Abstract
Chronic wounds are wounds that cannot heal properly due to various factors, such as underlying diseases, infection or reinjury, and improper healing of skin wounds and ulcers can cause a serious economic burden. Numerous studies have shown that extracellular vesicles (EVs) derived from stem/progenitor cells promote wound healing, reduce scar formation and have significant advantages over traditional treatment methods. EVs are membranous particles that carry various bioactive molecules from their cellular origins, such as cytokines, nucleic acids, enzymes, lipids and proteins. EVs can mediate cell-to-cell communication and modulate various physiological processes, such as cell differentiation, angiogenesis, immune response and tissue remodelling. In this review, we summarize the recent advances in EV-based wound healing, focusing on the signalling pathways that are regulated by EVs and their cargos. We discuss how EVs derived from different types of stem/progenitor cells can promote wound healing and reduce scar formation by modulating the Wnt/β-catenin, phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin, vascular endothelial growth factor, transforming growth factor β and JAK–STAT pathways. Moreover, we also highlight the challenges and opportunities for engineering or modifying EVs to enhance their efficacy and specificity for wound healing.
Keywords: Extracellular vesicles, Refractory wound, Stem/progenitor cells, Scar formation, Engineered extracellular vesicles, Wound healing, Scar, Signalling pathway
Graphical Abstract
Graphical Abstract.
Highlights.
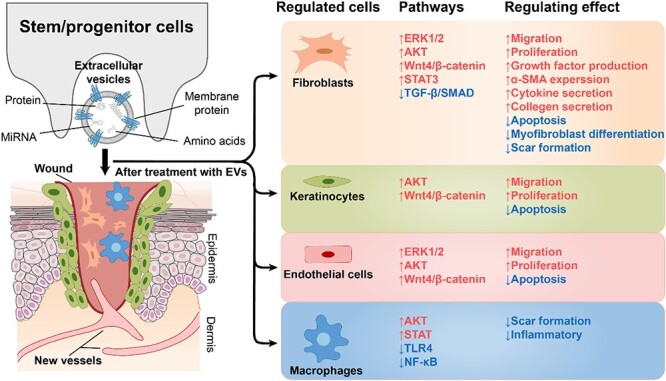
EVs derived from stem/progenitor cells can enhance wound healing and reduce scar formation by modulating various signalling pathways and inflammatory mediators.
EVs modulate various signalling pathways, such as Wnt/β-catenin, PI3K/AKT/mTOR and VEGF, to regulate inflammation, angiogenesis, cell proliferation and migration, and extracellular matrix remodelling.
Engineered EVs can optimize their therapeutic potential and target specific aspects of wound healing.
Background
Wound healing refers to the healing process of skin and other tissues after being broken or damaged by external forces, and includes the complex combination of regeneration of various tissues, granulation tissue hyperplasia and scar tissue formation [1, 2]. Wound care is one of the fastest growing segments of the modern health care market, with the global wound care market valued at US $17.49 billion in 2021. The market is projected to grow from USD 18.51 Billion in 2022 to USD 28.23 billion in 2029, progressing at a compound annual growth rate (CAGR) of 6.2% during the forecast period. The types of wounds have also changed. Wounds can be divided into acute or chronic wounds [3, 4]. At present, refractory chronic wounds have surpassed acute wounds, and have become a widespread medical burden worldwide, bringing pain, psychological pressure and loss of quality of life to millions of people [5]. The common clinical chronic wounds mainly manifest as pressure ulcers, diabetic foot ulcers and venous ulcers of the lower limbs. The global epidemic of chronic underlying diseases, such as ageing, heart disease and intractable infections, contributes to the development of chronic wounds [6, 7]. Traditional treatment options for chronic wound healing are now divided into nonsurgical and surgical treatments. Nonsurgical treatments include physical therapy, negative pressure wound therapy, gene therapy, new surgical dressing and application of growth factors [8–13]. Refractory wounds have multiple aetiologies and pathologies, but the clinical symptoms are quite similar, making diagnosis and proper treatment difficult [14]. How to prevent scar formation caused by refractory wounds is also a tricky problem and new treatment options are needed [15].
Extracellular vesicles (EVs) can be used in the diagnosis and treatment of many wounds and diseases and are a promising new therapy [16]. EVs are 30 nm–10 μm membranous particles that can be derived from almost all cell types, and that transfer various bioactive molecules between cells, thereby mediating a variety of physiological processes, such as cell differentiation and proliferation, blood vessel formation, stress response and immune response [17]. EVs are natural, nanotransport particles with biocompatibility, circulatory stability, low toxicity and low immunogenicity, so they can be used as efficient carriers of molecular cargo and are ideal therapeutic candidates for regenerative medicine [18]. In addition, compared with synthetic transporters, EVs are more stable, simpler to produce and lower in cost. Several studies have demonstrated that stem cell therapy has a significant effect in the treatment of trauma [19]. Recently, it has been found that stem cells exert their effects mainly through EVs, and EVs can bypass the potential tumourigenic risk of stem cells, adverse immune response and poor survival of stem cells in wounds [20]. In other words, EVs are more promising because they have better therapeutic effects than stem cells and can avoid many disadvantages of cell therapy. EVs derived from stem/progenitor cells have shown great potential in the treatment of refractory wounds and have been investigated for skin beauty treatment, relief of chronic skin wounds caused by diabetes and bedsores [21, 22]. There are even teams investigating the efficacy of EVs for many diseases, such as epidermolysis bullosa, dry eye, cardiovascular diseases, cancer, neurodegenerative diseases and COVID-19, showing the powerful generalizability of the therapeutic effects of EVs [23].
An engineered EV is a type of EV that has been modified or enhanced to improve its therapeutic potential for wound healing. Engineered EVs can be derived from different cell sources and loaded with various molecules, including growth factors, cytokines or drugs, to target specific signalling pathways or processes in wound healing. The use of engineered EVs in wound treatment is an emerging and promising field that has the potential to overcome some of the limitations of natural EVs or other regenerative therapies. More research is needed to optimize the engineering methods, characterization techniques, standardization protocols, delivery systems and safety assessments of engineered EVs for clinical applications. In this review, we discuss how EVs derived from different sources can regulate the Wnt/β-catenin, phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR), vascular endothelial growth factor (VEGF), transforming growth factor (TGF)-β and JAK–STAT signalling pathways to promote wound healing and reduce scar formation in refractory wounds. We also highlight the recent advances in engineering and modifying EVs to control their biotherapeutic performance and enhance their application prospects.
Review
Wound healing processes and challenges
Four phases of wound healing
(1) Haemostasis phase: within seconds of trauma, the blood and the fibrous proteins in the wound are quickly solidified into a clot, some of which dry to form scabs, and the clots and scabs play a role in the protection of the wound, preventing further blood loss and pathogen invasion [24]. Platelets secrete a variety of growth factors, such as fibroblast growth factor (FGF), platelet-derived growth factor and TGF, which recruit inflammatory cells to the wound, triggering the inflammatory phase of wound healing [25].
(2) Inflammatory phase: inflammation often occurs 24–36 h after injury, manifested as congestion, serous exudation and leukocyte swim out, resulting in local redness and swelling [26]. The main leukocytes involved are neutrophils, which change to macrophages after 3 days [27]. Neutrophils infiltrate the wound and secrete proteases and antimicrobials (such as reactive oxygen species) to remove damaged extracellular matrix (ECM) and pathogens [28, 29]. Released products such as nuclear factor-κB (NF-κB) and Toll-like receptor (TLR), which are required for cytokine transcription and pathogen recognition, respectively, attract macrophages and lymphocytes to the wound and activate them [30, 31]. Macrophages can initiate or end inflammation through phenotypic transformation. In the early stage of inflammation, classically activated macrophages (M1-type) are recruited at the wound site, expressing a broad spectrum of proinflammatory cytokines, such as interleukin-1β (IL-1β) [32]. In the later stage of inflammation, macrophages change to the M2 type, which mediates anti-inflammatory effects. By activating keratinocytes, fibroblasts and endothelial cells, M2-type macrophages promote tissue regeneration and initiate the proliferative phase of wound healing [33].
(3) Proliferation phase: this phase mainly depends on the activity of keratinocytes and fibroblasts to form new tissue and blood vessels in the wound area [34]. Keratinocytes are the main proliferating cells of the epidermis and can differentiate and replace ageing epidermal cells under physiological conditions [35]. The process of re-epithelialization of a wound begins with the proliferation of keratinocytes at the edge of the wound [36]. Fibroblasts also play a significant role, initiating extensive proliferation to secrete a variety of substances to form new ECM, including collagen type-III matrix, mucins, fibronectin and proteoglycans (such as hyaluronic acid) [37, 38]. The newly formed ECM promotes the formation of granulation tissue, and the damaged ECM is degraded by several enzymes, including matrix metalloenzymes (MMPs) and plasminogen activators [39–41]. Granulation tissue is composed mainly of fibroblasts, new blood vessels and type-III collagen, which will mature to form a scar [1]. Fibroblasts can also differentiate into α-smooth muscle actin (α-SMA)-rich myofibroblasts, which have a contractile function and pull the edges of the wound together [40]. Increased levels of growth factors such as VEGF, hepatocyte growth factor and FGF stimulate the intact blood vessels around the wound and degrade the partial basement membrane of blood vessels, thus making the vascular endothelial cells in this area proliferate and migrate to rebuild new blood vessels [42–45].
(4) Reconstruction phase: the delicate process of scar tissue remodelling, which involves the reconstruction of new blood vessels, cells and tissue, can take months or years to manifest itself, much longer than other stages of wound healing [46, 47]. Collagen decomposes, and type-III collagen, which constitutes immature ECM, is replaced by stronger mature type-I collagen, leading to ECM structure adjustment and wound-thickness reduction [48, 49]. Most new cells undergo apoptosis, leading to a decrease in dermal cells and blood vessel density [50]. Most of the newly formed capillaries recede, and a small part rebuilds to form a new network of blood vessels [51]. The connective tissue underneath the wound continues to contract, pulling the edges closer together [52].
Chronic wound aetiology and pathology
Chronic wounds fail to achieve anatomical and functional integrity through a normal, orderly and timely process of repair [53]. Clinically, the term mostly refers to wounds that cannot heal for more than 1 month and have no tendency to heal [54]. Here, the limit of 1 month is not absolute [55]. However, due to the complex aetiology of chronic wounds, it is difficult to define them simply, so there is no unified definition of chronic trauma at present [56]. The correct sequence, timing and regulation of each of the four phases of wound healing are critical, and any adverse effects of disease or complications can lead to chronic ulceration where the wound does not heal properly and enters a persistent state of inflammation [57]. Chronic wounds usually encompass venous insufficiency ulcers, peripheral arterial ulcers, diabetic ulcers, pressure ulcers, infectious ulcers, neoplastic ulcers and refractory wounds caused by connective tissue diseases such as leprosy [58–60]. The aetiology of chronic wounds is complex and various mechanisms have been proposed in existing research. (1) At the cellular level, increased levels of proinflammatory factors secreted by neutrophils make matrix metalloproteinases hyperactive, promote ECM breakdown and impair cell migration [61, 62]. Dysfunction of the transformation of fibroblasts into myofibroblasts leads to fibrous disease, hypertrophic scars and keloid formation [63]. The formation of scars on the skin, especially on the face, neck and hands, will increase the patient’s psychological stress [64]. (2) Underlying diseases, such as diabetes can lead to dysregulation of insulin levels, and excessive blood glucose can hinder macrophage polarization, resulting in macrophages maintaining a proinflammatory (M1-type) state and causing inflammation and cellular stress, which are difficult to terminate [65]. Moreover, due to the lack of polarization, macrophages cannot be transformed into the M2 type, resulting in stagnation of the healing process in the inflammatory phase and difficulty in entering the proliferative phase, making diabetic patients more prone to chronic skin injury [66]. Dehydration and malnutrition can also greatly affect cell proliferation and angiogenesis. Ageing and heart disease are common underlying conditions leading to the development of chronic wounds in an ageing society [67]. Loss of certain types of collagen, such as epidermolysis bullosa due to loss of collagen type VII, can lead to weak skin that is prone to blisters and nonhealing ulcers that must be remedied by artificial treatments [68]. (3) Reinjury or infection is a problem, and if there is persistent pathogen infection or wound reinjury at the wound site, this will hurt new cells and ECM in the process of proliferation, which easily leads to serious scar formation [69].
EVs as therapeutic agents
Definition, origin, composition, structure and function of EVs
EVs contain three main isoforms, i.e. exosomes, ectosomes and apoptotic bodies [70]. In fact, there is still research constantly refining the categories of EVs. Therefore, the EVs mentioned in many articles are generally understood as generalized EVs. To avoid confusion, EVs are used to refer to all vesicles secreted by cells in this review. EVs are formed by fusion of multivesicular bodies (MVBs) with plasma membranes. During endocytosis, the cell membrane buds inwards to form endosomes, which then bud inwards to form MVBs [71]. MVBs are assembled by either an endosomal sorting complex required for transport (ESCRT)-independent transport pathway or an ESCRT-dependent endosomal sorting complex [72]. In the ESCRT-independent pathway, sphingomyelin (present in endosomal membranes within lipid rafts) is converted into ceramide by sphingomyelinase [73]. Ceramide binds to form microdomains that drive intraluminal vesicles (ILVs) to form MVBs [74]. The ESCRT-dependent pathway requires ESCRT-protein complexes (specifically ESCRT-0, ESCRT-I, ESCRT-II and ESCRT-III) and related proteins [such as Vps4, Vta1 and programmed cell death 6 interacting protein (ALIX)] that contribute to ILV production [66, 75]. ESCRTs works together to twist the endosomal membrane and selectively transport the components that form ILVs. ESCRT-III component proteins are aggregated by ESCRT-II or ALIX, and the polymerization of these components results in neck contraction and cleavage to form ILVs [76]. Vsp4, an ATPase, dismantles ESCRTs and allows them to be recycled [76].
Farnesyltransferase is required for Ras protein activation, and Ras proteins and their downstream effectors, including Raf and the extracellular signal-regulating kinases RhoA and ADP-ribosylation factors 1 and 6 (ARF1 and ARF6), increase myosin contractility by phosphorylating myosin light chains, thereby encouraging MVB release [66, 77, 78]. Some of the formed MVBs bind to lysosomes and are degraded by the contained hydrolases [79]. Other MVBs do not fuse with lysosomes and are transported to the cell membrane with the help of cytoskeletal actin and microtubules [79]. This process is regulated by several proteins, such as the Rab protein, and cholesterol can also affect this process [80]. The contents of EVs are quite abundant, including various proteins, enzymes, transcription factors, lipids, ECM proteins, receptors, metabolites, nucleic acids and especially a wide variety of microRNAs (miRNAs) [81]. These contained bioactive molecules enable them to perform functions ranging from targeting/adhesion to metabolism to inflammation regulation. Some of these proteins are specifically enriched in EVs and can be used as markers for EV identification or isolation, such as ALIX, tetramer CD9, CD63, CD81, CD82 and tumor susceptibility gene 101 [82] (Figure 1). Exocarta (http://exocarta.org/index.html); The Urinary EV Protein Database (http://dir.nhlbi.nih.gov/papers/lkem/exosome/); and Vesiclepedia (http://www.microvesicles.org/) [83].
Figure 1.
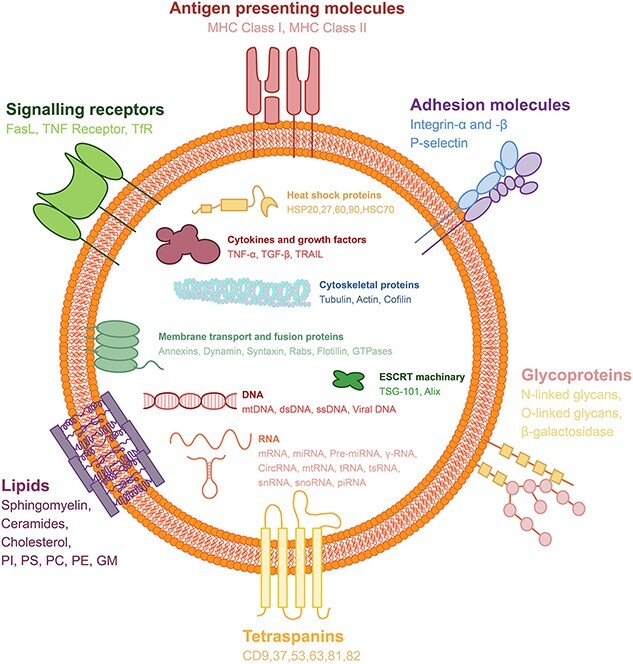
Schematic diagram of cellular EVs, their surface molecules and their contents. The surface of EVs is decorated with different types of molecules that play important roles in their biogenesis, trafficking and function. These molecules include: (1) antigen-presenting molecules, such as major histocompatibility complex (MHC) class I and II, which are involved in immune recognition and modulation; (2) adhesion molecules, such as integrin-α and β, and P-selectin, which mediate EV binding and uptake by target cells; (3) signalling receptors, such as Fas ligand (FasL), tumor necrosis factor (TNF) receptor and transferrin receptor (TfR), which trigger intracellular signalling pathways upon EV interaction; (4) glycoproteins, such as N-linked glycans, O-linked glycans and beta-galactosidase, which modulate EV stability, solubility and bioactivity; (5) lipids, such as sphingomyelin, ceramides, cholesterol, phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE) and ganglioside GM1, which affect EV membrane fluidity, curvature and charge; and (6) tetraspanins, such as CD9, CD37, CD53, CD63, CD81 and CD82, which are the most abundant and characteristic proteins on EV surfaces and participate in EV formation, sorting and targeting. The contents of EVs are enriched with different types of molecules that reflect the origin and function of the EV. These molecules include: (1) heat shock proteins, such as HSP20, HSP27, HSP60, HSP90 and HSC70, which are involved in protein folding, stability and protection from stress; (2) cytokines and growth factors, such as tumor necrosis factor-alpha (TNF-α), transforming growth factor-beta (TGF-β) and TNF-related apoptosis-inducing ligand (TRAIL), which modulate immune responses, inflammation and cell survival; (3) cytoskeletal proteins, such as tubulin, actin and cofilin, which regulate EV shape, size and release; (4) membrane transport and fusion proteins, such as annexins, dynamin, syntaxin, Rabs, flotillin and GTPases, which facilitate EV budding, trafficking and fusion with target membranes; (5) endosomal sorting complex required for transport (ESCRT) machinery, such as tumor susceptibility gene 101 (TSG-101) and ALIX, which mediate EV formation and sorting of cargo molecules; (6) DNA, such as mitochondrial DNA (mtDNA), double-stranded DNA (dsDNA), single-stranded DNA (ssDNA) and viral DNA, which can transfer genetic information and induce immune responses; and (7) RNA, such as messenger RNA (mRNA), microRNA (miRNA), precursor miRNA (pre-miRNA), gamma-RNA (γ-RNA), circular RNA (circRNA), mitochondrial RNA (mtRNA), transfer RNA (tRNA), transfer strand RNA (tsRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA) and piwi-interacting RNA (piRNA), which can regulate gene expression and cellular functions in target cells. EVs extracellular vesicles
Mechanisms of EV cargo selection
Research has shown that the type and content of EVs are highly dependent on specific conditions [84]. Stressors, drug treatments or the type of stem cells from which they are derived can significantly alter EV yield, size and composition, which are dependent on EV cargo sorting mechanisms, including ESCRT, tetraspanins and lipid-dependent mechanisms [80, 83]. For instance, mesenchymal stem cells (MSCs) cultured under serum-free priming conditions or hypoxia (1% O2) generate EVs rich in specific metabolites such as cholesterol, arginine, aspartic acid, adenosine, palmitate and isoleucine [85]. These molecules have anti-inflammatory activity, promote M2-macrophage polarization and regulate lymphocyte function [86]. The MSC-EVs obtained under these conditions can further reduce the formation of scars compared with those obtained under general conditions. As another example, treatment of MSCs with the stressor lipopolysaccharide induced the stem cells to be in an inflammatory state [87]. The resulting MSC-EVs after this treatment are found to contain higher levels of anti-inflammatory signalling factors such as IL-10, TGF-β and cyclooxygenase-2 (COX-2) (important in the bioproduction of prostaglandin E2) and are shown to be more effective in the treatment of chronic trauma [87]. An attractive research project is achieving the artificial regulation of EV generation and content [88]. The molecular composition of EVs is derived from cells, but differs from cells in that EVs contain a variety of specific proteins, lipids and RNAs and do not contain some of those present in the cytoplasm [89, 90]. This suggests that specific sorting mechanisms exist to control the entry of specific molecules into EVs. Research has shown that the sorting of specific proteins and RNA in outer secreted bodies is controlled in a variety of ways, although most ways are not fully understood. Through existing sorting methods, we can manipulate the contents of EVs according to different purposes to achieve a better therapeutic effect with fewer side effects.
EV-mediated modulation of wound healing
Commonly used EVs derived from various stem cells and their rich inclusions can be involved in different phases of wound healing. The therapeutic effect of EVs is mainly achieved by surface proteins and contents, including a variety of cytokines and miRNAs, and they play a key role in different phases of the wound healing process by regulating multiple signalling pathways and inflammatory mediators [23, 91]. The proliferation and migration of endothelial cells, angiogenesis and maturation, and the development of hair follicles and sebaceous glands are observed after EVs are injected into the wound [92–94]. EVs can also enhance the proliferation and migration of fibroblasts in a dose-dependent manner and increase collagen deposition [21]. Importantly, in general, the stronger the collagen synthesis ability of fibroblasts at the wound site, the more likely scar formation is to occur, which affects the skin appearance and is detrimental to the normal function of the skin, increasing the psychological burden of patients [95]. However, EVs can promote the proliferation and secretion of fibroblasts at the beginning of wound healing, inhibit the deposition of collagen at the end of healing and mediate the structural remodelling of newly formed tissue, achieving the ideal therapeutic effect of both promoting wound healing and inhibiting scar formation [96, 97]. Therefore, EV therapy has significant advantages and potential over traditional methods.
The sequence of wound healing steps is strict and explicit, and numerous signalling pathways are involved. Different pathways focus on different functions. For instance, Wnt/β-catenin has a variety of pathways to regulate collagen deposition; the PI3K/AKT/mTOR pathway is more inclined to activate endothelial cell, keratinocyte and fibroblast functions; the VEGF pathway focuses on promoting angiogenesis; and the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and JAK–STAT pathways focus on inhibiting excessive inflammatory responses in wounds [98–102]. The TGF-β pathway is mainly activated in the second phase of wound healing and has a strong ability to promote inflammation and collagen deposition [103]. In addition, detection of the degree of activation of different signalling pathways can be helpful in refining the classification of wounds and selecting a more ideal treatment plan. For instance, kinetic analysis of AKT, STAT-3, ERK1/2, Wnt, and β-catenin in cells at the site of injury can be performed to determine more appropriate targeted treatment.
Modulation of Wnt/β-catenin pathway: a dual regulator of wound healing and scar formation
The Wnt/β-catenin signalling pathway is a highly conserved pathway that regulates various aspects of development, stem cell maintenance and wound healing [104]. The activation of this pathway involves the binding of Wnt ligands to their receptors, which inhibits the degradation of β-catenin and allows it to translocate to the nucleus and activate Wnt-responsive genes [104]. EVs, which are membrane-bound particles released by various cell types, can modulate the Wnt/β-catenin pathway in wound healing by delivering Wnt ligands or other factors that affect β-catenin stability or activity [105]. EVs can act as both positive and negative regulators of the Wnt/β-catenin pathway depending on the stage of wound healing and the source of EVs [106, 107]. In this section, we will review how EVs derived from different stem/progenitor cells can influence the Wnt/β-catenin pathway in wound healing and discuss the potential applications and challenges of EV-based therapy for wound healing (Figure 2).
Figure 2.
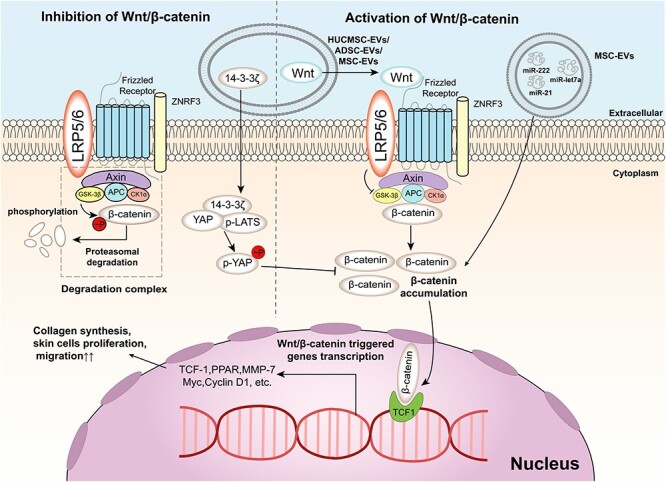
Wnt/β-catenin signalling pathway and its regulation by EVs in wound healing. The Wnt/β-catenin signalling pathway is a key pathway that regulates cell proliferation, differentiation and survival. The pathway is activated by the binding of Wnt ligands to Frizzled receptors and LRP5/6 co-receptors on the cell membrane, which inhibits the degradation of β-catenin by the destruction complex composed of Axin, APC and GSK3β. The stabilized β-catenin translocates to the nucleus and interacts with TCF1 to activate the transcription of target genes, such as MMP-7, Myc and Cyclin D1. EVs are small membrane-bound vesicles that can modulate the Wnt/β-catenin signalling pathway by delivering various molecules to target cells. For example, EVs can carry Wnt ligands or 14–3-3ζ protein to activate or inhibit the pathway, respectively. EVs can also deliver microRNAs (miRNAs) such as miR-let7a, miR-21 and miR-222 to activate the pathway by targeting its different components. EVs can also affect the crosstalk between the Wnt/β-catenin signalling pathway and other pathways, such as the Hippo/YAP pathway. The Hippo/YAP pathway is a negative regulator of the Wnt/β-catenin signalling pathway by phosphorylating yes-associated protein (YAP) and preventing its interaction with β-catenin. EVs can deliver phosphorylated YAP (p-YAP) or phosphorylated large tumor suppressor kinaseLATS (p-LATS) to target cells and suppress the Wnt/β-catenin signalling pathway. The regulation of the Wnt/β-catenin signalling pathway by EVs is diverse and dynamic in wound healing. For example, in the early stage of wound healing, EVs contain a dominant Wnt effect, activating the Wnt/β-catenin signalling pathway to promote collagen synthesis, skin cell proliferation and migration. In the late stage of wound healing, 14–3-3ζ contained in EVs plays an important role in inhibiting the Wnt/β-catenin signalling pathway, preventing excessive secretion and deposition of collagen causing scar formation. EVs extracellular vesicles
In the early stage of wound healing, EVs derived from human umbilical cord mesenchymal stem cells (HUCMSC-EVs) or adipose mesenchymal stem cells can promote re-epithelialization, angiogenesis and tissue regeneration by delivering Wnt4 and activating the Wnt/β-catenin pathway in endothelial cells, keratinocytes and fibroblasts [108–110]. These EVs can increase the expression of genes related to cell proliferation, migration and differentiation, such as N-cadherin, collagen I, CK19 and proliferating cell nuclear antigen (PCNA), while decreasing the expression of E-cadherin, which is associated with cell–cell adhesion and epithelial integrity [111, 112]. These effects can be reversed by treatment with a β-catenin inhibitor (ICG001), indicating that the therapeutic effect of EVs is mediated by the Wnt/β-catenin pathway [113]. In vivo experiments also demonstrated that adipose mesenchymal stem cell-derived EVs can home to the site of skin incision and significantly promote skin wound healing in mice [114].
In contrast, in the late stage of wound healing, HUCMSC-EVs or endothelial progenitor cell-derived EVs (EPC-EVs) inhibit excessive cell proliferation and collagen deposition by delivering 14–3-3ζ protein, which regulates the phosphorylation of YAP and its interaction with phosphorylated-LATS, resulting in the inhibition of the Wnt/β-catenin pathway in skin cells [107, 115, 116]. YAP is a transcriptional coactivator that interacts with TCF family transcription factors and enhances Wnt-responsive gene expression. The phosphorylation of YAP by p-LATS leads to its cytoplasmic retention and degradation, thus inhibiting the Wnt/β-catenin pathway. By regulating YAP or other factors, EVs can fine-tune the activity of β-catenin to balance wound healing and scar formation [117].
EVs have emerged as a promising tool for wound healing therapy because they can deliver various bioactive molecules to modulate key signalling pathways involved in wound healing. However, there remain challenges and limitations that need to be addressed before EV-based therapy can be translated into clinical practice. Some of these challenges include: (1) standardization of EV isolation and characterization methods; (2) optimization of EV dosage and administration routes; (3) evaluation of EV safety and immunogenicity; (4) identification of specific biomarkers for EV tracking and monitoring; and (5) engineering of EVs to enhance their targeting and loading capacity. Further research is needed to overcome these challenges and explore the full potential of EVs for wound healing [106].
Modulation of PI3K/AKT/mTOR pathway: a key driver of cell proliferation, migration and angiogenesis
The PI3K/AKT/mTOR signalling pathway is a key pathway that regulates cell growth, survival, migration and angiogenesis, which are essential for wound healing (Figure 3). This pathway can be activated by various cytokines and miRNAs that are delivered by EVs derived from different stem/progenitor cells [118]. The activation of this pathway can: stimulate the expression of growth factors such as VEGF, FGF and epidermal growth factor (EGF), which promote angiogenesis and tissue regeneration; enhance the proliferation and migration of endothelial cells, keratinocytes and fibroblasts, which are involved in re-epithelialization and granulation tissue formation [119]; and inhibit excessive inflammation by downregulating proinflammatory factors such as tumor necrosis factor alpha (TNF-α), IL-1β and IL-6 and upregulating anti-inflammatory factors such as IL-10 and signal transducer and activator of transcription 3 (STAT3) [120–123].
Figure 3.
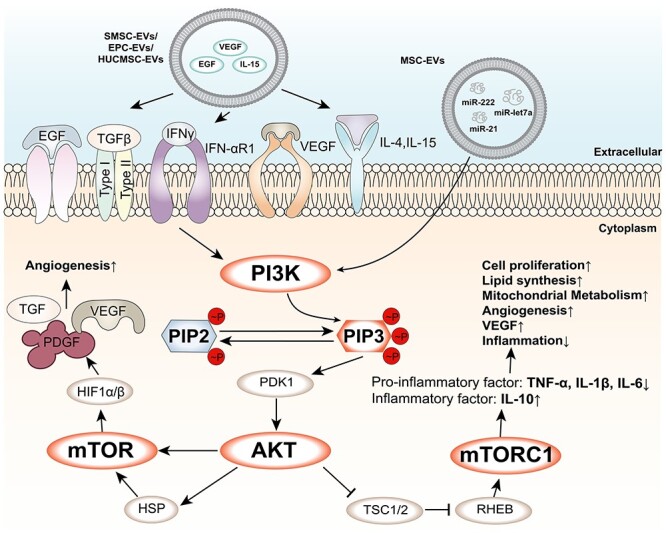
PI3K/AKT/mTOR signalling pathway and its regulation by EVs. The PI3K/AKT/mTOR signalling pathway is a central pathway that regulates cell growth, metabolism and survival. The pathway is activated by various growth factors and cytokines, such as epidermal growth factor (EGF), transforming growth factor-beta (TGF-β), interferon-gamma (IFN-γ), vascular endothelial growth factor (VEGF), interleukin-4 (IL-4) and IL-15, which bind to their respective receptors on the cell membrane and trigger the activation of phosphatidylinositol 3-kinase (PI3K). PI3K converts phosphatidylinositol 4,5-bisphosphate (PIP2) into phosphatidylinositol 3,4,5-trisphosphate (PIP3), which recruits and activates protein kinase B (AKT) and phosphoinositide-dependent kinase 1 (PDK1). AKT phosphorylates and inhibits tuberous sclerosis complex 1 and 2 (TSC1/2), which releases the inhibition of Ras homologue enriched in brain (RHEB). RHEB activates mammalian target of rapamycin complex 1 (mTORC1), which regulates various cellular processes, such as protein synthesis, lipid synthesis, mitochondrial metabolism, angiogenesis and inflammatory response. mTORC1 also activates hypoxia-inducible factor 1-alpha (HIF1α), which forms a heterodimer with HIF1β and induces the expression of target genes, such as TGF-β, platelet-derived growth factor (PDGF), and VEGF. EVs are small membrane-bound vesicles that can modulate the PI3K/AKT/mTOR signalling pathway by delivering various molecules to target cells. For example, EVs can carry EGF, IL-15 or VEGF to activate the pathway, or microRNAs (miRNAs) such as miR-let7a, miR-21 and miR-222 to activate the pathway by targeting its different components. EVs can also affect the crosstalk between the PI3K/AKT/mTOR signalling pathway and other pathways, such as the Wnt/β-catenin pathway or the NF-κB pathway. EVs extracellular vesicles
(1) Synovial mesenchymal stem cell EVs can deliver miR-222, miR-21 and miR-let7a, which can activate the Wnt/β-catenin and AKT/ERK/STAT3 pathways to promote angiogenesis [124]. (2) EPC-EVs can deliver VEGF-A and VEGF receptor 2 (VEGFR-2), which can activate the VEGF/PI3K/AKT/endothelial nitric oxide synthase (eNOS) pathway to promote angiogenesis [125]. (3) HUCMSC-EVs can deliver VEGF-A, IL-15 and EGF, which can activate the PI3K/AKT and mTOR pathways to regulate the expression of IGF-1 in DETCs that is beneficial to wound healing in diabetic mice [126]. (4) Human amniotic epithelial cell derived EVs can deliver miR-21-5p and miR-181a-5p, which can target phosphatase and tensin homolog (PTEN) and increase AKT phosphorylation in keratinocytes and fibroblasts [127]. (5) MSC-EVs can deliver miR-126-3p and miR-210-3p, which can target sprouty-related EVH1 domain-containing protein 1 (SPRED1) and protein tyrosine phosphatase 1B (PTP1B) and increase AKT phosphorylation in keratinocytes and fibroblasts [128–130].
These EVs can also increase YAP nuclear accumulation in keratinocytes and fibroblasts by delivering YAP mRNA or inhibiting LATS1/2 phosphorylation. YAP is a transcriptional coactivator that interacts with TEA domain transcription factor (TEAD) family transcription factors and enhances cell proliferation and migration gene expression [131, 132]. HUCMSC-EVs or MSC-EVs can also inhibit excessive inflammation by modulating the PI3K/AKT/mTOR pathway in macrophages or other immune cells by delivering various miRNAs and proteins that are involved in inflammation regulation [133]. For example, HUCMSC-EVs can deliver miR-181c, miR-1180, miR-183, miR-550b and miR-133a, which can induce M2 macrophage polarization and reduce proinflammatory cytokine production [134, 135]. MSC-EVs can deliver: miR-let7b and miR-181c, which can upregulate AKT and STAT3 phosphorylation and downregulate TLR4 expression in macrophages or other immune cells [136, 137]; heat shock protein (HSP) 90a protein, which can inhibit NF-kB activation and reduce proinflammatory cytokine production; and anti-inflammatory miRNAs such as miR-124a, miR-125b, miR-126 and miR-let7b, which can target various inflammatory mediators such as NF-kB, MAPK, TNF-α, IL-1β and IL-6 [138, 139].
Modulation of VEGF pathway: a potent stimulator of angiogenesis and tissue regeneration
The VEGF signalling pathway is a key pathway that regulates angiogenesis, which is the formation of new blood vessels from pre-existing ones. Angiogenesis is essential for wound healing, as it provides oxygen and nutrients to the wound site and facilitates the removal of waste products and inflammatory cells [140]. The VEGF signalling pathway is activated by the binding of VEGF ligands to VEGFRs on the surface of endothelial cells [141]. This binding triggers a cascade of downstream signalling pathways that mediate endothelial cell proliferation, migration, survival and permeability, as well as the expression of genes that promote angiogenesis [140]. The VEGF signalling pathway interacts with other pathways involved in wound healing, such as the MAPK and PI3K-AKT pathways, which are located downstream of VEGF signalling and modulate its effects [142]. EVs derived from different sources can modulate the VEGF signalling pathway in wound healing by delivering various cytokines, miRNAs and proteins that are involved in angiogenesis [143] (Figure 4). For instance, EPC-EVs can deliver VEGF-A and VEGFR-2, which activate the VEGF/PI3K/AKT/eNOS pathway and enhance the healing of soft tissue wounds by stimulating angiogenesis and collagen synthesis in fibroblasts and keratinocytes [125, 144, 145]. HUCMSC-EVs can deliver VEGF-A, IL-15 and EGF, which can activate the PI3K/AKT and mTOR pathways and regulate the expression of IGF-1 in DETCs that is beneficial to wound healing in diabetic mice [108, 146]. MSC-EVs can deliver miR-126-3p and miR-210-3p, which can target SPRED1 and PTP1B and increase AKT phosphorylation in keratinocytes and fibroblasts, thereby enhancing their proliferation and migration [147].
Figure 4.
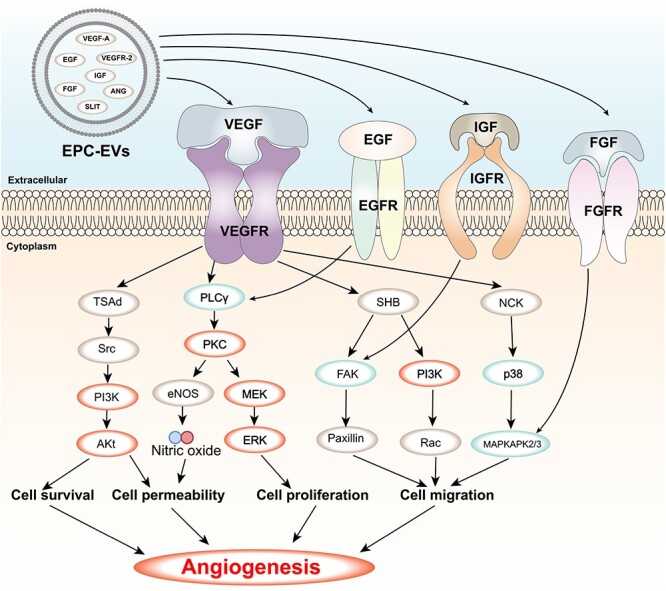
VEGF signalling pathway and its regulation by EVs contents. VEGF is a key factor that stimulates the formation of new blood vessels from existing ones or from the embryonic circulatory system. VEGF binds to its receptors (VEGFRs) on the surface of endothelial cells and activates various downstream pathways, such as Ras/MAPK, FAK/paxillin, PI3K/AKT and PLCγ/PKC, that regulate cell proliferation, migration, survival, permeability and angiogenesis. EVs are small membrane vesicles that are released by various cell types and contain bioactive molecules, such as proteins and RNAs. EVs can modulate the VEGF signalling pathway by delivering their contents to target cells or by interacting with VEGFRs on the cell surface. Some of the EVs contents that have been shown to affect the VEGF signalling pathway are SLIT, IGF, ANG, EGF, FGF, VEGFR-2 and VEGF-A. The figure shows the main components and effects of the VEGF signalling pathway and how EVs regulate this pathway through their contents. FGF Fibroblast growth factor, FGFR FGF receptor, IGF insulin-like growth factor, IGFR IGF receptor, EGF epidermal growth factor, EGFR EGF receptor, MAPKAPK2/3 mitogen-activated protein kinase-activated protein kinase 2/3, MEK MAPK/ERK kinase, ERK extracellular signal-regulated kinase, Ra, Ras-related C3 botulinum toxin substrate, PI3K phosphatidylinositol 3-kinase, p38 p38 MAPK, NCK non-catalytic region of tyrosine kinase adaptor protein, FAK focal adhesion kinase, SHB Src homology 2 domain-containing adapter protein B, eNOS endothelial nitric oxide synthase, PKC protein kinase C, PLCγ phospholipase C gamma, AKT protein kinase B, Src proto-oncogene tyrosine-protein kinase Src, TSAd T cell-specific adapter protein, VEGF vascular endothelial growth factor, VEGFR VEGF receptor, EVs extracellular vesicles
Modulation of TGF-β pathway: a major promoter of inflammation and collagen deposition
The TGF-β signalling pathway is a complex and multifunctional pathway that regulates various cellular processes, such as proliferation, differentiation, migration, apoptosis and ECM synthesis [148]. Among the TGF-β family, TGF-β1 plays a dominant role in skin wound healing and mainly mediates pathological healing, such as chronic inflammation and excessive scarring [149]. The TGF-β signalling pathway can be activated by various stimuli, including cytokines, growth factors and mechanical stress, and can interact with other pathways involved in wound healing, such as the JAK–STAT, MAPK/ERK, PI3K/AKT/mTOR and Wnt/β-catenin pathways [150]. The function of the TGF-β pathway is context-dependent and can be either beneficial or detrimental for wound healing depending on the stage, type and location of the wound [151]. EVs derived from different sources can modulate the TGF-β signalling pathway in wound healing by delivering various cytokines, miRNAs and proteins that are involved in inflammation and ECM remodelling [152] (Figure 5). For instance, HUCMSC-EVs can deliver miR-21, miR-125b, miR-23a and miR-145, which inhibit the TGF-β/SMAD2 pathway and reduce the expression of collagen and α-SMA in fibroblasts and myofibroblasts [153]. This action can prevent excessive scar formation and fibrosis in the late stage of wound healing. MSC-EVs can deliver proteins that can regulate MMP levels and the TGF-β signalling pathway, such as tissue inhibitor of metalloproteinases (TIMP)1 and TIMP2 [154]. This sequence of events modulates the balance between ECM synthesis and degradation and regulates α-SMA and collagen deposition in the wound site [155]. MSC-EV treatment can also aid in the contraction of newly formed scar tissue by altering the ratio of type III to type I collagen and MMP3 to TIMP1 [156, 157].
Figure 5.
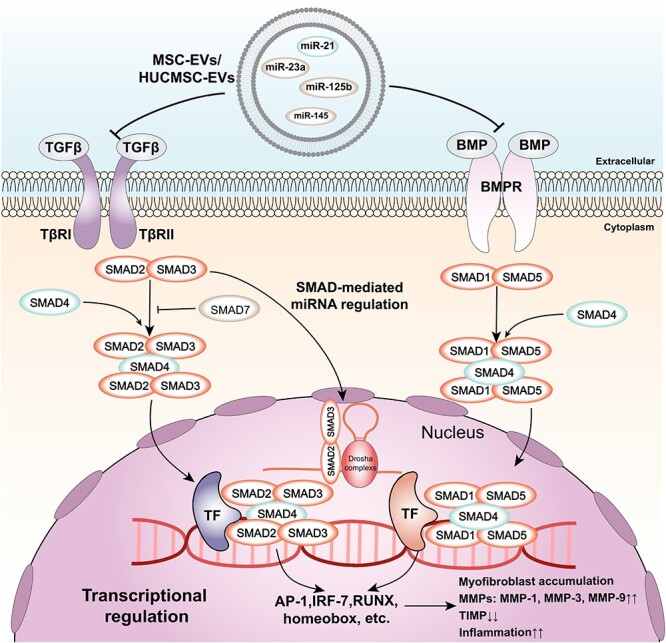
TGF-β signalling pathway and its regulation by EVs. The figure shows the major components and regulatory effects of TGF-β signalling and how cellular EVs regulate TGF-β signalling through inclusions. TGF-β and BMP bind to their respective receptors (TβRII and BMPR) and activate SMAD proteins (SMAD2/3 for TGFβ and SMAD1/5 for BMP). SMAD4 forms complexes with SMAD2/3 or SMAD1/5 and translocates to the nucleus, where it interacts with transcription factors (TF) to regulate gene expression. SMAD-mediated miRNA regulation involves Drosha complexes and SMAD2/3. The final effect of TGFβ signalling is myofibroblast accumulation, MMPs and TIMP expression, and an inflammatory condition. Exosome contents, such as miR-145, miR-125b, miR-23a and miR-21, can modulate TGFβ signalling by targeting different components of the pathway. TβRII TGF-β receptor type II, TGF-β transforming growth factor beta, BMPR bone morphogenetic protein receptor, BMP bone morphogenetic protein, TF transcription factor, AP-1 activator protein 1, IRF-7 interferon regulatory factor 7, RUNX runt-related transcription factor, MMPs matrix metalloproteinases, TIMP tissue inhibitor of metalloproteinases, miR microRNA, EVs extracellular vesicles
Modulation of JAK–STAT pathway: a key mediator of cytokine responses and macrophage polarization
The JAK–STAT signalling pathway is a key pathway that mediates cellular responses to various cytokines and growth factors, such as interleukins, interferons and colony-stimulating factors. The JAK–STAT pathway can regulate various aspects of wound healing, such as inflammation, angiogenesis, cell proliferation and migration, and ECM synthesis [158, 159]. The activation of the JAK–STAT pathway depends on the binding of cytokines or growth factors to their specific receptors, which leads to the phosphorylation of JAKs and STATs. The phosphorylated STATs then dimerize and translocate to the nucleus, where they regulate the transcription of target genes [160, 161]. EVs derived from different sources can modulate the JAK–STAT signalling pathway in wound healing by delivering various cytokines, miRNAs and proteins that are involved in inflammation and angiogenesis [162] (Figure 6). For example, EVs derived from fibrocytes can deliver anti-inflammatory miRNAs (miR-124a, miR-125b, miR-126 and miR-let7b) and HSP90a, which can promote the phosphorylation of STAT3 and the polarization of macrophages to the M2 type [163, 164]. This can reduce the production of proinflammatory cytokines such as TNF-α, IL-1β and IL-6 and enhance the secretion of anti-inflammatory cytokines such as IL-10 and TGF-β [165, 166]. EVs derived from MSCs can deliver miR-21, which can regulate the JAK–STAT pathway and promote the activation of Ras protein [167]. This can enhance the PI3K/AKT pathway and stimulate angiogenesis and collagen synthesis in the wound site.
Figure 6.
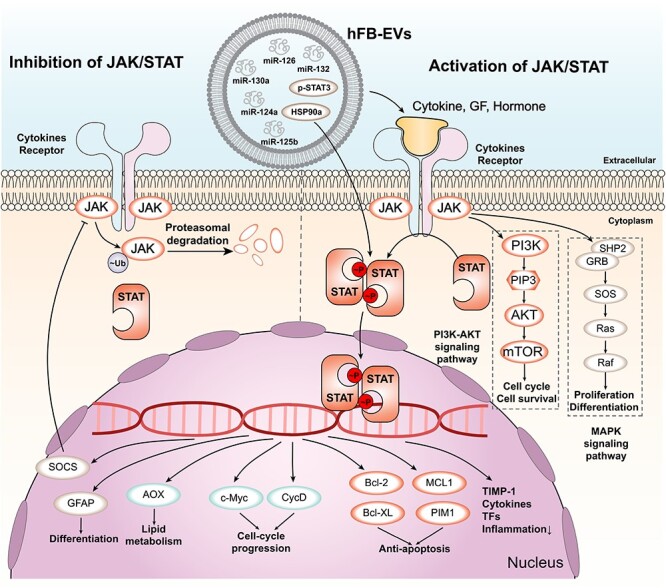
JAK/STAT signalling pathway and its regulation by EVs. The main components of the JAK/STAT signalling pathway are shown. Cytokines, growth factors (GF) and hormones bind to their receptors on the cell membrane, activating JAK kinases that phosphorylate (p) STAT proteins. p-STAT proteins form dimers and translocate to the nucleus, where they regulate the transcription of target genes. Ubiquitylated JAK proteins are degraded by proteasomes. The downstream target genes of p-STAT proteins include those involved in anti-apoptosis, cell-cycle progression, lipid metabolism and differentiation. Some examples of these genes are PIM1, MCL1, Bcl-XL, Bcl-2, CycD, c-Myc, AOX, GFAP, SOCS, TIMP-1, cytokines and transcription factors (TFs). The JAK/STAT signalling pathway interacts with other signalling pathways, such as the PI3K-AKT and MAPK pathways. The PI3K-AKT pathway regulates cell cycle and survival through AKT, PIP3, PI3K and mTOR. The MAPK pathway regulates proliferation and differentiation through Raf, Ras, SOS, SHP2, GRB2 and ERK. EVs are extracellular vesicles that contain various molecules, such as microRNAs (miRNAs), proteins and lipids. EVs can modulate the JAK/STAT signalling pathway by delivering or removing some of these molecules. For example, EVs can carry miR-124a, miR-130a, miR-125b, HSP90a, p-STAT3, miR-132 and miR-126 to target cells and affect their gene expression and signalling activity. EVs extracellular vesicles, STAT3 signal transducer and activator of transcription 3, TIMP-1 tissue inhibitor of metalloproteinases, MAPK mitogen-activated protein kinase, ERK extracellular signal-regulated kinase
Optimal EV therapy for various wound types
Different types of wounds have different requirements and objectives for optimal healing. Therefore, the choice of EV sources and contents should be tailored to the specific characteristics and challenges of each wound type [168]. Some examples of how exosome sources and contents can be matched to wound types are as follows (Table S1, see online supplementary material).
(1) For wounds that suffer from poor blood supply and oxygenation, such as diabetic ulcers, pressure ulcers and venous ulcers, EPC-EVs or MSC-EVs might be beneficial [169]. These EVs can contain VEGF-A and VEGFR-2, which are key molecules for stimulating the formation and maturation of new blood vessels in the wound area [170]. By enhancing angiogenesis, these EVs can improve the delivery of oxygen and nutrients to the wound site and facilitate wound healing [168, 171].
(2) For wounds that are prone to excessive inflammation and scar formation, such as burns, surgical wounds and lacerations, HUCMSC-EVs or MSC-EVs might be helpful. These exosomes can contain anti-inflammatory miRNAs, such as miR-181c, or proteins, such as interleukin-10 (IL-10), which modulate the inflammatory response and reduce the production of proinflammatory cytokines, such as TNF-α, IL-1β and IL-6 [108, 172, 173]. By reducing inflammation, these exosomes can prevent tissue damage and infection and promote wound resolution. Moreover, these exosomes can also inhibit the expression of collagen and the activation of the TGF-β pathway, which are involved in scar formation. By inhibiting scar formation, these exosomes preserve the function and appearance of the skin [174–176].
(3) For wounds that need to accelerate cell growth and movement, such as abrasions, contusions and punctures, human amniotic epithelial cell derived EVs or MSC-EVs might be effective. These exosomes can contain growth factors, such as EGF, or proteins that activate the PI3K/AKT/mTOR pathway, such as Wnt4 [171, 172, 177]. These molecules can stimulate the proliferation and migration of various cell types involved in wound healing, such as keratinocytes, fibroblasts and endothelial cells. By enhancing cell proliferation and migration, these exosomes promote re-epithelialization, granulation tissue formation and wound closure [174, 178, 179].
Engineered EVs
Enhancing the therapeutic potential of EVs through engineering
EVs have shown great potential for promoting wound healing and skin regeneration by enhancing angiogenesis, inflammation, keratinization, ECM remodelling and scar reduction. However, traditional EVs derived from natural sources have some limitations and shortcomings that can affect their therapeutic effect, such as low yield, impurity, lack of targeting and low drug delivery rate. These limitations can reduce the efficiency, specificity and safety of exosome therapy for wound healing [168, 180, 181]. To overcome these limitations, engineered EVs have been developed by modifying or enhancing the properties of natural EVs [182]. Engineered EVs can be derived from different cell sources, such as stem cells, and loaded with various molecules, such as growth factors, cytokines or drugs, to target specific pathways or processes in wound healing [183]. They can also be modified on their surface or membrane to improve their stability, biocompatibility, homing ability and delivery efficiency. Engineered EVs have shown promising results in preclinical and clinical studies for wound healing and skin regeneration [184] (Table S2, see online supplementary material). For example, they can trigger wound healing and skin regeneration in ischaemic wounds by delivering TGF-β and VEGF signals to the wound site [185]. They can also modulate the immune response and enhance the antimicrobial activity of macrophages in infected wounds by delivering interferon-gamma and nitric oxide [186, 187]. Engineered EVs are a potential solution to the limitations of traditional EVs for wound healing. They can offer more control over the biotherapeutic performance and delivery by manipulating their cargo or surface markers.
Characteristics, production methods and applications of engineered EVs
Engineered EVs are EVs that are modified to carry specific therapeutic agents or molecules. They can be used to deliver drugs or RNA molecules to target cells and tissues and to attenuate the oncogenic activity of cancer cells [188]. Engineered EVs can be obtained by modification of natural membrane vesicles, such as by electroporation, transfection or genetic engineering [189] (Figure 7). Electroporation is a technique that uses electric pulses to create pores in the membrane of EVs, allowing the entry of drugs or RNA molecules [190]. Transfection is a technique that uses liposomes or other agents to introduce DNA or RNA molecules into EVs [191]. Genetic engineering is a technique that uses viral vectors or the CRISPR–Cas9 system to modify the genes of EV-producing cells, resulting in altered expression or secretion of EV proteins or RNAs [192]. Engineered EVs have been explored for various clinical applications, such as cancer therapy, immunotherapy, gene therapy, tissue regeneration and diagnosis. For example, Codiak Biosciences has developed engineered EVs that carry a tumour antigen and a stimulatory molecule to activate T cells against cancer cells [193]. Engineered EVs can deliver therapeutic RNAs to target cells and tissues affected by rare diseases resulting from genetic mutations [194]. These engineered EVs can also carry anti-inflammatory and pro-regenerative factors to modulate the inflammatory and regenerative responses in cardiac and muscular disorders [195]. Engineered EVs have several advantages over conventional EVs or synthetic nanoparticles, such as biocompatibility, low immunogenicity, high stability, natural targeting ability and easy modification. Therefore, engineered EVs have promising prospects for biomedical applications [196]. However, there are also some challenges and limitations that need to be addressed, such as scalability, standardization, quality control, safety evaluation, regulatory approval and cost-effectiveness [197]. More research and development are needed to overcome these hurdles and to translate engineered EVs from the laboratory to the clinic.
Figure 7.
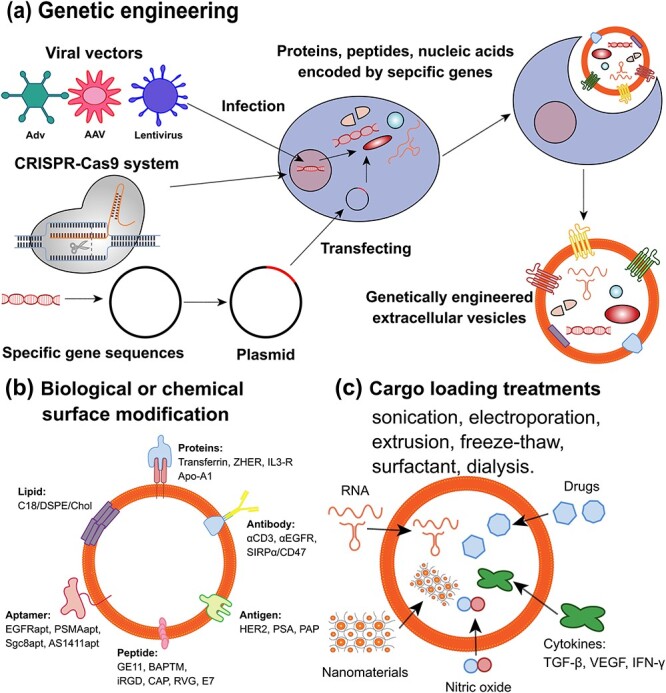
Different methods of EV engineering and their applications. EVs are small membrane-bound vesicles that can be engineered to modify their surface molecules, cargo contents or targeting specificity. Exosome engineering can be achieved by three main approaches: (a) genetic engineering, (b) surface modification, and (c) cargo loading. (a) Genetic engineering involves manipulating the genetic information of exosome-secreting cells using viral vectors, CRISPR-Cas9 system or plasmid transfection of specific gene fragments. This can result in the synthesis of specific proteins, peptides or nucleic acids that can be incorporated into EVs. For instance, EVs can be engineered to express fluorescent proteins, therapeutic genes or immunostimulatory molecules. (b) Surface modification involves attaching biological or chemical molecules to the surface of EVs using covalent or non-covalent bonds. This can alter the biophysical properties, stability or targeting ability of EVs. For example, EVs can be modified with proteins, antibodies, lipids, aptamers, peptides or antigens that can enhance their binding affinity, specificity or immunogenicity to target cells or tissues. (c) Cargo loading involves introducing exogenous molecules into the lumen of EVs using physical or chemical treatments. This can increase the functional diversity and efficacy of EVs. For instance, EVs can be loaded with RNA, nanomaterials, drugs, cytokines or gaseous molecules that can modulate gene expression, imaging contrast, drug delivery, inflammation or vasodilation in target cells or tissues, EGF epidermal growth factor, EVs extracellular vesicles, FGF fibroblast growth factor, VEGF vascular endothelial growth factor, TGF-β transforming growth factor-beta, IFN-γ interferon-gamma
Cost-effectiveness and feasibility of engineered EV therapy
Engineered EVs are modified or produced artificially to enhance their therapeutic or diagnostic potential. They can be classified into different types based on their origin (autologous or allogeneic), source (stem cell or nonstem cell), specificity (tissue-specific or nonspecific) and method (natural or artificial) [198, 199]. The current production and cost status of engineered EVs may vary depending on these factors, as well as the quality and quantity requirements for different applications [200]. Generally, engineered EVs may have higher yield and lower cost than traditional EVs if they are produced by artificial methods that do not require donor cells or complex isolation procedures, such as nanovesicles or exosome-mimetics [201]. However, they may have lower yields and higher costs than traditional EVs if they are produced by natural methods that require donor cells and multiple isolation and modification steps, such as hybrid EVs [199]. Moreover, engineered EVs may have lower yields and higher costs than traditional EVs if they are modified to introduce specific functions or targets, which can increase the complexity and difficulty of engineering [202]. Some methods that expand production and effect the cost of engineered EVs may include the following. (1) Developing standardized and scalable protocols for isolation, purification, characterization and modification of EVs from different sources and methods [203]. (2) Optimizing the culture conditions, engineering strategies and quality control measures for producing high-quality and high-purity EVs with desired properties and functions [204]. (3) Exploring novel biomaterials, nanotechnologies and bioengineering approaches for generating artificial or hybrid EVs with enhanced stability, specificity and efficacy [205]. (4) Reducing the production costs by using renewable or low-cost resources, such as plant-derived or microbial-derived EVs, or by recycling or reusing the waste materials from exosome production [206]. (5) Increasing the production efficiency by using automated or integrated systems, such as microfluidic devices or bioreactors, for continuous or large-scale production of EVs [204].
Engineered EVs have shown great promise in various biomedical applications, such as drug delivery, gene therapy, tissue regeneration, immunotherapy, diagnosis and biomarker discovery. They have several advantages over other nanocarriers or cell therapies, such as low toxicity, high biocompatibility, natural targeting ability, cargo loading capacity, editable surface structure and immune evasion [207, 208]. However, engineered EVs also face some challenges and limitations, such as lack of clear definition and classification, heterogeneity and variability of exosome characteristics and functions, potential immunogenicity and transmission of pathogens or malignancies, ethical and regulatory issues, and technical difficulties in engineering and manufacturing [197, 209]. Therefore, more research is needed to address these challenges and limitations, as well as to explore new applications and mechanisms of action of engineered EVs [197, 210]. Furthermore, more clinical trials are needed to evaluate the safety and efficacy of engineered EVs in human patients with various diseases. In summary, engineered EVs have a bright future in biomedicine as a novel and versatile delivery platform with multiple benefits [200].
EV therapy: a promising strategy
In recent years, research has found that EVs have good regulatory potential and play an important role in a variety of biological processes [211]. EVs can not only be used for chronic wound healing but also show great potential in many therapeutic areas, including cosmetic dermatology, cardiovascular dysfunction, cancer and neurodegenerative diseases [21, 75, 212]. These complex vesicles have received much attention in the field of biomarker research and are now seen as an alternative strategy for stem cell-based regenerative therapy, which is similar in treatment mechanism to stem cell therapy but with better efficacy, lower side effects, and is easier to artificially regulate and modify [213]. In addition to the direct use of EVs for wound treatment, EV-mediated drug delivery is also a good therapeutic approach with low toxicity, low immunogenicity and high engineering ability, and is expected to be used as cell-free therapy for a variety of diseases. Compared with the disadvantages of stem cell therapy, such as low cell survival rate, high immunogenicity and tumourigenicity, EVs as a cell-free therapy have significant advantages [214].
However, the current research on EVs for wound treatment focuses more on the research of EVs extracted from certain stem/progenitor cells applied to the wound site and whether they promote healing. How to achieve artificial modification of EVs, i.e. to obtain engineered EVs, is a more promising field. For instance, therapeutic drugs such as small molecules or nucleic acid drugs can be incorporated into EVs and then delivered to specific types of cells or tissues for targeted drug delivery. Targeted delivery increases the local concentration of the therapeutic agent and minimizes side effects [215]. EVs can be engineered through genetic or chemical methods to achieve targeted drug delivery [216]. There are many directions for engineering modification of EVs, which can target both the membrane components of EVs and the contents of EVs, including: (1) artificial incorporation of cargos [217]; (2) modification or regulation of surface lipids or proteins; and (3) changes in the content and composition of RNA and proteins [207]. The functions of EV membrane components are numerous and important. The surface proteins of EVs can mediate their targeting and adhesion to specific cells or tissues, which gives them an advantage over liposomes, which have lower targeting and adhesion abilities. [84]. The membrane component can avoid activating the immune clearance of the body, is well tolerated and will not induce toxicity [218]. The targeting of EVs to wound sites is also related to their membrane surface receptors. Engineered modification of the membrane components of EVs is designed to increase the local concentration of EVs at the lesion site, thereby reducing toxicity and side effects and maximizing therapeutic efficacy [139]. The engineering of the contents is more complex and promising, for instance, regulating the sorting mechanism of EV cargo, changing the type and volume of the contents, and achieving specific transport of therapeutic agents into EVs.
However, the current research is limited to characterization. Although surface engineering has been widely used for targeted drug delivery, how it affects EVs, their cell stability entry pathway and tissue distribution in vivo remain to be elucidated. Characterization of the sorting of EV contents is insufficient, which increases the difficulty for artificial regulation. More studies are needed to explore the sorting mechanism of the exosome cargo, i.e. how EVs selectively obtain intracellular components.
Conclusions
In this review article, we have summarized and discussed how EVs derived from different sources can promote wound healing and skin regeneration by modulating various signalling pathways and their key proteins. We focused on the Wnt/β-catenin, PI3K/AKT/mTOR, VEGF, TGF-β and JAK–STAT signalling pathways, which are involved in various aspects of wound healing, such as inflammation, angiogenesis, cell proliferation and migration, extracellular matrix remodelling and scar formation. We have also highlighted the recent advances in engineering and modifying EVs to optimize their biotherapeutic performance and delivery for wound healing applications. We have discussed the definition, source, production method and clinical application of engineered EVs, as well as the prospects and challenges of this emerging field. We believe that this review article provides a comprehensive and updated overview of the current state of knowledge on how EVs promote wound healing and skin regeneration and that it can inspire further research and innovation in this field.
Supplementary Material
Acknowledgement
This work was supported by grants from the National Natural Science Foundation of China (81902784), the CAMS Innovation Fund for Medical Sciences (CIFMS, 2019-I2M-5-004), Fund of Sichuan Provincial Department of Science and Technology (2022YFSY0058), the Research Funding (RCDWJS 2020-20), Research and Development Program (RD-02-202002) from West China School/Hospital of Stomatology Sichuan University.
Contributor Information
Bowen Yang, State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, Chinese Academy of Medical Sciences Research Unit of Oral Carcinogenesis and Management, West China Hospital of Stomatology, Sichuan University, No. 14, Section 3, Renmin South Road, Wuhou District, Chengdu 610041, China.
Yumeng Lin, State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, Chinese Academy of Medical Sciences Research Unit of Oral Carcinogenesis and Management, West China Hospital of Stomatology, Sichuan University, No. 14, Section 3, Renmin South Road, Wuhou District, Chengdu 610041, China.
Yibo Huang, State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, Chinese Academy of Medical Sciences Research Unit of Oral Carcinogenesis and Management, West China Hospital of Stomatology, Sichuan University, No. 14, Section 3, Renmin South Road, Wuhou District, Chengdu 610041, China.
Nanxi Zhu, State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, Chinese Academy of Medical Sciences Research Unit of Oral Carcinogenesis and Management, West China Hospital of Stomatology, Sichuan University, No. 14, Section 3, Renmin South Road, Wuhou District, Chengdu 610041, China.
Ying-Qiang Shen, State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, Chinese Academy of Medical Sciences Research Unit of Oral Carcinogenesis and Management, West China Hospital of Stomatology, Sichuan University, No. 14, Section 3, Renmin South Road, Wuhou District, Chengdu 610041, China.
Abbreviations
ALIX: Programmed cell death 6 interacting protein; ECM: Extracellular matrix; EGF: Epidermal growth factor; eNOS: Endothelial nitric oxide synthase; EPCs: Endothelial progenitor cells; ERK: Extracellular signal-regulated kinase; EVs: extracellular vesicles; FGF fibroblast growth factor; HUCMSC-EVs: Human umbilical cord mesenchymal stem cells derived extracellular vesicles; IL-1β: Interleukin-1β; ILVs: Intraluminal vesicles; MAPK: Mitogen-activated protein kinase; miRNAs: MicroRNAs; MMPs matrix metalloenzymes; MSCs: Mesenchymal stem cells; mTOR: Mammalian target of rapamycin; MVBs: Multivesicular bodies; NF-κB: Nuclear factor-κB; PI3K: Phosphoinositide 3-kinase; SMA: α-Smooth muscle actin; TGF: Transforming growth factor; TIMP-1: Tissue inhibitor of metalloproteinases; TNF-α: Tumor necrosis factor alpha; TLR: Toll-like receptor; TSG101: Tumor susceptibility gene 101; VEGF: Vascular endothelial growth factor; VEGFRs: Vascular endothelial growth factor receptors. CAGR: compound annual growth rate; COX-2: cyclooxygenase-2; PCNA: proliferating cell nuclear antigen; YAP: yes-associated protein; LATS: large tumor suppressor kinase; STAT3: signal transducer and activator of transcription 3;SPRED1: sprouty-related EVH1 domain-containing protein 1; PTP1B: protein tyrosine phosphatase 1B;TEAD: TEA domain transcription factor; PTEN: phosphatase and tensin homolog.
Authors’ contributions
BY and Y-QS conceived and designed the main ideas of this article. BY performed the literature review, wrote the first draft of the manuscript and revised it according to feedback. YML, YBH and NXZ provided feedback and revisions to improve the manuscript. Y-QS supervised the writing process and reviewed the manuscript several times to determine the final version. All authors read and approved the manuscript for publication.
Conflict of interests
The authors declare that they have no competing interests or connections, direct or indirect, that might raise the question of bias in the work reported or the conclusions, implications or opinions stated in this review. The authors have no pertinent commercial or other sources of funding for the individual author(s) or for the associated department(s) or organization(s), personal relationships, or direct academic competition related to this review. The authors have followed the guidelines of the journal for writing a conflict of interest statement and have obtained the relevant information from all co-authors.
Data availability
The data that support the findings of this review are available from the corresponding author upon reasonable request. The data include the web page context, the search query, the search results, and the conflict of interest statement. The data are stored in a secure database that complies with the journal's data sharing policy.
References
- 1. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99:665–706. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30475656. 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun J, Hua B, Livingston EW, Taves S, Johansen PB, Hoffman M, et al. Abnormal joint and bone wound healing in hemophilia mice is improved by extending factor IX activity after hemarthrosis. Blood. 2017;129:2161–71. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28039188. 10.1182/blood-2016-08-734053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aragona M, Dekoninck S, Rulands S, Lenglez S, Mascre G, Simons BD, et al. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat Commun. 2017;8:14684. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28248284. 10.1038/ncomms14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wound Care Market Size . Share & COVID-19 lmpact analysis, by type(advanced wound dressing,(antimicrobial dressings,alginate Dressings,Foam dressings,hydrocolloid dressings, and others),traditional WoundCare products, negative pressure wound therapy, bioactives (biological SkinEquivalents,growth Factors,and others),and others);by application(chronic wounds (diabetic foot Ulcers,Pressure Ulcers,leg Ulcers,andothers), and acute wounds (surgical Wounds,and others)); by end user(hospitals, Clinics,Homecare settings, and others), and regional forecast, 2022-2029. Market Research Report. 2022; 242. Retrieved from; https://www.fortunebusinessinsights.com/wound-care-market-103268. [Google Scholar]
- 5. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr266 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25473038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen CY, Yin H, Chen X, Chen TH, Liu HM, Rao SS, et al. Angstrom-scale silver particle-embedded carbomer gel promotes wound healing by inhibiting bacterial colonization and inflammation. Sci Adv. 2020;6(43). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/33097529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gimblet C, Meisel JS, Loesche MA, Cole SD, Horwinski J, Novais FO, et al. Cutaneous Leishmaniasis Induces a Transmissible Dysbiotic Skin Microbiota that Promotes Skin Inflammation. Cell Host Microbe. 2017;22:13–24 e14. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28669672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonora BM, Cappellari R, Mazzucato M, Rigato M, Grasso M, Menegolo M, et al. Stem cell mobilization with plerixafor and healing of diabetic ischemic wounds: a phase IIa, randomized, double-blind, placebo-controlled trial. Stem Cells Transl Med. 2020;9:965–73. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32485785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pang Q, Lou D, Li S, Wang G, Qiao B, Dong S, et al. Smart flexible electronics-integrated wound dressing for real-time monitoring and on-demand treatment of infected wounds. Adv Sci (Weinh). 2020;7:1902673. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32195091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heun Y, Pogoda K, Anton M, Pircher J, Pfeifer A, Woernle M, et al. HIF-1alpha dependent wound healing angiogenesis in vivo can be controlled by site-specific Lentiviral magnetic targeting of SHP-2. Mol Ther. 2017;25:1616–27. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28434868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hiragami F, Motoda H, Takezawa T, Takabayashi C, Inoue S, Wakatake Y, et al. Heat shock-induced three-dimensional-like proliferation of normal human fibroblasts mediated by pressed silk. Int J Mol Sci. 2009;10:4963–76. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20087471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Srifa W, Kosaric N, Amorin A, Jadi O, Park Y, Mantri S, et al. Cas9-AAV6-engineered human mesenchymal stromal cells improved cutaneous wound healing in diabetic mice. Nat Commun. 2020;11:2470. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32424320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Costa ML, Achten J, Bruce J, Tutton E, Petrou S, Lamb SE, et al. Effect of negative pressure wound therapy vs standard wound management on 12-month disability among adults with severe open fracture of the lower limb: the WOLLF randomized clinical trial. JAMA. 2018;319:2280–8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29896626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKenzie K, Fingerhut L, Walker S, Harrison A, Harrison JE. Classifying external causes of injury: history, current approaches, and future directions. Epidemiol Rev. 2012;34:4–16. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22045696. [DOI] [PubMed] [Google Scholar]
- 15. Wei, J. J., Kim, H. S., Spencer, C. A., Brennan-Crispi, D., Zheng, Y., Johnson, N. M., et al. , Activation of TRPA1 nociceptor promotes systemic adult mammalian skin regeneration, Sci Immunol, 2020, 5(50). Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32859683. 10.1126/sciimmunol.aba5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan JL, Lau SN, Leaw B, Nguyen HPT, Salamonsen LA, Saad MI, et al. Amnion epithelial cell-derived exosomes restrict lung injury and enhance endogenous lung repair. Stem Cells Transl Med. 2018;7:180–96. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29297621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whittaker TE, Nagelkerke A, Nele V, Kauscher U, Stevens MM. Experimental artefacts can lead to misattribution of bioactivity from soluble mesenchymal stem cell paracrine factors to extracellular vesicles. J Extracell Vesicles. 2020;9:1807674. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32944192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/32029601. 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu J, Zhang M, Gao Y, Qin X, Zhang T, Cui W, et al. Tetrahedral framework nucleic acids promote scarless healing of cutaneous wounds via the AKT-signaling pathway. Signal Transduct Target Ther. 2020;5:120. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32678073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han Z, Liu S, Pei Y, Ding Z, Li Y, Wang X, et al. Highly efficient magnetic labelling allows MRI tracking of the homing of stem cell-derived extracellular vesicles following systemic delivery. J Extracell Vesicles. 2021;10(3):e12054 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33489014.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim H, Lee JW, Han G, Kim K, Yang Y, Kim SH. Extracellular vesicles as potential Theranostic platforms for skin diseases and aging. Pharmaceutics. 2021;13(5). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/34065468. 10.3390/pharmaceutics13050760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pardo F, Villalobos-Labra R, Sobrevia B, Toledo F, Sobrevia L. Extracellular vesicles in obesity and diabetes mellitus. Mol Asp Med. 2018;60:81–91. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29175307. [DOI] [PubMed] [Google Scholar]
- 23. Cheng L, Hill AF. Therapeutically harnessing extracellular vesicles. Nat Rev Drug Discov. 2022;21:379–99. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35236964. [DOI] [PubMed] [Google Scholar]
- 24. Mills EW, Green R, Ingolia NT. Slowed decay of mRNAs enhances platelet specific translation. Blood. 2017;129:e38–48. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28213379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lienemann PS, Vallmajo-Martin Q, Papageorgiou P, Blache U, Metzger S, Kivelio AS, et al. Smart hydrogels for the augmentation of bone regeneration by endogenous mesenchymal progenitor cell recruitment. Adv Sci (Weinh). 2020;7:1903395. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32274319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beck IM, Vanden Berghe W, Vermeulen L, Yamamoto KR, Haegeman G, De Bosscher K. Crosstalk in inflammation: the interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases. Endocr Rev. 2009;30:830–82. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19890091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kourtzelis I, Li X, Mitroulis I, Grosser D, Kajikawa T, Wang B, et al. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat Immunol. 2019;20:40–9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30455459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hahm E, Li J, Kim K, Huh S, Rogelj S, Cho J. Extracellular protein disulfide isomerase regulates ligand-binding activity of alphaMbeta2 integrin and neutrophil recruitment during vascular inflammation. Blood. 2013;121:3789–800S3781-3715 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23460613.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99:1325–80. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30920354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21195345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chow NA, Jasenosky LD, Goldfeld AE. A distal locus element mediates IFN-gamma priming of lipopolysaccharide-stimulated TNF gene expression. Cell Rep. 2014;9:1718–28. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25482561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li X, Liu R, Su X, Pan Y, Han X, Shao C, et al. Harnessing tumor-associated macrophages as aids for cancer immunotherapy. Mol Cancer. 2019;18:177. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31805946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu X, Xu J, Zhang B, Liu J, Liang C, Meng Q, et al. The reciprocal regulation between host tissue and immune cells in pancreatic ductal adenocarcinoma: new insights and therapeutic implications. Mol Cancer. 2019;18:184. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31831007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang SY, Kim H, Kwak G, Yoon HY, Jo SD, Lee JE, et al. Development of biocompatible HA hydrogels embedded with a new synthetic peptide promoting cellular migration for advanced wound care management. Adv Sci (Weinh). 2018;5:1800852. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30479928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu B, Xia X, Zhu F, Park E, Carbajal S, Kiguchi K, et al. IKKalpha is required to maintain skin homeostasis and prevent skin cancer. Cancer Cell. 2008;14:212–25. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18772111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lang E, Polec A, Lang A, Valk M, Blicher P, Rowe AD, et al. Coordinated collective migration and asymmetric cell division in confluent human keratinocytes without wounding. Nat Commun. 2018;9:3665. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30202009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deng CC, Hu YF, Zhu DH, Cheng Q, Gu JJ, Feng QL, et al. Single-cell RNA-seq reveals fibroblast heterogeneity and increased mesenchymal fibroblasts in human fibrotic skin diseases. Nat Commun. 2021;12:3709. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34140509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoyle NP, Seinkmane E, Putker M, Feeney KA, Krogager TP, Chesham JE, et al. Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing. Sci Transl Med. 2017;8(415). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/29118260. 10.1126/scitranslmed.aal2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin X, Tang F, Jiang S, Khamis H, Bongers A, Whitelock JM, et al. A biomimetic approach toward enhancing angiogenesis: Recombinantly expressed domain V of human Perlecan is a bioactive molecule that promotes angiogenesis and vascularization of implanted biomaterials. Adv Sci (Weinh). 2020;7:2000900. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32995122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee TH, Yeh CF, Lee YT, Shih YC, Chen YT, Hung CT, et al. Fibroblast-enriched endoplasmic reticulum protein TXNDC5 promotes pulmonary fibrosis by augmenting TGFbeta signaling through TGFBR1 stabilization. Nat Commun. 2020;11:4254. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32848143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Slaby O, Svoboda M, Michalek J, Vyzula R. MicroRNAs in colorectal cancer: translation of molecular biology into clinical application. Mol Cancer. 2009;8:102. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19912656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yuge S, Nishiyama K, Arima Y, Hanada Y, Oguri-Nakamura E, Hanada S, et al. Mechanical loading of intraluminal pressure mediates wound angiogenesis by regulating the TOCA family of F-BAR proteins. Nat Commun. 2022;13:2594. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35551172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu L, Stevens J, Hilton MB, Seaman S, Conrads TP, Veenstra TD, et al. COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci Transl Med. 2014;6:242ra284. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24964992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ueki R, Uchida S, Kanda N, Yamada N, Ueki A, Akiyama M, et al. A chemically unmodified agonistic DNA with growth factor functionality for in vivo therapeutic application. Sci Adv. 2020;6:eaay2801. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32270033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hanna A, Shevde LA. Hedgehog signaling: modulation of cancer properies and tumor mircroenvironment. Mol Cancer. 2016;15:24. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26988232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. John AE, Graves RH, Pun KT, Vitulli G, Forty EJ, Mercer PF, et al. Translational pharmacology of an inhaled small molecule alphavbeta6 integrin inhibitor for idiopathic pulmonary fibrosis. Nat Commun. 2020;11:4659. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32938936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bozic M, Caus M, Rodrigues-Diez RR, Pedraza N, Ruiz-Ortega M, Gari E, et al. Protective role of renal proximal tubular alpha-synuclein in the pathogenesis of kidney fibrosis. Nat Commun. 2020;11:1943. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32327648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maller O, Drain AP, Barrett AS, Borgquist S, Ruffell B, Zakharevich I, et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat Mater. 2021;20:548–59. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33257795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Nieuwenhoven FA, Turner NA. The role of cardiac fibroblasts in the transition from inflammation to fibrosis following myocardial infarction. Vasc Pharmacol. 2013;58:182–8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22885638. [DOI] [PubMed] [Google Scholar]
- 50. Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17(12). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/27973441. 10.3390/ijms17122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kobayashi T, Liu X, Kim HJ, Kohyama T, Wen FQ, Abe S, et al. TGF-beta1 and serum both stimulate contraction but differentially affect apoptosis in 3D collagen gels. Respir Res. 2005;6:141. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16324212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–16. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17525249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lu Y, Wen H, Huang J, Liao P, Liao H, Tu J, et al. Extracellular vesicle-enclosed miR-486-5p mediates wound healing with adipose-derived stem cells by promoting angiogenesis. J Cell Mol Med. 2020;24:9590–604. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32666704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Babaei M, Rezaie J. Application of stem cell-derived exosomes in ischemic diseases: opportunity and limitations. J Transl Med. 2021;19:196. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33964940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Harding KG, Morris HL, Patel GK. Science, medicine and the future: healing chronic wounds. BMJ. 2002;324:160–3. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11799036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bellu, E., Medici, S., Coradduzza, D., Cruciani, S., Amler, E., & Maioli, M., Nanomaterials in skin regeneration and rejuvenation, Int J Mol Sci, 2021, 22(13). Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34209468. 10.3390/ijms22137095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bice BD, Stephens MR, Georges SJ, Venancio AR, Bermant PC, Warncke AV, et al. Environmental enrichment induces Pericyte and IgA-dependent wound repair and lifespan extension in a colon tumor model. Cell Rep. 2017;19:760–73. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28445727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ruggeri M, Bianchi E, Rossi S, Vigani B, Bonferoni MC, Caramella C, et al. Nanotechnology-based medical devices for the treatment of chronic skin lesions: from research to the clinic. Pharmaceutics. 2020;12(9). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/32867241. 10.3390/pharmaceutics12090815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Toutous Trellu L, Nkemenang P, Comte E, Ehounou G, Atangana P, Mboua DJ, et al. Differential diagnosis of skin ulcers in a mycobacterium ulcerans endemic area: data from a prospective study in Cameroon. PLoS Negl Trop Dis. 2016;10(4):e0004385. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/27074157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bansal A, Schmidt M, Rennegarbe M, Haupt C, Liberta F, Stecher S, et al. AA amyloid fibrils from diseased tissue are structurally different from in vitro formed SAA fibrils. Nat Commun. 2021;12:1013. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33579941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Miner JJ, Xia L, Yago T, Kappelmayer J, Liu Z, Klopocki AG, et al. Separable requirements for cytoplasmic domain of PSGL-1 in leukocyte rolling and signaling under flow. Blood. 2008;112:2035–45. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18550846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Michel P, Granica S, Magiera A, Rosinska K, Jurek M, Poraj L, et al. Salicylate and Procyanidin-rich stem extracts of Gaultheria procumbens L. inhibit pro-inflammatory enzymes and suppress pro-inflammatory and pro-oxidant functions of human neutrophils ex vivo. Int J Mol Sci. 2019;20(7). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/30970662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Evans MA, Smart N, Dube KN, Bollini S, Clark JE, Evans HG, et al. Thymosin beta4-sulfoxide attenuates inflammatory cell infiltration and promotes cardiac wound healing. Nat Commun. 2013;4:2081. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23820300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jiang D, Christ S, Correa-Gallegos D, Ramesh P, Kalgudde Gopal S, Wannemacher J, et al. Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin. Nat Commun. 2020;11:5653. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33159076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20042775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hu W, Liu C, Bi ZY, Zhou Q, Zhang H, Li LL, et al. Comprehensive landscape of extracellular vesicle-derived RNAs in cancer initiation, progression, metastasis and cancer immunology. Mol Cancer. 2020;19:102. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32503543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu M, Chu Y, Shang Q, Zheng Z, Li Y, Cao L, et al. Mesenchymal stromal cells pretreated with pro-inflammatory cytokines promote skin wound healing through VEGFC-mediated angiogenesis. Stem Cells Transl Med. 2020;9:1218–32 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32534464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sebastiano V, Zhen HH, Haddad B, Bashkirova E, Melo SP, Wang P, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra163. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25429056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen SH, Chou PY, Chen ZY, Lin FH. Electrospun water-borne polyurethane Nanofibrous membrane as a barrier for preventing postoperative Peritendinous adhesion. Int J Mol Sci. 2019;20(7). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/30939838. 10.3390/ijms20071625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sun Z, Yang S, Zhou Q, Wang G, Song J, Li Z, et al. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol Cancer. 2018;17:82. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29678180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yanatori I, Richardson DR, Dhekne HS, Toyokuni S, Kishi F. CD63 is regulated by iron via the IRE-IRP system and is important for ferritin secretion by extracellular vesicles. Blood. 2021;138:1490–503. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34265052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Feng Q, Luo Y, Zhang XN, Yang XF, Hong XY, Sun DS, et al. MAPT/tau accumulation represses autophagy flux by disrupting IST1-regulated ESCRT-III complex formation: a vicious cycle in Alzheimer neurodegeneration. Autophagy. 2020;16:641–58. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31223056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hofmann K, Tomiuk S, Wolff G, Stoffel W. Cloning and characterization of the mammalian brain-specific, Mg2+−dependent neutral sphingomyelinase. Proc Natl Acad Sci U S A. 2000;97:5895–900. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10823942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Janockova J, Slovinska L, Harvanova D, Spakova T, Rosocha J. New therapeutic approaches of mesenchymal stem cells-derived exosomes. J Biomed Sci. 2021;28:39. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sung BH, von Lersner A, Guerrero J, Krystofiak ES, Inman D, Pelletier R, et al. A live cell reporter of exosome secretion and uptake reveals pathfinding behavior of migrating cells. Nat Commun. 2020;11:2092. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32350252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gupta A, Pulliam L. Exosomes as mediators of neuroinflammation. J Neuroinflammation. 2014;11:68. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24694258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Giaccia AJ. Molecular radiobiology: the state of the art. J Clin Oncol. 2014;32:2871–8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25113768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. French KC, Antonyak MA, Cerione RA. Extracellular vesicle docking at the cellular port: extracellular vesicle binding and uptake. Semin Cell Dev Biol. 2017;67:48–55. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28104520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wendler F, Bota-Rabassedas N, Franch-Marro X. Cancer becomes wasteful: emerging roles of exosomes(dagger) in cell-fate determination. J Extracell Vesicles. 2013;2; Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24223259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kumar R, Tang Q, Muller SA, Gao P, Mahlstedt D, Zampagni S, et al. Fibroblast growth factor 2-mediated regulation of neuronal exosome release depends on VAMP3/Cellubrevin in hippocampal neurons. Adv Sci (Weinh). 2020;7:1902372 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32195080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Iraci N, Gaude E, Leonardi T, Costa ASH, Cossetti C, Peruzzotti-Jametti L, et al. Extracellular vesicles are independent metabolic units with asparaginase activity. Nat Chem Biol. 2017;13:951–5. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28671681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tai YL, Chu PY, Lee BH, Chen KC, Yang CY, Kuo WH, et al. Basics and applications of tumor-derived extracellular vesicles. J Biomed Sci. 2019;26:35. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31078138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27:172–88. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27979573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tao SC, Guo SC, Zhang CQ. Modularized extracellular vesicles: the Dawn of prospective personalized and precision medicine. Adv Sci (Weinh). 2018;5:1700449. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29619297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chase LG, Yang S, Zachar V, Yang Z, Lakshmipathy U, Bradford J, et al. Development and characterization of a clinically compliant xeno-free culture medium in good manufacturing practice for human multipotent mesenchymal stem cells. Stem Cells Transl Med. 2012;1:750–8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23197667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yoshida K, Nakashima A, Doi S, Ueno T, Okubo T, Kawano KI, et al. Serum-free medium enhances the immunosuppressive and Antifibrotic abilities of mesenchymal stem cells utilized in experimental renal fibrosis. Stem Cells Transl Med. 2018;7:893–905. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30269426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Maldonado-Lasuncion I, O'Neill N, Umland O, Verhaagen J, Oudega M. Macrophage-derived inflammation induces a transcriptome makeover in mesenchymal stromal cells enhancing their potential for tissue repair. Int J Mol Sci. 2021;22(2). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/33466704. 10.3390/ijms22020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Al-Dossary AA, Tawfik EA, Isichei AC, Sun X, Li J, Alshehri AA, et al. Engineered EV-mimetic nanoparticles as therapeutic delivery vehicles for high-grade serous ovarian cancer. Cancers (Basel). 2021;13(12). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/34203051. 10.3390/cancers13123075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lee H, Li C, Zhang Y, Zhang D, Otterbein LE, Jin Y. Caveolin-1 selectively regulates microRNA sorting into microvesicles after noxious stimuli. J Exp Med. 2019;216:2202–20. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31235510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Men Y, Yelick J, Jin S, Tian Y, Chiang MSR, Higashimori H, et al. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat Commun. 2019;10:4136. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31515491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pomatto M, Gai C, Negro F, Cedrino M, Grange C, Ceccotti E, et al. Differential therapeutic effect of extracellular vesicles derived by bone marrow and adipose mesenchymal stem cells on wound healing of diabetic ulcers and correlation to their cargoes. Int J Mol Sci. 2021;22(8). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/33917759. 10.3390/ijms22083851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yoshioka Y, Konishi Y, Kosaka N, Katsuda T, Kato T, Ochiya T. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles. 2013;2.. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xie C, Ji N, Tang Z, Li J, Chen Q. The role of extracellular vesicles from different origin in the microenvironment of head and neck cancers. Mol Cancer. 2019;18:83. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30954079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chen SY, Lin MC, Tsai JS, He PL, Luo WT, Herschman H, et al. EP4 antagonist-elicited extracellular vesicles from mesenchymal stem cells rescue cognition/learning deficiencies by restoring brain cellular functions. Stem Cells Transl Med. 2019;8:707–23. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30891948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. de Kerckhove M, Tanaka K, Umehara T, Okamoto M, Kanematsu S, Hayashi H, et al. Targeting miR-223 in neutrophils enhances the clearance of Staphylococcus aureus in infected wounds. EMBO Mol Med. 2018;10(10). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/30171089. 10.15252/emmm.201809024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287–96. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27471669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bjorge IM, Kim SY, Mano JF, Kalionis B, Chrzanowski W. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine - a new paradigm for tissue repair. Biomater Sci. 2017;6:60–78. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29184934. [DOI] [PubMed] [Google Scholar]
- 98. Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab. 2013;17:745–55. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23623748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Arenas DJ, Floess K, Kobrin D, Pai RL, Srkalovic MB, Tamakloe MA, et al. Increased mTOR activation in idiopathic multicentric Castleman disease. Blood. 2020;135:1673–84. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32206779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang C, Dai X, Wu S, Xu W, Song P, Huang K. FUNDC1-dependent mitochondria-associated endoplasmic reticulum membranes are involved in angiogenesis and neoangiogenesis. Nat Commun. 2021;12:2616. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33972548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yaeger R, Corcoran RB. Targeting alterations in the RAF-MEK pathway. Cancer Discov. 2019;9:329–41. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30770389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The cell is dead. Long live the cell! Trends Cell Biol. 2008;18:467–73. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18774295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yuan S, Chu H, Huang J, Zhao X, Ye ZW, Lai PM, et al. Viruses harness YxxO motif to interact with host AP2M1 for replication: a vulnerable broad-spectrum antiviral target. Sci Adv. 2020;6:eaba7910. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32923629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/beta-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7:3. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34980884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ma L, Chen C, Liu D, Huang Z, Li J, Liu H, et al. Apoptotic extracellular vesicles are metabolized regulators nurturing the skin and hair. Bioact Mater. 2023;19:626–41. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35600968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lou P, Liu S, Xu X, Pan C, Lu Y, Liu J. Extracellular vesicle-based therapeutics for the regeneration of chronic wounds: current knowledge and future perspectives. Acta Biomater. 2021;119:42–56. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33161186. [DOI] [PubMed] [Google Scholar]
- 107. Zhang B, Shi Y, Gong A, Pan Z, Shi H, Yang H, et al. HucMSC exosome-delivered 14-3-3zeta orchestrates self-control of the Wnt response via modulation of YAP during cutaneous regeneration. Stem Cells. 2016;34:2485–500. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27334574. [DOI] [PubMed] [Google Scholar]
- 108. Wei Q, Wang Y, Ma K, Li Q, Li B, Hu W, et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells facilitate diabetic wound healing through MiR-17-5p-mediated enhancement of angiogenesis. Stem Cell Rev Rep. 2022;18:1025–40. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33942217. [DOI] [PubMed] [Google Scholar]
- 109. Qian L, Pi L, Fang BR, Meng XX. Adipose mesenchymal stem cell-derived exosomes accelerate skin wound healing via the lncRNA H19/miR-19b/SOX9 axis. Lab Investig. 2021;101:1254–66. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34045678. [DOI] [PubMed] [Google Scholar]
- 110. Li C, An Y, Sun Y, Yang F, Xu Q, Wang Z. Adipose mesenchymal stem cell-derived exosomes promote wound healing through the WNT/beta-catenin Signaling pathway in dermal fibroblasts. Stem Cell Rev Rep. 2022;18:2059–73. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35471485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nikfarjam S, Rezaie J, Zolbanin NM, Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020;18:449. Retrieved from .https://www.ncbi.nlm.nih.gov/pubmed/33246476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003–11. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18056176. [DOI] [PubMed] [Google Scholar]
- 113. Akcora BO, Storm G, Bansal R. Inhibition of canonical WNT signaling pathway by beta-catenin/CBP inhibitor ICG-001 ameliorates liver fibrosis in vivo through suppression of stromal CXCL12. Biochim Biophys Acta Mol basis Dis. 2018;1864:804–18. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29217140. [DOI] [PubMed] [Google Scholar]
- 114. Xu P, Xin Y, Zhang Z, Zou X, Xue K, Zhang H, et al. Extracellular vesicles from adipose-derived stem cells ameliorate ultraviolet B-induced skin photoaging by attenuating reactive oxygen species production and inflammation. Stem Cell Res Ther. 2020;11:264. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32611371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wu P, Zhang B, Shi H, Qian H, Xu W. MSC-exosome: a novel cell-free therapy for cutaneous regeneration. Cytotherapy. 2018;20:291–301. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29434006. [DOI] [PubMed] [Google Scholar]
- 116. Dovrat S, Caspi M, Zilberberg A, Lahav L, Firsow A, Gur H, et al. 14-3-3 and beta-catenin are secreted on extracellular vesicles to activate the oncogenic Wnt pathway. Mol Oncol. 2014;8:894–911. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24721736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Deng F, Peng L, Li Z, Tan G, Liang E, Chen S, et al. YAP triggers the Wnt/beta-catenin signalling pathway and promotes enterocyte self-renewal, regeneration and tumorigenesis after DSS-induced injury. Cell Death Dis. 2018;9:153. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29396428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jere SW, Houreld NN, Abrahamse H. Role of the PI3K/AKT (mTOR and GSK3beta) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019;50:52–9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30890300. [DOI] [PubMed] [Google Scholar]
- 119. Castilho RM, Squarize CH, Gutkind JS. Exploiting PI3K/mTOR signaling to accelerate epithelial wound healing. Oral Dis. 2013;19:551–8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23379329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Acosta-Martinez M, Cabail MZ. The PI3K/Akt pathway in meta-inflammation. Int J Mol Sci. 2022;23(23). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/36499659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Xue JF, Shi ZM, Zou J, Li XL. Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed Pharmacother. 2017;89:1252–61. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28320092. [DOI] [PubMed] [Google Scholar]
- 122. Wang J, Lv X, Guo X, Dong Y, Peng P, Huang F, et al. Feedback activation of STAT3 limits the response to PI3K/AKT/mTOR inhibitors in PTEN-deficient cancer cells. Oncogene. 2021;10:8. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/33431808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Martin M, Schifferle RE, Cuesta N, Vogel SN, Katz J, Michalek SM. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J Immunol. 2003;171:717–25. Retrieved from .https://www.ncbi.nlm.nih.gov/pubmed/12847238. [DOI] [PubMed] [Google Scholar]
- 124. Campos A, Sharma S, Obermair A, Salomon C. Extracellular vesicle-associated miRNAs and Chemoresistance: a systematic review. Cancers (Basel). 2021;13(18). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/34572835. 10.3390/cancers13184608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Li L, Wang H, Zhang J, Chen X, Zhang Z, Li Q. Effect of endothelial progenitor cell-derived extracellular vesicles on endothelial cell ferroptosis and atherosclerotic vascular endothelial injury. Cell Death Discov. 2021;7:235. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34493702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ma Y, Zhou D, Zhang H, Tang L, Qian F, Su J. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote the proliferation of Schwann cells by regulating the PI3K/AKT Signaling pathway via transferring miR-21. Stem Cells Int. 2021;2021:1496101. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34552631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hu S, Wang Z, Jin C, Chen Q, Fang Y, Jin J, et al. Human amniotic epithelial cell-derived extracellular vesicles provide an extracellular matrix-based microenvironment for corneal injury repair. J Tissue Eng. 2022;13:20417314221122123. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/36093432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sohrabi B, Dayeri B, Zahedi E, Khoshbakht S, Nezamabadi Pour N, Ranjbar H, et al. Mesenchymal stem cell (MSC)-derived exosomes as novel vehicles for delivery of miRNAs in cancer therapy. Cancer Gene Ther. 2022;29:1105–16. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35082400. [DOI] [PubMed] [Google Scholar]
- 129. Li G, Wang B, Ding X, Zhang X, Tang J, Lin H. Plasma extracellular vesicle delivery of miR-210-3p by targeting ATG7 to promote sepsis-induced acute lung injury by regulating autophagy and activating inflammation. Exp Mol Med. 2021;53:1180–91. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34321587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hu Y, Zai H, Jiang W, Yao Y, Ou Z, Zhu Q. miR-126 in extracellular vesicles derived from Hepatoblastoma cells promotes the tumorigenesis of Hepatoblastoma through inducing the differentiation of BMSCs into cancer stem cells. J Immunol Res. 2021;2021:6744715. Retrieved from . https://www.ncbi.nlm.nih.gov/pubmed/34746322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Furth N, Aylon Y. The LATS1 and LATS2 tumor suppressors: beyond the hippo pathway. Cell Death Differ. 2017;24:1488–501. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28644436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Mana-Capelli S, McCollum D. Angiomotins stimulate LATS kinase autophosphorylation and act as scaffolds that promote hippo signaling. J Biol Chem. 2018;293:18230–41. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30266805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Li K, Yan G, Huang H, Zheng M, Ma K, Cui X, et al. Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J Nanobiotechnology. 2022;20:38. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35057811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, et al. Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burn-induced excessive inflammation. EBioMedicine. 2016;8:72–82. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27428420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ding Y, Bi L, Wang J. MiR-1180 promotes cardiomyocyte cell cycle re-entry after injury through the NKIRAS2-NFkappaB pathway. Biochem Cell Biol. 2020;98:449–57. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31955591. [DOI] [PubMed] [Google Scholar]
- 136. Mayr, C., Hemann, M. T., & Bartel, D. P., Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation, Science, 2007, 315, 1576–9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17322030. 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhang L, Li YJ, Wu XY, Hong Z, Wei WS. MicroRNA-181c negatively regulates the inflammatory response in oxygen-glucose-deprived microglia by targeting toll-like receptor 4. J Neurochem. 2015;132:713–23. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25545945. [DOI] [PubMed] [Google Scholar]
- 138. Mukaro VR, Quach A, Gahan ME, Boog B, Huang ZH, Gao X, et al. Small tumor necrosis factor receptor biologics inhibit the tumor necrosis factor-p38 signalling axis and inflammation. Nat Commun. 2018;9:1365. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29636466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Dou G, Tian R, Liu X, Yuan P, Ye Q, Liu J, et al. Chimeric apoptotic bodies functionalized with natural membrane and modular delivery system for inflammation modulation. Sci Adv. 2020;6:eaba2987. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32832662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611–25. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27461391. [DOI] [PubMed] [Google Scholar]
- 141. Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–27. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15585754. [DOI] [PubMed] [Google Scholar]
- 142. Weddell JC, Chen S, Imoukhuede PI. VEGFR1 promotes cell migration and proliferation through PLCgamma and PI3K pathways. NPJ Syst Biol Appl. 2018;4:1. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29263797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chang CH, Pauklin S. Extracellular vesicles in pancreatic cancer progression and therapies. Cell Death Dis. 2021;12:973. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34671031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Chen K, Li Y, Xu L, Qian Y, Liu N, Zhou C, et al. Comprehensive insight into endothelial progenitor cell-derived extracellular vesicles as a promising candidate for disease treatment. Stem Cell Res Ther. 2022;13:238. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35672766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Xia L, Fu GS, Yang JX, Zhang FR, Wang XX. Endothelial progenitor cells may inhibit apoptosis of pulmonary microvascular endothelial cells: new insights into cell therapy for pulmonary arterial hypertension. Cytotherapy. 2009;11:492–502. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19526390. [DOI] [PubMed] [Google Scholar]
- 146. Hansmann G, Chouvarine P, Diekmann F, Giera M, Ralser M, Mülleder M, et al. Human umbilical cord mesenchymal stem cell-derived treatment of severe pulmonary arterial hypertension. Nature Cardiovascular Research. 2022;1:568–76. 10.1038/s44161-022-00083-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bian X, Ma K, Zhang C, Fu X. Therapeutic angiogenesis using stem cell-derived extracellular vesicles: an emerging approach for treatment of ischemic diseases. Stem Cell Res Ther. 2019;10:158. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31159859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22992590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–38. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27108839. [DOI] [PubMed] [Google Scholar]
- 150. Patel NK, Nunez JH, Sorkin M, Marini S, Pagani CA, Strong AL, et al. Macrophage TGF-beta signaling is critical for wound healing with heterotopic ossification after trauma, JCI. Insight. 2022;7(20). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/36099022. 10.1172/jci.insight.144925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kiritsi D, Nystrom A. The role of TGFbeta in wound healing pathologies. Mech Ageing Dev. 2018;172:51–8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29132871. [DOI] [PubMed] [Google Scholar]
- 152. Zhang L, Wei W, Ai X, Kilic E, Hermann DM, Venkataramani V, et al. Extracellular vesicles from hypoxia-preconditioned microglia promote angiogenesis and repress apoptosis in stroke mice via the TGF-beta/Smad2/3 pathway. Cell Death Dis. 2021;12:1068. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34753919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Brennan MA, Layrolle P, Mooney DJ. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv Funct Mater. 2020;30(37). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/32952493. 10.1002/adfm.201909125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Jackson HW, Defamie V, Waterhouse P, Khokha R. TIMPs: versatile extracellular regulators in cancer. Nat Rev Cancer. 2017;17:38–53. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27932800. [DOI] [PubMed] [Google Scholar]
- 155. Gomes LR, Terra LF, Wailemann RA, Labriola L, Sogayar MC. TGF-beta1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer. 2012;12:26. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22260435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lai P, Weng J, Guo L, Chen X, Du X. Novel insights into MSC-EVs therapy for immune diseases. Biomark Res. 2019;7:6. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/30923617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Helissey C, Guitard N, Thery H, Goulinet S, Mauduit P, Girleanu M, et al. Two new potential therapeutic approaches in radiation cystitis derived from mesenchymal stem cells: extracellular vesicles and conditioned medium. Biology (Basel). 2022;11(7). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/36101361. 10.3390/biology11070980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Jere SW, Abrahamse H, Houreld NN. The JAK/STAT signaling pathway and photobiomodulation in chronic wound healing. Cytokine Growth Factor Rev. 2017;38:73–9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29032938. [DOI] [PubMed] [Google Scholar]
- 159. Lee JH, Lee CW, Park SH, Choe KM. Spatiotemporal regulation of cell fusion by JNK and JAK/STAT signaling during drosophila wound healing. J Cell Sci. 2017;130:1917–28. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28424232. [DOI] [PubMed] [Google Scholar]
- 160. Harrison DA. The Jak/STAT pathway, Cold Spring Harb Perspect Biol. 2012;4(3). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/22383755. 10.1101/cshperspect.a011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6:402. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34824210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Xie J, Wu X, Zheng S, Lin K, Su J. Aligned electrospun poly(L-lactide) nanofibers facilitate wound healing by inhibiting macrophage M1 polarization via the JAK-STAT and NF-kappaB pathways. J Nanobiotechnology. 2022;20:342. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35883095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Li Y, Tan J, Miao Y, Zhang Q. MicroRNA in extracellular vesicles regulates inflammation through macrophages under hypoxia. Cell Death Discov. 2021;7:285. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34635652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Tang D, Cao F, Yan C, Fang K, Ma J, Gao L, et al. Extracellular vesicle/macrophage Axis: potential targets for inflammatory disease intervention. Front Immunol. 2022;13:705472. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35769456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Asati V, Mahapatra DK, Bharti SK. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: structural and pharmacological perspectives. Eur J Med Chem. 2016;109:314–41. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26807863. [DOI] [PubMed] [Google Scholar]
- 166. Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18:189–218. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14737178. [DOI] [PubMed] [Google Scholar]
- 167. Li HW, Zeng HS. Regulation of JAK/STAT signal pathway by miR-21 in the pathogenesis of juvenile idiopathic arthritis. World J Pediatr. 2020;16:502–13. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/31641939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Narauskaite D, Vydmantaite G, Rusteikaite J, Sampath R, Rudaityte A, Stasyte G, et al. Extracellular vesicles in skin wound healing. Pharmaceuticals (Basel). 2021;14(8). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/34451909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Hade MD, Suire CN, Mossell J, Suo Z. Extracellular vesicles: emerging frontiers in wound healing. Med Res Rev. 2022;42:2102–25. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35757979. [DOI] [PubMed] [Google Scholar]
- 170. Hou Y, Li J, Guan S, Witte F. The therapeutic potential of MSC-EVs as a bioactive material for wound healing. Engineered Regeneration. 2021;2:182–94. [Google Scholar]
- 171. Cabral J, Ryan AE, Griffin MD, Ritter T. Extracellular vesicles as modulators of wound healing. Adv Drug Deliv Rev. 2018;129:394–406. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29408181. [DOI] [PubMed] [Google Scholar]
- 172. Bailey AJM, Li H, Kirkham AM, Tieu A, Maganti HB, Shorr R, et al. , MSC-derived extracellular vesicles to heal diabetic wounds: a systematic review and meta-analysis of preclinical animal studies, Stem Cell Rev Rep, 2022, 18, 968–79. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33893619. 10.1007/s12015-021-10164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Born LJ, Chang KH, Shoureshi P, Lay F, Bengali S, Hsu ATW, et al. HOTAIR-loaded mesenchymal stem/stromal cell extracellular vesicles enhance angiogenesis and wound healing. Adv Healthc Mater. 2022;11(5):e2002070. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33870645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Dai W, Dong Y, Han T, Wang J, Gao B, Guo H, et al. Microenvironmental cue-regulated exosomes as therapeutic strategies for improving chronic wound healing. NPG Asia Materials. 2022;14(1). 10.1038/s41427-022-00419-y. [DOI] [Google Scholar]
- 175. Vu NB, Nguyen HT, Palumbo R, Pellicano R, Fagoonee S, Pham PV. Stem cell-derived exosomes for wound healing: current status and promising directions. Minerva Med. 2021;112:384–400. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33263376. [DOI] [PubMed] [Google Scholar]
- 176. Xie Y, Yu L, Cheng Z, Peng Y, Cao Z, Chen B, et al. SHED-derived exosomes promote LPS-induced wound healing with less itching by stimulating macrophage autophagy. J Nanobiotechnology. 2022;20:239. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35597946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Yu Z, Hao R, Du J, Wu X, Chen X, Zhang Y, et al. A human cornea-on-a-chip for the study of epithelial wound healing by extracellular vesicles. iScience. 2022;25:104200. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35479406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Prasai A, Jay JW, Jupiter D, Wolf SE, El Ayadi A, Role of exosomes in dermal wound healing: a systematic review, J Invest Dermatol, 2022, 142, 662–678 e668. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34461128. 10.1016/j.jid.2021.07.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Li M, Wang T, Tian H, Wei G, Zhao L, Shi Y. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model, Artif cells Nanomed. Biotechnol. 2019;47:3793–803. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31556314. [DOI] [PubMed] [Google Scholar]
- 180. Rincon-Benavides MA, Mendonca NC, Cuellar-Gaviria TZ, Salazar-Puerta AI, Ortega-Pineda L, Blackstone BN, et al. Engineered Vasculogenic extracellular vesicles drive nonviral direct conversions of human dermal fibroblasts into induced endothelial cells and improve wound closure. Advanced Therapeutics. 2023;6:2200197. Retrieved from https://onlinelibrary.wiley.com/doi/abs/10.1002/adtp.202200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Lu S, Lu L, Liu Y, Li Z, Fang Y, Chen Z, et al. Native and engineered extracellular vesicles for wound healing, front Bioeng. Biotechnol. 2022;10:1053217. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/36568307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Kim S, Shin Y, Choi Y, Lim K-M, Jeong Y, Dayem AA, et al. Improved wound healing and skin regeneration ability of 3,2′-Dihydroxyflavone-treated mesenchymal stem cell-derived extracellular vesicles. Int J Mol Sci. 2023;24(8). 10.3390/ijms24086964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Chen J, Tan Q, Yang Z, Jin Y. Engineered extracellular vesicles: potentials in cancer combination therapy. J Nanobiotechnology. 2022;20:132. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35292030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Jayasinghe MK, Pirisinu M, Yang Y, Peng B, Pham TT, Lee CY, et al. Surface-engineered extracellular vesicles for targeted delivery of therapeutic RNAs and peptides for cancer therapy. Theranostics. 2022;12:3288–315. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35547755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. White MJV, Briquez PS, White DAV, Hubbell JA. VEGF-A, PDGF-BB and HB-EGF engineered for promiscuous super affinity to the extracellular matrix improve wound healing in a model of type 1 diabetes. NPJ Regen Med. 2021;6:76. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34795305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Wang Y, Zhao M, Liu S, Guo J, Lu Y, Cheng J, et al. Macrophage-derived extracellular vesicles: diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 2020;11:924. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33116121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Totemeyer S, Sheppard M, Lloyd A, Roper D, Dowson C, Underhill D, et al. IFN-gamma enhances production of nitric oxide from macrophages via a mechanism that depends on nucleotide oligomerization domain-2. J Immunol. 2006;176:4804–10. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16585574. [DOI] [PubMed] [Google Scholar]
- 188. Sahoo S, Kariya T, Ishikawa K. Targeted delivery of therapeutic agents to the heart. Nat Rev Cardiol. 2021;18:389–99. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33500578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51:1–12. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30872574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Pomatto MAC, Bussolati B, D'Antico S, Ghiotto S, Tetta C, Brizzi MF, et al. Improved loading of plasma-derived extracellular vesicles to encapsulate antitumor miRNAs. Mol Ther Methods Clin Dev. 2019;13:133–44. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30788382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Han Y, Jones TW, Dutta S, Zhu Y, Wang X, Narayanan SP, et al. Overview and update on methods for cargo loading into extracellular vesicles. Processes (Basel). 2021;9(2). Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/33954091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Li T, Zhang L, Lu T, Zhu T, Feng C, Gao N, et al. Engineered extracellular vesicle-delivered CRISPR/CasRx as a novel RNA editing tool. Adv Sci (Weinh). 2023;10(10):e2206517. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/36727818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Jang SC, Economides KD, Moniz RJ, Sia CL, Lewis N, McCoy C, et al. ExoSTING, an extracellular vesicle loaded with STING agonists, promotes tumor immune surveillance. Commun Biol. 2021;4:497. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33888863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Martini PGV, Guey LT. A new era for rare genetic diseases: messenger RNA therapy. Hum Gene Ther. 2019;30:1180–9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31179759. [DOI] [PubMed] [Google Scholar]
- 195. Lai J, Huang C, Guo Y, Rao L. Engineered extracellular vesicles and their mimics in cardiovascular diseases. J Control Release. 2022;347:27–43. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35508222. [DOI] [PubMed] [Google Scholar]
- 196. Fan Z, Jiang C, Wang Y, Wang K, Marsh J, Zhang D, et al. Engineered extracellular vesicles as intelligent nanosystems for next-generation nanomedicine. Nanoscale Horiz. 2022;7:682–714. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35662310. [DOI] [PubMed] [Google Scholar]
- 197. Estes S, Konstantinov K, Young JD. Manufactured extracellular vesicles as human therapeutics: challenges, advances, and opportunities. Curr Opin Biotechnol. 2022;77:102776. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/36041354. [DOI] [PubMed] [Google Scholar]
- 198. Zhang X, Zhang H, Gu J, Zhang J, Shi H, Qian H, et al. Engineered extracellular vesicles for cancer therapy. Adv Mater. 2021;33:e2005709. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33644908. [DOI] [PubMed] [Google Scholar]
- 199. Mentkowski KI, Snitzer JD, Rusnak S, Lang JK. Therapeutic potential of engineered extracellular vesicles. AAPS J. 2018;20:50. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29546642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748–59. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34211166. [DOI] [PubMed] [Google Scholar]
- 201. Zhang J, Ji C, Zhang H, Shi H, Mao F, Qian H, et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci Adv. 2022;8:eabj8207. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Mukherjee A, Bisht B, Dutta S, Paul MK. Current advances in the use of exosomes, liposomes, and bioengineered hybrid nanovesicles in cancer detection and therapy. Acta Pharmacol Sin. 2022;43:2759–76. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35379933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Corso G, Mager I, Lee Y, Gorgens A, Bultema J, Giebel B, et al. Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography. Sci Rep. 2017;7:11561. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28912498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Watson DC, Johnson S, Santos A, Yin M, Bayik D, Lathia JD, et al. Scalable isolation and purification of extracellular vesicles from Escherichia coli and other bacteria. J Vis Exp. 2021;(176). Retrieved from; https://www.ncbi.nlm.nih.gov/pubmed/34723953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Bellotti C, Lang K, Kuplennik N, Sosnik A, Steinfeld R. High-grade extracellular vesicles preparation by combined size-exclusion and affinity chromatography. Sci Rep. 2021;11:10550. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34006937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Xu R, Simpson RJ, Greening DW. A protocol for isolation and proteomic characterization of distinct extracellular vesicle subtypes by sequential centrifugal ultrafiltration. Methods Mol Biol. 2017;1545:91–116. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27943209. [DOI] [PubMed] [Google Scholar]
- 207. Zhang F, Guo J, Zhang Z, Duan M, Wang G, Qian Y, et al. Application of engineered extracellular vesicles for targeted tumor therapy. J Biomed Sci. 2022;29:14. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35189894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Man K, Brunet MY, Jones MC, Cox SC. Engineered extracellular vesicles: tailored-made nanomaterials for medical applications. Nanomaterials (Basel). 2020;10(9): Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32942556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. de Abreu RC, Fernandes H, da Costa Martins PA, Sahoo S, Emanueli C, Ferreira L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat Rev Cardiol. 2020;17:685–97. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32483304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Lyu Y, Guo Y, Okeoma CM, Yan Z, Hu N, Li Z, et al. Engineered extracellular vesicles (EVs): promising diagnostic/therapeutic tools for pediatric high-grade glioma. Biomed Pharmacother. 2023;163:114630. Retrieved from https://www.sciencedirect.com/science/article/pii/S0753332223004183. [DOI] [PubMed] [Google Scholar]
- 211. Zhuang J, Tan J, Wu C, Zhang J, Liu T, Fan C, et al. Extracellular vesicles engineered with valency-controlled DNA nanostructures deliver CRISPR/Cas9 system for gene therapy. Nucleic Acids Res. 2020;48:8870–82. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32810272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Gualerzi A, Kooijmans SAA, Niada S, Picciolini S, Brini AT, Camussi G, et al. Raman spectroscopy as a quick tool to assess purity of extracellular vesicle preparations and predict their functionality. J Extracell Vesicles. 2019;8:1568780. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30728924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Upadhya R, Madhu LN, Attaluri S, Gitai DLG, Pinson MR, Kodali M, et al. Extracellular vesicles from human iPSC-derived neural stem cells: miRNA and protein signatures, and anti-inflammatory and neurogenic properties. J Extracell Vesicles. 2020;9:1809064. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32944193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Cai T, Zhang Q, Wu B, Wang J, Li N, Zhang T, et al. LncRNA-encoded microproteins: a new form of cargo in cell culture-derived and circulating extracellular vesicles. J Extracell Vesicles. 2021;10(9):e12123. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34276900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Maugeri M, Nawaz M, Papadimitriou A, Angerfors A, Camponeschi A, Na M, et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat Commun. 2019;10:4333. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31551417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Liang, Y., Duan, L., Lu, J., & Xia, J., Engineering exosomes for targeted drug delivery, Theranostics, 2021, 11, 3183–95. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33537081. 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Subra C, Grand D, Laulagnier K, Stella A, Lambeau G, Paillasse M, et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J Lipid Res. 2010;51:2105–20. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/20424270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Cho K, Kook H, Kang S, Lee J. Study of immune-tolerized cell lines and extracellular vesicles inductive environment promoting continuous expression and secretion of HLA-G from semiallograft immune tolerance during pregnancy. J Extracell Vesicles. 2020;9:1795364. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32944184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this review are available from the corresponding author upon reasonable request. The data include the web page context, the search query, the search results, and the conflict of interest statement. The data are stored in a secure database that complies with the journal's data sharing policy.


