Abstract
Asymmetric de novo syntheses of euphol and tirucallol have been accomplished by way of a concise sequence of chemical steps featuring several modern stereoselective transformations. The preparative solution described for these complex problems in natural product synthesis departs significantly from biomimetic polyene cyclization chemistry that has been leveraged to address related tetracyclic triterpenoid targets. In particular, a diastereoselective Friedel–Crafts type cyclization was employed to establish a tetracycle bearing a stereodefined quaternary center at C9 (steroid numbering) that provided access to intermediates of relevance for introducing the C10 and C14 quaternary centers by sequential stereospecific 1,2-alkyl shifts (C9 → C10, and C15 → C14). Finally, the stereodefined C17-side chain was introduced in a single step by late-stage stereoselective conjugate addition to an intermediate possessing a D-ring enone. Notably, these de novo asymmetric syntheses are the first of their kind, providing completely synthetic access to enantiodefined euphane and tirucallane systems. Overall, each synthesis has been accomplished in fewer than twenty linear chemical steps from a simple Hajos–Parrish-derived ketone through a sequence that features just fifteen chromatographic operations.
Graphical Abstract
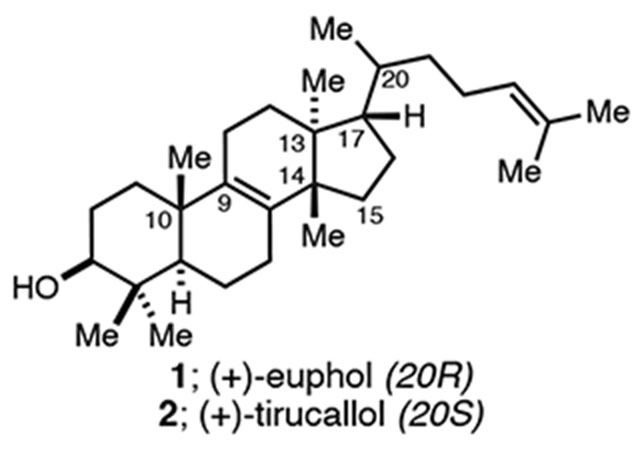
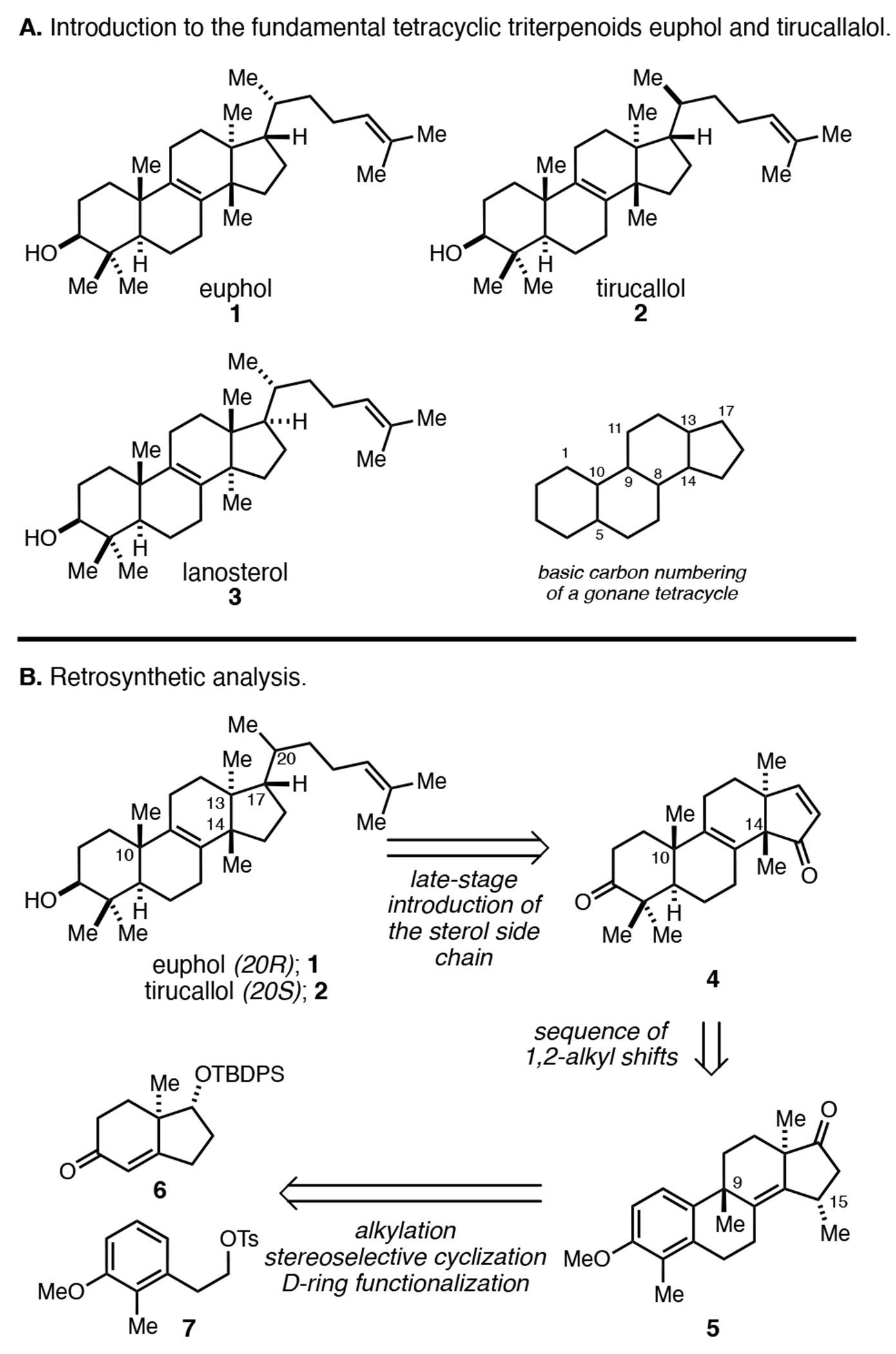
Tetracyclic triterpenoids derived from the cationic cyclization of 2,3-oxidosqualene represent a large family of natural products that have been found to possess a wide array of medicinally relevant properties.1 Among these, the fundamental tetracyclic triterpenoids euphol (1), tirucallol (2) and lanosterol (3; Figure 1A), are known not only for their activities as anti-cancer and anti-inflammatory agents, but also appreciated as precursors to scores of bioactive natural products including, in the case of lanosterol, cholesterol and the steroid hormones.2 While tetracyclic triterpenoids have played central roles in the development of organic chemistry, first from a structural elucidation perspective, then serving as targets for synthesis, these fundamental examples (1–3) remain unsolved problems for asymmetric de novo synthesis.3 Structural features that continue to represent obstacles for stereoselective syntheses of these targets include the presence of vicinal quaternary centers at C13 and C14, the central unsaturation at C8–C9, and the stereodefined and unsaturated side chain at C17. In fact, there has not yet been a successful multistep asymmetric synthesis of euphol, tirucallol, or lanosterol reported, the closest being: (1) Woodward’s conversion of (−)-cholesterol to (+)–lanosterol, and (2) Johnson’s relay synthesis of (+)-euphol and related semisynthesis of (+)–tirucallol.4,5 As a result, programs seeking to identify novel synthetic variants of these natural products as leads in medicinal chemistry are constrained to semisynthesis, where only modest structural/skeletal changes are straightforward to accomplish with available reaction technology.6 As such, in addition to addressing these enduring problems in de novo asymmetric synthesis, we became interested in establishing a concise synthetic entry to these systems that could one day enable medicinal exploration in a manner not feasible currently.7 Our efforts, also being guided by using natural product targets as a testing ground for recently developed reaction technology, has resulted in concise asymmetric syntheses of euphol (1) and tirucallol (2) that feature a stereoselective intramolecular Friedel–Crafts cyclization, tandem oxidative dearomatization/Wagner–Meerwein rearrangement, semi-pinacol rearrangement that forges both the C10 and C14 quaternary centers, and late-stage introduction of stereodefined and unsaturated C17-side chain common to scores of tetracyclic triterpenoid natural products by conjugate addition. Figure 1B illustrates the basic features of our retrosynthetic analysis that ultimately concludes with our plan of employing hydrindane 6 and tosylate 7 as readily available starting materials.
Figure 1.
Introduction.
Prior to presenting the asymmetric de novo synthesis of 1 and 2 central to this work, we offer a summary of prior studies by Johnson that ultimately resulted in a relay synthesis of (+)-1 and a semisynthesis of (+)-2.5 As illustrated in Figure 2A, cationic cyclization of (+/−)-8 was employed to generate the polycyclic product (+/−)-9; a structure containing a C8–C9 alkene and vicinal quaternary stereocenters at C13 and C14. While confirming the feasibility of the planned cationic cyclization, the process proceeded in only ~16% yield. Moving along, seven additional steps were used to convert this intermediate to (+/−)-10 in 9% yield. Instead of advancing racemic 10 to the targeted natural products, degradation of euphol acetate was used to secure sufficient quantities of (−)-10 to complete a relay-based synthesis of (+)-euphol (1) and a semisynthesis of (+)-tirucallol (2) (Figure 2B).
Figure 2.
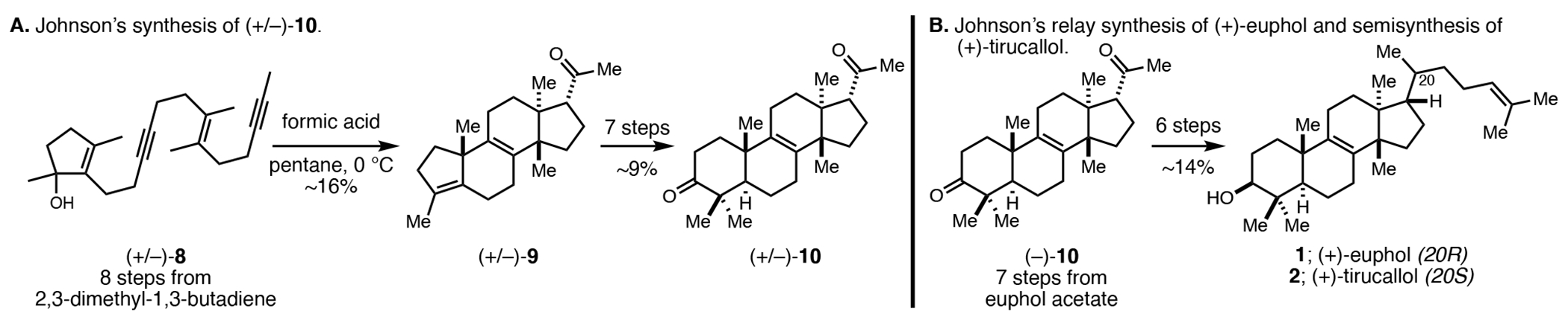
Summary of Johnson’s relay synthesis of euphol that exploits an acid-mediated cyclization of a dienediynol (8).
Our efforts to accomplish the asymmetric de novo synthesis of these tetracyclic triterpenoid targets began with base-mediated alkylation of enone 6 (derived from the Hajos–Parrish ketone) and the homobenzylic tosylate 7 (Figure 3).8 In short, α-alkylation of the conjugated enolate of 6 is followed by base-mediated equilibration to generate enone 11.9 With our next goal of accomplishing a stereoselective intramolecular Friedel–Crafts cyclization, we applied a two-step sequence that first introduces a C9 methyl group by 1,2-addition of MeMgBr to the ketone of 11. As we have previously shown, treatment of the tertiary alcohol product of this reaction with BF3•OEt2 results in a highly stereoselective cyclization that establishes the C9 stereocenter of 12 with ≥ 20:1 ds.10 Desilylation followed by oxidation to the enone provided access to 14 via the intermediacy of ketone 13. Notably, dienone 14 was thought to be an ideal intermediate for conversion to a tetracycle that possesses key structural features of euphane natural products.
Figure 3.
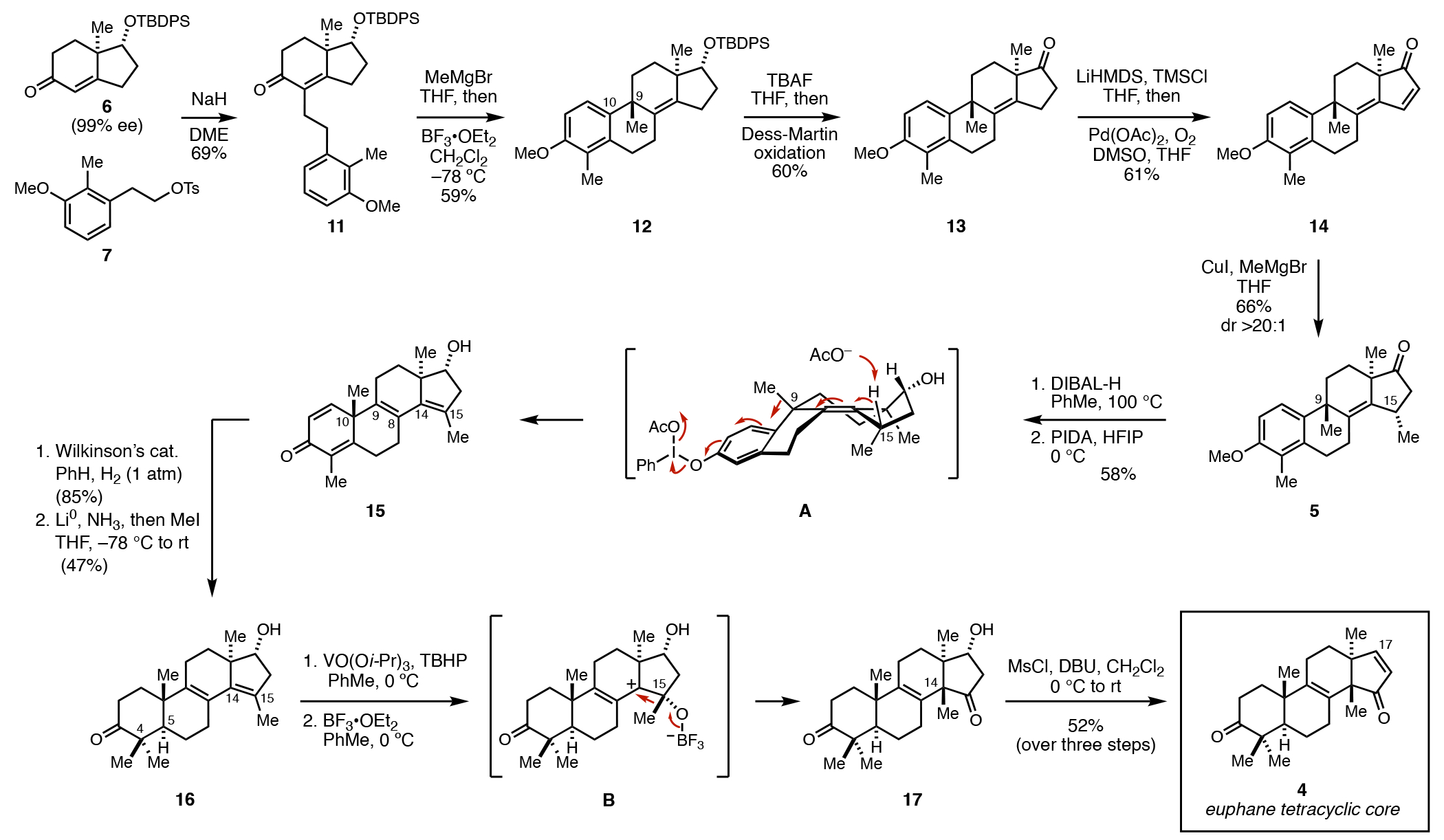
Synthesis of a tetracyclic enone (4) comprising the steroedefined euphol core from the Hajos–Parrish ketone-derived hydrindane 6.
First, the differential substitution of dienone 14 was leveraged to accomplish site-selective conjugate addition (MeMgBr, CuI), resulting in a product that possesses a C15α-methyl substituent (5). Moving forward, anisole demethylation with DIBAL also stereoselectively reduced the C17 ketone of 5, delivering a phenolic product that was smoothly converted to 15 by PIDA-mediated oxidative dearomatization followed by group-selective Wagner–Meerwein rearrangement as depicted in A. Through this two-step sequence, the methyl group previously located at C9 is shifted to C10, and a diene is generated that spans C9,C8,C14 and C15. This latter functionality was appreciated to be of great potential future value in setting the critical vicinal quaternary centers characteristic of euphane and tirucallane natural products.
With 15 in hand, chemoselective reduction of the C1–C2 alkene (Wilkinson’s catalyst, H2) was followed by dissolving metal reduction of the enone, and alkylation of the resulting enolate with MeI. The combined features present in the resulting product, a 17α-hydroxy group that was generated from the reductive demethylation of 5 and the aforementioned conjugated diene spanning the C and D rings that was generated naturally from the oxidative dearomatization process, were then exploited to accomplish initial chemo- and stereoselective hydroxy-directed epoxidation [VO(Oi-Pr)3, TBHP] and BF3•OEt2-mediated semi-Pinacol rearrangement.11 Notably, the intermediate generated from the stereoselective epoxidation positions the C15-methyl group on the β-face and sets the stage for precise installation of the C14 quaternary center in 17 by way of the allylic cation B. Finally, β-hydroxy ketone 17 was converted to the targeted enone 4 by the action of MsCl and DBU.
While compound 4 possesses the majority of structural features seen in euphane and tirucallane natural products, it lacks the stereodefined eight carbon side chain at C17. Noting that many triterpenoid natural products possess related side chains at this position, it was appreciated that approaches to the installation of this unit typically rely on several sequential chemical steps.12 Here, we sought a means to introduce the entire side chain in a single step by stereoselective conjugate addition of an appropriately functionalized cuprate to the D-ring enone of 4. Previous studies targeting an unrelated natural product have revealed that such a strategy might well be quite complex, owing to the fact that this type of conjugate addition establishes two stereocenters (one at C17 and one at C20).13 Preliminary studies with a simple cuprate derived from i-PrMgBr revealed that the stereochemical course of 1,4-addition to 4 was highly selective, delivering a product whereby C–C bond formation preferentially occurs on the undesired β-face at C17 (not depicted; see Supporting Information for details). To address this stereochemical issue, conjugate addition of the cuprate derived from 18 was conducted in the presence of TMSCl, resulting in an intermediated enolsilane that was subsequently oxidized to the corresponding enone by Saegusa–Ito oxidation.14 The result of this sequence was a 2:1 mixture of enones 19 and 20 that were separated by HPLC and subsequently converted to (+)-euphol (1) and (+)-tirucallol (2) by stereoselective reduction of the D-ring enone and C3 ketone (Li0, NH3 followed by NaBH4), followed by deoxygenation of the C15 ketone by Wolff–Kishner reduction.
Overall, these studies have resulted in the first de novo asymmetric total synthesis of any euphane or tirucallane natural product, doing so by completing the syntheses of the foundational tetracyclic triterpenoids (+)-euphol (1) and (+)-tirucallol (2). Key aspects of our synthetic design include: (1) stereoselective Friedel–Crafts cyclization that converts 11 to the tetracyclic intermediate 12 that possesses the critically important C9 quaternary stereocenter, (2) capitalizing on the differential substitution of the conjugated dienone 14 to introduce a methyl group that later winds up at the C14 quaternary center of the natural product targets, (3) PIDA-mediated oxidative dearomatization and group-selective Wagner–Meerwein rearrangement that establishes the C10 quaternary centers of the natural products and a conjugated diene spanning the C and D rings, (4) chemo- and stereoselective epoxidation of diene 16 that sets up subsequent semi-Pinacol rearrangement to install the C14 quaternary center, and (5) late-stage introduction of the stereodefined and unsaturated side chain at C17. We expect that aspects of this synthesis pathway will enable access to unique unnatural variants of euphanes and tirucallanes that would not be straightforward to access through established semisynthesis or relay synthesis-based approaches. Future efforts aiming to exploit such features in the design and synthesis of medically relevant natural product-inspired agents will be reported in due course.
Supplementary Material
Figure 4.
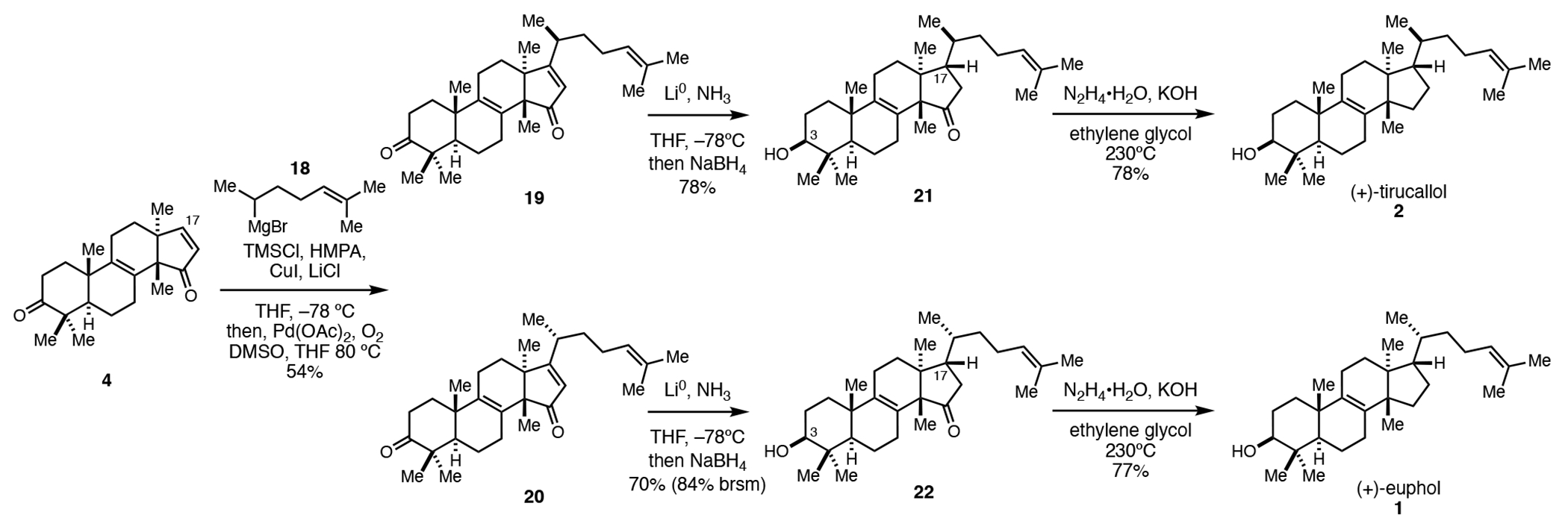
Introduction of the C17 side chain by conjugate addition and completion of the total syntheses of (+)-euphol and (+)-tirucallol.
ACKNOWLEDGMENT:
We gratefully acknowledge financial support of this work by the National Institutes of Health – NIGMS (R35 GM134725).
Footnotes
The authors declare no competing financial interest.
Data Availability Statement:
The data underlying this study are available in the published article and its Supporting Information.
REFERENCES:
- (1).(a) Ghosh S. Chapter 12 – Triterpenoids: Structural Diversity, Biosynthetic Pathway, and Bioactivity. Stud. Nat. Prod. Chem 2020, 67, 411–461. [Google Scholar]; (b) Hamid K; Alqahtani A; Kim M-S, Cho J-L; Cui PH; Li CG; Groundwater PW; Li GQ Tetracyclic Triterpenoids in Herbal Medicines and their Activities in Diabetes and its Complications. Curr. Top. Med. Chem 2015, 15, 2406–2430. [DOI] [PubMed] [Google Scholar]; (c) Ghiulai R; Rosca OJ; Antal DS; Mioc M; Mioc A; Racoviceanu R; Macasoi I; Olariu T; Dehelean C; Cretu OM; Voicu M; Soica C Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Efficiency in Wound Healing Approaches. Molecules. 2020. 25, 5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Silva VAO; Rosa MN; Tansini A; Oliveira RJS; Martinho M; Lima JP; Pianowski LF; Reis RM In vitro screening of cytotoxic activity of euphol from Euphorbia tirucalli on a large panel of human cancer-derived cell lines. Exp. Ther. Med 2018, 16, 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fernandez-Arche A; Saenz MT; Arroyo M; de la Puerta R; Garcia MD Topical anti-inflammatory effect of tirucallol, a triterpene isolated from Euphorbia lactea latex. Phytomedicine, 2010, 17, 146–148. [DOI] [PubMed] [Google Scholar]; (c) Risley JM Cholesterol Biosynthesis: Lanosterol to Cholesterol. J. Chem. Educ 2002, 79, 377–384. [Google Scholar]
- (3).(a) Lapworth A; Robinson R Crystal Structures of Vitamin D and Related Compounds. Nature, 1932, 129, 277–278. [Google Scholar]; (b) Rosenheim O; King H The Ring System of Sterols and Bile Acids. Nature 1932, 130, 315. [Google Scholar]; (c) Rosenheim O; King H The chemistry of the sterols, bile acids, and other cyclic constituents of natural fats and oils. Annu. Rev. Biochem 1934, 3, 87–110. [Google Scholar]; (d) Windaus A Concerning the constitution of cholesterol and biliary acid. Z. Physiol. Chem 1932, 213, 147–187. [Google Scholar]; (e) Fieser LF; Fieser M Steroids. Reinhold, NY, Chapman and Hall, London, 1959. xvii + 945 pp. [Google Scholar]; (f) Woodward RB; Sondheimer F; Taub D The Total Synthesis of Cholesterol. J. Am. Chem. Soc 1951, 73, 3548. [Google Scholar]; (g) Woodward RB; Sondheimer F; Taub D; Heusler K; McLamore WM The Total Synthesis of Steroids. J. Am. Chem. Soc 1952, 74, 4223–4251. [Google Scholar]; (h) Zeelan FJ Steroid Total Synthesis. Nat. Prod. Rep, 1994, 11, 607–612. [DOI] [PubMed] [Google Scholar]
- (4).Woodward RB; Patchett AA; Barton DHR; Ives DAJ; Kelly RB The synthesis of lanosterol (lanostadienol). J. Chem. Soc 1957, 1131–1144. [Google Scholar]
- (5).Bartlett WR; Johnson WS; Plummer MS; Small VR Jr. Biomimetic polyene cyclizations. Cationic cyclization of a substrate having an internal acetylenic bond. Synthesis of euphol and tirucallol. J. Org. Chem 1990, 55, 2215–2224. [Google Scholar]
- (6).(a) Romero-Morán LJ; Ramírez-Apan MT; Hernández-Ortega S; Martínez-Otero D; Delgado G Tris nor-Euphane-Type Triterpenoid and Other Constituents Isolated from Euphorbia tenquahuete Sessé & Moc.: Preparation and Cytotoxic Evaluation of Semisynthetic Derivatives of Euphol. ACS Omega. 2022, 7, 35077–35082. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Morais TR; Da Costa-Silva TA; Tempone AG; Borborema SET ; Scotti MT; De Sousa RMF; Araujo ACC; De Oliveira A; De Morais SAL; Sartorelli P; Lago JHG Antiparasitic Activity of Natural and Semi-Synthetic Tirucallane Triterpenoids from Schinus terebinthifolius (Anacarduaceae): Structure/Activity Relationships. Molecules 2014, 19, 5761–5776 [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sonoda Y; Ichinose K; Yoshimura T; Sato Y; Sasaki T Synthesis of Lanosterol Derivatives with a Functional Group at C-32, Including an Antineoplastic Sterol, 3β-Hydroxylanost-7-en-32-oic Acid. Chem. Pharm. Bull 1991, 39 100–103. [DOI] [PubMed] [Google Scholar]; (d) Yang X; Chen X-J; Yang Z; Xi Y-B; Wang L; Wu Y; Yan Y-B; Rao Y Synthesis, Evaluation, and Structure-Activity Relationship Study if Lanosterol Derivatives to Reverse Mutant-Crystallin-Induced Protein Aggregation. J. Med. Chem 2018, 61, 8693–8706. [DOI] [PubMed] [Google Scholar]
- (7).This goal has also driven our recent programs aimed at establishing general asymmetric entries to classic steroid skeletons. For examples, see:; (a) Kim WS; Du K; Eastman A; Hughes RP; Micalizio GC Synthetic nat- or ent-steroids in as few as five chemical steps from epichlorohydrin. Nature Chem. 2018, 10, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kim WS; Shait ZA; Nguyen SM; Schoepke E; Eastman A; Burris TP; Gaur AB; Micalizio GC A synthesis strategy for tetracyclic terpenoids leads to agonists of ERβ. Nature Commun. 2019, 10, 2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Caine D; Kotian PL; McGuiness MD Synthesis and Photochemical Rearrangements of Bicyclic Cross-Conjugated Cyclohexadienones Containing δ-Oxy Substituents J. Org. Chem 1991, 56, 6307–6313. [Google Scholar]
- (9).Cai ZY; Covey DF A facile total synthesis of ent-17β-estradiol and structurally related analogues. Steroids 2007, 72, 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Nicholson JM; Millham AB; Bucknam AR; Markham LE; Sailors XI; Micalizio GC General Enantioselective and Stereochemically Divergent Four-Stage Approach to Fused Tetracyclic Terpenoid Systems. J. Org. Chem 2022, 87, 3352–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11) (a).Hayakawa I; Matsumaru N; Sakakura A Toward the Synthesis of Paspaline-Type Indole-Terpenes: Stereoselective Construction of Core Scaffold with Contiguous Asymmetric Quaternary Carbon Centers. J. Org. Chem 2021, 86, 9802–9810. [DOI] [PubMed] [Google Scholar]; (b) Song Z-L; Fan C-A; Tu Y-Q Semipinacol Rearrangement in Natural Product Synthesis. Chem. Rev 2011, 111, 7523–7556. [DOI] [PubMed] [Google Scholar]; (c) Meinwald J; Labana SS; Chadha MS Peracid Reactions. III. The Oxidation of Bicyclo[2.2.1]heptadiene. J. Am. Chem. Soc 1963, 85, 582–585. [Google Scholar]; (d) House HO The Acid-catalyzed Rearrangement of the Stilbene Oxides. J. Am. Chem. Soc 1955, 77, 3070–3075. [Google Scholar]; (e) He J; Ling J; Chiu P Vinyl Epoxides in Organic Synthesis. Chem. Rev 2014, 114, 8037–8128. [DOI] [PubMed] [Google Scholar]
- (12).(a) Ohmori M; Yamada S; Takayama H Stereocontrolled Synthesis of Steroid Side Chain; Stereoselective Syntheses of Cholesterol and 25-Hydroxycholesterol. Tet. Lett 1982, 45, 4709–4712. [Google Scholar]; (b) Takahashi T; Ootake A; Tsuji J; Tachibana K Regio- and Stereoselective Introduction of 15-β hydroxy Group and Side Chains to Steroids by the Palladium-Catalyzed Reaction of 1,3-diene Monoepoxides with Carbonucleophiles. Tetrahedron 1985, 41, 5747–5754. [Google Scholar]; (c) Westover EJ; Covey DF First Synthesis of ent-desmosterol and its conversion to ent-deuterocholesterol. Steroids. 2003, 68, 159–166. [DOI] [PubMed] [Google Scholar]; (d) Rychnovsky SD; Mickus DE Synthesis of ent-Cholesterol, the Unnatural Enantiomer. J. Org. Chem 1992, 57, 2732–2736. [Google Scholar]
- (13).(a) Liu G; Mei G; Chen R; Yuan H; Yang Z; Li C.-c. Total Synthesis of Aplykurodinone-1. Org. Lett 2014, 16, 4380–4383. [DOI] [PubMed] [Google Scholar]; For an earlier five-step approach to installation of this side that is based on sequential conjugate addition, hydrogenation, deprotection, oxidation, and Wittig reaction, see:; (b) Zhang Y; Danishefsky SJ Total Synthesis of (+/−)-Aplykurodinone-1: Traceless Stereochemical Guidance. J. Am. Chem. Soc 2010, 132, 9567–9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).(a) Ito Y; Hirao T; Saegusa T Synthesis of α,β-unsaturated Carbonyl Compounds by Palladium(II)-Catalyzed Dehydrosilylation of Silyl Enol Ethers J. Org. Chem 1978, 43, 1011–1013. [Google Scholar]; (b) Shi Z; Zhang C; Tang C; Jiao N Recent Advances in Transition-metal Catalyzed Reactions Using Molecular Oxygen as the Oxidant. Chem. Soc. Rev 2012, 41, 3381–3430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.


