013). In contrast, in the Pso subgroup, there were no sex-related differences regarding the health-related quality of life or functional capacity.
Depressive Symptoms
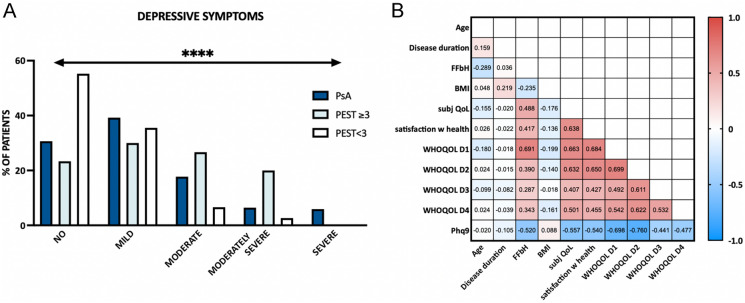
Overall prevalence of depressive symptoms was high; 54% and 69% of the queried patients with Pso and PsA, respectively, showed at least mild depressive symptoms, and 19.8% of the patients with Pso and 30.1% of the patients with PsA had moderate to severe depressive symptoms (Fig. 2a). In contrast, only 17% of the patients had a history of depression from medical records or were taking antidepressants. A total of 10.6% of patients received antidepressant medication.
Fig. 2.
Depressive symptoms and quality of life. a Depressive symptoms determined by PHQ-9. Patients with PsA shown in dark blue, patients with Pso with PEST ≥ 3 in light blue, patients with Pso with PEST < 3 in white. b Pearson’s correlations between clinical data, quality of life, and depressive symptoms. Numbers within the graph represent Pearson’s R values. Red color indicates positive correlation, blue negative correlation. BMI body mass index, QoL quality of life, FFbH Functional Questionnaire Hannover, PEST Psoriasis Epidemiology Screening Tool, PHQ-9 Patient health questionnaire 9 (depressive symptoms), PsA psoriatic arthritis, Pso psoriasis, WHOQOL D1 World Health Organization Quality of Life Domain 1 (= physical health-related QoL), D2 = mental HRQoL, D3 = social QoL, D4 = environmental QoL
Patients with Pso with a PEST score ≥ 3 reached on average 8.68 points in the PHQ-9 depression questionnaire, equivalent to mild depressive symptoms, compared to 4.63 points in patients with Pso with a PEST score < 3 (within normal range) and 7.88 points in patients with PsA (ANOVA p < 0.0001). Significantly more depressive symptoms could be detected in patients with pain (p = 0.011) and functional impairment (p < 0.001). Depressive symptoms were highly negatively correlated with all domains of quality of life (p < 0.001; Fig. 2b). There were no significant differences between male and female patients regarding depressive symptoms.
In order to identify predictors of depression, a binomial logistic regression model was calculated using a stepwise backwards elimination approach and adjusting for demographic factors (age, sex, disease duration, BMI, smoking status). The model was statistically significant, χ2 (9) = 41.42, p < 0.001, classifying 75.1% of cases correctly. Functional impairment (OR 4.50, p < 0.003), axial complaints (OR 2.80, p = 0.030), diagnosis of psoriatic arthritis (OR 2.69, p = 0.046), and number of joint regions with complaints (OR 1.10, p = 0.032) were independently associated with moderate to severe depressive symptoms (Table 4). Separate models for PsA and Pso can be found in Supplemental Table 1.
Table 4.
Binomial logistic regression analysis of factors associated with depressive symptoms (PHQ-9 > 10)
| Predictors | OR | 95% CI | B | p |
|---|---|---|---|---|
| Functional impairment (FFbH < 60%) | 4.50 | 1.64–12.35 | 1.51 | 0.003 |
| Axial complaints | 2.80 | 1.11–7.08 | 1.03 | 0.030 |
| Diagnosis PsA | 2.69 | 1.02–7.11 | 0.99 | 0.046 |
| No. of joint regions with complaints | 1.10 | 1.01–1.20 | 0.09 | 0.032 |
Multivariable logistic regression adjusted for age, sex, disease duration, smoking status, BMI. Functional capacity measured by FFbH questionnaire
BMI body mass index, CI confidence interval, FFbH Functional Questionnaire Hannover, OR odds ratio, PHQ-9 Patient health questionnaire 9, PsA psoriatic arthritis
Discussion
Within this study we investigated the burden of disease, functional status, quality of life, and depressive symptoms in a cohort of 300 patients with psoriatic disease and assessed predictors of functional impairment and depression.
Functional capacity in everyday life was significantly worse in PsA than in patients with Pso despite patients receiving DMARD therapy according to guidelines. While an obvious explanation may be the accrual of damage and disability over time, functional capacity was not associated with disease duration in this cohort. In a logistic regression analysis, we were able to show that a diagnosis of psoriatic arthritis, depressive symptoms, and age were independently associated with functional impairment in everyday life. Husted et al. had previously reported the number of actively inflamed joints to predict functional impairment in PsA [25]. Another study had identified age, joint damage, and baseline functional capacity as important predictors of physical function over time [26]. Functional impairment may lead to the inability to perform activities of daily living independently and need for assistance. It was shown that disability/functional impairment is associated with an increased risk of hospitalization [27]. Furthermore, functional impairment may lead to work disability with absenteeism, reduced effectiveness at work/loss of productivity, or unemployment and may thus pose a significant socioeconomic burden on patients and their families as well as on society in general [28]. To decrease the risk of disability, early diagnosis and initiation of treatment following a treat-to-target approach are essential [29]. This is further underlined by the fact that a reduced functional capacity in our study was highly significantly associated with a reduced quality of life and contributed significantly to depressive symptoms in our cohort.
We furthermore showed that patients with Pso with suspicion of psoriatic arthritis had a worse quality of life and more depressive symptoms than patients with Pso without a suspicion of PsA. Patients with suspicion of PsA had a significantly worse mental health-related quality of life compared to patients with confirmed PsA. However, subjective quality of life and health-related quality of life remained reduced in patients with confirmed PsA. Furthermore, diagnosis of PsA was independently associated with the prevalence of depressive symptoms in this cohort. Our data thus are in line with other published studies, which showed no changes in health-related quality of life in patients with PsA as well as a higher risk of depression for patients with PsA than Pso [8, 14, 30]. Vice versa, studies have also demonstrated that patients with psoriasis and with depression have an increased risk of developing PsA [31]. While the underlying pathophysiologic factors are not well understood, the development of both PsA and depression seems to be associated with chronic or systemic inflammation [31, 32]. Ogdie et al. described an association of multidomain presentations of psoriatic disease with worse disease activity, worse quality of life, and more depression [33]. In our study, factors independently associated with depressive symptoms were a diagnosis of PsA, axial complaints, and above all a reduced functional capacity. McDonough et al. as well as Haugeberg et al. had previously identified fatigue to independently predict depression in psoriatic disease/PsA, while in McDonough’s analysis employment was a protective factor [14, 34]. It has recently been shown that depressive symptoms reduce the probability of achieving a minimal disease activity (MDA) status [35]. Additionally, depression may contribute to morbidity and disability and has been associated with an increased risk of cardiovascular events such as myocardial infarctions and strokes in patients with psoriasis [36]. Depression was furthermore shown to increase the perception of pain [37]. Depression may thus have a far-reaching impact on patients’ lives with impaired quality of life, poor sleep, reduced social participation, social dysfunction, and work disability [38]. According to the World Health Organization (WHO), depression is a leading cause of disability worldwide [39]. Depression was furthermore shown to be associated with a high cost of illness [40]. The identification and treatment of depression are crucial as depression is furthermore associated with reduced treatment adherence and poor treatment response [41, 42].
We also observed a discrepancy between history of depressive symptoms on medical records and patients receiving antidepressants as well as the high prevalence of depressive symptoms at the timepoint of assessment. These data argue that depression is underdiagnosed and undertreated in patients with psoriasis. Similar findings were recently published for patients with rheumatoid arthritis [43]. Taking into account the negative effects of depression on quality of life, disease activity, and therapy adherence, patients should be screened for depressive symptoms and access to mental health services should be improved to facilitate diagnosis and concomitant therapy of depression.
Interestingly, we observed an association of female sex with worse quality of life and functional status only for PsA, but not for the skin psoriasis subgroup. Published data show that skin psoriasis affects quality of life in women to a higher degree than in men, though registry studies show that men tend to have higher PASI scores, i.e., more severe psoriasis [44]. The fact that we did not detect any sex-related differences in QoL in the psoriasis subgroup may attributed to the use of the WHOQOL-BREF questionnaire, which assesses general quality of life, instead of a dermatology-specific instrument, such as DLQI or Skindex-29. Sex-related differences in PsA and spondyloarthritides in general have recently gained renewed interest. The pattern of arthritis was shown to differ between sexes with women more frequently suffering from polyarticular joint involvement of PsA with, however, less frequent development of erosions or joint destruction [45]. Axial involvement and radiographic axial changes occur more frequently in male patients with PsA [46]. Despite men having a worse prognosis regarding disease progression, women more often report higher levels of pain, more disease burden and limitation in daily function, and more frequently develop work disability [47–49]. Women more frequently report pain and increased pain sensitivity is observed among women [50]. Thus, further studies regarding sex-related differences and adjustment of treatment strategies will be necessary to reduce impairment and improve outcomes and subjective quality of life.
Irrespective of sex, Coates et al. recently described a substantial impact and significant residual burden of PsA on social, emotional, and work-related aspects of life despite current DMARD therapy [11]. Also, Liu et al. reported a substantial disease burden on patients with PsA even after achieving low disease activity (LDA) states [51] with major contributors being pain, fatigue, and psychological aspects of disease. Thus, patients’ perspectives of their disease and physician assessment of disease activity may differ and current tools used in the assessment of disease activity may not adequately reflect patients’ perspectives. The residual burden of disease needs to be addressed and treatment targets redefined to improve quality of life and patient satisfaction with health. A more patient-centered holistic approach may be needed to address these issues.
Limitations of this study include the cross-sectional study design, which meant that patients were not followed over time to assess outcomes, and the questionnaire-based approach relying on patient-reported measures. Furthermore, data may not be fully generalizable because of the monocentric university hospital setting. An additional weakness of this study is the lack of data on comorbidities as these may influence disease burden and disease activity in patients with PsA [52, 53]. Furthermore, the question of direction of dependence of depressive symptoms and functional impairment was beyond the scope of this study. Further studies will be needed to answer this question.
Conclusion
This cross-sectional analysis assessed functional capacity, burden of disease, quality of life, and depressive symptoms in a cohort of 300 patients with psoriatic disease including 111 patients with Pso and 189 with PsA. We were able to show that depressive symptoms are predicted by functional impairment, axial complaints, diagnosis of psoriatic arthritis, and number of joint regions with complaints. We furthermore showed that functional impairment, quality of life, and depressive symptoms are mutually interdependent, necessitating early diagnosis and initiation of anti-inflammatory therapy to avoid long-term damage, disability, and mental health complications. However, further studies will be necessary to determine the directionality and exact relationship between functional impairment and depression.
The data presented in this manuscript furthermore indicate that depression is underdiagnosed and undertreated in patients with psoriatic disease, with possible negative effects on quality of life, disease activity, and therapy adherence. Patients with psoriatic disease should thus be screened for depressive symptoms and access to mental health services should be improved to facilitate early diagnosis and concomitant therapy of depression.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all patients who participated in this study.
Author Contributions
Nils Venhoff, Reinhard E. Voll, Jens Thiel, Stephanie Finzel, Christoph Schempp, Franziska Schauer, Dominique Endres, Ludger Tebartz van Elst designed and supervised the study and gave their critical input. Sonja Hiestand, Franziska Schauer, Christoph Schempp, Nils Venhoff, Natalie Frede, Nils Craig-Mueller, Markus Zeisbrich, Stephanie Finzel, Jens Thiel consented and cared for the patients enrolled in this study and provided clinical information. Natalie Frede, Sonja Hiestand and Nils Venhoff performed the data analysis. Natalie Frede and Nils Venhoff wrote the manuscript. All authors read and approved the final manuscript.
Funding
Parts of this study were financially supported by an unrestricted grant from Novartis Pharma GmbH, Germany. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. The Rapid Service Fee was funded by the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Nils Venhoff: Speaker honoraria: AbbVie, Novartis, UCB, Bristol-Myers Squibb, Pfizer; Advisory Boards: AbbVie, Novartis, UCB; Research grants: Bristol-Myers Squibb, Novartis, Pfizer. Jens Thiel: Speaker honoraria: GSK, BMS, AstraZeneca, Abbvie, UCB, Lilly; Advisory Boards: Novartis, GSK, AstraZeneca, Lilly. Grant/research support from: BMS, Novartis. Reinhard E. Voll: Speaker fees: AbbVie, Amgen, BMS, Boehringer-Ingelheim, GSK, Janssen-Cilag, Hexal, Novartis, Pfizer, Roche; Advisory boards: AbbVie, Amgen, Boehringer-Ingelheim, BMS, GSK, Janssen-Cilag, Hexal, Neutrolis, Novartis, Sanofi, Takeda; Unrestricted research grants: Amgen, BMS, Novartis, Pfizer. Natalie Frede received travel grants from AbbVie, Janssen, Sobi, Pfizer. Sonja Hiestand, Franziska Schauer, Dominique Endres, Ludger Tebartz van Elst, Markus Zeisbrich, Nils Craig-Mueller, Stephanie Finzel and Christoph Schempp have nothing to declare.
Ethical Approval
The study was conducted under the ethics protocol 190/17 (ethics committee of the University of Freiburg, Germany). Patients gave their written consent according to International Conference on Harmonization Good Clinical Practice (ICH GCP) guidelines. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
References
- 1.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960. doi: 10.1001/jama.2020.4006. [DOI] [PubMed] [Google Scholar]
- 2.Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251–65.e19. doi: 10.1016/j.jaad.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 3.Villani AP, Rouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(2):242–248. doi: 10.1016/j.jaad.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Haroon M, Kirby B, FitzGerald O. High prevalence of psoriatic arthritis in patients with severe psoriasis with suboptimal performance of screening questionnaires. Ann Rheum Dis. 2013;72(5):736–740. doi: 10.1136/annrheumdis-2012-201706. [DOI] [PubMed] [Google Scholar]
- 5.Eder L, Chandran V, Gladman DD. What have we learned about genetic susceptibility in psoriasis and psoriatic arthritis? Curr Opin Rheumatol. 2015;27(1):91–98. doi: 10.1097/BOR.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 6.Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74(6):1045–1050. doi: 10.1136/annrheumdis-2013-204858. [DOI] [PubMed] [Google Scholar]
- 7.Haugeberg G, Michelsen B, Kavanaugh A. Impact of skin, musculoskeletal and psychosocial aspects on quality of life in psoriatic arthritis patients: a cross-sectional study of outpatient clinic patients in the biologic treatment era. RMD Open. 2020;6(1):e001223. doi: 10.1136/rmdopen-2020-001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freites Nuñez D, Madrid-García A, Leon L, et al. Factors associated with health-related quality of life in psoriatic arthritis patients: a longitudinal analysis. Rheumatol Ther. 2021;8(3):1341–1354. doi: 10.1007/s40744-021-00349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wervers K, Luime JJ, Tchetverikov I, et al. Influence of disease manifestations on health-related quality of life in early psoriatic arthritis. J Rheumatol. 2018;45(11):1526–1531. doi: 10.3899/jrheum.171406. [DOI] [PubMed] [Google Scholar]
- 10.Bergman MJ, Zueger P, Patel J, et al. Clinical and economic benefit of achieving disease control in psoriatic arthritis and ankylosing spondylitis: a retrospective analysis from the OM1 registry. Rheumatol Ther. 2023;10(1):187–199. doi: 10.1007/s40744-022-00504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coates LC, Orbai AM, Azevedo VF, et al. Results of a global, patient-based survey assessing the impact of psoriatic arthritis discussed in the context of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire. Health Qual Life Outcomes. 2020;18(1):173. doi: 10.1186/s12955-020-01422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol. 2018;14(5):405–417. doi: 10.1080/1744666X.2018.1468252. [DOI] [PubMed] [Google Scholar]
- 13.Dowlatshahi EA, Wakkee M, Arends LR, Nijsten T. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134(6):1542–1551. doi: 10.1038/jid.2013.508. [DOI] [PubMed] [Google Scholar]
- 14.McDonough E, Ayearst R, Eder L, et al. Depression and anxiety in psoriatic disease: prevalence and associated factors. J Rheumatol. 2014;41(5):887–896. doi: 10.3899/jrheum.130797. [DOI] [PubMed] [Google Scholar]
- 15.Nazik H, Nazik S, Gul FC. Body image, self-esteem, and quality of life in patients with psoriasis. Indian Dermatol Online J. 2017;8(5):343–346. doi: 10.4103/idoj.IDOJ_503_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zięciak T, Rzepa T, Król J, Żaba R. Stigmatization feelings and depression symptoms in psoriasis patients. Psychiatr Pol. 2017;51(6):1153–1163. doi: 10.12740/PP/68848. [DOI] [PubMed] [Google Scholar]
- 17.Alpsoy E, Polat M, FettahlıoGlu-Karaman B, et al. Internalized stigma in psoriasis: a multicenter study. J Dermatol. 2017;44(8):885–891. doi: 10.1111/1346-8138.13841. [DOI] [PubMed] [Google Scholar]
- 18.Zhao SS, Miller N, Harrison N, Duffield SJ, Dey M, Goodson NJ. Systematic review of mental health comorbidities in psoriatic arthritis. Clin Rheumatol. 2020;39(1):217–225. doi: 10.1007/s10067-019-04734-8. [DOI] [PubMed] [Google Scholar]
- 19.Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371. doi: 10.1155/2017/9724371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohlmann T, Raspe H. Hannover Functional Questionnaire in ambulatory diagnosis of functional disability caused by backache. Rehabilitation (Stuttg) 1996;35(1):I–VIII. [PubMed] [Google Scholar]
- 21.Ibrahim GH, Buch MH, Lawson C, Waxman R, Helliwell PS. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27(3):469–474. [PubMed] [Google Scholar]
- 22.World Health Organization Programme on Mental Health. WHOQOL-BREF: introduction, administration, scoring and generic version of the assessment: field trial version. 1996. Geneva: WHO.
- 23.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The jamovi project. jamovi (Version 2.3) [Computer Software] (2023) Retrieved 05 Sept 2023 from https://www.jamovi.org.
- 25.Husted JA, Tom BD, Farewell VT, Schentag CT, Gladman DD. Description and prediction of physical functional disability in psoriatic arthritis: a longitudinal analysis using a Markov model approach. Arthritis Rheum. 2005;53(3):404–409. doi: 10.1002/art.21177. [DOI] [PubMed] [Google Scholar]
- 26.Leung YY, Ho KW, Li EK, et al. Predictors of functional deterioration in Chinese patients with psoriatic arthritis: a longitudinal study. BMC Musculoskelet Disord. 2014;15:284. doi: 10.1186/1471-2474-15-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown RT, Diaz-Ramirez LG, Boscardin WJ, Lee SJ, Williams BA, Steinman MA. Association of functional impairment in middle age with hospitalization, nursing home admission, and death. JAMA Intern Med. 2019;179(5):668–675. doi: 10.1001/jamainternmed.2019.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tillett W, de-Vries C, McHugh NJ. Work disability in psoriatic arthritis: a systematic review. Rheumatology (Oxford) 2012;51(2):275–283. doi: 10.1093/rheumatology/ker216. [DOI] [PubMed] [Google Scholar]
- 29.FitzGerald O, Ogdie A, Chandran V, et al. Psoriatic arthritis. Nat Rev Dis Primers. 2021;7(1):59. doi: 10.1038/s41572-021-00293-y. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Carazo JL, López-Estebaranz JL, Guisado C. Comorbidities and health-related quality of life in Spanish patients with moderate to severe psoriasis: a cross-sectional study (Arizona study) J Dermatol. 2014;41(8):673–678. doi: 10.1111/1346-8138.12465. [DOI] [PubMed] [Google Scholar]
- 31.Lewinson RT, Vallerand IA, Lowerison MW, et al. Depression is associated with an increased risk of psoriatic arthritis among patients with psoriasis: a population-based study. J Invest Dermatol. 2017;137(4):828–835. doi: 10.1016/j.jid.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Friedrich MJ. Research on psychiatric disorders targets inflammation. JAMA. 2014;312(5):474–476. doi: 10.1001/jama.2014.8276. [DOI] [PubMed] [Google Scholar]
- 33.Ogdie A, Hur P, Liu M, et al. Effect of multidomain disease presentations on patients with psoriatic arthritis in the corrona psoriatic arthritis/spondyloarthritis registry. J Rheumatol. 2021;48(5):698–706. doi: 10.3899/jrheum.200371. [DOI] [PubMed] [Google Scholar]
- 34.Haugeberg G, Hoff M, Kavanaugh A, Michelsen B. Psoriatic arthritis: exploring the occurrence of sleep disturbances, fatigue, and depression and their correlates. Arthritis Res Ther. 2020;22(1):198. doi: 10.1186/s13075-020-02294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong A, Ye JY, Cook RJ, Gladman DD, Chandran V. Depression and anxiety reduce the probability of achieving a state of sustained minimal disease activity in patients with psoriatic arthritis. Arthritis Care Res (Hoboken) 2022;74(9):1430–1434. doi: 10.1002/acr.24593. [DOI] [PubMed] [Google Scholar]
- 36.Egeberg A, Khalid U, Gislason GH, Mallbris L, Skov L, Hansen PR. Impact of depression on risk of myocardial infarction, stroke and cardiovascular death in patients with psoriasis: a Danish Nationwide Study. Acta Derm Venereol. 2016;96(2):218–221. doi: 10.2340/00015555-2218. [DOI] [PubMed] [Google Scholar]
- 37.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 38.Hammer-Helmich L, Haro JM, Jönsson B, et al. Functional impairment in patients with major depressive disorder: the 2-year PERFORM study. Neuropsychiatr Dis Treat. 2018;14:239–249. doi: 10.2147/NDT.S146098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedrich MJ. Depression is the leading cause of disability around the world. JAMA. 2017;317(15):1517. doi: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- 40.König H, Rommel A, Thom J, et al. The excess costs of depression and the influence of sociodemographic and socioeconomic factors: results from the German Health Interview and Examination Survey for Adults (DEGS) Pharmacoeconomics. 2021;39(6):667–680. doi: 10.1007/s40273-021-01000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renzi C, Picardi A, Abeni D, et al. Association of dissatisfaction with care and psychiatric morbidity with poor treatment compliance. Arch Dermatol. 2002;138(3):337–342. doi: 10.1001/archderm.138.3.337. [DOI] [PubMed] [Google Scholar]
- 42.Fortune DG, Richards HL, Kirby B, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch Dermatol. 2003;139(6):752–756. doi: 10.1001/archderm.139.6.752. [DOI] [PubMed] [Google Scholar]
- 43.Peterson S, Piercy J, Blackburn S, Sullivan E, Karyekar CS, Li N. The multifaceted impact of anxiety and depression on patients with rheumatoid arthritis. BMC Rheumatol. 2019;3:43. doi: 10.1186/s41927-019-0092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guillet C, Seeli C, Nina M, Maul LV, Maul JT. The impact of gender and sex in psoriasis: what to be aware of when treating women with psoriasis. Int J Womens Dermatol. 2022;8(2):e010. doi: 10.1097/JW9.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Queiro R, Tejón P, Coto P, et al. Clinical differences between men and women with psoriatic arthritis: relevance of the analysis of genes and polymorphisms in the major histocompatibility complex region and of the age at onset of psoriasis. Clin Dev Immunol. 2013;2013:482691. doi: 10.1155/2013/482691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee W, Reveille JD, Davis JC, Learch TJ, Ward MM, Weisman MH. Are there gender differences in severity of ankylosing spondylitis? Results from the PSOAS cohort. Ann Rheum Dis. 2007;66(5):633–638. doi: 10.1136/ard.2006.060293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallenius M, Skomsvoll JF, Koldingsnes W, et al. Work disability and health-related quality of life in males and females with psoriatic arthritis. Ann Rheum Dis. 2009;68(5):685–689. doi: 10.1136/ard.2008.092049. [DOI] [PubMed] [Google Scholar]
- 48.Passia E, Vis M, Coates LC, et al. Sex-specific differences and how to handle them in early psoriatic arthritis. Arthritis Res Ther. 2022;24(1):22. doi: 10.1186/s13075-021-02680-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gossec L, Walsh JA, Michaud K, et al. Women with psoriatic arthritis experience higher disease burden than men: findings from a real-world survey in the USA and Europe. J Rheumatol. 2023;50:192–196. doi: 10.3899/jrheum.220154. [DOI] [PubMed] [Google Scholar]
- 50.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu V, Fong W, Kwan YH, Leung YY. Residual disease burden in patients with axial spondyloarthritis and psoriatic arthritis despite low disease activity states in a multiethnic Asian population. J Rheumatol. 2021;48(5):677–684. doi: 10.3899/jrheum.200934. [DOI] [PubMed] [Google Scholar]
- 52.Lubrano E, Scriffignano S, Azuaga AB, Ramirez J, Cañete JD, Perrotta FM. Impact of comorbidities on disease activity, patient global assessment, and function in psoriatic arthritis: a cross-sectional study. Rheumatol Ther. 2020;7(4):825–836. doi: 10.1007/s40744-020-00229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cañete JD, Tasende JAP, Laserna FJR, Castro SG, Queiro R. The impact of comorbidity on patient-reported outcomes in psoriatic arthritis: a systematic literature review. Rheumatol Ther. 2020;7(2):237–257. doi: 10.1007/s40744-020-00202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.