Abstract
Background
Early detection of esophageal varices (EV) before the first attack of bleeding is crucial for primary prophylaxis. The current work aims to investigate the use of a combination of FibroScan and platelet count as noninvasive means to identify EV in patients with compensated cirrhosis.
Methods
Sixty-two patients with compensated hepatitis C virus (HCV)-related cirrhosis were divided into two groups with and without EV. All patients were exposed to complete history, physical examination, laboratory, and endoscopic evaluation. FibroScan was performed for all patients, and the two groups were compared.
Results
A statistically significant higher mean liver stiffness measurement (LSM) (KPa), lower mean platelet count to splenic diameter ratio (PSR), and higher mean fibrosis-4 (FIB4) score were noticed in those with EV with P < 0.0005. A cutoff value of ≥23.1 for LSM, ≥3.71 for FIB4, and ≥130 mm for splenic diameter have a sensitivity of 94%, 97%, and 97% and a specificity of 81%, 81%, and 68%, respectively, in the detection of varices. Platelet count of ≥112,500 (×103/dl) and of ≥771.33 for PSR have a sensitivity of 84% and 77% and a specificity of 87% and 90%, respectively, to rule out the presence of varices. LSM, FIB4 score, and splenic diameter are predictors of the presence of varices where platelet count and PSR are negative predictors.
Conclusion
The combination of LSM by transient elastography (TE), PSR, or platelet count can be used to detect a relevant category of patients with compensated cirrhosis who have a very low possibility of EV where endoscopy can be avoided.
Keywords: Cirrhosis, Esophageal varices, Transient elastography
Introduction
Cirrhosis is the end stage of most chronic liver diseases that lead to portal hypertension. Esophageal varices (EVs) are a dangerous sequelae of portal hypertension, and bleeding from varices is a serious complication occurring in about 40% of cirrhotic patients.1 The mortality from an acute attack of bleeding varices is still around 30% even after advances in treatment modalities.2
Now, at the time of diagnosis of cirrhosis, upper gastrointestinal endoscopy must be performed on all patients for screening for detection of EV to assess the need for prophylactic therapy.3
Early detection of EV before the first attack of bleeding is crucial because studies on primary prophylaxis showed clearly that we can reduce the risk of variceal bleeding by about 15%–50% for risky EV.2
Upper gastrointestinal endoscopy is the most accurate and reliable method to diagnose EV. In screening, it is still the gold standard method; however, it has its own limitations where conflicting evidence regarding interobserver agreement for endoscopic diagnosis, grading, or risk assessment of EV exists. Also, screening endoscopy may not be cost-effective.1 Easily assed noninvasive variables may be a more affordable approach for screening.
Several studies have been performed to predict and grade EV by noninvasive means assessing clinical signs, biochemical liver function, and variables related to liver fibrosis, portal hypertension, and hypersplenism.4
Liver stiffness measurement (LSM) performed by transient elastography (TE) has emerged as a novel, safe, noninvasive, and objective tool for assessment of advanced fibrosis/cirrhosis mainly in post-hepatitis C virus (HCV)-compensated cirrhosis.5
Based on preliminary data from a few previous studies,6 the recent Baveno VI consensus conference on the methodology of management of portal hypertension recommend that patients with platelet count above 150,000/mm3 and liver stiffness <20 kPa kPa have a low prevalence of risky varices, and upper gastrointestinal endoscopy is not advised.7 The guidelines also recommend long-term follow-up of these patients by annual TE and platelet count, and if platelets decrease or liver stiffness increases, screening endoscopy is advised.7
Although several studies have shown that patients with platelet count above 150,000/mm³ and TE <20 kPa are at low risk of EV and even lower risk of variceal hemorrhage;5,7 some studies did not reach that conclusion.8 So, these criteria are a matter of debate and have not been validated yet.
Aim of the study
The current work aims to investigate prospectively the available evidence on the validation of the combined use of platelet count and liver stiffness (TE measured by FibroScan) as noninvasive means to identify post-HCV-compensated cirrhotic patients without EV. The main endpoints are the presence of varices.
Material and methods
Patients
This study was performed with 62 patients presenting for routine follow-up at hepatology clinics. Thirty-one patients presented with varices, and the other 31 patients presented without varices based on their history.
Sample size
The sample size was calculated using PASS software version (2008).9 Sample size estimation depends on a previous similar study by de Franchis.7 In the study, The area under the receiver operating characteristic curve (AUROC) for liver stiffness measurement LSM and platelet count combined was 0.746, which is significantly better than 0.50. A sample of 31 from the positive group and 31 from the negative group achieve 95% power to detect a difference of 0.2460 between the area under the ROC curve (AUC)AUROC under the null hypothesis of 0.5000 and an AUC under the alternative hypothesis of 0.7460 using a two-sided z-test at a significance level of 0.05000. The data are continuous responses. The AUC is computed between false positive rates of 0.000 and 1.000. The ratio of the standard deviation of the responses in the negative group to the standard deviation of the responses in the positive group is 1.000.
Inclusion criteria
This is a prospective study that was conducted on patients with post-HCV- compensated cirrhosis, with no history of hematemesis and/or melena or upper gastrointestinal (GIT) endoscopy.
Compensated cirrhosis is defined by fibrosis-4 (FIB4) score ≥3.25 or FIB4 score 1.45 to 3.25 with FibroScan (LSM) ≥10 kPa according to the Baveno VI recommendations.
Fibrosis-4 (FIB-4): is a non-invasive score based on many laboratory parameters to estimate the degree of scarring in the liver studied in hepatitis C-related liver diseases.10
Interpretation: For HCV:
Exclusion criteria
All patients with LSM lower than 10 kPa; Child-Pugh B and C. ; Other causes of cirrhosis rather than HCV; including the presence of infection or fever; active and past alcohol use; previous variceal hemorrhage; previous beta-blocker, nitrates, or diuretics; previous endoscopic therapy, history of surgery for portal hypertension, splenectomy or transjugular intrahepatic portosystemic stent shunt placement (TIPS), splenic or portal vein thrombosis, hepatocellular carcinoma; and previous decompensation (as it increases the prevalence of varices, where screening upper endoscopy is mandatory,) were excluded from the study.
Methods
Patients were subjected to:
-
•
Relevant history: Including a standardized questionnaire covering the following : Name, age, sex of the patient. Infection with HCV. History of liver disease, cirrhosis. History of medical treatment. History of radiotherapy and chemotherapy.
-
•
Physical examination: Manifestations of hepatocellular failure, abdominal vein collaterals, hepatomegaly, and, splenomegaly were recorded.
-
•
Laboratory tests: Complete blood count (CBC), hepatic profile, renal function tests, hepatitis markers, serum metabolic panel (sodium, potassium, creatinine), and, tests for other causes of cirrhosis only if there was a suggestive clinical clue were done for all patients.
All patients with cirrhosis were evaluated according to the Child-Turcotte-Pugh (CTP).11
-
•
Abdominal ultrasonography: For evaluation of the state of the liver, splenic size, presence of porto-systemic collaterals or ascites.
-
•Endoscopic evaluation: Upper gastrointestinal endoscopy was done for all patients, and EV were graded according to the Japanese classification as follows:
-
•(FI): The veins can be depressed by the scope and are minimally elevated.
-
•(FII): The veins cannot be depressed by the scope occupying less than one-third of the esophageal lumen, and, are separated by normal mucosa.
-
•(FIII): The veins cannot be depressed by the scope occupying more than one-third of the esophageal lumen with or without red signs.
-
•
-
•
The presence of gastric varices and portal hypertensive gastropathy was recorded wherever appropriate.
-
•
Transient elastography: The process was done performed by an experienced gastroenterologist where TE was measured using a FibroScan device. The device is provided with the M probe (Echosens, Paris, France). Ten measurements were obtained with a success rate ≥60%. The interquartile range (IQR) should be less than 30% of the median (IQR/M ≤ 30%).14.
Statistical analysis
The data were coded, entered, and analyzed using the statistical package SPSS (Statistical Program for Social Science), version 17. Qualitative data were expressed as count and percent and compared using the Chi-square test. Quantitative data will be tested initially for normality using Kolmogorov-Smirnov or Shapiro-Wilk test. Normally distributed data were expressed as mean ± SD standard deviation and compared using an independent sample t-test. Non-normally distributed data were expressed as median IQR and compared using the Mann-Whitney U test. To assess the diagnostic accuracy (sensitivity, specificity, positive and negative predictive values) of the combined noninvasive index (liver stiffness measurement LSM and platelet count), receiver operating characteristic (ROC) curves were constructed, and the corresponding area under the ROC curve (AUROC) were was computed. Logistic regression analyses was were done performed to test for significant predictors of the outcome nominal variable. A pP- value of ≤0.050 was considered statistically significant.
Ethics
We obtained written consents from all patients who participated in the study or from their families, and the study was approved by the Mansoura Medical Ethics Committee (MMEC) of Faculty of medicine.
Results
Table 1 shows a statistically significant difference between those with and those without EV as regards LSM (KPa), splenic diameter, platelet count, platelet count to splenic diameter ratio (PSR), red blood cell count, lymphocyte count, total leukocytic count (TLC), Red cell distribution width - standard deviation (RDW-SD), Red cell distribution width - coefficient variation (RDW-CV), FIB4 score, International Normalization Ratio (INR), serum creatinine, serum bilirubin, and serum albumin. A statistically significantly higher mean LSM and FIB4 score (KPa) exists in those with EV with P < 0.0005. A lower mean PSR was noticed in those with EV with P < 0.0005. Patients with EV have a statistically significant higher mean splenic diameter (mm) and lower mean platelet count than those without varices with P < 0.0005 for both.
Table 1.
Comparisons of the demographic and laboratory parameters between the study populations.
| Parameter | Variceal group (n = 31) | Nonvariceal group (n = 31) | P |
|---|---|---|---|
| Age (years) | 60.8 ± 6.9 (61) | 57.4 ± 10.95 (61) | 0.374∗ |
| Sex (male/female): (count & percent) | 17 (54.8%)/14 (45.2) | 19 (61.3%) 12 (38.7%) | 0.607∗∗∗ |
| LSM (KPa) | 31.3 ± 7.2 (31.2) | 19 ± 11 (16.6) | <0.0005∗ |
| Splenic longitudinal diameter (mm) | 165.9 ± 24.4 (160) | 127.9 ± 29.2 (120) | <0.0005∗ |
| Platelet count (×103/dl) | 88.94 ± 37 (90) | 172.84 ± 63.8 (177) | <0.0005∗ |
| PSR | 560.5 ± 271.5 (511.8) | 1475.4 ± 728.4 (1527.3) | <0.0005∗ |
| FIB4 score | 6.58 ± 2.96 (5.77) | 3.04 ± 2.08 (2.12) | <0.0005∗ |
| RBCs (×103/dl) | 4.45 ± 0.46 (4.55) | 4.72 ± 0.64 (4.76) | 0.012∗ |
| Hb (gm/dl) | 12.97 ± 1.62 (13) | 13.79 ± 1.67 (13.9) | 0.058∗∗ |
| HCT | 39.38 ± 4.68 (39.5) | 41.11 ± 4.78 (41) | 0.154∗∗ |
| TLC (×103/dl) | 5.18 ± 1.69 (4.8) | 6.29 ± 1.84 (6.04) | 0.017∗∗ |
| RDW-SD | 47.23 ± 2.8 (47.2) | 46.05 ± 10.6 (43.2) | 0.001∗ |
| RDW-CV | 15.12 ± 1.8 (14.6) | 14.19 ± 2.4 (13.7) | 0.001∗ |
| MPV | 11.71 ± 1.3 (11.3) | 11.22 ± 1.12 (10.9) | 0.110∗ |
| Prothrombin time (INR) | 1.09 ± 0.07 (1.09) | 1.04 ± 0.04 (1.03) | 0.007∗ |
| Creatinine (mg/dl) | 0.83 ± 0.19 (0.86) | 0.68 ± 0.12 (0.69) | 0.001∗∗ |
| ALT (IU/L) | 60.04 ± 35.28 (48) | 50.9 ± 38.2 (35.73) | 0.094∗ |
| AST (IU/L) | 64.84 ± 29.62 (56) | 56.43 ± 36.56 (44) | 0.066∗ |
| Total serum bilirubin (mg/dl) | 0.99 ± 0.24 (1) | 0.75 ± 0.27 (0.77) | 0.001∗∗ |
| Direct serum bilirubin (mg/dl) | 0.51 ± 0.14 (0.5) | 0.37 ± 0.14 (0.35) | 0.001∗∗ |
| Serum albumin (gm/dl) | 3.81 ± 0.33 (3.7) | 4.02 ± 0.3 (4) | 0.007∗ |
| Blood urea nitrogen (BUN) (mg/dl) | 26.97 ± 9.12 (24) | 24.23 ± 7.05 (22) | 0.244∗ |
| Alkaline phosphatase (IU/L) | 61.23 ± 13.27 (61) | 58.94 ± 13.11 (57) | 0.499∗ |
LSM, liver stiffness measurement; PSR, platelet count to splenic diameter ratio; RBC, red blood cell count; TLC, Total leukocytic count; HCT, Haematocrit; MPV, Mean platelet volume; RDW-SD, Red cell distribution width - standard deviation; RDW-CV, Red cell distribution width - coefficient variation; AST, Aspartate aminotransferase; ALT, Alanine Aminotransfera.
∗ significant, ∗∗ highly significant.
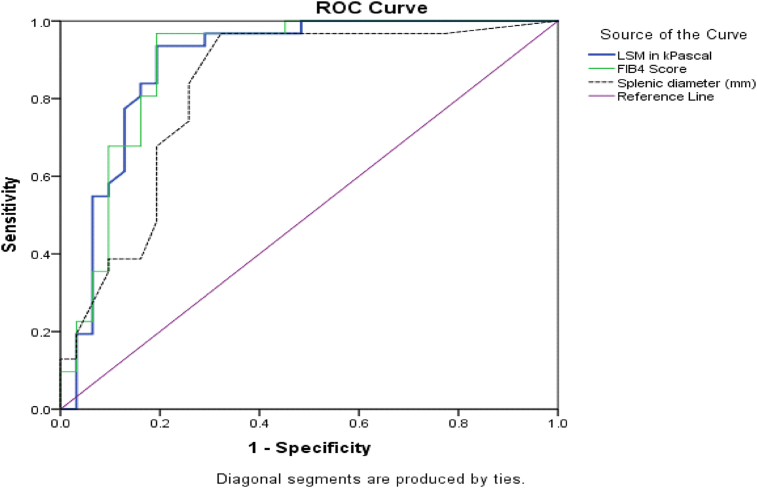
Table 2 shows that a cutoff value ≥23.1 for LSM, ≥3.71 for FIB4, and ≥130 mm for splenic diameter has a sensitivity of 94%, 97%, and 97% and a specificity of 81%, 81%, and 68%, respectively, to diagnose the presence of varices. The ROC curve illustrates sensitivity against specificity with a high AUC for both LSM and FIB4 (0.890) and splenic diameter (0.824) (Fig. 1).
Table 2.
Cutoff values of liver stiffness, FIB4 score, and splenic diameter in diagnosis of esophageal varices.
| Variable | Cutoff value | AUC | 95% CI | SE | Sensitivity% | specificity% | P | Pairwise comparison of ROC curves |
||
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | ||||||||
| Liver stiffness (LS) (KPascal) | ≥23.1 | 0.890 | 0.800–0.980 | 0.046 | 94% | 81% | <0.0005 | 1 | 0.3624 | 0.3582 |
| FIB4 | ≥3.71 | 0.890 | 0.801–0.979 | 0.045 | 97% | 81% | <0.0005 | |||
| Splenic long. diameter (mm) | ≥130 | 0.824 | 0.714–0.934 | 0.056 | 97% | 68% | <0.0005 | |||
AUC, area under the curve; CI, confidence interval; ROC, receiver operating characteristic; P, significance of each cutoff; p1, LS vs FIB4; p2, LS vs SD; p3, FIB4 vs SD; SE, standard error.
Fig. 1.
The ROC curve of LSM, FIB4 score, and splenic diameter (mm) in the diagnosis of esophageal varices.
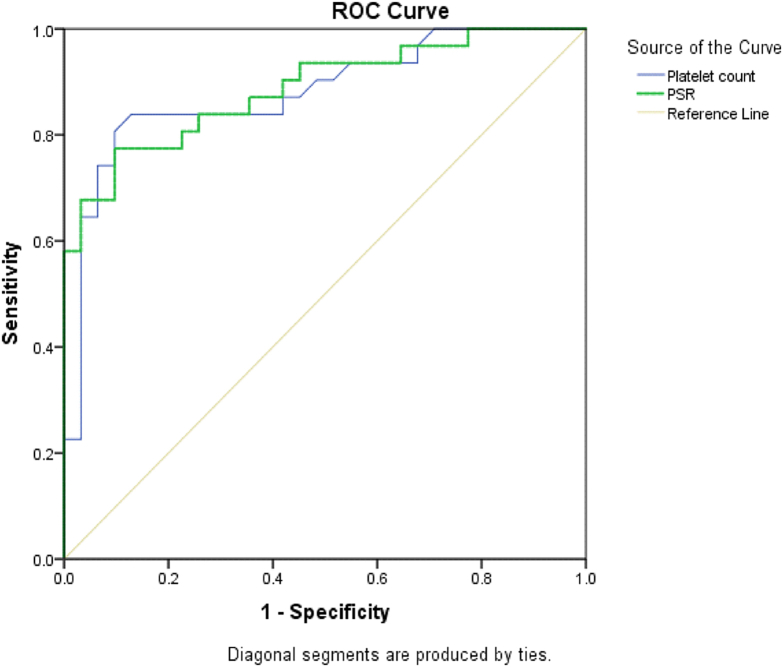
Table 3 shows that platelet count ≥112,500 (×103/dl) and PSR of ≥771.33 have a sensitivity of 84% and 77% and a specificity of 87% and 90%, respectively, to rule out the presence of varices. Fig. 2 illustrates sensitivity against specificity with a high AUC for both PSR (0.887) and platelet count (0.880).
Table 3.
Cutoff value of platelet count and PSR score in ruling out diagnosis of esophageal varices.
| Variable | Cutoff value | AUC | 95% CI | SE | Sensitivity% | Specificity% | P | Pairwise comparison of ROC curves |
|---|---|---|---|---|---|---|---|---|
| Platelet count (×103/dl) | ≥112,500 | 0.880 | 0.792–0.969 | 0.045 | 84% | 87% | <0.0005 | 0.9095 |
| PSR | ≥771.33 | 0.887 | 0.804–0.969 | 0.042 | 77% | 90% | <0.0005 |
Bold values are represents the important significant items.
Fig. 2.
The ROC curve of platelet count and PSR in ruling out esophageal varices.
A point-biserial correlation was run between variceal status and each of the diagnostic parameters (LSM, FIB4 score, splenic diameter, platelet count, and PSR). There was a statistically significant correlation between the presence of EV and each of the five diagnostic parameters (P < 0.0005) (Table 4).
Table 4.
Point-biserial correlation and standard logistic regression analysis between variceal status and diagnostic parameters.
| Parameter | rpb | p∗ | B | OR (95% CI) | p∗∗ |
|---|---|---|---|---|---|
| LSM | 0.5573 | <0.0005 | 0.161 | 1.175 (1.081–1.276) | <0.0005 |
| FIB4 score | 0.5754 | <0.0005 | 0.710 | 2.033 (1.412–2.929) | <0.0005 |
| Splenic diameter | 0.5833 | <0.0005 | 0.472 | 1.603 (1.272–2.020) | <0.0005 |
| Platelet count | −0.6329 | <0.0005 | −0.350 | 0.966 (0.949–0.983) | <0.0005 |
| PSR | −0.6459 | <0.0005 | −0.004 | 0.996 (0.994–0.998) | <0.0005 |
rpb, Point-biserial correlation coefficient; B, logistic regression coefficient; OR, odds ratio.
p∗, by point–biserial correlation. p∗∗, by standard (simple) logistic regression analysis.
Bold values are represents the important significant items.
Also, a standard logistic regression analysis showed that LSM, FIB4 score, and splenic diameter are predictors of the presence of varices; each one-unit increase in these 3 parameters increases the risk by 1.175, 2.033, and 1.603, respectively. On the other hand, platelet count and PSR are negative predictors of the presence of varices.
The logistic regression model was statistically significant, χ2 = 29.222, P < 0.0005. The model explained 50.1% (Nagelkerke R2) of the variance in variceal status and correctly classified 85.5% of cases. Sensitivity was 87.1%, specificity was 83.9%, positive predictive value was 84.4%, and the negative predictive value was 86.7%.
Of the three predictor variables, LSM was statistically significant as shown in Table 5. With every one unit increase in LSM, patients had 1.2 times higher odds to exhibit EV. The other four parameters (FIB4, Splenic diameter, PLT, and PSR) were not entered in the model because of their highly significant correlation with LSM.
Table 5.
Predictors of the likelihood of the presence of esophageal varices.
| Predictor | B | SE | Wald | p | OR (95% CI) |
|---|---|---|---|---|---|
| LSM | 0.170 | 0.044 | 15.218 | <0.0005 | 1.186 (1.088–1.291) |
| Age (years) | 0.083 | 0.047 | 3.137 | 0.077 | 1.087 (0.991–1.192) |
| Sex | −0.840 | 0.687 | 1.496 | 0.221 | 0.432 (0.112–1.659) |
| Constant | −8.704 | ||||
Bold values are represents the important significant items.
Discussion
In decompensated cirrhosis, scores that are validated for prediction, prognosis, and risk stratification of EV have very limited role in the setting of compensated cirrhosis.12,13 Endoscopy is impractical for repeated follow-up of patients with decompensated cirrhosis. Hence, several noninvasive methods have been suggested to replace screening endoscopy such as LSM.14 Besides, studies suggest that LSM combined with spleen size and platelet count can accurately identify cirrhotic patients with varices needing intervention.15
In April 2015, the Baveno VI conference on the methods of diagnosis and treatment of portal hypertension recommended that in patients with compensated cirrhosis, upper gastrointestinal endoscopy should be avoided when liver stiffness is <20 KPa and platelets count >150,000/mm³.7 The study showed that the combination of simple parameters such as LSM with TE, platelet count, spleen diameter, and PSR allows noninvasive and early identification of high-risk patients for developing varices.
In our study, the included population was homogeneous as regards the severity and etiology of the underlying liver disease (compensated cirrhosis). The initial step in our approach to risk prediction was targeting a population where this may lead to the detection of patients at earlier stages of the disease and where the prevalence of varices is low.
In our approach, we did not include intensive modeling with wide variable selection. The approach was restricted either to one LSM by TE or two (LSM by TE and platelet count) variables or to a combination of variables that was validated in many studies as PSR. This reduces the chances of overfitting or overoptimism in model development and performance.
The present study showed a statistically significant higher mean LSM (KPa) in those with EV than that in those without varices. It also showed a correlation between the presence of EV and liver stiffness values. Previously, a correlation between the presence of EV and liver stiffness was reported.16,17 In the present study, AUROC of LSM for diagnosing the presence of EV was 0.890, and cutoff values ≥23.1 KPa were used to diagnose the presence of varices. Previously, AUROCs of LSM for diagnosing the presence of EV have been reported to range from 0.74 to 0.85 and cutoffs from 13.9 to 21.5 KPa.16,17
The sensitivity of LSM for prediction of EV was high (94%) but with a low specificity (81%). Previously, LSM has high sensitivity (76%–95%) for the prediction of EV but with a lower specificity (43%–78%).16,17 Our results agreed with a study carried out in Egypt in which they evaluated 32 patients with HCV-related cirrhosis to assess the ability of FibroScan to predict the presence and grade of EV. They showed that the FibroScan values were significantly higher in the presence of EV. The sensitivity and specificity were 95% and 67%, respectively, with a 29.7 KPa cutoff value.18 Karatzas et al concluded that noninvasive methods, specifically elastography, have given alternatives for surveillance of certain cirrhotic populations and avoiding screening esophago gastroduodenoscopy (EGD) in large groups of cirrhotic patients.19
In patients with liver cirrhosis, a recent meta-analysis performed by Li et al showed pooled sensitivity and specificity of 84% and 68%, respectively, for the detection of large varices when they evaluated the accuracy of LSM in the prediction of EV with positive and negative likelihood ratios of 2.58 and 0.24, respectively.20 These results were similar to those of another meta-analysis, with a pooled sensitivity and specificity of 81% and 71%, respectively.20
The difference between our results and these studies as regard LSM can be attributed to the following reasons: First, the majority of these studies were conducted in single centers retrospectively; second, small patient number; third, the included patients were with different cirrhosis-related etiologies; and lastly, the prevalence of the disease. Finally, Jia li, et al. concluded a reliable model to prognosticate Gastroesophageal varices (GOV) and variceal hemorrhage and used to stratify not only the high-risk Chronic Hepatitis B (CHB) patients but also patients with other CLDs for developing GOV and variceal bleeding.21
In clinical practice, the diagnostic validities of simple blood markers and TE were suboptimal despite being useful in the prediction of high-risk EV. Therefore, a combination of noninvasive markers was proposed to improve the prediction of the presence of EV.
FIB-4 proposed a simple noninvasive test for liver fibrosis. A FIB-4 score between 1.45 and 3.25 allows correct detection of patients with moderate or significant fibrosis, respectively. Its value comes from several aspects: first, its simplicity; second, it is inexpensive; third, results are immediately available.10,11
The present study showed a statistically significant higher mean FIB-4 in those with EV as compared to that in those without varices. The AUROC of FIB-4 in the diagnosis of EV was 0.890, and a cutoff value ≥3.71 has a sensitivity of 97% and a specificity of 81%. Previously, the serum biomarkers have AUROCs ranging from 0.57 to 0.86, with higher sensitivity (51–92%) than specificity (53–76%). In a large study that retrospectively compared a panel of serum biomarkers (platelet count, AST/ALT ratio, APRI, Lok index, Forns index, FIB-4, and Fibro index), the combination of Forns index (cutoff = 8.8) and Lok index (cutoff = 1.5) had the best diagnostic performance with a 90% negative predictive value for predicting clinically relevant EV in more than 500 patients with chronic liver diseases.22
The present study showed a statistically significant higher splenic diameter in those with EV as compared to those without varices and showed that AUROC of 0.824 and a cutoff value ≥130 mm with a sensitivity of 97% and a specificity of 68% can help diagnose the presence of varices. Thomopoulos et al agreed with these findings where they proved that splenic diameter ≥13.5 cm has a sensitivity of 95% and specificity of 37% in the prediction of the presence of EV, so it can serve as a good predictor for the presence of EV.23
The present study showed a statistically significantly lower platelet count in those with EV as compared to those without varices and showed that AUROCs of 0.880 and a cutoff value ≥112,500 (×103/dl) with a sensitivity of 84% and a specificity of 87% can rule out the presence of varices. These findings go hand in hand with those of Thomopoulos et al, Garcia-Tsao et al, and Pilette et al; however, most patient populations included in these studies did not have a uniform etiology.23, 24, 25 On the other hand, Sedrak et al reported that no association between platelet count and size of varices was detected.26 This could be partly attributed to the matched platelet count in their studied groups. Moreover, it is worth noting that portal hypertension is not the sole factor responsible for thrombocytopenia.26
It was deemed that the PSR is the appropriately used parameter as splenomegaly is implicated in thrombocytopenia of cirrhosis where the size of the spleen is inversely correlated with platelet count. This ratio normalizes platelet count to sequestration of the spleen as platelet count alone cannot be attributed to portal hypertension and may be misleading. The present study showed a statistically significantly lower PSR in those with EV as compared to those without varices and showed that AUROCs of 0.887 and a cutoff value ≥771.33 with a sensitivity of 77% and a specificity of 90% can help diagnose the presence of varices. These findings agreed with many studies such as those by Tarantino et al and Zaman et al;27,28 however, several PSR studies failed to reproduce the aforementioned encouraging results.29 Similarly, in a study by Kazemi et al, the performances of TE did not differ from those of PSR in the detection of large EV.17 However, the combination of LSM with platelet count and spleen diameter increased the diagnostic accuracy.
In the present study, a point-biserial correlation was run between variceal status and each of the diagnostic parameters (LSM, FIB4 score, splenic diameter, platelet count, and PSR). A statistically significant correlation between the presence of EV and each of the five diagnostic parameters (P < 0.0005) was found. A standard logistic regression analysis showed that LSM, FIB4 score, and splenic diameter are predictors of the presence of varices; each one-unit increase in these 3 parameters increases the risk by 1.175, 2.033, and 1.603, respectively. On the other hand, platelet count and PSR are negative predictors of the presence of varices.
In our study, the model of logistic regression was statistically significant, χ2 = 29.222, P < 0.0005. The model explained 50.1% (Nagelkerke R2) of the variance in variceal status and correctly classified 85.5% of cases. The sensitivity was 87.1% with 83.9% specificity. The positive and negative predictive values were 84.4% and 86.7%, respectively.
Finally, our study is not without limitations. The study may be limited by the small number of cases, the FIB-4 may be affected by the inflammatory parameters, and the restriction of the cause of cirrhosis to HCV alone surmises that the data cannot be generalized to cirrhosis due to other causes.
Conclusion
Our results suggest that the combination of LSM with TE, platelet count, or PSR could be used to identify a relevant percentage of patients with Child-Pugh. A compensated cirrhosis that have a very low risk of EV in whom endoscopy could be avoided. These data also suggest that noninvasive biomarkers are effective in the prediction of EV in HCV-related cirrhosis. These markers may help the physicians to initiate appropriate primary pharmacological prophylaxis in areas where endoscopy is not easily accessible.
Disclosure of competing interest
The authors have none to declare.
References
- 1.D'Amico G., Morabito A. Noninvasive markers of esophageal varices: another round, not the last. Hepatology. 2004;39:30–34. doi: 10.1002/hep.20018. [DOI] [PubMed] [Google Scholar]
- 2.Jamal M.M., Samarasena J.B., Hashemzadeh M. Decreasing in-hospital mortality for oesophageal variceal hemorrhage in the USA. Eur J Gastroenterol Hepatol. 2008;20(10):947–955. doi: 10.1097/MEG.0b013e32830280c7. [DOI] [PubMed] [Google Scholar]
- 3.D'Amico G., De Franchis R. Upper digestive bleeding in cirrhosis: post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599–612. doi: 10.1053/jhep.2003.50385. [DOI] [PubMed] [Google Scholar]
- 4.Galal G.M., Amin N.F., Abdel Hafeez H.A., El-Baz M.A.H. Can serum fibrosis markers predict medium/large oesophageal varices in patients with liver cirrhosis? Arab J Gastroenterol. 2011;12(2):62–67. doi: 10.1016/j.ajg.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Castera L., Forns X., Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Ding N.S., Nguyen T., Iser D.M., et al. Liver stiffness plus platelet count can be used to exclude high-risk oesophageal varices. Liver Int. 2015;36:240–245. doi: 10.1111/liv.12916. [DOI] [PubMed] [Google Scholar]
- 7.de Franchis R. Expanding consensus in portal hypertension. J Hepatol. 2015;63(3):743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Gomez M.L., Herrera E.L., De la Revilla Negro J., et al. Are noninvasive test good enough to predict gastrointestinal varices in patients with compensated advanced chronic liver disease? J Hepatol. 2016;64:S731. [Google Scholar]
- 9.Paternostro R., Schwarzer R., Ferlitsch M., et al. Exclusion of varices via transient elastography combined with platelet count according to the Baveno VI guidelines can only be made for large but not small varices. J Hepatol. 2016;64:S283. [Google Scholar]
- 10.Vallet-Pichard A., Mallet V., Nalpas B., Verkarre V., et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 11.Child C.G., Turcotte J.G. In: The Liver and Portal Hypertension. Child C.G., editor. W. B. Saunders Co.; Philadelphia: 1964. Surgery and portal hypertension; pp. 50–64. [Google Scholar]
- 12.Pugh R.N., Murray-Lyon I.M., Dawson J.L., et al. Transection of the esophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 13.D'Amico G., Garcia-Tsao G., Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Abraldes J.G., Araujo I.K., Turon F., Berzigotti A. Diagnosing and monitoring cirrhosis: liver biopsy, hepatic venous pressure gradient and elastography. Gastroenterol Hepatol. 2012;35:488–495. doi: 10.1016/j.gastrohep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Kim B.K., Han K.H., Park J.Y., et al. A liver stiffness measurement-based, noninvasive prediction model for high-risk esophageal varices in B-viral liver cirrhosis. Am J Gastroenterol. 2010;105:1382–1390. doi: 10.1038/ajg.2009.750. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W., Wang L., Wang L., et al. Liver stiffness measurement, better than APRI, Fibroindex, Fib-4, and NBI gastroscopy, predicts portal hypertension in patients with cirrhosis. Cell Biochem Biophys. 2015;71:865. doi: 10.1007/s12013-014-0275-z. [DOI] [PubMed] [Google Scholar]
- 17.Kazemi F.A., Kettaneh G.N., Kontchou E., et al. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol. 2006;45:230–235. doi: 10.1016/j.jhep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Calvaruso V., Bronte F., Conte E., Simone F., Craxì, et al. Modified spleen stiffness measurement by transient elastography is associated with presence of large oesophageal varices in patients with compensated hepatitis C virus cirrhosis. J Viral Hepat. 2013;20(12):867–874. doi: 10.1111/jvh.12114. [DOI] [PubMed] [Google Scholar]
- 19.Karatzas A., Konstantakis C., Aggeletopoulou I., Kalogeropoulou C., Thomopoulos K., Triantos C. Νon-invasive screening for esophageal varices in patients with liver cirrhosis. Ann Gastroenterol. 2018;31(3):305–314. doi: 10.20524/aog.2018.0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li T., Qu Y., Yang B., Xue Y., Wang L. Evaluation of large esophageal varices in cirrhotic patients by transient elastography:a meta-analysis. Rev Esp Enferm Dig. 2016;108:464–472. doi: 10.17235/reed.2016.3980/2015. [DOI] [PubMed] [Google Scholar]
- 21.Jia-Li Ma, Ling-Ling He, Yu Jiang, et al. New model predicting gastroesophageal varices and variceal hemorrhage in patients with chronic liver disease. Ann Hepatol. 2020;19(3):287–294. doi: 10.1016/j.aohep.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Sebastiani G., Tempesta D., Fattovich G., et al. Prediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: results of a multicenter, large-scale study. J Hepatol. 2010;53:630–638. doi: 10.1016/j.jhep.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Thomopoulos K.C., Labropoulou-Karatza C., Mimidis K.P., Katsakoulis E.C., et al. Non-invasive predictors of the presence of large oesophageal varices in patients with cirrhosis. Dig Liver Dis. 2003;35(7):473–478. doi: 10.1016/s1590-8658(03)00219-6. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Tsao G., Escorsell A., Zakko M., et al. Predicting the presence of significant portal hypertension and varices in compensated cirrhotic patients. Hepatology. 1997;26:360A. [Google Scholar]
- 25.Pilette C., Oberti F., Aubé C., et al. Non-invasive diagnosis of esophageal varices in chronic liver diseases. J Hepatol. 1999;31(5):867. doi: 10.1016/s0168-8278(99)80288-8. [DOI] [PubMed] [Google Scholar]
- 26.Sedrak H., Khalifa R., Elkafrawy A., Elewa H. Noninvasive predictors of large esophageal varices: is there an emerging role of aspartate aminotransferase-to-platelet ratio index in hepatocellular carcinoma? Egypt J Intern Med. 2015;27:139–146. [Google Scholar]
- 27.Tarantino G., Citro V., Esposito P., Giaquinto S., de Leone A., et al. Blood ammonia levels in liver cirrhosis: a clue for the presence of portosystemic collateral veins. BMC Gastroenterol. 2009;9:21. doi: 10.1186/1471-230X-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaman A., Hapke R., Flora K., Rosen H.R., Benner K. Factors predicting the presence of esophageal or gastric varices in patients with advanced liver disease. Am J Gastroenterol. 1999;94:3292–3296. doi: 10.1111/j.1572-0241.1999.01540.x. [DOI] [PubMed] [Google Scholar]
- 29.Qamar A.A., Grace N.D., Groszmann R.J., Garcia-Tsao G., Bosch J., et al. Platelet count is not a predictor of the presence or development of Gastroesophageal varices in cirrhosis. Hepatology. 2000;47:153–159. doi: 10.1002/hep.21941. [DOI] [PubMed] [Google Scholar]