Abstract
Background
Human papillomavirus‐associated oropharyngeal squamous cell carcinomas are a distinct subgroup of tumours that may have a better prognosis than traditional tobacco/alcohol‐related disease. Iatrogenic complications, associated with conventional practice, are estimated to cause mortality of approximately 2% and high morbidity. As a result, clinicians are actively investigating the de‐escalation of treatment protocols for disease with a proven viral aetiology.
Objectives
To summarise the available evidence regarding de‐escalation treatment protocols for human papillomavirus‐associated, locally advanced oropharyngeal squamous cell carcinoma.
Search methods
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials; PubMed; EMBASE; CINAHL; Web of Science; Cambridge Scientific Abstracts; ICTRP and additional sources for published and unpublished trials. The date of the most recent search was 25 June 2013.
Selection criteria
Randomised controlled trials investigating de‐escalation treatment protocols for human papillomavirus‐associated, locally advanced oropharyngeal carcinoma. Specific de‐escalation categories were: 1) bioradiotherapy (experimental) versus chemoradiotherapy (control); 2) radiotherapy (experimental) versus chemoradiotherapy (control); and 3) low‐dose (experimental) versus standard‐dose radiotherapy (control). The outcomes of interest were overall and disease‐specific survival, treatment‐related morbidity, quality of life and cost.
Data collection and analysis
Three authors independently selected studies from the search results and extracted data. We planned to use the Cochrane 'Risk of bias' tool to assess study quality.
Main results
We did not identify any completed randomised controlled trials that could be included in the current version of this systematic review. We did, however, identify seven ongoing trials that will meet our inclusion criteria. These studies will report from 2014 onwards. We excluded 30 studies on methodological grounds (seven randomised trials with post hoc analysis by human papillomavirus status, 11 prospective trials and 12 ongoing studies).
Authors' conclusions
There is currently insufficient high‐quality evidence for, or against, de‐escalation of treatment for human papillomavirus‐associated oropharyngeal carcinoma. Future trials should be multicentre to ensure adequate power. Adverse events, morbidity associated with treatment, quality of life outcomes and cost analyses should be reported in a standard format to facilitate comparison with other studies.
Plain language summary
Medical treatments for throat cancer (oropharyngeal cancer) that is associated with human papillomavirus (HPV) infection
Recent studies suggest a connection between a virus (human papillomavirus) and throat cancer (oropharyngeal cancer) in some patients. This review has been conducted to assess potential new treatments that have emerged as a result of this information.
When diagnosed, throat cancers can be at an advanced stage and radiotherapy (which uses beams of radiation to kill cancer cells) or chemotherapy (drugs which kill cancer cells) are the most frequently used treatments. Both have side effects and may result in a decreased ability to talk, eat or drink. Newer therapies (biological) are now emerging that will help the immune system to fight cancer.
So far, high‐quality evidence to assess these new treatment protocols is lacking, but may be available after 2014 as several ongoing studies are completed. Important outcomes to measure will be the likelihood of survival from the various treatments, as well as side effects and quality of life in the longer term. This review will be updated to include this new evidence as it becomes available.
This review is currently up to date to June 2013.
Background
Description of the condition
Oropharyngeal squamous cell carcinomas (SCCs) may arise from the tonsil, base of tongue, soft palate and posterior pharyngeal wall region. They are relatively infrequent, with an incidence of about 0.8 to 3.8 per 100,000 population per annum. However, in the head and neck this is now the most prevalent site for carcinomas and the number of cases appears to be rising (Dwivedi 2009; Evans 2010). The oropharynx plays an essential role in swallowing, speech and protecting the airway as it is situated at the bifurcation of the respiratory and digestive tract. Treatment modalities are influenced heavily by the aim of reducing the risk of functional disability where possible. Most tumours are locally advanced at the time of diagnosis, which may complicate the choice of primary treatment (Choi 2009; Mendenhall 2011).
Human papillomavirus (HPV) is a major carcinogen, with an estimated 4.8% of total worldwide cancers in 2008 linked to the virus (de Martel 2012). Almost all cervical cancers (99.7%) are causally associated with HPV (Walboomers 1999). The association between high‐risk (carcinogenic) HPV and oropharyngeal SCC is now evident from data collected by independent controlled studies. The virus now fulfils epidemiological criteria for disease causality, especially in non‐smokers (Sudhoff 2011). A recent meta‐analysis of the world literature demonstrated that the proportion of oropharyngeal SCC caused by HPV has increased from 40.5% in studies recruiting before the year 2000 to 72.2% in studies reporting after 2005 (Mehanna 2012). Although the majority of systematic reviews are based on retrospective case series, they consistently report a significant improvement in survival when a tumour is associated with HPV (Licitra 2006; Petrelli 2013; Ragin 2007).
A significant proportion of oropharyngeal SCCs (40% to 60%) have HPV16 DNA integrated within their genomic DNA, with minor contributions made by other oncogenic HPV subtypes (Gillison 2004). These HPV DNA sequences are transcriptionally active, which strongly suggests a functional influence within the host cancer cell (Van Houten 2001; Wiest 2002). The roles of the two HPV16 oncoproteins E6 and E7 have been studied extensively and include inhibition of p53 and pRb (retinoblastoma) tumour suppressor proteins (Hoffman 2010). Expression of the E6 protein will cause inhibition of p53, leading to loss of cell cycle arrest and apoptosis when DNA damage is detected within the cell. Expression of the E7 protein may cause degradation of pRb, leading to unopposed progression through the cell cycle. Laboratory studies have demonstrated that repression of E6 and E7 will lead to activation of the p53 and pRb pathways, decreased cellular proliferation and cellular growth arrest (Goodwin 2000; Wells 2000). This situation is quite different to HPV‐negative oropharyngeal SCC, where an irreversible p53 mutation will normally be present and may contribute to the poorer survival observed in this patient cohort (Oliver 2002).
Description of the intervention
The current management of oropharyngeal SCC is dictated by the stage of the disease as well as clinician and patient preference. Early‐stage disease (T1 N0 and T2 N0) can be treated by a single modality, such as surgery or radiotherapy. The standard of care for locally advanced disease may include primary chemoradiotherapy with or without selective neck dissection or primary surgical resection (with or without reconstruction) with adjuvant chemoradiotherapy (Mehanna 2011).
Biotherapy is an emerging treatment (sometimes called biological therapy or immunotherapy) that modifies the immune system of the body to target cancer cells, decrease side effects or both. Epidermal growth factor receptors (EGFRs) are expressed at high levels in the majority of head and neck SCC. Clinical data would indicate that EGFR over‐expression has an adverse effect on survival and local‐regional control after radiotherapy (Chung 2011). Activation of the EGF receptor in response to a ligand results in phosphorylation of the tyrosine kinase domain, leading to a cascade of events within the cell. This can result in cell proliferation, transcription of growth factors such as pro‐angiogenic molecules and anti‐apoptosis. Blockade of this pathway has become an important therapeutic target (Modjtahedi 2009). Restriction of EGFR signalling by means of either antibody blockage of EGF binding or small molecule inhibition of the intracellular tyrosine kinase region has been shown to increase radiation sensitivity in vitro (Haddad 2008).
Chemotherapy is the administration of cytotoxic medication that targets rapidly dividing cancer cells, disrupting growth and destroying them. The current evidence would suggest that use of primary chemoradiation is more prevalent than surgery due to the perceived reduction in treatment‐related morbidity (Gregoire 2010). Chemotherapy can be used in combination with radiotherapy (concurrent) to increase radiosensitivity or before radiotherapy (neoadjuvant) to reduce the tumour size. The combination of two or three chemotherapeutic medications can be more effective against the tumour as different agents interrupt the life cycle of malignant cells at different stages. The unwanted side effect of this approach may be increased toxicity, which can be exacerbated by the parallel use of radiotherapy (Gregoire 2007). Temporary side effects of chemotherapy include lethargy, nausea/vomiting, hair loss, susceptibility to infection, anaemia, diarrhoea, constipation or mucositis. The majority of these effects are mild but occasionally some can be severe. Less commonly, severe or permanent side effects can include cardiac impairment, peripheral neuropathy, nephrotoxicity or ototoxicity.
Radiotherapy works by disrupting the DNA of rapidly dividing cells so that the normal repair mechanisms (which are less effective in cancer cells) are impeded and the cells die. Non‐malignant tissue may also be affected by the ionising radiation, and in the head and neck region this includes the salivary glands and oral mucosa. In a small number of cases, radiotherapy can instigate oncogenic change and is therefore no longer used for benign conditions (Mendenhall 2011).
How the intervention might work
De‐escalation with bioradiation
Cetuximab is a humanised monoclonal mouse antibody that binds to and inhibits the EGF receptor. Along with the small molecule tyrosine kinase inhibitors (e.g. erlotinib) this class of drug is widely utilised in head and neck SCC (Bourhis 2010). The efficacy and safety of cetuximab in combination with radiotherapy was studied in a randomised controlled trial of 424 patients with locally advanced squamous cell carcinoma of the head and neck versus radiation alone (Bonner 2010). Overall survival at five years was significantly improved in the cetuximab plus radiotherapy group (45.6% versus 36.4%). Furthermore, the cetuximab arm showed no evidence of an increased rate of mucositis, dysphagia or gastrostomy dependence, and no evidence of a worsening of quality of life relative to radiotherapy alone. This is in contrast to the literature on concurrent platinum‐based chemoradiotherapy, which suggests that certain short and long‐term complications are greatly increased relative to primary radiotherapy (Murphy 2009; Nguyen 2009).
The Bonner 2010 trial also described many of the clinical parameters associated with improved outcome in the cetuximab arm (oropharyngeal sub‐site, advanced N stage, low T stage, high Karnofsky performance status, male gender and young age) and noted that this same profile is often linked to a diagnosis of HPV‐associated disease. Pre‐treatment tumour specimens were not available for HPV stratification, so a direct testing of this hypothesis is not possible.
Vermorken 2008 conducted the first study to demonstrate a benefit in overall survival with cetuximab added to cisplatin/5‐fluorouracil used to treat recurrent or metastatic head and neck SCC (or both) (10.1 versus 7.4 months, hazard ratio (HR) = 0.797, P = 0.036). Grade 3 or 4 adverse events were similar for both groups (82% experimental arm versus 76% control arm (P = 0.19)).
The RTOG 0522 trial recently reported data from 321 oropharyngeal SCC patients randomised to receive cetuximab in combination with cisplatin and radiotherapy (RTOG 0522). Although the trial was stopped prematurely after a median follow‐up of 29 months, the data published would suggest a potential disadvantage towards the HPV cohort in terms of overall and disease‐free survival (HR overall survival 1.24 (0.57 to 2.71)); HR disease‐free survival 1.48 (0.84 to 2.61)). The triplet regimen was also associated with higher rates of mucositis and skin reactions. However, as the trial did not include prospective HPV testing, it is unlikely to provide a clear answer regarding optimal management. In 2013, Pajares et al performed a retrospective analysis of HPV‐associated patients with locally advanced HNSCC (eight out of 52 patients), which showed no significant difference between the use of bioradiation versus chemoradiation (Pajares 2013). The TREMPLIN study investigated induction chemotherapy followed by chemoradiotherapy or bioradiotherapy for larynx preservation (without HPV stratification) and showed no significant difference in either arm (Lefebvre 2013).
Recent data indicate an inverse relationship between EGFR expression and high‐risk HPV16 (Hong 2010; Kumar 2008). This information would seem rather paradoxical, given the results from the Bonner trial, but may fit the clinical data supplied by RTOG 0522. Nevertheless, for HPV‐associated oropharyngeal carcinoma, the prospect remains that targeted bioradiation (cetuximab + radiotherapy) may reduce the burden of acute/late toxicity while maintaining long‐term survival.
De‐escalation with bioradiation in HPV‐associated oropharyngeal SCC would imply radiotherapy with either concurrent biotherapy (experimental) or chemotherapy (control).
De‐escalation of chemoradiation
The majority of randomised trials dedicated to oropharyngeal carcinoma have compared chemoradiation to primary radiotherapy. The GORTEC trial compared concurrent carboplatin/5‐fluorouracil to standard fractionation radiotherapy and revealed an improved five‐year survival in the chemoradiation group (Denis 2004). This finding was confirmed by a large meta‐analysis involving data from 17,346 head and neck SCC cases (Pignon 2009). In this study, a subgroup analysis for oropharyngeal carcinoma estimated a 6.5% absolute benefit in survival at five years with concurrent chemotherapy, and the benefit appears superior for platinum monotherapy when compared to other chemotherapy regimens. Cisplatin was the first member of a class of platinum‐containing anti‐cancer drugs, which now also includes carboplatin. The platinum complexes interact with DNA by preferentially attaching to the guanine nucleobase, causing cross‐linkage bonds and ultimately programmed cell death. The principal mechanism of action of the taxane class of drugs (docetaxel and paclitaxel) is the disruption of microtubule function. Two phase III trials in locally advanced head and neck SCC have now demonstrated a survival benefit when a taxane is combined with cisplatin/5‐fluorouracil (TPF) during the induction chemotherapy phase (Hitt 2005; Posner 2007). Finally, the PARADIGM phase III trial recently investigated the benefit of adding induction chemotherapy (TPF) prior to concurrent chemoradiotherapy (cisplatin) in HNSCC and found no obvious benefit for patients. However, this study had various methodological deficiencies that included early termination, a lack of HPV stratification and inclusion of non‐oropharyngeal cases in the analysis (Haddad 2013).
De‐escalation of chemoradiation in HPV‐associated oropharyngeal SCC would imply either radiotherapy alone (experimental) or radiotherapy with concurrent chemotherapy (control).
De‐escalation of radiotherapy
The two main types of non‐conventional (altered) fractionation are accelerated and hyper‐fractionation. Accelerated fractionation uses a similar overall dose to conventional treatment but over a reduced time period. This fractionation regime has been developed to counteract tumour cell repopulation during the course of therapy (squamous cell cancers of the head and neck can double the number of cancerous cells in three days). The hyperfractionated regime utilises daily multiple attenuated doses over a similar duration to conventional fractionation to give a larger total dose, e.g. twice‐daily fractions of 1.1 to 1.2 Gy/fraction to a total dose of 74 to 80 Gy. Further developments in radiotherapy include intensity‐modulated radiotherapy (IMRT) and image‐guided radiotherapy (IGRT), which may improve precision and reduce side effects (Gregoire 2007; Nutting 2011).
Treatment of oropharyngeal SCC with radiotherapy has traditionally been given at a dose of approximately 2.0 Gy per day, five days a week to a total dose of 60 to 70 Gy. The precise method of dividing up the treatment dose, or fractionation, has changed over the years and varies from institution to institution. This is a consequence of ongoing research looking at the biological interaction between the cancer cell and healthy surrounding tissue and has been recently reviewed (Dirix 2010). Meta‐analyses indicate that altered fractionation schedules may translate into survival gains (Bourhis 2006) and six fractions per week is the now the recommended standard endorsed by National Comprehensive Cancer Network (NCCN 2010).
De‐escalation of radiotherapy in HPV‐associated oropharyngeal SCC would imply biotherapy given concurrently with either low‐dose IMRT (< 60 Gy (experimental)) or standard dose IMRT (60 to 70 Gy (control)).
Why it is important to do this review
The data now suggest that HPV‐associated oropharyngeal SCCs are a distinct subgroup of tumours and this recognition is particularly important because the prognosis may be better than for the traditional tobacco or alcohol‐related tumours (Gillison 2004).
At present, the treatment for locally advanced oropharyngeal carcinoma is very intensive (usually by chemoradiation) and can lead to chronic complications. Many patients require insertion of a percutaneous gastrostomy tube, intravenous fluids, or both, with the potential for infection or electrolyte imbalance. Iatrogenic complications are estimated to cause mortality of around 2%, which clinicians reluctantly accept as the majority of treated patients will be cured (Lee 2011).
As a result, there is growing but guarded interest in conducting safe de‐escalation studies that attempt to maintain the extremely positive prognosis of patients with HPV‐associated oropharyngeal squamous cell carcinoma (SCC) while limiting both short‐term and subsequent long‐term toxicities.
Objectives
Primary objective
To summarise the available evidence regarding de‐escalation treatment protocols for human papillomavirus‐associated, locally advanced oropharyngeal squamous cell carcinoma.
Primary outcomes are overall survival, treatment‐related morbidity and side effects.
Secondary objective
To determine the implications of treatment modalities in terms of quality of life, disease‐free survival and costs.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing interventions for locally advanced oropharyngeal SCC with HPV status established at baseline (before the study commences).
Types of participants
Patients with carcinoma in the oropharynx sub‐site were included (as defined by the World Health Organization classification C09, C10). Oral cavity (C01‐C02, C03, C04, C05‐C06), hypopharynx (C13), nasopharynx (C11) and larynx (C32) lesions were excluded (WHO 2000).
Cancers were primary squamous cell carcinomas arising from the oropharyngeal mucosa, diagnosed to be HPV16‐positive by polymerase chain reaction or DNA/RNA in‐situ hybridisation and displaying p16 activity (a surrogate marker of viral activity) utilising immunohistochemistry (Schache 2011; Westra 2009). Studies that stratified by a single assay (DNA polymerase chain reaction (PCR)/DNA in situ hybridisation (ISH) or p16 immunohistochemistry) were still included in the analysis but were subject to subgroup analysis.
Types of interventions
The specific de‐escalation treatment categories were 1) bioradiotherapy (experimental) versus chemoradiotherapy (control); 2) radiotherapy (experimental) versus chemoradiotherapy (control); and 3) low‐dose (experimental) versus standard‐dose radiotherapy (control). We considered any mode of administration. The treatments received and compared were the primary treatments for the tumour and patients did not undergo prior intervention other than diagnostic biopsy.
Types of outcome measures
Primary outcomes
Overall survival
Treatment‐related morbidity and side effects
Secondary outcomes
Patient‐reported quality of life
Disease‐specific survival
Local or regional control rates (or both)
Distant control rates
Costs
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The date of the search was 25 June 2013.
Electronic searches
We searched the following databases from their inception for published, unpublished and ongoing trials: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2013, Issue 6); PubMed; EMBASE; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; ISRCTN; ClinicalTrials.gov; ICTRP; Google Scholar and Google. In searches prior to 2013, we also searched BIOSIS Previews 1926 to 2012.
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by The Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, we searched PubMed, TRIPdatabase, The Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. We searched for conference abstracts using the Cochrane Ear, Nose and Throat Disorders Group Trials Register.
Data collection and analysis
Selection of studies
Three authors (LM, DM and AM) independently screened the results of the search to identify studies that broadly met the inclusion criteria. We reviewed those studies selected in full text and applied the inclusion criteria independently. Any conflict was resolved by referral to a senior author.
Data extraction and management
Three review authors (LM, DM and AM) independently extracted data using a specially designed data extraction form. We piloted the data extraction forms on several papers and modified them as required before use. We discussed any disagreements in full and consulted a senior review author as appropriate. Where necessary, we contacted the authors for clarification or missing information.
For each trial we recorded the following data.
Year of publication, country of origin and source of study funding.
Details of the participants, including demographic characteristics and criteria for inclusion and exclusion.
Details of the type of intervention, timing and duration.
Details of treatment‐related morbidity, categorised as acute (< 90 days after treatment) or late (> 90 days) and classified according to the Common Terminology Criteria for Adverse Events (CTCAE 2009).
Details of quality of life outcome assessments assessed by the European Organisation for Research and Treatment of Cancer (EORTC) QLQ‐HN35 or EuroQol 2011 questionnaires (EORTC 2013/EuroQol 2011).
Details of all other outcomes reported, including cost, method of assessment and time intervals.
HPV status for each patient before the treatment commenced.
Assessment of risk of bias in included studies
Had studies suitable for inclusion been identified, LM, DM and AM would have independently undertaken assessment of the risk of bias of the included trials with the following taken into consideration, as guided by the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective outcome reporting; and
other sources of bias.
We planned to use the Cochrane 'Risk of bias' tool in RevMan 5.2 (RevMan 2012), which involves describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry (low risk of bias, high risk of bias or unclear risk of bias).
Assessment of heterogeneity
We planned to inspect heterogeneity by graphical display of estimated intervention effects from individual studies, together with 95% confidence intervals. More formally, we would have assessed heterogeneity of effects between studies using a Chi² test, available in RevMan 5.2 (RevMan 2012), with a significance level of P < 0.05. In that case, we planned to use the I² statistic to quantify the degree of inconsistency among results of included studies (I² > 50% indicates substantial heterogeneity, I² > 75% indicates considerable heterogeneity (Handbook 2011)).
Data synthesis
We would have attempted meta‐analyses if studies had been available with similar comparisons and had reported the same outcome types. We planned to extract data from included studies and enter data into RevMan 5.2 for statistical analysis (RevMan 2012). In the event of incomplete data, we intended to contact the study authors to obtain further information. We planned to seek statistical advice where necessary.
We would have approached survival data in one of two ways, depending on the data available. For dichotomous data (including the proportion surviving at one, three and five years), we would have estimated intervention effects of individual studies as relative effects (risk ratios (RR)). For survival data, we would have estimated intervention effects of individual studies as hazard ratios (HR). If hazard ratios were not quoted in studies, we planned to calculate them from available summary statistics such as observed events, expected events, variance, confidence intervals, P values or survival curves (Parmar 1998; Tierney 2007).
If the data provided were in the form of means and standard deviations (e.g. the quality of life questionnaire EORTC QLQ‐HN35), we intended to display effects on outcomes using the mean difference scale. However, if the quality of life outcomes were provided using an alternative questionnaire, the data may still have been pooled by utilising the standardised mean difference with 95% CIs and using an intention‐to‐treat analysis.
Subgroup analysis and investigation of heterogeneity
For oropharyngeal cancer, where possible, we would have evaluated different stage lesions in subgroup analyses. We intended to assess clinical heterogeneity by examining the types of participants, interventions and outcomes in each study. If appropriate, we intended to calculate pooled estimates using a random‐effects model (Mantel‐Haenszel method for dichotomous data outcomes and generic inverse‐variance method for survival data outcomes (Handbook 2011)).
Sensitivity analysis
We planned to undertake a sensitivity analysis to examine the effects of allocation concealment, randomisation, quality of follow‐up and blind outcome assessment (if appropriate).
Results
Description of studies
Results of the search
Our search in June 2013 identified a total of 617 references, which dropped to 503 after removal of duplicates. From these a review of the titles and abstracts highlighted 37 studies as being potentially relevant. See Figure 1 for a flow chart depicting the search history.
1.
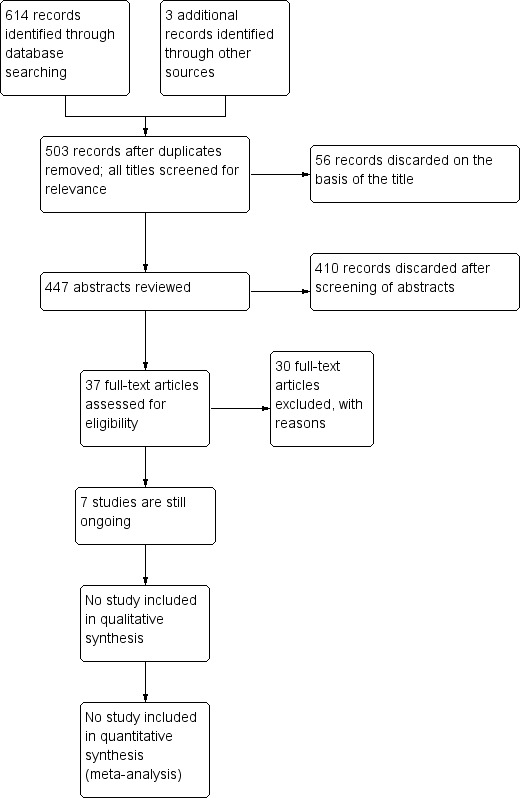
Study flow diagram.
Included studies
After a full review of the relevant articles, no study met the full inclusion criteria.
Excluded studies
We excluded 30 studies in total after a review of the full‐text article (see Characteristics of excluded studies for exclusion details and Table 1, Table 2 and Table 3 for study details).
1. Description of excluded studies (non‐randomised studies).
| Trial | Description |
| Argiris 2011 | A prospective clinical trial without randomisation investigating serum/tissue biomarkers, which may determine the success or failure of cetuximab therapy in 16 patients with locally advanced oropharyngeal SCC. HPV status was noted at baseline |
| Chen 2013 | A case‐control study evaluating the responsiveness of HPV‐positive and HPV‐negative oropharyngeal cancer to intensity‐modulated radiotherapy (IMRT), using axial imaging obtained daily during the course of image‐guided radiotherapy (IGRT). HPV status was noted at baseline |
| Fakhry 2008 | A prospective study without randomisation investigating 62 patients with locally advanced oropharyngeal SCC who were treated by induction chemotherapy with intravenous paclitaxel and carboplatin followed by concomitant weekly intravenous paclitaxel and standard fractionation radiation therapy. Statistical models were used to compare the risk of death or recurrence among patients stratified by HPV status (a post hoc analysis from pre‐treatment biopsy samples) |
| Gilbert 2012 | A prospective study without randomisation, which evaluated the tolerability and clinical efficacy of combined oxaliplatin and pemetrexed as an induction chemotherapy regimen in 27 patients with locally advanced oropharyngeal SCC. A minority of patients consented to HPV status determination by a post hoc analysis |
| Kies 2010 | A prospective trial without randomisation investigating the efficacy of combining cetuximab with chemotherapy in 41 patients with locally advanced oropharyngeal SCC. Statistical models were used to compare the risk of death or recurrence among patients stratified by HPV status (a post hoc analysis from pre‐treatment biopsy samples) |
| Le 2012 | A prospective study without randomisation, which investigated biomarkers in 274 patients with locally advanced oropharyngeal SCC. The patients were recruited from an existing randomised trial comparing radiotherapy/cisplatin with tirapazamine/cisplatin. Statistical models were used to compare the risk of death or recurrence among patients stratified by p16 IHC status (a post hoc analysis from pre‐treatment biopsy samples is an ongoing phase II trial that is open‐label and non‐randomised). The study has so far enrolled 7 patients diagnosed as having stage III‐IV primary oropharyngeal SCC with HPV status ascertained at baseline. Patients will undergo an attenuated chemoradiotherapy regimen with the first results due in September 2015 |
| O'Sullivan 2012 | A prospective study without randomisation, which investigated 358 patients with locally advanced oropharyngeal SCC. The patients were recruited from an existing randomised trial comparing altered fractionation radiotherapy with chemoradiotherapy. Statistical models were used to compare the risk of death or recurrence among patients stratified by p16 status (a post hoc analysis from pre‐treatment biopsy samples) |
| Psyrri 2011 | A prospective study without randomisation, which investigated the clinical outcomes of 38 patients with stage III‐IV HNSCC. Patients were recruited from an existing phase II trial (Eastern Cooperative Oncology Group 2303) of induction chemotherapy with weekly cetuximab, paclitaxel and carboplatin x 6 followed by chemoradiotherapy with weekly cetuximab. Statistical models were used to compare the risk of death or recurrence among patients stratified by p16 IHC status (a post hoc analysis from pre‐treatment biopsy samples) |
| Semrau 2012 | A prospective study without randomisation, which investigated 52 patients with locally advanced oropharyngeal SCC. The patients received either concomitant boost (69.2 Gy) or conventionally fractionated (70 Gy) radiotherapy, with concurrent paclitaxel/carboplatin. Statistical models were used to compare the risk of death or recurrence among patients stratified by HPV status (a post hoc analysis from pre‐treatment biopsy samples) |
| Snietura 2011 | A prospective study without randomisation, which investigated 66 patients with oropharyngeal SCC. The purpose of the study was to analyse the influence of HPV infection on the outcome of a randomised clinical trial (p‐CAIR) of conventional versus 7 days per week postoperative radiotherapy. Statistical models were used to compare the risk of death or recurrence among patients stratified by HPV status (a post hoc analysis from pre‐treatment biopsy samples) |
| Thibaudeau 2011 | A prospective cohort study of 169 patients with locally advanced oropharyngeal SCC treated with chemoradiation therapy. Statistical models were used to compare the risk of death or recurrence among patients stratified by HPV status (a post hoc analysis from pre‐treatment biopsy samples) |
HNSCC: head and neck squamous cell carcinoma HPV: human papillomavirus IHC: immunohistochemistry SCC: squamous cell carcinoma
2. Description of excluded studies (RCTs with post hoc analysis by HPV status).
| Trial | Description |
| DAHANCA 5 | This double‐blind, placebo‐controlled study investigated the clinical outcomes of 331 patients with early and late‐stage HNSCC. Participants were recruited from an existing nationwide cohort (Danish Head and Neck Cancer Group). All participants were treated by conventional radiotherapy +/‐ nimorazole. Statistical models were used to compare the risk of death or recurrence among patients stratified by p16 IHC status (a post hoc analysis from pre‐treatment biopsy samples) |
| DAHANCA 6 & 7 | This trial investigated the clinical outcomes of 794 patients with early and late‐stage HNSCC. Participants were recruited from an existing nationwide cohort (Danish Head and Neck Cancer Group) who were all treated by either conventional or accelerated radiotherapy. Statistical models were used to compare the risk of death or recurrence among patients stratified by p16 IHC status (a post hoc analysis from pre‐treatment biopsy samples) |
| RTOG 0129 | 323 patients with locally advanced oropharyngeal SCC were included in this study. The patients were recruited from an existing randomised trial comparing standard fractionation radiotherapy with accelerated fractionation radiotherapy. Statistical models were used to compare the risk of death or recurrence among patients stratified by p16 IHC status (a post hoc analysis from pre‐treatment biopsy samples) |
| RTOG 0522 | This study investigated the concurrent use of cetuximab with cisplatin and radiation in 895 patients with locally advanced HNSCC. The study was terminated early at the third interim analysis because there was a less than 10% chance the study would be positive for the primary endpoint (only data from the experimental arm were available for analysis). Statistical models were used to compare the risk of death or recurrence among patients stratified by p16 status (a post hoc analysis from pre‐treatment biopsy samples) |
| RTOG 9003 | 190 patients with locally advanced oropharyngeal SCC were included in this study. The patients were recruited from an existing randomised trial comparing 4 different radiotherapy protocols. Statistical models were used to compare the risk of death or recurrence among patients stratified by p16 IHC status (a post hoc analysis from pre‐treatment biopsy samples) |
| TAX 324 | 111 patients with locally advanced stage oropharyngeal SCC. The patients were recruited from an existing randomised trial (TAX 324) comparing induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel. Statistical models were used to compare the risk of death or recurrence among patients stratified by HPV status (a post hoc analysis from pre‐treatment biopsy samples) |
| TROG 02.02 | 185 patients with stage III‐IV oropharyngeal SCC. The patients were recruited from an existing phase III randomised trial (Trans‐Tasman Radiation Oncology Group 02.02) comparing concurrent radiotherapy and cisplatin with or without tirapazamine. Statistical models were used to compare the risk of death or recurrence among patients stratified by HPV status (a post hoc analysis from pre‐treatment biopsy samples) |
HNSCC: head and neck squamous cell carcinoma HPV: human papillomavirus IHC: immunohistochemistry SCC: squamous cell carcinoma
3. Description of excluded studies (trials still ongoing).
| Trial | Description |
| Chera 2012 | A phase I prospective trial investigating de‐intensification chemoradiation protocols in 40 patients with either early or late‐stage oropharyngeal SCC. HPV‐positive status formed part of the inclusion criteria. The study design is not appropriate for inclusion as it was not randomised and incorporated a planned surgical intervention (neck dissection) 1 to 3 months after completion of medical therapy |
| Eisbruch 2012 | A phase II trial that is open‐label and non‐randomised. The study will enrol 36 patients diagnosed as having stage III‐IV oropharyngeal SCC with HPV status ascertained at baseline. Patients will undergo an attenuated chemoradiotherapy regimen with the first results due in January 2021 |
| Huang 2012 | A prospective study without randomisation, which analysed the temporal regression of cervical lymph nodes following primary radiotherapy or chemoradiation therapy (CRT) in 317 patients with N2‐N3 oropharyngeal SCC. Statistical models will be used to compare the risk of death or recurrence among patients stratified by p16 status (a post hoc analysis from pre‐treatment biopsy samples) |
| Mehrotra 2012 | A phase II trial that is open‐label and non‐randomised. The study has so far enrolled 2 patients diagnosed with stage III‐IV primary oropharyngeal SCC with HPV status ascertained at baseline. Patients who respond to induction chemotherapy will undergo an attenuated radiotherapy dose schedule |
| Merlano 2013 | A phase III study comparing chemoradiation against induction chemotherapy followed by bioradiation (radiotherapy + cetuximab). The main outcome of the trial is overall survival and secondary endpoints are response rate, progression‐free survival, role of biomolecular prognostic factors (EGFR, HPV) and toxicity. Initial results will be available in 2014. DNA PCR and p16 IHC will determine HPV status but there is no stratification on this basis (as reported by the lead author) |
| Quon 2012 | A prospective trial without randomisation evaluating de‐escalation treatment in early and late‐stage SCC of the oropharynx. HPV status will be established at baseline for 60 patients. Treatment is dictated by the staging of disease at presentation and will consist of de‐escalated daily fractionated radiation therapy alone (63 Gy) or concurrent weekly cisplatin de‐escalated chemoradiation therapy. Nodal metastases will receive 70 Gy in 35 fractions |
| REALISTIC 2012 | A phase I dose escalation trial of a Listeria monocytogenes based vaccine in patients with oropharyngeal SCC. The study is non‐randomised and aims to recruit 161 patients who have recently completed standard‐protocol chemoradiotherapy or surgery and have confirmed HPV16 status |
| Seiwert 2011 | Patients with locoregionally advanced head and neck cancer were treated with cetuximab, carboplatin, paclitaxel induction chemotherapy for 2 cycles. Patients were then randomised to A: cetuximab, 5‐FU, hydroxyurea and hyperfractionated week‐on, week‐off radiotherapy (72 to 74 Gy) (CetuxFHX), or B: cetuximab, cisplatin, accelerated radiation with concomitant boost (72 Gy) (CetuxPX). Primary endpoints were 1‐ and 2‐year progression‐free and overall survival. The lead author has reported that HPV status will be determined by post hoc analysis |
| Siu 2009 | A phase III trial (NCIC‐CTG) that is multicentre, randomised and open‐label in design. The study will enrol 320 patients with locally advanced HNSCC who will be randomised to standard fractionation radiotherapy (70 Gy in 7 weeks) with concurrent cisplatin chemotherapy or accelerated fractionation radiotherapy (70 Gy in 6 weeks) with the molecular targeting agent panitumumab (similar in activity spectrum to cetuximab but with a reduced dermatological/allergy profile). The primary outcome will be progression‐free survival. Primary data collection is due to be completed in March 2015. The lead author has reported that HPV status will be determined by post hoc analysis (p16 IHC/HPV DNA PCR) |
| Takenaka 2013 | A phase II trial that is open‐label and non‐randomised. The study will enrol 39 patients diagnosed to have stage III‐IV oropharyngeal SCC with HPV status ascertained at baseline by PCR +/‐ p16 immunohistochemistry. All enrolled patients will undergo an attenuated radiotherapy treatment dose |
| Teknos 2010 | A phase I, prospective, non‐randomised trial involving 38 participants with locally advanced oropharyngeal SCC. The study will investigate the side effects and optimal dose range for vorinostat when given together with cisplatin and radiation therapy. HPV status established at baseline |
| Yao 2013 | A phase II prospective trial without randomisation involving 37 participants with stage III‐IV HNSCC. The study will investigate the effect of erlotinib combined with docetaxel and radiotherapy. HPV status established at baseline |
EGFR: epidermal growth factor receptor HNSCC: head and neck squamous cell carcinoma HPV: human papillomavirus IHC: immunohistochemistry PCR: polymerase chain reaction SCC: squamous cell carcinoma
Ongoing studies
Seven studies are currently ongoing (see Characteristics of ongoing studies).
De‐escalation with bioradiation
De‐ESCALaTE 2012 is a multicentre, randomised, controlled, open‐label, phase III trial. The planned sample size is 304 patients with stage III‐IV oropharyngeal SCC with HPV status established at baseline. HPV‐positive patients will be randomised to receive either cisplatin + radiotherapy (arm A) or cetuximab + radiotherapy (arm B). The primary outcome will be early and late toxicity assessed two years after treatment. Quality of life, dysphagia and disease‐free survival outcomes will also be assessed at this time point. Primary data collection is due to be completed in February 2015.
TROG‐12.01 is a multicentre, randomised, controlled, open‐label, phase III trial. Two hundred patients with locally advanced oropharyngeal SCC and HPV status established at baseline will be recruited. The study will compare treatment‐related side effects (both acute and longer‐term) between the cisplatin and cetuximab regimens. Both treatments will be given with the same dose of radiation therapy over seven weeks. The primary outcome will be symptom severity. Secondary measures will be acute and late toxicity, cost‐effectiveness and disease‐free survival. Primary data collection is set for completion in May 2019.
RTOG 1016 is a multicentre, randomised, controlled, open‐label, phase III trial. The study will recruit 706 patients with early or late‐stage oropharyngeal SCC and ascertain HPV status at baseline. Patients will be randomised to receive either cetuximab or cisplatin in combination with IMRT. The primary outcome will be five‐year overall survival. Secondary measures are progression‐free survival and acute/late toxicity. Primary data collection is set for completion in June 2020.
De‐escalation of chemoradiation
DFCI 2010 is a randomised, controlled, open‐label, phase II trial (dual centre: Dana Farber Cancer Institute, Boston/MD Anderson Cancer Centre, Houston). The study will enrol 128 patients diagnosed with stage III‐IV locally advanced HNSCC with HPV status ascertained at baseline. Patients will be allocated to one of two induction therapy groups: paclitaxel/carboplatin/cetuximab (PCC) or cetuximab/docetaxel/cisplatin/fluorouracil (C‐TPF). The groups will then undergo further randomisation to receive radiotherapy or concurrent chemoradiotherapy. The primary outcome will be two‐year progression‐free survival. Secondary outcomes will be acute/late toxicity and quality of life. The primary data collection date is July 2015.
De‐escalation of radiotherapy
ECOG 1308 is a multicentre, randomised, controlled, open‐label, phase II trial. The study will enrol patients diagnosed with stage III‐IV oropharyngeal SCC who have HPV status ascertained at baseline. Ninety HPV‐positive patients will first undergo induction chemotherapy before randomisation to receive either low‐dose or standard‐dose IMRT in combination with cetuximab. The primary outcome will be two‐year progression‐free survival. Secondary outcomes will be acute and late toxicity, objective response of the primary tumour and quality of life. The primary data collection date is January 2014.
Cohen 2010 is a multicentre, randomised, double‐blinded, phase II trial. The study will enrol patients diagnosed with stage III‐IV locally advanced SCC who have HPV status ascertained at baseline. Eighty HPV‐positive patients will first undergo induction chemotherapy with cisplatin, paclitaxel, cetuximab +/‐ everolimus before randomisation to receive either low‐dose or standard‐dose IMRT in combination with cetuximab. The primary outcome will be response to induction chemotherapy. Primary data collection is due to be completed in May 2016.
Quarterback 2012 is a multicentre, randomised, controlled, single‐blinded, phase III trial comparing two doses of definitive radiation therapy given with induction and concurrent chemotherapy in HPV‐positive oropharynx, unknown primary or nasopharynx cancer. The planned sample size is 365 patients with stage III‐IV oropharyngeal/nasopharyngeal/unknown primary SCC with HPV status established at baseline. Eligible, consented and registered patients will receive three cycles of docetaxel cisplatin and 5‐FU (TPF) induction chemotherapy. After three cycles, the patients will be assessed for clinical, radiographic and pathological response to TPF. Patients with a clinical or radiographic complete or partial remission will be randomised in the second phase of this study, where patients will undergo a 2:1 randomisation to reduced (56 Gy) or standard (70 Gy) dose radiotherapy with weekly carboplatin and cetuximab (Erbitux) or carboplatin only, respectively. Toxicity will be assessed by symptom scores, quality of life and serious adverse event monitoring. The primary endpoint of the trial is equivalent local regional control and progression‐free survival at three years. Primary data collection is due to be completed in June 2019.
Risk of bias in included studies
Our review of the literature found no studies that met the full inclusion criteria.
Effects of interventions
No studies could be included in this current version of the review.
Discussion
We undertook this systematic review to summarise the evidence for treatment de‐escalation protocols for human papillomavirus (HPV)‐associated oropharyngeal carcinomas. We analysed 503 articles and found seven ongoing studies that met the inclusion criteria. The first reports will be available in the year 2014 (ECOG 1308).
Recent epidemiological evidence from the US confirms the importance of this clinical question by suggesting that HPV‐associated oropharyngeal SCC is rising at an exponential rate. If published trends continue, the annual number of HPV‐positive oropharyngeal carcinomas will surpass the annual number of cervical cancers by the year 2020 (Chaturvedi 2011). This may reflect the situation in other countries where similar cervical screening programmes exist.
At present, there is no evidence to support a change in clinical practice regarding treatment based on HPV tumour status. However, it is clear that HPV status may inform predictions on prognosis when combined with other factors, such as stage of disease, co‐morbidity and smoking status (Licitra 2006; Ragin 2007; Petrelli 2013). Clinicians should enrol their patients into the existing clinical studies to ensure that they recruit quickly and that the results are available in a timely fashion.
Summary of main results
There were no completed studies that met the full inclusion criteria for this review.
Quality of the evidence
This review of the medical literature produced a number of potentially relevant studies. Most studies were either retrospective or prospective with inadequate randomisation of HPV status at baseline.
The seven randomised prospective studies identified are currently ongoing and are due to report from 2014 onwards. The overall risk of bias is unclear at present.
Potential biases in the review process
The search for this topic is comprehensive and fully up to date, allowing for identification of all appropriate studies.
Agreements and disagreements with other studies or reviews
There are currently no other reviews in this category.
Authors' conclusions
Implications for practice.
No current high‐quality evidence is available to inform practice when treating human papillomavirus‐associated, locally advanced oropharyngeal squamous cell carcinoma. Seven ongoing randomised controlled trials will report from 2014 onwards. At present, there is no evidence to support a change in clinical practice regarding treatment based on HPV tumour status.
Implications for research.
Well‐designed trials that are randomised, adequately powered and have suitable follow‐up are required to assess the effectiveness of de‐escalation treatment protocols in HPV‐associated disease. Of particular interest will be adverse events and morbidity and standardised reporting will be important in this respect.
Ideally, studies would be multicentre, with a stringent protocol, comparing a single or dual modality treatment and with HPV status assessed at baseline by multiple analytic techniques, e.g. polymerase chain reaction/HPV16 DNA or RNA in situ hybridisation/p16 immunohistochemistry.
All trials should be reported according to rigorous standards of clarity and transparency, with full reporting of data (CONSORT 2010).
Finally, health‐related quality of life is an important outcome and should form a core determinant of any future study.
Acknowledgements
We acknowledge the very helpful contributions made by the editorial team of the Cochrane ENT Disorders Group.
Appendices
Appendix 1. Search strategies
| CENTRAL | PubMed | EMBASE (Ovid) | CINAHL (EBSCO) |
| #1 MeSH descriptor Oropharyngeal Neoplasms #2 MeSH descriptor Head and Neck Neoplasms explode all trees #3 MeSH descriptor Otorhinolaryngologic Neoplasms explode all trees #4 MeSH descriptor Neoplasms explode all trees #5 cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour* OR malignan* OR SCC* #6 #4 OR #5 #7 MeSH descriptor Oropharynx explode all trees #8 oropharyn* OR mesopharyn* OR tonsil* OR "head and neck" OR "head neck" OR "head‐neck" OR "head‐and‐neck" #9 #7 OR #8 #10 #6 AND #9 #11 HNSCC OR SCCHN OR OP‐SCC OR OPSCC #12 #1 OR #2 OR #3 OR #10 OR #11 #13 MeSH descriptor Tumor Virus Infections explode all trees #14 MeSH descriptor “Papillomaviridae” explode all trees #15 MeSH descriptor “Papilloma/virology” explode all trees #16 hpv* OR papillomavir* OR (papilloma AND vir*) #17 #13 OR #14 OR #15 OR #16 #18 #12 AND #17 #19 MeSH descriptor Radiotherapy explode all trees #20 radiotherap* OR radiat* OR irradiat* OR fraction* OR IMRT OR radioimmuno* #21 MeSH descriptor Oropharyngeal Neoplasms explode all trees with qualifier RT #22 MeSH descriptor Drug Therapy explode all trees #23 chemo* OR drug NEXT therap* OR anticarcinogenic OR anti NEXT carcinogenic OR anticancer* OR anti NEXT cancer* OR antineoplastic* OR anti NEXT neoplastic* OR combination AND therap* #24 MeSH descriptor Oropharyngeal Neoplasms explode all trees with qualifier DT #25 MeSH descriptor Biological therapy explode all trees #26 MeSH descriptor Receptor, Epidermal Growth Factor explode all trees with qualifier AI #27 biotherap* OR biological NEXT therap* OR immunotherap* OR immunisa* OR immuniza* OR immunomodula* OR vaccin* OR ((cell OR gene OR dna OR tissue) AND therap*) OR ((egfr OR (epidermal AND growth AND factor AND receptor*) AND inhibit*)) #28 #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 #29 #18 AND #28 | #1 "Oropharyngeal Neoplasms"[Mesh:NoExp] #2 ("Head and Neck Neoplasms"[Mesh]) #3 "Otorhinolaryngologic Neoplasms"[Mesh] #4 "Neoplasms"[Mesh] #5 (cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour* OR malignan* OR SCC*) #6 (#4 OR #5) #7 "Oropharynx" [Mesh] #8 (oropharyn* OR mesopharyn* OR tonsil* OR "head and neck" OR "head neck" OR "head‐neck" OR "head‐and‐neck") #9 (#7 OR #8) #10 (#6 AND #9) #11 (HNSCC OR SCCHN OR OP‐SCC OR OPSCC) #12 (#1 OR #2 OR #3 OR #10 OR #11) #13 "Papillomaviridae"[Mesh] #14 "Tumor Virus Infections"[Mesh] #15 "Papilloma/virology"[Mesh] #16 (hpv* OR papillomavir* OR (papilloma AND vir*)) #17 (#13 OR #14 OR #15 OR #16) #18 (#11 AND #17) #19 "Radiotherapy"[Mesh] #20 (radiotherap* OR radiat* OR irradiat* OR fraction* OR IMRT OR radioimmuno*) #21 "Drug Therapy"[Mesh] #22 (("drug therap*" OR (chemo* AND therap*) OR anticarcinogenic OR "anti carcinogenic" OR anticancer* OR "anti cancer*" OR antineoplastic* OR "anti neoplastic*" OR (combination AND therap*)) #23 (( "Oropharyngeal Neoplasms/drug therapy"[Mesh] OR "Oropharyngeal Neoplasms/radiotherapy"[Mesh] )) #24 "Biological Therapy"[Mesh] #25 ("Receptor, Epidermal Growth Factor/antagonists and inhibitors"[Mesh]) #26 (biother* OR "biological therap*" OR immunotherap* OR immunisa* OR immuniza* OR immunomodula* OR vaccin* OR ((cell OR gene OR dna OR tissue) AND therap*) OR ((egfr OR (epidermal AND growth AND factor AND receptor*) AND inhibit*))) #27 (#19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26) #28 (#18 AND #27) |
1 exp oropharynx tumor/ 2 exp "head and neck tumor"/ 3 exp neoplasm/ 4 (cancer* or carcinoma* or neoplas* or tumor* or tumour* or malignan* or SCC*).tw. 5 3 or 4 6 exp oropharynx/ 7 (oropharyn* or mesopharyn* or tonsil* or "head and neck" or "head neck" or "head‐neck" or "head‐and‐neck").tw. 8 6 or 7 9 5 and 8 10 (HNSCC or SCCHN or OP‐SCC or OPSCC).tw. 11 1 or 2 or 9 or 10 12 exp tumor virus/ 13 exp papilloma virus/ 14 (hpv* or papillomavir* or (papilloma* and vir*)).tw. 15 12 or 13 or 14 16 11 and 15 17 exp radiotherapy/ 18 (radiotherap* or radiat* or irradiat* or fraction* or IMRT or radioimmuno*).tw. 19 exp oropharynx tumor/dt, rt [Drug Therapy, Radiotherapy] 20 exp drug therapy/ 21 ((chemo* or drug NEXT therap* or anticarcinogenic or anti NEXT carcinogenic or anticancer* or anti NEXT cancer* or antineoplastic* or anti NEXT neoplastic* or combination) and therap*).tw. 22 exp biological therapy/ 23 (biotherap* or biological NEXT therap* or immunotherap* or immunisa* or immuniza* or immunomodula* or vaccin* or ((cell or gene or dna or tissue) and therap*) or ((egfr or (epidermal and growth and factor and receptor*)) and inhibit*)).tw. 24 17 or 18 or 19 or 20 or 21 or 22 or 23 25 16 and 24 |
S1 (MH "Head and Neck Neoplasms+") S2 (MH "Otorhinolaryngologic Neoplasms+") S3 (MH "Neoplasms+") S4 TX cancer* or carcinoma* or neoplas* or tumor* or tumour* or malignan* or SCC* S5 S3 OR S4 S6 (MH "Oropharynx+") S7 TX oropharyn* or mesopharyn* or tonsil* or "head and neck" or "head neck" or "head‐neck" or "head‐and‐neck" S8 S6 OR S7 S9 S5 AND S8 S10 TX HNSCC or SCCHN or OP‐SCC or OPSCC S11 S1 OR S2 OR S9 OR S10 S12 (MH "Tumor Virus Infections+") S13 (MH "Papillomaviruses") S14 TX hpv* or papillomavir* or (papilloma* and vir*) S15 S12 OR S13 OR S14 S16 S11 AND S15 S17 (MH "Radiotherapy+") S18 TX radiotherap* or radiat* or irradiat* or fraction* or IMRT or radioimmuno* S19 (MH "Drug Therapy+") S20 (MH "Otorhinolaryngologic Neoplasms+/DT/RT") S21 TX "drug therap*" OR (chemo* AND therap*) OR anticarcinogenic OR "anti carcinogenic" OR anticancer OR "anti cancer" OR antineoplastic* OR "anti neoplastic*" OR (combination AND therap*) S22 (MH "Biological Therapy+") S23 (MH "Epidermal Growth Factors/AI") S24 TX biother* OR "biological therap*" OR immunotherap* OR immunisa* OR immuniza* OR immunomodula* OR vaccin* OR ((cell OR gene OR dna OR tissue) AND therap*) OR ((EGFR OR (epidermal AND growth AND factor AND receptor*)) AND inhibit*) S25 S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 S26 S16 AND S25 |
| CAB Abstracts (Ovid) | Web of Science | BIOSIS Previews (Web of Knowledge) | ISRCTN (mRCT) |
| 1 exp "head and neck cancer"/ 2 exp neoplasms/ 3 (cancer* or carcinoma* or neoplas* or tumor* or tumour* or malignan* or SCC*).tw. 4 2 or 3 5 (Otorhinolaryn* or oropharyn* or mesopharyn* or tonsil* or "head and neck" or "head neck" or "head‐neck" or "head‐and‐neck").tw. 6 4 and 5 7 (HNSCC or SCCHN or OP‐SCC or OPSCC).tw. 8 1 or 6 or 7 9 exp Papillomaviridae/ 10 (hpv* or papillomavir* or (papilloma* and (vir* or shope))).tw. 11 (("tumor virus" and infec*) or (fibroma and shope) or ("tumour virus" and infect*)).tw. 12 9 or 10 or 11 13 8 and 12 14 exp radiotherapy/ 15 (radiotherap* or radiat* or irradiat* or fraction* or IMRT or radioimmuno*).tw. 16 exp drug therapy/ 17 ((chemo* or drug NEXT therap* or anticarcinogenic or anti NEXT carcinogenic or anticancer* or anti NEXT cancer* or antineoplastic* or anti NEXT neoplastic* or combination) and therap*).tw. 18 (biotherap* or biological NEXT therap* or immunotherap* or immunisa* or immuniza* or immunomodula* or vaccin* or ((cell or gene or dna or tissue) and therap*) or ((egfr or (epidermal and growth and factor and receptor*)) and inhibit*)).tw. 19 14 or 15 or 16 or 17 or 18 20 13 and 19 |
#1 TS=(cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour* OR malignan* OR SCC*) #2 TS=(Otorhinolaryn* or oropharyn* or mesopharyn* or tonsil* or "head and neck" or "head neck" or "head‐neck" or "head‐and‐neck" ) #3 WC=(Otorhinolaryngology) #4 #3 OR #2 #5 #4 AND #1 #6 TS=(HNSCC or SCCHN or OP‐SCC or OPSCC ) #7 #6 OR #5 #8 TS=(("tumor virus" AND infec*) OR (fibroma AND shope) OR ("tumour virus" AND infect*)) #9 TS=(hpv* or papillomavir* or (papilloma* and (vir* OR shope))) #10 #9 OR #8 #11 #10 AND #7 #12 TS=(radiotherap* or radiat* or irradiat* or fraction* or IMRT or radioimmuno*) #13 TS=(((chemo* or drug) NEAR therap*) or ((anticarcinogenic or anti) NEAR carcinogenic) or ((anticancer* or anti) NEAR cancer*) or ((antineoplastic* or anti) next neoplastic*) or (combination and therap*)) #14 TS=(((biotherap* or biological) NEAR therap*) or immunotherap* or immunisa* or immuniza* or immunomodula* or vaccin* or ((cell or gene or dna or tissue) and therap*) or (egfr or (epidermal and growth and factor and receptor*) and inhibit*) ) #15 #14 OR #13 OR #12 #16 #15 AND #11 |
#1 TS=(cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour* OR malignan* OR SCC*) #2 TS=(Otorhinolaryn* or oropharyn* or mesopharyn* or tonsil* or "head and neck" or "head neck" or "head‐neck" or "head‐and‐neck" ) #3 SU=(Otorhinolaryngology) #4 #3 OR #2 #5 #4 AND #1 #6 TS=(HNSCC or SCCHN or OP‐SCC or OPSCC ) #7 #6 OR #5 #8 TS=(("tumor virus" AND infec*) OR (fibroma AND shope) OR ("tumour virus" AND infect*)) #9 TS=(hpv* or papillomavir* or (papilloma* and (vir* OR shope))) #10 #9 OR #8 #11 #10 AND #7 #12 TS=(radiotherap* or radiat* or irradiat* or fraction* or IMRT or radioimmuno*) #13 TS=(((chemo* or drug) NEAR therap*) or ((anticarcinogenic or anti) NEAR carcinogenic) or ((anticancer* or anti) NEAR cancer*) or ((antineoplastic* or anti) next neoplastic*) or (combination and therap*)) #14 TS=(((biotherap* or biological) NEAR therap*) or immunotherap* or immunisa* or immuniza* or immunomodula* or vaccin* or ((cell or gene or dna or tissue) and therap*) or (egfr or (epidermal and growth and factor and receptor*) and inhibit*) ) #15 #14 OR #13 OR #12 #16 #15 AND #11 |
(hpv OR papilloma* OR papiloma*) AND (HNSCC or SCCHN or OP‐SCC or OPSCC) OR (hpv OR papilloma* OR papiloma*) AND (Otorhinolaryn* or oropharyn* or mesopharyn* or tonsil* or "head and neck" or "head neck" or "head‐neck" or "head‐and‐neck”) AND (cancer* or carcinoma* or neoplas* or tumor* or tumour* or malignan* or SCC*) |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Argiris 2011 | ALLOCATION: Not a randomised controlled trial |
| Chen 2013 | ALLOCATION: Not a randomised controlled trial |
| Chera 2012 | ALLOCATION: Not a randomised controlled trial |
| DAHANCA 5 | ALLOCATION: Randomised PARTICIPANTS: Patients recruited to this RCT were subjected to a post hoc analysis by investigating HPV status in pre‐treatment biopsy samples |
| DAHANCA 6 & 7 | ALLOCATION: Randomised PARTICIPANTS: Patients recruited to this RCT were subjected to a post hoc analysis by investigating HPV status in pre‐treatment biopsy samples |
| Eisbruch 2012 | ALLOCATION: Not a randomised controlled trial |
| Fakhry 2008 | ALLOCATION: Not a randomised controlled trial |
| Gilbert 2012 | ALLOCATION: Not a randomised controlled trial |
| Huang 2012 | ALLOCATION: Not a randomised controlled trial |
| Kies 2010 | ALLOCATION: Not a randomised controlled trial |
| Le 2012 | ALLOCATION: Not a randomised controlled trial |
| Mehrotra 2012 | ALLOCATION: Not a randomised controlled trial |
| Merlano 2013 | ALLOCATION: Randomised PARTICIPANTS: Patients recruited to this RCT were subjected to a post hoc analysis by investigating HPV status in pre‐treatment biopsy samples (study not yet completed) |
| O'Sullivan 2012 | ALLOCATION: Not a randomised controlled trial |
| Psyrri 2011 | ALLOCATION: Not a randomised controlled trial |
| Quon 2012 | ALLOCATION: Not a randomised controlled trial |
| REALISTIC 2012 | ALLOCATION: Not a randomised controlled trial |
| RTOG 0129 | ALLOCATION: Randomised PARTICIPANTS: Patients recruited to this RCT were subjected to a post hoc analysis by investigating HPV status in pre‐treatment biopsy samples |
| RTOG 0522 | ALLOCATION: Randomised PARTICIPANTS: Patients recruited to this RCT were subjected to a post hoc analysis by investigating HPV status in pre‐treatment biopsy samples |
| RTOG 9003 | ALLOCATION: Randomised PARTICIPANTS: Patients recruited to this RCT were subjected to a post hoc analysis by investigating HPV status in pre‐treatment biopsy samples |
| Seiwert 2011 | ALLOCATION: Randomised PARTICIPANTS: Patients recruited to this RCT were subjected to a post hoc analysis by investigating HPV status in pre‐treatment biopsy samples (study not yet completed) |
| Semrau 2012 | ALLOCATION: Not a randomised controlled trial |
| Siu 2009 | ALLOCATION: Randomised PARTICIPANTS: Patients recruited to this RCT were subjected to a post hoc analysis by investigating HPV status in pre‐treatment biopsy samples (study not yet completed) |
| Snietura 2011 | ALLOCATION: Not a randomised controlled trial |
| Takenaka 2013 | ALLOCATION: Not a randomised controlled trial |
| TAX 324 | ALLOCATION: Randomised PARTICIPANTS: Patients recruited to this RCT were subjected to a post hoc analysis by investigating HPV status in pre‐treatment biopsy samples |
| Teknos 2010 | ALLOCATION: Not a randomised controlled trial |
| Thibaudeau 2011 | ALLOCATION: Not a randomised controlled trial |
| TROG 02.02 | ALLOCATION: Randomised PARTICIPANTS: Patients recruited to this RCT were subjected to a post hoc analysis by investigating HPV status in pre‐treatment biopsy samples |
| Yao 2013 | ALLOCATION: Not a randomised controlled trial |
HPV: human papillomavirus IMRT: intensity‐modulated radiation therapy RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Cohen 2010.
| Trial name or title | 'Selection of chemoradiotherapy based on response to induction chemotherapy ‐ a randomised phase II study in locally advanced squamous cell carcinoma of the head and neck' |
| Methods | Interventional, multicentre, phase II trial, randomised, double‐blind |
| Participants | 80 adult patients with local advanced stage III‐IV head and neck squamous cell carcinoma. HPV status ascertained at baseline |
| Interventions | Drug: everolimus escalating dose
Phase I portion (2 21‐day cycles): cisplatin (100 mg/m2 day 1), paclitaxel (175 mg/m2, day 1), cetuximab (400 mg/m2 loading dose day 1 then 250 mg/m2 weekly), everolimus escalating dose Drug: everolimus or placebo Phase II portion (2 28‐day cycles): cisplatin (100 mg/m2 day 1), paclitaxel (175 mg/m2, day 1), cetuximab (400 mg/m2 loading dose day 1 then 250 mg/m2 weekly), everolimus dose determined in phase I HPV‐positive patients who have a positive response to induction chemotherapy will undergo an attenuated radiation field |
| Outcomes | Primary: response rates to induction chemotherapy consisting of cisplatin/paclitaxel/cetuximab +/‐ everolimus Secondary: study will determine the maximum administered dose (MAD), maximum tolerated dose (MTD), dose‐limiting toxicity (DLT) and safety of everolimus with cisplatin/paclitaxel/cetuximab induction chemotherapy |
| Starting date | May 2010 |
| Contact information | Dr Ezra Cohen, University of Chicago (ecohen@medicine.bsd.uchicago.edu) |
| Notes | Primary data collection due for completion in May 2016 |
De‐ESCALaTE 2012.
| Trial name or title | 'Determination of epidermal growth factor receptor‐inhibitor (cetuximab) versus standard chemotherapy (cisplatin) early and late toxicity events in human papillomavirus‐positive oropharyngeal squamous cell carcinoma: a randomised controlled trial' |
| Methods | Interventional, multicentre, phase III trial, randomised, open‐label |
| Participants | 1. 304 adult patients with stage III‐IVa oropharyngeal squamous cell carcinoma 2. Clinical multidisciplinary team decision to treat with primary curative chemoradiotherapy 3. Medically fit Eastern Co‐operative Oncology Group (ECOG) 0, 1 or 2 4. Adequate cardiovascular, haematological, renal and hepatic function 5. Using adequate contraception (male and female participants); must take contraceptive measures during and for at least 3 months after treatment |
| Interventions | Experimental group: cetuximab initial dose of 400 mg/m2, administered intravenously, 1 week before start of radiotherapy followed by 7 weekly doses of 250 mg/m2, administered intravenously during radiotherapy Control group: 3 doses of cisplatin 100 mg/m2, administered intravenously, on days 1, 22 and 43 of radiotherapy |
| Outcomes | Primary outcome:
Early and late toxicity (grade 3‐5 CTCAE 2009); time point(s): 2 years from end of treatment
Secondary outcomes: 1. Acute severe toxicity; time point(s): 3 months from end of treatment 2. Late severe toxicity; time point(s): 2 years from end of treatment 3. Quality of life; time point(s): 2 years from end of treatment 4. Dysphagia; time point(s): 2 years from end of treatment 5. Cost effectiveness; time point(s): 2 years from end of treatment 6. Overall survival, recurrence and metastasis; time point(s): 2 years from end of treatment |
| Starting date | 9 October 2012 |
| Contact information | h.mehanna@bham.nhs.uk and m.t.fulton‐lieuw@warwick.ac.uk |
| Notes | Primary data collection due for completion in February 2015 |
DFCI 2010.
| Trial name or title | 'Paclitaxel, carboplatin and cetuximab (PCC) versus cetuximab, docetaxel, cisplatin and fluorouracil (C‐TPF) in previously untreated patients with locally advanced head and neck squamous cell carcinoma (HNSCC)' |
| Methods | Interventional, phase II RCT, dual‐site, open‐label |
| Participants | The study will enrol 128 patients diagnosed with stage III‐IV locally advanced HNSCC with HPV status ascertained at baseline |
| Interventions | Patients will be allocated to 1 of 2 induction therapy groups: paclitaxel/carboplatin/cetuximab (PCC) or cetuximab/docetaxel/cisplatin/fluorouracil (C‐TPF). The groups will then undergo further randomisation to receive radiotherapy or concurrent chemoradiotherapy |
| Outcomes | Primary outcome: 2‐year progression‐free survival Secondary outcomes: acute/late toxicity and quality of life |
| Starting date | The primary data collection date is July 2015 |
| Contact information | Dr Vali A. Papadimitrakopulou (vpapadim@mdanderson.org) |
| Notes | Primary data collection due for completion in July 2015 |
ECOG 1308.
| Trial name or title | 'A phase II trial of induction chemotherapy followed by cetuximab with low dose versus standard dose IMRT in patients with HPV‐associated resectable squamous cell carcinoma of the oropharynx' |
| Methods | Open‐label randomised controlled trial |
| Participants | 90 adult patients diagnosed to have local advanced oropharyngeal carcinoma (stage III/IV). HPV status ascertained at baseline by both p16 immunohistochemistry and HPV16 in situ hybridisation |
| Interventions | Experimental group: patients undergo low‐dose intensity‐modulated radiotherapy (IMRT) 5 days per week for approximately 5 weeks (27 fractions). Patients also receive cetuximab IV over 1 to 2 hours once weekly for 6 weeks |
| Outcomes | Primary outcome: 2‐year progression‐free survival Secondary outcome: toxicity, overall survival, objective response of primary tumour and nodal disease, quality of life (assessed at baseline,1, 6, 12 and 24 months after treatment) |
| Starting date | 17 March 2010 |
| Contact information | Dr Shanthi Marur, Eastern Co‐operative Oncology Group (smarur1@jhmi.edu) |
| Notes | Primary data collection due for completion in January 2014 |
Quarterback 2012.
| Trial name or title | 'The Quarterback Trial: a randomized phase III clinical trial comparing reduced and standard radiation therapy doses for locally advanced HPV16 positive oropharynx cancer' |
| Methods | Interventional, multicentre, phase III trial, randomised, single‐blinded (outcomes assessor) |
| Participants | 365 patients with stage III‐IV oropharyngeal/nasopharyngeal/unknown primary SCC. HPV status ascertained at baseline by both p16 immunohistochemistry and HPV consensus PCR |
| Interventions | Eligible, consented and registered patients will receive 3 cycles of docetaxel cisplatin and 5‐FU (TPF) induction chemotherapy. After 3 cycles, the patients will be assessed for clinical, radiographic and pathological response to TPF. Patients with a clinical or radiographic complete or partial remission will be randomised in the second phase of this study, where patients will undergo a 2:1 randomisation to reduced (56 Gy) or standard (70 Gy) dose radiotherapy with weekly carboplatin and cetuximab (Erbitux) or carboplatin only, respectively. Patients not meeting the response criteria will be treated with standard‐dose CRT. Patients not completing 3 cycles of TPF for reasons of toxicity, progressive disease, choice or other medical necessity will be treated with standard‐dose CRT or surgery depending on their primary site and overall medical condition and will be followed for survival |
| Outcomes | Toxicity will be assessed by symptom scores, quality of life and serious adverse event monitoring. The primary endpoint of the trial is equivalent local regional control and PFS at 3 years |
| Starting date | September 2012 |
| Contact information | Dr Marshal Posner (marshall.posner@mssm.edu) |
| Notes | Primary data collection due for completion in June 2019 |
RTOG 1016.
| Trial name or title | 'Phase III trial of radiotherapy plus cetuximab versus chemoradiotherapy in HPV‐associated oropharynx cancer (RTOG‐1016)' |
| Methods | Interventional, multicentre, phase III trial, randomised, open‐label |
| Participants | 706 adult patients with early or late‐stage oropharyngeal carcinoma. HPV status ascertained at baseline by both p16 immunohistochemistry and HPV16 in situ hybridisation. Patients are stratified according to T stage (T1‐2 versus T 3‐4), N stage (N0‐2a versus N2b‐3), Zubrod performance status (0 versus 1) and smoking history (≤ 10 pack‐years versus > 10 pack‐years). Patients are randomised to 1 of 2 treatment arms |
| Interventions | Experimental group: 1 week prior to radiotherapy, patients receive cetuximab IV over 2 hours (400 mg/m2). Patients then receive cetuximab IV over 1 hour once weekly (250 mg/m2) for 7 weeks. Patients undergo IMRT once daily on days 1 to 4 and twice daily on day 5 weekly for 6 weeks (70Gy 35 fractions). Control group: patients undergo IMRT as above and also receive cisplatin (100 mg/m2) IV over 1 to 2 hours on days 1 and 22 |
| Outcomes | Primary: 5‐year overall survival Secondary: progression‐free survival, local‐regional failure, distant metastasis, acute toxicities (CTCAE 2009) and overall toxicity burden at end of treatment and at 1, 3 and 6 months after completion of treatment; late toxicities at 1, 2 and 5 years |
| Starting date | June 2011 |
| Contact information | andy.trotti@moffitt.org |
| Notes | Primary data collection due for completion in June 2020 |
TROG‐12.01.
| Trial name or title | 'A randomised trial of weekly cetuximab and radiation versus weekly cisplatin and radiation in good prognosis locoregionally advanced HPV‐associated oropharyngeal squamous cell carcinoma (TROG12.01)' |
| Methods | Interventional, multicentre, phase III trial, randomised, open‐label |
| Participants |
200 adult patients with locally advanced oropharyngeal carcinoma. HPV status ascertained at baseline 1. Adequate haematological, renal and hepatic function 2. ECOG performance status score of 0 to 1 3. Participants capable of childbearing are using adequate contraception and intend to continue use of contraception for at least 6 months following completion of treatment |
| Interventions | Experimental group: IMRT (70 Gy in 35 fractions, 5 days a week over 7 weeks) with weekly cetuximab (400 mg/m2 loading dose IV prior to radiation, followed by weekly cetuximab 250 mg/m2 for the duration of the radiotherapy) Control group: IMRT (70 Gy in 35 fractions, 5 days a week over 7 weeks) with weekly cisplatin (40 mg/m2 IV for the duration of the radiotherapy) |
| Outcomes | Primary outcome: early symptom severity as measured by M.D. Anderson Symptom Inventory ‐ Head and Neck Module (MDASI‐HN) from baseline to week 20 Secondary outcomes (time point from 2 to 5 years after treatment): 1. Clinician‐assessed acute and late toxicity (CTCAE 2009) 2. Late symptom severity 3. Quality of life 4. Dysphagia 5. Cost‐effectiveness 6. Overall survival, recurrence and metastasis |
| Starting date | May 2013 |
| Contact information | janani.sivasuthan@petermac.org |
| Notes | Primary data collection due for completion in May 2019 |
CRT: chemoradiation therapy HPV: human papillomavirus IMRT: intensity‐modulated radiotherapy IV: intravenous
Differences between protocol and review
At the request of the Cochrane ENT Group editorial team we recruited further authors with expertise in systematic research methodology (RCD and KR).
Contributions of authors
Liam Masterson (LM) conceived, designed and managed the review.
LM, Daniel Moualed (DM) and Ajmal Masood (AM) screened the titles and abstracts. DM and AM organised retrieval of papers.
LM, DM and AM screened the retrieved papers against the inclusion criteria, appraised the quality of the papers and extracted data.
LM, DM and AM all obtained additional data on published studies.
LM entered data into RevMan 5 (RevMan 2012) and was responsible with DM and AM for data management in the review and analysis of the data.
Raghav C Dwivedi (RCD), Kirsty Rhodes (KR), Richard Benson (RB), Jane Sterling (JS), Holger Sudhoff (HS), Piyush Jani (PJ) and Peter Goon (PG) provided a methodological and clinical perspective.
Sources of support
Internal sources
Cambridge University Hospitals NHS Trust, UK.
External sources
LM and PG received a grant from Cancer Research UK (A14962) in support of this work, UK.
Declarations of interest
Liam Masterson: none known. Daniel Moualed: none known. Ajmal Masood: none known. Raghav C Dwivedi: none known. Richard Benson: none known. Jane C Sterling: none known. Kirsty M Rhodes: none known. Holger Sudhoff: none known. Piyush Jani: none known. Peter Goon: none known.
New
References
References to studies excluded from this review
Argiris 2011 {published data only}
- Argiris A, Lee SC. Serum biomarkers as potential predictors of antitumor activity of cetuximab‐containing therapy for locally advanced head and neck cancer. Oral Oncology 2011;47(10):961‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen AM, Li J, Beckett LA, Zhara T, Farwell G, Lau DH, et al. Differential response rates to irradiation among patients with human papillomavirus positive and negative oropharyngeal cancer. Laryngoscope 2012;123(1):152‐7. [DOI: 10.1002/lary.23570] [DOI] [PubMed] [Google Scholar]
Chera 2012 {published data only}
- Chera B, Green R. De‐intensification of radiation and chemotherapy for low‐risk human papillomavirus‐related oropharyngeal squamous cell carcinoma. http://clinicaltrials.gov/ct2/show/record/NCT01530997 2012. [NCT01530997]
DAHANCA 5 {published data only}
- Lassen P, Eriksen JG, Hamilton‐Dutoit S, Tramm T, Alsner J, Overgaard J, Danish Head and Neck Cancer Group (DAHANCA). HPV‐associated p16‐expression and response to hypoxic modification of radiotherapy in head and neck cancer. Radiotherapy and Oncology 2010;94:30‐5. [DOI] [PubMed] [Google Scholar]
DAHANCA 6 & 7 {published data only}
- Lassen P, Eriksen JG, Krogdahl A, Therkildsen MH, Overgaard M, Specht L, et al. Danish Head and Neck Cancer Group (DAHANCA). The influence of HPV‐associated p16‐expression on accelerated fractionated radiotherapy in head and neck cancer: evaluation of the randomised DAHANCA 6&7 trial. Radiotherapy and Oncology 2011;100:49‐55. [DOI] [PubMed] [Google Scholar]
Eisbruch 2012 {published data only}
- Eisbruch A. Reduced‐intensity therapy for advanced oropharyngeal cancer in non‐smoking human papilloma virus (HPV)‐16 positive patients. http://clinicaltrials.gov/show/NCT01649414 2012. [NCT01649414]
Fakhry 2008 {published data only}
- Fakhry C, Westra WH. Improved survival of patients with human papillomavirus‐positive head and neck squamous cell carcinoma in a prospective clinical trial. Journal of the National Cancer Institute 2008;100(4):261‐9. [DOI] [PubMed] [Google Scholar]
Gilbert 2012 {published data only}
- Gilbert J, Murphy B. Phase 2 trial of oxaliplatin and pemetrexed as an induction regimen in locally advanced head and neck cancer. Cancer 2012;118(4):1007‐13. [DOI] [PubMed] [Google Scholar]
Huang 2012 {published data only}
- Huang S, O'Sullivan B. Temporal regression of cervical lymph node in N2‐N3 head‐and‐neck cancer treated with primary radiation therapy +/‐ chemotherapy: stratified by HPV status. International Journal of Radiation Oncology, Biology, Physics 2012;1:S205. [DOI] [PubMed] [Google Scholar]
Kies 2010 {published data only}
- Kies MS, Holsinger FC. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. Journal of Clinical Oncology 2010;28(1):8‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Le 2012 {published data only}
- Le QT, Fisher R. Prognostic and predictive significance of plasma HGF and IL‐8 in a phase III trial of chemoradiation with or without tirapazamine in locoregionally advanced head and neck cancer. Clinical Cancer Research 2012;18(6):1798‐807. [DOI: 10.1158/1078-0432.CCR-11-2094] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehrotra 2012 {published data only}
- Mehrotra B, Schwartz DL, Frank D, Saltman B, Roy R, Lebowicz YZ, et al. Phase II trial of neoadjuvant chemotherapy for HPV‐associated squamous cell carcinoma of the oropharynx followed by reduced‐dose radiotherapy/chemoradiotherapy for responders or standard dose chemoradiotherapy for non‐responders. Journal of Clinical Oncology 2012;30:Abstract TPS5601. [Google Scholar]
Merlano 2013 {published and unpublished data}
- Merlano M, Denaro N. The phase III study interceptor in HNC: preliminary report on toxicity. Oral Oncology. 2013; Vol. 49:S21‐2.
O'Sullivan 2012 {published and unpublished data}
- O'Sullivan B, Huang SH. Outcomes of HPV‐related oropharyngeal cancer patients treated by radiotherapy alone using altered fractionation. Radiotherapy and Oncology 2012;103(1):49‐56. [DOI] [PubMed] [Google Scholar]
Psyrri 2011 {published data only}
- Psyrri A, Ghebremichael MS. P16 protein status and response to treatment in a prospective clinical trial (ECOG 2303) of patients with head and neck squamous cell carcinoma (HNSCC). Journal of Clinical Oncology 2011;29(15 Suppl):Abstract e16032. [Google Scholar]
Quon 2012 {published data only}
- Quon H, Blackford A. Phase II study of radiation therapy dose de‐intensification for HPV‐associated oropharyngeal carcinoma. International Journal of Radiation Oncology, Biology, Physics 2012;84(3):S208. [NCT01088802] [Google Scholar]
REALISTIC 2012 {published and unpublished data}
- Jones T. A phase I, dose escalation trial of recombinant listeria monocytogenes (Lm)‐ based vaccine encoding human papilloma virus serotype 16 target antigens (ADXS11‐001) in patients with HPV‐16 +ve oropharyngeal carcinoma. Liverpool Cancer Trials Unit (http://www.lctu.org.uk/trial/trial_info.asp?id=73&tgcode=2&menuid=30) 2012 (accessed 3 February 2014). [ ISRCTN47069182]
RTOG 0129 {published data only}
- Ang KK. Human papillomavirus and survival of patients with oropharyngeal cancer. New England Journal of Medicine 2010;363(1):24‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
RTOG 0522 {published data only}
- Ang KK, Zhang QE. A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III‐IV head and neck squamous cell carcinomas. Journal of Clinical Oncology 2011;29(15 Suppl):Abstract 5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
RTOG 9003 {published data only}
- Gillison ML, Zhang Q, Jordan R, Xiao W, Westra WH, Trotti A, et al. Tobacco smoking and increased risk of death and progression for patients with p16‐positive and p16‐negative oropharyngeal cancer. Journal of Clinical Oncology 2012;10:2102‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seiwert 2011 {published data only}
- Seiwert TY, Haraf DJ. A randomized phase II trial of cetuximab‐based induction chemotherapy followed by concurrent cetuximab, 5‐FU, hydroxyurea, and hyperfractionated radiation (CetuxFHX), or cetuximab, cisplatin, and accelerated radiation with concomitant boost (CetuxPX) in patients with locoregionally advanced head and neck cancer (HNC). Journal of Clinical Oncology 2011;29(15 Suppl):Abstract 5519. [Google Scholar]
Semrau 2012 {published data only}
- Semrau R, Duerbaum H, Temming S, Huebbers C, Stenner M, Drebber U, et al. Prognostic impact of human papillomavirus status, survivin, and epidermal growth‐factor receptor expression on survival in patients treated with radiochemotherapy for very advanced nonresectable oropharyngeal cancer. Head and Neck 2012 Oct 5 [Epub ahead of print]. [DOI: 10.1002/hed.23126] [DOI] [PubMed]
Siu 2009 {published data only}
- Siu L, Waldron J. Radiation therapy and cisplatin or panitumumab in treating patients with locally advanced stage III or stage IV head and neck cancer. http://clinicaltrials.gov/show/NCT00820248 2009. [NCT00820248]
Snietura 2011 {published data only}
- Snietura M, Piglowski W. Impact of HPV infection on the clinical outcome of p‐CAIR trial in head and neck cancer. European Archives of Oto‐Rhino‐Laryngology 2011;268(5):721‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takenaka 2013 {published data only}
- Takenaka Y. Phase II study of de‐intensified treatment (radiation alone) for human papilloma virus‐associated oropharyngeal cancer. http://apps.who.int/trialsearch/trial.aspx?trialid=JPRN‐UMIN000008953 2013.
TAX 324 {published data only}
- Posner MR, Lorch JH. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Annals of Oncology 2011;22(5):1071‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Teknos 2010 {published data only}
- Teknos T. Vorinostat in the treatment of advanced staged oropharyngeal squamous cell carcinoma. http://www.clinicaltrials.gov/ct2/show/NCT01064921 2010. [NCT01064921]
Thibaudeau 2011 {published data only}
- Thibaudeau E, Fortin B, Coutlée F. HPV prevalence and prognostic value in a prospective cohort of 255 patients with locally advanced HNSCC: a single‐centre experience. International Journal of Otolaryngology 2013 Apr 24 [Epub ahead of print]. [DOI: 10.1155/2013/437815] [DOI] [PMC free article] [PubMed]
TROG 02.02 {published data only}
- Rischin D, Young RJ. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. Journal of Clinical Oncology 2010;28(27):4142‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yao 2013 {published data only}
- Yao M. Erlotinib, docetaxel, and radiation therapy in stage III or stage IV squamous cell carcinoma of the head and neck. http://clinicaltrials.gov/ct2/show/NCT00720304 2008. [NCT00720304]
References to ongoing studies
Cohen 2010 {published and unpublished data}
- Cohen E. Selection of chemoradiotherapy based on response to induction chemotherapy ‐ a randomized phase II study in locally advanced squamous cell carcinoma of head and neck. http://clinicaltrials.gov/show/NCT01133678 2010. [NCT01133678]
De‐ESCALaTE 2012 {published and unpublished data}
- Mehanna H. De‐ESCALaTE: Determination of epidermal growth factor receptor‐inhibitor (cetuximab) versus standard chemotherapy (cisplatin) early and late toxicity events in human papillomavirus‐positive oropharyngeal squamous cell carcinoma: a randomised controlled trial. http://www.cancerresearchuk 2011.
DFCI 2010 {published and unpublished data}
- Papadimitrakopoulou V. Paclitaxel, carboplatin and cetuximab (PCC) versus cetuximab, docetaxel, cisplatin and fluorouracil (C‐TPF) in previously untreated patients with locally advanced head and neck squamous cell carcinoma (HNSCC). http://www.clinicaltrials.gov/ct2/show/NCT01154920 2010. [NCT01154920]
ECOG 1308 {published and unpublished data}
- Marur S, Comis RL. A phase II trial of induction chemotherapy followed by cetuximab (Erbitux) with low dose vs. standard dose IMRT in patients with HPV‐associated resectable squamous cell carcinoma of the oropharynx. http://clinicaltrials.gov/show/NCT01084083 2010. [NCT01084083] [DOI] [PMC free article] [PubMed]
Quarterback 2012 {published data only}
- Posner M. The Quarterback Trial: a randomized phase III clinical trial comparing reduced and standard radiation therapy doses for locally advanced HPV16 positive oropharynx cancer. http://clinicaltrials.gov/show/NCT01706939. New York: ClinicalTrials.gov, 2012. [NCT01706939]
RTOG 1016 {published data only (unpublished sought but not used)}
- Trotti A. Phase III trial of radiotherapy plus cetuximab versus chemoradiotherapy in HPV‐associated oropharynx cancer. http://clinicaltrials.gov/ct2/show/NCT01302834 2011. [NCT01302834]
TROG‐12.01 {published and unpublished data}
- Rischin D, Sivasuthan J. A randomised trial of weekly cetuximab and radiation versus weekly cisplatin and radiation in good prognosis locoregionally advanced HPV‐associated oropharyngeal squamous cell carcinoma. http://clinicaltrials.gov/ct2/show/NCT01855451 2013. [NCT01855451]
Additional references
Bonner 2010
- Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5‐year survival data from a phase 3 randomised trial, and relation between cetuximab‐induced rash and survival. Lancet Oncology 2010;11(1):21‐8. [DOI] [PubMed] [Google Scholar]
Bourhis 2006
- Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, et al. Meta‐Analysis of Radiotherapy in Carcinomas of Head and Neck (MARCH) Collaborative Group. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta‐analysis. Lancet 2006;368(9538):843‐54. [DOI] [PubMed] [Google Scholar]
Bourhis 2010
- Bourhis J, Lefebvre JL, Vermorken JB. Cetuximab in the management of locoregionally advanced head and neck cancer: expanding the treatment options?. European Journal of Cancer 2010;46(11):1979‐89. [DOI] [PubMed] [Google Scholar]
Chaturvedi 2011
- Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of Clinical Oncology 2011;29(32):4294‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2009
- Choi WH, Hu KS, Culliney B. Cancer of the oropharynx. In: Harrison LB, Sessions RB, Hong WK editor(s). Head and Neck Cancer: A Multidisciplinary Approach. 3rd Edition. Philadelphia: Lippincott, William & Wilkins, 2009:285‐335. [Google Scholar]
Chung 2011
- Chung CH, Zhang Q, Hammond EM, Trotti AM, Wang H, Spencer S, et al. Integrating epidermal growth factor receptor assay with clinical parameters improves risk classification for relapse and survival in head‐and‐neck squamous cell carcinoma. International Journal of Radiation Oncology, Biology, Physics 2011;81(2):331‐8. [DOI: 10.1016/j.ijrobp.2010.05.024] [DOI] [PMC free article] [PubMed] [Google Scholar]
CONSORT 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Annals of Internal Medicine 2010;152(11):1‐7. [DOI] [PubMed] [Google Scholar]
CTCAE 2009
- National Institutes of Health, National Cancer Institute. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events. 4th Edition. US Department of Health and Human Services, 2009. [Google Scholar]
de Martel 2012
- Martel C, Ferlay F, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncology 2012;13(6):607‐15. [DOI] [PubMed] [Google Scholar]
Denis 2004
- Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, et al. Final results of the 94‐01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced‐stage oropharynx carcinoma. Journal of Clinical Oncology 2004;22(1):69‐76. [DOI] [PubMed] [Google Scholar]
Dirix 2010
- Dirix P, Nuyts S. Evidence‐based organ‐sparing radiotherapy in head and neck cancer. Lancet Oncology 2010;11:85‐91. [DOI] [PubMed] [Google Scholar]
Dwivedi 2009
- Dwivedi RC, Rhys‐Evans PH, Patel SG. Tumours of the oropharynx. In: Montgomery P, Rhys‐Evans PH, Gullane PJ editor(s). Principles and Practice of Head and Neck Surgery and Oncology. 2nd Edition. London: Informa, 2009:192‐232. [Google Scholar]
EORTC 2013
- Singer S, Arraras J, Baumann I, Boehm A, Chie WC, Galalae R, et al. on behalf of the EORTC Quality of Life and the EORTC Head and Neck Cancer Groups. Quality of life in head and neck cancer patients receiving multimodal or targeted therapy ‐ update of the EORTC QLQ‐H&N35, Phase I. Head and Neck 2013;35(9):1331‐8. [DOI: 10.1002/hed.23127] [DOI] [PubMed] [Google Scholar]
EuroQol 2011
- Rabin R, Oemar M, Oppe M. EQ‐5D‐3L User Guide. 4th Edition. EuroQol Group, 2011. [Google Scholar]
Evans 2010
- Evans M, Powell NG. The changing aetiology of head and neck cancer: the role of human papillomavirus. Clinical Oncology 2010;22(7):538‐46. [DOI] [PubMed] [Google Scholar]
Gillison 2004
- Gillison ML. Human papillomavirus‐associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Seminars in Oncology 2004;31(6):744‐54. [DOI] [PubMed] [Google Scholar]
Goodwin 2000
- Goodwin EC, DiMaio D. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proceedings of the National Academy of Sciences of the United States of America 2000;97:12513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gregoire 2007
- Grégoire V, Neve W, Eisbruch A, Leed N, Weyngaerte D, Gestele D. Intensity‐modulated radiation therapy for head and neck carcinoma. Oncologist 2007;12:555‐64. [DOI] [PubMed] [Google Scholar]
Gregoire 2010
- Gregoire V, Lefebvre JL, Licitra L, Felip E, EHNS‐ESMO‐ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS‐ESMO‐ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2010;21:184‐6. [DOI] [PubMed] [Google Scholar]
Haddad 2008
- Haddad RI, Shin DM. Recent advances in head and neck cancer. New England Journal of Medicine 2008;359:1143‐54. [DOI] [PubMed] [Google Scholar]
Haddad 2013
- Haddad R, O'Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncology 2013;14(3):257‐64. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hitt 2005
- Hitt R, López‐Pousa A, Martínez‐Trufero J, Escrig V, Carles J, Rizo A, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. Journal of Clinical Oncology 2005;23(34):8636‐45. [DOI] [PubMed] [Google Scholar]
Hoffman 2010
- Hoffmann M, Ihloff AS, Gorogh T, Weise JB, Fazel A, Krams M. p16(INK4a) overexpression predicts translational active human papillomavirus infection in tonsillar cancer. International Journal of Cancer 2010;127:1595–602. [DOI] [PubMed] [Google Scholar]
Hong 2010
- Hong A, Dobbins T, Lee CS, Jones D, Jackson E, Clark J, et al. Relationships between epidermal growth factor receptor expression and human papillomavirus status as markers of prognosis in oropharyngeal cancer. European Journal of Cancer 2010;46(11):2088‐96. [DOI: 10.1016/j.ejca.2010.04.016] [DOI] [PubMed] [Google Scholar]
Kumar 2008
- Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, et al. EGFR, p16, HPV Titer, Bcl‐xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. Journal of Clinical Oncology 2008;26(19):3128‐37. [DOI: 10.1200/JCO.2007.12.7662] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2011
- Lee MK, Nalliah RP, Kim MK, Elangovan S, Allareddy V, Kumar‐Gajendrareddy P, et al. Prevalence and impact of complications on outcomes in patients hospitalized for oral and oropharyngeal cancer treatment. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 2011;112(5):581‐91. [DOI] [PubMed] [Google Scholar]
Lefebvre 2013
- Lefebvre JL, Pointreau Y, Rolland F, Alfonsi M, Baudoux A, Sire C, et al. Induction chemotherapy followed by either chemoradiotherapy or bioradiotherapy for larynx preservation: the TREMPLIN randomized phase II study. Journal of Clinical Oncology 2013;31(7):853‐9. [DOI] [PubMed] [Google Scholar]
Licitra 2006
- Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, Artusi R. High‐risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. Journal of Clinical Oncology 2006;24(36):5630‐6. [DOI] [PubMed] [Google Scholar]
Mehanna 2011
- Mehanna H. Oropharyngeal cancer. In: Roland N, Paleri V, MacKenzie K, Clarke P, Homer J, Pracy P, et al. editor(s). Head and Neck Cancer. 4th Edition. London: British Association of Otorhinolaryngology Head and Neck Surgery, 2011:137‐46. [Google Scholar]
Mehanna 2012
- Mehanna H, Beech T, Nicholson T, El‐Hariry I, McConkey C, Paleri V, et al. The prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer: a systematic review and meta‐analysis of trends by time and region. Head and Neck 2012 Jan 20 [Epub ahead of print]. [DOI: 10.1002/hed.22015] [DOI] [PubMed]
Mendenhall 2011
- Mendenhall WM, Werning JW, Pfister DG. Treatment of head and neck cancer. In: Vita VT Jr, Lawrence TS, Rosenberg SA editor(s). Cancer: Principles and Practice of Oncology. 9th Edition. Philadelphia: Lippincott, Williams & Wilkins, 2011:729‐80. [Google Scholar]
Modjtahedi 2009
- Modjtahedi H, Essapen S. Epidermal growth factor receptor inhibitors in cancer treatment: advances, challenges and opportunities. Anticancer Drugs 2009;20(10):851‐5. [DOI] [PubMed] [Google Scholar]
Murphy 2009
- Murphy BA, Gilbert J. Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Seminars in Radiation Oncology 2009;19(1):35‐42. [DOI] [PubMed] [Google Scholar]
NCCN 2010
- National Comprehensive Cancer Network. NCCN Guidelines for Treatment of Cancer by Site (Head & Neck Cancer). http://www.nccn.org/professionals/physician_gls/f_guidelines.asp 2010.
Nguyen 2009
- Nguyen NP, Frank C, Moltz CC, Vos P, Smith HJ, Nguyen PD, et al. Analysis of factors influencing aspiration risk following chemoradiation for oropharyngeal cancer. British Journal of Radiology 2009;82:675–80. [DOI] [PubMed] [Google Scholar]
Nutting 2011
- Nutting C, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid‐sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncology 2011;12(2):127‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliver 2002
- Oliver M, Eeles R, Holstein M, Khan MA, Harris CC, Hainaut P. The IARC TP53 database: new online mutation analysis and recommendations to users. Human Mutation 2002;19:607–14. [DOI] [PubMed] [Google Scholar]
Pajares 2013
- Pajares B, Perez‐Villa L, Trigo JM, Toledo MD, Alvarez M, Jimenez B, et al. Concurrent radiotherapy plus epidermal growth factor receptor inhibitors in patients with human papillomavirus‐related head and neck cancer. Clinical & Translational Oncology 2013 Nov 6 [Epub ahead of print]. [PUBMED: 24193865] [DOI] [PubMed]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Petrelli 2013
Pignon 2009
- Pignon JP, Maître A, Maillard E, Bourhis J. Meta‐analysis of chemotherapy in head and neck cancer (MACH‐NC): an update on 93 randomized trials and 17,346 patients. Radiotherapy and Oncology 2009;92:4‐14. [DOI] [PubMed] [Google Scholar]
Posner 2007
- Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. TAX 324 Study Group. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. New England Journal of Medicine 2007;357(17):1705‐15. [DOI] [PubMed] [Google Scholar]
Ragin 2007
- Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta‐analysis. International Journal of Cancer 2007;121:1813‐20. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schache 2011
- Schache AG, Liloglou T, Risk JM, Filia A, Jones TM, Sheard J, et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clinical Cancer Research 2011;17:6262‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sudhoff 2011
- Sudhoff HH, Schwarze HP, Winder D, Steinstraesser L, Gormer M, Stanley M, et al. Evidence for a causal association for HPV in head and neck cancers. European Archives of Oto‐Rhino‐Laryngology 2011;268:1541–7. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Houten 2001
- Houten VM, Snijders PJ, Brekel MW, Kummer JA, Meijer CJ, Leeuwen B, et al. Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. International Journal of Cancer 2001;93:232–5. [DOI] [PubMed] [Google Scholar]
Vermorken 2008
- Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum‐based chemotherapy plus cetuximab in head and neck cancer. New England Journal of Medicine 2008;359(11):1116‐27. [DOI] [PubMed] [Google Scholar]
Walboomers 1999
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. Journal of Pathology 2009;189(1):12‐9. [DOI] [PubMed] [Google Scholar]
Wells 2000
- Wells SI, Francis DA, Karpova AY, Dowhanick JJ, Benson JD, Howley PM. Papillomavirus E2 induces senescence in HPV‐positive cells via pRB and p21(CIP) dependent pathways. EMBO Journal 2000;19:5762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Westra 2009
- Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head and Neck Pathology 2009;3:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2000
- WHO. International Classification of Diseases for Oncology (ICD‐O). 3rd Edition. Geneva: World Health Organization, 2000. [Google Scholar]
Wiest 2002
- Wiest T, Schwarz E, Enders C, Flechtenmacher C, Bosch FX. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene 2002;21:1510–7. [DOI] [PubMed] [Google Scholar]


