Abstract
Pituitary stalk interruption syndrome (PSIS) is a rare disorder characterized by the imaging triad of thinned or absent pituitary stalk, ectopic posterior pituitary lobe, and hypoplastic or absent anterior lobe. Patients typically present with 1 or more anterior pituitary lobe hormone deficits, most commonly growth hormone or gonadotropin, but patients may achieve normal stature and secondary sexual characteristics. Here, we present a case of a young female patient presenting with amenorrhea, normal stature, and sexual development, and an imaging triad of PSIS. To our knowledge, this is the first case of PSIS to present with hyperprolactinemia and an otherwise normal pituitary hormone profile.
Keywords: Pituitary, Pituitary stalk interruption syndrome, Amenorrhea, Prolactin, Genetic, Migration anomaly
Introduction
Pituitary stalk interruption syndrome (PSIS) is an uncommon congenital abnormality with uncertain cause. Diagnostic imaging criteria for diagnosis include a triad of, a thin or interrupted pituitary stalk, aplasia, or hypoplasia of the anterior pituitary lobe, and absent or ectopic posterior pituitary (neurohypophysis) [1]. Most patients present at or around puberty with delayed growth/short stature and absence of secondary sexual characteristics. Here, we present a case of a 20-year-old female with normal stature and secondary sexual characteristics and with a chief complaint of amenorrhea.
Case report
A 17-year-old female presented to the women's health clinic with a persistent complaint of amenorrhea. At this time, her prolactin levels were elevated >35 ng/mL on 2 separate occasions (reference range 5-23). All additional labs including thyroid function tests, and gonadotrophic hormones were within normal range. A noncontrast-enhanced MRI performed at this time indicated that the neurohypophysis (pituitary bright spot) was not well seen and a recommendation for a contrast-enhanced sella MRI was made to further evaluate this finding.
She was, however, lost to follow-up and presented with similar complaints approximately 3 years later at age 20. Her physical examination was unrevealing. She was of normal adult height and weight. Laboratory findings revealed an elevated prolactin of 33 ng/mL (reference range 5-23 ng/mL). The hormone panel showed: Follitropin 6.2; Lutropin, 7.8 mIU/mL; and TSH 2.36 mcIU/mL, all within normal limits, see Table 1 for reference ranges. Because she was of normal adult height, Growth Hormone (GH) was not assessed. Inhibin and anti-Mullerian hormone panels were not performed. An MRI was reordered to evaluate for possible prolactin-secreting microadenoma.
Table 1.
Table of clinical laboratory values showing “Test” performed, Normal range and Lab Value with units provided.
| Test | Normal range | Lab value |
|---|---|---|
| FSH | 1.7 – 21.5 | 6.2 mIU/L |
| LH | 1.0 – 95.6 | 7.8 IU/L |
| Prolactin | 4.8 – 23.3 | 33.0 ng/ml |
| TSH | 0.45 – 5.33 | 2.36 mU/L |
| Glucose | 70 - 120 | 88 mg/dL |
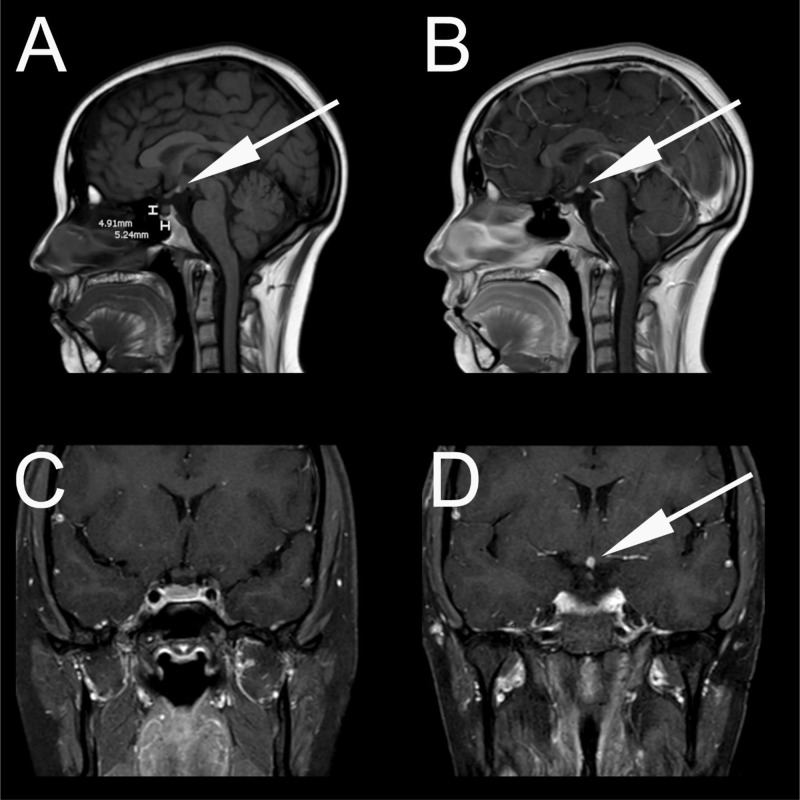
Contrast enhanced thin-slice MRI of the sella was performed in both coronal and sagittal planes (1.5T Intera; Philips Medical Systems, Best, The Netherlands; 3 mm slice thickness) which was unrevealing for pituitary microadenoma. However, the neurohypophysis was not visualized in the dorsal sella on sagittal non-contrast T1 weighted MRI (Fig. 1A). A T1 hyperintense focus was seen at the level of the hypothalamus on sagittal noncontrast and contrast-enhanced T1 weight imaging (Figs. 1A and B). Sagittal T1 image (Fig. 1A), also showed hypoplasia of the anterior pituitary measuring 4.9 mm in height. Contrast-enhanced sagittal and coronal T1 weighted images show an absence of the pituitary stalk confirming a diagnosis of pituitary stalk interruption syndrome (PSIS) (Figs. 1B–D).
Fig. 1.
(A) Sagittal unenhanced T1 weighted MRI showing ectopic posterior pituitary at the level of the hypothalamus. Note that the anterior pituitary is mildly atrophic, measuring 4.9 mm craniocaudally. (B) Sagittal contrast-enhanced T1 weighted MRI confirming the posterior pituitary at level of the hypothalamus. No pituitary stalk was identified. (C) Coronal contrast enhanced T1 weighted MRI at the level of the optic chiasm showing absence of the pituitary stalk. (D) Coronal contrast-enhanced T1 weighted MRI at the level of the hypothalamus showing ectopic posterior pituitary. No pituitary stalk is visualized.
Discussion
Pituitary Stalk Interruption Syndrome (PSIS) is a rare type of congenital hypopituitarism with an incidence of 0.5/100,000 live births [2]. To better understand the underlying pathogenesis of this syndrome, a brief review of normal pituitary development is required. The posterior pituitary lobe (PPL) arises from the downward growth of the diencephalon. The PPL is formed by hypothalamic magnocellular neurons arising from the paraventricular and supraoptic nuclei which send their axons into the infundibulum and PPL (neurohypophysis) forming the hypothalamohypophyseal tract [3]. These neurons secrete antidiuretic hormone (ADH) and oxytocin directly into the systemic circulation, regulating blood pressure and milk production/secretion, respectively. This secretion is considered regulated in part by pituicytes, glial cells, that also reside in the PPL [4].
The anterior pituitary lobe (adenohypophysis), on the other hand, is comprised of 5 types of endocrine cells which are indirectly regulated by the hypothalamus. These regulating hormones are secreted by the hypothalamus into the median eminence and regulate the release of 5 neuroendocrine hormones [5,6]. Only 1 anterior pituitary hormone, prolactin, is solely under inhibitory control (mediated by dopamine). Growth hormone, in contrast, has both releasing and inhibiting factors (somatostatin inhibits). The remainder of the anterior pituitary hormones are regulated by stimulating hormones that trigger from the anterior pituitary, for example, gonadotropin releasing hormone.
In PSIS, the axonal extension of neurons from the hypothalamus to the posterior sella fails to occur. In this setting, the neurohypophysis may be ectopic or absent and the infundibulum or stalk hypoplastic or absent. The adenohypophysis is also said to be atrophic but can also be absent. The first cases of PSIS were described in 1987 by Fujisawa et al. [7], who described the clinical manifestations and imaging findings of 10 patients with pituitary stalk transection. Despite having an ectopic location of the posterior pituitary lobe, patients typically have normal levels of antidiuretic hormone and oxytocin. However, because there is no pituitary stalk, releasing factors arising from the hypothalamus are not able to communicate with the anterior pituitary lobe, resulting typically in low growth hormone and gonadotropins among others. As a result, the clinical presentation of PSIS typically presents with short stature and hypogonadism and is typically diagnosed around puberty, although cases of variable presentation and time of onset have been previously described [2]. Paradoxically, it has been demonstrated that in patients with surgical transection of the stalk, there is a concomitant elevation of prolactin [8]. Indeed, PSIS may result in amenorrhea in adolescent and young adult females despite having undergone normal pubertal development [9].
The diagnosis of PSIS is made with a combination of physical exam findings, laboratory testing, and magnetic resonance imaging. In the case described above, our patient was of normal stature for an adult female with an elevation of prolactin. The remainder of her pituitary hormone profile to include anterior pituitary hormone was normal. In PSIS, there is typically at least 1 anterior pituitary deficiency, usually growth hormone, with deficiencies in thyroid hormone and gonadotrophic hormones also being common [10].
While there are several causes of hyperprolactinemia in girls and young women, the most common cause, once medications and drugs are excluded is prolactinoma [11]. MRI plays a confirmatory role in this diagnosis and can differentiate between pituitary adenoma and PSIS. In PSIS, a characteristic triad of thin or interrupted pituitary stalk, aplasia, or hypoplasia of the anterior pituitary lobe, and absent or ectopic posterior pituitary (neurohypophysis) may be seen [12]. When evaluating the sella and pituitary for PSIS, the normal stalk thickness should be 2 mm with less than 1 mm being considered thinned, while normal adenohypophysis should measure not more than 10 mm in height in the menstruating female [13]. The average anterior pituitary height for a 20-year-old female is 6.5 mm (our patient measured 4.9 mm) in keeping with the imaging triad of PSIS [14]. The imaging triad does not necessarily need to be complete in order to make a diagnosis of PSIS. Additionally, ophthalmic abnormalities are relatively common in PSIS and are seen in up to 30% of cases, which was not seen in our patient [15]. Additional imaging that could further improve diagnosis include thin-slice T2 weighted imaging (FIESTA sequence) or 3Dthin-slice T1 post contrast imaging. FIESTA and 3D postcontrast T1 imaging are not a routine part of sella imaging but in hindsight, this may give a better evaluation of the pituitary stalk in select cases.
The majority of PSIS cases are sporadic with only about 5% being related to genetic causes [10,15]. Since the initial characterization of PSIS, there have been numerous genes linked to hereditary forms of PSIS to include SOX3, HEX1, LHX4, and many others [16], [17]–18]. In the genetic causes of PSIS, the multiplicity of genes involved is thought to account for the heterogeneity in clinical presentation. Prior to genetic analysis, birth trauma, perinatal hypoxia, dystocia, and breech presentation were all postulated as possible causes of PSIS, however, PSIS were also seen in patients with a normal birth history arguing against these mechanisms as the sole possible causes.
Early hormone replacement is the mainstay of treatment in most cases of PSIS. This helps to ensure normal or near normal maturation in adult height and secondary sexual characteristics. Variations in response to treatment have been observed in patients with PSIS. In our case, since the hormone profile was normal apart from prolactin, a Depo-Provera shot followed by oral Annovera was started.
PSIS has a heterogeneous clinical, biological, and radiological presentation, most likely due, in part, to multiple genetic or antenatal/perinatal causes of PSIS that are not yet fully understood. PSIS manifests either as isolated or combined pituitary hormone deficiency with variable age at onset and varying degrees of clinical severity. In the past, the defining characteristic of PSIS was short stature (growth hormone deficiency) with variable degrees of additional anterior and/or posterior pituitary dysfunction. More recently, it has been discovered that not all patients with PSIS have short stature and abnormal secondary sexual characteristics. Indeed, patients may appear to develop as normally developed adults with only mild changes in the pituitary hormone profile. Our case is interesting and unique because the patient has the imaging triad of PSIS but only 1 posterior lobe hormone derangement, prolactin with resultant amenorrhea. To our knowledge, this combination of imaging, laboratory, and clinical findings has not yet been reported in the literature.
Patient consent
Written informed consent for the publication of this case was obtained from the patient.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Fatima T, Chandio SH, Muzzafar K, Mumtaz H, Jahan N. Pituitary stalk interruption syndrome. Cureus. 2020;12(9) doi: 10.7759/cureus.10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nawaz A, Azeemuddin M, Shahid J. Pituitary stalk interruption syndrome presenting in a euthyroid adult with short stature. Radiol Case Rep. 2018;13(2):503–506. doi: 10.1016/j.radcr.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris GW. Neural control of the pituitary gland. Physiol Rev. 1948;28(2):139–179. doi: 10.1152/physrev.1948.28.2.139. [DOI] [PubMed] [Google Scholar]
- 4.Hatton GI. Pituicytes, glia and control of terminal secretion. J Exp Biol. 1988;139:67–79. doi: 10.1242/jeb.139.1.67. [DOI] [PubMed] [Google Scholar]
- 5.Schally AV, Arimura A, Kastin AJ. Hypothalamic regulatory hormones. Science. 1973;179(4071):341–350. doi: 10.1126/science.179.4071.341. [DOI] [PubMed] [Google Scholar]
- 6.Burgus R, Guillemin R. Hypothalamic releasing factors. Annu Rev Biochem. 1970;39:499–526. doi: 10.1146/annurev.bi.39.070170.002435. [DOI] [PubMed] [Google Scholar]
- 7.Fujisawa I, Kikuchi K, Nishimura K, Togashi K, Itoh K, Noma S, et al. Transection of the pituitary stalk: development of an ectopic posterior lobe assessed with MR imaging. Radiology. 1987;165(2):487–489. doi: 10.1148/radiology.165.2.3659371. [DOI] [PubMed] [Google Scholar]
- 8.Turkington RW, Underwood LE, Van Wyk JJ. Elevated serum prolactin levels after pituitary-stalk section in man. N Engl J Med. 1971;285(13):707–710. doi: 10.1056/NEJM197109232851302. [DOI] [PubMed] [Google Scholar]
- 9.Corvest V, Lemaire P, Brailly-Tabard S, Brauner R. Puberty and inhibin B in 35 adolescents with pituitary stalk interruption syndrome. Front Pediatr. 2020;8:304. doi: 10.3389/fped.2020.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynaud R, Abarel F, Saveneau A, Kaffel N, Castinetti F, LeComte P, et al. Pituitary stalk interruption syndrome in 83 patients: novel HESX1 mutation and severe hormonal prognosis in malformative forms. Eur J Endocrinol. 2011;164(4):457–465. doi: 10.1530/EJE-10-0892. [DOI] [PubMed] [Google Scholar]
- 11.Mancini T, Casanueva FF, Giustina A. Hyperprolactinemia and prolactinomas. Endocrinol Metab Clin North Am. 2008;37(1):67–99. doi: 10.1016/j.ecl.2007.10.013. viii. [DOI] [PubMed] [Google Scholar]
- 12.Argyropoulou M, Perignon F, Brauner R, Brunelle F. Magnetic resonance imaging in the diagnosis of growth hormone deficiency. J Pediatr. 1992;120(6):886–891. doi: 10.1016/s0022-3476(05)81955-9. [DOI] [PubMed] [Google Scholar]
- 13.Elster AD. Imaging of the sella: anatomy and pathology. Semin Ultrasound CT MR. 1993;14(3):182–194. doi: 10.1016/s0887-2171(05)80079-4. [DOI] [PubMed] [Google Scholar]
- 14.Tsunoda A, Okuda O, Sato K. MR height of the pituitary gland as a function of age and sex: especially physiological hypertrophy in adolescence and in climacterium. AJNR Am J Neuroradiol. 1997;18(3):551–554. [PMC free article] [PubMed] [Google Scholar]
- 15.Brauner R, Bignon-Topalovic J, Bashamboo A, McElreavey K. Pituitary stalk interruption syndrome is characterized by genetic heterogeneity. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0242358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods KS, Cundall M, Turton J, Rizotti K, Mehta A, Palmer R, et al. Over- and underdosage of SOX3 is associated with infundibular hypoplasia and hypopituitarism. Am J Hum Genet. 2005;76(5):833–849. doi: 10.1086/430134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machinis K, Pantel J, Netchine I, Leger J, Camand OJ, Sobrier ML, et al. Syndromic short stature in patients with a germline mutation in the LIM homeobox LHX4. Am J Hum Genet. 2001;69(5):961–968. doi: 10.1086/323764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melo ME, Marui S, Carvalho LR, Arnhold IJP, Leite CC, Mendonca BB, et al. Hormonal, pituitary magnetic resonance, LHX4 and HESX1 evaluation in patients with hypopituitarism and ectopic posterior pituitary lobe. Clin Endocrinol (Oxf) 2007;66(1):95–102. doi: 10.1111/j.1365-2265.2006.02692.x. [DOI] [PubMed] [Google Scholar]