Abstract
Prostate cancer (PC) and colorectal cancer (CRC) are two of the leading causes of cancer-related mortality. The incidence of synchronous neoplasms in patients with CRC is increasing, though synchronous PC and CRC remains a rare occurrence in clinical practice. Early diagnosis, accurate staging, and characterization of tumors are essential for selecting patient-tailored therapy. The origin of metastatic disease in synchronous cases presents a challenge for conventional imaging modalities, but advances in molecular imaging have addressed this limitation. Positron emission tomography/computed tomography (PET/CT) is now the preferred modality for assessing synchronous cases. The authors present a 72-year-old male patient with the rare occurrence of two coexisting primary cancers. At first, fluorine-18 fluorodeoxyglucose (18F-FDG) PET/CT detected the first colorectal primary tumor extension along with evidence of heterogeneous 18F-FDG activity within an enlarged prostate, warranting further evaluation. Subsequently, gallium-68 prostate-specific membrane antigen (68 Ga-PSMA) PET/CT imaging revealed the second prostate primary cancer with evidence of bone metastases. Adoption of a dual PET/CT approach in cases where biopsy is impractical can achieve accurate staging results during the initial diagnostic workup.
Keywords: PET/CT, Synchronous cancers, FDG, PSMA, Dual PET/CT approach, Multiple primary neoplasms
Introduction
The utilization of positron emission tomography/computed tomography (PET/CT) in the diagnosis of various cancer subtypes is becoming prevalent and is increasingly acknowledged as an essential element in detecting synchronous tumors [1–3]. Occasionally, this may require using dual PET/CT modalities to more accurately define the extent of each tumor. The primary benefits of this approach are threefold: initial staging, identifying the source of metastases, and determining therapy response. The distinction of the primary origin of metastatic disease contributes to accurate staging and implies important diagnostic and therapeutic challenges. The development of multiple synchronous tumors is thought to be caused by similar mechanisms. The issue is believed to be influenced by mutual environmental and genetic factors. Therefore, it is likely that multiple factors are responsible for the development of synchronous tumors.
Although rare, multiple primary synchronous tumors are being increasingly detected, with reported incidence rates of more than 10% [4]. This has been observed in recent years, as fluorine-18 fluorodeoxyglucose (18F-FDG) PET/CT has become a major part of assessing malignant tumors. It is noteworthy that prostate cancer (PC) and colorectal cancer (CRC) are extremely rare coinciding with multiple primary tumors that are only reported with an incidence rate of less than 1% [5, 6]. To the best of our knowledge, there is only one existing case report comparing the findings between 18F-FDG PET/CT and gallium-68 prostate-specific membrane antigen (68 Ga-PSMA) PET/CT in a patient with synchronous CRC and PC [7]. Adopting the approach of dual PET/CT modalities compares the results of both 68 Ga-PSMA PET/CT and 18F-FDG PET/CT scans. This can help reach accurate staging results for both tumors.
Case Report
A 72-year-old male patient with a history of benign prostatic hyperplasia and no family history of cancer was found to have melena and moderate anemia with a hemoglobin of 9.7 g/dL (normal range for males 13.8–17.2 g/dL). Carcinoembryonic antigen (CEA) testing was performed and found to be mildly elevated at 8 ng/ml (reference values below 2.5 ng/mL). A subsequent colonoscopy and biopsy revealed moderately differentiated adenocarcinoma of the rectum, while a pelvic magnetic resonance imaging (MRI) showed a T4bN2b rectal tumor invading the anterior peritoneal reflection with extramural vascular invasion and potential invasion of prostate gland (Fig. 1). As a result of the findings on the MRI, a prostate-specific antigen (PSA) test was ordered, resulting in high baseline values of 59.9 ng/ml (reference range for age 4.5–5.5 ng/ml).
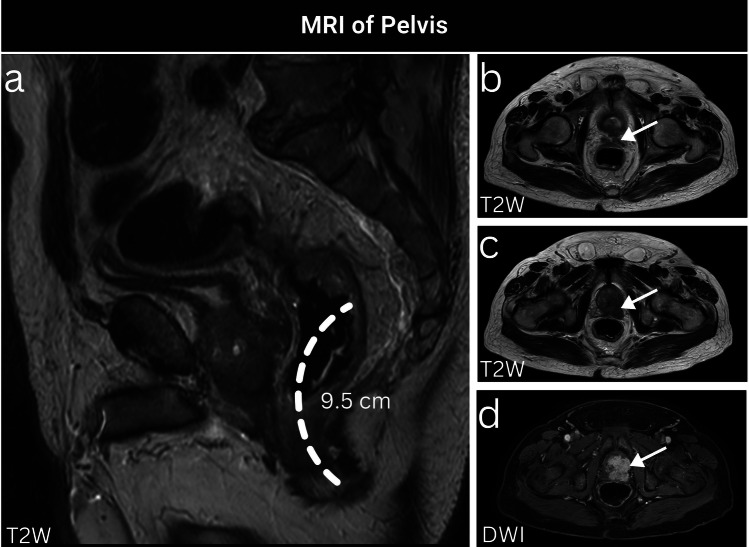
Fig. 1.
A 72-year-old male patient, recently diagnosed with CRC, had a pelvic MRI ordered for primary tumor staging, which revealed a mid-rectal tumor located approximately 9.5 cm from the anal verge (a). This tumor is evidently straddling and invading the anterior peritoneal reflection (b, arrow). Additionally, there is current evidence of potential direct prostatic invasion, as evidenced by prominent hypointensity on T2-weighted images (c, arrow) and hyperintensity on diffusion-weighted imaging with involvement of both seminal vesicles (d, arrow)
18F-FDG PET/CT was conducted (Fig. 2a–k) and confirmed the presence of hypermetabolic primary rectal malignancy (SUVmax of 24.8) with involvement of the lower para-aortic and aortocaval lymph nodes (Fig. 2a, b, d, e, arrowheads). Moreover, there was heterogeneously increased 18F-FDG uptake in the peripheral zone of the prostate (SUVmax of 4.3) and the left seminal vesicle (Fig. 2c, curved arrow). This prompted a prostatic biopsy, which revealed prostatic acinar adenocarcinoma with a Gleason score of 8.
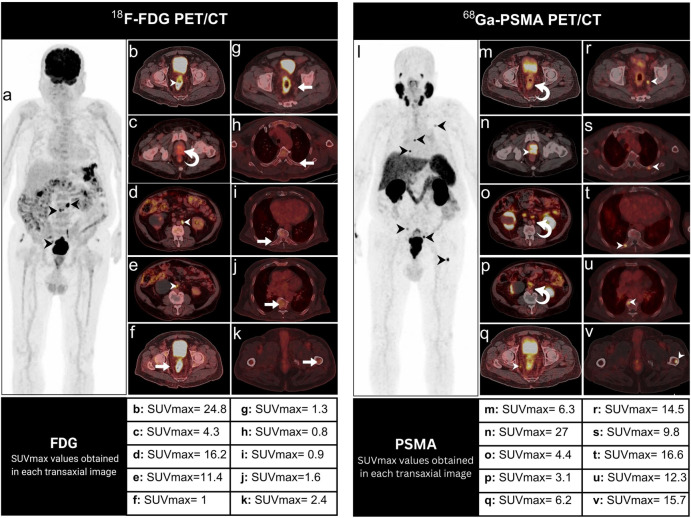
Fig. 2.
A 72-year-old male patient with synchronous CRC and PC. 18F-FDG maximum intensity projection (MIP) (a), axial 18F-FDG PET/CT images (b–k) were acquired and examined. The study revealed hypermetabolic rectal malignancy (b, arrowheads), with involvement of the hypermetabolic lower para-aortic and aortocaval lymph nodes (a, d, e, arrowheads). This was evident in concordance with heterogeneous 18F-FDG-avid foci noted within the peripheral zone of the prostate and the left seminal vesicle (c, curved arrow) necessitating further evaluation. In result, 68 Ga-PSMA MIP (l), axial 68 Ga-PSMA PET/CT images (m–v) were acquired after 10 days, and showed abnormally increased uptake in the prostate, and bilateral seminal vesicles (n, arrowhead), left posterior 5th rib, right 9th rib near the costovertebral junction, T7 vertebra, proximal shaft of the left femur, and internal iliac lymph nodes (q–v, arrowheads). Additionally, only heterogeneous uptake in the rectal tumor and its associated lower para-aortic and aortocaval lymph nodes was evident (m, o, p, curved arrows). It is noteworthy that all the 68 Ga-PSMA PET/CT findings, except for the heterogeneous prostate and seminal vesicles, were not visible on 18F-FDG PET/CT (f–k, arrows)
According to our guidelines for high-risk PC workup, 68 Ga-PSMA PET/CT (Fig. 2l–v) was performed to accurately stage the PC. A 68 Ga-PSMA PET/CT performed 10 days after the 18F-FDG PET/CT scan showed abnormally increased uptake in the prostate (SUVmax of 27), bilateral seminal vesicles, left posterior 5th rib, right 9th rib near the costovertebral junction, T7 vertebra, proximal shaft of the left femur, and internal iliac lymph nodes (Fig. 2n, q–v, arrowheads). All the 68 Ga-PSMA PET/CT findings, except for the heterogeneous prostate and seminal vesicles, were not visible on 18F-FDG PET/CT (Fig. 2f–k, arrows). On the other hand, 68 Ga-PSMA PET/CT only showed heterogeneous uptake in the rectal tumor (SUVmax of 6.3), and its associated lower para-aortic and aortocaval lymph nodes (Fig. 2m, o, p, curved arrows).
Finally, the patient was diagnosed with synchronous primary rectal adenocarcinoma with abdominal lymph node metastases, as well as primary acinar prostate adenocarcinoma with pelvic lymph node metastases and bone metastases. He was scheduled to receive palliative chemotherapy with XELOX (capecitabine plus oxaliplatin).
Discussion
CRC is the third most commonly diagnosed cancer worldwide and is the second leading cause of cancer-related mortalities [8]. Similarly, prostate cancer (PC) is the second most prevalent cancer globally and ranks as the fifth most significant cause of cancer-related deaths worldwide [9]. In Jordan, CRC is the most common cancer among men, with a prevalence rate of 13.1% among Jordanian men with cancer in 2018 [10]. PC followed in fourth place with a 7.6% prevalence rate among Jordanian male cancer patients [10]. Despite the high frequency of those cancers in Jordan and worldwide, their coexistence as independent primary tumors is an uncommon occurrence, with an incidence rate between 0.1 and 0.2% [4, 5]. Nevertheless, the detection of synchronous multiple primary tumors is rising in incidence due to the surge in cancer risk factors, longer life expectancies, progress of diagnostic imaging modalities, and enhanced clinical awareness [11].
In many cases, elevated PSA in rectal cancer patients is caused by direct invasion of the prostate by the tumor, causing a release of free PSA. However, the tumor was negative for both SATB2 and CDX2, negating the possibility of metastasis or direct invasion [12]. As the delayed identification of the second primary tumor is typically due to inadequate suspicion [13], physicians should be aware of the possibility of synchronous primary CRC and PC since it could lead to a challenging management plan [14].
18F-FDG remains the most robust radiotracer in the detection of most cancers and their metastases, and while it is not routinely recommended for the staging of primary CRC, it is exceedingly valuable in the detection of its metastases with a sensitivity that can reach up to 94.6% on a per patient basis [15]. However, it has proven to be of limited utility in the diagnosis of PC, with some papers reporting a sensitivity as low as 4% and higher sensitivity rates found only in the advanced stages of PC [7]. 68 Ga-PSMA has proven itself superior to all other radiotracers in the imaging of PC, with an exceptional tumor-to-background ratio leading to 74% sensitivity and 96% specificity in initial staging and re-staging at biochemical recurrence, even at low PSA values [16–19]. Our findings were similar to a previous case report that compared the findings between 18F-FDG PET/CT and 68 Ga-PSMA PET/CT in a patient with synchronous CRC and PC [7]. The patient demonstrated increased uptake in the rectum and a pre-sacral lymph node on the 18F-FDG PET/CT scan, but these findings were not appreciated on the 68 Ga-PSMA PET/CT scan, in contrast to the prostate and an iliac crest lesion, which showed intense uptake on 68 Ga-PSMA PET/CT but minimal uptake on 18F-FDG PET/CT [7], illustrating the variance in avidity of each tracer.
This variance supports one potential application of 18F-FDG and 68 Ga-PSMA PET imaging in patients with synchronous colorectal and PC: the potential ability to determine the origin of suspected metastases based on the avidity of uptake with the different tracers if the biopsy was not possible or impractical.
Author Contribution
Akram Al-Ibraheem participated in the study conception and design. Rahma Hammoudeh participated in the manuscript initial drafting. Rahma Hammoudeh and Ahmed Saad Abdlkadir contributed equally to this manuscript and share first authorship. Rahma Hammoudeh, Nour Kasasbeh, and Ahmed Saad Abdlkadir participated in data collection. Akram Al-Ibraheem, Rahma Hammoudeh, Nour Kasasbeh, and Ahmed Saad Abdlkadir participated in the data analysis and interpretation of results. Akram Al-Ibraheem, Ahmed Saad Abdlkadir, and Malik E. Juweid participated in manuscript finalization. All authors read and approved the final manuscript.
Data Availability
The current study data are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Akram Al-Ibraheem, Rahma Hammoudeh, Nour Kasasbeh, Ahmed Saad Abdlkadir, and Malik E. Juweid declare that they have no conflict of interest.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to Participate
Informed consent was obtained from the patient.
Consent for Publication
Not applicable.
Footnotes
Akram Al-Ibraheem contributed to this study as the first author.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hiraoka A, Hirooka M, Ochi H, Koizumi Y, Shimizu Y, Shiraishi A, et al. Importance of screening for synchronous malignant neoplasms in patients with hepatocellular carcinoma: impact of FDG PET/CT. Liver Int. 2013;33:1085–1091. doi: 10.1111/liv.12161. [DOI] [PubMed] [Google Scholar]
- 2.Malik V, Johnston C, Donohoe C, Claxton Z, Lucey J, Ravi N, et al. (18)F-FDG PET-detected synchronous primary neoplasms in the staging of esophageal cancer: incidence, cost, and impact on management. Clin Nucl Med. 2012;37:1152–1158. doi: 10.1097/RLU.0b013e31827083ba. [DOI] [PubMed] [Google Scholar]
- 3.Raveenthiran S, Esler R, Yaxley J, Kyle S. The use of (68)Ga-PET/CT PSMA in the staging of primary and suspected recurrent renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2019;46:2280–2288. doi: 10.1007/s00259-019-04432-2. [DOI] [PubMed] [Google Scholar]
- 4.Luciani A, Balducci L. Multiple primary malignancies. Semin Oncol. 2004;31:264–273. doi: 10.1053/j.seminoncol.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Sturludottir M, Martling A, Carlsson S, Blomqvist L. Synchronous rectal and prostate cancer–the impact of MRI on incidence and imaging findings. Eur J Radiol. 2015;84:563–567. doi: 10.1016/j.ejrad.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 6.Wu A, He S, Li J, Liu L, Liu C, Wang Q, et al. Colorectal cancer in cases of multiple primary cancers: clinical features of 59 cases and point mutation analyses. Oncol Lett. 2017;13:4720–4726. doi: 10.3892/ol.2017.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kichloo A, Amir R, Aljadah M, Wani F, Solanki S, Singh J, et al. FDG-PET versus PSMA-PET: a patient with prostate cancer. J Investig Med High Impact Case Rep. 2020;8:2324709620941313. doi: 10.1177/2324709620941313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338–344. doi: 10.1136/gutjnl-2022-327736. [DOI] [PubMed] [Google Scholar]
- 9.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 10.Jordanian Ministry of Health. Jordan Cancer Registry: cancer incidence in Jordan. 2018 [database on the Internet]. https://moh.gov.jo/ebv4.0/root_storage/ar/eb_list_page. Accessed 4 May 2023.
- 11.Pan SY, Huang CP, Chen WC. Synchronous/metachronous multiple primary malignancies: review of associated risk factors. Diagnostics. 2022;12:1940. [DOI] [PMC free article] [PubMed]
- 12.Dabir PD, Svanholm H, Christiansen JJ. SATB2 is a supplementary immunohistochemical marker to CDX2 in the diagnosis of colorectal carcinoma metastasis in an unknown primary. APMIS. 2018;126:494–500. doi: 10.1111/apm.12854. [DOI] [PubMed] [Google Scholar]
- 13.Lin C, Jin K, Hua H, Lin J, Zheng S, Teng L. Synchronous primary carcinomas of the rectum and prostate: Report of three cases. Oncol Lett. 2011;2:817–819. doi: 10.3892/ol.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu V, Sidiqi B, Kobritz M, Purchla J, Herman JM, Gamma NAL, et al. Synchronous prostate and rectal cancers across a large distributed healthcare system: implications for national accreditation program for rectal cancer (NAPRC) guidelines. J Clin Oncol. 2022;40:e15591-e. doi: 10.1200/JCO.2022.40.16_suppl.e15591. [DOI] [Google Scholar]
- 15.Agarwal A, Marcus C, Xiao J, Nene P, Kachnic LA, Subramaniam RM. FDG PET/CT in the management of colorectal and anal cancers. AJR Am J Roentgenol. 2014;203:1109–1119. doi: 10.2214/AJR.13.12256. [DOI] [PubMed] [Google Scholar]
- 16.Hope TA, Goodman JZ, Allen IE, Calais J, Fendler WP, Carroll PR. Metaanalysis of (68)Ga-PSMA-11 PET accuracy for the detection of prostate cancer validated by histopathology. J Nucl Med. 2019;60:786–793. doi: 10.2967/jnumed.118.219501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirmas N, Al-Ibraheem A, Herrmann K, Alsharif A, Muhsin H, Khader J, et al. [(68)Ga]PSMA PET/CT improves initial staging and management plan of patients with high-risk prostate cancer. Mol Imaging Biol. 2019;21:574–581. doi: 10.1007/s11307-018-1278-8. [DOI] [PubMed] [Google Scholar]
- 18.Al-Ibraheem A, Abuhijla F, Salah S, Shahait M, Khader J, Mohamad I, et al. The influence of 68Ga-prostate-specific membrane antigen PET/computed tomography on prostate cancer staging and planning of definitive radiation therapy. Nucl Med Commun. 2021;42:811–817. doi: 10.1097/MNM.0000000000001394. [DOI] [PubMed] [Google Scholar]
- 19.Cerci JJ, Fanti S, Lobato EE, Kunikowska J, Alonso O, Medina S, et al. Diagnostic performance and clinical impact of (68)Ga-PSMA-11 PET/CT imaging in early relapsed prostate cancer after radical therapy: a prospective multicenter study (IAEA-PSMA Study) J Nucl Med. 2022;63:240–247. doi: 10.2967/jnumed.120.261886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The current study data are available from the corresponding author on reasonable request.