Abstract
Background
Both measured and predicted effective orifice area (EOA) indexed to the body surface area (EOAi) have been suggested to define prosthesis-patient mismatch (PPM) in patients undergoing transcatheter aortic valve replacement (TAVR). The impact of PPM on clinical outcomes may accumulate with extended follow-up and vary according to the definition used.
Aims
We aimed to investigate the long-term clinical impact of PPM in patients undergoing TAVR.
Methods
Patients in a prospective TAVR registry were stratified by the presence of moderate (0.65-0.85 or 0.55-0.70 cm2/m2 if obese) or severe (≤0.65 or ≤0.55 cm2/m2 if obese) PPM according to echocardiographically measured EOAi (measured PPM), predicted EOAi based on published EOA reference values for each valve model and size (predicted PPMTHV), or predicted EOAi based on EOA reference values derived from computed tomography measurements of aortic annulus dimensions (predicted PPMCT).
Results
In an analysis of 2,463 patients, the frequency of measured PPM (moderate: 27.0%; severe: 8.7%) was higher than the frequency of predicted PPMTHV (moderate: 11.3%; severe: 1.2%) or predicted PPMCT (moderate: 12.0%; severe: 0.1%). During a median follow-up of 429 days, 10-year mortality was comparable in patients with versus without measured PPM or predicted PPMCT. In contrast, patients with moderate predicted PPMTHV had a lower risk of 10-year all-cause mortality compared with those without PPM (adjusted hazard ratio: 0.73, 95% confidence interval: 0.55-0.96).
Conclusions
The use of predicted versus measured EOAi results in a lower estimate of PPM severity. We observed no increased risk of death in patients with PPM over a median follow-up time of 429 days. ClinicalTrials.gov: NCT01368250.
Introduction
Prosthesis-patient mismatch (PPM) is a condition in which the effective orifice area (EOA) of a normally functioning prosthesis is too small relative to the patient’s body surface area (BSA), resulting in a high residual transprosthetic pressure gradient. In surgical series, PPM has been associated with adverse clinical outcomes after surgical aortic valve replacement (SAVR)1,2,3. However, the impact of PPM in the transcatheter aortic valve replacement (TAVR) population remains controversial because of differences in the methods used to ascertain PPM4.
In SAVR series, PPM has been based on predicted EOA, calculated by dividing the EOA reference value, indicated by the manufacturer for the prosthesis model and size, by the BSA. In contrast, previous TAVR studies have mainly defined PPM using EOA measured directly by transthoracic echocardiography (measured PPM)5,6,7,8. Recent TAVR studies have suggested that the use of measured EOA overestimates the frequency of PPM compared to the use of predicted EOA and that predicted EOA more accurately determines the frequency of PPM and may, hence, be more useful to assess the impact on outcomes9,10,11. Available evidence is, however, limited in terms of duration of follow-up and the spectrum of transcatheter heart valve (THV) types included. Therefore, the present study aimed to systematically evaluate the long-term clinical outcomes of patients stratified according to PPM, assessed by measured and predicted EOA in a prospective TAVR registry including different valve types and generations.
Methods
Study design and population
The Bern TAVI Registry is a prospective TAVR registry enrolling consecutive patients undergoing TAVR for severe, symptomatic aortic stenosis at Bern University Hospital, Switzerland, which forms part of the nationwide SwissTAVI Registry (ClinicalTrials.gov: NCT01368250)12. The present analysis included patients who underwent TAVR with balloon-expandable (SAPIEN XT, SAPIEN 3, SAPIEN 3 Ultra [Edwards Lifesciences]) or self-expanding devices (CoreValve, Evolut R/PRO/PRO Plus [Medtronic]) between August 2007 and June 2022. For the purpose of the present study, patients who underwent intervention but had no device implanted, those who were treated with non-study devices, and patients with incomplete information for the assessment of PPM (body mass index [BMI], BSA, or measured EOA at discharge) were excluded. The registry was approved by the Bern Cantonal Ethics Committee, and patients provided written informed consent to participate.
Definition of PPM
PPM was classified on the basis of EOA indexed to BSA (EOAi) as none (EOAi>0.85 cm2/m2), moderate (EOAi>0.65 and ≤0.85 cm2/m2) or severe (EOAi ≤0.65 cm2/m2) in the non-obese population, and as none (EOAi>0.70 cm2/m2), moderate (EOAi>0.55 and ≤0.70 cm2/m2), or severe (EOAi ≤0.55 cm2/m2) in the obese population (BMI ≥30 kg/m2)13.
Assessment of EOA
Comprehensive transthoracic echocardiography using a Philips iE33 machine (Philips Healthcare) was performed by a board-certified cardiologist and echocardiography specialist before TAVR and prior to discharge. Measured EOA was calculated using the continuity equation indexed to BSA14,15; the EOA was calculated using the left ventricular stroke volume, derived as the outer-to-outer border of the stented valve, multiplied by the pulsed-wave Doppler time-velocity integral of flow at that location. Predicted EOA was assessed using two different derived methods. The first method was based on the reference values of EOA indicated by the published data for each size and type of implanted THV (predicted EOATHV) (Supplementary Table 1). The second method was used in patients who underwent TAVR with SAPIEN 3/3 Ultra, CoreValve, or Evolut R/PRO/PRO Plus and was based on the reference values of EOA derived from aortic annulus dimensions measured by preprocedural computed tomography (CT; predicted EOACT) (Supplementary Table 2). The reference values for predicted EOA were derived from published data, which were calculated using data from pooled cohorts of the randomised clinical trials9,10,11. Preprocedural CT examinations were independently re-evaluated by dedicated imaging specialists, and the measurements were integrated into the database16.
Data collection and clinical endpoints
All baseline clinical, procedural, and follow-up data were prospectively recorded in a dedicated database, held at the Clinical Trials Unit at the University of Bern, Switzerland. In the SwissTAVI Registry, regular follow-up is standardised at 30 days, 1 year, 5 years, and 10 years17. A clinical events committee independently adjudicated all adverse events according to the Valve Academic Research Consortium (VARC) definitions18,19. An independent clinical trials unit is responsible for central data monitoring to verify the completeness and accuracy of the data and for statistical analysis. The outcomes of interest in the present study included all-cause and cardiovascular mortality, structural valve deterioration, and unplanned repeat aortic valve intervention at 1, 5, and 10 years after TAVR. Structural valve deterioration was defined according to the VARC criteria between 2007 and 2013 and has since been defined according to the VARC-2 criteria18,19. Unplanned repeat aortic valve intervention was defined as a composite of valve-in-valve procedure, balloon valvuloplasty, surgical revision, or paravalvular leak closure.
Statistical analysis
Categorical variables are presented as frequencies and percentages, and the differences between groups were evaluated with the chi-square test or Fisher’s exact test. Continuous variables are presented as mean values±standard deviation (SD) and compared between groups using an F test from an analysis of variance (ANOVA). Risk ratios with 95% confidence intervals (95% CI) from Poisson regressions were provided where appropriate. Time-to-event curves were constructed using the Kaplan-Meier method. Univariable and multivariable Cox proportional hazards models were used to calculate hazard ratios (HR) and 95% CIs for the clinical outcomes. Multivariable adjustment was performed with predefined baseline variables potentially related to clinical outcomes, including age, sex, and the Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM). It was anticipated that traditional Cox proportional hazards models may overestimate event rates when competing with death in this elderly population with relevant comorbidities. To account for this limitation, the Fine and Gray method was used to model the cumulative incidence function of the outcomes of interest in the present study and to determine the subdistribution HR (sHR) under competing risk of death or, in the case of cardiovascular death, under competing risk of non-cardiovascular death20,21. All statistical tests were 2-sided and p-values<0.05 were considered significant. Statistical analyses were performed using Stata v17 (StataCorp).
Results
Study population and frequency of PPM
Among 3,586 consecutive patients enrolled into the prospective Bern TAVI Registry, 2,463 patients were available for the analysis of measured PPM and predicted PPM defined by predicted EOATHV (predicted PPMTHV); 1,570 patients were available for the analysis of predicted PPM based on predicted EOACT (predicted PPMCT) (Supplementary Figure 1). The frequency of moderate and severe PPM according to measured EOAi was 27.0% and 8.7%, respectively. According to predicted EOATHV, the frequency of moderate and severe PPMTHV was 11.3% and 1.2%, respectively. Using predicted EOACT, the frequency of moderate and severe predicted PPMCT was 12.0% and 0.1%, respectively (Table 1, Central illustration). The frequency of moderate and severe PPM was lower in the obese population compared with the non-obese population regardless of the method of assessment, with the exception of severe measured PPM, which was comparable between the groups (8.9% vs 8.7%; p=0.865) (Table 1). Supplementary Table 3 shows the frequency of PPM according to THV type. Balloon-expandable valves had a higher frequency of moderate or severe PPM, whereas severe predicted PPMTHV was observed only in self-expanding valves.
Table 1. Frequency of PPM according to the method of the definition of PPM.
| All patients (N=2,463) | Patients with BMI <30 kg/m2 (N=1,902) | Patients with BMI ≥30 kg/m2 (N=561) | p-value | |
|---|---|---|---|---|
| Measured PPM | ||||
| Measured EOAi, cm2/m2 | 0.95±0.29 | 0.98±0.29 | 0.82±0.24 | <0.001 |
| Moderate or severe measured PPM | 879 (35.7) | 699 (36.8) | 180 (32.1) | 0.045 |
| Moderate measured PPM | 664 (27.0) | 534 (28.1) | 130 (23.2) | 0.023 |
| Severe measured PPM | 215 (8.7) | 165 (8.7) | 50 (8.9) | 0.865 |
| Predicted PPMTHV | ||||
| Predicted EOAiTHV, cm2/m2 | 0.96±0.15 | 0.99±0.15 | 0.84±0.11 | <0.001 |
| Moderate or severe predicted PPMTHV | 308 (12.5) | 262 (13.8) | 46 (8.2) | <0.001 |
| Moderate predicted PPMTHV | 279 (11.3) | 235 (12.4) | 44 (7.8) | 0.003 |
| Severe predicted PPMTHV | 29 (1.2) | 27 (1.4) | 2 (0.4) | 0.043 |
| Predicted PPMCT | N=1,570 | N=1,220 | N=350 | |
| Predicted EOAiCT, cm2/m2 | 0.97±0.15 | 1.0±0.15 | 0.84±0.11 | <0.001 |
| Moderate or severe predicted PPMCT | 190 (12.1) | 160 (13.1) | 30 (8.6) | 0.020 |
| Moderate predicted PPMCT | 189 (12.0) | 159 (13.0) | 30 (8.6) | 0.025 |
| Severe predicted PPMCT | 1 (0.1) | 1 (0.1) | 0 (0.0) | 1.000 |
| Values are mean±standard deviation or n (%). BMI: body mass index; EOAi: effective orifice area indexed to body surface area; EOAiCT: predicted EOA based on the normal reference values of EOA derived from aortic annulus dimensions measured by preprocedural computed tomography; EOATHV: predicted EOA based on the normal reference values of EOA for each size and type of implanted transcatheter heart valve; PPM: prosthesis-patient mismatch; PPMCT: PPM defined by predicted EOA derived from preprocedural computed tomography; PPMTHV: PPM defined by predicted EOA for each size and model of implanted transcatheter heart valve | ||||
Central illustration. Measured and predicted PPM after TAVR.
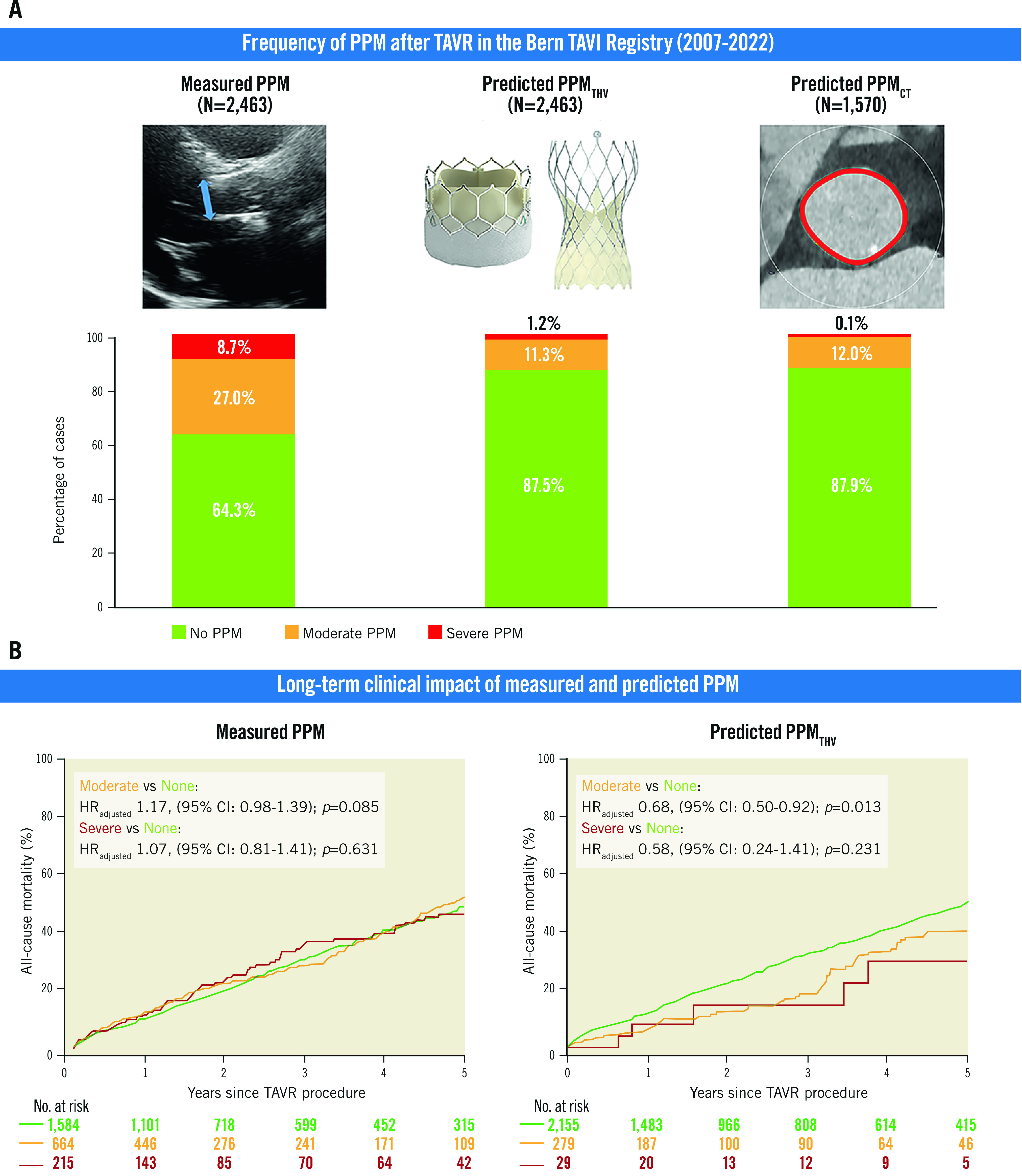
Frequency of PPM according to type and severity after TAVR (A), and cumulative event curves for all-cause mortality according to type and severity of PPM (B). Hazard ratios and p-values were calculated with the use of Cox proportional hazards models. CI: confidence interval; EOA: effective orifice area; HRadjusted: adjusted hazard ratio; PPM: prosthesis-patient mismatch; PPMCT: PPM defined by the normal reference values of EOA derived from aortic annulus area/perimeter measured by preprocedural computed tomography; PPMTHV: PPM defined by the normal reference values of EOA for each size and model of implanted transcatheter heart valve; TAVR: transcatheter aortic valve replacement; THV: transcatheter heart valve
Baseline and procedural characteristics according to measured PPM and predicted PPMTHV are shown in Supplementary Table 4. Overall, 1,154 patients (46.9%) were female, the mean age of the cohort was 82±6 years, and the STS-PROM was 5.0±3.9%; 22.8% of patients were obese. TAVR was performed by transfemoral access in 93.6% of patients, and the distribution of balloon-expandable and self-expanding devices was 63.0% and 37.0%, respectively. Valve-in-valve procedures were performed in 129 patients (5.2%). Patients with moderate or severe measured PPM more commonly had balloon-expandable valves than those without PPM (67.0% vs 71.5% vs 58.8%; p<0.001). In contrast, all patients with severe predicted PPMTHV had self-expanding valves with a small valve size (≤23 mm).
Postprocedural haemodynamics
Postprocedural haemodynamics in patients with measured PPM and predicted PPMTHV are summarised in Supplementary Table 5. Patients with moderate or severe PPM, defined by either method, had higher mean prosthetic gradients (13.4±6.4 mmHg vs 11.5±4.4 mmHg vs 9.1±4.0 mmHg in measured PPM and 13.6±6.7 mmHg vs 13.4±5.4 mmHg vs 9.7±4.3 mmHg in predicted PPMTHV; both p-values<0.001) and a higher prevalence of high residual gradient (mean prosthesis gradient ≥20 mmHg: 14.6% vs 5.2% vs 1.1% in measured PPM and 10.3% vs 12.0% vs 2.1% in predicted PPMTHV; both p-values<0.001). Measured EOAi was lower in patients with severe or moderate predicted PPMTHV compared to those with no predicted PPMTHV (0.75±0.19 cm2/m2 vs 0.82±0.29 cm2/m2 vs 0.96±0.26 cm2/m2; p<0.001). There was no significant difference in the rate of moderate or severe paravalvular regurgitation. Although the stroke volume index and left ventricular ejection fraction were lower in patients with moderate and severe measured PPM compared with those with no PPM (29.8±10.8 mL/m2 vs 32.3±8.3 mL/m2 vs 40.0±11.7 mL/m2; p<0.001 and 52.4±15.8% vs 54.1±13.5% vs 57.1±12.9%; p<0.001, respectively), these differences were not observed in the analysis of predicted PPMTHV.
Clinical outcomes
Clinical outcomes at 1, 5, and 10 years according to PPM by any definition are summarised in Table 2, Table 3, Table 4, and Supplementary Table 6-Supplementary Table 8. During follow-up, 864 patients died. The median follow-up time for the overall population was 429 (interquartile range 363-1,724) days.
Table 2. Clinical outcomes according to the method for the definition of PPM.
| Measured PPM | ||||||||
|---|---|---|---|---|---|---|---|---|
| None | Moderate | Severe | Moderate vs none | Severe vs none | ||||
| (N=1,584) | (N=664) | (N=215) | Adjusted HR (95% CI) | p-value | Adjusted HR (95% CI) | p-value | ||
| At 1 year | All-cause death | 154 (10.5) | 76 (12.2) | 24 (11.8) | 1.20 (0.91-1.58) | 0.198 | 1.16 (0.75-1.78) | 0.509 |
| Cardiovascular death | 94 (6.5) | 51 (8.4) | 16 (8.1) | 1.33 (0.94-1.87) | 0.106 | 1.29 (0.76-2.19) | 0.352 | |
| Structural valve deterioration | 23 (1.7) | 10 (1.7) | 5 (2.5) | 1.02 (0.49-2.15) | 0.956 | 1.54 (0.58-4.05) | 0.384 | |
| Repeat aortic valve intervention | 11 (0.7) | 5 (0.8) | 4 (1.9) | 1.07 (0.37-3.08) | 0.90 | 2.52 (0.80-7.94) | 0.114 | |
| At 5 years | All-cause death | 413 (52.8) | 178 (58.3) | 58 (51.5) | 1.17 (0.98-1.39) | 0.085 | 1.07 (0.81-1.41) | 0.631 |
| Cardiovascular death | 275 (40.0) | 128 (46.9) | 40 (39.7) | 1.26 (1.02-1.56) | 0.029 | 1.13 (0.81-1.58) | 0.473 | |
| Structural valve deterioration | 30 (4.6) | 15 (6.2) | 9 (11.4) | 1.37 (0.74-2.55) | 0.319 | 1.92 (0.91-4.05) | 0.088 | |
| Repeat aortic valve intervention | 15 (2.3) | 6 (2.5) | 5 (5.8) | 1.12 (0.43-2.89) | 0.818 | 2.11 (0.76-5.83) | 0.149 | |
| At 10 years | All-cause death | 493 (84.3) | 204 (89.7) | 67 (94.6) | 1.17 (1.00-1.38) | 0.055 | 1.19 (0.92-1.54) | 0.187 |
| Cardiovascular death | 344 (77.2) | 148 (82.7) | 48 (92.7) | 1.25 (1.03-1.52) | 0.023 | 1.26 (0.93-1.71) | 0.131 | |
| Structural valve deterioration | 34 (8.2) | 16 (8.3) | 9 (9.3) | 1.17 (0.64-2.12) | 0.614 | 1.89 (0.90-3.96) | 0.091 | |
| Repeat aortic valve intervention | 17 (3.8) | 7 (11.3) | 5 (3.8) | 0.99 (0.41-2.41) | 0.985 | 2.09 (0.77-5.70) | 0.149 | |
| Predicted PPMTHV | ||||||||
| None | Moderate | Severe | Moderate vs none | Severe vs none | ||||
| (N=2,155) | (N=279) | (N=29) | Adjusted HR (95% CI) | p-value | Adjusted HR (95% CI) | p-value | ||
| At 1 year | All-cause death | 237 (11.8) | 15 (5.9) | 2 (7.9) | 0.53 (0.32-0.90) | 0.019 | 0.57 (0.14-2.28) | 0.422 |
| Cardiovascular death | 149 (7.5) | 10 (4.0) | 2 (7.9) | 0.58 (0.30-1.10) | 0.094 | 0.92 (0.23-3.73) | 0.910 | |
| Structural valve deterioration | 33 (1.7) | 5 (2.0) | 0 | 1.13 (0.44-2.91) | 0.800 | - | - | |
| Repeat aortic valve intervention | 15 (0.7) | 5 (1.9) | 0 | 2.34 (0.85-6.46) | 0.101 | - | - | |
| At 5 years | All-cause death | 600 (49.5) | 44 (38.8) | 5 (33.0) | 0.68 (0.50-0.92) | 0.013 | 0.58 (0.24-1.41) | 0.231 |
| Cardiovascular death | 407 (38.0) | 32 (30.6) | 4 (24.6) | 0.72 (0.50-1.03) | 0.073 | 0.68 (0.25-1.81) | 0.438 | |
| Structural valve deterioration | 48 (4.1) | 6 (3.2) | 0 | 1.00 (0.43-2.35) | 0.994 | - | - | |
| Repeat aortic valve intervention | 19 (1.4) | 7 (5.0) | 0 | 2.77 (1.16-6.63) | 0.022 | - | - | |
| At 10 years | All-cause death | 702 (86.8) | 56 (82.0) | 6 (66.5) | 0.73 (0.55-0.96) | 0.022 | 0.54 (0.24-1.22) | 0.139 |
| Cardiovascular death | 494 (80.3) | 41 (72.8) | 5 (62.3) | 0.74 (0.54-1.03) | 0.071 | 0.62 (0.26-1.49) | 0.284 | |
| Structural valve deterioration | 52 (8.5) | 7 (9.6) | 0 | 1.07 (0.48-2.36) | 0.870 | - | - | |
| Repeat aortic valve intervention | 20 (3.6) | 9 (20.6) | 0 | 3.41 (1.55-7.52) | 0.002 | - | - | |
| Data are event counts with Kaplan-Meier failure rates (%) counting only the first event of each type per patient. Patients were censored at last valid contact with events assessed and adjudicated. Adjusted hazard ratios and p-values after adjusting for age, gender and STS-PROM. CI: confidence interval; HR: hazard ratio; PPM: prosthesis-patient mismatch; PPMTHV: prosthesis-patient mismatch defined by the normal reference values of effective orifice area for each size and model of implanted transcatheter heart valve; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; THV: transcatheter heart valve | ||||||||
Table 3. Clinical outcomes according to predicted PPMCT.
| Predicted PPMCT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | Moderate | Severe | Moderate vs none | Severe vs none | ||||||||
| (N=1,380) | (N=189) | (N=1) | Crude HR (95% CI) | p-value |
Adjusted HR (95% CI) |
p-value | Crude HR (95% CI) | p-value |
Adjusted HR (95% CI) |
p-value | ||
| At 1 year | All-cause death | 141 (10.3) | 13 (6.9) | 0 | 0.66 (0.38-1.17) | 0.155 | 0.66 (0.37-1.18) | 0.164 | - | - | - | - |
| Cardiovascular death | 84 (6.3) | 9 (4.9) | 0 | 0.77 (0.39-1.53) | 0.455 | 0.79 (0.39-1.58) | 0.500 | - | - | - | - | |
| Structural valve deterioration | 23 (1.8) | 5 (2.7) | 0 | 1.58 (0.60-4.15) | 0.356 | 1.45 (0.54-3.86) | 0.459 | - | - | - | - | |
| Repeat aortic valve intervention | 14 (1.0) | 2 (1.1) | 0 | 1.03 (0.23-4.53) | 0.968 | 0.97 (0.22-4.36) | 0.968 | - | - | - | - | |
| At 5 years | All-cause death | 334 (44.8) | 30 (48.6) | 0 | 0.90 (0.62-1.31) | 0.584 | 0.89 (0.61-1.29) | 0.528 | - | - | - | - |
| Cardiovascular death | 219 (33.3) | 21 (38.2) | 0 | 0.99 (0.63-1.55) | 0.950 | 1.01 (0.64-1.59) | 0.966 | - | - | - | - | |
| Structural valve deterioration | 27 (2.9) | 7 (8.2) | 0 | 2.08 (0.90-4.79) | 0.086 | 1.91 (0.82-4.44) | 0.134 | - | - | - | - | |
| Repeat aortic valve intervention | 15 (1.4) | 2 (1.1) | 0 | 0.99 (0.23-4.34) | 0.991 | 0.97 (0.22-4.31) | 0.963 | - | - | - | - | |
| At 10 years | All-cause death | 380 (84.5) | 30 (48.6) | 0 | 0.90 (0.62-1.31) | 0.584 | 0.90 (0.61-1.31) | 0.571 | - | - | - | - |
| Cardiovascular death | 259 (78.5) | 21 (38.2) | 0 | 0.99 (0.63-1.55) | 0.950 | 1.02 (0.65-1.60) | 0.945 | - | - | - | - | |
| Structural valve deterioration | 27 (2.9) | 7 (8.2) | 0 | 2.08 (0.90-4.79) | 0.086 | 1.91 (0.82-4.44) | 0.134 | - | - | - | - | |
| Repeat aortic valve intervention | 16 (5.8) | 2 (1.1) | 0 | 0.99 (0.23-4.34) | 0.991 | 0.93 (0.21-4.17) | 0.929 | - | - | - | - | |
| Data are event counts with Kaplan-Meier failure rates (%) counting only the first event of each type per patient. Patients were censored at the last valid contact with events assessed and adjudicated. Adjusted hazard ratios and p-values after adjusting for age, gender and STS-PROM. CI: confidence interval; HR: hazard ratio; PPMCT: prosthesis-patient mismatch defined by the normal reference values of effective orifice area derived from preprocedural computed tomography; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality | ||||||||||||
Table 4. Clinical outcomes according to the method of the definition of PPM competing risk analysis.
| Measured PPM | ||||||||
|---|---|---|---|---|---|---|---|---|
| None | Moderate | Severe | Moderate vs none | Severe vs none | ||||
| (N=1,584) | (N=664) | (N=215) | Adjusted sHR (95% CI) | p-value | Adjusted sHR (95% CI) | p-value | ||
| At 1 year | Cardiovascular death | 94 (6.4) | 51 (8.2) | 16 (7.9) | 1.33 (0.94-1.87) | 0.103 | 1.28 0.75-2.18) | 0.364 |
| Structural valve deterioration | 23 (1.6) | 10 (1.6) | 5 (2.5) | 1.01 (0.48-2.14) | 0.969 | 1.54 (0.58-4.07) | 0.383 | |
| Repeat aortic valve deterioration | 11 (0.7) | 5 (0.8) | 4 (1.9) | 1.07 (0.38-3.06) | 0.897 | 2.52 (0.79-5.99) | 0.148 | |
| At 5 years | Cardiovascular death | 322 (33.5) | 147 (38.0) | 43 (34.2) | 1.18 (0.97-1.44) | 0.089 | 1.08 (0.78-1.49) | 0.648 |
| Structural valve deterioration | 35 (3.0) | 17 (3.6) | 9 (5.8) | 1.16 (0.65-2.07) | 0.626 | 1.80 (0.86-3.77) | 0.119 | |
| Repeat aortic valve deterioration | 16 (1.3) | 8 (1.6) | 5 (3.1) | 1.19 (0.51-2.78) | 0.688 | 2.14 (0.76-5.99) | 0.148 | |
| At 10 years | Cardiovascular death | 402 (64.0) | 174 (69.7) | 55 (68.5) | 1.18 (0.99-1.41) | 0.068 | 1.18 (0.90-1.56) | 0.232 |
| Structural valve deterioration | 40 (4.7) | 19 (5.4) | 9 (7.9) | 1.15 (0.66-1.98) | 0.627 | 1.60 (0.77-3.32) | 0.206 | |
| Repeat aortic valve deterioration | 19 (2.3) | 9 (2.7) | 5 (4.6) | 1.12 (0.51-2.49) | 0.772 | 1.83 (0.67-5.01) | 0.241 | |
| Predicted PPMTHV | ||||||||
| None | Moderate | Severe | Moderate vs none | Severe vs none | ||||
| (N=2,155) | (N=279) | (N=29) | Adjusted sHR (95% CI) | p-value | Adjusted HR (95% CI) | p-value | ||
| At 1 year | Cardiovascular death | 149 (7.4) | 10 (3.9) | 2 (7.4) | 0.58 (0.31-1.11) | 0.099 | 0.96 (0.24-3.85) | 0.956 |
| Structural valve deterioration | 33 (1.6) | 5 (2.0) | 0 | 1.16 (0.44-3.03) | 0.761 | - | - | |
| Repeat aortic valve deterioration | 15 (0.7) | 5 (1.9) | 0 | 2.37 (0.87-6.47) | 0.091 | - | - | |
| At 5 years | Cardiovascular death | 473 (35.7) | 35 (25.9) | 4 (24.6) | 0.71 (0.50-1.00) | 0.050 | 0.61 (0.22-1.68) | 0.344 |
| Structural valve deterioration | 54 (3.4) | 7 (3.9) | 0 | 1.11 (0.50-2.45) | 0.799 | - | - | |
| Repeat aortic valve deterioration | 21 (1.3) | 8 (4.1) | 0 | 2.97 (1.31-6.76) | 0.009 | - | - | |
| At 10 years | Cardiovascular death | 581 (66.9) | 45 (54.0) | 5 (51.9) | 0.72 (0.53-0.97) | 0.030 | 0.62 (0.25-1.52) | 0.294 |
| Structural valve deterioration | 60 (5.1) | 8 (6.1) | 0 | 1.15 (0.55-2.39) | 0.719 | - | - | |
| Repeat aortic valve deterioration | 23 (2.1) | 10 (7.9) | 0 | 3.51 (1.65-7.46) | 0.001 | - | - | |
| For competing risks of death, or in case of cardiovascular death, of non-cardiovascular death, subdistributions of the hazard ratio (sHR) with 95% confidence intervals (95% CI) are reported.. Adjusted hazard ratios and p-values after adjusting for age, gender and STS-PROM. PPM: prosthesis-patient mismatch; PPMTHV: prosthesis-patient mismatch defined by the normal reference values of effective orifice area for each size and model of implanted transcatheter heart valve; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; THV: transcatheter heart valve | ||||||||
Table 2 and Supplementary Table 6 show clinical outcomes according to measured PPM. All-cause death at 1, 5, and 10 years occurred in 10.5%, 52.8% and 84.3% of patients with no measured PPM, in 12.2%, 58.3%, and 89.7% in patients with moderate measured PPM, and in 11.8%, 51.5% and 94.6% of patients with severe measured PPM (Central illustration). There was no significant difference in all-cause mortality between groups. Cardiovascular mortality at 10 years was higher in patients with moderate measured PPM compared with those with no measured PPM (82.7% vs 77.2%; adjusted HR [HRadj] 1.25, 95% CI: 1.03-1.52; p=0.023), while there was no significant difference in cardiovascular mortality between patients with severe and no measured PPM. Rates of structural valve deterioration and repeat aortic valve intervention at 10 years were comparable between groups (Figure 1). The rate of persisting heart failure symptoms (New York Heart Association [NYHA] Class III/IV) is shown in Supplementary Table 7.
Figure 1. Cumulative event curves for clinical outcomes stratified by the method for the definition of PPM.
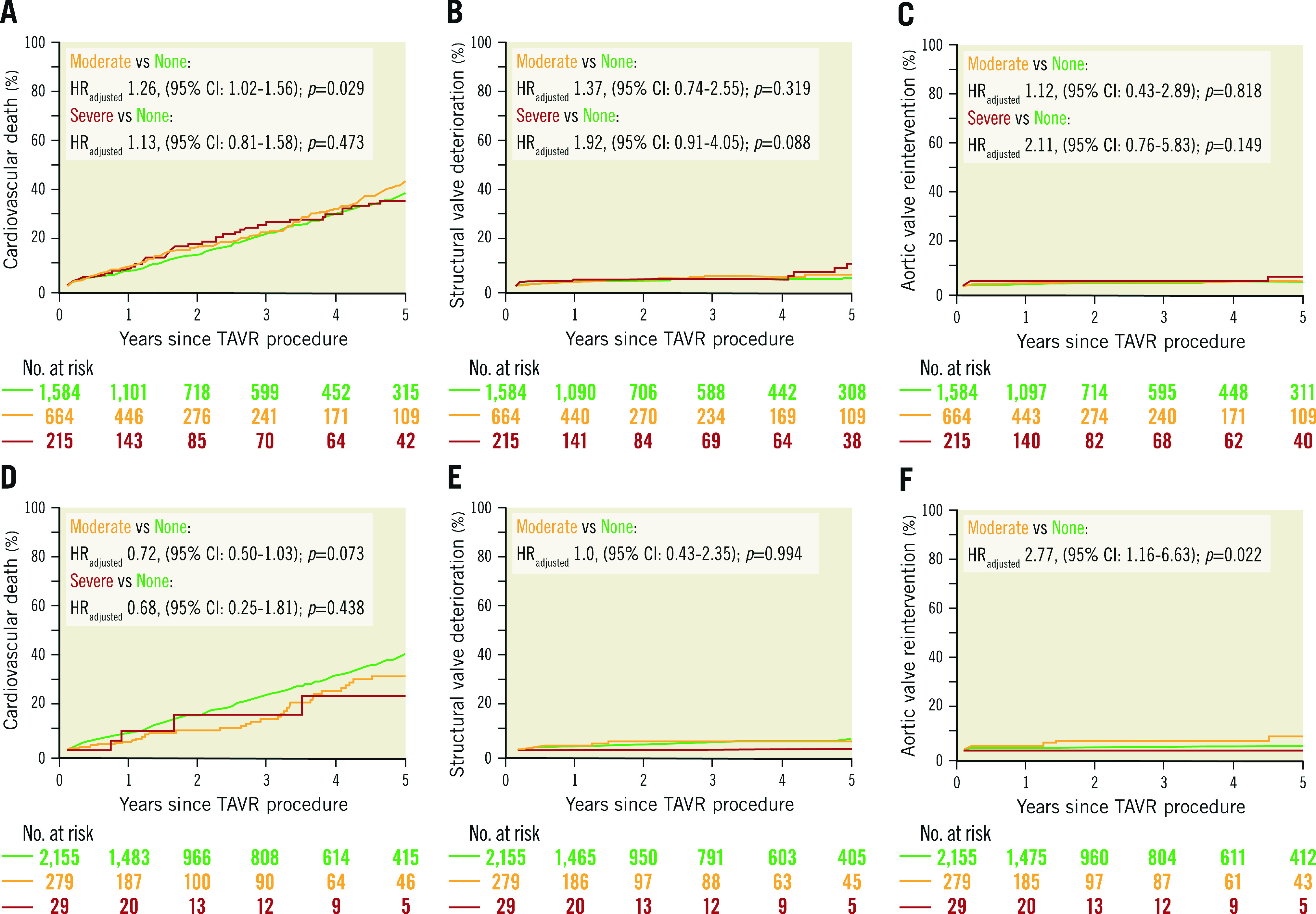
A, B & C) Clinical outcomes according to measured PPM. D, E & F) Clinical outcomes according to predicted PPMTHV. Hazard ratios and p-values were calculated with the use of Cox proportional hazards models. CI: confidence interval; HRadjusted: adjusted hazard ratio; PPM: prosthesis-patient mistmatch; PPMTHV: PPM defined by the normal reference values of EOA for each size and model of implanted transcatheter heart valve; TAVR: transcatheter aortic valve replacement
Clinical outcomes at 1, 5, and 10 years according to predicted PPMTHV are summarised in Table 2 and Supplementary Table 6. All-cause death at 1, 5 and 10 years occurred in 11.8%, 49.5% and 86.8% of patients with no predicted PPMTHV, in 5.9%, 38.8% and 82.0% of patients with moderate predicted PPMTHV, and in 7.9%, 33.0% and 66.5% of patients with severe predicted PPMTHV (Central illustration). Patients with moderate predicted PPMTHV had a lower risk of 10-year all-cause death (HRadj 0.73, 95% CI: 0.55-0.96; p=0.022) compared to patients with no predicted PPMTHV. In contrast, mortality was comparable between patients with severe and no predicted PPMTHV. Moderate predicted PPMTHV was associated with an increased risk of repeat aortic valve intervention at 10 years (HRadj 3.41, 95% CI: 1.55-7.52; p=0.002), while the rates of structural valve deterioration and persisting heart failure symptoms were comparable between groups (Table 2, Supplementary Table 6, Supplementary Table 7, Figure 1).
Table 3 shows the clinical outcomes according to predicted PPMCT. There were no differences in all-cause or cardiovascular mortality between groups.
Competing risk analysis
In the competing risk survival analysis for outcomes, moderate and severe measured PPM were not associated with an increased risk of cardiovascular death (moderate: sHRadj 1.18, 95% CI: 0.99-1.41; p=0.068 and severe: sHRadj 1.18, 95% CI: 0.90-1.56; p=0.232, respectively), structural valve deterioration (moderate: sHRadj 1.15, 95% CI: 0.66-1.98; p=0.627 and severe: sHRadj 1.60, 95% CI: 0.77-3.32; p=0.206, respectively), or repeat aortic valve intervention (moderate: sHRadj 1.12, 95% CI: 0.51-2.49; p=0.772 and severe: sHRadj 1.83, 95% CI: 0.67-5.01; p=0.241, respectively). The clinical impact of moderate predicted PPMTHV was consistent with the main analysis (Table 4, Supplementary Table 8, Figure 2).
Figure 2. Cumulative event curves for clinical outcomes stratified by the method for definition of PPM in a competing analysis.
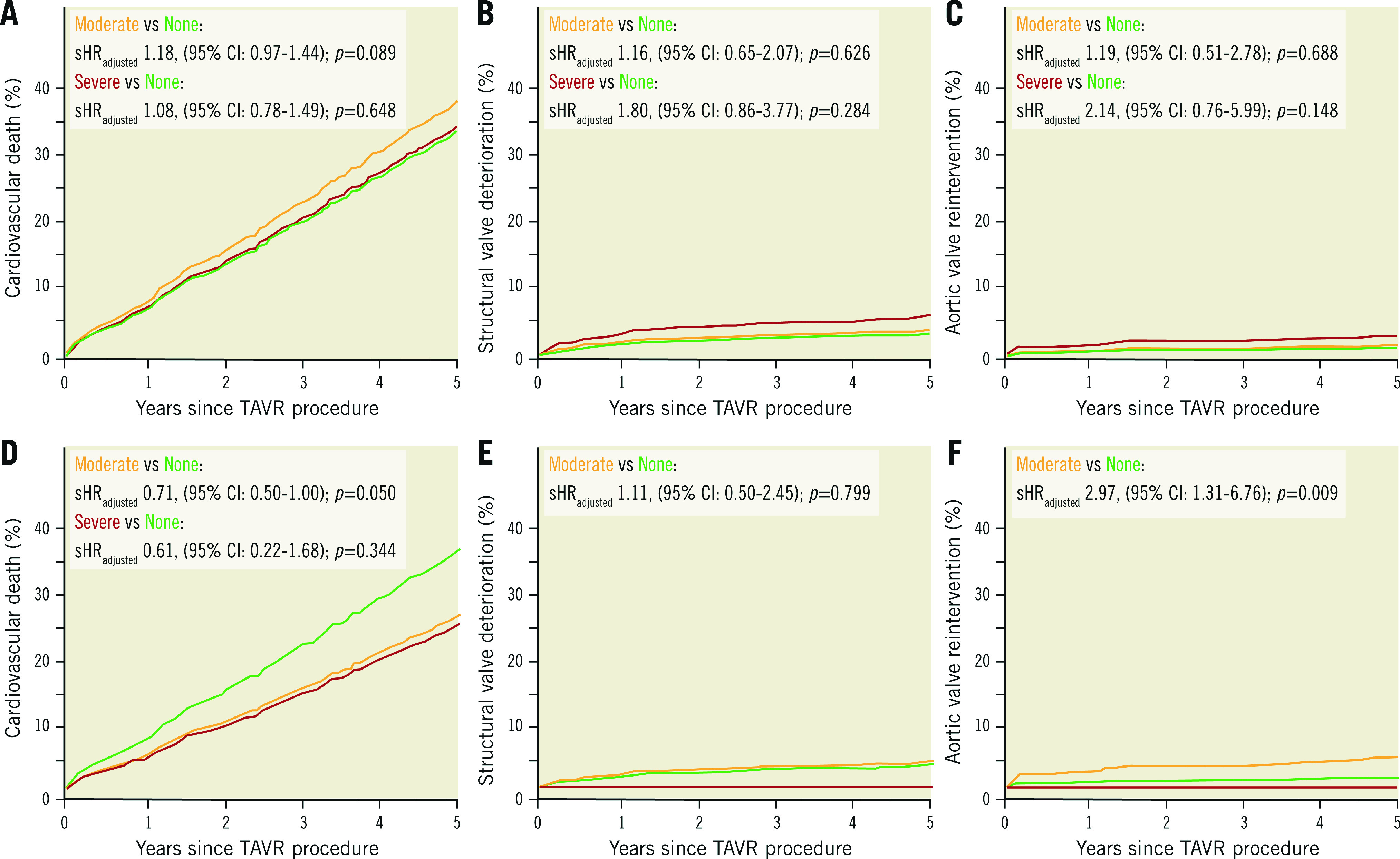
A, B & C) Clinical outcomes according to measured PPM. D, E & F) Clinical outcomes according to predicted PPMTHV. For competing risks of death, or in case of cardiovascular death, of non-cardiovascular death, subdistributions of the hazard ratio (sHR) with 95% confidence intervals (95% CI) are reported. HRadjusted: adjusted hazard ratio; PPM: prosthesis-patient mistmatch; PPMTHV: PPM defined by the normal reference values of EOA for each size and model of implanted transcatheter heart valve; TAVR: transcatheter aortic valve replacement
Discussion
The main findings of the present study can be summarised as follows: 1) predicted EOAi results in lower estimates of PPM severity as compared to measured EOAi in patients after TAVR. 2) There was no consistent signal for an increased risk of death over the course of 10 years in patients with PPM, irrespective of the definition.
Recent studies have suggested that the use of measured EOA overestimates the frequency and severity of PPM compared to the use of predicted EOA. Consistent with these previous studies, we found higher frequencies of moderate and severe PPM using measured EOA compared to predicted EOA10,11. The discrepancy in the frequency of PPM according to different definitions relates to inaccuracies in the assessment of EOA: 1) indexing of EOA to BSA may underestimate the EOAi and lead to an overestimation of the frequency and severity of PPM in obese patients10; 2) higher transvalvular gradients and smaller EOAs are documented by Doppler echocardiography compared to cardiac catheterisation due to the pressure recovery phenomenon22; 3) the geometric assumption that the cross-sectional area of the left ventricular outflow tract is circular results in an underestimation of EOAi and overestimation of PPM when using 2-dimensional echocardiography as compared with CT23,24; and 4) measured EOA is flow dependent, and a low-flow state may lead to underestimation of the EOA, resulting in pseudo-severe PPM11. In addition, it has been suggested that predicted PPM has a stronger association with haemodynamic outcomes compared to measured PPM10. Nevertheless, rates of residual gradients above 20 mmHg were relatively low in our analysis even in patients with PPM. Furthermore, overestimation of the frequency and severity of PPM may lead to a misinterpretation of the impact of PPM in TAVR populations. In the present study, we found substantial differences in mortality depending on the methods used to assess PPM (e.g., all-cause mortality at 5 years: 58.3% and 51.5% in moderate and severe measured PPM versus 38.8% and 33.0% in moderate and severe predicted PPMTHV). Using the predicted PPM definition could avoid overestimation of the mortality risk.
Several studies investigating the clinical impact of measured PPM in patients undergoing TAVR yielded conflicting results5,6,7,8. A recent meta-analysis of 81,969 TAVR patients concluded that patients with moderate/severe PPM as defined by measured EOA had a modestly increased risk of mortality compared with patients without PPM (HR 1.09, 95% CI: 1.04-1.14; p<0.001)25. Of note, the estimation of cumulative incidence using the Kaplan-Meier method and predicted risk using Cox regression models, which are commonly used in TAVR studies, can lead to biased risk estimates which ignore competing risks, especially in elderly populations with multiple comorbidities26,27. Indeed, in the present analysis, cardiovascular death occurred more frequently in patients with moderate measured PPM compared to those without PPM, whereas the effect was no longer significant under competing risk of non-cardiovascular death. Investigators should consider the presence of competing risks when conducting time-to-event analyses, especially in comorbid populations with long-term follow-up.
Interestingly, patients with moderate predicted PPMTHV and PPMCT had a lower 10-year mortality compared to those with no PPM in the present study. Similar trends have been observed in previous studies. In the analysis from the PARTNER 2 trial and registry, moderate predicted PPMTHV and PPMCT had a trend towards lower mortality up to 5 years after TAVR as compared to those without PPM (36.9% vs 41.4% and 38.7% vs 41.4%, respectively)11. Previous studies were unable to evaluate the impact of severe predicted PPM on clinical outcomes because of the modest numbers of patients with severe predicted PPM10,11. In line with previous studies, severe predicted PPMTHV was extremely rare in the present analysis. Our findings suggest that predicted PPM may not adversely impact prognosis, irrespective of severity, in a TAVR population. In contrast, in the present analysis, repeat aortic valve interventions were more frequently performed in patients with moderate predicted PPMTHV than in those with no PPM. The high residual gradients may lead to impaired forward haemodynamics, accelerated bioprosthesis degeneration and the need for reintervention. Further studies are needed to investigate the impact of high residual gradients and PPM after TAVR on repeat aortic valve interventions and to determine whether performing a reintervention reduces the risk of adverse outcomes in this setting1.
There is a growing demand to establish a standardised assessment method for PPM in patients undergoing TAVR. The two methods of predicted EOA are arguably more robust parameters to determine the true frequency of PPM in TAVR populations but require critical assessment. First, predicted EOA ignores differences in actual EOAs resulting from a flexible range of device expansion. Our group previously reported that, particularly in patients with considerable device landing zone calcification and annulus ellipticity, suboptimal THV-sizing is not uncommon28. The use of a uniform cut-off value of predicted EOATHV may under- or overestimate the severity of PPM in patients with complex aortic root anatomies. Second, predicted EOACT is based on preprocedural CT. Fukui et al reported that the post-TAVR left ventricular outflow tract area is significantly larger than the pre-TAVR native aortic annulus and that PPM defined by post-TAVR CT was associated with an increased risk of all-cause mortality24. A tailored approach including both post-TAVR CT and echocardiography may be key to the management of patients with suspected PPM. Finally, the predicted EOA does not take into account flow variability. Abbas et al evaluated the impact of low-flow status on clinical outcomes in patients with and without severe measured PPM and showed that severe measured PPM with low-flow status was associated with cardiac death after TAVR regardless of the implanted valve size or post-TAVR transvalvular gradient29. More recently, the PARTNER trials have proposed new reference values of predicted EOAs for each size and generation of balloon-expandable valves according to the flow status (low or normal flow)30.
Limitations
The results of the present study should be interpreted in light of several limitations. First, more than 15% of patients were excluded because of inadequate data for PPM assessment, which may have introduced a degree of selection bias. However, we provide comprehensive data on more than 2,400 patients who were assessed for PPM severity using 3 methods from a large prospective registry that adheres to high standards of data quality, with rigorous data collection, standardised follow-up and independent adjudication of events. Second, since this was a retrospective analysis based on prospectively collected data, the possibility of residual confounding cannot be excluded despite rigorous statistical techniques. Third, although the present study is the largest and longest to investigate the clinical impact of predicted PPM, the low prevalence of severe predicted PPM and the relatively short median follow-up time warrant cautious interpretation of the results. Fourth, although the occurrence of structural valve deterioration was systematically recorded and adjudicated, the definitions of structural valve deterioration were not in accordance with the current VARC criteria, which may have led to under- or overreporting of structural valve deterioration in the cohort. Finally, the present cohort predominantly included octogenarians, and the results may not be generalisable to younger patients with fewer comorbidities and longer life expectancy.
Conclusions
The use of predicted, as compared to measured, EOAi downgrades the severity of PPM in patients after TAVR. In a competing risk analysis, we found no increased risk of death in patients with PPM over a median follow-up time of 429 days, regardless of the EOA definition. Further study is needed to evaluate the impact of PPM on long-term clinical outcomes after TAVR in younger patients.
Impact on daily practice
Unlike SAVR studies, most TAVR studies have evaluated PPM using measured EOA and may overestimate the frequency and severity of PPM. PPM was not associated with an increased risk of 10-year mortality in TAVR under competing risk of death or, in cardiovascular death, under competing risk of non-cardiovascular death by any method of measurement. The impact of PPM on long-term clinical outcomes should be investigated in younger populations.
Supplementary data
Predicted EOA based on transcatheter heart valve type and size.
Predicted EOA based on aortic annulus dimensions by preprocedural computed tomography.
Frequency of PPM according to device type.
Baseline and procedural characteristics according to measured and predicted PPM.
Post-TAVR valve haemodynamics.
Crude hazard ratios for clinical outcomes according to the method for the definition of PPM.
Residual heart failure symptoms according to the method for the definition of PPM.
Crude hazard ratios for clinical outcomes according to the method for the definition of PPM in a competing risk analysis.
Study flowchart.
Acknowledgments
Conflict of interest statement
S. Windecker reports research, travel or educational grants to the institution without personal remuneration from Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Braun, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardinal Health, CardioValve, Cordis Medical, Corflow Therapeutics, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Farapulse Inc., Fumedica, Guerbet, Idorsia, Inari Medical, InfraRedx, Janssen-Cilag, Johnson & Johnson, MedAlliance, Medicure, Medtronic, Merck Sharp & Dohme, Miracor Medical, Novartis, Novo Nordisk, Organon, OrPha Suisse, Pharming Tech. Pfizer, Polares Medical, Regeneron, Sanofi-Aventis, Servier, SINOMED, Terumo, Vifor, and V-Wave. He served as an advisory board member and/or a member of the steering/executive group for trials funded by Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Boston Scientific, Biotronik, Bristol-Myers Squibb, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares Medical, Recardio, SINOMED, Terumo, and V-Wave, with payments to the institution but no personal payments. He is also a member of the steering/executive committee group of several investigator-initiated trials that receive funding from industry without impact on his personal remuneration. T. Pilgrim reports research grants to the institution from Edwards Lifesciences, Boston Scientific, and Biotronik; and personal fees from Biotronik, Boston Scientific, Medtronic, Abbott, and HighLife SAS. D. Reineke reports travel expenses from Abbott, Edwards Lifesciences, and Medtronic. S. Stortecky reports research grants to the institution from Edwards Lifesciences, Medtronic, Boston Scientific, and Abbott; and personal fees from Boston Scientific, Teleflex, and BTG. F. Praz reports travel expenses from Abbott, Edwards Lifesciences, and Polares Medical. J. Lanz reports speaker fees to the institution from Edwards Lifesciences and Abbott; and served as advisory board member for Abbott. T. Okuno reports speaker fees from Abbott. D. Heg has no personal conflicts; his employer, CTU Bern, University of Bern, has a staff policy of not accepting honoraria or consultancy fees. However, CTU Bern is involved in the design, conduct, or analysis of clinical studies funded by not-for-profit and for-profit organisations. In particular, pharmaceutical and medical device companies provide direct funding to some of these studies. For an up-to-date list of CTU Bern’s conflicts of interest see http://www.ctu.unibe.ch/research/declaration_of_interest/index_eng.html. The other authors have no conflicts of interest to declare.
Abbreviations
- EOA
effective orifice area
- EOACT
predicted EOA based on the normal reference values of EOA derived from aortic annulus dimensions measured by preprocedural computed tomography
- EOAi
effective orifice area indexed to body surface area
- EOATHV
predicted EOA based on the normal reference values of EOA for each size and type of implanted transcatheter heart valve
- PPM
prosthesis-patient mismatch
- PPMCT
PPM defined by predicted EOA derived from preprocedural computed tomography
- PPMTHV
PPM defined by predicted EOA for each size and model of implanted transcatheter heart valve
- TAVR
transcatheter aortic valve replacement
Contributor Information
Daijiro Tomii, Department of Cardiology, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland.
Taishi Okuno, Department of Cardiology, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland.
Dik Heg, CTU Bern, University of Bern, Bern, Switzerland.
Masaaki Nakase, Department of Cardiology, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland.
Jonas Lanz, Department of Cardiology, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland.
Fabien Praz, Department of Cardiology, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland.
Stefan Stortecky, Department of Cardiology, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland.
David Reineke, Department of Cardiovascular Surgery, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland.
Stephan Windecker, Department of Cardiology, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland.
Thomas Pilgrim, Department of Cardiology, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland.
References
- Flameng W, Herregods MC, Vercalsteren M, Herijgers P, Bogaerts K, Meuris B. Prosthesis-patient mismatch predicts structural valve degeneration in bioprosthetic heart valves. Circulation. 2010;121:2123–9. doi: 10.1161/CIRCULATIONAHA.109.901272. [DOI] [PubMed] [Google Scholar]
- Head SJ, Mokhles MM, Osnabrugge RL, Pibarot P, Mack MJ, Takkenberg JJ, Bogers AJ, Kappetein AP. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: a systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur Heart J. 2012;33:1518–29. doi: 10.1093/eurheartj/ehs003. [DOI] [PubMed] [Google Scholar]
- Fallon JM, DeSimone JP, Brennan JM, O’Brien S, Thibault DP, DiScipio AW, Pibarot P, Jacobs JP, Malenka DJ. The Incidence and Consequence of Prosthesis-Patient Mismatch After Surgical Aortic Valve Replacement. Ann Thorac Surg. 2018;106:14–22. doi: 10.1016/j.athoracsur.2018.01.090. [DOI] [PubMed] [Google Scholar]
- Ternacle J, Abbas AE, Pibarot P. Prosthesis-Patient Mismatch After Transcatheter Aortic Valve Replacement: Has It Become Obsolete? JACC Cardiovasc Interv. 2021;14:977–80. doi: 10.1016/j.jcin.2021.03.039. [DOI] [PubMed] [Google Scholar]
- Pibarot P, Weissman NJ, Stewart WJ, Hahn RT, Lindman BR, McAndrew T, Kodali SK, Mack MJ, Thourani VH, Miller DC, Svensson LG, Herrmann HC, Smith CR, Rodés-Cabau J, Webb J, Lim S, Xu K, Hueter I, Douglas PS, Leon MB. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: a PARTNER trial cohort--a analysis. J Am Coll Cardiol. 2014;64:1323–34. doi: 10.1016/j.jacc.2014.06.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann HC, Daneshvar SA, Fonarow GC, Stebbins A, Vemulapalli S, Desai ND, Malenka DJ, Thourani VH, Rymer J, Kosinski AS. Prosthesis-Patient Mismatch in Patients Undergoing Transcatheter Aortic Valve Replacement: From the STS/ACC TVT Registry. J Am Coll Cardiol. 2018;72:2701–11. doi: 10.1016/j.jacc.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Okuno T, Pilgrim T, Heg D, Stortecky S, Praz F, Windecker S, Lanz J. Prosthesis-Patient Mismatch Based on Energy Loss Index After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2020;13:2584–6. doi: 10.1016/j.jcin.2020.07.031. [DOI] [PubMed] [Google Scholar]
- Tang GHL, Sengupta A, Alexis SL, Bapat VN, Adams DH, Sharma SK, Kini AS, Kodali SK, Ramlawi B, Gada H, Vora AN, Forrest JK, Kaple RK, Liu F, Reardon MJ. Outcomes of Prosthesis-Patient Mismatch Following Supra-Annular Transcatheter Aortic Valve Replacement: From the STS/ACC TVT Registry. JACC Cardiovasc Interv. 2021;14:964–76. doi: 10.1016/j.jcin.2021.03.040. [DOI] [PubMed] [Google Scholar]
- Hahn RT, Leipsic J, Douglas PS, Jaber WA, Weissman NJ, Pibarot P, Blanke P, Oh JK. Comprehensive Echocardiographic Assessment of Normal Transcatheter Valve Function. JACC Cardiovasc Imaging. 2019;12:25–34. doi: 10.1016/j.jcmg.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Ternacle J, Guimaraes L, Vincent F, Côté N, Côté M, Lachance D, Clavel MA, Abbas AE, Pibarot P, Rodés-Cabau J. Reclassification of prosthesis-patient mismatch after transcatheter aortic valve replacement using predicted vs. measured indexed effective orifice area. Eur Heart J Cardiovasc Imaging. 2021;22:11–20. doi: 10.1093/ehjci/jeaa235. [DOI] [PubMed] [Google Scholar]
- Ternacle J, Pibarot P, Herrmann HC, Kodali S, Leipsic J, Blanke P, Jaber W, Mack MJ, Clavel MA, Salaun E, Guzzetti E, Annabi MS, Bernier M, Beaudoin J, Khalique OK, Weissman NJ, Douglas P, Bax J, Dahou A, Xu K, Alu M, Rogers E, Leon M, Thourani VH, Abbas AE, Hahn RT. Prosthesis-Patient Mismatch After Aortic Valve Replacement in the PARTNER 2 Trial and Registry. JACC Cardiovasc Interv. 2021;14:1466–77. doi: 10.1016/j.jcin.2021.03.069. [DOI] [PubMed] [Google Scholar]
- Stortecky S, Franzone A, Heg D, Tueller D, Noble S, Pilgrim T, Jeger R, Toggweiler S, Ferrari E, Nietlispach F, Taramasso M, Maisano F, Grünenfelder J, Muller O, Huber C, Roffi M, Carrel T, Wenaweser P, Windecker S. Temporal trends in adoption and outcomes of transcatheter aortic valve implantation: a SwissTAVI Registry analysis. Eur Heart J Qual Care Clin Outcomes. 2019;5:242–51. doi: 10.1093/ehjqcco/qcy048. [DOI] [PubMed] [Google Scholar]
- VARC-3 WRITING COMMITTEE: Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, Bax JJ, Leipsic JA, Blanke P, Blackstone EH, Finn MT, Kapadia S, Linke A, Mack MJ, Makkar R, Mehran R, Popma JJ, Reardon M, Rodes-Cabau J, Van Mieghem NM, Webb JG, Cohen DJ, Leon MB. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J Am Coll Cardiol. 2021;77:2717–46. doi: 10.1016/j.jacc.2021.02.038. [DOI] [PubMed] [Google Scholar]
- Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, Khandheria BK, Levine RA, Marx GR, Miller FA, Nakatani S, Quiñones MA, Rakowski H, Rodriguez LL, Swaminathan M, Waggoner AD, Weissman NJ, Zabalgoitia M American Society of Echocardiography’s Guidelines and Standards Committee; Task Force on Prosthetic Valves; American College of Cardiology Cardiovascular Imaging Committee; Cardiac Imaging Committee of the American Heart Association; European Association of Echocardiography; European Society of Cardiology; Japanese Society of Echocardiography; Canadian Society of Echocardiography; American College of Cardiology Foundation; American Heart Association; European Association of Echocardiography; European Society of Cardiology; Japanese Society of Echocardiography; Canadian Society of Echocardiography. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22:975–1014; quiz 1082-4. doi: 10.1016/j.echo.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Lancellotti P, Pibarot P, Chambers J, Edvardsen T, Delgado V, Dulgheru R, Pepi M, Cosyns B, Dweck MR, Garbi M, Magne J, Nieman K, Rosenhek R, Bernard A, Lowenstein J, Vieira ML, Rabischoffsky A, Vyhmeister RH, Zhou X, Zhang Y, Zamorano JL, Habib G. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:589–90. doi: 10.1093/ehjci/jew025. [DOI] [PubMed] [Google Scholar]
- Okuno T, Brugger N, Asami M, Heg D, Siontis GCM, Winkel MG, Lanz J, Gräni C, Huber A, Stortecky S, George I, Kodali S, Pilgrim T, Windecker S, Khalique OK, Praz F. Clinical impact of mitral calcium volume in patients undergoing transcatheter aortic valve implantation. J Cardiovasc Comput Tomogr. 2021;15:356–65. doi: 10.1016/j.jcct.2020.10.003. [DOI] [PubMed] [Google Scholar]
- Okuno T, Demirel C, Tomii D, Heg D, Häner J, Siontis GCM, Lanz J, Räber L, Strotecky S, Fürholz M, Praz F, Windecker S, Pilgrim T. Long-term risk of unplanned percutaneous coronary intervention after transcatheter aortic valve replacement. EuroIntervention. 2022;18:797–803. doi: 10.4244/EIJ-D-22-00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, Krucoff MW, Mack M, Mehran R, Miller C, Morel MA, Petersen J, Popma JJ, Takkenberg JJ, Vahanian A, van Es, Vranckx P, Webb JG, Windecker S, Serruys PW. Standardized endpoint definitions for Transcatheter Aortic Valve Implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–69. doi: 10.1016/j.jacc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés-Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–54. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics. 1988;16:1141–54. [Google Scholar]
- Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med. 1993;12:737–51. doi: 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- Abbas AE, Mando R, Kadri A, Khalili H, Hanzel G, Shannon F, Al-Azizi K, Waggoner T, Kassas S, Pilgrim T, Okuno T, Camacho A, Selberg A, Elmariah S, Bavry A, Ternacle J, Christensen J, Gheewala N, Pibarot P, Mack M. Comparison of Transvalvular Aortic Mean Gradients Obtained by Intraprocedural Echocardiography and Invasive Measurement in Balloon and Self-Expanding Transcatheter Valves. J Am Heart Assoc. 2021;10:e021014. doi: 10.1161/JAHA.120.021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney J, Sellers SL, Blanke P, Pibarot P, Hahn RT, Dvir D, Douglas PS, Weissman NJ, Kodali SK, Thourani VH, Jilaihawi H, Khalique O, Smith CR, Kueh SH, Ohana M, Grover R, Naoum C, Crowley A, Jaber WA, Alu MC, Parvataneni R, Mack M, Webb JG, Leon MB, Leipsic JA. CT-Defined Prosthesis-Patient Mismatch Downgrades Frequency and Severity, and Demonstrates No Association With Adverse Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2017;10:1578–87. doi: 10.1016/j.jcin.2017.05.031. [DOI] [PubMed] [Google Scholar]
- Fukui M, Garcia S, Lesser JR, Gössl M, Tang L, Caye D, Newell M, Hashimoto G, Lopes BBC, Stanberry LI, Enriquez-Sarano M, Pibarot P, Hahn R, Sorajja P, Cavalcante JL. Prosthesis-patient mismatch defined by cardiac computed tomography versus echocardiography after transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2021;15:403–11. doi: 10.1016/j.jcct.2021.01.001. [DOI] [PubMed] [Google Scholar]
- Sá MP, Jacquemyn X, Van den, Tasoudis P, Dokollari A, Torregrossa G, Sicouri S, Clavel MA, Pibarot P, Ramlawi B. Impact of Prosthesis-Patient Mismatch After Transcatheter Aortic Valve Replacement: Meta-Analysis of Kaplan-Meier-Derived Individual Patient Data. JACC Cardiovasc Imaging. 2023;16:298–310. doi: 10.1016/j.jcmg.2022.07.013. [DOI] [PubMed] [Google Scholar]
- Holmes DR, Brennan JM, Rumsfeld JS, Dai D, O’Brien SM, Vemulapalli S, Edwards FH, Carroll J, Shahian D, Grover F, Tuzcu EM, Peterson ED, Brindis RG, Mack MJ STS/ACC TVT Registry. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313:1019–28. doi: 10.1001/jama.2015.1474. [DOI] [PubMed] [Google Scholar]
- Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133:601–9. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T, Heg D, Lanz J, Praz F, Gräni C, Langhammer B, Reineke D, Räber L, Wenaweser P, Pilgrim T, Windecker S, Stortecky S. Heart valve sizing and clinical outcomes in patients undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2021;98:E768–79. doi: 10.1002/ccd.29700. [DOI] [PubMed] [Google Scholar]
- Abbas AE, Ternacle J, Pibarot P, Xu K, Alu M, Rogers E, Hahn RT, Leon M, Thourani VH. Impact of Flow on Prosthesis-Patient Mismatch Following Transcatheter and Surgical Aortic Valve Replacement. Circ Cardiovasc Imaging. 2021;14:e012364. doi: 10.1161/CIRCIMAGING.120.012364. [DOI] [PubMed] [Google Scholar]
- Akinmolayemi O, Ozdemir D, Pibarot P, Zhao Y, Leipsic J, Douglas PS, Jaber WA, Weissman NJ, Blanke P, Hahn RT. Clinical and Echocardiographic Characteristics of Flow-Based Classification Following Balloon-Expandable Transcatheter Heart Valve in PARTNER Trials. JACC Cardiovasc Imaging. 2023;16:1–9. doi: 10.1016/j.jcmg.2022.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Predicted EOA based on transcatheter heart valve type and size.
Predicted EOA based on aortic annulus dimensions by preprocedural computed tomography.
Frequency of PPM according to device type.
Baseline and procedural characteristics according to measured and predicted PPM.
Post-TAVR valve haemodynamics.
Crude hazard ratios for clinical outcomes according to the method for the definition of PPM.
Residual heart failure symptoms according to the method for the definition of PPM.
Crude hazard ratios for clinical outcomes according to the method for the definition of PPM in a competing risk analysis.
Study flowchart.


