Highlights
-
•
RHDV2_Bg12 originated in a hare through recombination at the RdP-VP60 junction.
-
•
Structural genes come from RHDV2, the non-structural ones from a new hare lagovirus.
-
•
Rabbits orally inoculated with RHDV2_Bg12 neither developed RHD nor seroconverted.
-
•
RHDV2_Bg12 has no longer been identified despite active surveillance on lagomorphs.
Keywords: Lagovirus, RHDV2, Recombination, Animal experiments, Virus selection
Abstract
The genus Lagovirus, belonging to the family Caliciviridae, emerged around the 1980s. It includes highly pathogenic species, rabbit hemorrhagic disease virus (RHDV/GI.1) and European brown hare syndrome virus (EBHSV/GII.1), which cause fatal hepatitis, and nonpathogenic viruses with enteric tropism, rabbit calicivirus (RCV/GI.3,4) and hare calicivirus (HaCV/GII.2). Lagoviruses have evolved along two independent genetic lineages: GI (RHDV and RCV) in rabbits and GII (EBHSV and HaCV) in hares. To be emphasized is that genomes of lagoviruses, like other caliciviruses, are highly conserved at RdRp-VP60 junctions, favoring intergenotypic recombination events at this point. The recombination between an RCV (genotype GI.3), donor of non-structural (NS) genes, and an unknown virus, donor of structural (S) genes, likely led to the emergence of a new lagovirus in the European rabbit, called RHDV type 2 (GI.2), identified in Europe in 2010. New RHDV2 intergenotypic recombinants isolated in rabbits in Europe and Australia originated from similar events between RHDV2 (GI.2) and RHDV (GI.1) or RCV (GI.3,4). RHDV2 (GI.2) rapidly spread worldwide, replacing RHDV and showing several lagomorph species as secondary hosts. The recombination events in RHDV2 viruses have led to a number of viruses with very different combinations of NS and S genes. Recombinant RHDV2 with NS genes from hare lineage (GII) was recently identified in the European hare. This study investigated the first RHDV2 (GI.2) identified in Italy in European hare (RHDV2_Bg12), demonstrating that it was a new virus that originated from the recombination between RHDV2, as an S-gene donor and a hare lagovirus, not yet identified but presumably nonpathogenic, as an NS gene donor. When rabbits were inoculated with RHDV2_Bg12, neither deaths nor seroconversions were recorded, demonstrating that RHDV2_Bg12 cannot infect the rabbit. Furthermore, despite intensive and continuous field surveillance, RHDV2_Bg12 has never again been identified in either hares or rabbits in Italy or elsewhere. This result showed that the host specificity of lagoviruses can depend not only on S genes, as expected until today, but potentially also on some species-specific NS gene sequences. Therefore, because RHDV2 (GI.2) infects several lagomorphs, which in turn probably harbor several specific nonpathogenic lagoviruses, the possibility of new speciation, especially in those other than rabbits, is real. RHDV2 Bg_12 demonstrated this, although the attempt apparently failed.
1. Introduction
Together with the European brown hare syndrome virus (EBHSV, genotype GII.1), rabbit hemorrhagic disease virus (RHDV, genotypes GI.1 and GI.2) belongs to the still emerging genus of lagoviruses, which is itself part of the Caliciviridae family [Ohlinger et al., 1990; Parra et al., 1990; Green et al., 2000; Vinje et al., 2019]. RHD and EBHS manifest as fatal hepatitis, with nearly 100% and 50% mortality rates in adults, respectively. The infection is subclinical in young animals <2 months old [Marcato et al., 1991; Abrantes et al., 2012; Capucci et al., 2017a]. Both viruses emerged around the 1980s. EBHSV (GII.1) was first identified in Scandinavia [Gavier-Widen et al., 1991], whereas RHDV (GI.1) emerged in China [Liu et al., 1984; Eden et al., 2015], spreading within a few years to Asia, Europe, Central America, and Africa [Morisse et al., 1991]. RHD has caused enormous economic damage to rabbit breeding and decimated wild rabbit populations, with indirect negative consequences for animal species, such as the lynx in Spain, for which the rabbit is an essential part of the food chain [Cooke, 2002]. In contrast, RHDV was introduced in Australia in 1996 as a biocontrol agent to reduce the enormous negative impact of wild rabbit populations as alien species on the country's ecology and agriculture [Mutze et al., 1998]. The genus Lagovirus also includes viruses that are genetically related to RHDV (GI.1 and GI.2) and EBHSV (GII.1) but not pathogenic (NP-lagovirus), commonly named rabbit calicivirus (RCV/GI.3,4) [Capucci et al., 1996; Strive et al., 2009; Le Gall-Recule et al., 2011b] and hare calicivirus (HaCV/GII.2), respectively [Droillard et al., 2018; Mahar et al., 2019; Cavadini et al., 2021]. These NP-lagoviruses are enteric viruses and induce specific antibodies that interfere to varying degrees with infections with pathogenic lagovirus, ranging from complete protection from RHD [Capucci et al., 1996] to almost no protection at all [Strive et al., 2009].
The epidemiological status of lagoviruses has become even more complicated since 2010 when a new viral emersion occurred again in rabbits, but this time in Europe [Le Gall-Recule et al., 2011a; Dalton et al., 2012]. The new virus was named RHDV type 2 (RHDV2/GI.2) [Le Gall-Recule et al., 2013] because, although genetically related to RHDV (GI.1), the average nucleotide identity based on VP60 sequences is 82.4%, and compared to other lagoviruses, it constitutes a new phylogenetic group. RHDV2 (GI.2) exhibits three distinctive phenotypic notable traits: (a) a significantly different immunogenic and antigenic capsid surface from that of RHDV, which makes RHDV2 a new serotype (different and specific RHDV2 vaccines are therefore required for complete protection from RHD); (b) the capacity to cause RHD even in young rabbits only a few weeks old [Le Gall-Recule et al., 2011a]; and (c) a broader host spectrum. Although the rabbit remains the primary host, RHDV2 also infects and causes RHD-like disease in other lagomorph species [Puggioni et al., 2013; Camarda et al., 2014; Le Gall-Recule et al., 2017; Velarde et al., 2017, 2021; Asin et al., 2022]. These distinctive phenotypic characteristics determined at least two main consequences: (i) the spread of RHDV2 (GI.2) in rabbit populations worldwide, including Australia [Hall et al., 2015], within a few years, resulting in almost complete replacement of RHDV (GI.1), and (ii) the acquisition of endemic status in North and Central America, never achieved by RHD despite several isolated outbreaks detected since 2000 [McIntosh et al., 2007; Asin et al., 2021, 2022; Williams et al., 2021].
Lagoviruses are small, round, nonenveloped viruses with a single-stranded, positive sense, polyadenylated RNA genome of ∼7.5 kb. The viral capsid consists of 90 subunits, each made up of two VP60 molecules, containing both genomic RNA (gRNA) and 3′-coterminal subgenomic RNA (sgRNA) of ∼2.5 kb [Meyers et al., 1991b; Wang et al., 2013]. The genome includes seven non-structural genes upstream of the capsid protein, all encoded in one polyprotein (ORF1), whereas the 3′-terminal ORF2 encodes a minor structural protein (VP10) [Meyers et al., 1991b; Mahar et al., 2021]. There is consistent homology between the 5′-terminal nucleotide sequences of gRNA and sgRNA [Meyers et al., 1991a], and the RNA-dependent RNA polymerase (RdRp)-VP60 junction is highly conserved within the genus, both genetic features facilitating genomic recombination events during the virus replication inside the cell. Viruses in this genus have been classified into two proposed genogroups: GI encompasses all viruses related to rabbit haemorrhagic disease virus (RHDV/GI.1, RHDV2/GI.2, RCV/GI.3,4), while GII includes a variety of highly divergent viruses, all related to European brown hare syndrome virus, both pathogenic (EBHSV/GII.1) and non-pathogenic (HaCV/GII.2 and GII.X) [Le Pendu et al., 2017]. Indeed, the diversity in the last viruses is huge compared to those in the GI.
In connection with the increased effectiveness of lagomorph disease surveillance systems, especially in Europe and Australia, and the rapid worldwide spread of RHDV2 (GI.2), the number of available lagovirus genome sequences has increased exponentially over the last decade. Consequently, researchers agreed on the need to classify the genomes of the lagovirus into the two proposed genogroups, six genotypes (GI.1–4 and GII.1–2), and several variants [Le Pendu et al., 2017] to bring order to the increasing genetic complexity. At the same time, the number of complete deposited genome sequences made up mainly of the SP sequence VP60, is steadily increasing. All these facts led to the discovery that, as in other genera of Caliciviridae [Bull et al., 2005, 2007; Coyne et al., 2006], genomic recombination occurs quite frequently in lagoviruses, especially at the junction between non-structural (NS) and structural (S) genes, effectively generating new genomes and thus new evolutionary possibilities [Lopes et al., 2015]. Recombinant lagoviruses are defined by the nomenclature [RdRp genotype]P-[capsid genotype] [Mahar et al., 2021]. Confirming the predominant presence of RHDV2 in the field, all currently identified genomes from rabbit isolates have the S genes of RHDV2. In contrast, NS genes come from distinct genotypes and variants of both RHDV (GI.1) and nonpathogenic RCV (GI.3,4) [Hu et al., 2017; Hall et al., 2018; Mahar et al., 2018; Silverio et al., 2018]. Furthermore, most recombinant genomes originated in rabbits but were also detected in hares [Le Gall-Recule et al., 2017; Velarde et al., 2017; Hall et al., 2018; Mahar et al., 2021]. More recently, new RHDV2 recombinants with genomes GII.1P-GI.2 originated in Germany from the European hare [Szillat et al., 2020], showing that their non-structural genes come from a hare-associated lineage. Interestingly, an epidemiological survey on the diffusion of RHDV2 recombinant with RCV-A1 (GI.4eP-GI.2 and GI.4cP-GI.2) in distinct regions of Australia showed that both recombinants were replacing the original RHDV2 (GI.1bP-GI.2), demonstrating that NS genes can significantly act on the fitness of the virus [Mahar et al., 2021]. Furthermore, knowledge acquired on genome recombination in RHDV2 promoted retrospective studies on old RHDV isolates from France and Sweden, which successfully demonstrated the occurrence of recombination events at the junction of RdRp and VP60 genes, thus indicating a possible gain in the fitness of recombinant viruses [Lopes et al., 2017; Abrantes et al., 2020b]. Finally, a study of RHDV2 genomes collected in France at the time of the emergence of the first cases demonstrated that NS genes of RHDV2 were closely related to those of RCV E1 (GI.3) and that a recombination GI.3P-GI.2 could be at the origin of RHDV2 emergence [Abrantes et al., 2020a].
The first identification of an RHDV2 strain in a European hare (RHDV2_Bg12) was in an individual found dead in Italy in 2012 during passive surveillance. A previous study reported the case and the preliminary characterization and sequencing of the VP60 [Velarde et al., 2017]. In the current study, we further demonstrate that RHDV2_Bg12 originated by recombination at the junction RdRp-VP60 between an RHDV2 (GI.2) donor of S genes and an unknown hare lagovirus as the donor of NS genes. Unexpectedly, rabbits inoculated with RHDV2_Bg12 were not susceptible to infection.
2. Materials and methods
2.1. Origin, preparation, and storage of samples
Liver samples were collected from two adult wild European hares (Lepus europaeus) found dead during passive surveillance monitoring plans. One hare originated from the province of Bergamo (Lombardy, northern Italy) in 2012 (Hare_Bg12), whereas a second one was from the area of Barcelona (Catalonia, northeastern Spain) in 2014 (Hare_Bcn14). At necropsy, both hares showed a general state of good preservation and lesions consistent with an EBHS-like disease, then confirmed by virological and histopathological methods as being caused by RHDV2 (GI.2) [Velarde et al., 2017]. In the first preliminary characterization, the VP60 of the two strains (RHDV2_Bg12 KT 308115 and RHDV2_Bcn14 – KT308116) were characterized, and they showed an amino acid identity of 98.77%. In addition, a previously characterized highly virulent RHDV2 strain (RHDV2_Ta14 – MH 746804) [Capucci et al., 2017b] identified in rabbits was used as challenge virus in experimental trials. Such strain has an amino acid identity of 98.60% with the VP60 of RHDV2_Bg12. Livers were homogenized at 10% (w/v) in phosphate-buffered saline (PBS; pH 7.4) and centrifuged at 10,000 × g for 15 min at 4 °C to remove cellular debris. Finally, the samples were mixed with an equal volume of glycerol and stored at – 25 °C [Velarde et al., 2017]. These preparations were used in this study for diagnostic purposes and further viral characterization as well as for being used in experimental trials as inoculum.
2.2. Genomic characterization
To obtain the complete genome sequence of the RHDV2_Bg12 strain, RNA was extracted from the hare liver and amplified by reverse transcription-polymerase chain reaction (RT-PCR) as described previously [Velarde et al., 2017]. To sequence the region of the genome upstream the VP60 gene, already sequenced, five overlapping amplicons, including the amplicon spanning the RdRp-VP60 junction, were obtained (Table 1), gel purified, and sequenced. To obtain the 3′-end of the genome, cDNA synthesis was performed using oligo-dTadapter primer followed by PCR using RHDV2–1676F/Adapter primer [Cavadini et al., 2021] (Table 1). The DNA sequences were determined with an ABI Prism 3500 Series Genetic Analysers in both directions (Applied Biosystems, Foster City, CA, USA) using the PCR primers and the Big Dye Terminator v3.1 (Life Technologies) as recommended by the manufacturer. Contig assembling and genome sequence analysis were done using Seqman NGen DNASTAR version 11.2.1 (DNASTAR, Madison, WI, USA). A pair of primers, EBHSVL-1005F and EBHSVL-1342R, was designed to amplify a portion of the p37 gene of RHDV2_Bg12 and used to analyze 38 EBHSV strains, originally identified by sandwich ELISA [WOAH, 2023] in hares in Italy between 2011 and 2023. Then, another pair of primers 4420F/RHDV2–116R, was also used to amplify and sequence RHDV2 collection strains. This pair of primers (Table 1) was designed to amplify and sequence the region spanning the RdRp-VP60 junction of RHDV2 strains and thus define the type of recombination. The reverse primer binds to the RHDV2 sequence of the vp60 gene, while the forward primer is a degenerate primer able to bind to the RdRp sequences of all different genotypes. A total of 175 strains were selected from outbreaks that occurred in both intensive and rural farms and equally distributed according to geographical location (North, Central and South Italy) and year of detection (2012–2020).
Table 1.
Oligonucleotides used in this study: the nucleotide positions are based on EBHSV strain GenBank accession KC832839 or (*) on RHDV2 strain GenBank accession KM878681.
| Primer | Sequence 5′–3′ | Position | Amplification size (nt) |
|---|---|---|---|
| Primers for amplification and sequencing the non structural portion of the genome | |||
| HaCV-AF | 5′-ATTATGGCGGTTGCGTCGCG-3′ | 3–17 | 641 |
| HaCV-AR | 5′-CTCGGGCAGCTGCGATCATGTC-3′ | 643–622 | |
| EBHS-2744R | 5′-TAGGCCTCCACACGTGTGGAT-3′ | 2737–2716 | 2215 |
| EBHS-500F | 5′-GGCATAATGGACAAGTTTGTTG-3′ | 499–523 | |
| EBHS-1018F | 5′-TGGGACAGGGTTATGTGTGC-3′ | 1018–1037 | 2161 |
| EBHS-3166R | 5′-TCGTCCTGTTTAAGCTGTCTG-3′ | 3179–3159 | |
| EBHS-3059F | 5′-CAACAYGCYCACGACATGAC-3′ | 3053–3071 | 1466 |
| EBHSV-4519R | 5′- CAACACAAGGTGACATTGTGGAGTC −3′ | 4519–4495 | |
| Primers for amplification and sequencing the ORF2 region | |||
| RHDV2–1676F* | 5′-GACCGTCCACAAGCACGCTT-3′ | 6980–6999 | 500 |
| Adapter | 5′-GACTCGAGTCGACATCG-3′ | — | |
| Primers for amplification and sequencing the region spanning the RdRp-VP60 junction | |||
| RHDV-4420F* | 5′-GTYGGCRTTGACATGACMTC-3′ | 4429–4448 | 984 |
| RHDV2–116R* | 5′-CTAGTAGTGGCCACAACACC-3′ | 5393–5412 | |
| Primers for amplification a portion of p37 of RHDV2_Bg12 | |||
| EBHSVL-1005F | 5′-TCTGATTGTTGATGCCATTAAGAGC-3′ | 990–1014 | 383 |
| EBHSVL-1342R | 5′-CACTGACAATTTCCAACTGAGACAA-3′ | 1372–1348 | |
2.3. Phylogenetic and recombination analysis
Phylogenetic analysis was performed using a data set of 142 full genome lagovirus sequences representing the six genotypes based on the VP60 sequences: GI.1 (n. 68), GI.2 (n. 33), GI.3 (n.4), GI.4 (n.16), GII.1 (n.17) and GII.2 (n. 4), available in GenBank and aligned with MEGA X [Kumar et al., 2018]. The analysis was carried out separately for the following partitions in the alignment: nucleotide positions (a) 1–5239, non-structural gene sequences, (b) 5240–6869, structural gene VP60. The evolutionary history was inferred by using the Maximum Likelihood method and General Time Reversible model. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. Support for each cluster was obtained from 1000 bootstrap replicates. Evolutionary analyses were conducted in MEGA X.
The dataset of 142 full genome lagovirus sequences was screened for potential recombination using six methods (RDP, GENECOV, Bootscan, MaxChi, Chimaera, SiScan) implemented in the RdP4 (V. 4.100) [Martin et al., 2015]. The sequence similarity plotting software SimPlot [Lole et al., 1999] was used for depicting the recombinant strains.
2.4. Animal experiments
All the trials were performed in a Biosecurity Level 3-designated area. All animal works were approved by the Ministry of Health (Decreto 21/2014-B) and conducted according to the requirements of national (DM 4/3/2014 n. 26) and European (2010/63/EU) laws regarding the care and use of animals.
The two RHDV2 isolates used to infect rabbits came from liver homogenates with 50% glycerol stored at −25 °C and diluted 1:10 in PBS shortly before inoculation. The trials were performed with adult seronegative New Zealand rabbits, bought from a commercial farm authorized for the production of experimental rabbits. After infection, rabbits were observed daily until the experiment's end, during which any variation in body temperature, drinking and feeding rates, or behavior was registered. Blood samples for detecting specific anti-RHDV2 antibodies by cELISA were taken 7 and 23 days postinfection (p.i.). Dead rabbits were subjected to necropsy, and liver tissue samples were taken to confirm the diagnosis of RHD using sandwich ELISA. An RT-PCR was performed by amplifying a portion of the p37 and VP60 genes [WOAH, 2023] as further viral characterization.
First preliminary trial (Fig. 1A): three rabbits were infected with 1.5 ml liver homogenate of RHDV2_Bg12 throughout the oronasal (os) route. Twenty-three days later, the three rabbits received 1 ml of the same inoculum intravenously (iv), while a fourth rabbit was added and inoculated os as above. Finally, the susceptibility of these four healthy rabbits was tested by inoculating them with the Spanish strain RHDV2_Bcn14 (KT308116) at 40 days p.i., two rabbits by os (1.5 ml inoculum) and two by iv (1 ml inoculum), respectively.
Fig. 1.
Schematic representation of the experimental trials. A) First preliminary trial; B) Second trial. On the horizontal bars are indicated the days since the beginning of the trial (blood sampling at −3 days before 1st infection) to the end of the experiments (respectively 49 and 33 p.i.). Time points for blood samplings and the different infections are indicated, also including the number and identification of each infected rabbit, route of infection, dose and type of inoculum. The dot lines and the upside-down rabbit figures at days 43 and 45 p.i. indicate respectively in Section A) the death of rabbits #87 and #90 after challenge with the Spanish isolate RHDV2_Bcn14, and in Section B) the death of rabbits #91 and #92 after challenge with RHDV2_TA14.
Second trial (Fig. 1B): a new group of eight rabbits was challenged with three different viral preparations: (i) four rabbits, two of which were inoculated by os and two by iv, with a mixture of the stock preparations at a ratio of three volumes of RHDV2_Bg12 and one volume of RHDV2_Bcn14; (ii) two rabbits, 1.5 ml by os, with RHDV2_Bg12, with a mixture of three volumes RHDV2_Bg12 and one of PBS; and (iii) the last two rabbits, 1.5 ml by os, with one volume of RHDV2_Bcn14, and three volumes of PBS. All preparations were kept in agitation for 1 h at room temperature before being diluted 1/10 in PBS and used for rabbit infection. The primary purpose of this last experiment was to verify if the low level of specific IgM found in the hare liver infected with RHDV2_Bg12 (see Results) could interfere with RHDV2_Bcn14 infectivity. Finally, survived rabbits were challenged at 24 days p.i. with 1.5 ml by os of a highly virulent RHDV2 strain (RHDV2_Ta14) [Capucci et al., 2017b]. The infection trial endpoints for animals that survived were fixed at 9 days after final infection (i.e., 49 days and 33 days after the beginning of the first and second trial, respectively). A graphic summary of the different animal experiments performed is reported in Fig. 1.
2.5. Sandwich enzyme-linked immunosorbent assay (ELISA)
The sandwich ELISA specific for RHDV2 was performed as described previously for RHDV [Capucci et al., 1995; WOAH, 2023]. The Nunc Maxisorp plate was adsorbed with rabbit anti-RHDV2 IgG at 2 μg/ml. Liver homogenates were diluted to base 3 in ELISA buffer starting at 1:10, and RHDV2 was detected using mouse RHDV2-specific mAbs [Velarde et al., 2017]. Finally, HRP-labeled rabbit anti-mouse IgG was used in the last step of the reaction. OPD was used for the detection of mAbs binding to RHDV2.
2.6. Competition and isotype ELISA
Competition ELISA (cELISA RHDV2) and isotype ELISA (IsoELISA) for RHDV2 were performed as described previously [Cooke et al., 2000; Velarde et al., 2017]. Briefly, the cELISA plate was adsorbed with rabbit IgG purified from a hyperimmune RHDV2 serum. Subsequently, the test serum was incubated with the pretitrated RHDV2 antigen, and the reaction between the virus and rabbit antibodies in the serum occurred in the liquid phase. Finally, an HRP-conjugated anti-RHDV2 mAb detected the amount of virus captured on the solid phase during the first incubation. Each serum was analyzed at an initial dilution of 1:10 and diluted in 4-base thrice (i.e., 1:10, 1:40, 1:160, and 1:640 dilution). A serum is classified as positive for RHDV2 antibodies when the ratio of its OD value at the 1:10 dilution to the OD value of the negative control serum at the same dilution is <0.75. The titer of a positive serum is equal to the dilution at which the ratio of its OD value to the OD value of the negative control serum at the 1:10 dilution is within 0.4–0.6.
IsoELISA comprises three ELISAs that detect IgG, IgM, and IgA against RHDV or RHDV2 [Cooke et al., 2000; WOAH, 2023]. For detecting IgM and IgA (ELISAs used in this study), reverse ELISA with an anti-IgM or anti-IgA mAb adsorbed to the solid phase was used. This approach is necessary to avoid the predominant competition of IgG for antigens that usually occurs in a direct ELISA. For both ELISA tests, the serum is diluted in 4-base from 1:40–1:163, 840 and incubated with RHDV2 added at a predetermined dilution. In the last step, an HRP-conjugated anti-RHDV2 mAb was added [Velarde et al., 2017]. A serum is classified as positive when the OD value at the 1:40 dilution is greater than twice the OD value of the negative control serum at the same dilution. The titer of a positive serum is equal to the last dilution at which it is still positive using the above calculation.
2.7. Western blotting
Polyacrylamide gel electrophoresis was performed with the Invitrogen system, treating the samples with NuPage LDS Sample solution and sample reducing agent. After heat denaturation, the samples were loaded onto precast gels (NuPage 4%–12% Bis-Tris gel), and gel running was performed using NuPage MOPS SDS Running buffer at 150 mA. Protein electrotransfer was performed using Bio-Rad Trans-blot Turbo and Trans-Blot Turbo Mini 0.2 µm polyvinylidene fluoride membranes according to the manufacturer's instructions. The membrane was saturated by incubation with PBS (pH 7.4) containing 1% bovine serum albumin and 0.1% Tween 20 for 30 min at room temperature under continuous agitation. Monoclonal antibody (mAb) 5G3, highly specific for the denatured VP60 of lagoviruses [Capucci et al., 1995,1996], was added at a previously established concentration, and the membrane was incubated under the same conditions for an additional hour. The membrane was washed thrice for 5 min each with PBS (pH 7.4) with 0.1% Tween 20 and incubated with horseradish peroxidase (HRP)-conjugated goat IgG anti-mouse IgG (IZSLER in-house product) for an additional hour. Finally, the membrane was developed using the One-Step™ Ultra TMB-Blotting solution (Invitrogen) after a cycle of four washes
2.8. Electron microscopy (EM)
Negative staining EM examination (nsEM) was conducted on liver homogenates using the Airfuge method [Lavazza et al., 1990]. The samples are frozen, thawed twice, and centrifuged at 5500 × g for 20 min and 12,800 × g for 10 min for clarification. The second supernatant (82 μl) was ultracentrifuged (Airfuge, Beckman Coulter, Inc., Life Sciences, Indianapolis, IN, USA) for 15 min at 21 psi (82,000 × g). The Airfuge was fitted with an A100 rotor holding six 175 μl test tubes in which specific adapters were put for 3 mm grids, which allowed direct pelleting of viral particles on carbon-coated Formvar copper grids. These were stained using 2% sodium phosphotungstate (NaPt; pH 6.8) for 1.5 min and observed with a TEM FEI Tecnai G2 Biotwin (Thermo Fisher, FEI, Hillsboro, OR, USA) operating at 85 kV. The identification and description of the lagovirus particles observed at × 26,500 were achieved based on their typical morphological characteristics.
3. Results
3.1. Genomic and phylogenetic studies
As reported previously [Velarde et al., 2017], BLAST analysis performed with the VP60 sequence of RHDV2_Bg12 (KT308115) presented 97.2% nt identity with the Italian reference RHDV2 strain Ud11, identify in North of Italy in 2011 (JQ929052) and 98.3% nt identity with the RHDV2 strain Sr12–2 (KC741409) identified in the Italian hare (Lepus corsicanus) in Sicily in 2012. Furthermore, in light of recent findings on multiple recombinant events among RHDV2 strains [Coyne et al., 2006; Lopes et al., 2015; Le Gall-Recule et al., 2017; Hall et al., 2018; Mahar et al., 2018], the complete genome sequence of RHDV2_Bg12 was analyzed to check for the possible presence of such events. The organization of the complete genome was identical to other lagoviruses, i.e., two overlapping open reading frames were present: ORF1 (1–7009 nt) and ORF2 (6990–7343 nt). The total length of the sequenced gRNA obtained was 7428 bp, not including the 5′-untranslated region and the first ATG; thus, it was ∼20 nucleotides shorter than the complete genome sequences in GenBank. Surprisingly, BLAST analysis performed on the ORF1 nucleotide sequence encoding for NS proteins showed the highest identity with EBHSV (GII.1), particularly with the EBHSV strain (KC832839) identified in Sweden in 1982 (87% identity; Table 2). Compared to HaCV (GII.2), the nonpathogenic lagovirus found in the hare (KR230102) [Cavadini et al., 2021], the identity of NS genes decreased to 81% and even less (78.8%) with a recombinant RHDV2 (GII.1P-GI.2) recently identified in a German hare (RHDV/GER-NW/EI17–1. L03577/2019, LR899142) [Szillat et al., 2020], with NS genes from the hare lagovirus lineage (GII). Finally, the NS genes identity of RHDV2_Bg12 further decreased to ∼70% compared to RHDV (GI.1) and RCV (GI.3) genomes included in GenBank. Conversely, RHDV2_Bg12 VP60 gene BLAST analysis showed the highest nucleotide identity (99%) with the type strain from Spain RHDV2-N11 first isolated from young rabbits in the autumn of 2011 in north-eastern Spain (KM878681) [Dalton et al., 2012].
Table 2.
Nucleotide and amino acid identity values relative to the NS genes/proteins of the hare RHDV2_Bg12 strain compared to the representative lagoviruses sequences in GenBank.
The phylogenetic analysis inferred with VP60 nucleotide sequences from 142 lagovirus available in GenBank confirmed previous data by placing the RHDV2_Bg12 strain in the RHDV2 (GI.2) cluster (Fig. 2A). On the contrary, the phylogenetic analysis tree of the sequences corresponding to the portion of the genome coding for the non-structural proteins (NSP) from the same 142 lagovirus strains placed the RHDV2_Bg12 in the hare lagovirus GII cluster (Fig. 2B), suggesting a recombination event between an RHDV2 (GI.2) strain and a hare lagovirus strain not yet identified (GII.X).
Fig. 2.
Maximum Likelihood (ML) phylogenetic trees performed for: A) the structural genes VP60 (n = 142 sequences; nucleotides 5240–6869; nucleotide substitutions model GTR+G + I) and B) the non-structural genes (n = 142 sequences; nucleotides 1–5239; nucleotide substitutions model GTR+G + I). The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. Support for each cluster was obtained from 1.000 bootstrap replicates. Bootstrap values >60% are shown. Genotype clusters, which do not include virus variants sequenced in this study, were collapsed and annotated accordingly.
SimPlot (Fig. 3) and RDP analysis were performed to gain further knowledge on such recombination events. Both approaches showed a recombination breakpoint close to the VP60 starting codon as described for other recombinants RHDV2 [Lopes et al., 2015; Mahar et al., 2018; Silverio et al., 2018, 2021], with a beginning between nucleotides 5219–5267 in alignment of the sequences (C.I. of 99%). The evidence of a single recombination event was supported by a strong P value using different methods implemented in the RDP software (RDP 1.64 × 10−4; GENECOV 1.751 × 10−108; BootScan 4.76 × 10−158; MaxChi 1.34 × 10−31; Chimaera 7 × 10−34; SiScan 2.92 × 10−52). As previously shown by BLAST analysis of the NS genes, the closest major parent is a GII.1 strain (KC832839), whereas the closest minor parent is the Spain prototype GI.2 strain (KM878681).
Fig. 3.
Similarity plot of the RHDV2_Bg12 recombinant strain. The horizontal axis represents the nucleotide position of the genome, whereas the vertical axis represents the similarity to the two putative parental strains. The black plot indicates the closest major parent, EBHSV strain KC832839, donor of the NS genes, whereas the gray plot indicates RHDV2_N11 KM87868 strain, the closest minor parent, donor of the S gene. A window size of 200 bp with a step size of 20 bp was used.
Finally, in order to investigate whether and to what extent RHDV2_Bg12 was actively circulating in rabbit populations in northern Italy, sequencing of a 984 bp region was performed over the RdRp-VP60 junction on 175 RHDV2 collection strains. Still, the RHDV2_Bg12 strain was not detected in any of the samples tested. Similarly, the examination of 38 EBHSV collection strains by RT-PCR with specific primers designed on the p37 gene of the NS region of RHDV2_Bg12 did not show any positive amplification. Therefore, the demonstration that RHDV2_Bg12 was the only case of RHDV2 found in the European hare in northern Italy suggested that RHDV2_Bg12 could not spread further in either hares or rabbits to any significant extent.
3.2. Experimental infections of rabbits
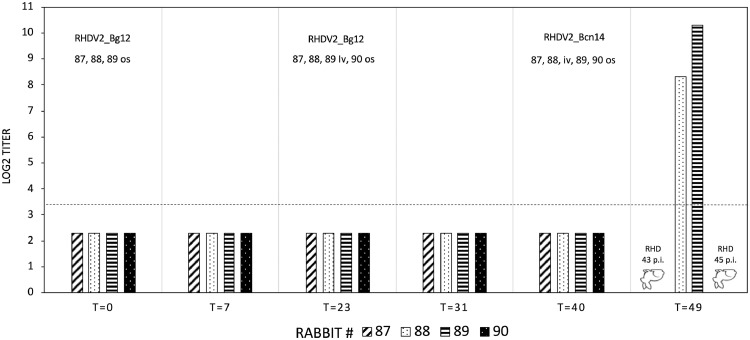
Because the genome of isolate RHDV2_Bg12 (GII.X/GI.2) was never found before, laboratory rabbits were inoculated to amplify the virus for further studies. Surprisingly, at 8 days p.i., the three rabbits inoculated os were healthy, showed no signs of disease, and remained seronegative for RHDV2 (Fig. 4). Identical results were obtained when the same three rabbits were reinfected iv with RHDV2_Bg12, and a fourth additional rabbit was infected os. On day 40, the four rabbits still seronegative for RHDV2 were challenged with the Spanish hare isolate RHDV2_Bcn14 (GI.3/GI.2) using both infection routes. This time, the expected results were achieved: all rabbits had increased body temperature, stopped feeding, decreased drinking and showed apathy and depression. Two died, and two definitely survived, showing an evident seroconversion at the endpoint of the experiment (49 days p.i.) (Fig. 4). Necropsy examination and virological ELISA performed on the liver of the two dead rabbits confirmed that death was due to RHD, an acute “hemorrhagic” form in the rabbit dead at 3 days p.i. (#87, iv infected) and a more subacute/chronic form (e.g., mainly hepatomegaly and pallor, splenomegaly, and icterus) in the rabbit dead at 5 days p.i. (#90, os infected). Finally, both RT-PCR amplifying the gene coding the VP60 of RHDV2 and the NS p37 of RHDV2_Bg12 excluded the replication of the latter in rabbits inoculated with RHDV2_Bcn14, confirming this last one as the cause of RHD (Fig. 5).
Fig. 4.
Summary of the serological results of the first experimental trial. Titres on the ordinate are expressed as Log2, and the dot-line represents the threshold of positivity (Log2 = 3.3). On the abscissa, the days post the beginning of the trial are reported (T=). Infections were performed as indicated in the upper section of the figure in correspondence to different time points. The upside-down rabbit figures indicate the death of rabbits #87 and #90, respectively, at days 43 and 45 p.i. due to RHD.
Fig. 5.
RT-PCR amplification of a portion of p37 and VP60 genes [WOAH, 2023]. RNA amplification from hare liver homogenates used as inoculum for infection (lane 1: Hare_RHDV2_Bg12 and lane 2: Hare_RHDV2_Bcn14) and rabbit liver homogenates experimentally infected (lane 3: rabbit #87 dead at 3 days p.i. and lane 4: rabbit #90 dead at 5 days p.i.). NTC, not-template control; MW, S100 molecular weight markers.
Considering the unexpected results, a second confirmatory infection trial was performed using RHDV2_Bg12 and RHDV2_Bcn14 isolates. In this experiment, four rabbits were infected, two by os and two by iv, with an inoculum made of a mixture of the two isolates to test whether the liver homogenate of the RHDV2_Bg12 hare might contain factors (see also below) interfering with its infectivity. Four more rabbits were contemporarily infected by os with one or the other strain mixed with PBS (Fig. 1). Although no mortality was observed in any inoculated rabbits, the six rabbits infected with the inoculum containing isolate RHDV2_Bcn14 seroconverted with medium-high titers in cELISA and IgM ELISA. Conversely, the remaining two rabbits infected by os only with RHDV2_Bg12 showed no seroconversion (Fig. 6). Finally, all the eight rabbits in this traial were then challenged with a highly pathogenic strain, i.e., RHDV2_Ta14 (MH746804) which lead to death due to acute RHD of the two rabbits previously infected with the sole RHDV2_Bg12 (#91 and 92). The other six seropositive rabbits showed no sign of disease, and ELISA titers did not change.
Fig. 6.
Serological results of the second experimental trial. On the ordinate, the titres, expressed as Log2, for RHDV2 antibodies detected by c-ELISA and IgM ELISA. The dot-line represents the threshold of positivity (Log2 = 3.3). On the abscissae, the days post the beginning of the trial are reported (T=). Infections were performed as indicated in the upper section of the figure in correspondence to the different time points. RHD indicates the death with clinical signs and lesions consistent with RHD of rabbits #91 and #92, respectively, at days 26 and 27 p.i..
In summary, these results, especially the absence of seroconversion and unaltered susceptibility to further RHDV2 challenge, strongly suggested that the rabbit is not susceptible to infection by the RHDV2_Bg12 hare isolate.
3.3. Structural analysis of RHDV2_Bg12
Faced with an unexpected but interesting result, isolate RHDV2_Bg12 was characterized for those aspects that could negatively interfere with the outcome of experimental infection. The sandwich ELISA in Fig. 7, based on two mAbs recognizing distinct epitopes on the surface of RHDV2, showed that the RHDV2_Bg12 concentration in the liver homogenate was very similar to that of RHDV2_Bcn14 and both about thrice lower than the average amount detected in the liver of rabbits that died of acute RHD. Furthermore, the similarity in the shape of virus dilution curves indicated the antigenic integrity of RHDV2_Bg12 and RHDV2_Bcn14.
Fig. 7.
ELISA sandwich performed with two RHDV2-specific mAbs on liver homogenates. The liver of a rabbit that died of acute RHD  , the liver of the Hare_Bcn14
, the liver of the Hare_Bcn14  , and the liver of the Hare_Bg12
, and the liver of the Hare_Bg12  . Abscissae indicate the inverse of the dilutions of the homogenates (40 means 1/40), whereas ordinates indicate the OD values (absorbance 492 nm = A492).
. Abscissae indicate the inverse of the dilutions of the homogenates (40 means 1/40), whereas ordinates indicate the OD values (absorbance 492 nm = A492).
The structural appearance of the virions observed with Western blotting and nsEM, both obtained using raw liver homogenates, are shown in Fig. 8A and B, respectively. Despite the difficulties and limitations of analyzing a crude and thus “dirty” homogenate, it was possible to identify in all three samples viral capsids with the typical morphology of lagoviruses, i.e., most of the particles were 32–35 roundparticles with small, short external projections. However, Western blotting, while confirming a similar virus concentration in the two homogenates, showed that whereas VP60 of the capsids of RHDV2_Bcn14 and RHDV2_Ud11, used as reference strains of acute RHD cases were intact, about half of the VP60 of RHDV2_Bg12 capsid was degraded to the VP30 fragment, corresponding to the inner capsid shell (S-domain of VP60) [Capucci et al., 1991; Granzow et al., 1996]. As shown previously, such degraded RHDV particles are related to the onset of the specific IgM response and the viral clearance. Thus, the homogenates were tested for anti-RHDV antibodies using specific ELISA isotype tests. No specific IgG or IgM was detected in the RHDV2_Bcn14 homogenate, whereas there was only a low amount of specific IgM, corresponding to a serum titer of ∼1:320, in the RHDV2_Bg12 homogenate. Overall, results showed that RHDV2_Bg12, although isolated from a case in which the specific IgM response was beginning, has retained a reasonably intact structure and most likely also its infectious power.
Fig. 8.
(A) Western blotting was performed on liver homogenates using mAb 5G3, recognizing a continuous epitope on the C-terminal half of the VP60 of lagoviruses. Lane 1, RHDV2_Ud11; lane 2, RHDV2_Bg12; lane 3, RHDV2_Bcn14. The samples were diluted at 1:300 (no. 1) and 1:30 (nos. 2 and 3). (B) nsEM of lagovirus particles detected in liver homogenates identified in (1) rabbits in Italy during the first RHDV2 outbreak (RHDV2_Ud11), (2) the hare from Italy (RHDV2_Bg12), and (3) the hare from Spain (RHDV2_Bcn14), respectively. NaPt 2%, TEM FEI Tecnai G2 Spirit operating at 85 kV. Bars, 200 nm.
4. Discussion
After the emergence of RHDV2, it was discovered that recombination of the genome at the junction between RdRp and VP60 also frequently occurs in the genus Lagovirus, giving rise to new viral genomes [Lopes et al., 2015]. This was presumably linked to the rapid and vast spread of RHDV2, which significantly increased the probability of a rabbit being simultaneously infected with RHDVs and RCVs [Le Gall-Recule et al., 2011b; Dalton et al., 2012; Hall et al., 2015; Asin et al., 2021; Ben Chehida et al., 2021]. In a previous study [Velarde et al., 2017], we characterized and described the isolate RHDV2_Bg12, which likely represents the first detection of RHDV2 in European hares in Europe, then followed by other cases in France [Le Gall et al., 2017] and Australia [Hall et al., 2017]. In this study, its complete genome was sequenced, and the genomic analysis concluded that the genome of RHDV2_Bg12 originated from recombination between an RHDV2 (genotype GI.2), as the donor of S gene and a hare lagovirus (genotype GII.X), yet unidentified, as the donor of NS genes.
Considering that the highest NS genes identity of RHDV2_Bg12 is 86.6% with one of the first EBHSV (genotype GII.1) isolates in Sweden and that the identity decreases with respect to HaCV (genotype GII.2) and the recent new GII.1 NS genes isolates in hares in Germany [Szillat et al., 2020], the genotype-level classification of RHDV2_Bg12 according to criteria proposed by Le Pendu et al. [2017] is difficult to attribute. Regardless of genome classification, the NS genes of these viruses are highly divergent from known lagoviruses, clustering basal to EBHSV (GII.1), but still highly distant from this clade (Fig. 2). As RHDV2_Bg12 clusters between EBHSV and HaCV variants, it is likely that the parental virus from which these NS genes came is a virus of hares, and it is difficult to tell whether this parental virus is benign/non-pathogenic. However, it seems likely since it was previously undetected. Overall, this work does show that there is a great diversity of unsampled lagoviruses in hares.
Since the parental strains were not identified in the Hare_Bg12, it is unlikely that the sampled hare was where the recombination event occurred. Concerning the NS genome portion, making any assumptions about the donor virus is impossible. However, previous extensive research on lagoviruses in the European hare in northern Italy allowed the authors to discover only HaCV (GII.2), the hare nonpathogenic lagovirus, whose genomic identity is 81% with NS genes of RHDV2_Bg12 [Cavadini et al., 2021]. Adding that the VP60 of RHDV2_Bg12 showed maximum identity with the Iberian RHDV2_N11 [Dalton et al., 2012] and the RHDV2_Sr12 [Camarda et al., 2014] isolated in Sicily, it can be concluded that the recombinant RHDV2_Bg12 originated before its first identification.
Where it first originated is undetermined: it could be just supposed that it was not in northern Italy, considering the extensive surveillance and systematic testing of found dead hares. A couple of hypotheses on the country/area of origin of the recombinant strain could be proposed. The first is France, considering i) the high presence of European hare and wild rabbit populations living in sympatry as a likely determinant factor for increased frequency of cross-species infection with RHDV2 [Le Gall-Recule et al., 2017]; ii) the quite short period elapsed between the first identification of RHDV2 and its spreading from France to neighbouring countries. The second is the Iberian Peninsula, since the VP60 of RHDV2_Bg12 is highly genetically correlated with that of RHDV2_N11, one of the first Spanish isolates characterised by a low pathogenic phenotype i.e., 0% case fatality rate in the adult rabbits and 21% in the kits [Dalton et al., 2012]. The fact remains that regardless of the origin, it is certainly unknown by which routes and ways it arrived in Italy.
The overall results of inoculating rabbits with RHDV2_Bg12 were the absence of infection/disease, with the immune system maintaining a naïve state and thus full susceptibility to RHD. Of course, the main experiment to ascertain the degree of infectivity of the RHDV2_Bg12 homogenate would have been to inoculate European hares. However, this type of experimental trials on hares are difficult to perform and still to be conducted. This is due to several reasons: the difficulty in complying with all the standards set by European and national rules for animal experiments, including the need for special authorization to infect wild animals, the difficulty in finding hares adapted to live in captivity, especially in cages placed in enclosures located in highly contained BL3 areas, without causing them suffering and distress. Alternatively, the RHDV2_Bg12 homogenate was thoroughly analyzed to identify any cause that might interfere with virus replication. First, the hare infected by RHDV2_Bg12 died of an EBHS-like disease coinciding with the onset of IgM response, presumably 4–6 days p.i. [Capucci et al., 1991], however, the amount of virus in the homogenate was still in the same order as in acute cases of RHD, and about half of the viral capsid still retained the classic cup shape, which refers to intact VP60. Therefore, even considering that (a) previous experimental inoculations of rabbits with RHDV isolates from “chronic” cases of RHD (i.e., high level of specific IgM and smooth capsid with VP60 completely degraded to VP30) induced of acute RHD (Capucci and Lavazza, unpublished data) and (b) the general condition of the body of the hare Bg12 found dead in the field was quite good, it was concluded that there is no reason to assume that RHDV2_Bg12 (GII.XP-GI.2) is not infectious per se.
The European hare appears to be the only host of RHDV2_Bg12 to date; thus, it could be considered a genotype within the lagovirus genus, largely different from those recognised till now. This also suggests that the host spectrum of lagovirus might depend not only on VP60, as believed until today, but also on NSP. However, further experiments are required to confirm this. RHDV2 recombinant variants detected so far are obviously able to replicate in rabbits regardless of whether the NS genes evolved in the pathogenic or nonpathogenic lineage. In particular, some recombinants of RHDV2 originating and circulating in the rabbit have also been isolated in dead hares (NS genes GI.1a, GI.1b, and GI.4), demonstrating that NSPs evolved in the rabbit may allow permissive and efficient replication even in a different lagomorph species, such as the European hare [Le Gall-Recule et al., 2017; Velarde et al., 2017; Hall et al., 2018; Mahar et al., 2021]. However, this does not prove that all NS genes evolved in rabbits can allow a recombinant RHDV2 (GI.XP-GI.2) to replicate in hares simply because epidemiological evidence allows only permissive ones to be identified. Moreover, RHDV2 could have originated from recombination with a nonpathogenic RCV, a donor of NS genes of genotype GI.3 [Abrantes et al., 2020a]. Furthermore, recombinants of RHDV2 recently identified in Australia (NS genes GI.4c and GI.4e) have replaced the former dominant parent RHDV2 (GI.1b-GI.2) [Mahar et al., 2021]. Finally, recombinants of RHDV2 have been identified in two hares with the hare lineage (GII.1P-GI.2) NS genes as distinct from RHDV2_Bg12 but also from other strains belonging to the EBHSV (GII.1) lineage [Szillat et al., 2020]. Note that these strains were identified in only two hares found dead in 2014 and 2019 in the same region about 100 km apart, but even if likely circulating, they have never been found again.
All this considered, it could be theoretically speculated that what occurred in rabbits could also happen in hares, i.e., an RHDV2 recombined with NS GII could increase its fitness for hares compared to RHDV2 with the NS gene GI evolved in rabbits. The results of the infection trials showed that a hare virus RHDV2_Bcn14, which has GI-derived NSPs, was able to infect and kill rabbits where the Bg_12 hare virus with that same capsid genotype was not, and this would further support the theory that GI NSPs are required for infection in rabbits. Since RHDV2_Bg12 was detected in 2012 and work presented here shows that neither this recombinant nor the parental strain that gave rise to the NS genes has been detected before or since, it is clear that these NS genes have not given rise to a fitter virus. In conclusion, whether NS genes can have a relevant action on the fitness of lagoviruses, including determining the host spectrum, cannot be actually defined since data is not strong enough to support this finding, but it is worthy of investigation in the future. In fact, considering that the rabbit and the hare are two distinct lagomorph species, some of the numerous molecular interactions between NSPs and host cellular macromolecules may have evolved differently and thus become species-specific over time, which could be the case with NSPs of RHDV2_Bg12.
Assuming that RHDV2_Bg12 represented a possibility of the emergence of a new lagovirus genotype in the European hare, the attempt failed. In fact, despite extensive field surveillance conducted over the past 10 years in Europe, it has never been identified again. Indeed, the RHDV2_Bg12 case is reminiscent of the Michigan RCV (MRCV) outbreak in a rabbit farm in the United States in 2001, during which some rabbits died with RHD clinical signs [Bergin et al., 2009]. Phylogenetic analyses indicated that MRCV was a lagovirus belonging to a genetic lineage distinct from RHDV and RCV. However, experimental inoculation of MRCV in rabbits yielded ambiguous results, and neither MRCV nor a related genome was ever identified again. The failure of the definitive emergence of MRCV and RHDV2_Bg12 should be likely related to epidemiological context in which they emerged i.e., in North America, the limited presence of the European rabbit and in Northern Italy, the presence of fragmented populations of hares which undergo hunting pressure and significant yearly fluctuations in age composition and densities. Considering that the European hare is a secondary host of RHDV2, likely a “spillover”, the likelihood of RHDV2 G.IIXP-GI.2 infecting rabbits seems relatively remote. However, this could happen if the newly acquired GII NS genes consistently change the makeup of RHDV2 to such an extent that it can increase its ability to spread within the European hares' population and thus the likelihood of a back-transmission to the rabbit.
Since the emergence of RHDV (GI.1) and EBHSV (GII.1) in the 1980s, virological and serological investigations and experimental reproduction of RHD and EBHS have shown that the primary hosts of the two viruses were the European rabbit and the brown hare, respectively [Lavazza et al., 1996, 2015]. Thanks to several phylogenetic studies further proof was the separate genetic evolution of RHDV (GI.1) and EBHSV (GII.1), which presumably began centuries ago, together with the related nonpathogenic lagoviruses that are also species-specific. This evolutionary picture of lagoviruses changed significantly with the emergence of RHDV2 (GI.2). The main reason for this was the broader host spectrum that RHDV2 (GI.2) exhibited during its rapid spread worldwide in rabbit populations, presumably in connection with a more overall specificity of histoblood group antigens acting as anchors on the cell for the lagovirus [Lopes et al., 2018]. The recombination events in RHDV2 viruses have led to a number of viruses with very different combinations of NS and S genes i.e., genes from different rabbit and hare lineages are combining to form a new virus with two evolutionary histories. As the rabbit is the primary host of RHDV2 and likely the most widespread lagomorphs in nature, almost all identified recombination events were intergenotypic (GI.1 or GI.3 or GI.4-GI.2). However, there are increasing identifications of new inter-genogroup recombinant lagoviruses (GII.1P-GI.2) in European hares [Szillat et al., 2020]. As in the rabbit, RHDV2 recombines with NS genes of hare lagoviruses, retaining its pathogenicity, confirming that the pathogenic phenotype of a lagovirus is associated with SPs (presumably VP60). NS genes of these new RHDV2 recombinant genomes of European hares, the one detected in Germany [Szillat et al., 2020] and RHDV2_Bg12, have never been detected before, suggesting that in hares, besides pathogenic EBHSV, other unrecognized lagovirus could be present. Thus, although such viral strains may be apparently not epidemiologically important, it is still of interest to report and characterize them in terms of cataloging the diversity of existing lagoviruses, and to better elucidate their evolutionary steps.
In conclusion, the increasing availability of complete genome sequences improved the knowledge of the genus Lagovirus. Indeed, other than genotyping, it becomes evident the importance to fully characterize and understand the phenotype of viruses, including the pathotype for which experimental trials are still relevant, especially in the case of newly emerging strains for which no epidemiological data are available, as in the case of RHDV2_Bg12 or the recombinant RHDV2 strains (GII.1P-GI.2) recently identified by Szillat et al. (2020) in European hare in Germany.
Altogether, genetic and epidemiological data on lagoviruses suggest that, driven by the continued worldwide spread of RHDV2 (GI.2), there are field conditions for the emergence and evolution in lagomorphs of new viral entities. This would recommend keeping the level of general surveillance on the lagoviruses as high as possible to precisely depict their evolution.
Data availability
Full genone sequences are available in GenBank under accession numbers KT308115. The raw data supporting the conclusions of this article will be made available on request.
Credit authorship contribution statement
Patrizia Cavadini: genomic analysis, discussion of the results and revision of the draft article.
Tiziana Trogu: animal experimentation, discussion of the results and revision of the draft article.
Roser Velarde: made available the Spanish RHDV2 strain, discussion of the results and revision of the draft article.
Antonio Lavazza: animal experimentation, electron microscopy, discussion of the results and revision of the draft article.
Lorenzo Capucci: structural and antigenic analysis of the viruses, drafting of the article - review and editing
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This work was partially supported by the Project Prima-Lagmed – Italian Ministry of University and Research CUP B84I18010580005
Acknowledgements
We wish to thank Mrs Francesca Merzoni, Mrs Alessadra Previdi and Silvia Brodini for the laboratory technical work.
Data availability
Data will be made available on request.
References
- Abrantes J., Droillard C., Lopes A.M., Lemaitre E., Lucas P., Blanchard Y., et al. Recombination at the emergence of the pathogenic rabbit haemorrhagic disease virus Lagovirus europaeus/GI.2. Sci. Rep. 2020;10:14502–14504. doi: 10.1038/s41598-020-71303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrantes J., Lopes A.M., Lemaitre E., Ahola H., Banihashem F., Droillard C., et al. Retrospective analysis shows that most RHDV GI.1 strains circulating since the late 1990s in France and Sweden were recombinant GI.3P-GI.1d strains. Genes (Basel) 2020;11:910. doi: 10.3390/genes11080910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrantes J., van der Loo W., Le Pendu J., Esteves P.J. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): a review. Vet. Res. 2012;43:12. doi: 10.1186/1297-9716-43-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asin J., Nyaoke A.C., Moore J.D., Gonzalez-Astudillo V., Clifford D.L., Lantz E.L., et al. Outbreak of rabbit hemorrhagic disease virus 2 in the southwestern United States: first detections in southern California. J. Vet. Diagn. Invest. 2021;33:728–731. doi: 10.1177/10406387211006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asin J., Rejmanek D., Clifford D.L., Mikolon A.B., Henderson E.E., Nyaoke A.C., et al. Early circulation of rabbit haemorrhagic disease virus type 2 in domestic and wild lagomorphs in southern California, USA (2020-2021) Transbound Emerg. Dis. 2022;69:e394–e405. doi: 10.1111/tbed.14315. [DOI] [PubMed] [Google Scholar]
- Ben Chehida F., Lopes A.M., Corte-Real J.V., Sghaier S., Aouini R., Messadi L., et al. Multiple introductions of rabbit hemorrhagic disease virus lagovirus europaeus/GI.2 in Africa. Biology (Basel) 2021;10:883. doi: 10.3390/biology10090883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergin I.L., Wise A.G., Bolin S.R., Mullaney T.P., Kiupel M., Maes R.K. Novel calicivirus identified in rabbits, Michigan, USA. Emerg. Infect. Dis. 2009;15:1955–1962. doi: 10.3201/eid1512.090839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R.A., Hansman G.S., Clancy L.E., Tanaka M.M., Rawlinson W.D., White P.A. Norovirus recombination in ORF1/ORF2 overlap. Emerg. Infect. Dis. 2005;11:1079–1085. doi: 10.3201/eid1107.041273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R.A., Tanaka M.M., White P.A. Norovirus recombination. J. Gen. Virol. 2007;88:3347–3359. doi: 10.1099/vir.0.83321-0. [DOI] [PubMed] [Google Scholar]
- Camarda A., Pugliese N., Cavadini P., Circella E., Capucci L., Caroli A., et al. Detection of the new emerging rabbit haemorrhagic disease type 2 virus (RHDV2) in Sicily from rabbit (Oryctolagus cuniculus) and Italian hare (Lepus corsicanus) Res. Vet. Sci. 2014;97:642–645. doi: 10.1016/j.rvsc.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Capucci L., Cavadini P., Lavazza A. Elsevier Inc; 2017. Rabbit Hemorrhagic Disease Virus and European Brown Hare Syndrome Virus. [DOI] [Google Scholar]
- Capucci L., Cavadini P., Schiavitto M., Lombardi G., Lavazza A. Increased pathogenicity in rabbit haemorrhagic disease virus type 2 (RHDV2) Vet. Rec. 2017;180:426. doi: 10.1136/vr.104132. [DOI] [PubMed] [Google Scholar]
- Capucci L., Frigoli G., Ronshold L., Lavazza A., Brocchi E., Rossi C. Antigenicity of the rabbit hemorrhagic disease virus studied by its reactivity with monoclonal antibodies. Virus Res. 1995;37:221–238. doi: 10.1016/0168-1702(95)00033-m. [DOI] [PubMed] [Google Scholar]
- Capucci L., Fusi P., Lavazza A., Pacciarini M.L., Rossi C. Detection and preliminary characterization of a new rabbit calicivirus related to rabbit hemorrhagic disease virus but nonpathogenic. J. Virol. 1996;70:8614–8623. doi: 10.1128/JVI.70.12.8614-8623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capucci L., Scicluna M.T., Lavazza A. Diagnosis of viral haemorrhagic disease of rabbits and the European brown hare syndrome. Rev. Sci. Tech. 1991;10:347–370. doi: 10.20506/rst.10.2.561. [DOI] [PubMed] [Google Scholar]
- Cavadini P., Molinari S., Merzoni F., Vismarra A., Posautz A., Gil V.A., Chiari M., Giannini F., Capucci L., Lavazza A. Widespread occurrence of the non-pathogenic hare calicivirus (HaCV Lagovirus GII.2) in captive-reared and free-living wild hares in Europe. Transbound Emerg. Dis. 2021;68(2):509–518. doi: 10.1111/tbed.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke B.D., Robinson A.J., Merchant J.C., Nardin A., Capucci L. Use of ELISAs in field studies of rabbit haemorrhagic disease (RHD) in Australia. Epidemiol. Infect. 2000;124:563–576. doi: 10.1017/s0950268899003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke B.D. Rabbit haemorrhagic disease: field epidemiology and the management of wild rabbit populations. Rev. Sci. Tech. 2002;21:347–358. doi: 10.20506/rst.21.2.1337. [DOI] [PubMed] [Google Scholar]
- Coyne K.P., Reed F.C., Porter C.J., Dawson S., Gaskell R.M., Radford A.D. Recombination of Feline calicivirus within an endemically infected cat colony. J. Gen. Virol. 2006;87:921–926. doi: 10.1099/vir.0.81537-0. [DOI] [PubMed] [Google Scholar]
- Dalton K.P., Nicieza I., Balseiro A., Muguerza M.A., Rosell J.M., Casais R., et al. Variant rabbit hemorrhagic disease virus in young rabbits. Spain. Emerg. Infect Dis. 2012;18:2009–2012. doi: 10.3201/eid1812.120341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droillard C., Lemaitre E., Chatel M., Guitton J.S., Marchandeau S., Eterradossi N., Le Gall-Reculé G. First complete genome sequence of a hare calicivirus strain isolated from Lepus europaeus. Microbiol. Resour. Announc. 2018;7:e01224. doi: 10.1128/MRA.01224-18. –18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden J., Read A.J., Duckworth J.A., Strive T., Holmes E.C. Resolving the origin of rabbit hemorrhagic disease virus: insights from an investigation of the viral stocks released in Australia. J. Virol. 2015;89:12217–12220. doi: 10.1128/JVI.01937-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavier-Widen D., Morner T. Epidemiology and diagnosis of the European brown hare syndrome in Scandinavian countries: a review. Rev. Sci. Tech. 1991;10:453–458. doi: 10.20506/rst.10.2.555. [DOI] [PubMed] [Google Scholar]
- Granzow H., Weiland F., Strebelow H.G., Liu C.M., Schirrmeier H. Rabbit hemorrhagic disease virus (RHDV): ultrastructure and biochemical studies of typical and core-like particles present in liver homogenates. Virus Res. 1996;41:163–172. doi: 10.1016/0168-1702(96)01285-3. [DOI] [PubMed] [Google Scholar]
- Green K.Y., Ando T., Balayan M.S., Berke T., Clarke I.N., Estes M.K., et al. Taxonomy of the caliciviruses. J. Infect. Dis. 2000;181(2):322. doi: 10.1086/315591. Suppl. [DOI] [PubMed] [Google Scholar]
- Hall R.N., Mahar J.E., Haboury S., Stevens V., Holmes E.C., Strive T. Emerging Rabbit Hemorrhagic Disease Virus 2 (RHDVb) Australia. Emerg. Infect. Dis. 2015;21:2276–2278. doi: 10.3201/eid2112.151210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R.N., Mahar J.E., Read A.J., Mourant R., Piper M., Huang N., et al. A strain-specific multiplex RT-PCR for Australian rabbit haemorrhagic disease viruses uncovers a new recombinant virus variant in rabbits and hares. Transbound Emerg. Dis. 2018;65:e444–e456. doi: 10.1111/tbed.12779. [DOI] [PubMed] [Google Scholar]
- Hall R.N., Peacock D.E., Kovaliski J., Mahar J.E., Mourant R., Piper M., Strive T. Detection of RHDV2 in European brown hares (Lepus europaeus) in Australia. Vet. Rec. 2017;180:121. doi: 10.1136/vr.104034. [DOI] [PubMed] [Google Scholar]
- Hu B., Wang F., Fan Z., Song Y., Abrantes J., Zuo Y., et al. Recombination between G2 and G6 strains of rabbit hemorrhagic disease virus (RHDV) in China. Arch. Virol. 2017;162:269–272. doi: 10.1007/s00705-016-3082-6. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavazza A., Cavadini P., Barbieri I., Tizzani P., Pinheiro A., Abrantes J., et al. Field and experimental data indicate that the eastern cottontail (Sylvilagus floridanus) is susceptible to infection with European brown hare syndrome (EBHS) virus and not with rabbit haemorrhagic disease (RHD) virus. Vet. Res. 2015;46:13–14. doi: 10.1186/s13567-015-0149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavazza A., Pascucci S., Gelmetti D. Rod-shaped virus-like particles in intestinal contents of three avian species. Vet. Rec. 1990;126:581. [PubMed] [Google Scholar]
- Lavazza A., Scicluna M.T., Capucci L. Susceptibility of hares and rabbits to the European brown hare syndrome virus (EBHSV) and rabbit haemorrhagic disease virus (RHDV) under experimental conditions. Zentralbl Veterinarmed B. 1996;43:401–410. doi: 10.1111/j.1439-0450.1996.tb00332.x. [DOI] [PubMed] [Google Scholar]
- Le Gall-Recule G., Lavazza A., Marchandeau S., Bertagnoli S., Zwingelstein F., Cavadini P., et al. Emergence of a new lagovirus related to Rabbit Haemorrhagic Disease Virus. Vet. Res. 2013;44:81. doi: 10.1186/1297-9716-44-81. -81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall-Recule G., Lemaitre E., Bertagnoli S., Hubert C., Top S., Decors A., et al. Large-scale lagovirus disease outbreaks in European brown hares (Lepus europaeus) in France caused by RHDV2 strains spatially shared with rabbits (Oryctolagus cuniculus) Vet. Res. 2017;48:70. doi: 10.1186/s13567-017-0473-y. -y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall-Recule G., Zwingelstein F., Boucher S., Le Normand B., Plassiart G., Portejoie Y., et al. Detection of a new variant of rabbit haemorrhagic disease virus in France. Vet. Rec. 2011;168:137–138. doi: 10.1136/vr.d697. [DOI] [PubMed] [Google Scholar]
- Le Gall-Recule G., Zwingelstein F., Fages M., Bertagnoli S., Gelfi J., Aubineau J., et al. Characterisation of a non-pathogenic and non-protective infectious rabbit lagovirus related to RHDV. Virology. 2011;410:395–402. doi: 10.1016/j.virol.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Le Pendu J., Abrantes J., Bertagnoli S., Guitton J., Le Gall-Recule G., Lopes A.M., et al. Proposal for a unified classification system and nomenclature of lagoviruses. J. Gen. Virol. 2017;98:1658–1666. doi: 10.1099/jgv.0.000840. [DOI] [PubMed] [Google Scholar]
- Liu S.J., et al. A new viral disease in rabbits. J. Vet. Diagn. Investig. 1984;16:253–255. [Google Scholar]
- Lole K.S., Bollinger R.C., Paranjape R.S., Gadkari D., Kulkarni S.S., Novak N.G., Ingersoll R., Sheppard H.W., Ray S.C. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 1999;73(1):152–160. doi: 10.1128/JVI.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes A.M., Breiman A., Lora M., Le Moullac-Vaidye B., Galanina O., Nystrom K., et al. Host-specific glycans are correlated with susceptibility to infection by lagoviruses, but not with their virulence. J. Virol. 2018;92:e01759. doi: 10.1128/JVI.01759-17. -17Print 2018 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes A.M., Dalton K.P., Magalhaes M.J., Parra F., Esteves P.J., Holmes E.C., et al. Full genomic analysis of new variant rabbit hemorrhagic disease virus revealed multiple recombination events. J. Gen. Virol. 2015;96:1309–1319. doi: 10.1099/vir.0.000070. [DOI] [PubMed] [Google Scholar]
- Lopes A.M., Silverio D., Magalhaes M.J., Areal H., Alves P.C., Esteves P.J., et al. Characterization of old RHDV strains by complete genome sequencing identifies a novel genetic group. Sci. Rep. 2017;7:13599–13602. doi: 10.1038/s41598-017-13902-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar J.E., Hall R.N., Shi M., Mourant R., Huang N., Strive T., et al. The discovery of three new hare lagoviruses reveals unexplored viral diversity in this genus. Virus Evol. 2019;5:vez005. doi: 10.1093/ve/vez005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar J.E., Jenckel M., Huang N., Smertina E., Holmes E.C., Strive T., et al. Frequent intergenotypic recombination between the non-structural and structural genes is a major driver of epidemiological fitness in caliciviruses. Virus Evol. 2021;7:veab080. doi: 10.1093/ve/veab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar J.E., Read A.J., Gu X., Urakova N., Mourant R., Piper M., et al. Detection and circulation of a novel Rabbit Hemorrhagic Disease Virus in Australia. Emerg. Infect. Dis. 2018;24:22–31. doi: 10.3201/eid2401.170412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcato P.S., Benazzi C., Vecchi G., Galeotti M., Della Salda L., Sarli G., et al. Clinical and pathological features of viral haemorrhagic disease of rabbits and the European brown hare syndrome. Rev. Sci. Tech. 1991;10:371–392. doi: 10.20506/rst.10.2.560. [DOI] [PubMed] [Google Scholar]
- Martin D.P., Murrell B., Golden M., Khoosal A., Muhire B. RDP4: detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015;1(1):vev003. doi: 10.1093/ve/vev003. March 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh M.T., Behan S.C., Mohamed F.M., Lu Z., Moran K.E., Burrage T.G., et al. A pandemic strain of calicivirus threatens rabbit industries in the Americas. Virol. J. 2007;4:96. doi: 10.1186/1743-422X-4-96. -96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers G., Wirblich C., Thiel H.J. Genomic and subgenomic RNAs of rabbit hemorrhagic disease virus are both protein-linked and packaged into particles. Virology. 1991;184:677–686. doi: 10.1016/0042-6822(91)90437-g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers G., Wirblich C., Thiel H.J. Rabbit hemorrhagic disease virus–molecular cloning and nucleotide sequencing of a calicivirus genome. Virology. 1991;184:664–676. doi: 10.1016/0042-6822(91)90436-f. [DOI] [PubMed] [Google Scholar]
- Morisse J.P., Le Gall G., Boilletot E. Hepatitis of viral origin in Leporidae: introduction and aetiological hypotheses. Rev. Sci. Tech. 1991;10:269–310. [PubMed] [Google Scholar]
- Mutze G., Cooke B., Alexander P. The initial impact of rabbit hemorrhagic disease on European rabbit populations in South Australia. J. Wildl. Dis. 1998;34:221–227. doi: 10.7589/0090-3558-34.2.221. [DOI] [PubMed] [Google Scholar]
- Ohlinger V.F., Haas B., Meyers G., Weiland F., Thiel H.J. Identification and characterization of the virus causing rabbit hemorrhagic disease. J. Virol. 1990;64:3331–3336. doi: 10.1128/JVI.64.7.3331-3336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra F., Prieto M. Purification and characterization of a calicivirus as the causative agent of a lethal hemorrhagic disease in rabbits. J. Virol. 1990;64:4013–4015. doi: 10.1128/JVI.64.8.4013-4015.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puggioni G., Cavadini P., Maestrale C., Scivoli R., Botti G., Ligios C., et al. The new French 2010 Rabbit Hemorrhagic Disease Virus causes an RHD-like disease in the Sardinian Cape hare (Lepus capensis mediterraneus) Vet. Res. 2013;44:96. doi: 10.1186/1297-9716-44-96. -96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverio D., Lopes A.M., Melo-Ferreira J., Magalhaes M.J., Monterroso P., Serronha A., et al. Insights into the evolution of the new variant rabbit haemorrhagic disease virus (GI.2) and the identification of novel recombinant strains. Transbound Emerg. Dis. 2018;65:983–992. doi: 10.1111/tbed.12830. [DOI] [PubMed] [Google Scholar]
- Strive T., Wright J.D., Robinson A.J. Identification and partial characterisation of a new Lagovirus in Australian wild rabbits. Virology. 2009;384:97–105. doi: 10.1016/j.virol.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Szillat K.P., Hoper D., Beer M., Konig P. Full-genome sequencing of German rabbit haemorrhagic disease virus uncovers recombination between RHDV (GI.2) and EBHSV (GII.1) Virus Evol. 2020;6:veaa080. doi: 10.1093/ve/veaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velarde R., Abrantes J., Lopes A.M., Estruch J., Corte-Real J.V., Esteves P.J., et al. Spillover event of recombinant Lagovirus europaeus/GI.2 into the Iberian hare (Lepus granatensis) in Spain. Transbound Emerg. Dis. 2021;68:3187–3193. doi: 10.1111/tbed.14264. [DOI] [PubMed] [Google Scholar]
- Velarde R., Cavadini P., Neimanis A., Cabezon O., Chiari M., Gaffuri A., et al. Spillover events of infection of brown hares (Lepus europaeus) with Rabbit Haemorrhagic Disease Type 2 Virus (RHDV2) caused sporadic cases of an european brown hare syndrome-like disease in Italy and Spain. Transbound Emerg. Dis. 2017;64:1750–1761. doi: 10.1111/tbed.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinje J., Estes M.K., Esteves P., Green K.Y., Katayama K., Knowles N.J., et al. ICTV virus taxonomy profile: caliciviridae. J. Gen. Virol. 2019;100:1469–1470. doi: 10.1099/jgv.0.001332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Xu F., Liu J., Gao B., Liu Y., Zhai Y., et al. Atomic model of rabbit hemorrhagic disease virus by cryo-electron microscopy and crystallography. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.B.A., Edmonds S.E., Kerr S.R., Broughton-Neiswanger L.E., Snekvik K.R. Clinical and pathologic findings in an outbreak in rabbits of natural infection by rabbit hemorrhagic disease virus 2 in the northwestern United States. J. Vet. Diagn. Invest. 2021;33:732–735. doi: 10.1177/10406387211022466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Organisation for Animal Health . World Organisation for Animal Health; Paris: 2023. Manual of Diagnostic Tests and Vaccines For Terrestrial Animals.https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.07.02_RHD.pdf Section 3.7. Lagomorpha Chapter 3.7.2. Rabbit Haemorrhagic Disease Version adopted in MayLast Access: 01/09/2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Full genone sequences are available in GenBank under accession numbers KT308115. The raw data supporting the conclusions of this article will be made available on request.
Data will be made available on request.