Abstract
Cancer disease is regarded as one of the most significant public health issues, regardless of economic standards. Medicinal plants are now regarded as a natural source of anticancer medicines due to their antioxidant and anti-mutagenic actions. Cucurbitaceae is considered to be one of the most economically significant families. One family species is Citrullus colocynthis (L.), which has a high concentration of many active secondary chemical metabolites. Various C. colocynthis plant extracts showed cytotoxicity against some cancer cells. This study aims to identify the C. colocynthis fruit components and determine whether they have anticancer action against MIA PaCa-2 and A431 cells. High-Performance Liquid Chromatography/Quadrupole Time of Flight/Mass Spectrometry (HPLC/QTOF/MS); the technique was accustomed to investigate the compounds of the ethyl acetate (EtOAc) fruit extract. Anticancer activity was investigated on both MIAPaCa-2 and A-431 cell lines. DPPH assay for antioxidant activity was carried out. Molecular modelling was employed to help understand the molecular basis for the observed anticancer activity. 24 compounds were tentatively identified by comparing the extract’s fragmentation pattern in positive mode against reference compounds spectra and literature. The EtOAc extract of C. colocynthis had effective positive results on cancer cells (MIAPaCa-2 and A-431) and was characterized by slight or no harmful effect on normal (healthy) cells. For the DPPH assay, EtOAc and BuOH extracts exhibited high antioxidant activity (86 and 76%, respectively) compared with the oxidative potential of the standard compound (Caffeic acid, 98%). One of the major cucurbitacin derivatives that LC/MS tentatively identified in the EtOAc extract was Cucurbita-5(10),6,23-triene-3β,25-diol. During this study, docking experiments and MD simulations were carried out, which suggested the anti-pancreatic cancer activity of C. colocynthis extract to be attributed to EGFR inhibition by Cucurbita-5(10),6,23-triene-3β,25-diol. Therefore, expansion of this type of research should be encouraged in the hope of obtaining natural therapeutics for cancerous tumors in the future, having the advantage of being cheaper, safer, and with fewer side effects.
Subject terms: Biochemistry, Plant sciences
Introduction
Cancer is perceived as one of the top leading causes of death worldwide1,2. It is a heterogenous disease characterized by aberrant cell proliferation and invasiveness of abnormal cells which spread to neighboring tissues3. Medicinal plants have been widely used as a source of natural compounds for the treatment of cancer4. Besides their antitumor activity, natural compounds have many advantages owing to their low side effects, low cost, and ease of availability5,6.
The Cucurbitaceae family is considered one of the most economically significant families. It has 122 genera and 940 species, all found throughout the world's tropical and subtropical regions7. Due to their beneficial effects as dietary and therapeutic agents, many members of the Cucurbitaceae family are making significant contributions as domesticated species8. Most plants in this family are tolerant of dry seasons but intolerant of wet, frost-sensitive, and poorly drained soils9.
Citrullus colocynthis (L.) is a plant belonging to the family Cucurbitaceae. Its synonyms are Cucumis colocynthis and Colocynthis vulgaris. Other common names for this plant included in Cambridge English dictionary are bitter apple, and bitter gourd, while in Arabic it is known as Hanzal and Handal6,10. Mazher et al.4 previously reported C. colocynthis to have a high concentration of phenolics, flavonoids, glycosides, fatty acids, tocopherol, alkaloids, volatile chemicals, proteins, and amino acids.
C. colocynthis has been eaten and processed for its medical uses. It was traditionally used as a remedy mainly for diabetes mellitus along with a variety of diseases including gastrointestinal, musculoskeletal, neurological, cardiovascular, and respiratory disorders4. Their fruit extracts are astringent and treat jaundice, tumors, asthma, and urinary tract diseases. A study by Hussain et al.8 showed that fruit rind can treat bronchitis, arthritis, TB, and constipation. Additionally, the primary active ingredient in the fruits of C. colocynthis, colocynthin or cucurbitacin E-2-O-glucoside, has cathartic, antihistaminic, anticholinergic, negative chronotropic, and negative inotropic properties7.
Various C. colocynthis plant extracts showed cytotoxicity against some cancer cells11–14. However, very limited data are available on the activity of C.colocynthis fruit extracts on human pancreatic cancer cell lines (MIAPaca-2) and human skin cancer cells (A-431). Accordingly, the aim of this study is to evaluate the effect of C.colocynthis on pancreatic and skin cancer cells. The HPLC/QTOF/MS/MS technique to identify the possible compounds in the extract along with molecular docking and MD simulations to find the primary compound responsible for theobserved biological activities.
Materials and methods
In this study, all methods followed the relevant guidelines/ regulations/ legislation.
Preparation of plant extract
Around June 2022, the C. colocynthis (L.) fruits were purchased from a local Egyptian market. After the plant material was dried in an oven at 40 °C for five days, it was ground into powder using an electric mill. Until needed, the powdered sample was stored in an airtight container. A soxhlet extracted about 450 g of the powdered dry fruits over 72 h using solvents (2.5 L each) with increasing polarity. The plant extracts were concentrated in a rotary evaporator at 40–60 °C, and the finished extracts were stored in the fridge. Hexane (3 g), dichloromethane (8 g), ethyl acetate (13 g), butanol (11 g), and aqueous layer (21 g) were the five fractions that were obtained.
HPLC–ESI–QTOF–MS/MS
HPLC–ESI–QTOF–MS/MS technique is used to look into the secondary metabolites of C. colocynthis fruit ethyl acetate extract. 50 mg of extract was dissolved in 1 mL of mobile phase A (water 0.1% formic acid), vortexed for 2 min, ultrasonically disrupted for 10 min, and centrifuged for 5 min at 10,000 rpm. The gas temperature and drying gas flow were 200 OC and 8 l/min, respectively. The injection had a 3 g/l concentration. The multi-step linear gradient was applied to mobile phase A (water 0.1%formic acid) at a flow rate of 3 ml/min for 45 min, starting at (90–10) percent of mobile phase B (Acetonitrile 0.1%formic acid). Columns: Agilent Technologies pre-Zorbax RP-18 column (dimensions: 150 mm 3 mm, dp = 2.7 m). For separation, a column at a temperature of 200 °C was utilized. The instrument used for chromatographic separation at the Faculty of Pharmacy- Fayoum University was the 6530 Q-TOF LC/MS (Agilent Technologies) equipped with an autosampler (G7129A), a quaternary pump (G7104C), and a column comp (G7116A). With a capillary voltage of 4500 V, ESI's ( +) ionization mode was used to acquire mass spectra. The mass spectra were captured between 50 and 3000 m/z. The collision energy was set to 10 V, while the skimmer and fragmentation voltages were set to 65 and 130 V, respectively.
Biological activity
Cytotoxicity
Cell culture
Human pancreatic cancer cell lines (MIAPaCa-2) and human skin cancer cells (A-431) were obtained from the American Type Culture Collection (ATCC). The cells were then cultured in T-25 flasks (Jet Biofil Flask) using 4–6 mL of DMEM-high glucose medium containing 10% fetal bovine serum (Gibson, 26140-079) and 1% antibiotic of penicillin–streptomycin (Gibson, 15140-122). They were raised at 37 °C in a 5% CO2 environment. The subculture's cells were washed twice with 1 ml PBS after removing the covering media when they were 80% confluence. The cells were pre-incubated for 1–2 min with trypsin–EDTA 0.25% (Gibson, 25200-056), and then the trypsin effect was countered by adding 3 ml of DMEM medium. They were put into 15 ml Falcon tubes and centrifuged at 1700 rpm for 7 min. The cells were pelleted, the supernatant medium was removed, the fresh medium was added, and the cells were placed in incubation flasks. 1 ml of freezing media (5–10% DMSO with 90–95% FBS) was used for freezing.
Using the MTT assay, determine cell toxicity
The colorimetric [3-(4, 5-dimethylthiazol-2yl-)-2,5-diphenyl tetrazolium bromide] tetrazolium reduction assay (MTT) was used to assess the vitality of the cells. A 96-well plate was used to seed the MIAPaCa-2 and A-431 cell lines, and 48 h of incubation were required. After incubation, the cells were treated to a final concentration of 100 ppm of the tested medicines in triplicates. 180 µl of the new medium was introduced to each well after the existing medium had been removed after 48 h. The MTT assay described by Mosmann 15 evaluated cytotoxicity. After treatment, the medium is removed from each well, and 20 µl of MTT (Sigma, Germany) is added. Each well is then incubated for 4 h at 37 °C in the dark. Following incubation, 200 µl of DMSO was added in place of the MTT solution. The cells were then placed in a shaking incubator for 10 min. An ELISA plate reader measured absorbance at 570 nm after adding glycine buffer.
Determination of IC50 values
Several concentrations of highly active samples that exhibited 60% cytotoxicity on various cancer cell lines were generated for dose–response investigations. The findings were utilized to determine the IC50 values of each sample in comparison with doxorubicin, a positive control.
DPPH assay
In the wells of a 96-well plate, 20 µl of extract that had been suitably diluted in DMSO was combined with 180 µl of DPPH in methanol (4 mg/ml) for the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging experiment. After 15 min of darkness, the plate was placed in a Multiskan automatic kinetic microplate reader (Labsystems Multiskan RC reader) to measure the solution's absorbance at 540 nm. Standard (Trolox solutions in DMSO) and appropriate blanks (DMSO) were run simultaneously. The EC50 (the concentration that reduces DPPH absorbance by 50%) was determined by testing the extracts at different concentrations. This approach closely resembles that of earlier researchers16. Testing was done in triplicate.
Molecular docking
Based on the literature, EGFR was selected as a drug target for pancreatic cancer17. The X-ray crystal structure of EGFR and its co-crystallized ligand (PDB ID: 5X2A)18 was obtained from the Protein Data Bank. The protein and the 3D structure of Cucurbita-5(10),6,23-triene-3β,25-diol were prepared using the structure preparation wizard in MOE (version 2019.01)19, and docking was performed using GOLD (version 5.8)20,21 using the ChemPLP scoring function as previously described22,23. PyMol was used for the generation of all figures24.
Molecular dynamics
A 100 ns MD simulations was carried out using the PMEMD.cuda code of the AMBER Molecular Dynamics package25 for EGFR-Cucurbita-5(10),6,23-triene-3β,25-diol complex following the same previously described protocol of minimization, heating, density equilibration and production26. The trajectories were analyzed using CPPTraj27. Plots and visual inspection of the trajectories were done using XMgrace28 and VMD29, respectively.
Ethics approval and consent to participate
The study was commenced after ethical clearance was secured from the National Research Centre Ethical Committee, Egypt, with protocol number (6441112202).
Results
Viability assay by MTT
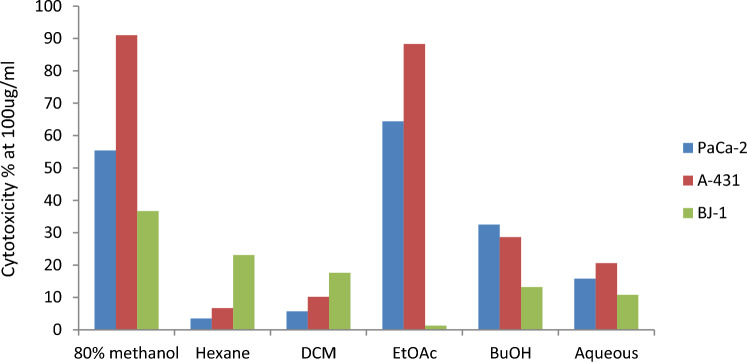
The viability of the MIAPaCa-2 and A-431 cell lines was evaluated using the MTT test after treatment with 100 µg/ml of C. colocynthis extracts. As seen in Fig. 1, the EtOAc fraction showed the highest cytotoxic effect, inhibiting PaCa-2 and A-431 by 54.4 and 68.3%, respectively. On the other hand, the extract showed only 1.3% inhibition on normal cells (BJ-1), indicating the extract to be selectively cytotoxic to cancer cells and not normal cells.
Figure 1.
In vitro cytotoxic activity % (mean ± SD) of C.colocynthis extracts against pancreatic (PaCa-2) and skin (A-431) cancer cells, 48-h post-treatment as compared with (BJ-1) a normal cell line.
Additionally, the EtOAc extract demonstrated an IC50 value of 17.4 0.12 and 13.1 0.21 µg/ml of PaCa-2 and A-431 cancer cells, respectively. The cytotoxicity is nearly 2.3 and 1.3 folds more potent than the IC50 value obtained with the positive control drug, doxorubicin (21 1.2 and 37.6 1.5 µg/ml, respectively) (Table 1).
Table 1.
In vitro cytotoxic activity (IC50 µg/ml) of C. colocynthis EtOAc extract and doxorubicin as a positive control against pancreatic (PaCa-2) and skin (A-431) cancer cells, 48-h post-treatment.
| IC 50 µg/ml | Cell line | |
|---|---|---|
| EtOAc extract | Doxorubicin | |
| 12.4 ± 0.12 | 28.3 ± 1.2 | PaCa-2 |
| 19.1 ± 0.21 | 24.9 ± 1.5 | A-431 |
DPPH test
Comparing the oxidative potential of the reference chemical (Caffeic acid, 98%) utilized in this study to the antioxidant activity of the EtOAc and BuOH extracts revealed their strong antioxidant activity in the DPPH experiment. The antioxidative activity of the EtOAc and BuOH extracts was significantly high (86% and 76%, respectively) with relative EC50 values of 1.0 and 0.62 mg/ml, respectively (Fig. 2).
Figure 2.
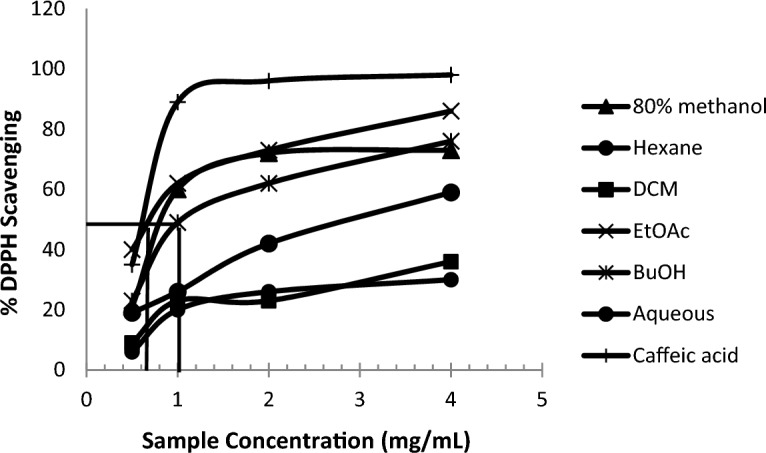
Concentration–response curves for the DPPH radical scavenging activity of Caffeic acid (positive control), different fractions of C.colocynthis.
HPLC/ESI/MS/MS annotations of the main compounds of C.colocynthis ethyl acetate fruit extract
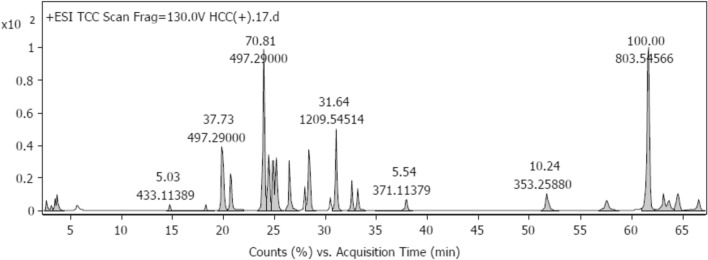
In the current study, the chemical composition of the EtOAc extract was analyzed utilizing the positive ionization mode of the LC–ESI–QTOF–MS/MS method. By comparing the fragmentation pattern in the positive mode (Fig. 3) against the spctra of the reference compounds and literature, a total of 24 molecules shown in Table 2 were tentatively identified. The extraction solvent greatly impacts the chemistry of the materials under study. The limit of detection for each peak of chemicals was calculated using the Rt and whole MS spectra.
Figure 3.
HPLC/MS/MS base peak chromatogram of C.colocynthis fruits in positive ionization mode.
Table 2.
Metabolites tentatively identified from C.colocynthis (L.) fruits ethyl acetate extract via positive mode HPLC-ESI-QTOF-MS/MS.
| No. | Tentative identification | Molecular formula | Rt | Ionization ESI(+ /−) | Molecular weight | Theoretical (m/z) [M − H]+ | Observed (m/z) | MS/MS production |
|---|---|---|---|---|---|---|---|---|
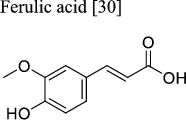
| 1 |
Ferulic acid30
|
C10H10O4 | 0.11 | [M − H]+ | 194.057 | 195.065 | 194.9014 | 145.0324, 149.0614, 177.0544, 195.065 |
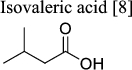
| 2 |
Isovaleric acid8
|
C5H10O2 | 0.2 | [M − H]+ | 102.068 | 103.076 | 103.937 | 58.0646, 61.0393, 103.076 |
| 3 |
1,4-Dideoxy-1,4-imino-d arabinitol8
|
C5H11NO3 | 0.7 | [M − H]+ | 133.073 | 134.081 | 134.942 | 58.968, 84.962, 16.932 |
| 4 |
4-Hydroxy-l-threonine8
|
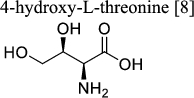
C4H9NO4 | 0.8 | [M − H]+ | 135.053 | 136.061 | 136.22 | 58.968, 71.242, 123.43 |
| 5 |
Lysine31
|
C6H14N2O2 | 1.86 | [M − H]+ | 146.1055 | 147.9543 | 147.1133 | 84.9573, 96.9986, 125.9832 |
| 6 |
Syringic acid30
|
C9H10O5 | 2.74 | [M − H]+ | 198.893 | 199.06 | 198.893 | 83.983, 138.05, 166.961, 182 |
| 7 |
Chrysin30
|
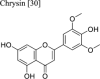
C15H10O4 | 3.15 | [M − H]+ | 254.058 | 255.066 | 255.971 | 143.0493, 209.0600, 253.0493 |
| 8 |
l-Carnitine8
|
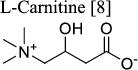
C7H16NO3 | 4.35 | [M − H]+ | 162.113 | 163.121 | 162.937 | 58.968, 84.957, 115.932 |
| 9 |
Cinnamic acid32
|
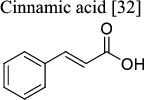
C9H8O2 | 5.14 | [M − H]+ | 148.052 | 149.06 | 149.035 | 56.964, 84.957, 125.983 |
| 10 |
Vanillic acid30
|
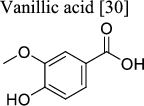
C8H8O4 | 7.06 | [M − H]+ | 168.04 | 169.05 | 169.08 | 64.017, 127.07, 143.99 |
| 11 |
Protocatechuic acid 4-glucoside30
|
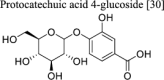
C13H16O9 | 8.38 | [M − H]+ | 316.079 | 317.087 | 317.68 | 61.01, 81.52, 113.0 |
| 12 |
Ascorbic acid30
|
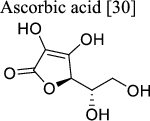
C6H8O6 | 8.77 | [M − H]+ | 176.032 | 177.04 | 177.075 | 64.02, 84.96, 134.96 |
| 13 |
Pantothenic acid8
|
C9H17NO5 | 10.2 | [M − H]+ | 219.11 | 220.12 | 220.959 | 66.02, 110.01, 134.99 |
| 14 |
Apigenin30
|
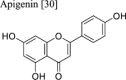
C15H10O5 | 11.51 | [M − H]+ | 270.05 | 271.06 | 271.86 | 55.98, 70.01, 116.93 |
| 15 |
Caffeic acid32
|
C9H8O4 | 11.93 | [M − H]+ | 180.042 | 181.05 | 182 | 84.96, 123.03, 148.07 |
| 16 |
Lariciresinol-glucopyranoside33
|
C26H34O11 | 12.52 | [M − H]+ | 522.202 | 523.21 | 523.981 | 82.026, 138.008, 186.99 |
| 17 |
|
C16H32O2 | 13.55 | [M − H]+ | 256.24 | 257.25 | 257.08 | 60.043, 143.99, 184.92 |
| 18 |
Cucurbita-5(10),6,23-triene-3β,25-diol
|
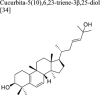
C30H48O2 | 16.14 | [M − H]+ | 440.36 | 441.37 | 442.13 | 70.01, 299.80, 392.86 |
| 19 |
Apigenin-7-rutinoside33
|
C27H30O14 | 17.16 | [M − H]+ | 578.155 | 579.16 | 579.28 | 55.05, 176.99, 318.89 |
| 20 |
Kaempferol 3-O-(6”-O-acetyl) glycoside
|
C23H22O12 | 24.41 | [M − H]+ | 490.103 | 491.111 | 491.1615 | 101.05, 181.06, 245.09 |
| 21 |
Myricetin-3-O-glucoside30
|
C21H20O13 | 26.17 | [M − H]+ | 480.090 | 481.098 | 481.3263 | 84.96, 143.99, 239.09, 317.179 |
| 22 |
Chicoric acid30
|
C22H18O12 | 27.05 | [M − H]+ | 474.079 | 475.087 | 475.305 | 445.18, 453.20, 473.20 |
| 23 |
m-Coumaric acid33
|
C9H8O3 | 34.59 | [M − H]+ | 164.047 | 165.055 | 165.0688 | 56.99, 84.96, 109.06 |
Data acquisition and analysis were performed using Agilent LC–ESI–QTOF/MS Mass Hunter workstation software (Qualitative Analysis, version B.06.00, Agilent 2012) and Mass Bank Data Base.
Molecular docking
EGFR was previously reported to be over-expressed in pancreatic cancer, representing an attractive therapeutic target17. Accordingly, we attempted to check the binding mode of the major compound of C. colocynthis extract to EGFR. The structure of EGFR complexed with the N8-phenyl-9H-purine-2,8-diamine reversible inhibitor, SKLB(3) (PDB code: 5X2A)18, was used to check the binding mode of Cucurbita-5(10),6,23-triene-3β,25-diol. To check the reliability of the resultant docked poses of the docking protocol, the co-crystallized ligand, SKLB (3), was docked into the active site of EGFR. Overlay of the docked pose and the experimentally determined position showed a similar binding mode with a root mean square deviation (RMSD) value of 0.78 Å (Fig. 4A). SKLB (3) occupies the ATP-binding cleft of EGFR, which is mainly lined by hydrophobic residues, with both ends of the aperture lined by polar residues. SKLB (3) completely occupied the aperture, forming several hydrophobic interactions and a few direct and water-mediated H-bonds. Substituents on the N-9 position of purine are sandwiched between Leu718, Val726, and Leu844 sidechains (Fig. 4A). These three residues form what has been previously reported as a "hydrophobic clamp," playing a crucial role in the EGFR binding of reversible inhibitors18.
Figure 4.
The ATP binding site of EGFR (PDB: 5X2A) (green, cartoon). (A) Overlay of crystallized coordinates (grey, sticks) and docked pose (magenta, sticks) of SKLB(3) with a ChemPLP score of 70.94. (B) Binding mode of Cucurbita-5(10),6,23-triene-3β,25-diol (magenta, sticks) having a ChemPLP score of 63.31.
The binding orientation of Cucurbita-5(10),6,23-triene-3β,25-diol is similar to SKLB (3). Its hydrophobic cyclopentaphenanthrene ring, representing the core scaffold of the compound, is sandwiched between the three "hydrophobic clamp" residues (Fig. 4B). The twist in the binding pose of the cyclopentaphenanthrene scaffold makes the cyclopentyl ring mimic the N-9 substituent of SKLB (3), which is believed to be crucial for EGFR tight binding18. Additionally, it is anchored from each end with a H-bond interaction between (1) the hydroxyl group of the heptenyl chain and the backbone carbonyl of Pro794 from one end and (2) the 3-hydroxyl group of the cyclopentaphenanthrene ring and the sidechain of Met790 from another end.
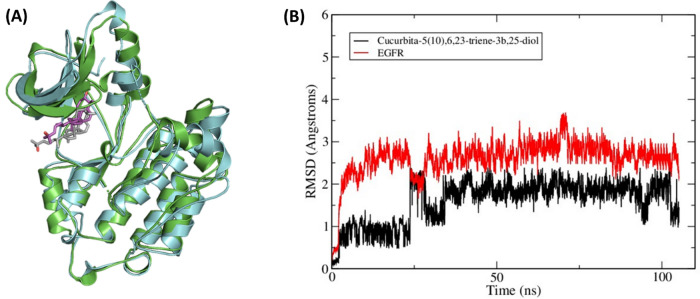
To confirm the stable binding of Cucurbita-5(10),6,23-triene-3β,25-diol to EGFR, a 100-ns MD simulation for the complex was carried out using AMBER software package25. The resultant trajectory was visually examined which showed the retention of Cucurbita-5(10),6,23-triene-3β,25-diol within the bidning site of EGFR (Fig. 5A). The initial docking binding mode was confirmed by RMSD analysis of the ligand non-hydrogen atoms which showed little deviation from the initial geometry with a plateau observed at 2 Å after 40 ns (Fig. 5B). Similarly, EGFR maintained its conformation with little deviations observed in the RMSD of the protein’s backbone atoms, with a plateau observed at 2.5 Å after 40 ns. This stable binding mode suggests that the observed anti-pancreatic cancer activity of C. colocynthis extract is attributed to EGFR inhibition by Cucurbita-5(10),6,23-triene-3β,25-diol.
Figure 5.
(A) Overlay of the initial and a representative frame from the MD simulation for Cucurbita-5(10),6,23-triene-3β,25-diol bound to EGFR. The initial EGFR coordinates are shown as green cartoon with Cucurbita-5(10),6,23-triene-3β,25-diol as grey sticks while the representative MD frame is shown as cyan cartoon with Cucurbita-5(10),6,23-triene-3β,25-diol as magenta sticks (B). Plot of root-mean-square deviations (RMSD) of the entire trajectory with frames sampled every 20 ps. The plot depicts RMSD values based on EGFR backbone atoms (red) and ligand heavy atoms (black) between the trajectory frames and the starting geometry.
Discussion
Cancer is regarded as one of the most significant public health issues, regardless of the economic standards1,2. Medicinal plants are now regarded as a natural source of anticancer medicines due to their antioxidant and anti-mutagenic activities along their low side effects, low cost, and ease of availability. Accordingly, medicinal plants are viewed as an attaractive option for cancer treatment4.
This study focused on evaluating the cytotoxic effect of C.colocynthis on pancreatic and skin cancer cells. The EtOAc extract of C. colocynthis demonstrated significantly favorable effects on cancer cells (PaCa-2 and A-431) while having little to no negative effects on healthy (normal) cells. This cytotoxic selectivity represents a major advantage for the EtOAc extract of C. colocynthis over other chemotherapeutic agents that lack this selectivity and kill both cancer and normal cells. Our results appear in agreement with previous studies that showed C. colocynthis plant extracts to have cytotoxic effects against some cancer cells1,2. Perven et al.12 reported that the ethanolic fruit extract had cytotoxic effects on the HEp-2 and L929 cancer cell lines in Wistar mice. Alkaloids from fruit extracts had cytotoxic effects against human breast cancer cell lines (MCF-7, HepG-2)11. The leaf extracts showed cytotoxic effects and induced apoptosis on the ER-MDAMB-231 and MCF7 cell lines which were used to model human breast cancer8.
Chemical analysis of the EtOAc extract of C. colocynthis was conducted by comparing the fragmentation patterns in the positive mode to reference compounds' spectra available in literature. Accordingly, a total of 24 compounds were identified with Cucurbita-5(10),6,23-triene-3,25-diol, a derivative of cucurbitacin (a highly oxygenated tetracyclic triterpene) identified as a major component in the extract. Molecular docking experiments showed Cucurbita-5(10), 6,23-triene-3,25-diol to bind effectively in the binding site of EGFR with a 100-ns MD simulation further proving the stability of this complex. The modelling studies strongly suggest Cucurbita-5(10), 6,23-triene-3,25-diol binding to EGFR to be responsible for the observed anticancer activity of the EtOAc extract of C. colocynthis. Our results align with previous studies reporting the role of Cucurbita-5(10),6,23-triene-3,25-diol in cancer treatment34,35.
In conclusion, the results show a selective cytotoxic effect of the the EtOAc extract of C. colocynthis towards pancreatic and skin cancer with Cucurbita-5(10), 6,23-triene-3,25-diol being a major component contributing to this observed effect. This suggests C. colocynthis to be a cheap and safe therapeutic to be used in pancreatic and skin cancers.
Author contributions
H.D.H.: design of the work, data analysis, interpretation of data, writing, editing, and revision of the manuscript draft and the corresponding author. E.R.: acquisition, data analysis, and revision of the manuscript draft. Y.M.M.: conducted and analyzed the molecular docking part, writing, editing, and revision of the manuscript. All authors have approved the submitted version. All authors read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
The data and materials are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shokrzadeh M, Azadbakht M, Ahangar N, Hashemi A, Saravi SS. Cytotoxicity of hydro-alcoholic extracts of Cucurbita pepo and Solanum nigrum on HepG2 and CT26 cancer cell lines. Pharmacogn. Mag. 2010;6(23):176. doi: 10.4103/0973-1296.66931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tagne RS, Telefo BP, Nyemb JN, Yemele DM, Njina SN, Goka SM, Lienou LL, NwaboKamdje AH, Moundipa PF, Farooq AD. Anticancer and antioxidant activities of methanol extracts and fractions of some Cameroonian medicinal plants. Asian Pac. J. Trop. Med. 2014;7S1:442–447. doi: 10.1016/S1995-7645(14)60272-8. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 4.Mazher M, Ishtiaq M, Mushtaq W, Maqbool M, Zahid N, Husain T, et al. Comprehensive review of phytochemistry and bioactivities of Citrullus colocynthis (L.) Schrad. Pharm. Res. 2020;4(4):000218. [Google Scholar]
- 5.Khan T, Ali M, Khan A, Nisar P, Jan SA, Afridi S, Shinwari ZK. Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules. 2019;10(1):47. doi: 10.3390/biom10010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mabberley DJ. Mabberley's Plant-Book: A Portable Dictionary of Plants, Their Classification and Uses. 3. Cambridge University Press; 2008. [Google Scholar]
- 7.Attar UA, Ghane SG. Optimized extraction of anticancer compound-cucurbitacin I and LC–MS identification of major metabolites from wild Bottle gourd (Lagenaria siceraria (Molina) Standl.) S. Afr. J. Bot. 2018;119:181–187. doi: 10.1016/j.sajb.2018.09.006. [DOI] [Google Scholar]
- 8.Hussain AI, Rathore HA, Sattar MZ, Chatha SA, Sarker SD, Gilani AH. Citrullus colocynthis (L.) Schrad (bitter apple fruit): A review of its phytochemistry, pharmacology, traditional uses and nutritional potential. J. Ethnopharmacol. 2014;155(1):54–66. doi: 10.1016/j.jep.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Khan M, Khan M, Al-Hamoud K, Adil SF, Shaik MR, Alkhathlan HZ. Diversity of Citrullus colocynthis (L.) Schrad seeds extracts: Detailed chemical profiling and evaluation of their medicinal properties. Plants (Basel). 2023;12(3):567. doi: 10.3390/plants12030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egyptian Herbal Monograph. Citrullus colocynthis, 147–163. EDA, Egypt (Herbal Monograph on Wild Medicinal Plants in Egypt (edaegypt.gov.eg)) (2022).
- 11.Mukherjee A, Patil SD. Effects of alkaloid rich extract of Citrullus colocynthis fruits on Artemia salina and human cancerous (MCF-7 AND HEPG-2) cells. J. Pharma Sci. Tech. 2012;1:15–19. [Google Scholar]
- 12.Perveen S, Ashfaq H, Ambreen S, Ashfaq I, Kanwal Z, Tayyeb A. Methanolic extract of Citrullus colocynthis suppresses growth and proliferation of breast cancer cells through regulation of cell cycle. Saudi J. Biol. Sci. 2021;28(1):879–886. doi: 10.1016/j.sjbs.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbas AO, Alaqil AA, Kamel NN, Moustafa ES. Citrullus colocynthis seed ameliorates layer performance and immune response under acute oxidative stress induced by paraquat injection. Animals (Basel). 2022;12(8):945. doi: 10.3390/ani12080945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trammell SAJ, Charles B. Targeted, LCMS-based metabolomics for quantitative measurement of NAD+ metabolites. Comput. Struct. Biotechnol. J. 2013;4:1–9. doi: 10.5936/csbj.201301012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Clarke G, Ting T, Wiart C, Fry J. High correlation of 2,2- diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, ferric reducing activity potential and total phenolics content indicates redundancy in use of all three assays to screen for antioxidant activity of extracts of plants from the Malaysian rainforest. Antioxidants. 2013;2:1–10. doi: 10.3390/antiox2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliveira-Cunha M, Newman WG, Siriwardena AK. Epidermal growth factor receptor in pancreatic cancer. Cancers (Basel). 2011;24:1513–1526. doi: 10.3390/cancers3021513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu S, Zhao P, Yang J, Ma R, Yan X, Yang S. Structural insights into drug development strategy targeting EGFR T790M/C797S. Oncotarget. 2018;9:13652–13665. doi: 10.18632/oncotarget.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molecular Operating Environment (MOE), Version 2019.01. (Chemical Computing Group ULC, 2019).
- 20.Jones G, Willett P, Glen RC. Molecular recognition of receptor sites using a genetic algorithm with a description of desolvation. J. Mol. Biol. 1995;245:43–53. doi: 10.1016/S0022-2836(95)80037-9. [DOI] [PubMed] [Google Scholar]
- 21.Jones G, Willett P, Glen RC, Leach AR, Taylor R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997;267:727–748. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- 22.Mohsen AMY, Mandour YM, Sarukhanyan E, Breitinger U, Villmann C, Banoub MM, et al. J. Nat. Prod. 2016;79:2997–3005. doi: 10.1021/acs.jnatprod.6b00479. [DOI] [PubMed] [Google Scholar]
- 23.El-Zohairy MA, Zlotos DP, Berger MR, Adwan HH, Mandour YM. Discovery of novel CCR5 ligands as anticolorectal cancer agents by sequential virtual screening. ACS Omega. 2021;6:10921–10935. doi: 10.1021/acsomega.1c00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The PyMOL Molecular Graphics System, Version 2.0 (Schrödinger, LLC, 2015).
- 25.Case DA, Aktulga HM, Belfon K, Ben-Shalom IY, Brozell SR, Cerutti DS, Cheatham TE, III, Cisneros GA, Cruzeiro VWD, Darden TA, Duke RE, Giambasu G, Gilson MK, Gohlke H, Goetz AW, Harris R, Izadi S, Izmailov SA, Jin C, Kasavajhala K, Kaymak MC, King E, Kovalenko A, Kurtzman T, Lee TS, LeGrand S, Li P, Lin C, Liu J, Luchko T, Luo R, Machado M, Man V, Manathunga M, Merz KM, Miao Y, Mikhailovskii O, Monard G, Nguyen H, O’Hearn KA, Onufriev A, Pan F, Pantano S, Qi R, Rahnamoun A, Roe DR, Roitberg A, Sagui C, Schott-Verdugo S, Shen J, Simmerling CL, Skrynnikov NR, Smith J, Swails J, Walker RC, Wang J, Wei H, Wolf RM, Wu X, Xue Y, York DM, Zhao S, Kollman PA. Amber 2021. University of California; 2021. [Google Scholar]
- 26.Mandour YM, Zlotos DP, Alaraby Salem M. A multi-stage virtual screening of FDA-approved drugs reveals potential inhibitors of SARS-CoV-2 main protease. J. Biomol. Struct. Dyn. 2022;40:2327–2338. doi: 10.1080/07391102.2020.1837680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roe DR, Cheatham TE. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013;9:3084–3095. doi: 10.1021/ct400341p. [DOI] [PubMed] [Google Scholar]
- 28.Turner, P. J. XMGRACE (Version 5.1.19). (Center for Coastal and Land-Margin Research, Oregon Graduate Institute of Science and Technology, 2005).
- 29.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 30.Meriem B, Boulanouar B, Abdelaziz G, Angel G, Raúl D, Mosad AG. Fatty acid and amino acid composition of citrullus colocynthis seeds growing in Algeria. Egypt. J. Chem. 2021;64(8):4727–4737. [Google Scholar]
- 31.Darwish RS, Abdulmunem OA, Khairy A, Ghareeb DA, Yassin AM, Abdulmalek SA, et al. Comparative metabolomics reveals the cytotoxic and anti-inflammatory discriminatory chemical markers of raw and roasted colocynth fruit (Citrullus colocynthis L.) RSC Adv. 2021;11:37049–37062. doi: 10.1039/D1RA07751A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abu-Reidah IM, Arráez-Román D, Quirantes-Piné R, Fernández-Arroyo S, Segura-Carretero A, Fernández-Gutiérrez A. HPLC–ESI-Q–TOF–MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract. Food Res. Int. 2012;46:108–117. doi: 10.1016/j.foodres.2011.11.026. [DOI] [Google Scholar]
- 33.Ul Haq F, Ali A, Khan MN, Shah SMZ, Kandel RC, Aziz N, Adhikari A, Choudhary MI, Ur-Rahman A, El-Seedi HR, Musharraf SG. Metabolite profiling and quantitation of cucurbitacins in Cucurbitaceae plants by liquid chromatography coupled to tandem mass spectrometry. Sci. Rep. 2019;9:15992. doi: 10.1038/s41598-019-52404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alghasham AA. Cucurbitacins: A promising target for cancer therapy. Int. J. Health Sci. 2013;7(1):77–89. doi: 10.12816/0006025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasir M, Sultana B, Nigam PS, Owusu-Apenten R. Antioxidant and genoprotective activity of selected cucurbitaceae seed extracts and LC–ESIMS/MS identification of phenolic components. Food Chem. 2016;199:307–313. doi: 10.1016/j.foodchem.2015.11.138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials are available from the corresponding author upon request.