Summary
SARS-CoV-2 Omicron BA.2.75 has diversified into multiple subvariants with additional spike mutations and several are expanding in prevalence, particularly CH.1.1 and BN.1. Here, we investigated the viral receptor affinities and neutralization evasion properties of major BA.2.75 subvariants actively circulating in different regions worldwide. We found two distinct evolutionary pathways and three newly identified mutations that shaped the virological features of these subvariants. One phenotypic group exhibited a discernible decrease in viral receptor affinities, but a noteworthy increase in resistance to antibody neutralization, as exemplified by CH.1.1, which is apparently as resistant as XBB.1.5. In contrast, a second group demonstrated a substantial increase in viral receptor affinity but only a moderate increase in antibody evasion, as exemplified by BN.1. We also observed that all prevalent SARS-CoV-2 variants in the circulation presently, except for BN.1, exhibit profound levels of antibody evasion, suggesting this is the dominant determinant of virus transmissibility today.
Subject areas: SARS-CoV-2, BA.2.75 subvariants, evolutionary trajectories, convergent evolution, neutralizing monoclonal antibody, mRNA vaccine, receptor-binding affinity, antibody evasion
Graphical abstract
Highlights
-
•
BA.2.75 continues to evolve into multiple subvariants, including CH.1.1 and BN.1
-
•
CH.1.1 exhibits a level of antibody evasion comparable to that of XBB.1.5
-
•
Among BA.2.75 sublineages, BN.1 has the highest viral receptor-binding affinity
-
•
BA.2.75 subvariants either increase receptor affinity or heighten antibody evasion
Introduction
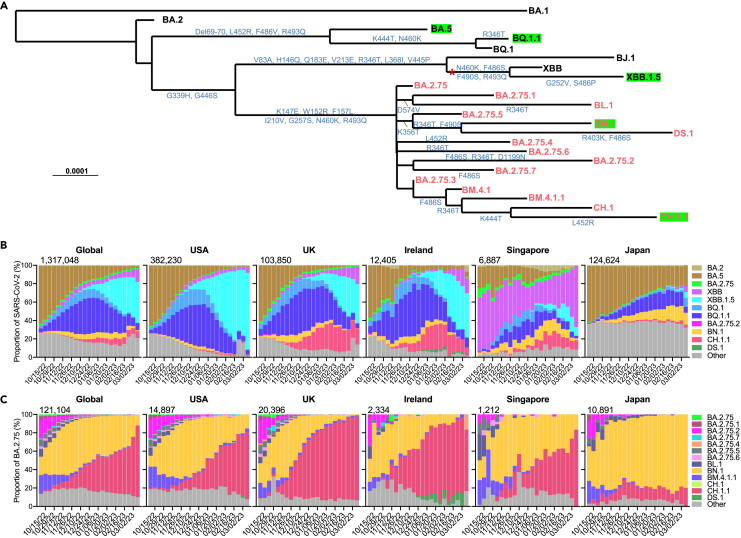
The Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to evolve, giving rise to several dominant subvariants worldwide. One particularly notable subvariant is designated as BA.2.751 (Figure 1A). Since its detection in India in early May 2022, the Omicron BA.2.75 subvariant has rapidly spread to over 108 countries, competing with the predominant BQ and XBB subvariants, and as of March 2023 its progenies are now responsible for over 8.54% of new SARS-CoV-2 cases worldwide (Figure 1B). Instead of observing the emergence of a singular dominant form, the recently circulating BA.2.75-derived subvariants remain relatively genetically diverse and demonstrate different evolutionary pathways.
Figure 1.
Spike alterations and prevalence of BA.2.75 subvariants
(A) Phylogenetic tree of selected BA.2.75 subvariants and current variants of concern (VOCs). The mutations on the branches showed the spike amino acid alterations of each variant. The recombination event for XBB from BJ.1 and BA.2.75 is denoted by the asterisk. The BA.2.75 subvariants are highlighted in red, and green boxes indicate current globally dominant variants with a frequency over 2%.
(B and C) Proportions of SARS-CoV-2 VOCs (B) and frequencies of BA.2.75 subvariants among BA.2.75 (C) in GISAID from October 2022 to March 2023. The cumulative number of sequences in the denoted time period is displayed at the upper right corner of each graph. See also Figure S1.
The current most frequently observed subvariants of BA.2.75 are CH.1.1, which has additional R346T, K444T, L452R, and F486S mutations, and BN.1, which has additional R346T, K356T, and F490S mutations, in their respective spikes (Figure 1A). Globally, CH.1.1 and BN.1 account for 77.2% and 11.1% of recent infections by BA.2.75 progenies, respectively. Interestingly, CH.1.1 appears to be more dominant among BA.2.75 subvariants in European and American countries, including the USA, the UK, and Ireland, whereas BN.1 is more dominant among BA.2.75 infections in Asian countries, such as Japan (Figure 1C). Another BA.2.75 subvariant drawing attention is DS.1, which has additional R403K and F486S mutations beyond BN.1 and was rapidly rising in Ireland weeks ago, where XBB.1.5, BQ.1.1, and CH.1.1 co-circulate. Other subvariants derived from BA.2.75 are also noteworthy, as they also carry spike mutations, including R346T, K444 T/M, L452R, F486S, or F490S (Figure 1A), which have been reported to impair monoclonal antibody (mAb) or polyclonal serum neutralization.2,3,4,5
The spike proteins of the Omicron BA.2.75 sublineage feature multiple convergent mutations that were previously observed in other Omicron variants, such as R346T, K444T, L452R, F486S, and F490S,2,3,6 as well as three newly identified mutations, R403K, K356T, and D574V (Figure S1). The expansion of these spike mutations observed in the new BA.2.75 subvariants therefore raise concerns about their impact on the effectiveness of current vaccines and antibody therapies. Here, our study addresses this concern and provides additional insight into SARS-CoV-2 evolutionary trajectory.
Results
Divergent receptor-binding affinities
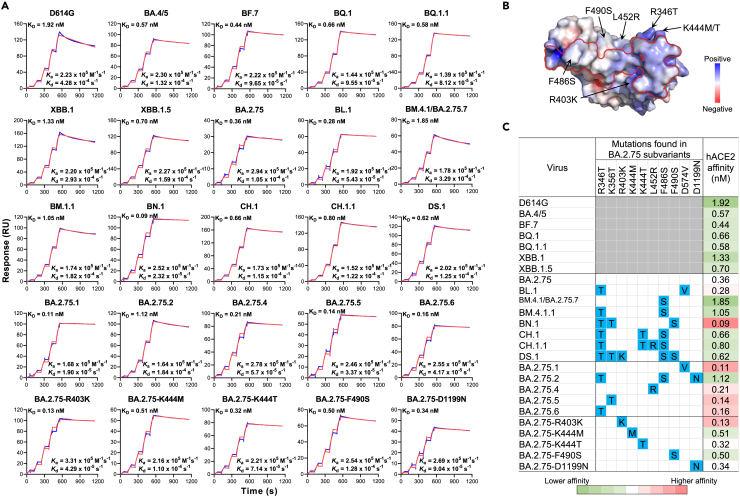
Viral entry into the cell begins with binding to a receptor. Therefore, transmission advantages of BA.2.75 subvariants may be associated, in part, with their binding affinity to the relevant viral receptor. Here, we measured the binding affinity between human angiotensin-converting enzyme 2 (hACE2) and each spike of several major BA.2.75 subvariants, as well as other important Omicron subvariants and viruses with select single mutations, by surface plasmon resonance (SPR) (Figure 2). Overall, BA.4/5, BF.7, BQ.1, BQ.1.1, and XBB.1.5 exhibited higher binding affinity to the receptor compared with the D614G strain, as we previously observed.2,6 Among BA.2.75 subvariants, several displayed lower binding affinities compared with parental BA.2.75 following the acquisition of more spike mutations, including CH.1, CH.1.1, and DS.1 (Figure 2A). Interestingly, when BA.2.75 carries an additional mutation R346T (BA.2.75.6), K356T (BA.2.75.5), L452R (BA.2.75.4), D574V (BA.2.75.1), or R403K, the hACE2-binding affinity was enhanced, even though these mutations are not in direct contact with the binding interface (Figures 2B and 2C). However, it remains unknown why BL.1, which carries the combination of R346T and D574N, did not show further enhanced binding affinity to the receptor compared with BA.2.75 with the individual mutation R346T or D574V. In contrast, other single mutations (K444M, K444T, F490S, and D1199N) did not dramatically alter binding affinity. Similar to F486V found in BA.4/5, F486S was also shown to greatly reduce binding affinity.3 Remarkably, BN.1, likely through the combination of R346T and K356T, had the highest binding affinity among BA.2.75 subvariants tested (4.0-fold that of BA.2.75). In short, the receptor-binding affinity for BA.2.75 subvariants has evolved in two distinct directions: one exhibiting increased affinity compared with BA.2.75, as observed in BN.1, whereas another demonstrating slightly decreased affinity, as observed for CH.1.1 and DS.1 (Figure 2C).
Figure 2.
Binding affinities of SARS-CoV-2 spike proteins with human angiotensin-converting enzyme 2 (hACE2) as measured by SPR
(A) Characterization of the binding between spike proteins with hACE2 tested by SPR. The raw and fitted curves are represented by blue and red lines, respectively.
(B) The electrostatic surface potential of the RBD in top view, with red and blue corresponding to negative and positive charges, respectively. The red line on the RBD surface indicates the footprint of ACE2. Black arrows indicate the surrounding mutations found in BA.2.75 subvariants.
(C) The summarized profile of viral receptor affinities of spike proteins. Spike mutations found in each of the indicated subvariants in addition to BA.2.75 are highlighted in blue. Enhanced ACE2 affinities compared with that of BA.2.75 are highlighted in red, whereas reduced affinities are in green. The results shown are representative of those obtained in two independent experiments.
Evasion of neutralization by monoclonal antibodies
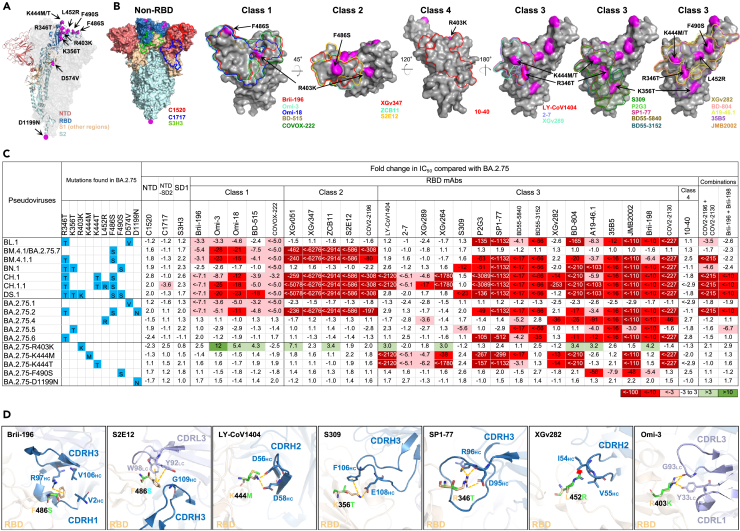
To investigate the antibody evasion properties of BA.2.75 sublineage, we generated vesicular stomatitis virus (VSV)-pseudotyped viruses of each subvariant and the R403K, K444M, K444T, F490S, and D1199N point mutants in the background of BA.2.75 (denoted BA.2.75-R403K, BA.2.75-K444M, BA.2.75-K444T, BA.2.75-F490S, and BA.2.75-D1199N, respectively) (Figure 3A). We then assessed their neutralization profile to a panel of 30 mAbs that had retained good potency against both D614G and BA.2.75 parental virus by targeting multiple epitopes on the viral spike. Among these mAbs, 27 were directed to the four epitope classes in the receptor-binding domain (RBD), including Brii-196 (amubarvimab),7 Omi-3,8 Omi-18,8 BD-515,9 COVOX-222,10 XGv051,11 XGv347,12 ZCB11,13 S2E12,14 COV2-2196 (tixagevimab),15 LY-CoV1404 (bebtelovimab),16 2-7,17 XGv289,12 XGv264,11 S309 (sotrovimab),18 P2G3,19 SP1-77,20 BD55-5840,21 BD55-3152,21 XGv282,12 BD-804,22 A19–46.1,23 35B5,24 JMB2002,25 Brii-198 (romlusevimab),7 COV2-2130 (cilgavimab),15 and 10-40.26 The other three mAbs, C1520,27 C1717,27 and S3H3,28 target the N-terminal domain (NTD), NTD-SD2 (subdomain 2), and SD1 (subdomain 1), respectively. In Figure 3B, the footprints of those mAbs with structural information available were drawn on the spike or the RBD, with the key mutations found in the BA.2.75 subvariants highlighted.
Figure 3.
Resistance of pseudotyped BA.2.75 subvariants to neutralization by monoclonal antibodies (mAbs)
(A) Key spike mutations found in BA.2.75 subvariants. Mutations are highlighted in magenta.
(B) Footprints of NTD-, NTD-SD2-, and SD1-directed neutralizing mAbs on spike, and RBD class 1 to class 4—directed neutralizing mAbs on RBD. Mutations found in BA.2.75 subvariants are highlighted in magenta.
(C) Fold change in IC50 values of BA.2.75 subvariants relative to BA.2.75, with resistance to neutralization highlighted in red and sensitization in green. Spike mutations found in each of the indicated subvariants in addition to BA.2.75 are highlighted in blue.
(D) Structural modeling of the impact on mAbs for the F486S, K444M, K346T, K356T, L452R, and R403K mutations. Clash is shown as the red asterisk; the interactions are shown as yellow dashed lines. See also Table S1.
The raw IC50 (the 50% inhibitory concentration) values for each mAb against each pseudovirus are summarized in Table S1, and the fold changes in IC50 values compared with that of BA.2.75 are presented in Figure 3C. Overall, the non-RBD mAbs and class 4 RBD mAb (C1520, C1717, S3H3, and 10-40) generally did not have impaired neutralization activity against the BA.2.75 subvariants, with the exception of C1717, which had a 3.6-fold drop in neutralizing CH.1.1. Class 1, 2, and 3 RBD mAbs exhibited diverse neutralization profiles. BM.4.1 and BA.2.75.7 (denoted as BM.4.1/BA.2.75.7 as they share an identical spike) partially or completely escaped neutralization by both class 1 and class 2 RBD mAbs because of the F486S mutation. BA.2.75.1 resisted RBD class 1 mAbs, due to the D574V mutation. BA.2.75.4, BA.2.75.5, and BA.2.75.6 demonstrated substantial resistance to some of the RBD class 3 mAbs after acquiring L452R, K356T, and R346T, respectively. The single mutation D1199N found in BA.2.75.2 did not alter the neutralization profile of BA.2.75, whereas the mutations K444M/T and F490S both impaired the neutralizing activity of some class 3 RBD mAbs.
BN.1 also exhibited relative resistance to some class 3 RBD mAbs, due to R346T, K356T, and F490S. The most strikingly resistant of the subvariants were BM.4.1.1, CH.1, CH.1.1, DS.1, and BA.2.75.2, which showed neutralization resistance to class 1 and 2 RBD mAbs and most class 3 RBD mAbs. CH.1 and CH.1.1 were most evasive to neutralization by this panel of mAbs, as only 2 of 27 RBD-directed mAbs retained unchanged potency against these subvariants, followed by DS.1, which impaired neutralization by 21 of 27 RBD antibodies. Surprisingly, we observed that a mutation unique to DS.1, R403K, in fact slightly sensitized BA.2.75 to neutralization by 10 of 26 class 1, 2, and 3 RBD mAbs tested (Figure 3C).
We also studied several antibodies and cocktails that were previously used clinically against BA.2.75 subvariants, including COV2-2130 and COV2-2196 (also known as Evusheld), the combination of Brii-196 (amubarvimab) and Brii-198 (romlusevimab), and LY-CoV1404 (bebtelovimab). Evusheld was rendered inactive or greatly impaired against BA.2.75.2, BM.4.1.1, CH.1, CH.1.1, and DS.1, which all have the R346T mutation paired with F486S (Figure 3C). Brii-196 + Brii-198, which was already greatly impaired against BA.2.75, further lost activity against BA.2.75.2, BA.2.75.5, CH.1, CH.1.1, and DS.1. Bebtelovimab was knocked out by the BA.2.75 single mutants carrying the K444 M/T mutations, as well as by CH.1 and CH.1.1.
Structural modeling of mAb-binding impairment in BA.2.75 subvariants
We conducted structural modeling to further investigate how mutations in the circulating BA.2.75 subvariants confer resistance or sensitization to mAbs against different epitopes (Figure 3D). One of these mutations, F486S, disrupted a common cation-π interaction with R97 of Brii-196 from VH3-53 gene class,29 as well as the interactions with Y92 and W98 for S2E12. The other RBD mutations, R346T, K356T, K444M/T, L452R, and F490S, are located on the outer surface of the RBD and within the epitope cluster of class 3 mAbs, which likely explains their loss of neutralizing activity. Specifically, the K444M and K444T mutations abolished two salt bridges interacting with D56 and D58 in CDRH2 of LY-CoV1404,6 and the K356T mutation weakened S309 by breaking the salt bridge and cation-π interaction. Additionally, the K356T mutation may introduce an N-glycan at N354 in RBD, which would reduce the accessibility of the surrounding residues due to steric hindrance, thereby conferring a degree of resistance to the RBD class 3 mAbs. R346T, L452R, and F490S mutations have previously been observed in BA.4.6,2,30 BA.4/5,3,21 and Lambda,31,32 respectively. The R346T mutation removed the salt bridge and several hydrogen bonds with D95 and R96 in SP1-77, the L452R mutation created steric hindrance with I54 in XGv282, and F490S disturbed the cation- π interaction with R74 in XGv2826 (Figure 3D). R403K mutation, a novel substitution sensitizing BA.2.75 to some RBD-directed mAbs, could retain the interaction with G93 and form an extra hydrogen bond with Y33 in Omi-3 (Figure 3D).
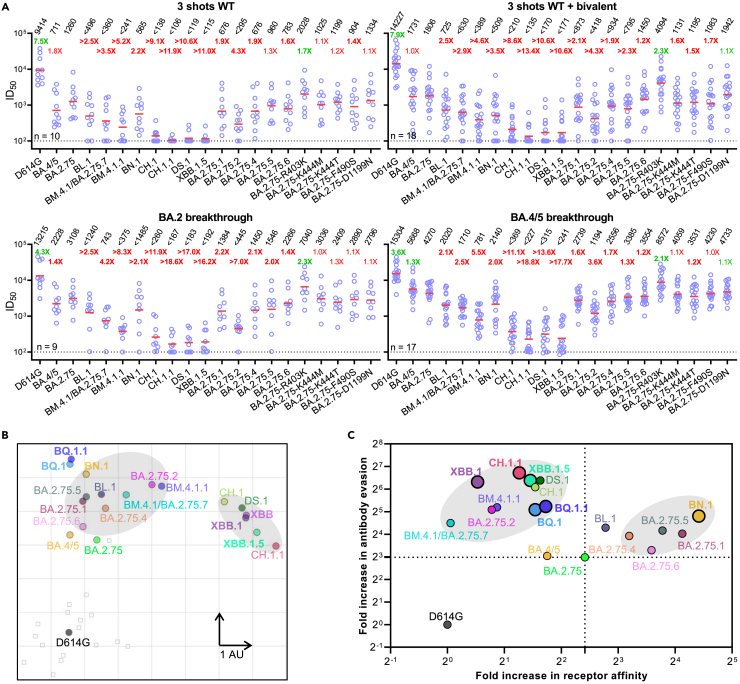
Enhanced evasion of serum neutralization
Given the increased evasion of BA.2.75 subvariants to mAb neutralization and the structural changes within multiple key epitopes, we next asked whether these subvariants were also capable of evading neutralization by sera from humans with prior immunity to SARS-CoV-2. We measured the neutralization resistance profiles of the BA.2.75 subvariants to sera from four different clinical cohorts: individuals who had received three doses of the wild-type mRNA vaccines (“3 shots WT”), three doses of the wild-type mRNA vaccines followed by one shot of the bivalent mRNA vaccines (“3 shots WT + bivalent”), and patients who had a BA.2 or BA.4/5 breakthrough infection after vaccination (“BA.2” and “BA.4/5 breakthrough”, respectively; Table S2). Their neutralization ID50 titers (50% inhibitory dilution) against D614G, BA.4/5, BA.2.75, and BA.2.75 subvariants are presented in Figure 4A. Consistent with our previous findings, BA.2.75 was 7.5-fold more resistant to the “3 shots WT” sera neutralization, whereas BA.4/5 was 1.8-fold more resistant than BA.2.75.1 The neutralization ID50 titers of this cohort were significantly lower against the new BA.2.75 subvariants than against BA.2.75, with the exception of BA.2.75.5. CH.1.1 showed the biggest drop in susceptibility to neutralization, of 11.9-fold, in the “3 shots WT” cohort. In addition, BL.1, BM.4.1/BA.2.75.7, BM.4.1.1, BN.1, CH.1, CH.1.1, and DS.1 significantly impaired the neutralization potency of the boosted sera by 2.1- to 11.0-fold, among which BM.4.1.1, CH.1, CH.1.1, and DS.1 were more neutralization evasive than BA.2.75.2. The D1199N single mutation, as well as the K444M, K444T, and F490S mutations, did not strongly alter the neutralization resistance of BA.2.75 to the “3 shots WT” sera (0.9- to 1.4-fold changes in ID50 titers). Strikingly, concordant with increased mAb neutralization, R403K sensitized BA.2.75 to serum neutralization by 1.7-fold.
Figure 4.
Neutralization of pseudotyped BA.2.75 subvariants by polyclonal sera from four clinical cohorts
(A) Neutralization of pseudotyped D614G and Omicron subvariants by sera from four different clinical cohorts. “3 shots WT” refers to individuals who received three doses of a COVID-19 WT mRNA vaccine, “3 shots WT + bivalent” refers to individuals vaccinated with three doses of the WT mRNA vaccine and subsequently one dose of a WA1/BA.5 bivalent mRNA vaccine, and breakthrough refers to individuals who received COVID-19 vaccines and were infected. The results are representative of those obtained in two independent experiments and shown as dots with geometric mean (red line). Values above the dots denote the raw geometric mean ID50 values, and the sample size (n) for each group is shown on the lower left. The limit of detection is 100 (dotted line). Comparisons were made against BA.2.75, and the fold changes in ID50 values are shown, with resistance to neutralization highlighted in red and sensitization in green. Statistically significant fold changes (p < 0.05, determined by using two-tailed Wilcoxon matched-pairs signed-rank tests) are highlighted in bold.
(B) Antigenic map based on the neutralization data of “3 shots WT + bivalent” vaccinee sera. SARS-CoV-2 variants are shown as colored circles, and sera are shown as gray squares. The x and y axes represent antigenic units (AU) with one unit corresponding to a 2-fold serum dilution of the neutralization titer.
(C) Changes to receptor-binding affinity and antibody evasion of Omicron BA.2.75 subvariants. The x axis illustrates the fold change in ACE2-binding affinity of the Omicron subvariants relative to the D614G strain. The y axis represents the relative immune evasion capability of Omicron subvariants in comparison to the D614G strain (fold change in geometric mean ID50 over “3 shots WT + bivalent” cohort). Black dashed lines correspond to equivalence to BA.2.75. See also Table S2.
A similar trend was also observed for the 3 shots WT + bivalent cohort and the BA.2 and BA.4/5 breakthrough cohorts. BL.1, BM.4.1/BA.2.75.7, BM.4.1.1, BN.1, and BA.2.75.2 impaired the neutralization potency of sera moderately more than BA.2.75, whereas CH.1, CH.1.1, and DS.1 exhibited substantially stronger antibody evasion to serum neutralization, similar to the current predominant Omicron subvariant XBB.1.5.
To visualize the antigenic relationship of the BA.2.75 subvariants, we used antigenic cartography33 of the bivalent vaccine-boosted serum neutralization results to construct a graphical map to display the antigenic distances among D614G, BA.4/5, XBB, XBB.1, XBB.1.5, BQ.1, BQ.1.1, and BA.2.75 subvariants (Figures 4B and S2). We chose bivalent vaccine-boosted serum samples as they exhibit relatively high neutralization titers and are more relevant to vaccine administration. In this rendering, each antigenic unit (AU) of distance in any direction corresponds to a 2-fold change in ID50 titer. BA.2.75 and BA.4/5 displayed a similar antigenic distance to the bivalent vaccine-boosted sera. The point mutations, R346T in BA.2.75.6, K356T in BA.2.75.5, L452R in BA.2.75.4, F486S in BM.4.1/BA.2.75.7, and D574V in BA.2.75.1, each increased the antigenic distance from the boosted sera compared with parental BA.2.75 by about 0.3, 1.14, 0,92, 1.47, and 1.02 AU, respectively, suggesting their importance in mediating resistance to polyclonal antibody neutralization. The combination of R346T, F486S, and D1199N (BA.2.75.2) was an average of 5.07 AU from bivalent sera, which is 1.96 AU further away than BA.4/5, suggesting an advantage of BA.2.75.2 over BA.4/5 in evading serum antibodies. BN.1 and BL.1 were 4.8 and 4.29 AU from boosted sera, respectively, which is at least 0.44 AU closer than BQ.1 and BQ.1.1, whereas BM.4.1.1 exhibited a similar distance to that of BQ.1 and BQ.1.1. Interestingly, CH.1, CH.1.1, and DS.1 were 6.09, 6.75, and 6.40 AU from bivalent vaccine-boosted sera, which is similar to the 6.39 AU for XBB.1.5, illustrating the competitive resistance advantage of CH.1.1, DS.1, and XBB.1.5. Finally, we note that these BA.2.75 subvariants have diverged into two separate antigenic clusters, one set (e.g., BN.1 and BA.2.75.1) grouping with BQ subvariants and another (e.g., CH.1.1 and DS.1) grouping with XBB subvariants (Figure 4B). These two clusters are antigenically quite distinct, highlighting the significant antigenic differences between these viruses.
Evolution of BA.2.75 subvariant phenotypes
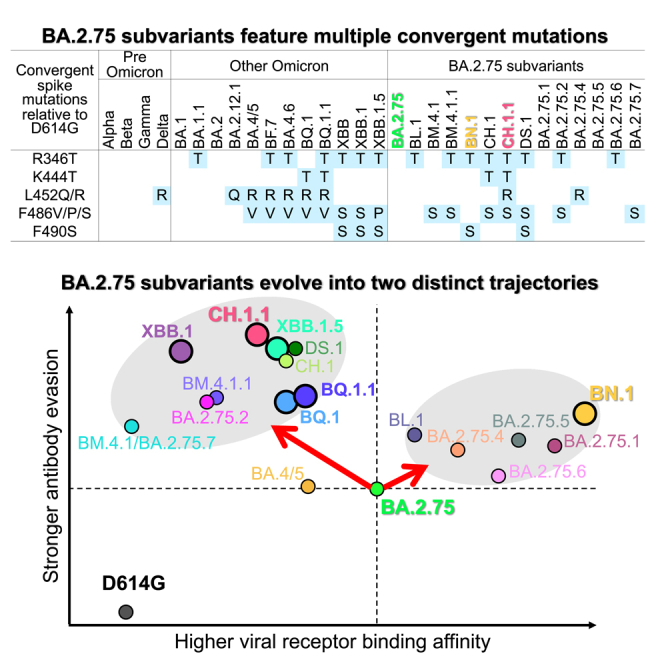
Lastly, we sought to examine whether there were common trends in receptor-binding and antibody evasion phenotypes across the BA.2.75 sublineage, by plotting the fold increases in hACE2-binding affinity versus the fold increases in antibody evasion to sera from the bivalent boosted cohort (Figure 4C). Two general phenotypic combinations were evident: one group including CH.1.1 and DS.1 with substantially higher neutralization resistance but decreased ACE2 affinity and another group including BN.1 with substantially higher ACE2 affinity but only moderately increased neutralization resistance. Placed in the context of other Omicron subvariants (e.g., XBB.1.5 and BQ.1.1), it is quite apparent that viral strains that are currently dominant in the circulation are primarily those with highest antibody evasion properties, again indicating this particular phenotype as the principal determinant of transmissibility in the population today (Figure 4C).
Discussion
To gain a deeper understanding of the evolution of the emerging Omicron BA.2.75 sublineage, we systematically evaluated antigenic and viral receptor-binding properties of major subvariants within this sublineage. Our experimental and in silico analyses revealed several critical mutations, including three not previously described (K356T, R403K, and D574V) (Figure S1), which conferred different degrees of antibody resistance and receptor-binding affinity (Figures 2, 3, and 4). Five RBD mutations, R346T, K356T, K444 M/T, and F490S, impaired the neutralization activities of some class 3 RBD mAbs, F486S led to a large loss of neutralizing activities of classes 1 and 2 RBD mAbs, and D574V conferred resistance to all of the class 1 RBD mAbs tested. Our results indicate that the K356T mutation could potentially introduce an additional N-linked glycan at the N354 site, thereby enhancing the ability of BA.2.75.5 to evade RBD class 3 antibodies. Additionally, the D574V mutation may influence the “up” and “down” dynamics of the RBD. This process might be modulated by changes in the local conformation of SD1.34,35 Notably, the BA.2.75 subvariants with K444 M/T and R346T paired with F486S evaded authorized antibodies bebtelovimab and Evusheld, respectively, which poses a new threat to individuals who need them therapeutically or prophylactically. Interestingly, we made the novel observation that R403K sensitizes BA.2.75 to neutralization by some class 1, 2, and 3 RBD mAbs (Figure 3), while it substantially increases the receptor affinity. This affinity increase could be a compensatory mechanism to regain the fitness loss in receptor binding caused by mutations at F486 in the DS.1 subvariant, mechanistically similar to the R493Q mutation observed in BA.4/5.3
In addition to R403K, our study shows that R346T, K356T, and D574V not only contributed to neutralization profile changes but also enhanced receptor-binding affinity, which sheds light on the continued co-evolution of immune evasion and factors affecting transmissibility of the virus (Figures 2, 3, and 4). This higher receptor-binding affinity could potentially compensate for lower antibody evasion properties and allow for expansion, as exemplified by BN.1. However, other transmissibility-related factors, such as cell-type tropism, syncytial formation, and viral load/titer in epithelial cells, need further investigation. These factors may co-evolve with immune evasion and receptor-binding affinity, potentially affecting viral transmissibility in the human population.
Overall, our investigations have shown that the evolutionary trajectory of BA.2.75 subvariants has diverged in two different directions: substantially higher neutralization resistance but slightly reduced ACE2 affinity, as seen in CH.1.1 and DS.1, and substantially higher ACE2 affinity but only moderately increased neutralization resistance, as seen in BN.1 (Figure 4C). Globally, and particularly in the US, UK, Ireland, and Singapore, BN.1 is being outcompeted by CH.1.1 (Figure 1C), although it remains unclear why the same has yet to occur in some Asian countries such as Japan.
BA.2.75 subvariants CH.1, CH.1.1, and DS.1 now rival XBB.1.5 in their resistance to monoclonal antibodies (Figure 3C) and polyclonal sera (Figure 4A). XBB.1.5 is slightly more antibody evasive than BQ.1 and BQ.1.1.6 It appears that highest level of resistance to antibody neutralization has been achieved by three Omicron sublineages (XBB.1/XBB.1.5, BQ.1/BQ.1.1, and CH.1.1), each utilizing a distinct mutational pathway to converge on a common phenotype (Figure 1A). Seemingly, the most dominant SARS-CoV-2 strains presently are also most evasive to antibody neutralization (Figure 4C). This correlation suggests that the ability to escape from antibody pressure is perhaps the dominant determinant of transmissibility in the population today.
Limitations of the study
Instead of live viruses, we used VSV-based pseudoviruses for the neutralization test. Although pseudovirus neutralization tests offer advantages, such as increased efficiency and enhanced biosafety, it could still be beneficial to confirm our findings with authentic virus evaluations. However, past studies have shown strong associations between pseudovirus and live virus neutralization tests in assessing antibody responses to SARS-CoV-2.3,36,37 Additionally, we may need to consider non-spike genes that can potentially influence biological functions related to virus transmissibility.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| C1520 | Wang et al.27 | N/A |
| C1717 | Wang et al.27 | N/A |
| S3H3 | Hong et al.28 | N/A |
| Brii-196 | Ju et al.7 | N/A |
| Omi-3 | Nutalai et al.8 | N/A |
| Omi-18 | Nutalai et al.8 | N/A |
| BD-515 | Cao et al.9 | N/A |
| COVOX-222 | Dejnirattisai et al.10 | N/A |
| XGv051 | Wang et al.11 | N/A |
| XGv347 | Wang et al.12 | N/A |
| ZCB11 | Zhou et al.13 | N/A |
| S2E12 | Tortorici et al.14 | N/A |
| COV2-2196 | Zost et al.15 | N/A |
| LY-CoV1404 | Westendorf et al.16 | N/A |
| 2–7 | Liu et al.17 | N/A |
| XGv289 | Wang et al.12 | N/A |
| XGv264 | Wang et al.11 | N/A |
| S309 | Pinto et al.18 | N/A |
| P2G3 | Fenwick et al.19 | N/A |
| SP1-77 | Luo et al.20 | N/A |
| BD55-5840 | Cao et al.21 | N/A |
| BD55-3152 | Cao et al.21 | N/A |
| XGv282 | Wang et al.12 | N/A |
| BD-804 | Du et al.22 | N/A |
| A19–46.1 | Wang et al.23 | N/A |
| 35B5 | Wang et al.24 | N/A |
| JMB2002 | Yin et al.25 | N/A |
| Brii-198 | Ju et al.7 | N/A |
| COV2-2130 | Zost et al.15 | N/A |
| 10–40 | Liu et al.26 | N/A |
| Bacterial and virus strains | ||
| VSV-G pseudotyped ΔG-luciferase | Kerafast | Cat#EH1020-PM |
| Biological samples | ||
| 3 Shots WT sera | Wang et al.6 | N/A |
| 3 Shots WT + Bivalent sera | Wang et al.6 | N/A |
| BA.2 breakthrough sera | Wang et al.6 | N/A |
| BA.4/5 breakthrough sera | Wang et al.6 | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Polyethylenimine (PEI) | Polysciences Inc. | Cat#23966-100 |
| hACE2 | Wang et al.3 | N/A |
| D614G S2P | Wang et al.3 | N/A |
| BA.4/5 S2P | Wang et al.3 | N/A |
| BF.7 S2P | Wang et al.2 | N/A |
| BQ.1 S2P | Wang et al.6 | N/A |
| BQ.1.1 S2P | Wang et al.6 | N/A |
| XBB.1 S2P | Wang et al.6 | N/A |
| XBB.1.5 S2P | This paper | N/A |
| BA.2.75 S2P | Wang et al.1 | N/A |
| BL.1 S2P | This paper | N/A |
| BM.4.1/BA.2.75.7 S2P | This paper | N/A |
| BM.4.1.1 S2P | This paper | N/A |
| BN.1 S2P | This paper | N/A |
| CH.1 S2P | This paper | N/A |
| CH.1.1 S2P | This paper | N/A |
| DS.1 S2P | This paper | N/A |
| BA.2.75.1 S2P | This paper | N/A |
| BA.2.75.2 S2P | This paper | N/A |
| BA.2.75.4 S2P | This paper | N/A |
| BA.2.75.5 S2P | This paper | N/A |
| BA.2.75.6 S2P | This paper | N/A |
| BA.2.75-R403K S2P | This paper | N/A |
| BA.2.75-K444M S2P | This paper | N/A |
| BA.2.75-K444T S2P | This paper | N/A |
| BA.2.75-F490S S2P | This paper | N/A |
| BA.2.75-D1199N S2P | This paper | N/A |
| Critical commercial assays | ||
| Luciferase Assay System | Promega | Cat#E4550 |
| QuikChange Lightning Site-Directed Mutagenesis Kit | Agilent | Cat#210518 |
| QuikChange Lightning Multi Site-Directed Mutagenesis Kit | Agilent | Cat#210516 |
| T4 DNA Ligase | New England Biolabs | Cat#M0202S |
| Series S sensor chip CM5 | Cytiva | Cat#BR100530 |
| His-capture kit | Cytiva | Cat#28995056 |
| Experimental models: cell lines | ||
| HEK293T | ATCC | Cat#CRL-3216; RRID: CVCL_0063 |
| Vero-E6 | ATCC | Cat#CRL-1586; RRID: CVCL_0574 |
| Expi293 cells | Thermo Fisher Scientific | Cat#A14527 |
| Recombinant DNA | ||
| pCMV3-D614G | Wang et al.3 | N/A |
| pCMV3-BA.4/5 | Wang et al.3 | N/A |
| pCMV3-BA.2.75 | Wang et al.1 | N/A |
| pCMV3-XBB.1.5 | This paper | N/A |
| pCMV3-BL.1 | This paper | N/A |
| pCMV3-BM.4.1/BA.2.75.7 | This paper | N/A |
| pCMV3-BM.4.1.1 | This paper | N/A |
| pCMV3-BN.1 | This paper | N/A |
| pCMV3-CH.1 | This paper | N/A |
| pCMV3-CH.1.1 | This paper | N/A |
| pCMV3-DS.1 | This paper | N/A |
| pCMV3-BA.2.75.1 | This paper | N/A |
| pCMV3-BA.2.75.2 | This paper | N/A |
| pCMV3-BA.2.75.4 | This paper | N/A |
| pCMV3-BA.2.75.5 | This paper | N/A |
| pCMV3-BA.2.75.6 | This paper | N/A |
| pCMV3-BA.2.75-R403K | This paper | N/A |
| pCMV3-BA.2.75-K444M | This paper | N/A |
| pCMV3-BA.2.75-K444T | This paper | N/A |
| pCMV3-BA.2.75-F490S | This paper | N/A |
| pCMV3-BA.2.75-D1199N | This paper | N/A |
| pcDNA3-sACE2-WT(732)-IgG1 | Chan et al.38 | RRID: Addgene_154104 |
| Software and algorithms | ||
| GraphPad Prism 9 | GraphPad Software Inc | https://www.graphpad.com/scientific-software/prism/ |
| PyMOL v.2.3.2 | Schrödinger, LLC | https://pymol.org/2/#page-top |
| Biacore T200 Evaluation Software (Version 1.0) | Cytiva | NA |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, David D. Ho (dh2994@cumc.columbia.edu).
Materials availability
All requests for resources and reagents should be directed to and will be fulfilled by the lead contact, David D. Ho (dh2994@cumc.columbia.edu). This includes selective cell lines, plasmids, antibodies, viruses, serum, and proteins. All reagents will be made available on request after completion of a Material Transfer Agreement.
Experimental model and study participant details
Sample collection
The sera samples were all collected at Columbia University Irving Medical Center or at the University of Michigan through the Immunity-Associated with SARS-CoV-2 Study (IASO), and the collections were conducted under protocols reviewed and approved by the Institutional Review Board of Columbia University or the Institutional Review Board of the University of Michigan Medical School.39 All subjects provided written informed consent. Sera from individuals who received three doses of either the mRNA-1273 or BNT162b2 vaccines are described in the text as “3 shots WT”. Sera from individuals who received three doses of either the mRNA-1273 or BNT162b2 vaccines followed by a bivalent mRNA vaccine are described in the text as “3 shots WT + bivalent”. Sera from individuals who were infected by an Omicron subvariant (BA.2) following vaccinations were collected from December 2021 to May 2022 and are described in the text as “BA.2 breakthrough”. Sera from individuals who received vaccinations and were subsequently infected by an Omicron subvariant (BA.4/5) were collected from July 2022 to August 2022 and are described in the text as “BA.4/5 breakthrough”. To confirm prior SARS-CoV-2 infection status, anti-nucleoprotein (NP) ELISA tests were performed on all serum samples, as well as DNA sequencing to determine the variant involved in breakthrough cases. In this study, neither sex nor gender of the participants are anticipated to exert a significant influence on the outcomes. Clinical information including age and gender on the different cohorts of study subjects is provided in Table S2.
Cell lines
Vero-E6 (CRL-1586) cells and HEK293T (CRL-3216) cells were obtained from the ATCC and cultured at 37°C with 5% CO2 in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Expi293 (A14527) cells were bought from Thermo Fisher Scientific and maintained in Expi293 Expression Medium following the manufacturer’s instructions. Vero-E6 cells are from African green monkey kidneys. HEK293T cells and Expi293 cells are of female origin.
Method details
Plasmids
The antibody sequences for the heavy chain variable (VH) and the light chain variable (VL) domains were synthesized by GenScript, and then cloned into the gWiz vector to produce antibody expression plasmids. For the packaging plasmids for pseudoviruses, mutations were made by using the QuikChange II XL site-directed mutagenesis kit (Agilent) on the BA.2.75 construct that we previously generated.1 For the soluble spike expression plasmids, the 2P substitutions (K986P, V987P) and a “GSAS” substitution in the furin cleavage site (682-685aa) were introduced in the ectodomain (1-1208aa in WA1) of each of the spikes by using the QuikChange Lightning Multi Site-Directed Mutagenesis Kit (Agilent) and then fused with a 8x His-tag at the C-terminus using T4 DNA Ligase (NEB) as previously described.40 All constructs were verified using Sanger sequencing prior to use.
Protein expression and purification
The gWiz-antibody, paH-spike, or pcDNA3-sACE2-WT(732)-IgG1 (Addgene plasmid #154104)38 plasmid was transfected into Expi293 cells using PEI at a ratio of 1:3, and then the supernatants were collected after five days. The antibodies and human ACE2 fused to a Fc tag were purified with Protein A Sepharose (Cytiva) following the manufacturer’s instructions. For SPR analysis, the human ACE2 protein was further purified with Superdex 200 Increase 10/300 GL column. Spike proteins were purified using Ni-NTA resin (Invitrogen) following the manufacturer’s instructions. The molecular weight and purity were checked by running the proteins on SDS-PAGE prior to use.
Surface plasmon resonance (SPR)
The CM5 chip was immobilized with anti-His antibodies using the His Capture Kit (Cytiva) to capture the spike protein through the C-terminal His-tag. Serially diluted human ACE2-Fc protein was then flowed over the chip in HBS-EP+ buffer (Cytiva). Binding affinities were measured with the Biacore T200 system at 25°C in the single-cycle mode. Data was analyzed by the Evaluation Software using the 1:1 binding model.
Pseudovirus production
Pseudotyped SARS-CoV-2 (pseudoviruses) were produced in the vesicular stomatitis virus (VSV) background, in which the native VSV glycoprotein was replaced by SARS-CoV-2 and its variants, as previously described.3 Briefly, plasmids containing the appropriate spike were transfected into HEK293T cells with PEI. After 24 h, VSV-G pseudotyped ΔG-luciferase (G∗ΔG-luciferase, Kerafast) was added, and then washed with culture medium three times before being cultured in fresh medium for another 24 h. Pseudoviruses were then harvested, centrifuged, and then aliquoted and stored at −80°C.
Pseudovirus neutralization assay
Each SARS-CoV-2 pseudovirus was titrated before use in the neutralization assay. Serially diluted heat-inactivated sera or antibodies were added in 96-well plates, starting at 1:100 dilution for sera and 10 μg/mL for antibodies. Then, pseudoviruses were added and incubated at 37 °C for 1 h. In each plate, wells containing only pseudoviruses were included as controls. Vero-E6 cells were then added at a density of 3 × 104 cells per well and incubate at 37 °C for an additional 10 h. Cells were lysed and luminescence was determined by the Luciferase Assay System (Promega) and SoftMax Pro v.7.0.2 (Molecular Devices) according to the manufacturers’ instructions. Data were analyzed in GraphPad Prism v.9.3.
Phylogenetic analysis
The genome sequences for each BA.2.75 subvariants were obtained from GISAID database (Accession: EPI_ISL_14217529, EPI_ISL_16926267, EPI_ISL_15050799, EPI_ISL_14908101, EPI_ISL_14536676, EPI_ISL_16581575, EPI_ISL_16939789, EPI_ISL_14492159, EPI_ISL_15611014, EPI_ISL_14434640, EPI_ISL_16040351, EPI_ISL_16434652, EPI_ISL_16954277, EPI_ISL_14536591 and EPI_ISL_13521515) to build the phylogenetic tree. The sequences were aligned by Muscle v3.8.31, and the low-quality sequencing sites with ‘N’ or ‘-’ were removed. The Maximum Likelihood tree was built in MEGA11 by Tamura-Nei model with 500 of bootstrap replication.
Antibody footprint and mutagenesis analysis
All the structures were downloaded from Protein DataBank (7XIX (BA.2 Spike), 8ASY (BA.2.75 RBD with ACE2), 7WK9 (S3H3), 7UAR (C1717), 7UAP (C1520), 7ZF3 (Omi-3), 7ZFB (Omi-18), 7CDI (Brii-196), 7OR9 (COVOX-222), 7WED (XGv347), 7K45 (S2E12), 7SD5 (10–40), 7XCO (S309), 7WRV (JMB2002), 7WRZ (BD55-5840), 7WR8 (BD55-3152), 7WM0 (35B5), 7WLC (XGv282), 7WE9 (XGv289), 7UPY (SP1-77), 7U0D (A19–46.1), 7QTK (P2G3), 7MMO (LY-CoV1404), 7LSS (2–7), 7EYA (BD-804)) for analysis. The interface residues of each antibody were obtained by running the InterfaceResidues script from PyMOLwiki in PyMOL, and the edge of these residues was defined as the footprint after checking manually in the structure. Mutagenesis analyses were conducted in PyMOL. All the structure analysis figures were generated in PyMOL v.2.3.2 (Schrödinger, LLC).
Antigenic cartography
The antigenic map was generated using the Racmacs package (https://acorg.github.io/Racmacs/, version 1.1.35) in R with 2000 optimization steps, a dilution step size of zero, and the minimum column basis parameter set to “none”. All distances between virus and serum positions on the map were optimized so that distances correspond to the fold decrease in neutralizing ID50 titer, relative to the maximum titer for each serum. Each unit of distance in any direction in the antigenic map corresponds to a 2-fold change in the ID50 titer.
Quantification and statistical analysis
Neutralization ID50 and IC50 values were determined by fitting a five-parameter dose-response curve using GraphPad Prism v.9.3. Statistical significance was evaluated using two-tailed Wilcoxon matched-pairs signed-rank tests in GraphPad Prism v.9.3.
Acknowledgments
This study was supported financially by the SARS-CoV-2 Assessment of Viral Evolution Program, NIAID, NIH (Subcontract No. 0258-A709-4609 under Federal Contract No. 75N93021C00014), and the Gates Foundation (Project No. INV019355) awarded to D.D.H. and through the National Institutes of Health Collaborative Influenza Vaccine Innovation Center (75N93019C00051) awarded to A.G. We acknowledge Michael T. Yin and Magdalena E. Sobieszczyk for providing serum samples. We thank Carmen Gherasim, David Manthei, Emily Stoneman, Victoria Blanc, Pamela Bennett-Baker, Savanna Sneeringer, Lauren Warsinske, Theresa Kowalski-Dobson, Alyssa Meyers, Zijin Chu, Hailey Kuiken, Lonnie Barnes, Ashley Eckard, Kathleen Lindsey, Dawson Davis, Aaron Rico, Casey Juntila, Daniel Raymond, Mayurika Patel, Nivea Vydiswaran, Julie Gilbert, and William J. Fitzsimmons from the IASO study team for providing serum samples. We thank all who contributed their data to GISAID.
Author contributions
L.L. and D.D.H. conceived this project. Q.W. and L.L. constructed the spike expression plasmids. Q.W., I.A.M., and L.L. conducted pseudovirus neutralization assays. Q.W., Z.L. and L.L. purified SARS-CoV-2 spike proteins, performed SPR assay, and conducted structural analyses. Y.G. and Z.S. conducted bioinformatic analyses and generated antigenic map. Q.W. managed the project. J.Y. and M.L. expressed and purified antibodies. R.V., A.S.L., and A.G. provided clinical samples. L.L. and D.D.H. directed and supervised the project. Q.W., Z.L., Y.G., I.A.M., S.I., L.L., and D.D.H. analyzed the results and wrote the manuscript.
Declaration of interests
S.I, J.Y., L.L., and D.D.H. are inventors on patent applications (WO2021236998) or provisional patent applications (63/271,627) filed by Columbia University for a number of SARS-CoV-2 neutralizing antibodies described in this manuscript. Both sets of applications are under review. D.D.H. is a co-founder of TaiMed Biologics and RenBio, consultant to WuXi Biologics and Brii Biosciences, and board director for Vicarious Surgical. Aubree Gordon serves on a scientific advisory board for Janssen Pharmaceuticals. Other authors declare no competing interests.
Published: October 18, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.108254.
Contributor Information
Lihong Liu, Email: ll3411@cumc.columbia.edu.
David D. Ho, Email: dh2994@cumc.columbia.edu.
Supplemental information
Data and code availability
-
•Data
- Data reported in this paper will be shared by the lead contact upon request.
-
•Code
- This paper does not report original code.
-
•All other items
- All the structures for structural modeling were downloaded from Protein DataBank (PDB) and the IDs of the structures are provided in method details. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Wang Q., Iketani S., Li Z., Guo Y., Yeh A.Y., Liu M., Yu J., Sheng Z., Huang Y., Liu L., Ho D.D. Antigenic characterization of the SARS-CoV-2 Omicron subvariant BA.2.75. Cell Host Microbe. 2022;30:1512–1517.e4. doi: 10.1016/j.chom.2022.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q., Li Z., Ho J., Guo Y., Yeh A.Y., Mohri H., Liu M., Wang M., Yu J., Shah J.G., et al. Resistance of SARS-CoV-2 omicron subvariant BA.4.6 to antibody neutralisation. Lancet Infect. Dis. 2022;22:1666–1668. doi: 10.1016/S1473-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q., Guo Y., Iketani S., Nair M.S., Li Z., Mohri H., Wang M., Yu J., Bowen A.D., Chang J.Y., et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4, & BA.5. Nature. 2022;608:603–608. doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starr T.N., Greaney A.J., Stewart C.M., Walls A.C., Hannon W.W., Veesler D., Bloom J.D. Deep mutational scans for ACE2 binding, RBD expression, and antibody escape in the SARS-CoV-2 Omicron BA.1 and BA.2 receptor-binding domains. bioRxiv. 2022;1 doi: 10.1101/2022.09.20.508745. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y., Jian F., Wang J., Yu Y., Song W., Yisimayi A., Wang J., An R., Chen X., Zhang N., et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature. 2023;614:521–529. doi: 10.1038/s41586-022-05644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q., Iketani S., Li Z., Liu L., Guo Y., Huang Y., Bowen A.D., Liu M., Wang M., Yu J., et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186:279–286.e8. doi: 10.1016/j.cell.2022.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 8.Nutalai R., Zhou D., Tuekprakhon A., Ginn H.M., Supasa P., Liu C., Huo J., Mentzer A.J., Duyvesteyn H.M.E., Dijokaite-Guraliuc A., et al. Potent cross-reactive antibodies following Omicron breakthrough in vaccinees. Cell. 2022;185:2116–2131.e18. doi: 10.1016/j.cell.2022.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y., Yisimayi A., Bai Y., Huang W., Li X., Zhang Z., Yuan T., An R., Wang J., Xiao T., et al. Humoral immune response to circulating SARS-CoV-2 variants elicited by inactivated and RBD-subunit vaccines. Cell Res. 2021;31:732–741. doi: 10.1038/s41422-021-00514-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dejnirattisai W., Zhou D., Supasa P., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Tuekprakhon A., Nutalai R., et al. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell. 2021;184:2939–2954.e9. doi: 10.1016/j.cell.2021.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L., Fu W., Bao L., Jia Z., Zhang Y., Zhou Y., Wu W., Wu J., Zhang Q., Gao Y., et al. Selection and structural bases of potent broadly neutralizing antibodies from 3-dose vaccinees that are highly effective against diverse SARS-CoV-2 variants, including Omicron sublineages. Cell Res. 2022;32:691–694. doi: 10.1038/s41422-022-00677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K., Jia Z., Bao L., Wang L., Cao L., Chi H., Hu Y., Li Q., Zhou Y., Jiang Y., et al. Memory B cell repertoire from triple vaccinees against diverse SARS-CoV-2 variants. Nature. 2022;603:919–925. doi: 10.1038/s41586-022-04466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou B., Zhou R., Tang B., Chan J.F.W., Luo M., Peng Q., Yuan S., Liu H., Mok B.W.Y., Chen B., et al. A broadly neutralizing antibody protects Syrian hamsters against SARS-CoV-2 Omicron challenge. Nat. Commun. 2022;13:3589. doi: 10.1038/s41467-022-31259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tortorici M.A., Beltramello M., Lempp F.A., Pinto D., Dang H.V., Rosen L.E., McCallum M., Bowen J., Minola A., Jaconi S., et al. Ultrapotent human antibodies protect against SARS-CoV-2 challenge via multiple mechanisms. Science. 2020;370:950–957. doi: 10.1126/science.abe3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zost S.J., Gilchuk P., Case J.B., Binshtein E., Chen R.E., Nkolola J.P., Schäfer A., Reidy J.X., Trivette A., Nargi R.S., et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584:443–449. doi: 10.1038/s41586-020-2548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westendorf K., Žentelis S., Wang L., Foster D., Vaillancourt P., Wiggin M., Lovett E., van der Lee R., Hendle J., Pustilnik A., et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022;39 doi: 10.1016/j.celrep.2022.110812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., Luo Y., Chan J.F.W., Sahi V., Figueroa A., et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 18.Pinto D., Park Y.J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A., et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 19.Fenwick C., Turelli P., Ni D., Perez L., Lau K., Herate C., Marlin R., Lana E., Pellaton C., Raclot C., et al. Patient-derived monoclonal antibody neutralizes SARS-CoV-2 Omicron variants and confers full protection in monkeys. Nat. Microbiol. 2022;7:1376–1389. doi: 10.1038/s41564-022-01198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo S., Zhang J., Kreutzberger A.J.B., Eaton A., Edwards R.J., Jing C., Dai H.Q., Sempowski G.D., Cronin K., Parks R., et al. An antibody from single human VH-rearranging mouse neutralizes all SARS-CoV-2 variants through BA.5 by inhibiting membrane fusion. Sci. Immunol. 2022;7 doi: 10.1126/sciimmunol.add5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao Y., Yisimayi A., Jian F., Song W., Xiao T., Wang L., Du S., Wang J., Li Q., Chen X., et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608:593–602. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du S., Liu P., Zhang Z., Xiao T., Yasimayi A., Huang W., Wang Y., Cao Y., Xie X.S., Xiao J. Structures of SARS-CoV-2 B.1.351 neutralizing antibodies provide insights into cocktail design against concerning variants. Cell Res. 2021;31:1130–1133. doi: 10.1038/s41422-021-00555-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L., Zhou T., Zhang Y., Yang E.S., Schramm C.A., Shi W., Pegu A., Oloniniyi O.K., Henry A.R., Darko S., et al. Ultrapotent antibodies against diverse and highly transmissible SARS-CoV-2 variants. Science. 2021;373 doi: 10.1126/science.abh1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Chen X., Tan J., Yue S., Zhou R., Xu Y., Lin Y., Yang Y., Zhou Y., Deng K., et al. 35B5 antibody potently neutralizes SARS-CoV-2 Omicron by disrupting the N-glycan switch via a conserved spike epitope. Cell Host Microbe. 2022;30:887–895.e4. doi: 10.1016/j.chom.2022.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin W., Xu Y., Xu P., Cao X., Wu C., Gu C., He X., Wang X., Huang S., Yuan Q., et al. Structures of the Omicron spike trimer with ACE2 and an anti-Omicron antibody. Science. 2022;375:1048–1053. doi: 10.1126/science.abn8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L., Iketani S., Guo Y., Reddem E.R., Casner R.G., Nair M.S., Yu J., Chan J.F.W., Wang M., Cerutti G., et al. An antibody class with a common CDRH3 motif broadly neutralizes sarbecoviruses. Sci. Transl. Med. 2022;14:eabn6859. doi: 10.1126/scitranslmed.abn6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z., Muecksch F., Cho A., Gaebler C., Hoffmann H.H., Ramos V., Zong S., Cipolla M., Johnson B., Schmidt F., et al. Analysis of memory B cells identifies conserved neutralizing epitopes on the N-terminal domain of variant SARS-Cov-2 spike proteins. Immunity. 2022;55:998–1012.e8. doi: 10.1016/j.immuni.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong Q., Han W., Li J., Xu S., Wang Y., Xu C., Li Z., Wang Y., Zhang C., Huang Z., Cong Y. Molecular basis of receptor binding and antibody neutralization of Omicron. Nature. 2022;604:546–552. doi: 10.1038/s41586-022-04581-9. [DOI] [PubMed] [Google Scholar]
- 29.Yuan M., Liu H., Wu N.C., Lee C.C.D., Zhu X., Zhao F., Huang D., Yu W., Hua Y., Tien H., et al. Structural basis of a shared antibody response to SARS-CoV-2. Science. 2020;369:1119–1123. doi: 10.1126/science.abd2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jian F., Yu Y., Song W., Yisimayi A., Yu L., Gao Y., Zhang N., Wang Y., Shao F., Hao X., et al. Further humoral immunity evasion of emerging SARS-CoV-2 BA.4 and BA.5 subvariants. Lancet Infect. Dis. 2022;22:1535–1537. doi: 10.1016/S1473-3099(22)00642-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Q., Ye S.B., Zhou Z.J., Li J.Y., Lv J.Z., Hu B., Yuan S., Qiu Y., Ge X.Y. Key mutations on spike protein altering ACE2 receptor utilization and potentially expanding host range of emerging SARS-CoV-2 variants. J. Med. Virol. 2023;95:e28116. doi: 10.1002/jmv.28116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura I., Kosugi Y., Wu J., Zahradnik J., Yamasoba D., Butlertanaka E.P., Tanaka Y.L., Uriu K., Liu Y., Morizako N., et al. The SARS-CoV-2 Lambda variant exhibits enhanced infectivity and immune resistance. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2021.110218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith D.J., Lapedes A.S., de Jong J.C., Bestebroer T.M., Rimmelzwaan G.F., Osterhaus A.D.M.E., Fouchier R.A.M. Mapping the antigenic and genetic evolution of influenza virus. Science. 2004;305:371–376. doi: 10.1126/science.1097211. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J., Cai Y., Xiao T., Lu J., Peng H., Sterling S.M., Walsh R.M., Jr., Rits-Volloch S., Zhu H., Woosley A.N., et al. Structural impact on SARS-CoV-2 spike protein by D614G substitution. Science. 2021;372:525–530. doi: 10.1126/science.abf2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou T., Tsybovsky Y., Gorman J., Rapp M., Cerutti G., Chuang G.Y., Katsamba P.S., Sampson J.M., Schön A., Bimela J., et al. Cryo-EM Structures of SARS-CoV-2 Spike without and with ACE2 Reveal a pH-Dependent Switch to Mediate Endosomal Positioning of Receptor-Binding Domains. Cell Host Microbe. 2020;28:867–879.e5. doi: 10.1016/j.chom.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt F., Weisblum Y., Muecksch F., Hoffmann H.H., Michailidis E., Lorenzi J.C.C., Mendoza P., Rutkowska M., Bednarski E., Gaebler C., et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J. Exp. Med. 2020;217 doi: 10.1084/jem.20201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan K.K., Dorosky D., Sharma P., Abbasi S.A., Dye J.M., Kranz D.M., Herbert A.S., Procko E. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science. 2020;369:1261–1265. doi: 10.1126/science.abc0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon V., Kota V., Bloomquist R.F., Hanley H.B., Forgacs D., Pahwa S., Pallikkuth S., Miller L.G., Schaenman J., Yeaman M.R., et al. PARIS and SPARTA: Finding the Achilles' Heel of SARS-CoV-2. mSphere. 2022;7 doi: 10.1128/msphere.00179-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•Data
- Data reported in this paper will be shared by the lead contact upon request.
-
•Code
- This paper does not report original code.
-
•All other items
- All the structures for structural modeling were downloaded from Protein DataBank (PDB) and the IDs of the structures are provided in method details. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.