Abstract
Coronary microvascular dysfunction (CMD) is a common complication of ST-segment elevation myocardial infarction (STEMI) and can lead to adverse cardiovascular events. This is a non-randomized, observational, prospective study of STEMI patients with multivessel disease who underwent primary PCI, grouped based on whether they underwent balloon pre-dilatation stenting or direct stenting of the culprit lesion. Coronary physiology measurements were performed 3 months post-PCI including coronary flow reserve (CFR) and index of microcirculatory resistance (IMR) measurements at the culprit vessel. The primary endpoint was the prevalence of CMD at 3 months, defined as IMR ≥ 25 or CFR < 2.0 with a normal fractional flow reserve. Secondary endpoints included major adverse cardiovascular events (MACE) at 12 months. Two hundred ten patients were enrolled; most were men, 125 (59.5%), with a median age of 65 years. One hundred twelve (53.2%) underwent balloon pre-dilatation before stenting, and 98 (46.7%) underwent direct stenting. The prevalence of CMD at 3 months was lower in the direct stenting group than in the balloon pre-dilatation stenting group (12.24% vs. 40.18%; p < 0.001). Aspiration thrombectomy and administration of intracoronary glycoprotein IIb/IIIa inhibitors were associated with lower odds of CMD (OR = 0.175, p = 0.001 and OR = 0.113, p = 0.001, respectively). Notably, MACE in patients who underwent direct stenting was lower than in those who underwent balloon pre-dilatation before stenting (14.29% vs. 26.79%; p = 0.040). In STEMI patients with multivessel disease, direct stenting of the culprit lesion, aspiration thrombectomy and administration of intracoronary glycoprotein IIb/IIIa inhibitors were associated with a lower prevalence of CMD at 3 months and lower incidence of MACE at 12 months compared with balloon pre-dilatation stenting.
This trial is registered at https://ichgcp.net/clinical-trials-registry/NCT05406297.
Subject terms: Cardiology, Interventional cardiology
Introduction
Prompt revascularization with primary percutaneous coronary intervention (PCI) of the occluded epicardial coronary artery is the standard of care for patients with ST-segment elevation acute myocardial infarction (STEMI)1. Although contemporary PCI can achieve patency in most cases, the restoration of coronary microcirculation and, subsequently, myocardial perfusion did not recover up to 50% of the STEMI patients2. Up to 15.5% of STEMI patients continue to experience persistent anginal symptoms after primary PCI, and the presence of coronary microvascular dysfunction (CMD) has been independently associated with worse angina status in this patient population3.
CMD is a term used to describe a range of anatomical and functional changes within the coronary microcirculation that reduce coronary blood flow to cardiomyocytes and result in myocardial ischemia4. CMD can be diagnosed using a variety of invasive and non-invasive techniques5. The thermodilution-based assessment of coronary flow reserve (CFR) and index of microcirculatory resistance (IMR) with a pressure–temperature sensor coronary guidewire is a highly reproducible, easily performed, and is the most common invasive method for diagnosing CMD6,7. Previous studies have shown a robust association between CFR, IMR and poor outcomes in patients with STEMI8.
The pathogenesis of CMD following revascularization in STEMI patients remains unclear. Recent hypotheses have linked CMD to distal embolization of thrombotic debris following mechanical fragmentation of the occlusion during pre-dilatation9. The presence of CMD is related to larger infarct size, higher peak enzyme level, and worse prognosis10 Direct stenting of the culprit occlusion, without pre-dilatation, may attenuate downstream embolization and, by extension, CMD11.
The purpose of this research was to determine whether there is a correlation between the technique of primary PCI used and the prevalence of CMD after reperfusion.
Methods
Study design
This is a prospective, single-blinded, non-randomized, observational, single-center trial conducted in the Hospital of Lithuanian University of Health Sciences Kauno klinikos, Kaunas, Lithuania. This study enrolled STEMI patients with multivessel coronary artery disease who underwent primary PCI according to the European Society of Cardiology (ESC) guidelines12. The PCI strategy was left to the discretion of the treating interventional cardiologist. Three months later, the patients underwent staged revascularization, followed by an invasive coronary physiology evaluation for CMD.
Study inclusion and exclusion criteria
Participants were adults aged 40 years and older with STEMI (ST elevation ≥ 2 mm in ≥ 2 contiguous chest leads or ≥ 1 mm in ≥ 2 contiguous limb leads) who had received dual antiplatelet therapy (acetylsalicylic acid 300 mg and ticagrelor 180 mg or clopidogrel 600 mg) at least 30 min prior to primary PCI of the culprit vessel and subsequently underwent staged PCI of the non-culprit vessel 3 months later.
To exclude the potential influence of pre-existing microvascular obstruction, patients with a history of acute coronary syndrome were excluded. Patients who did not have a non-culprit coronary lesion and thus did not require a follow-up angiogram were also excluded. Patients with a serious comorbid illness such as sepsis, autoimmune disease, end-stage liver disease, end-stage renal failure, or solid organ cancer were excluded. Patients with severe valvular heart disease were excluded for significantly variable coronary physiology, and those with coronary artery bypass grafts were excluded because of altered coronary circulation13. Patients who underwent primary fibrinolysis, were allergic to contrast media, or were unable to tolerate adenosine triphosphate were also excluded.
Primary percutaneous coronary intervention
Primary PCI was performed using 6-Fr guiding catheters via radial or femoral arterial approaches. Patients were anticoagulated with a heparin bolus (70–100 U/kg) administered either intravenously, or directly into the coronary artery via the guiding catheter. The route of heparin administration was up to the discretion of the operator and was recorded prospectively. Two interventional cardiologists blinded to treatment allocation and study data independently assessed angiographic variables such as the TIMI flow score at baseline and at the completion of the primary PCI procedure. The treating operator independently determined whether to pursue balloon pre-dilatation stenting (balloon pre-dilatation followed by stenting) or direct stenting (stenting without balloon pre-dilatation). In accordance with standard procedural practices, all patients in both groups underwent post-dilation following stent implantation. This routine step ensures optimal stent apposition and expansion, minimizing the risks associated with potential stent under-expansion or malapposition. Iopromide (Ultravist, Bayer HealthCare Pharmaceuticals, Leverkusen, Germany) was used as the contrast agent. The study team documented information such as stent diameter and length, maximum inflation pressure, and the amount of contrast agent. The decision to use an aspiration catheter (Thrombuster II manual thrombus aspiration catheter, Kaneka Inc., Osaka, Japan) during the primary PCI was determined by the treating physician and was prospectively documented. Per institutional guidelines, an intracoronary glycoprotein IIb/IIIa inhibitor was administered if a TIMI flow score of 3 was not achieved after epicardial revascularization of the culprit artery during the initial presentation with STEMI. Relative contraindications included age > 80 years, a low hemoglobin, history of hemorrhagic stroke or bleeding requiring blood transfusion; the decision to administer glycoprotein IIb/IIIa inhibitor was ultimately determined by the treating physician and prospectively documented.
Coronary physiology assessment
All coronary physiology measurements were performed 3 months after the STEMI by an experienced interventionalist who was blinded to the revascularization technique employed during primary PCI. CFR, fractional flow reserve (FFR), and IMR were assessed using the CoroFlow system (Coroventis Research AB, Uppsala, Sweden). After undergoing successful staged PCI, nitroglycerin was administered through the guiding catheter, and a coronary pressure/temperature sensor-tipped guidewire (Pressure Wire X; Abbott Vascular, Santa Clara, CA, United States) was equalized to the guide catheter pressure with the pressure sensor positioned at the tip of the catheter at the aortic sinus, then advanced to the distal two-thirds of the infarct related artery. Maximal hyperemia was induced by repeated intracoronary adenosine boluses. After achieving maximal hyperemia, three milliliters of normal saline were administered through the guiding catheter and IMR was calculated. If the measurements obtained from the first three administrations were inconsistent, the measurements were repeated to ensure accuracy CFR was determined as the difference between the baseline and hyperemic mean transit time (Tmn). IMR was computed by multiplying the distal coronary pressure during maximal hyperemia by the hyperemic Tmn. The ratio of mean distal (d) to mean proximal (p) coronary artery pressure (P) during maximal hyperemia was used to calculate FFR (FFR = Pd/Pa).
Data collection and echocardiographic imaging
Patient demographics, medical history, clinical course, laboratory values, angiographic characteristics, and follow-up data were collected prospectively. Left ventricular ejection fraction (LVEF) was assessed by acquiring 2-dimensional and 3-dimensional images using ultrasound (EPIQ 7, Phillips Ultrasound, Inc., Washington, USA) at 24 h and 1-year post-STEMI. These images were acquired by a trained cardiovascular imaging technician who was blinded to the study data and followed the guidelines established by the European Association of Cardiovascular Imaging (EACVI)14.
Study endpoints
The primary endpoint was the presence of CMD three months after STEMI. The secondary endpoint was the rate of major adverse cardiovascular events (MACE) within 12 months of follow-up.
Definitions
STEMI was defined according to the fourth universal definition of myocardial infarction15. Door-to-wire time was defined as the time (in minutes) from the first medical contact at the facility to the time of advancement of the PCI wire. Dyslipidemia was defined as a fasting total cholesterol level > 70 mg/dl (1.8 mmol/l) or the use of lipid-lowering medications16. Hypertension was defined as a blood pressure ≥ 140/90 mmHg or the use of blood pressure-lowering medication17. Diabetes mellitus was defined as a fasting plasma glucose level ≥ 7.0 mmol/l, or the use of blood glucose-lowering medication18. MACE was defined as the composite endpoint of cardiovascular death, non-fatal myocardial infarction, target vessel revascularization, recurrent hospitalization due to decompensated heart failure, and stroke (ischemic or hemorrhagic). Renal function was assessed by calculating the glomerular filtration rate using the Cockcroft-Gault equation.
Successful PCI was defined as the implantation of a second-generation drug-eluting stent to the target lesions, resulting in visual reduction of the lesion to less than 20% stenosis, and restoration of coronary blood flow equivalent to both TIMI 2 and TIMI 3 Flow levels. Normal values for FFR, CFR, and IMR were defined as > 0.80, ≥ 2.0, and < 25 U, respectively19,20. Microcirculatory dysfunction was defined as IMR ≥ 25 or a CFR < 2.07,19–21. While an IMR > 40 immediately after PCI in STEMI patients has been shown to predict MACE, coronary physiology measurements in this study were performed 3 months after PCI, hence this threshold was not applicable22. Nevertheless, a sensitivity analysis examining the incidence of MACE at 12 month follow-up in patients with an IMR < 25, 25 ≤ IMR ≤ 40, and IMR > 40 was performed (Supplemental Fig. 1).
Statistical analysis
Continuous variables were determined to be skewed and therefore were presented as median values with quartile ranges. Categorical variables were presented as frequency and percentage. Wilcoxon Rank Sum, Chi-Square, or Fisher's Exact tests were used to assess baseline differences and outcomes between the study groups, as appropriate. Stepwise selection was used to create multivariable logistic regression models to investigate procedural factors associated with CMD. Kaplan–Meier analysis was used to assess MACE-free survival rates, and differences were evaluated using the log-rank test. A probability level of p < α (where α is the significance level set at 0.05) was assumed to determine statistical significance. Data processing was performed using IBM SPSS Statistics 27.
Ethics approval and consent to participate
We conducted this study in compliance with the ethical standards of the Regional Bioethics Committee of Kaunas, Lithuania (the permission number is BE-2-5) and the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects. Clinical Trials registration number: NCT05406297, concurrently registered. All subjects gave their informed consent to participate, and an information letter was given to them.
Results
Study population characteristics
This study enrolled 210 patients, of whom 98 patients (46.7%) underwent direct stenting and 112 (53.2%) underwent balloon pre-dilatation before stenting. The median age of the patients was 65 years, falling within an interquartile range of 58 to 76 years. Of the total, 125 patients, accounting for 59.5%, were male. This gender distribution was similar among those who underwent direct stenting and those who received balloon pre-dilatation prior to stenting. Both groups had similar body mass indices, body surface areas, and culprit vessels. Fifty-one (24.3%) patients had diabetes, and 109 (51.9%) patients were current or former smokers, with similar distributions between the two groups. Other risk factors, including arterial hypertension, dyslipidemia, Killip classification, CHADS2-VASc score, and history of heavy alcohol use, stroke, or coronary artery disease, were also similar between the two groups (Table 1).
Table 1.
Characteristics of ST-elevation myocardial infarction patients classified by percutaneous coronary intervention technique.
| Characteristic | Overall (n = 210) | Direct stenting (n = 98) | Balloon pre-dilatation stenting (n = 112) | P-value |
|---|---|---|---|---|
| Sex (Female) | 85 (40.48%) | 35 (35.71%) | 50 (44.64%) | 0.240 |
| Age (years) | 65.0 [58, 76] | 67.0 [58.25, 76.0] | 63.50 [56.0, 75.0] | 0.477 |
| Body mass index (kg/m2) | 27.39 [24.56, 30.69] | 27.71 [25.32, 30.60] | 26.38 [24.29, 31.10] | 0.443 |
| Body surface area (m2) | 1.93 [1.81, 2.10] | 1.94 [1.81, 2.10] | 1.92 [1.83, 2.12] | 0.768 |
| Primary diagnosis | ||||
| Anterior STEMI | 116 (55.24%) | 53 (54.08%) | 63 (56.25%) | 0.860 |
| Inferior STEMI | 94 (44.76%) | 45 (45.92%) | 49 (43.75%) | |
| Arterial hypertension | 123 (58.57%) | 58 (59.18%) | 65 (58.04%) | 0.978 |
| History of coronary artery disease | 59 (28.10%) | 27 (27.55%) | 32 (28.57%) | 0.992 |
| History of PCI | 26 (12.38%) | 11 (11.22%) | 15 (13.39%) | 0.790 |
| History of stroke | 27 (12.86%) | 11 (11.22%) | 15 (13.39%) | 0.649 |
| History of diabetes mellitus | 51 (24.29%) | 21 (21.43%) | 30 (26.79%) | 0.458 |
| History of dyslipidemia | 119 (56.67%) | 60 (61.22%) | 59 (52.68%) | 0.268 |
| Smoker (former/current) | 109 (51.90%) | 51 (52.04%) | 58 (51.79%) | 1 |
| History of alcohol abuse | 20 (9.52%) | 8 (8.16%) | 12 (10.71%) | 0.695 |
| Baseline CHADS2-VASc score | 3 [2, 4] | 3 [2, 4] | 3 [2, 4] | 0.851 |
| KILLIP class | ||||
| I | 62 (29.52%) | 27 (27.55%) | 35 (31.25%) | 0.519 |
| II | 111 (52.86%) | 53 (54.08%) | 58 (51.79%) | |
| III | 27 (12.86%) | 15 (15.31%) | 12 (10.71%) | |
| IV | 10 (4.76%) | 3 (3.06%) | 7 (6.25%) | |
STEMI ST elevation myocardial infarction, PCI percutaneous coronary intervention.
Laboratory and echocardiographic findings
Complete blood counts, creatinine clearance, initial troponin and peak troponin levels were similar between the two groups. The direct stenting group had a slightly higher total cholesterol (5.1 mmol/l vs 4.4 mmol/l; p = 0.035) and low-density lipoprotein cholesterol (3.5 mmol/l vs 3.2 mmol/l; p = 0.047). The creatinine clearance rate (40.5 ml/min vs 38.2 ml/min; p = 0.121) and initial troponin levels were similar between the two study groups. There was no difference in LVEF after primary PCI between the two groups (42.0% vs. 40%; p = 0.914), while the LVEF of the direct stenting group was higher at 12 months (48% vs. 45%; p = 0.003) (Table 2).
Table 2.
Laboratory and echocardiographic parameters of patients with ST-elevation myocardial infarction, categorized by percutaneous coronary intervention technique.
| Parameters | Overall (n = 210) | Direct stenting (n = 98) | Balloon pre-dilatation stenting (n = 112) | P-value |
|---|---|---|---|---|
| Laboratory test | ||||
| Hemoglobin (g/l) | 136.0 [119.0, 148.0] | 137.0 [121.0, 146.8] | 135.0 [118.0, 148.3] | 0.631 |
| White blood cell count (109/l) | 9.86 [8.22, 12.09] | 9.78 [8.09, 12.07] | 10.05 [8.38, 12.10] | 0.742 |
| Platelets (× 109/l) | 240.5 [204.0, 273.0] | 242.0 [212.5, 273.8] | 240.0 [200.0, 271.5] | 0.635 |
| Total cholesterol (mmol/l) | 4.64 [3.75, 5.79] | 5.06 [4.07, 6.01] | 4.43 [3.60, 5.57] | 0.035 |
| Low-density lipoprotein (mmol/l) | 3.26 [2.37, 4.31] | 3.51 [2.66, 4.46] | 3.17 [2.23, 4.08] | 0.047 |
| High-density lipoprotein (mmol/l) | 1.12 [0.92, 1.35] | 1.11 [0.94, 1.36] | 1.13 [0.91, 1.32] | 0.568 |
| Triglycerides (mmol/l) | 1.16 [0.82, 1.65] | 1.11 [0.82, 1.72] | 1.17 [0.85, 1.57] | 0.896 |
| Creatinine clearance (mL/min) | 39.5 [34.95, 47.5] | 40.45 [35.28, 48.68] | 38.20 [34.80, 47.10] | 0.121 |
| Basal troponin I (µg/l) | 2.19 [0.81, 3.71] | 2.22 [0.96, 3.89] | 2.18 [0.76, 3.29] | 0.427 |
| Peak troponin I (µg/l) | 45.0 [27.0, 64.0] | 42.0 [26.25, 67.75] | 46.0 [28.0, 62.0] | 0.830 |
| High-sensitivity C-reactive protein (mg/l) | 3.80 [1.85, 10.52] | 4.22 [1.85, 10.06] | 3.66 [1.94, 10.77] | 0.872 |
| Echocardiographic parameters | ||||
| Post-PCI left ventricular ejection fraction (%) | 40.0 [36.25, 45.75] | 42.0 [38.5, 45.0] | 40.0 [35.75, 46.25] | 0.913 |
| 12-month left ventricular ejection fraction (%) | 45.0 [40.0, 50.0] | 48.0 [40.0, 55.0] | 45.0 [35.0, 50.0] | 0.003 |
CMD coronary microvascular dysfunction, PCI percutaneous coronary intervention.
Procedural characteristics and coronary physiology findings
Pain-to-door time (278.5 min vs. 348 min; p = 0.166) and door-to-balloon time (39.0 min vs. 41.5 min; p = 0.318) TIMI flow before and after PCI were similar between the two groups. The left anterior descending artery was the culprit artery in 118 (56.2%) patients at a similar rate in both groups (53 (54.1%) vs 65 (58.0%); p = 0.771). Intracoronary heparin was utilized in 104 (49.5%) patients, intracoronary glycoprotein IIb/IIIa inhibitor was utilized in 46 (21.9%) patients, and aspiration thrombectomy was performed on 52 (24.8%) patients. The rates of these interventions were similar between the groups undergoing direct stenting and balloon pre-dilatation before stenting: 57.1% vs. 42.9%; p = 0.054, 21.4% vs. 22.3%; p = 1, and 25.5% vs. 24.1%; p = 0.940, respectively. Furthermore, there were no significant differences in the study groups regarding contrast dose, stent diameter and length, or maximal inflation pressure (Table 3).
Table 3.
Coronary angiography and physiology parameters of ST-elevation myocardial infarction patients, categorized by percutaneous coronary intervention technique.
| Parameters | Overall (n = 210) | Direct stenting (n = 98) | Balloon pre-dilatation stenting (n = 112) | P-value |
|---|---|---|---|---|
| Angiographic | ||||
| Pain-to-door time (minutes) | 314 [107.75, 592.25] | 348.0 [112.0, 677.75] | 278.5 [108.75, 460.25] | 0.166 |
| Door‐to‐balloon (minutes) | 40 [29.25, 52.0] | 41.5 [31.0, 51.75] | 39.0 [29.0, 52.25] | 0.318 |
| Pre-PCI TIMI flow | ||||
| 0 | 130 (61.90%) | 55 (56.12%) | 75 (66.96%) | 0.305 |
| 1 | 8 (3.81%) | 3 (3.06%) | 5 (4.46%) | |
| 2 | 44 (20.95%) | 25 (25.51%) | 19 (16.96%) | |
| 3 | 28 (13.33%) | 15 (15.31%) | 13 (11.61%) | |
| Post-PCI TIMI flow | ||||
| 0 | 2 (0.95%) | 1 (1.02%) | 1 (0.89%) | 0.824 |
| 1 | 1 (0.48%) | 0 (0.0%) | 1 (0.89%) | |
| 2 | 22 (10.48%) | 10 (10.20%) | 12 (10.71%) | |
| 3 | 185 (88.1%) | 87 (88.78%) | 98 (87.50%) | |
| Culprit vessel | ||||
| Left anterior descending artery | 118 (56.19%) | 53 (54.08%) | 65 (58.04%) | 0.771 |
| Circumflex artery | 49 (23.33%) | 25 (25.51%) | 24 (21.43%) | |
| Right coronary artery | 43 (20.48%) | 20 (20.41%) | 23 (20.54%) | |
| Number of diseased vessels | ||||
| 2-Vessel disease | 123 (58.57%) | 56 (57.14%) | 67 (59.82%) | 0.801 |
| 3-Vessel disease | 87 (41.43%) | 42 (42.86%) | 45 (40.18%) | |
| Intracoronary interventions | ||||
| Intracoronary heparin infusion | 104 (49.52%) | 56.0 (57.14%) | 48.0 (42.86%) | 0.054 |
| Intracoronary glycoprotein IIb/IIIa inhibitor | 46 (21.9%) | 21.0 (21.43%) | 25.0 (22.32%) | 1 |
| Aspiration thrombectomy | 52 (24.76%) | 25.0 (25.51%) | 27.0 (24.11%) | 0.940 |
| Stent diameter (millimeters) | 3.0 [3.0, 3.5] | 3.0 [3.0, 3.5] | 3.0 [3.0, 3.5] | 0.699 |
| Stent length (millimeters) | 24.0 [19.0, 26.0] | 24.0 [19.0, 26.0] | 24.0 [19.0, 26.0] | 0.293 |
| Maximal stent pressure (atm) | 14.0 [14.0, 16.0] | 15.0 [14.0, 17.0] | 14.0 [13.0, 16.0] | 0.160 |
| Contrast dose (milliliters) | 100.0 [90.0, 110.0] | 100.0 [90.0, 110.0] | 100.0 [90.0, 111.25] | 0.262 |
| Coronary physiology at 3-month follow-up | ||||
| Coronary flow reserve | 2.81 [2.54, 2.98] | 2.87 [2.65, 3.14] | 2.70 [2.16, 2.95] | 0.003 |
| Fractional flow reserve | 0.92 [0.87, 0.97] | 0.92 [0.87, 0.97] | 0.92 [0.86, 0.97] | 0.452 |
| Index of microvascular resistance | 20 [15.0, 29.0] | 19.5 [14.0, 22.0] | 22.0 [15.0, 42.0] | 0.001 |
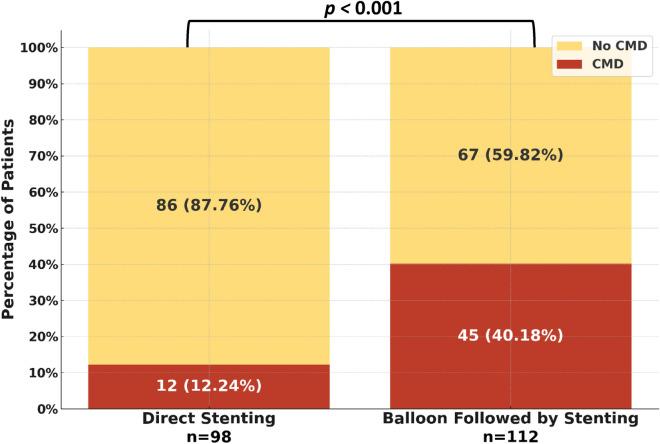
| Coronary microvascular dysfunction | 57 (27.14%) | 12.0 (12.24%) | 45.0 (40.18%) | < .001 |
PCI percutaneous coronary intervention.
Three months post-primary PCI, the FFR values exhibited no significant difference between patients who underwent direct stenting and those who were subject to balloon pre-dilatation before stenting (0.92 vs 0.92; p = 0.452). Conversely, CFR values demonstrated a significant increase (2.87 vs 2.70; p < 0.001), and the IMR values displayed a notable decrease (19.5 vs 22.0; p = 0.001) in the patients who underwent direct stenting compared to those who underwent balloon pre-dilatation. Furthermore, there was a lower prevalence of CMD in the direct stenting group (12.2% vs 40.2%; p < 0.001) (Table 3, Fig. 1).
Figure 1.
Prevalence of coronary microvascular dysfunction displayed by percutaneous coronary intervention technique.
Major adverse cardiovascular events at 12 months
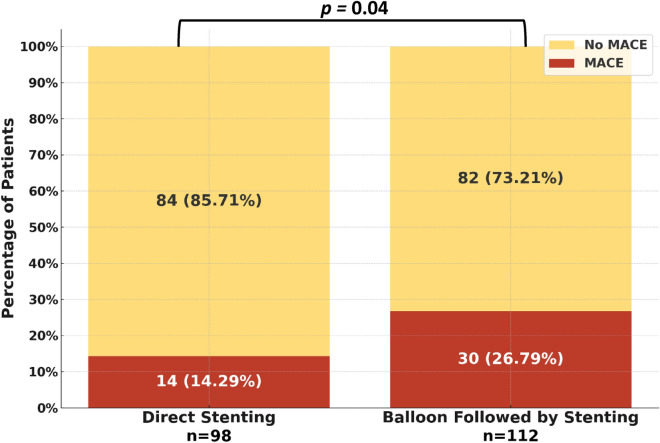
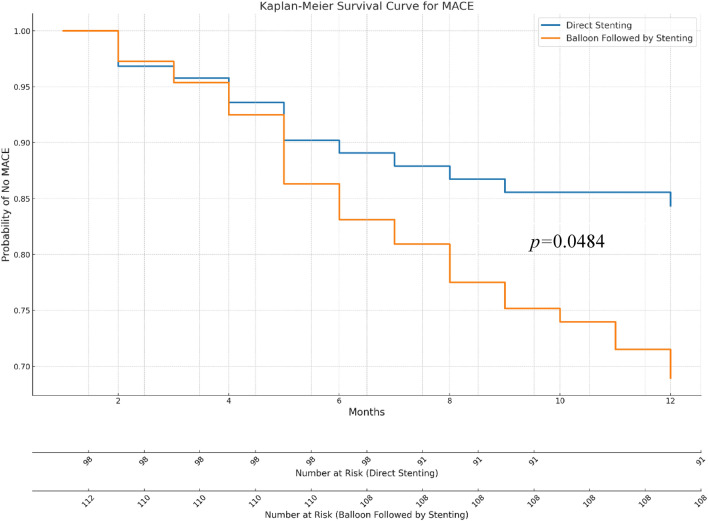
At 12 month follow-up, the incidence of MACE in patients who underwent direct stenting was lower than in those who underwent balloon pre-dilatation before stenting (14.3% vs. 26.8%; p = 0.040) (Table 4, Fig. 2). This difference was driven largely by a decreased incidence of stroke in patients who underwent direct stenting (0% vs 6.3%; p = 0.033). The Kaplan–Meier curve revealed an increased incidence of MACE in patients who underwent balloon pre-dilatation stenting (log-rank p = 0.048), most noticeably starting at 6 months post-PCI (Fig. 3).
Table 4.
Twelve-month clinical outcomes of patients presenting with ST-elevation myocardial infarction, categorized by percutaneous coronary intervention technique.
| Overall (n = 210) | Direct stenting (n = 98) | Balloon pre-dilatation stenting (n = 112) | P-value | |
|---|---|---|---|---|
| Ischemic or hemorrhagic stroke | 7 (3.33%) | 0 (0%) | 7 (6.25%) | 0.033 |
| Nonfatal MI | 10 (4.76%) | 3 (3.06%) | 7 (6.25%) | 0.448 |
| Cardiovascular death | 6 (2.86%) | 1 (1.02%) | 5 (4.46%) | 0.280 |
| Target vessel revascularization | 10 (4.76%) | 6 (6.12%) | 4 (3.57%) | 0.588 |
| Decompensated HF requiring hospitalization | 16 (7.62%) | 5 (5.10%) | 11 (9.82%) | 0.305 |
| MACE | 44 (20.95%) | 14 (14.29%) | 30 (26.79%) | 0.040 |
MI myocardial infarction, HF heart failure, MACE the composite of stroke, nonfatal myocardial infarction, revascularization, heart failure hospitalization, and cardiovascular death.
Figure 2.
Rates of major adverse cardiac events displayed by percutaneous coronary intervention technique.
Figure 3.
Kaplan–Meier event-free survival curve for occurrence of major adverse cardiac events grouped by percutaneous coronary intervention technique.
A sensitivity analysis examining the incidence of MACE at 12 months according to IMR category revealed a lower incidence of MACE in patients with an IMR < 25, when compared to those with an 25 ≤ IMR ≤ 40 (p < 0.001) and those with an IMR > 40 (p < 0.001). These was no difference in the incidence of MACE at 12 months in patients with an 25 ≤ IMR ≤ 40 and those with an IMR > 40 (p = 0.352) (Supplemental Fig. 1).
Multivariable logistic analysis
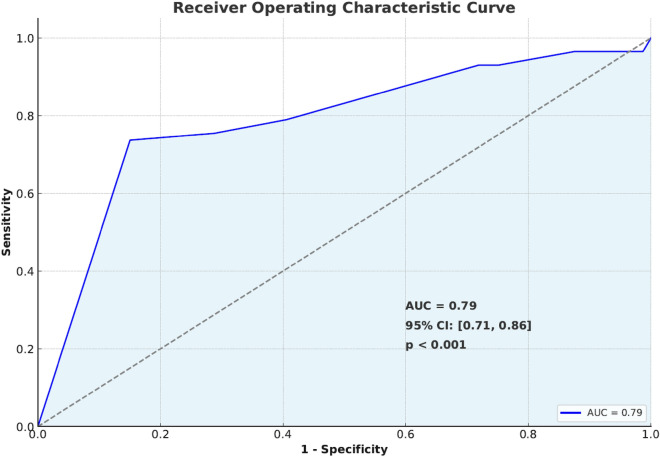
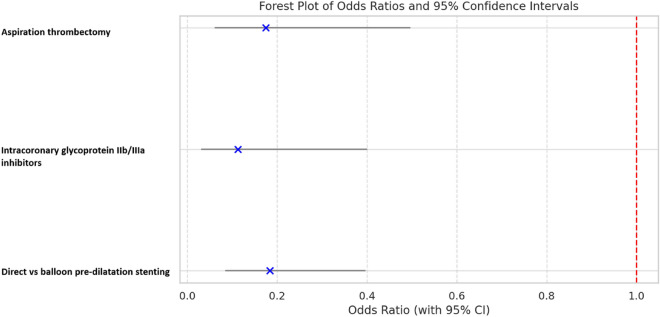
The multivariable logistic regression analysis revealed that direct stenting, aspiration thrombectomy, and utilization of glycoprotein IIb/IIIa jointly yielded a receiver operator characteristic area under the curve of 0.79 (0.71–0.86), indicating a good predictive ability in the binary logistic multivariable analysis (Fig. 4). In this particular model, we were able to determine that the use of direct stenting as opposed to balloon pre-dilatation before stenting, the utilization of aspiration thrombectomy, and the administration of intracoronary glycoprotein IIb/IIIa were associated with decreased odds of CMD (odds ratio (OR): 0.184, 95% confidence interval (CI): 0.085–0.396, p < 0.001), (OR: 0.175, 95% CI: 0.062–0.495, p = 0.001), and (OR: 0.113, 95% CI: 0.032–0.399, p = 0.001) (respectively (Table 5, Fig. 5).
Figure 4.
Receiver Operating Characteristic curve for the model of coronary microvascular dysfunction in ST-elevation myocardial infarction patients. AUC area under the receiver operating characteristic curve.
Table 5.
Multivariable binary logistic analysis for prediction of coronary microvascular dysfunction after ST-segment elevation myocardial infarction event.
| Effect | Odds ratio | 95% Confidence limits | P-value | |
|---|---|---|---|---|
| Direct stenting vs balloon pre-dilatation stenting | 0.184 | 0.085 | 0.396 | < .001 |
| Aspiration thrombectomy | 0.175 | 0.062 | 0.495 | 0.001 |
| Glycoprotein IIb/IIIa | 0.113 | 0.032 | 0.399 | 0.001 |
Figure 5.
Forest plot for the outcome of coronary microvascular dysfunction.
Discussion
This prospective, single-blinded, cohort study is one of the largest studies examining the relationship between PCI technique and CMD in STEMI patients. Direct stenting was associated with a lower prevalence of CMD and adverse cardiac events when compared to balloon pre-dilatation before stenting. Both aspiration thrombectomy and administration of glycoprotein IIb/IIIa inhibitors were associated with a lower prevalence of CMD. Unlike prior studies that assessed CMD during the acute phase of STEMI, we performed coronary physiology testing three months after STEMI23,24 because of altered coronary physiology during the acute phase of STEMI25–27. Ríos-Navarro et al., in their experimental study, demonstrated a near-complete resolution of CMD 30 days post-reperfusion26. This hypothesis found further empirical validation in the research conducted by Demirkiran et al., wherein a marked optimization of CMD indices was observed within the 30-day post-STEMI period. Specifically, data demonstrated a decrement in IMR metrics from a pre-established 38.8 to a subsequent 25.6, concomitant with an augmentation in CFR from an initial measurement of 2.16 to a later 3.7727. Current guidelines establish primary PCI as the gold standard for treating STEMI patients, but do not specify whether to pursue direct stenting or balloon predilatation1.
In this study, multivariable logistic analysis showed that direct stenting was associated with approximately fivefold lower odds of CMD compared with balloon pre-dilatation stenting (Table 5, Fig. 5). There are several potential explanations for this observation. First, balloon manipulation within the culprit lesion may cause distal embolization of fragmented thrombus or atheromatous debris, aggravating microvascular occlusion and leading to prolonged myocardial ischemia28. Webb and colleagues observed that in saphenous venous graft lesions, direct stenting resulted in less distal embolization than predilatation followed by stenting, likely because thrombus and friable material were entrapped behind the stent struts29. Kalayci and colleagues similarly found that STEMI patients treated with direct stenting were less likely to exhibit visible distal embolization (4.4% vs 7.4%; p = 0.014) and were more likely to have complete resolution of ST segment elevation (68.9% vs 59.6%; p < 0.001) than those treated with balloon pre-dilatation stenting30. Second, inflation of the balloon within the culprit lesion may release atherogenic plaque components which further activate the coagulation cascade31. Third, predilatation may result in arterial wall endothelial dissection and subsequent rapid thrombosis. Fourth, pre-dilatation may propagate endothelial damage, triggering an inflammatory response and limiting the appropriate endothelialization of the stented vessel, thereby increasing the risk of stent thrombosis or neointimal hyperplasia31. Direct stenting also has the potential to reduce radiation exposure and healthcare expenditures by reducing procedure time32.
However, direct stenting also has limitations, including difficulty estimating the caliber of the coronary artery, which may result in inadequate stent expansion, difficulty or failure to deliver or optimally position the stent due to inadequate visualization of the lesion margins33.
Previous studies investigating the relationship between PCI techniques and CMD have shown contradictory results. He and colleagues investigated the impact of stenting technique on CMD and did not find that direct stenting had any advantages over balloon predilatation. However, the authors utilized cardiac magnetic resonance (CMR) rather than invasive coronary physiology to assess CMD. Further, they performed CMR 1 week after STEMI, which may be too early to obtain a reliable assessment of coronary microcirculation26,34. Kim and colleagues failed to demonstrate any impact of PCI technique on microcirculation. While this study was randomized, it only included 38 patients in each arm, coronary physiology was ascertained immediately after primary PCI, and only IMR was used to assess CMD, rather than both IMR and CFR23. Several other randomized controlled trials investigating direct stenting and its effect on myocardial perfusion have been conducted, but are outdated (conducted between the late 1990s and early 2000s) and do not reflect current clinical practice or modern techniques to assess CMD35–39.
A study by Scarparo and colleagues found that among STEMI patients who had a higher thrombus burden (TIMI grade flow 0–1), those who were treated with direct stenting had a lower incidence of all-cause mortality at 15 years (hazard ratio (HR) 0.65, 95% CI 0.50–0.84, p = 0.001) and MACE at 10 years (HR 0.71, 95% CI 0.55–0.92, p = 0.010), when compared with those who were treated with balloon pre-dilatation before stenting40. McCormick and colleagues found that balloon pre-dilatation stenting was independently associated with one year mortality OR 2.42, 95 CI 1.08–5.45, p = 0.032) compared with direct stenting. Neither study was randomized41. A randomized study by Cuisset and colleagues found a lower IMR with direct stenting compared with balloon pre-dilatation before stenting (13 ± 3 vs. 24 ± 14; p < 0.01); however, this study was small (50 patients) and only included patients with stable angina42.
The present study also revealed that aspiration thrombectomy during primary PCI was associated with an approximately fivefold decrease in the prevalence of CMD (Table 5, Fig. 5). The use of an aspiration thrombectomy catheter during primary PCI is still being debated in the medical community, with investigations yielding contradictory data24,43–45. According to the Thrombectomy Trialists Collaboration study, direct stenting with aspiration thrombectomy during primary PCI did not enhance clinical outcomes or myocardial reperfusion parameters43. This is appropriately reflected in the current guidelines, which state that routine use of aspiration thrombectomy is not encouraged1. However, the Thrombectomy Trialists Collaboration study used myocardial blush to assess for CMD, rather than invasive thermodilution, CMR, or another quantifiable physiologic index. Hoole and colleagues conducted a randomized clinical pilot trial in which they performed a series of IMR measurements during different stages of primary PCI, followed by CMR analysis at 24 h and three-month follow-up. They found a trend toward less microcirculatory damage in patients who underwent aspiration thrombectomy; however, this did not reach statistical significance. The authors did acknowledge that the results should be interpreted with caution as only 26 patients were included in the CMR analysis, resulting in an underpowered study. Furthermore, IMR was only obtained during primary PCI, which may not be reliable because of altered coronary physiology during STEMI24.
The MUltidevice Thrombectomy in Acute ST-Segment Elevation Acute Myocardial Infarction trial, which was the largest randomized trial to evaluate the impact of aspiration thrombectomy on CMD, included 208 STEMI patients and assessed CMD via CMR at 3 months. The aspiration thrombectomy group had a lower prevalence of CMD (11.4% vs. 26.7%, p = 0.02)46. Similarly, the Thrombectomy With Export Catheter in Infarct-Related Artery During Primary Percutaneous Coronary Intervention—EXPIRA trial found that aspiration thrombectomy led to a smaller percentage of left-ventricular myocardium with microvascular obstruction (31.5% vs. 72.9%, p = 0.0005), when assessed by CMR during the acute phase of STEMI47. Another randomized trial conducted by Zajdel and colleagues found that aspiration thrombectomy was associated with less infarcted myocardium with microvascular obstruction on CMR at 6 months (9.0% vs. 26.9%, p = 0.009), although this analysis only included 45 patients45.
Finally, our study revealed that administration of an intracoronary glycoprotein IIb/IIIa inhibitor (eptifibatide) during primary PCI was associated with an approximately fivefold decreased risk of CMD (Table 5, Fig. 5). Intracoronary administration of glycoprotein IIb/IIIa inhibitors allows for higher drug concentrations, and therefore, increased drug activity in the target vessel48. Platelet aggregation, which plays a role in microvascular obstruction, is attenuated by glycoprotein IIb/IIIa inhibitors49. In a rodent model, administration of a glycoprotein IIb/IIIa inhibitor during STEMI preserved the structural and functional integrity of the microvascular endothelium via a process involving nitric oxide50. Akpek and colleagues conducted a randomized controlled trial and found that patients who received an intracoronary glycoprotein IIb/IIIa inhibitor had better TIMI flow after primary PCI compared with those who received placebo51.
Limitations
The main limitation of the study was that it was an observational, not randomized, and single center study. However, prior randomized studies examining this topic have suffered from high rates of patient cross-over, limiting the interpretability of their results23,52,37. Despite the study's relatively small sample size (2010 patients), it is one of the largest prospective studies evaluating invasive CMD testing in patients presenting with STEMI. While the door-to-balloon times in the current study adhered to the ESC guidelines, we observed extended pain-to-door times12. This is often attributed to patient factors and a matter of public health awareness. However, in a sub-analysis, we demonstrated that the prevalence of CMD was lower in patients receiving direct stenting irrespective of their pain-to-door time (Supplemental Table 1). We were unable to assess the presence of CMD prior to STEMI and could only ascertain the prevalence of CMD at 3 months. Intracoronary glycoprotein IIb/IIIa inhibitors were administered if a TIMI flow score of 3 was not achieved after epicardial revascularization, which is a potential confounder in our analysis. Due to ethical concerns, our study only included patients with multivessel coronary artery disease who required staged PCI at 3-month follow-up, thus limiting the generalizability of our findings. While the study found that direct stenting was associated with a decreased incidence of MACE, this was driven by a reduction in the incidence of stroke. Intriguingly, prior studies have shown that patients with CMD have a higher prevalence of atrial fibrillation8. We hypothesized that these strokes might be cardioembolic in nature, given the increased rates of new-onset atrial fibrillation in patients who underwent balloon pre-dilatation. However, this trend was not statistically significant (15.18% vs 6.12%; p = 0.061), and larger studies will be needed to confirm our findings.
Conclusions
In conclusion, our study suggests that direct stenting, aspiration thrombectomy, and intracoronary injection of glycoprotein IIb/IIIa inhibitors each is associated with a lower prevalence of CMD at 3 months and lower incidence of MACE at 12 months when compared with balloon pre-dilatation before stenting. Further research is needed to confirm our results in larger, randomized trials conducted across multiple institutions.
Supplementary Information
Author contributions
A.A.: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing. A.Haq.: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing—original draft, Writing—review & editing. R.U.: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing—review & editing. A.Ham.: Conceptualization, Methodology, Resources, Supervision, Validation, Writing—review & editing. E.S.B.: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing—review & editing. I.G.: Data curation, Formal analysis, Validation, Visualization, Writing—review & editing. T.-Y.T.: Data curation, Formal analysis, Investigation, Visualization, Writing—original draft, Writing—review & editing. V.T.: Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Writing—review & editing. K.B.: Investigation, Resources, Writing—review & editing. S.R.: Investigation, Supervision, Writing—review & editing. P.W.S.: Project administration, Validation, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
The study was sponsored by the Lithuanian University of Health Sciences.
Data availability
The datasets used in this study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-47343-x.
References
- 1.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P, Collet J-P, Kristensen SD, Aboyans V, Baumbach A, Bugiardini R, Coman IM, Delgado V, Fitzsimons D, Gaemperli O, Gershlick AH, Gielen S, Harjola V-P, Katus HA, Knuuti J, Kolh P, Leclercq C, Lip GYH, Morais J, Neskovic AN, Neumann F-J, Niessner A, Piepoli MF, Richter DJ, Shlyakhto E, Simpson IA, Steg PG, Terkelsen CJ, Thygesen K, Windecker S, Zamorano JL, Zeymer U, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh T, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Zamorano JL, Chettibi M, Hayrapetyan HG, Metzler B, Ibrahimov F, Sujayeva V, Beauloye C, Dizdarevic-Hudic L, Karamfiloff K, Skoric B, Antoniades L, Tousek P, Terkelsen PJ, Shaheen SM, Marandi T, Niemelä M, Kedev S, Gilard M, Aladashvili A, Elsaesser A, Kanakakis IG, Merkely B, Gudnason T, Iakobishvili Z, Bolognese L, Berkinbayev S, Bajraktari G, Beishenkulov M, Zake I, Lamin HB, Gustiene O, Pereira B, Xuereb RG, Ztot S, Juliebø V, Legutko J, Timóteo AT, Tatu-Chiţoiu G, Yakovlev A, Bertelli L, Nedeljkovic M, Studenčan M, Bunc M, García de Castro AM, Petursson P, Jeger R, Mourali MS, Yildirir A, Parkhomenko A, Gale CP. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2017;2017(39):119–177. doi: 10.1016/j.rec.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Bulluck H, Foin N, Tan JW, Low AF, Sezer M, Hausenloy DJ. Invasive assessment of the coronary microcirculation in reperfused ST-segment–elevation myocardial infarction patients. Circul. Cardiovasc. Interv. 2017 doi: 10.1161/circinterventions.116.004373. [DOI] [PubMed] [Google Scholar]
- 3.Montone RA, Vetrugno V, Santacroce G, Del Buono MG, Meucci MC, Camilli M, Galli M, Leone AM, D’Amario D, Buffon A, Aurigemma C, Burzotta F, Trani C, Niccoli G, Crea F. Recurrence of angina after ST-segment elevation myocardial infarction: The role of coronary microvascular obstruction. Eur. Heart J. Acute Cardiovasc. Care. 2019;10:624–632. doi: 10.1177/2048872619880661. [DOI] [PubMed] [Google Scholar]
- 4.Kaski J-C, Crea F, Gersh BJ, Camici PG. Reappraisal of ischemic heart disease. Circulation. 2018;138:1463–1480. doi: 10.1161/CIRCULATIONAHA.118.031373. [DOI] [PubMed] [Google Scholar]
- 5.Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options. J. Am. Coll. Cardiol. 2018;72:2625–2641. doi: 10.1016/j.jacc.2018.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, Neumann F-J, Sechtem U, Banning AP, Bonaros N, Bueno H, Bugiardini R, Chieffo A, Crea F, Czerny M, Delgado V, Dendale P, Flachskampf FA, Gohlke H, Grove EL, James S, Katritsis D, Landmesser U, Lettino M, Matter CM, Nathoe H, Niessner A, Patrono C, Petronio AS, Pettersen SE, Piccolo R, Piepoli MF, Popescu BA, Räber L, Richter DJ, Roffi M, Roithinger FX, Shlyakhto E, Sibbing D, Silber S, Simpson IA, Sousa-Uva M, Vardas P, Witkowski A, Zamorano JL, Achenbach S, Agewall S, Barbato E, Bax JJ, Capodanno D, Cuisset T, Deaton C, Dickstein K, Edvardsen T, Escaned J, Funck-Brentano C, Gersh BJ, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Prescott E, Saraste A, Storey RF, Svitil P, Valgimigli M, Windecker S, Aboyans V, Baigent C, Collet J-P, Dean V, Delgado V, Fitzsimons D, Gale CP, Grobbee D, Halvorsen S, Hindricks G, Iung B, Jüni P, Katus HA, Landmesser U, Leclercq C, Lettino M, Lewis BS, Merkely B, Mueller C, Petersen S, Petronio AS, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa-Uva M, Touyz RM, Benkhedda S, Metzler B, Sujayeva V, Cosyns B, Kusljugic Z, Velchev V, Panayi G, Kala P, Haahr-Pedersen SA, Kabil H, Ainla T, Kaukonen T, Cayla G, Pagava Z, Woehrle J, Kanakakis J, Tóth K, Gudnason T, Peace A, Aronson D, Riccio C, Elezi S, Mirrakhimov E, Hansone S, Sarkis A, Babarskiene R, Beissel J, Maempel AJC, Revenco V, de Grooth GJ, Pejkov H, Juliebø V, Lipiec P, Santos J, Chioncel O, Duplyakov D, Bertelli L, Dikic AD, Studenčan M, Bunc M, Alfonso F, Bäck M, Zellweger M, Addad F, Yildirir A, Sirenko Y, Clapp B. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2019;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 7.Ford TJ, Stanley B, Sidik N, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, McCartney P, Corcoran D, Collison D, Rush C, Sattar N, McConnachie A, Touyz RM, Oldroyd KG, Berry C. 1-year outcomes of angina management guided by invasive coronary function testing (CorMicA) JACC Cardiovasc. Interv. 2020;13:33–45. doi: 10.1016/j.jcin.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldujeli A, Patel R, Grabauskyte I, Hamadeh A, Lieponyte A, Tatarunas V, Khalifeh H, Briedis K, Skipskis V, Aldujeili M, Jarasuniene D, Rana S, Unikas R, Haq A. The impact of trimethylamine N-Oxide and coronary microcirculatory dysfunction on outcomes following ST-elevation myocardial infarction. J. Cardiovasc. Dev. Dis. 2023;10:197. doi: 10.3390/jcdd10050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niccoli G, Scalone G, Lerman A, Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur. Heart J. 2015;37:1024–1033. doi: 10.1093/eurheartj/ehv484. [DOI] [PubMed] [Google Scholar]
- 10.De Maria GL, Alkhalil M, Wolfrum M, Fahrni G, Borlotti A, Gaughran L, Dawkins S, Langrish JP, Lucking AJ, Choudhury RP, Porto I, Crea F, Dall’Armellina E, Channon KM, Kharbanda RK, Banning AP. Index of microcirculatory resistance as a tool to characterize microvascular obstruction and to predict infarct size regression in patients with STEMI undergoing primary PCI. JACC Cardiovasc. Imaging. 2019;12:837–848. doi: 10.1016/j.jcmg.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Saad M, Stiermaier T, Fuernau G, Pöss J, de Waha-Thiele S, Desch S, Thiele H, Eitel I. Impact of direct stenting on myocardial injury assessed by cardiac magnetic resonance imaging and prognosis in ST-elevation myocardial infarction. Int. J. Cardiol. 2019;283:88–92. doi: 10.1016/j.ijcard.2018.11.141. [DOI] [PubMed] [Google Scholar]
- 12.Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J-P, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, Wijns W, Glineur D, Aboyans V, Achenbach S, Agewall S, Andreotti F, Barbato E, Baumbach A, Brophy J, Bueno H, Calvert PA, Capodanno D, Davierwala PM, Delgado V, Dudek D, Freemantle N, Funck-Brentano C, Gaemperli O, Gielen S, Gilard M, Gorenek B, Haasenritter J, Haude M, Ibanez B, Iung B, Jeppsson A, Katritsis D, Knuuti J, Kolh P, Leite-Moreira A, Lund LH, Maisano F, Mehilli J, Metzler B, Montalescot G, Pagano D, Petronio AS, Piepoli MF, Popescu BA, Sádaba R, Shlyakhto E, Silber S, Simpson IA, Sparv D, Tavilla G, Thiele H, Tousek P, Van Belle E, Vranckx P, Witkowski A, Zamorano JL, Roffi M, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh TA, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Sousa-Uva M, Simpson IA, Zamorano JL, Pagano D, Freemantle N, Sousa-Uva M, Chettibi M, Sisakian H, Metzler B, İbrahimov F, Stelmashok VI, Postadzhiyan A, Skoric B, Eftychiou C, Kala P, Terkelsen CJ, Magdy A, Eha J, Niemelä M, Kedev S, Motreff P, Aladashvili A, Mehilli J, Kanakakis I-G, Becker D, Gudnason T, Peace A, Romeo F, Bajraktari G, Kerimkulova A, Rudzītis A, Ghazzal Z, Kibarskis A, Pereira B, Xuereb RG, Hofma SH, Steigen TK, Witkowski A, de Oliveira EI, Mot S, Duplyakov D, Zavatta M, Beleslin B, Kovar F, Bunc M, Ojeda S, Witt N, Jeger R, Addad F, Akdemir R, Parkhomenko A, Henderson R. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2018;40:87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 13.Guieu R, Deharo J-C, Maille B, Crotti L, Torresani E, Brignole M, Parati G. Adenosine and the cardiovascular system: The good and the bad. J. Clin. Med. 2020;9:1366. doi: 10.3390/jcm9051366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J-U. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2015;16:233–271. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth universal definition of myocardial infarction (2018) Circulation. 2018 doi: 10.1161/cir.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 16.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O, Mueller C, Drexel H, Aboyans V, Corsini A, Doehner W, Farnier M, Gigante B, Kayikcioglu M, Krstacic G, Lambrinou E, Lewis BS, Masip J, Moulin P, Petersen S, Petronio AS, Piepoli MF, Pintó X, Räber L, Ray KK, Reiner Ž, Riesen WF, Roffi M, Schmid J-P, Shlyakhto E, Simpson IA, Stroes E, Sudano I, Tselepis AD, Viigimaa M, Vindis C, Vonbank A, Vrablik M, Vrsalovic M, Zamorano JL, Collet J-P, Koskinas KC, Casula M, Badimon L, John Chapman M, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O, Windecker S, Aboyans V, Baigent C, Collet J-P, Dean V, Delgado V, Fitzsimons D, Gale CP, Grobbee D, Halvorsen S, Hindricks G, Iung B, Jüni P, Katus HA, Landmesser U, Leclercq C, Lettino M, Lewis BS, Merkely B, Mueller C, Petersen S, Petronio AS, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa-Uva M, Touyz RM, Nibouche D, Zelveian PH, Siostrzonek P, Najafov R, van de Borne P, Pojskic B, Postadzhiyan A, Kypris L, Špinar J, Larsen ML, Eldin HS, Viigimaa M, Strandberg TE, Ferrières J, Agladze R, Laufs U, Rallidis L, Bajnok L, Gudjónsson T, Maher V, Henkin Y, Gulizia MM, Mussagaliyeva A, Bajraktari G, Kerimkulova A, Latkovskis G, Hamoui O, Slapikas R, Visser L, Dingli P, Ivanov V, Boskovic A, Nazzi M, Visseren F, Mitevska I, Retterstøl K, Jankowski P, Fontes-Carvalho R, Gaita D, Ezhov M, Foscoli M, Giga V, Pella D, Fras Z, de Isla LP, Hagström E, Lehmann R, Abid L, Ozdogan O, Mitchenko O, Patel RS. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 2019;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 17.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, De Backer G, Heagerty AM, Agewall S, Bochud M, Borghi C, Boutouyrie P, Brguljan J, Bueno H, Caiani EG, Carlberg B, Chapman N, Cífková R, Cleland JGF, Collet J-P, Coman IM, de Leeuw PW, Delgado V, Dendale P, Diener H-C, Dorobantu M, Fagard R, Farsang C, Ferrini M, Graham IM, Grassi G, Haller H, Hobbs FDR, Jelakovic B, Jennings C, Katus HA, Kroon AA, Leclercq C, Lovic D, Lurbe E, Manolis AJ, McDonagh TA, Messerli F, Muiesan ML, Nixdorff U, Olsen MH, Parati G, Perk J, Piepoli MF, Polonia J, Ponikowski P, Richter DJ, Rimoldi SF, Roffi M, Sattar N, Seferovic PM, Simpson IA, Sousa-Uva M, Stanton AV, van de Borne P, Vardas P, Volpe M, Wassmann S, Windecker S, Zamorano JL, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh TA, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa-Uva M, Zamorano JL, Tsioufis C, Lurbe E, Kreutz R, Bochud M, Rosei EA, Jelakovic B, Azizi M, Januszewics A, Kahan T, Polonia J, van de Borne P, Williams B, Borghi C, Mancia G, Parati G, Clement DL, Coca A, Manolis A, Lovic D, Benkhedda S, Zelveian P, Siostrzonek P, Najafov R, Pavlova O, De Pauw M, Dizdarevic-Hudic L, Raev D, Karpettas N, Linhart A, Olsen MH, Shaker AF, Viigimaa M, Metsärinne K, Vavlukis M, Halimi J-M, Pagava Z, Schunkert H, Thomopoulos C, Páll D, Andersen K, Shechter M, Mercuro G, Bajraktari G, Romanova T, Trušinskis K, Saade GA, Sakalyte G, Noppe S, DeMarco DC, Caraus A, Wittekoek J, Aksnes TA, Jankowski P, Polonia J, Vinereanu D, Baranova EI, Foscoli M, Dikic AD, Filipova S, Fras Z, Bertomeu-Martínez V, Carlberg B, Burkard T, Sdiri W, Aydogdu S, Sirenko Y, Brady A, Weber T, Lazareva I, Backer TD, Sokolovic S, Jelakovic B, Widimsky J, Viigimaa M, Pörsti I, Denolle T, Krämer BK, Stergiou GS, Parati G, Trušinskis K, Miglinas M, Gerdts E, Tykarski A, de Carvalho RM, Dorobantu M, Chazova I, Lovic D, Filipova S, Brguljan J, Segura J, Gottsäter A, Pechère-Bertschi A, Erdine S, Sirenko Y, Brady A. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 18.Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2013;37:S81–S90. [DOI] [PubMed]
- 19.Demir OM, Boerhout CKM, de Waard GA, van de Hoef TP, Patel N, Beijk MAM, Williams R, Rahman H, Everaars H, Kharbanda RK, Knaapen P, van Royen N, Piek JJ, Perera D. Comparison of Doppler flow velocity and thermodilution derived indexes of coronary physiology. JACC Cardiovasc. Interv. 2022;15:1060–1070. doi: 10.1016/j.jcin.2022.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Buono MG, Montone RA, Camilli M, Carbone S, Narula J, Lavie CJ, Niccoli G, Crea F. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases. J. Am. Coll. Cardiol. 2021;78:1352–1371. doi: 10.1016/j.jacc.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fearon WF, Kobayashi Y. Invasive assessment of the coronary microvasculature: The index of microcirculatory resistance. Circ Cardiovasc. Interv. 2017;10(12):e005361. doi: 10.1161/CIRCINTERVENTIONS.117.005361. [DOI] [PubMed] [Google Scholar]
- 22.Fearon WF, Low AF, Yong AS, McGeoch R, Berry C, Shah MG, Ho MY, Kim H-S, Loh JP, Oldroyd KG. Prognostic value of the index of microcirculatory resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127:2436–2441. doi: 10.1161/CIRCULATIONAHA.112.000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim BG, Cho SW, Seo J, Kim GS, Jin M-N, Lee HY, Byun YS, Kim BO. Effect of direct stenting on microvascular dysfunction during percutaneous coronary intervention in acute myocardial infarction: A randomized pilot study. J. Int. Med. Res. 2022;50:030006052211278. doi: 10.1177/03000605221127888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoole SP, Jaworski C, Brown AJ, McCormick LM, Agrawal B, Clarke SC, West NEJ. Serial assessment of the index of microcirculatory resistance during primary percutaneous coronary intervention comparing manual aspiration catheter thrombectomy with balloon angioplasty (IMPACT study): A randomised controlled pilot study. Open Heart. 2015;2:e000238. doi: 10.1136/openhrt-2015-000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samady H, Lepper W, Powers ER, Wei K, Ragosta M, Bishop GG, Sarembock IJ, Gimple L, Watson DD, Beller GA, Barringhaus KG. Fractional flow reserve of infarct-related arteries identifies reversible defects on noninvasive myocardial perfusion imaging early after myocardial infarction. J. Am. Coll. Cardiol. 2006;47:2187–2193. doi: 10.1016/j.jacc.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 26.Ríos-Navarro C, Hueso L, Miñana G, Núñez J, Ruiz-Saurí A, Sanz MJ, Cànoves J, Chorro FJ, Piqueras L, Bodí V. Coronary serum obtained after myocardial infarction induces angiogenesis and microvascular obstruction repair. Role of hypoxia-inducible factor-1A. Revista Española de Cardiología (English Edn.) 2018;71:440–449. doi: 10.1016/j.rec.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Demirkiran A, Robbers LFHJ, van der Hoeven NW, Everaars H, Hopman LHGA, Janssens GN, Berkhof HJ, Lemkes JS, van de Bovenkamp AA, van Leeuwen MAH, Nap A, van Loon RB, de Waard GA, van Rossum AC, van Royen N, Nijveldt R. The dynamic relationship between invasive microvascular function and microvascular injury indicators, and their association with left ventricular function and infarct size at 1-month after reperfused ST-segment–elevation myocardial infarction. Circul. Cardiovasc. Interv. 2022;15:892–902. doi: 10.1161/CIRCINTERVENTIONS.122.012081. [DOI] [PubMed] [Google Scholar]
- 28.Papapostolou S, Andrianopoulos N, Duffy SJ, Brennan AL, Ajani AE, Clark DJ, Reid CM, Freeman M, Sebastian M, Selkrig L, Yudi MB, Noaman SQ, Chan W. Long-term clinical outcomes of transient and persistent no-reflow following percutaneous coronary intervention (PCI): A multicentre Australian registry. EuroIntervention. 2018;14:185–193. doi: 10.4244/EIJ-D-17-00269. [DOI] [PubMed] [Google Scholar]
- 29.Webb JG, Carere RG, Virmani R, Baim D, Teirstein PS, Whitlow P, McQueen C, Kolodgie FD, Buller E, Dodek A, Mancini GBJ, Oesterle S. Retrieval and analysis of particulate debris after saphenous vein graft intervention. J. Am. Coll. Cardiol. 1999;34:468–475. doi: 10.1016/S0735-1097(99)00196-5. [DOI] [PubMed] [Google Scholar]
- 30.Kalayci A, Oduncu V, Karabay CY, Erkol A, Tanalp AC, Tanboga IH, Candan O, Gecmen C, Izgi IA, Kirma C. Outcomes of direct stenting in patients with ST-elevated myocardial infarction. Herz. 2017;43:447–454. doi: 10.1007/s00059-017-4581-2. [DOI] [PubMed] [Google Scholar]
- 31.Limbruno U, De Carlo M, Pistolesi S, Micheli A, Sonia Petronio A, Camacci T, Fontanini G, Balbarini A, Mariani M, De Caterina R. Distal embolization during primary angioplasty: Histopathologic features and predictability. Am. Heart J. 2005;150:102–108. doi: 10.1016/j.ahj.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Cosansu K, Ureyen C, Vatan M, Agac M, Kilic H, Akdemir R. Impact of direct stenting on clinical outcomes for small vessel coronary artery disease in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction. Adv. Interv. Cardiol. 2019;15:404–411. doi: 10.5114/aic.2019.90214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann F-J, Gick M. Direct stenting in ST-elevation myocardials infarction: Convenient, but not improving outcomes. Eur. Heart J. 2018;39:2480–2483. doi: 10.1093/eurheartj/ehy353. [DOI] [PubMed] [Google Scholar]
- 34.He J, Kong L-C, Zeng J-T, Shi B-Z, An D-A-L, Chen B-H, Ding S, Li Z, Yang F, Yang Y-N, Yan F-H, Xiu J-C, Wang H-W, Xu J-R, Ge H, Pu J. Comparison of direct stenting with conventional strategy on myocardial impairments in ST-segment elevation myocardial infarction: A cardiac magnetic resonance imaging study. Int. J. Cardiovasc. Imaging. 2020;36:1167–1175. doi: 10.1007/s10554-020-01812-w. [DOI] [PubMed] [Google Scholar]
- 35.Loubeyre C, Morice M-C, Lefèvre T, Piéchaud J-F, Louvard Y, Dumas P. A randomized comparison of direct stenting with conventional stent implantation in selected patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2002;39:15–21. doi: 10.1016/S0735-1097(01)01701-6. [DOI] [PubMed] [Google Scholar]
- 36.Sabatier R, Hamon M, Zhao QM, Burzotta F, Lecluse E, Valette B, Grollier G. Could direct stenting reduce no-reflow in acute coronary syndromes? A randomized pilot study. Am. Heart J. 2002;143:1027–1032. doi: 10.1067/mhj.2002.122509. [DOI] [PubMed] [Google Scholar]
- 37.Ballarino MA, Moreyra E, Damonte A, Sampaolesi A, Woodfield S, Pacheco G, Caballero G, Picabea E, Baccaro J, Tapia L, Lascano ER. Multicenter randomized comparison of direct vs. conventional stenting: The DIRECTO trial. Catheter. Cardiovasc. Interv. 2003;58:434–440. doi: 10.1002/ccd.10404. [DOI] [PubMed] [Google Scholar]
- 38.Gasior M, Gierlotka M, Lekston A, Wilczek K, Zebik T, Hawranek M, Wojnar R, Szkodzinski J, Piegza J, Dyrbus K, Kalarus Z, Zembala M, Polonski L. Comparison of outcomes of direct stenting versus stenting after balloon predilation in patients with acute myocardial infarction (DIRAMI) Am. J. Cardiol. 2007;100:798–805. doi: 10.1016/j.amjcard.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 39.Ozdemir R, Sezgin AT, Barutcu I, Topal E, Gullu H, Acikgoz N. Comparison of direct stenting versus conventional stent implantation on blood flow in patients with ST-segment elevation myocardial infarction. Angiology. 2006;57:453–458. doi: 10.1177/0003319706290620. [DOI] [PubMed] [Google Scholar]
- 40.Scarparo P, Improta R, Wilschut J, Kardys I, Den Dekker WK, Daemen J, Zijlstra F, Van Mieghem NM, Diletti R. Very long-term clinical outcomes after direct stenting in patients presenting with ST-segment elevation myocardial infarction. Cardiovasc. Revascularization Med. 2022;41:144–150. doi: 10.1016/j.carrev.2022.01.014. [DOI] [PubMed] [Google Scholar]
- 41.McCormick LM, Brown AJ, Ring LS, Gajendragadkar PR, Dockrill SJ, Hansom SP, Giblett JP, Gilbert TJ, Hoole SP, West NE. Direct stenting is an independent predictor of improved survival in patients undergoing primary percutaneous coronary intervention for ST elevation myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care. 2014;3:340–346. doi: 10.1177/2048872614530864. [DOI] [PubMed] [Google Scholar]
- 42.Cuisset T, Hamilos M, Melikian N, Wyffels E, Sarma J, Sarno G, Barbato E, Bartunek J, Wijns W, De Bruyne B. Direct stenting for stable angina pectoris is associated with reduced periprocedural microcirculatory injury compared with stenting after pre-dilation. J. Am. Coll. Cardiol. 2008;51:1060–1065. doi: 10.1016/j.jacc.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 43.Mahmoud KD, Jolly SS, James S, Džavík V, Cairns JA, Olivecrona GK, Renlund H, Gao P, Lagerqvist B, Alazzoni A, Kedev S, Stankovic G, Meeks B, Frøbert O, Zijlstra F. Clinical impact of direct stenting and interaction with thrombus aspiration in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention: Thrombectomy Trialists Collaboration. Eur. Heart J. 2018;39:2472–2479. doi: 10.1093/eurheartj/ehy219. [DOI] [PubMed] [Google Scholar]
- 44.Meier D, Fournier S, Masci PG, Eeckhout E, Antiochos P, Tzimas G, Stoyanov N, Muenkaew M, Monney P, Schwitter J, Muller O, Harbaoui B. Impact of manual thrombectomy on microvascular obstruction in STEMI patients. Catheter. Cardiovasc. Interv. 2020;97:1141–1148. doi: 10.1002/ccd.28907. [DOI] [PubMed] [Google Scholar]
- 45.Zajdel W, Miszalski-Jamka T, Zalewski J, Legutko J, Żmudka K, Paszek E. Cardiac magnetic resonance shows improved outcomes in patients with an ST-segment elevation myocardial infarction and a high thrombus burden treated with adjuvant aspiration thrombectomy. J. Clin. Med. 2022;11:5000. doi: 10.3390/jcm11175000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Carlo M, Aquaro GD, Palmieri C, Guerra E, Misuraca L, Giannini C, Lombardi M, Berti S, Petronio AS. A prospective randomized trial of thrombectomy versus no thrombectomy in patients with ST-segment elevation myocardial infarction and thrombus-rich lesions. JACC Cardiovasc. Interv. 2012;5:1223–1230. doi: 10.1016/j.jcin.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Sardella G, Mancone M, Bucciarelli-Ducci C, Agati L, Scardala R, Carbone I, Francone M, Di Roma A, Benedetti G, Conti G, Fedele F. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size. J. Am. Coll. Cardiol. 2009;53:309–315. doi: 10.1016/j.jacc.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 48.Elbadawi A, Gasioch G, Elgendy IY, Mahmoud AN, Ha LD, Ashry HA, Shahin H, Hamza MA, Abuzaid AS, Saad M. Intracoronary eptifibatide during primary percutaneous coronary intervention in early versus late presenters with ST segment elevation myocardial infarction: A randomized trial. Cardiol. Ther. 2016;5:203–213. doi: 10.1007/s40119-016-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubboli A, Patti G. What is the role for glycoprotein IIB/IIIA inhibitor use in the catheterization laboratory in the current Era? Curr. Vasc. Pharmacol. 2018;16:451–458. doi: 10.2174/1570161116666180117102422. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Tao GZ. Effects of tirofiban on the reperfusion-related no-reflow in rats with acute myocardial infarction. J. Geriatr. Cardiol. 2013;10:52–58. doi: 10.3969/j.issn.1671-5411.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akpek M, Sahin O, Sarli B, Baktir AO, Saglam H, Urkmez S, Ergin A, Oguzhan A, Arinc H, Kaya MG. Acute effects of intracoronary tirofiban on no-reflow phenomena in patients with ST-segment elevated myocardial infarction undergoing primary percutaneous coronary intervention. Angiology. 2014;66:560–567. doi: 10.1177/0003319714545780. [DOI] [PubMed] [Google Scholar]
- 52.Tan HC, Lim YT, Rosli TLA, Sim KH, Tan KH, Lee CH, Ismail O, Azman W. Direct stenting compared to conventional stenting in diabetic patients undergoing elective angioplasty for coronary artery disease (DECIDE): A multicenter, open label, randomized, controlled efficacy study. Am. Heart J. 2004;148:1007–1011. doi: 10.1016/j.ahj.2004.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in this study are available from the corresponding author on reasonable request.