Summary
Neuropeptides are packed into large dense core vesicles (LDCVs) that are transported from the soma out into their processes. Limited information exists regarding mechanisms regulating LDCV trafficking, particularly during challenges to bodily homeostasis. Addressing this gap, we used 2-photon imaging in an ex vivo preparation to study LDCVs trafficking dynamics in vasopressin (VP) neurons, which traffic and release neuropeptide from their dendrites and axons. We report a dynamic bidirectional trafficking of VP-LDCVs with important differences in speed and directionality between axons and dendrites. Acute, short-lasting stimuli known to alter VP firing activity and axonal/dendritic release caused modest changes in VP-LDCVs trafficking dynamics. Conversely, chronic/sustained systemic osmotic challenges upregulated VP-LDCVs trafficking dynamic, with a larger effect in dendrites. These results support differential regulation of dendritic and axonal LDCV trafficking, and that changes in trafficking dynamics constitute a novel mechanism by which peptidergic neurons can efficiently adapt to conditions of increased hormonal demand.
Subject areas: Natural sciences, Biological sciences, Biochemistry, Neuroscience, Molecular neuroscience, Cellular neuroscience, Cell biology, Functional aspects of cell biology
Graphical abstract
Highlights
-
•
Quantification of LDCV dynamics in dendrites and axons of VP neurons
-
•
Different baseline VP-LDCVs trafficking dynamics between dendrites and axons
-
•
Acute changes in firing activity modestly altered VP-LDCVs trafficking dynamics
-
•
Chronic osmotic challenges upregulated LDCV trafficking in dendrites and axons
Natural sciences; Biological sciences; Biochemistry; Neuroscience; Molecular neuroscience; Cellular neuroscience; Cell biology; Functional aspects of cell biology
Introduction
A fundamental property of all neurons is the vesicular release of neurotransmitter or neuropeptide via action potential-triggered exocytosis. These vesicles fall into two broad categories based on their appearance under electron microscopy: smaller clear core vesicles (CCVs), which are typically packaged with classic neurotransmitters such as glutamate or acetylcholine, and large dense core vesicles (LDCVs) which usually contain bigger molecules, particularly neuropeptides. Much of what is currently known about CNS neurotransmission, including neurotransmitter synthesis, packaging, factors regulating their release, and mechanisms modulating synaptic strengths, originated from studies limited to CCVs.1,2 Neurotransmitter signals within CCVs are loaded into synaptic terminals by neurotransmitter-specific vesicular transporters (such as VGluT or VAchT).3 In contrast, LDCVs bud off at the trans-golgi and thus do not require vesicular transporter proteins.4 Thus, due to a lack of peptide reuptake machinery,5 neuropeptides must constantly be generated, packaged, and trafficked from the nucleus to the releasing sites, constituting a critical process for the normal function of central neuropeptidergic neurons. Notably, however, and compared to CCVs, the fundamental processes regulating LDCV biology, including trafficking dynamics, activity-dependent release, and replenishment following sustained activation, remain largely understudied.
Oxytocin (OT) and vasopressin (VP) magnocellular neurosecretory cells (MNCs) located in the hypothalamic supraoptic (SON) and paraventricular (PVN) nuclei are considered classical examples of central neuropeptidergic neurons. These neurons’ axons bundle and project ventrally through the median eminence into the posterior pituitary. In response to physiological challenges such as an increase in plasma osmolality or during lactation,6,7,8 MNCs neuropeptide cargo is released into the systemic circulation in an action potential frequency- and pattern-dependent manner.9,10,11,12
In addition to axonal transport and release, LDCVs containing OT and VP are also transported to proximal and distal dendrites, from where neuropeptides are released intranuclearly, to mediate autocrine, paracrine, and hormonal actions.13,14,15 Dendritic release of OT and VP is a well-established phenomenon,13,14,16,17,18,19 demonstrated to play fundamental roles in helping coordinate and sustain the rhythmic activity of these neurons in response to physiological demand.20,21 Notably, neuropeptide release from dendritic LDCVs can occur independently of axonal release,19,22,23 and a growing body of evidence indicates that the mechanisms regulating dendritic release, including the necessity of action potentials,24 the engagement of glutamate NMDA receptors (NMDARs),19,24 and the process of priming,18,22 differ significantly from those regulating axonal release.25,26,27 While much of the work on dendritic release of OT and VP has focused on elucidating mechanisms that regulate the process of exocytosis, little is known about those implicated in vesicular trafficking from the nucleus to the farthest reaches of MNC processes, including dendrites. This is functionally relevant for this system given that, as stated earlier, release of OT and VP into the bloodstream to mediate actions at peripheral targets prevents these neurons from recycling vesicle product at the synapse, resulting in constant demand for de novo peptide synthesis and trafficking.28,29 Thus, MNCs constitute an ideal central neuropeptidergic model to study precise mechanisms regulating LDCV trafficking dynamics, particularly within dendritic compartments.
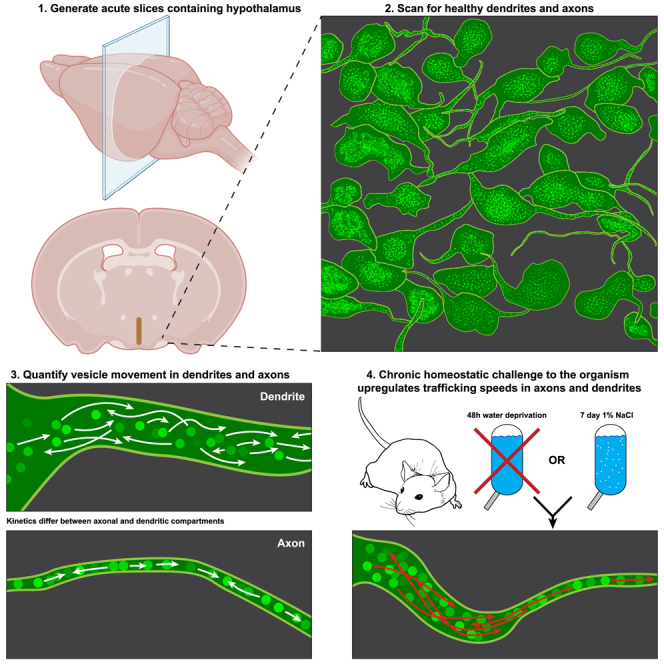
In this study, we performed high-resolution 2 photon imaging in hypothalamic slices obtained from a transgenic rat expressing EGFP tethered to the C-terminal of VP.30 Thus, in this rat model EGFP is fused directly to VP and stored within the LDCVs, enabling visualization of LDCV in both axonal and dendritic processes. Using this approach, we quantitatively assessed for the first time LDCV trafficking properties in axons and dendrites of VP MNCs under basal conditions, in response to acute changes in neuronal activity, and following systemic physiological challenges that imposed a chronic and sustained demand for VP activity and neuropeptide release.
Results
Basal characteristics of VP-LDCV trafficking properties
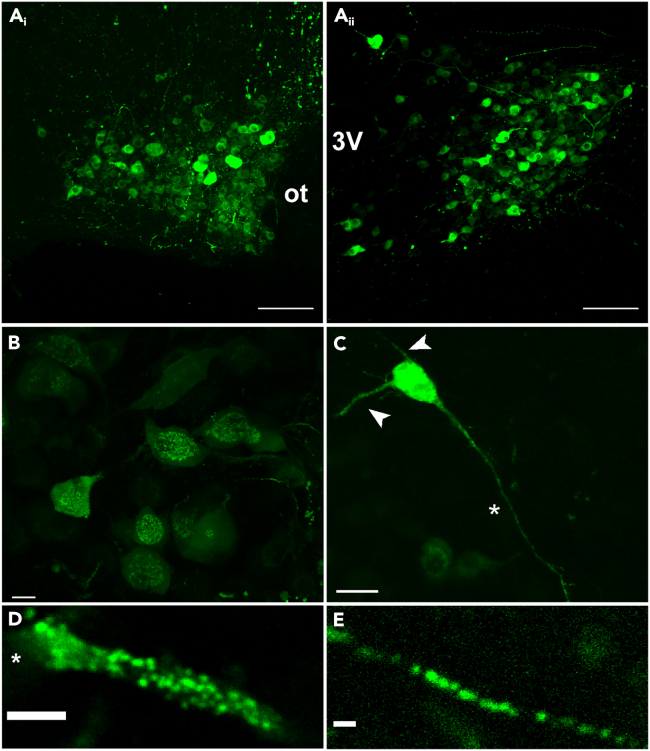
LDCVs containing EGFP-VP were observed as fluorescent spherical puncta in all SON and PVN neuronal compartments including soma, axons, and dendrites (Figure 1A, Video S1). A total number of 11,505 LDCVs in 68 processes from 37 rats were analyzed in this study. In somata, pools of LDCVs were visualized clustered around nuclei and, for the most part, they were inert (Figure 1B). LDCVs were readily observed in processes, and their density varied highly between neurons (Figures 1C–1E). Compared to somata, LDCVs in axons and dendrites were often very active, moving bidirectionally in an anterograde and retrograde manner.
Figure 1.
Representative examples of VP-containing LDCVs in various neuronal compartments of SON and PVN EGFP-VP neurons
(A) 2-photon images showing EGFP-VP neurons in the entire SON (i) and PVN (ii) at 20x magnification with optic tract (ot) and 3rd ventricle (3V) as landmarks for SON and PVN, respectively. Scale bars = 100 μm.
(B) Higher magnification image (40x) of the SON showing the distribution of EGFP-VP-LDCVs in the soma and processes. Scale bar = 10 μm.
(C) z stack projection of a single EGFP-VP neuron showing short dendrites without spines (arrows), contrasted with the narrow, long projecting axon (asterisk). Scale bar = 25 μm.
(D) A cropped VP dendrite containing LDCVs at 60x magnification, where movement could be tracked. Soma is cropped to the left (asterisk). Scale bar = 2 μm.
(E) A cropped VP axon containing LDCVs at 60x magnification, where movement could be tracked. Scale bar = 1 μm.
LDCVs traffic bidirectionally in a multi-order branch stemming from the edge of a soma (highlighted in red).
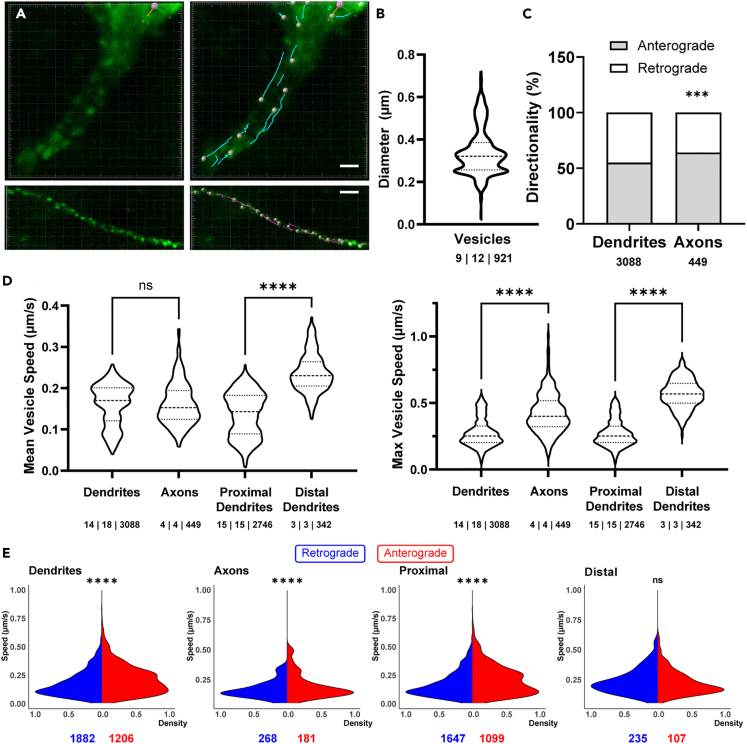
To quantitatively assess LDCVs movement, we targeted process segments visible in a single z-plane with a minimum length of 15 μm, containing a high density of LDCVs (Figure 2A, Video S2). LDCVs had an average puncta diameter of 0.34 ± 0.004 μm (Figure 2B). No differences were observed in the size of dendritic compared to axonal LDCVs. In both cases, LDCVs were found to travel both anterogradely and retrogradely, with the proportion of anterograde:retrograde movement directionality being significantly higher in axons compared to dendrites (Figure 2C; 64.2% ± 3.4% [n = 5 processes] and 55% ± 0.7% anterograde [n = 18 processes] for axons and dendrites, respectively, p < 0.001). The mean basal speed of LDCVs movement in all processes was 0.19 ± 0.001 μm/s, with a mean max speed of 0.37 ± 0.004 μm/s. In many cases, however, we observed LDCVs traveling at 1–1.5 μm/s. A detailed quantification of LDCVs movement under basal conditions for the pooled LDCV data, as well as a breakdown based on morphological compartment (in axons vs. dendrites), and proximity to soma along the dendrite (proximal dendrites [<15 μm from soma] vs. distal dendrites [>50 μm from soma]), is presented in Figure 2 and summarized in Table 1. While we observed no difference in the mean speed between dendrites and axons (p > 0.05), the max LDCV speed was slower in the former (p < 0.0001). Notably, LDCVs traveled significantly faster in distal when compared to proximal dendritic segments (p < 0.0001 for both mean and max speed) (Figure 2D).
Figure 2.
Characterization of VP-LDCVs trafficking properties under basal conditions
(A) Single-frame image from a 2-photon time series capture of VP-LDCV movement in an apical dendrite (top) and an axon (bottom) of a VP neuron, showing both the raw image (left) and the Imaris analysis overlay (right). Scale bars: 2 μm.
(B) Violin plot showing the mean VP-LDCV diameter.
(C) Summary of directionality of all tracked LDCVs under baseline conditions. The incidence of LDCVs traveling anterogradely in axons is statistically higher than that in dendrites (∗∗∗p < 0.003, chi-squared test). Numbers below each group represent n values (LDCVs).
(D) Violin plots of mean (left) and max (right) speeds of VP-LDCVs. ∗∗∗∗p < 0.0001, one way ANOVA, Šidák multiple comparisons test.
(E) Mean speed density distribution of VP-LDCVs traveling in retrograde and anterograde direction. Numbers below each group represent n values (LDCVs).
Table 1.
Basal properties of LDCV speeds
| Group | Dendrites | Axons | Proximal Dendrites | Distal Dendrites |
|---|---|---|---|---|
| n | 14 | 18 | 3088 | 4 | 4 | 449 | 11 | 15 | 2746 | 3 | 3 | 342 |
| Mean Speed (μm/s) | 0.19 ± 0.001 | 0.16 ± 0.004 | 0.20 ± 0.002 | 0.23 ± 0.004 |
| Max Speed (μm/s) | 0.37 ± 0.004 | 0.42 ± 0.012 | 0.35 ± 0.004 | 0.56 ± 0.011 |
The time series of LDCV movement is duplicated with raw data (left) and Imaris analysis overlay (right).
In addition to overall speed of movement, we also compared the distribution of LDCV mean speed traveling anterogradely versus retrogradely (Figure 2E). The distribution of LDCVs traveling anterograde versus retrograde was statistically significant in all comparisons except for trafficking in distal dendrites (dendrites p < 0.0001; axons p < 0.0001; proximal dendrites p < 0.0001; distal dendrites p > 0.05). The mean speed of LDCVs traveling anterograde compared to retrograde was faster in dendrites (p < 0.0001), while it was significantly slower in axons (p < 0.0001). When examining a breakdown of proximal and distal dendrites, proximal dendrites showed faster anterograde speeds compared to retrograde (p < 0.0001) while there was no difference in mean directionality speed in distal dendrites (see Table 2 for full anterograde vs. retrograde breakdown).
Table 2.
Basal speeds of anterograde and retrograde vesicles
| Anterograde | n | Retrograde | n | unpaired t test (Ant vs. Ret) | |
|---|---|---|---|---|---|
| Dendrites | |||||
| Mean | 0.20 ± 0.003 | 1882 | 0.1839 ± 0.003 | 1206 | ∗∗∗∗p < 0.0001 |
| Max | 0.35 ± 0.009 | 0.44 ± 0.01 | ∗∗∗∗p < 0.0001 | ||
| Axons | |||||
| Mean | 0.14 ± 0.005 | 268 | 0.20 ± 0.007 | 181 | ∗∗∗∗p < 0.0001 |
| Max | 0.45 ± 0.01 | 0.36 ± 0.01 | ∗p < 0.05 | ||
| Proximal Dendrites | |||||
| Mean | 0.22 ± 0.003 | 1647 | 0.18 ± 0.003 | 1099 | ∗∗∗∗p < 0.0001 |
| Max | 0.35 ± 0.01 | 0.42 ± 0.01 | ∗∗∗∗p < 0.0001 | ||
| Distal Dendrites | |||||
| Mean | 0.24 ± 0.007 | 235 | 0.26 ± 0.01 | 107 | p > 0.05 |
| Max | 0.55 ± 0.01 | 0.59 ± 0.02 | p > 0.05 | ||
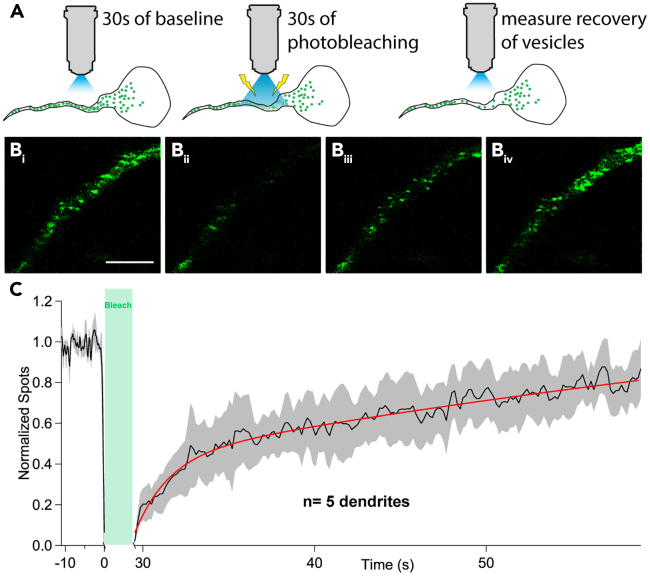
Kinetics of dendritic LDCV replenishment
Next, we evaluated the kinetics of dendritic LDCV replenishment. To this end, we used fluorescence recovery after photobleaching (FRAP). Namely, we photobleached dendritic compartments for 30 s and then measured the rate of LDCV recovery over time (Figures 3A and 3B). We plotted LDCV spots/frame as normalized to baseline measurements (Figure 3C). The resulting curve could be confidently fit with a double exponential (fast component: τ = 1.89 ± 0.2 s; amplitude = 0.41 ± 0.03; slow component τ = 77.2 ± 70.9 s; amplitude = 1.2 ± 0.8). This indicates that dendritic LDCV replenishment occurs relatively rapidly, and that the observed trafficking activity reflected newly trafficked LDCVs, rather than same LDCVs “patrolling” back and forth along the longitudinal axis of the dendrite.
Figure 3.
VP-LDCV trafficking kinetics following fluorescence recovery after photobleaching (FRAP)
(A) Diagrammatic depiction of protocol used for FRAP.
(B) Image of a dendrite undergoing the FRAP protocol showing baseline prior to photobleaching (i), immediately after photobleaching (ii), 15 s after photobleaching (iii), and 60 s after photobleaching (iv), when LDCV recovery has plateaued. Scale bar = 5 μm.
(C) Plot of mean spots/frame normalized to the average of the baseline (n = 5 dendrites). The recovery curve was confidently fit to a double exponential (red line, τfast = 1.89 ± 0.2s and τslow = 77.2 ± 70.9s).
Is basal LDCV trafficking regulated in an activity-dependent manner?
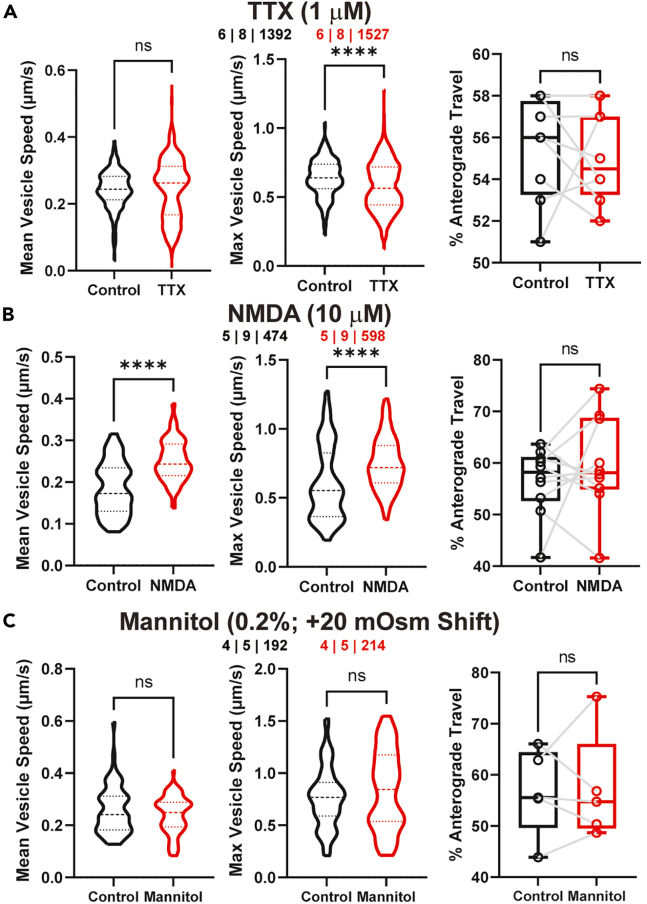
To determine to what extent LDCV trafficking in dendrites is dependent on the basal activity levels of VP neurons, we examined firsts whether silencing the neurons would affect trafficking dynamics. As shown in Figure 4A (see also Table 3), we found than bath application of TTX (1 μM) induced no significant changes in mean speed (p > 0.05). Conversely, the max speed decreased significantly (p < 0.0001). Additionally, trafficking directionality (i.e., % anterograde travel) was not significant after TTX application (p > 0.05, n = 8 processes).
Figure 4.
Changes in VP-LDCV trafficking dynamics in response to acute changes in VP neuronal activity
(A–C) Plots of dendritic LDCV mean speed (left), max speed (middle), and incidence of anterograde travel (right) under basal conditions and in response to (A) TTX (1 μM), (B) NMDA (10 μM), and (C) Mannitol (0.2%). Numbers at the top center of each panel represent n values (animals | dendrites | LDCVs). %Anterograde travel individual points represent 1 process. ∗∗∗∗p < 0.0001, unpaired t test (Mean and Max vesicle speed), paired t test (%Anterograde Travel).
Table 3.
Speeds of LDCVs affected by acute challenges in dendrites
| Group | Control | TTX | %Δ | p values |
|---|---|---|---|---|
| n | 6 | 8 | 1392 | 6 | 8 | 1527 | ||
| Mean Speed (μm/s) | 0.24 ± 0.003 | 0.24 ± 0.002 | 0.50% | p > 0.05 |
| Max Speed (μm/s) | 0.63 ± 0.003 | 0.57 ± 0.007 | −9.60% | ∗∗∗∗p < 0.0001 |
| Control | NMDA | %Δ | p values | |
|---|---|---|---|---|
| n | 5 | 9 | 474 | 5 | 9 | 598 | ||
| Mean Speed (μm/s) | 0.18 ± 0.01 | 0.25 ± 0.004 | 38.90% | ∗∗∗∗p < 0.0001 |
| Max Speed (μm/s) | 0.60 ± 0.03 | 0.75 ± 0.02 | 25.00% | ∗∗∗∗p < 0.0001 |
| Control | Mannitol | %Δ | p values | |
|---|---|---|---|---|
| n | 4 | 5 | 192 | 4 | 5 | 214 | ||
| Mean Speed (μm/s) | 0.26 ± 0.01 | 0.24 ± 0.01 | −7.70% | p > 0.05 |
| Max Speed (μm/s) | 0.76 ± 0.04 | 0.83 ± 0.05 | 9.21% | p > 0.05 |
We next examined whether an acute increase in VP neuronal activity known to evoke dendritic release of VP affected LDCV trafficking properties. Glutamate is a key excitatory transmitter in SON neurons, and activation of both synaptic and extrasynaptic NMDARs has been shown to promote spiking activity and evoke dendritic release of VP.19,24,31,32 We therefore evaluated the effects of an acute NMDA application on LDCV trafficking properties. We found that bath application of NMDA (10 μM, 3–5 min) upregulated mean trafficking speed (p < 0.0001) and max speed (p < 0.0001), without affecting trafficking directionality (p > 0.05, n = 9 processes) (Figure 4B; Table 3.)
VP neuronal activity and dendritic release of VP can also be stimulated and triggered by an acute osmotic stimulation.33,34 We therefore tested whether a local osmotic challenge affected trafficking dynamics. We used a +20 mOsm challenge as it is physiologically relevant for VP secretion and has been previously shown to increase VP release ex vivo.24,33 We found that increasing ACSF osmolality from 301 ± 2 to 320 ± 3 mOsm (mannitol 0.2%, 3–5 min) failed to significantly alter LDCV trafficking mean speed, max speed, or directionality (p > 0.05, n = 5 processes) (Figures 4C; Table 3).
Chronic stimulation of VP neurons and their effect on LDCV trafficking properties
In addition to acute stimuli known to trigger exocytosis of readily releasable pools of VP-LDCVs, MNCs are plastic neurons whose activity changes in response to systemic whole-body homeostatic challenges like dehydration and salt loading (SL).35,36,37,38 Acute stimuli reported earlier had a detectable yet modest effects on the trafficking properties of VP neurons; we therefore hypothesized that peptide trafficking could be more robustly altered in response to long-term homeostatic challenges to the VP system.
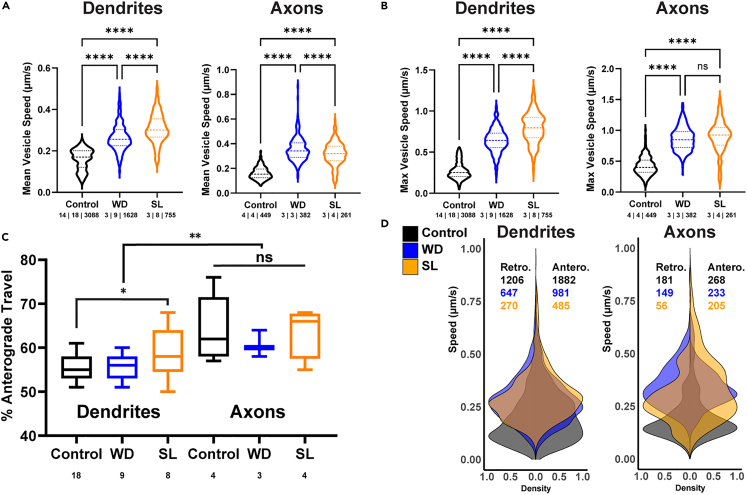
We tested two discrete prolonged osmotic homeostatic challenges to VP MNCs: A 48-h water deprivation (WD) and a 7-day SL regiment wherein normal drinking water was replaced with water containing 1% NaCl. These are widely used models for studying the effects of chronic osmotic challenge39,40,41 on the upregulation of VP synthesis and release,30,42,43,44 as well as on the downstream consequences on whole animal physiology.45,46,47 Under both WD and SL challenges, the mean and max trafficking speeds increased significantly and robustly relative to controls (p < 0.0001 in both cases Figures 5A and 5B, Video S3), in both dendrites and axons, demonstrating increases in speed between 42.6% and 131.1% (Table 4). Notably, in dendrites (but not axons), SL evoked a more robust effect in both mean and max LDCV speed when compared to WD (p < 0.001 for both parameters, Figures 5A and 5B). Furthermore, SL (but not WD) significantly increased the percentage of anterograde LDCV travel in dendrites (p < 0.05) but not in axons (p > 0.05) (Figure 5C).
Figure 5.
Changes in VP-LDCV trafficking dynamics in response to chronic osmotic challenges
(A) Violin plots of mean LDCV speed in dendrites (left) and axons (right) under euhydrated control conditions, 48 h of water deprivation (WD), and 7 days salt loading (SL). ∗∗∗p < 0.0001, One Way ANOVA, Šidák multiple comparisons test.
(B) Violin plots of max LDCV speed in dendrites (left) and axons (right) under euhydrated control conditions, 48 h of water deprivation (WD), and 7 days salt loading (SL). Max LDCV speed of dendrites (left) and axons (right) compared under control conditions, WD, and SL. ∗∗∗p < 0.0001, One Way ANOVA, Šidák multiple comparisons test.
(C) Summary of directionality of all tracked LDCVs under control euhydrated conditions, WD, and SL in dendrites and axons (∗∗p < 0.01, two-way ANOVA). The only within-groups difference was between control and SL dendrites (∗p < 0.05, Šidák multiple comparisons test). Numbers below each group represent n values (processes).
(D) Kernel density distributions of mean speed of VP-LDCV anterograde vs. retrograde traveling in control euhydrated, WD, and SL conditions from dendrites (left) and axons (right). Numbers in each plot represent n values, color coded to correspond to experimental group (LDCVs).
Table 4.
Basal speeds of LDCVs affected by chronic challenges in dendrites and axons
| Dendrites |
Axons |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean speed (μm/s) | %Δ | p value | n | Mean speed (μm/s) | %Δ | p value | |
| Control | 14 | 18 | 3088 | 0.188 ± 0.001 | – | 4 | 4 | 449 | 0.164 ± 0.004 | – | ||
| WD | 3 | 9 | 1628 | 0.29 ± 0.003 | 54.2% | ∗∗∗∗p < 0.0001 | 3 | 3 | 382 | 0.34 ± 0.005 | 111.6% | ∗∗∗∗p < 0.0001 |
| SL | 3 | 8 | 755 | 0.323 ± 0.005 | 71.8% | ∗∗∗∗p < 0.0001 | 3 | 4 | 261 | 0.33 ± 0.009 | 107.3% | ∗∗∗∗p < 0.0001 |
| WD:SL %Δ | – | – | 11.4% | ∗∗∗∗p < 0.0001 | – | – | −2.0% | ∗∗∗∗p < 0.0001 |
| Max speed (μm/s) | %Δ | Max speed (μm/s) | %Δ | |||||
|---|---|---|---|---|---|---|---|---|
| Control | 0.367 ± 0.004 | – | 0.424 ± 0.004 | – | ||||
| WD | 0.72 ± 0.006 | 96.2% | ∗∗∗∗p < 0.0001 | 0.861 ± 0.01 | 103.1% | ∗∗∗∗p < 0.0001 | ||
| SL | 0.848 ± 0.01 | 131.1% | ∗∗∗∗p < 0.0001 | 0.9369 ± 0.03 | 121.0% | ∗∗∗∗p < 0.0001 | ||
| WD:SL %Δ | – | – | 17.8% | ∗∗∗∗p < 0.0001 | – | – | 8.8% | p > 0.05 |
A time series from two different dendrites comparing LDCV movement in control vs. SL conditions. Note the up-tempo movement of SL compared to control.
Finally, we plotted kernel density functions of anterograde and retrograde movement in the three experimental conditions (control, WD, and SL) to evaluate the distribution of LDCV speeds. As shown in Figure 5D, the plots indicate that the LDCV mean speed distribution was significantly different between anterograde and retrograde travel in control (p < 0.0001), WD (p < 0.01), and SL (p < 0.0001) cases, supporting a higher speed in the anterograde direction. A full breakdown of mean and max speeds comparing anterograde and retrograde movement is in Table 5.
Table 5.
Speeds of anterograde and retrograde vesicles affected by chronic challenges
| n | Anterograde (mm/s) | n | Retrograde (mm/s) | p values (Ant vs. Ret) | p values (Between groups) | ||
|---|---|---|---|---|---|---|---|
| Dendrites (Mean Speed) | |||||||
| Control | 1882 | 0.20 ± 0.003 | 1206 | 0.1839 ± 0.003 | ∗∗∗∗p < 0.0001 | C v. WD Antero: | ∗∗∗∗p < 0.0001 |
| WD | 981 | 0.29 ± 0.004 | 647 | 0.29 ± 0.004 | p > 0.05 | C v. SD Antero: | ∗∗∗∗p < 0.0001 |
| SL | 485 | 0.32 ± 0.006 | 270 | 0.27 ± 0.006 | ∗∗∗∗p < 0.0001 | WD v. SD Antero: | ∗∗∗∗p < 0.0001 |
| C v. WD Retro: | ∗∗∗∗p < 0.0001 | ||||||
| C v. SD Retro: | ∗∗∗∗p < 0.0001 | ||||||
| WD v. SD Retro: | p > 0.05 | ||||||
| Dendrites (Max Speed) | |||||||
| Control | 1882 | 0.35 ± 0.009 | 1206 | 0.44 ± 0.01 | ∗∗∗∗p < 0.0001 | C v. WD Antero: | ∗∗∗∗p < 0.0001 |
| WD | 981 | 0.73 ± 0.009 | 647 | 0.71 ± 0.01 | p > 0.05 | C v. SD Antero: | ∗∗∗∗p < 0.0001 |
| SL | 485 | 0.80 ± 0.01 | 270 | 0.83 ± 0.02 | p > 0.05 | WD v. SD Antero: | ∗∗∗∗p < 0.0001 |
| C v. WD Retro: | ∗∗∗∗p < 0.0001 | ||||||
| C v. SD Retro: | ∗∗∗∗p < 0.0001 | ||||||
| WD v. SD Retro: | ∗∗∗∗p < 0.0001 | ||||||
| Axons (Mean Speed) | |||||||
| Control | 268 | 0.14 ± 0.005 | 181 | 0.20 ± 0.007 | ∗∗∗∗p < 0.0001 | C v. WD Antero: | ∗∗∗∗p < 0.0001 |
| WD | 233 | 0.35 ± 0.007 | 149 | 0.35 ± 0.008 | p > 0.05 | C v. SD Antero: | ∗∗∗∗p < 0.0001 |
| SL | 205 | 0.35 ± 0.01 | 56 | 0.27 ± 0.01 | ∗∗∗p < 0.001 | WD v. SD Antero: | p > 0.05 |
| C v. WD Retro: | ∗∗∗∗p < 0.0001 | ||||||
| C v. SD Retro: | ∗∗∗∗p < 0.0001 | ||||||
| WD v. SD Retro: | p > 0.05 | ||||||
| Axons (Max Speed) | |||||||
| Control | 268 | 0.45 ± 0.01 | 181 | 0.36 ± 0.01 | ∗p < 0.05 | C v. WD Antero: | ∗∗∗∗p < 0.0001 |
| WD | 233 | 0.86 ± 0.01 | 149 | 0.87 ± 0.02 | p > 0.05 | C v. SD Antero: | ∗∗∗∗p < 0.0001 |
| SL | 205 | 0.98 ± 0.03 | 56 | 0.78 ± 0.04 | ∗∗p < 0.01 | WD v. SD Antero: | ∗∗∗∗p < 0.0001 |
| C v. WD Retro: | ∗∗∗∗p < 0.0001 | ||||||
| C v. SD Retro: | ∗∗∗∗p < 0.0001 | ||||||
| WD v. SD Retro: | p > 0.05 | ||||||
Discussion
In this study, we employed high-resolution 2-photon imaging in a transgenic EGFP-VP rat to characterize dynamic trafficking of LDCVs in axons and dendrites of VP MNCs under basal conditions, as well as in response to acute and chronic challenges known to increase VP firing activity and to evoke both systemic and dendritic release of VP. To the best of our knowledge, this constitutes the first study to monitor trafficking dynamics of LDCV in VP neurons in real time, addressing adaptive changes in their dynamics in response to conditions of enhanced hormonal demand.
Highly dynamic trafficking of VP-LDCVs in axons and dendrites under basal conditions
Our approach allowed us to readily identify VP-containing LDCVs in dendrites and axons of VP MNCs and quantitatively assess velocity and directionality of their trafficking in these different neuronal compartments. We found that VP-LDCVs in both axons and dendrites displayed rapid movements in both anterograde and retrograde directions. Notably, we found important differences in directionality and velocity between axons and dendrites, as well as different dendritic segments (i.e., proximal vs. distal). For example, the max velocity was slower in dendrites compared to axons, whereas both the mean and max trafficking velocities were faster in distal compared to proximal dendritic segments. In dendrites, anterograde trafficking displayed faster velocities compared to retrograde trafficking whereas the opposite was true for axons. Distal dendrites anterograde vs. retrograde speeds were statistically insignificant.
Trafficking of LDCVs in dendrites did not display a directionality preference, with approximately half traveling in the anterograde and the other half in retrograde directions. While anterograde traveling was significantly higher in axons, the percentage of those traveling retrogradely was still high (∼35%) compared to other neuronal types.48,49,50 While we currently lack an explanation for this, we need to consider that, in the slice preparation, axons are cut off from their terminals at the posterior pituitary; thus, vesicles simply may switch direction when reaching a dead end.51 Nonetheless, retrograde traveling has also been reported in intact axons both in vivo and cell culture conditions.48,50,52
The continuous abundant trafficking of LDCVs in VP MNCs stands as a possible mechanism by which neurons ensure resupply of neuropeptide to active sites of release, both at axonal terminals and dendrites. In addition to classical axonal release of VP into the peripheral circulatory system, VP neurons also release their LDCV cargo content from somatodendritic compartments, playing an important role in intra-hypothalamic communication. Multiple studies demonstrate these diverse modes of release can be regulated independently and subserve different functional roles.13,15,17,24 Still, whether compartment-specific differences in cargo trafficking properties, and/or their modulation by physiologically relevant challenges, existed in VP neurons remained until now unknown. Thus, in this context, our reported differences in LDCV trafficking behavior between dendrites and axons further support the importance of compartment-specific regulation of neuropeptide dynamics in VP MNCs.
A similar highly dynamic bidirectional trafficking of LDCVs was previously shown in other neuronal types (e.g., Neuropeptide Y LDCVs), both in cell cultures and in vivo.48,50,52 This likely represents the presence of microtubules with mixed polarity53 and an assortment of motor proteins in the processes of VP neurons traveling at various speeds.
Compared to previous reports in other neurons, the trafficking speeds reported in our studies were slower, but still within the range previously reported (from 0.7 to 1.5 μm/s).48,51,52,54,55 Several factors could contribute to these differences in basal velocities. Firstly, almost all previous studies were performed either in cell cultures54,55 or in vivo in mice52 or drosophila.51 Thus, it is possible that trafficking kinetics are different in an acute slice preparation, even though our recordings were obtained close to physiological temperatures. This limitation should be acknowledged, as temperature is a factor that critically affects various processes’ kinetics. Nonetheless, the slice offers the advantage of spatiotemporal resolution, stable imaging, and a paradigm in which much of the other work in VP neuron physiology can be more directly compared to. Particularly, it is an approach that retains ex vivo adaptive remodeling of the system (synaptic plasticity, firing properties, ion channel function, etc.) in response to in vivo challenges (e.g., chronic osmotic stimulation, lactation, pregnancy).24,56,57,58,59,60,61 Another important difference is that we found LDCVs in VP neurons to be larger (∼300 nm) compared to those previously reported in similar studies.52,62,63 Still, whether larger LDCVs may also contribute to slower speeds is currently unknown. Lastly, LDCVs in VP neurons are densely packed and ubiquitous, as they are the primary vesicle product of these neurons, containing approximately 85,000 molecules/vesicle of the VP peptide.26,64 This contrasts with cortical or CA1 pyramidal neurons which primarily package and release smaller CCVs containing glutamate.65 Thus, the larger size and higher density of LDCVs may also contribute to the lower LDCV speeds observed in VP MNCs.
Finally, we wanted to address to what extent the basal velocity and/or directionality of VP-LDCV trafficking was activity dependent. We found that blocking action potential firing with TTX did not affect directionality and only slightly (though significantly, ∼10%) diminished the max speed of travel. This would suggest that the basal dynamics of LDCVs trafficking in VP neurons are largely independent of the basal degree of VP neuronal activity. This observation aligns with previous studies showing that dendritic release can occur independently of action potentials.23,24,66 In addition, it is possible that the lack of TTX effect may reflect a low level of basal activity of VP neurons in the slice preparation compared to in vivo.
We acknowledge that in some cases, as previously reported in other cells,51,52 we observed LDCVs that would change direction or freeze mid-trajectory, which were not included in our analysis. Given, however, the large volume of vesicles analyzed, excluding these likely had a minor impact on the overall data reported. Nonetheless, this further supports the complexity and dynamic nature of LDCV trafficking in VP neurons.
VP-LDCVs speeds are scarcely modulated by acute, transient increases in firing activity but accelerate under chronic sustained challenges such as WD and SL
A critical question regarding LDCV trafficking in general is whether and how this phenomenon is regulated. In the case of VP neurons, we wanted to specifically address whether conditions and mechanisms that increase VP neuronal activity and dendritic/axonal release of the neuropeptide are accompanied by changes in VP-LDCV dynamics. Transient activation of glutamate NMDARs and an acute hyperosmotic stimulation are both known to increase the firing activity of VP neurons.31,35,36,67,68,69 However, we recently showed that NMDAR-evoked firing, even for the same number or frequency of action potentials, evoked a much more robust dendritic release of VP.24 In line with these previous findings, we report here that bath-applied NMDA significantly increased dendritic VP-LDCV mean and max trafficking velocities (∼40% and ∼25%, respectively, p < 0.001). Conversely, an acute increase of 20 mOsm in the ACSF (mannitol 0.2%), an approach previously shown to increase VP firing activity,34,68 as well as dendritic release of VP19,24 in the slice preparation failed to affect VP-LDCV velocities. Neither NMDA nor mannitol affected VP-LDCV trafficking directionality. Thus, these results suggest that an acute increase in the demands for dendritic release of VP could be sufficient to engage changes in dendritic VP-LDCV trafficking. To further explore this, rats were subjected to two discrete prolonged osmotic demands for VP hormone release, namely a 48 h WD and 7 days of 1% SL. Both these challenges are well characterized, and while both lead to an increase in plasma osmolality and VP synthesis and release,70,71,72 SL has been reported to induce more pronounced changes in these parameters, compared to WD.46 Moreover, it is important to highlight that numerous previous studies have shown that adaptive remodeling of the system in response to in vivo challenges (e.g., chronic osmotic stimulation, lactation, pregnancy) is still reflected in the ex vivo slice preparation (e.g., synaptic plasticity, firing properties, intrinsic properties, ion channel functioning, etc.),24,56,57,58,59,60,61 further supporting that the slice preparation, despite its limitations (like any other approach) is an efficient model to study adaptive changes in the magnocellular system in response to systemic physiological changes. We found that VP-LDCV trafficking robustly upregulated under these prolonged osmotic challenges, and that in dendrites (but not axons) larger effects were observed in SL compared to WD rats. Thus, we found both the mean and max dendritic and axonal speeds to be significantly increased in WD and SL, but only in dendrites did SL evoke significantly larger effects than WD. Notably, changes in directionality (i.e., higher proportion of anterogradely traveling LDCVs) were observed only in dendrites, and only in response to SL. Finally, changes in VP-LDCV trafficking dynamics in response to chronic osmotic stimulation were much more pronounced compared to acute increases in VP activity/release (e.g., a max speed increase of 25% after acute NMDA versus 96% and 131% increase in WD or SL, respectively, compared to baseline). The molecular mechanisms by which an increased hormonal demand can result in changes in VP-LDCV trafficking dynamics are at present unknown. Work from different laboratories over the past decades has clearly demonstrated that systemic VP release is dependent on the degree and pattern of firing activity of these neurons,9,10,11 both of which are also dependent on the osmotic state of the animal.73,74 Moreover, abundant data support a high degree of activity and release of VP both in water-deprived and salt-loaded rats.43,44,75,76 While we did not measure simultaneous LDCV dynamics and VP release, based on the wealth of previous studies we can confidently state that the increased velocity of LDCV under sustained osmotic stimulation correlates with an increased activity and release of VP in the system.
Additionally, a recent study showed that microtubule density is upregulated in VP neurons after SL, supporting a contribution of microtubules to the osmotic response.77 This restructuring and expansion of the microtubule network could provide further avenues for active transport, accounting, at least in part, for the upregulated LDCV transport kinetics that we report in this study. Another possibility is that microtubule-mediated transport is known to be regulated through post-translational modifications of tubulins or motor proteins.52,78,79,80,81,82 This could reflect the presence of several post-translational modifications to microtubules, allowing for a wide variety of active transport properties, functions, and destinies for trafficked LDCVs.81,82 Still, whether this mechanism contributes to upregulating VP-LDCV trafficking during osmotic challenges remains to be determined.
Together, these results support the notion that in response to conditions of increased hormonal demand, VP neurons upregulate LDCV trafficking both in dendrites and axons to maintain appropriate levels of readily releasable pools of neuropeptide during states of increased demands, particularly during prolonged stimulation. A large body of evidence showed that, under these challenging conditions, the magnocellular neurosecretory system experiences a phenomenal degree of structural/functional plasticity, including adoption of specific firing patterns,74,83,84 changes in the neuronal-glial-vascular microenvironment,38,57,85 and transcriptomic changes as well,46,86 all of which are necessary for the optimal facilitation of hormone release and reestablishment of fluid/electrolyte homeostasis. Results from the present study suggest that adaptive changes in LDCV trafficking dynamics constitute another important factor contributing to the overall VP adaptive responses to cope with systemic challenges to homeostasis.
Limitations of the study
Only male subjects were used in this study; thus, the influence of sex on the results of the study cannot be determined. Furthermore, this study was performed using an acute slice preparation of the neurons. Trafficking properties observed in this preparation may not reflect kinetics as they exist in vivo.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Tetrodotoxin (TTX) | Alomone Labs | Cat#T-550 |
| Mannitol | Sigma-Alderich | Cat#M9647 |
| N-methyl-D-aspartic acid (NMDA) | Sigma-Alderich | Cat#M3262 |
| Experimental models: Organisms/strains | ||
| Rattus norvegicus: eGFP-VP | Ueta et al.30 | N/A |
| Software and algorithms | ||
| Imaris | Bitplane Imaris/Oxford Instruments | RRID:SCR_007370 |
| GraphPad Prism | GraphPad Software Inc. | RRID:SCR_002798 |
| ggplot2 | R-plot | RRID:SCR_014601 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Javier E. Stern (jstern@gsu.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
All studies described herein were approved by the Georgia State University Institutional Animal Care and Use Committee (IACUC) and align with values and guidelines for animal research outlined by the American Veterinary Medical Association.
Young adult (150-300g) transgenic male Wistar rats containing eGFP-VP were used for all experiments, and randomly assigned to each experimental group.30 These animals received ad libitum food and water and were housed on a standard 12:12 light cycle. For water deprivation experiments, water bottles were removed from cages 48 h prior to experiments. For salt-loading experiments, standard drinking water was replaced with water containing 1% NaCl for seven days before experiment.
Methods details
Acute slice preparation
On the day of the experiment, the GFP rats were anesthetized (Euthasol, Virbac, ANADA #200-071, Fort Worth, TX, USA, 50 mg kg−1 i.p.) and then perfused transcardially with 30 mL of ice-cold sucrose artificial cerebrospinal fluid (ACSF) (NaCl replaced by equal-osmol sucrose)87 containing the following (in mM): 210 sucrose, 2.5 KCl, 1 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 20 D-Glucose, 0.4 ascorbic acid, and 2.0 CaCl2; pH 7.2; 300–305 mosmol l−1. Rats were then rapidly decapitated, and brains were subsequently dissected, mounted in the chamber of a vibrotome (Leica VT1200s, Leica Microsystems, Buffalo Grove, IL, USA), and submerged in the same sucrose solution and bubbled constantly with 95% O2/5% CO2 gas. Coronal slices containing the hypothalamic SON and PVN were cut at 240 μm thickness and placed in a holding chamber containing ACSF bubbled with 95% O2/5% CO2. The ACSF is identical in composition to the sucrose solution, but with 210 mM sucrose replaced by 119 mM NaCl and 2 mM Na+-Pyruvate. Slices rested for a minimum of 45 min in total before any experiment.
2 photon microscopy
Slices were placed on the stage of an Olympus BX51WI upright microscope (Bruker, Billerica, MA, USA). The stage was perfused (∼2 mL/min) with ACSF bubbled continuously with 95% O2/5% CO2 and warmed to 32°C. eGFP-VP neurons were excited with a 2 photon (2P) MaiTai Laser (Spectra-Physics, Milpitas, CA, USA) tuned to 860 nm in and imaged with a 60x Nikon LUMPLFLN60×W objective. Slices were scanned for quality processes (either an axon or dendrite) that displayed LDCV movement suitable for imaging. When imaging PVN, we targeted exclusively magnocellular VP neurons by avoiding eGFP-positive neurons with small somas, thus trafficking in parvocellular VP neurons was not evaluated. Desirable characteristics included healthy, viable eGFP-positive neurons (as assessed by characteristic morphology under DIC illumination88), located at least two cell layers below the surface of the slice, with processes containing moving LDCVs, low process background fluorescence, and processes that were straight and remain in the same z-plane (i.e., processes that aren’t tortuous). Conversely, cells at the surface of the slice often displayed inert LDCV movement along with visual characteristic of damaged cells under DIC (swollen somata, dull and loosely defined edges, visible nucleus).88 Given these cells were likely damaged by the slicing procedure, we did not image them.
Some neurons had inert LDCV movement and thus we did not evaluate these neurons due to concerns about cell viability. With regards to dendrites, we exclusively imaged first order dendrites,89,90,91,92,93 favoring neurons with thick apical dendrites as these tended to have lower background fluorescence and thus made analysis more accurate. We only imaged processes with a minimum of 15 μm visible in a single z-plane. All dendrite measurements labeled “proximal” refer to initial dendrite segments at the point where they join with the soma. Distal dendrite measurements are segments greater than 50 μm from the soma. Axons were identified based on their very narrow width (i.e., the same size as a single vesicle diameter) and long length (hundreds of microns). All axon measurements were imaged at least 20 μm from the soma and care was taken to ensure that any imaged process was not severed from its soma. During acquisition, we employed a 3x digital zoom and cropped the process to maximize spatiotemporal resolution. We scanned using resonant galvo mode with 16x frame averaging. Laser power, photomultiplier tube (PMT) sensitivity, field of view, and scan speed all varied between trials to maximize spatiotemporal resolution without photobleaching the specimen. Imaging acquisition occurred for 1–2 min per process. Trials in which acute pharmacological challenges were presented to the same process, imaging settings and acquisition time remained consistent before and after drug application. The goal of these studies was to measure LDCV kinetics, thus maximizing acquisition of quality time series to ensure accurate tracking was prioritized over maintaining consistent acquisition parameters across trials.
Quantification and statistical analysis
VP magnocellular neurosecretory neurons from both SON and PVN were evaluated. While SON contains only magnocellular type neurons, PVN contains both magnocellular and parvocellular VP neurons which have distinct morphological and electrophysiological properties.94,95,96,97 When evaluating VP neurons from PVN, we targeted magnocellular neurons located in the lateral magnocellular subdivision, which contains the majority of VP magnocellular neurons.98 Given the parity of magnocellular VP neurons from SON and PVN, data obtained from these VP neurons were pooled together for analysis.
Images were imported and analyzed in Imaris (Andor Technology, Belfast, Northern Ireland) for spot detection and tracking. First, images were background subtracted and a mask was created for each time series to isolate the imaged dendrite or axon and eliminate potential spot detection outside of the target. A reference point was placed in the center of the dendritic or axonal hillock so that measurements could be calculated in relation to this reference point. Next, we ran the ‘spots’ pipeline function to detect LDCVs and track movement. The ‘spots quality’ parameter was adjusted between trials to maximize accurate detection of particle movement. However, this remained the same in trials where the same process was being analyzed, such as before and after an acute pharmacological application. The following parameters remained the same: Track Spots: Yes, Estimated XY Diameter: 0.3 μm, Background Subtraction: Yes, Algorithm: Autoregressive Motion, Max Distance: 0.4, Max Gap Size: 1. Only LDCVs that could be observed for a minimum of 10 s and displaced a minimum of 2 μm in distance were included for analysis. Tracks were checked manually for accuracy. All data was exported as.csv files for further statistical analysis in MATLAB and PRISM. Mean and max speed were determined from the files. Directionality was calculated using the statistic “Distance from Origin Reference Frame”. This parameter calculates distance of each spot from the reference point for each frame that the LDCV is tracked. The first value was subtracted from the final value. If this value was positive, the LDCV was classified as anterograde movement; if this value was negative, the LDCV was classified as retrograde movement. The positive and negative values were reassigned 1 or -1 respectively for easier analysis. Statistics and most graphics generated with GraphPad Prism (Boston, MA, USA). Error bars represent standard error of the mean (SEM) where applicable. Distribution density analyses were performed on mean speed data binned into 50 bins and subsequent kernel density plot generation was performed using R Statistical Software (v4.2.2)99 and the ggplot2 R package (v3.3.5).100 Table and figure n value formatting is as follows: animals | processes | LDCVs, unless otherwise noted. For anterograde vs. retrograde comparisons, only vesicle count is reported to avoid redundancy. Figure legend denotes statistical test used; asterisks in figures denote significance level (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
Acknowledgments
Funding: NIH K99HL168434 (M.K.K.), DFG AL 2466/2-1 (F.A.), R01 NS115209 (D.N.C.), R01 NINDS 094640 and R01 HL162575-01 (J.E.S). K.D. is supported by 2CI Neurogenomics Fellowship (GSU), a Brains & Behavior Fellowship (GSU), and a Kenneth W. and Georganne F. Honeycutt Fellowship (GSU).
Author contributions
Conceptualization, J.E.S. and M.K.K.; Methodology, J.E.S., M.K.K., and F.A.; Formal Analysis, M.K.K. and K.D.; Investigation, M.K.K. and F.A.; Resources, J.E.S.; Writing – Original Draft, J.E.S. and M.K.K.; Writing – Review & Editing, J.E.S., M.K.K., F.A., K.D., and D.N.C.; Visualization, M.K.K. and K.D.; Supervision, J.E.S. and D.N.C.; Project Administration, J.E.S.; Funding Acquisition, M.K.K. and J.E.S.
Declaration of interests
The authors declare no competing interests.
Published: October 18, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.108243.
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Guedes-Dias P., Holzbaur E.L.F. Axonal transport: Driving synaptic function. Science. 2019;366 doi: 10.1126/science.aaw9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y., Shuai K., Sun Y., Zhu L., Wu X.-M. Advances in the study of axon–associated vesicles. Front. Mol. Neurosci. 2022;15 doi: 10.3389/fnmol.2022.1045778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henny P., Jones B.E. Vesicular glutamate (VGlut), GABA (VGAT), and acetylcholine (VACht) transporters in basal forebrain axon terminals innervating the lateral hypothalamus. J. Comp. Neurol. 2006;496:453–467. doi: 10.1002/cne.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C., Kim K. Neuropeptides. WormBook. 2008:1–36. doi: 10.1895/wormbook.1.142.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo A.F. Overview of neuropeptides: awakening the senses? Headache. 2017;57:37–46. doi: 10.1111/head.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moos F., Poulain D.A., Rodriguez F., Guerné Y., Vincent J.D., Richard P. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp. Brain Res. 1989;76:593–602. doi: 10.1007/BF00248916. [DOI] [PubMed] [Google Scholar]
- 7.Stricker E.M., Hosutt J.A., Verbalis J.G. Neurohypophyseal secretion in hypovolemic rats: inverse relation to sodium appetite. Am. J. Physiol. 1987;252:R889–R896. doi: 10.1152/ajpregu.1987.252.5.R889. [DOI] [PubMed] [Google Scholar]
- 8.Leng G., Brown C.H., Russell J.A. Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog. Neurobiol. 1999;57:625–655. doi: 10.1016/S0301-0082(98)00072-0. [DOI] [PubMed] [Google Scholar]
- 9.Dutton A., Dyball R.E. Phasic firing enhances vasopressin release from the rat neurohypophysis. J. Physiol. 1979;290:433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bicknell R.J., Leng G. Relative efficiency of neural firing patterns for vasopressin release in vitro. Neuroendocrinology. 1981;33:295–299. doi: 10.1159/000123248. [DOI] [PubMed] [Google Scholar]
- 11.Cazalis M., Dayanithi G., Nordmann J.J. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J. Physiol. 1985;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong W.E. The neurophysiology of neurosecretory cells. J. Physiol. 2007;585:645–647. doi: 10.1113/jphysiol.2007.145755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludwig M. Dendritic release of vasopressin and oxytocin. J. Neuroendocrinol. 1998;10:881–895. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig M., Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 15.Brown C.H., Ludwig M., Tasker J.G., Stern J.E. Somato-dendritic vasopressin and oxytocin secretion in endocrine and autonomic regulation. J. Neuroendocrinol. 2020;32 doi: 10.1111/jne.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pow D.V., Morris J.F. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 17.Ludwig M., Sabatier N., Bull P.M., Landgraf R., Dayanithi G., Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- 18.Tobin V.A., Douglas A.J., Leng G., Ludwig M. The Involvement of Voltage-Operated Calcium Channels in Somato-Dendritic Oxytocin Release. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Son S.J., Filosa J.A., Potapenko E.S., Biancardi V.C., Zheng H., Patel K.P., Tobin V.A., Ludwig M., Stern J.E. Dendritic Peptide Release Mediates Interpopulation Crosstalk between Neurosecretory and Preautonomic Networks. Neuron. 2013;78:1036–1049. doi: 10.1016/j.neuron.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurbin A., Orcel H., Alonso G., Moos F., Rabié A. The vasopressin receptors colocalize with vasopressin in the magnocellular neurons of the rat supraoptic nucleus and are modulated by water balance. Endocrinology. 2002;143:456–466. doi: 10.1210/endo.143.2.8643. [DOI] [PubMed] [Google Scholar]
- 21.Sabatier N., Richard P., Dayanithi G. Activation of multiple intracellular transduction signals by vasopressin in vasopressin-sensitive neurones of the rat supraoptic nucleus. J. Physiol. 1998;513:699–710. doi: 10.1111/j.1469-7793.1998.699ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobin V.A., Hurst G., Norrie L., Dal Rio F.P., Bull P.M., Ludwig M. Thapsigargin-induced mobilization of dendritic dense-cored vesicles in rat supraoptic neurons. Eur. J. Neurosci. 2004;19:2909–2912. doi: 10.1111/j.1460-9568.2004.03388.x. [DOI] [PubMed] [Google Scholar]
- 23.Sabatier N., Caquineau C., Dayanithi G., Bull P., Douglas A.J., Guan X.M.M., Jiang M., Van der Ploeg L., Leng G. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J. Neurosci. 2003;23:10351–10358. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitra S., Zhang M., Cauley E., Stern J.E. NMDA receptors potentiate activity-dependent dendritic release of neuropeptides from hypothalamic neurons. J. Physiol. 2019;597:1735–1756. doi: 10.1113/JP277167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemos J.R., Ortiz-Miranda S.I., Cuadra A.E., Velázquez-Marrero C., Custer E.E., Dad T., Dayanithi G. Modulation/physiology of calcium channel sub-types in neurosecretory terminals. Cell Calcium. 2012;51:284–292. doi: 10.1016/j.ceca.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leng G., Ludwig M. Neurotransmitters and peptides: whispered secrets and public announcements. J. Physiol. 2008;586:5625–5632. doi: 10.1113/jphysiol.2008.159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown C.H. Magnocellular Neurons and Posterior Pituitary Function. Compr. Physiol. 2016;6:1701–1741. doi: 10.1002/cphy.c150053. [DOI] [PubMed] [Google Scholar]
- 28.Laurent P., Ch’ng Q., Jospin M., Chen C., Lorenzo R., de Bono M. Genetic dissection of neuropeptide cell biology at high and low activity in a defined sensory neuron. Proc. Natl. Acad. Sci. USA. 2018;115:E6890–E6899. doi: 10.1073/pnas.1714610115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim T., Gondré-Lewis M.C., Arnaoutova I., Loh Y.P. Dense-core secretory granule biogenesis. Physiology. 2006;21:124–133. doi: 10.1152/physiol.00043.2005. [DOI] [PubMed] [Google Scholar]
- 30.Ueta Y., Fujihara H., Serino R., Dayanithi G., Ozawa H., Matsuda K.i., Kawata M., Yamada J., Ueno S., Fukuda A., Murphy D. Transgenic expression of enhanced green fluorescent protein enables direct visualization for physiological studies of vasopressin neurons and isolated nerve terminals of the rat. Endocrinology. 2005;146:406–413. doi: 10.1210/en.2004-0830. [DOI] [PubMed] [Google Scholar]
- 31.Nissen R., Hu B., Renaud L.P. Regulation of spontaneous phasic firing of rat supraoptic vasopressin neurones in vivo by glutamate receptors. J. Physiol. 1995;484:415–424. doi: 10.1113/jphysiol.1995.sp020674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliet S.H., Bourque C.W. Properties of supraoptic magnocellular neurones isolated from the adult rat. J. Physiol. 1992;455:291–306. doi: 10.1113/jphysiol.1992.sp019302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliet S.H., Bourque C.W. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature. 1993;364:341–343. doi: 10.1038/364341a0. [DOI] [PubMed] [Google Scholar]
- 34.Kusano K., House S.B., Gainer H. Effects of Osmotic Pressure and Brain-Derived Neurotrophic Factor on the Survival of Postnatal Hypothalamic Oxytocinergic and Vasopressinergic Neurons in Dissociated Cell Culture. J. Neuroendocrinol. 1999;11:145–152. doi: 10.1046/j.1365-2826.1999.00296.x. [DOI] [PubMed] [Google Scholar]
- 35.Leng G., Brown C.H., Bull P.M., Brown D., Scullion S., Currie J., Blackburn-Munro R.E., Feng J., Onaka T., Verbalis J.G., et al. Responses of magnocellular neurons to osmotic stimulation involves coactivation of excitatory and inhibitory input: an experimental and theoretical analysis. J. Neurosci. 2001;21:6967–6977. doi: 10.1523/JNEUROSCI.21-17-06967.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawasaki M., Yamaguchi K., Saito J., Ozaki Y., Mera T., Hashimoto H., Fujihara H., Okimoto N., Ohnishi H., Nakamura T., Ueta Y. Expression of immediate early genes and vasopressin heteronuclear RNA in the paraventricular and supraoptic nuclei of rats after acute osmotic stimulus. J. Neuroendocrinol. 2005;17:227–237. doi: 10.1111/j.1365-2826.2005.01297.x. [DOI] [PubMed] [Google Scholar]
- 37.Fujio T., Fujihara H., Shibata M., Yamada S., Onaka T., Tanaka K., Morita H., Dayanithi G., Kawata M., Murphy D., Ueta Y. Exaggerated Response of Arginine Vasopressin-Enhanced Green Fluorescent Protein Fusion Gene to Salt Loading without Disturbance of Body Fluid Homeostasis in Rats. J. Neuroendocrinol. 2006;18:776–785. doi: 10.1111/j.1365-2826.2006.01476.x. [DOI] [PubMed] [Google Scholar]
- 38.Roy R.K., Althammer F., Seymour A.J., Du W., Biancardi V.C., Hamm J.P., Filosa J.A., Brown C.H., Stern J.E. Inverse neurovascular coupling contributes to positive feedback excitation of vasopressin neurons during a systemic homeostatic challenge. Cell Rep. 2021;37 doi: 10.1016/j.celrep.2021.109925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neupane C., Sharma R., Pai Y.H., Lee S.Y., Jeon B.H., Kim H.-W., Stern J.E., Park J.B. High Salt Intake Recruits Tonic Activation of NR2D Subunit-Containing Extrasynaptic NMDARs in Vasopressin Neurons. J. Neurosci. 2021;41:1145–1156. doi: 10.1523/JNEUROSCI.1742-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satoh K., Oti T., Katoh A., Ueta Y., Morris J.F., Sakamoto T., Sakamoto H. In vivo processing and release into the circulation of GFP fusion protein in arginine vasopressin enhanced GFP transgenic rats: response to osmotic stimulation. FEBS J. 2015;282:2488–2499. doi: 10.1111/febs.13291. [DOI] [PubMed] [Google Scholar]
- 41.Antunes-Rodrigues J., Ruginsk S.G., Mecawi A.S., Margatho L.O., Reis W.L., Ventura R.R., da Silva A.L., Vilhena-Franco T., Elias L.L.K. In: Neurobiology of Body Fluid Homeostasis: Transduction and Integration Frontiers in Neuroscience. De Luca L.A., Menani J.V., Johnson A.K., editors. CRC Press/Taylor & Francis; 2014. Neuroendocrinology of Hydromineral Homeostasis. [Google Scholar]
- 42.Di S., Jiang Z., Wang S., Harrison L.M., Castro-Echeverry E., Stuart T.C., Wolf M.E., Tasker J.G. Labile Calcium-Permeable AMPA Receptors Constitute New Glutamate Synapses Formed in Hypothalamic Neuroendocrine Cells during Salt Loading. eNeuro. 2019;6 doi: 10.1523/ENEURO.0112-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levi D.I., Wyrosdic J.C., Hicks A.-I., Andrade M.A., Toney G.M., Prager-Khoutorsky M., Bourque C.W. High dietary salt amplifies osmoresponsiveness in vasopressin-releasing neurons. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morita M., Kita Y., Notsu Y. Mechanism of AVP release and synthesis in chronic salt-loaded rats. J. Pharm. Pharmacol. 2001;53:1703–1709. doi: 10.1211/0022357011778106. [DOI] [PubMed] [Google Scholar]
- 45.Yoshimura M., Ohkubo J., Katoh A., Ohno M., Ishikura T., Kakuma T., Yoshimatsu H., Murphy D., Ueta Y. A c-fos-monomeric red fluorescent protein 1 fusion transgene is differentially expressed in rat forebrain and brainstem after chronic dehydration and rehydration. J. Neuroendocrinol. 2013;25:478–487. doi: 10.1111/jne.12022. [DOI] [PubMed] [Google Scholar]
- 46.Greenwood M.P., Mecawi A.S., Hoe S.Z., Mustafa M.R., Johnson K.R., Al-Mahmoud G.A., Elias L.L.K., Paton J.F.R., Antunes-Rodrigues J., Gainer H., et al. A comparison of physiological and transcriptome responses to water deprivation and salt loading in the rat supraoptic nucleus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308:R559–R568. doi: 10.1152/ajpregu.00444.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.da Silveira L.T.G., Junta C.M., Monesi N., de Oliveira-Pelegrin G.R., Passos G.A., Rocha M.J.A. Time course of c-fos, vasopressin and oxytocin mRNA expression in the hypothalamus following long-term dehydration. Cell. Mol. Neurobiol. 2007;27:575–584. doi: 10.1007/s10571-007-9144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Wit J., Toonen R.F., Verhaagen J., Verhage M. Vesicular trafficking of semaphorin 3A is activity-dependent and differs between axons and dendrites. Traffic. 2006;7:1060–1077. doi: 10.1111/j.1600-0854.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 49.Park J.J., Cawley N.X., Loh Y.P. A bi-directional carboxypeptidase E-driven transport mechanism controls BDNF vesicle homeostasis in hippocampal neurons. Mol. Cell. Neurosci. 2008;39:63–73. doi: 10.1016/j.mcn.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Jong E.K., Vinet J., Stanulovic V.S., Meijer M., Wesseling E., Sjollema K., Boddeke H.W.G.M., Biber K. Expression, transport, and axonal sorting of neuronal CCL21 in large dense-core vesicles. Faseb. J. 2008;22:4136–4145. doi: 10.1096/fj.07-101907. [DOI] [PubMed] [Google Scholar]
- 51.Wong M.Y., Zhou C., Shakiryanova D., Lloyd T.E., Deitcher D.L., Levitan E.S. Neuropeptide delivery to synapses by long-range vesicle circulation and sporadic capture. Cell. 2012;148:1029–1038. doi: 10.1016/j.cell.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knabbe J., Nassal J.P., Verhage M., Kuner T. Secretory vesicle trafficking in awake and anaesthetized mice: differential speeds in axons versus synapses. J. Physiol. 2018;596:3759–3773. doi: 10.1113/JP276022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rolls M.M. Principles of microtubule polarity in linear cells. Dev. Biol. 2022;483:112–117. doi: 10.1016/j.ydbio.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bittins C.M., Eichler T.W., Hammer J.A., Gerdes H.-H. Dominant-negative myosin Va impairs retrograde but not anterograde axonal transport of large dense core vesicles. Cell. Mol. Neurobiol. 2010;30:369–379. doi: 10.1007/s10571-009-9459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwinter D.M., Lo K., Mafi P., Silverman M.A. Dynactin regulates bidirectional transport of dense-core vesicles in the axon and dendrites of cultured hippocampal neurons. Neuroscience. 2009;162:1001–1010. doi: 10.1016/j.neuroscience.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 56.Stern J.E., Hestrin S., Armstrong W.E. Enhanced neurotransmitter release at glutamatergic synapses on oxytocin neurones during lactation in the rat. J. Physiol. (Lond.) 2000;526:109–114. doi: 10.1111/j.1469-7793.2000.t01-1-00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oliet S.H., Piet R., Poulain D.A. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- 58.Teruyama R., Lipschitz D.L., Wang L., Ramoz G.R., Crowley W.R., Bealer S.L., Armstrong W.E. Central blockade of oxytocin receptors during mid-late gestation reduces amplitude of slow afterhyperpolarization in supraoptic oxytocin neurons. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1167–E1171. doi: 10.1152/ajpendo.90620.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choe K.Y., Han S.Y., Gaub P., Shell B., Voisin D.L., Knapp B.A., Barker P.A., Brown C.H., Cunningham J.T., Bourque C.W. High Salt Intake Increases Blood Pressure via BDNF-Mediated Downregulation of KCC2 and Impaired Baroreflex Inhibition of Vasopressin Neurons. Neuron. 2015;85:549–560. doi: 10.1016/j.neuron.2014.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Potapenko E.S., Biancardi V.C., Zhou Y., Stern J.E. Altered astrocyte glutamate transporter regulation of hypothalamic neurosecretory neurons in heart failure rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;303:R291–R300. doi: 10.1152/ajpregu.00056.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fleming T.M., Scott V., Naskar K., Joe N., Brown C.H., Stern J.E. State-dependent changes in astrocyte regulation of extrasynaptic NMDA receptor signalling in neurosecretory neurons. J. Physiol. 2011;589:3929–3941. doi: 10.1113/jphysiol.2011.207340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grimaud B., Frétaud M., Terras F., Bénassy A., Duroure K., Bercier V., Trippé-Allard G., Mohammedi R., Gacoin T., Del Bene F., et al. In Vivo Fast Nonlinear Microscopy Reveals Impairment of Fast Axonal Transport Induced by Molecular Motor Imbalances in the Brain of Zebrafish Larvae. ACS Nano. 2022;16:20470–20487. doi: 10.1021/acsnano.2c06799. [DOI] [PubMed] [Google Scholar]
- 63.Persoon C.M., Moro A., Nassal J.P., Farina M., Broeke J.H., Arora S., Dominguez N., van Weering J.R., Toonen R.F., Verhage M. Pool size estimations for dense-core vesicles in mammalian CNS neurons. EMBO J. 2018;37 doi: 10.15252/embj.201899672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nordmann J.J., Morris J.F. Method for quantitating the molecular content of a subcellular organelle: hormone and neurophysin content of newly formed and aged neurosecretory granules. Proc. Natl. Acad. Sci. USA. 1984;81:180–184. doi: 10.1073/pnas.81.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borges-Merjane C., Kim O., Jonas P. Functional Electron Microscopy, “Flash and Freeze,” of Identified Cortical Synapses in Acute Brain Slices. Neuron. 2020;105:992–1006.e6. doi: 10.1016/j.neuron.2019.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ludwig M., Bull P.M., Tobin V.A., Sabatier N., Landgraf R., Dayanithi G., Leng G. Regulation of activity-dependent dendritic vasopressin release from rat supraoptic neurones. J. Physiol. 2005;564:515–522. doi: 10.1113/jphysiol.2005.083931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiong J.-J., Hatton G.I. Differential responses of oxytocin and vasopressin neurons to the osmotic and stressful components of hypertonic saline injections: a Fos protein double labeling study. Brain Res. 1996;719:143–153. doi: 10.1016/0006-8993(95)01466-7. [DOI] [PubMed] [Google Scholar]
- 68.Ludwig M., Horn T., Callahan M.F., Grosche A., Morris M., Landgraf R. Osmotic stimulation of the supraoptic nucleus: central and peripheral vasopressin release and blood pressure. Am. J. Physiol. 1994;266:E351–E356. doi: 10.1152/ajpendo.1994.266.3.E351. [DOI] [PubMed] [Google Scholar]
- 69.Ludwig M., Callahan M.F., Neumann I., Landgraf R., Morris M. Systemic osmotic stimulation increases vasopressin and oxytocin release within the supraoptic nucleus. J. Neuroendocrinol. 1994;6:369–373. doi: 10.1111/j.1365-2826.1994.tb00595.x. [DOI] [PubMed] [Google Scholar]
- 70.Ludwig M., Williams K., Callahan M.F., Morris M. Salt loading abolishes osmotically stimulated vasopressin release within the supraoptic nucleus. Neurosci. Lett. 1996;215:1–4. doi: 10.1016/s0304-3940(96)12956-6. [DOI] [PubMed] [Google Scholar]
- 71.Tweedle C.D., Hatton G.I. Ultrastructural comparisons of neurons of supraoptic and circularis nuclei in normal and dehydrated rats. Brain Res. Bull. 1976;1:103–121. doi: 10.1016/0361-9230(76)90054-X. [DOI] [PubMed] [Google Scholar]
- 72.Wakerley J.B., Poulain D.A., Brown D. Comparison of firing patterns in oxytocin- and vasopressin-releasing neurones during progressive dehydration. Brain Res. 1978;148:425–440. doi: 10.1016/0006-8993(78)90730-8. [DOI] [PubMed] [Google Scholar]
- 73.Summy-Long J.Y., Kadekaro M. Role of circumventricular organs (CVO) in neuroendocrine responses: interactions of CVO and the magnocellular neuroendocrine system in different reproductive states. Clin. Exp. Pharmacol. Physiol. 2001;28:590–601. doi: 10.1046/j.1440-1681.2001.03491.x. [DOI] [PubMed] [Google Scholar]
- 74.Bourque C.W., Renaud L.P. Activity patterns and osmosensitivity of rat supraoptic neurones in perfused hypothalamic explants. J. Physiol. 1984;349:631–642. doi: 10.1113/jphysiol.1984.sp015178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keil L.C., Barbella Y.R., Dundore R.L., Wurpel J.N., Severs W.B. Vasopressin release induced by water deprivation: effects of centrally administered saralasin. Neuroendocrinology. 1983;37:401–405. doi: 10.1159/000123583. [DOI] [PubMed] [Google Scholar]
- 76.Thornton S.N., Leng G., Bicknell R.J., Chapman C., Purdew T. Vasopressin, but not oxytocin, is released in response to water deprivation in conscious goats. J. Endocrinol. 1986;110:335–340. doi: 10.1677/joe.0.1100335. [DOI] [PubMed] [Google Scholar]
- 77.Hicks A.-I., Barad Z., Sobrero A., Lean G., Jacob-Tomas S., Yang J., Choe K.Y., Prager-Khoutorsky M. Effects of salt loading on the organisation of microtubules in rat magnocellular vasopressin neurones. J. Neuroendocrinol. 2020;32 doi: 10.1111/jne.12817. [DOI] [PubMed] [Google Scholar]
- 78.Schlager M.A., Hoogenraad C.C. Basic mechanisms for recognition and transport of synaptic cargos. Mol. Brain. 2009;2:25. doi: 10.1186/1756-6606-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akhmanova A., Hammer J.A. Linking molecular motors to membrane cargo. Curr. Opin. Cell Biol. 2010;22:479–487. doi: 10.1016/j.ceb.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirokawa N., Niwa S., Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 81.Janke C., Chloë Bulinski J. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2011;12:773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 82.Sirajuddin M., Rice L.M., Vale R.D. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat. Cell Biol. 2014;16:335–344. doi: 10.1038/ncb2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brimble M.J., Dyball R.E. Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. J. Physiol. 1977;271:253–271. doi: 10.1113/jphysiol.1977.sp011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stern J.E., Armstrong W.E. Changes in the Electrical Properties of Supraoptic Nucleus Oxytocin and Vasopressin Neurons during Lactation. J. Neurosci. 1996;16:4861–4871. doi: 10.1523/JNEUROSCI.16-16-04861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Du W., Stern J.E., Filosa J.A. Neuronal-Derived Nitric Oxide and Somatodendritically Released Vasopressin Regulate Neurovascular Coupling in the Rat Hypothalamic Supraoptic Nucleus. J. Neurosci. 2015;35:5330–5341. doi: 10.1523/JNEUROSCI.3674-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen S., Xu H., Dong S., Xiao L. Morpho-Electric Properties and Diversity of Oxytocin Neurons in Paraventricular Nucleus of Hypothalamus in Female and Male Mice. J. Neurosci. 2022;42:2885–2904. doi: 10.1523/JNEUROSCI.2494-21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aghajanian G.K., Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- 88.Sakmann B., Neher E., editors. Single-Channel Recording. Springer US; 1995. [DOI] [Google Scholar]
- 89.Armstrong W.E., Schöler J., McNeill T.H. Immunocytochemical, Golgi and electron microscopic characterization of putative dendrites in the ventral glial lamina of the rat supraoptic nucleus. Neuroscience. 1982;7:679–694. doi: 10.1016/0306-4522(82)90074-4. [DOI] [PubMed] [Google Scholar]
- 90.Armstrong W.E. Morphological and electrophysiological classification of hypothalamic supraoptic neurons. Prog. Neurobiol. 1995;47:291–339. doi: 10.1016/0301-0082(95)80005-S. [DOI] [PubMed] [Google Scholar]
- 91.Smith B.N., Armstrong W.E. Tuberal supraoptic neurons—I. Morphological and electrophysiological characteristics observed with intracellular recording and biocytin filling in vitro. Neuroscience. 1990;38:469–483. doi: 10.1016/0306-4522(90)90043-4. [DOI] [PubMed] [Google Scholar]
- 92.Randle J.C., Bourque C.W., Renaud L.P. Serial reconstruction of lucifer yellow-labeled supraoptic nucleus neurons in perfused rat hypothalamic explants. Neuroscience. 1986;17:453–467. doi: 10.1016/0306-4522(86)90259-9. [DOI] [PubMed] [Google Scholar]
- 93.Lefranc G. [Neurohistologic study of the supraoptic and paraventricular nuclei in guinea pigs and cats by the Golgi triple impregnation technic] C. R. Acad. Hebd. Seances Acad. Sci. D. 1966;263:976–979. [PubMed] [Google Scholar]
- 94.Stern J.E. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J. Physiol. (Lond.) 2001;537:161–177. doi: 10.1111/j.1469-7793.2001.0161k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tasker J.G., Dudek F.E. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J. Physiol. 1991;434:271–293. doi: 10.1113/jphysiol.1991.sp018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luther J.A., Daftary S.S., Boudaba C., Gould G.C., Halmos K.C., Tasker J.G. Neurosecretory and non-neurosecretory parvocellular neurones of the hypothalamic paraventricular nucleus express distinct electrophysiological properties. J. Neuroendocrinol. 2002;14:929–932. doi: 10.1046/j.1365-2826.2002.00867.x. [DOI] [PubMed] [Google Scholar]
- 97.Sofroniew M.V. Vasopressin- and neurophysin-immunoreactive neurons in the septal region, medial amygdala and locus coeruleus in colchicine-treated rats. Neuroscience. 1985;15:347–358. doi: 10.1016/0306-4522(85)90217-9. [DOI] [PubMed] [Google Scholar]
- 98.Swanson L.W., Sawchenko P.E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 99.R Core Team . 2021. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 100.Wickham H. Springer Science & Business Media; 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LDCVs traffic bidirectionally in a multi-order branch stemming from the edge of a soma (highlighted in red).
The time series of LDCV movement is duplicated with raw data (left) and Imaris analysis overlay (right).
A time series from two different dendrites comparing LDCV movement in control vs. SL conditions. Note the up-tempo movement of SL compared to control.
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.