Abstract
Mitral regurgitation is the second most frequent heart valve disease in Europe and the most frequent in the US. Although surgery is the therapy of choice when intervention is indicated, transcatheter mitral valve repair or replacement are alternatives for patients who are not eligible for surgery. However, the development of transcatheter mitral valves is slower than expected. Although several transcatheter heart valves have been developed, only one has been commercialised. Indeed, most of these devices are being evaluated in clinical studies, with promising initial results. In this review, we propose an overview on transcatheter mitral valve replacement for the treatment of native mitral valve disease, from indication to results, including patients with severe annular calcification, and we provide you with a glimpse into the future of these therapies.
Introduction
The development of percutaneous therapies during the last decade has revolutionised the treatment of valvular heart diseases. Nevertheless, the impact of these therapies on clinical practice is not equal for all valve diseases. While transcatheter aortic valve implantation (TAVI) has become the preferred therapy for most patients with aortic stenosis1,2, transcatheter mitral valve implantation (TMVI) therapies have undergone a longer development process.
Mitral regurgitation (MR) is the second most common heart valve disease in Europe3. Surgery has been shown to improve survival in patients with symptomatic MR1,2. However, up to 50% of patients with MR and an indication for intervention may not receive treatment because they are either not eligible for or refuse surgery3,4,5. Minimally invasive transcatheter repair techniques can bridge this gap with safe and effective interventions6,7 and promising outcomes, but edge-to-edge repair techniques are limited to suitable anatomies6,8,9. TMVI might serve as an alternative minimally invasive treatment option that overcomes the anatomical constraints of percutaneous repair. Furthermore, TMVI has the potential to treat mixed mitral valve (MV) disease10.
However, the development of transcatheter MVs is slower than anticipated. Multiple devices specifically designed for this purpose are being evaluated in early feasibility studies as well as pivotal trials. Nonetheless, no dedicated device has been approved for commercial use in the US for the treatment of native MR and only one device, the Tendyne Transcatheter Mitral Valve Replacement (TMVR; Abbott Structural) system, has received a CE (European conformity) mark. Moreover, trials designated to evaluate the safety and efficacy of these dedicated devices face high rates of rejection11,12,13. Epidemiological, clinical, anatomical and device-related challenges have slowed down the development of TMVI compared to TAVI (Table 1)14. First, patients with MV disease are younger and have less comorbid conditions, resulting in a lower surgical risk than those with aortic stenosis3,15. Second, surgical MV repair is associated with better survival than replacement, and a percutaneous option is commercially available and widely adopted in clinical practice1,2,16. Third, the anatomical and functional complexity of the MV and its relationship with crucial adjacent structures pose a challenge for the development of TMVI technology17. Indeed, left ventricular outflow tract (LVOT) obstruction is a potential complication of TMVI, and it is associated with up to 50% mortality18. Fourth, the forces of valve migration are greater, and the mitral annulus is not circular and is larger and less frequently calcified than the aortic valve19. Whereas the fixation of aortic devices is passive and mainly assured by the radial force of the transcatheter heart valve (THV), mitral THVs require a mechanism of active fixation to counteract the forces of valve embolisation. Fifth, MV disease is more frequently associated with other heart valve diseases, particularly tricuspid regurgitation (TR)15 and atrial fibrillation. Lastly, both transseptal and transapical access are used for percutaneous mitral therapies, but most dedicated devices have been developed for the transapical route. This is because the size of the delivery system required limits the feasibility of the transseptal approach in certain cases. Additionally, the transapical route offers a more direct and coaxial access to the mitral valve, further favouring its use. However, experience from TAVI has shown an excess of mortality associated with the transapical approach, and this is higher in patients with left ventricular dysfunction20, which is common in patients with chronic MR. Similarly, superior survival was found in patients who underwent a mitral valve-in-valve procedure using transseptal access compared with transapical access in an analysis of patients enrolled in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry21.
Table 1. Challenges for the development of TMVI.
| TMVI candidates | TAVI candidates | |
|---|---|---|
| Clinical and epidemiological factors | ||
| Age | Younger | Older |
| Comorbidities | Less frequent | More frequent |
| Surgical risk | Lower | Higher |
| Alternative to replacement | Yes, repair (of choice) | None |
| Multiple valve disease | Frequent (TR) | Less frequent |
| Entities | Two: FMR and DMR | One: calcific AS |
| Anatomical factors | ||
| Components of the valve | Mitral valve, LV, LA, subvalvular apparatus | Aortic valve |
| Configuration of the valve | Asymmetrical – 2 leaflets | Symmetrical – 3 leaflets |
| Morphology of annulus | Saddle-shaped | Circular |
| Dimensions of annulus | Larger | Smaller |
| Calcifications | Less frequent | Frequent |
| Structures in proximity | Circumflex artery, coronary sinus, LVOT | Coronary arteries |
| Components of the valve | Mitral valve, LV, LA, subvalvular apparatus | Aortic valve |
| Physiological factors | ||
| Forces of valve embolisation | High (systolic pressure gradient) | Low (diastolic pressure gradient) |
| Device-related factors | ||
| Mechanisms of fixation | Active | Passive |
| Approach | Mainly transapical | Mainly transfemoral |
| Rate of degeneration | High | Low |
| Impact of paravalvular leak | High | Low |
| Risk of valve thrombosis | High | Low |
| AS: aortic stenosis; DMR: degenerative mitral regurgitation; FMR: functional mitral regurgitation; LA: left atrium; LV: left ventricle; LVOT: left ventricular outflow tract; TAVI: transcatheter aortic valve implantation; TR: tricuspid regurgitation; TMVI: transcatheter mitral valve implantation | ||
Among patients with native MV disease, patients with severe mitral annular calcification (MAC) pose a particular challenge. These patients are considered to be at high surgical risk, are frequently denied surgery and are thus excluded from most studies evaluating new mitral THVs. They are also poor candidates for percutaneous mitral repair therapies due to the mixed MV disease found frequently in this subset. This unmet clinical need has been partially addressed by the use of aortic THVs. Indeed, while calcification precludes the function of certain mechanisms of active fixation used in dedicated devices22, it might serve as an anchoring zone for balloon-expandable aortic THVs23.
In this review, we provide an overview of TMVI therapies for the treatment of native MV disease using both dedicated mitral devices as well as aortic devices. Additionally, we will provide a glimpse of what the future of this therapy may hold.
Anatomy, prevalence of disease and candidates for TMVI
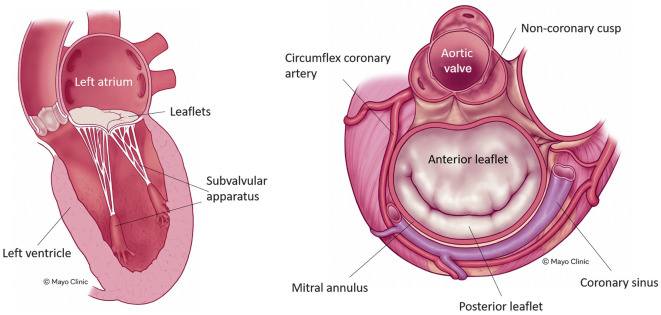
The MV is a heterogenous apparatus located between the left ventricle and the left atrium. It consists of several components including the mitral annulus, two unequally distributed leaflets with the anterior leaflet larger than the posterior leaflet, a subvalvular apparatus (including the papillary muscles and chordae), the left ventricle, and the left atrium17. All these components work together to facilitate the proper function of the MV, and any alteration to these components can result in MV disease (Figure 1). The mitral annulus is saddle-shaped and multiplanar, and it has a contractile function that helps to ensure the proper coaptation of both leaflets24. Its dimensions change across the cardiac cycle, with a mean diameter >36 mm, which is larger than the aortic valve19. The circumflex artery and the coronary sinus are located close to the mitral annulus, and the MV is in direct proximity to the left ventricular outflow tract19 (Figure 1).
Figure 1. The mitral valve apparatus.
The main components of the mitral valve apparatus and the anatomical relationships are shown. Note the close relationship between the mitral valve and the left ventricular outflow tract. Reproduced with permission from the Mayo Clinic.
Mitral regurgitation is the second most common heart valve disease in Europe4,15. Its prevalence increases with age, with a prevalence rate of up to 9% in individuals >75 years old25. Currently, the most frequent aetiology of MR is degenerative3,15. There are 2 types of MR: degenerative (DMR) and functional (FMR). DMR results from the alteration of the MV itself (leaflets and/or chordae). FMR is due to left ventricle or left atrium dysfunction leading to annular dilatation, papillary muscle displacement and tethering of the leaflets and, finally, a deficit of coaptation of both leaflets. Although MV repair is the therapy of choice in both cases when the indication of surgery is retained1,2, only approximately one-third of patients are finally operated on, and among them, one-third undergo surgical MV replacement3. These patients might be candidates for TMVI.
MAC is the deposit of calcium around the mitral annulus26, which typically occurs more frequently in the posterior aspect of the annulus17 (Figure 2). It is noteworthy that only 1% of patients with MAC exhibit circumferential calcification of the annulus, making them eligible candidates for TMVI27. Severe MAC can lead to mitral stenosis and/or regurgitation. While the diagnosis of mitral regurgitation is essentially similar to patients with no calcification of the mitral annulus, the diagnosis of MAC-associated mitral stenosis can be challenging28. Indeed, the elevated transmitral gradient observed in these patients might be due to the loss of left atrium compliance (which occurs in patients with preserved left ventricular function heart failure − frequently observed in patients with MAC) and local acceleration forces without real mitral stenosis28.
Figure 2. Multimodality imaging of mitral annular calcification.

Transoesophageal (A) and computed tomography (B) images showing a severe mitral annular calcification.
The prevalence of severe calcification of the mitral annulus varies between 5-42%29 across studies, mainly depending on the population studied and the method used for the detection of calcifications. It is more commonly observed in elderly people29.
Patients with MAC are generally poor candidates for surgery due to technical difficulties. Techniques used to overcome the challenges associated with severe MAC, such as decalcification, device implantation in an intra-atrial position or an extracardiac valved conduit are associated with a high risk of complications. Therefore, TMVI may be a good option for these patients. However, the rate of rejection due to anatomical constraints is high, and only a proportion of these patients are ultimately eligible30.
Screening before TMVI
As stated before, the MV apparatus differs from the aortic valve. Likewise, screening, patient selection, and preprocedural planning for TMVI differ from those of TAVI. A multimodal combination of advanced imaging techniques, like echocardiography (transthoracic and transoesophageal) and 3-dimensional computed tomography (CT), is essential for the evaluation of patient eligibility.
From a clinical perspective, it is necessary to evaluate if patients are in a physical condition to tolerate an anterolateral minithoracotomy for transapical access and the risk of futility, since common advanced heart valve disease is observed in these patients. Furthermore, strict anticoagulation post-TMVI is required, precluding patients with absolute contraindications.
Echocardiography
Pivotal for the assessment of patients with suspected MR is transthoracic echocardiography complemented by 2-dimensional and 3-dimensional transoesophageal echocardiography (TOE). This basic diagnostic tool allows for distinct quantification of the MR with different synergistic measurement techniques. In addition, the MV anatomy and pathology can be fully described, elucidating the MR aetiology, which is crucial for further therapeutic considerations31. Besides these MV-specific assessments, a careful evaluation of the anatomy of the entire heart is key for a successful procedure. In particular, accurate characterisation of the left atrial and ventricular geometry, dimensions and function is crucial for the evaluation of TMVI eligibility, since ongoing trials exclude patients with severely dilated ventricles and severely reduced function. Especially in patients with ischaemic cardiomyopathy, analysis of the myocardial structure can reveal scars, which might limit transapical access if located unfavourably10,32.
Moreover, the relationship to the LVOT needs further examination33. Albeit precise anatomical assessment of the LVOT and simulation of the neo-LVOT post-TMVI is the domain of computed tomography, an evaluation of potential preprocedural dynamic obstructions of the LVOT, at rest and during a Valsalva manoeuvre, is recommended in echocardiography31.
Preprocedural risk assessment requires further assessment of right ventricular size and function as well as concomitant TR32. An echocardiographic estimation of pulmonary artery pressure should be verified and, in some patients, specified invasively.
Cardiac computed tomography
Multimodal imaging is the cornerstone of successful TMVI planning. Although echocardiography remains pivotal in preprocedural assessment, periprocedural guidance and postprocedural follow-up, additional computed tomography is absolutely essential for preprocedural planning.
ASSESSMENT OF MV PROPERTIES
MV annular dimensions can be measured directly in a saddle-shaped model as the MV area and circumference. Simplified, the mitral annulus can be esteemed to be “D-shaped” by excluding the anterior horn via a virtual straight line connecting the medial and lateral fibrous trigones (Figure 3A-Figure 3D)34. Despite the exact characterisation of the mitral annulus itself, a careful evaluation of the subvalvular apparatus of the MV is essential. The measurement of the papillary muscle to mitral annulus distance is especially important for sufficient planning of prosthetic valve sizing and anchoring.
Figure 3. Preprocedural assessment in CT for TMVI.
Essential in the preprocedural TMVI planning is the assessment of the mitral valve geometry. Established parameters are the anterior-posterior (A) and lateral-medial diameters (B), the intertrigonal distance (C), as well as annular circumference and area (D) in systole and diastole. Quantification of the aortomitral angle is a basic instrument for the risk calculation of postinterventional LVOT obstruction (E). A virtual model of the heart and ribcage allows for the determination of the ideal intercostal space for transapical access (F). CT: computed tomography; LVOT: left ventricular outflow tract; TMVI: transcatheter mitral valve implantation
Precise evaluation of the amount and the distribution of mitral annular calcification is another advantage of the X-ray-based CT35 (Figure 4A). Nonetheless, non-severe, particularly non-circumferential, MAC can result in poor device sealing causing paravalvular leakage − despite using dedicated devices − which is associated with poor outcomes33. Most unfavourably, device embolisation or migration can also occur. Based on 72 patients undergoing TMVI from the MAC registry, a MAC score was derived incorporating information on average calcium thickness, degree of annular circumference involved, calcification at one or both fibrous trigones, and calcification of one or both leaflets. This score was proven to predict device embolisation during TMVI36 when using aortic THVs. Figure 4B show the analysis of dimensions of the mitral annulus in patients with MAC.
Figure 4. Preprocedural assessment in CT for ViMAC TMVI using aortic devices.
The following parameters must be evaluated: the extension and severity of calcification (A), annulus dimensions (B), and the risk of LVOT (C,D,E). The dimensions of the neo-LVOT after simulation of the transcatheter heart valve is the most validated parameter for estimating the risk of LVOT obstruction. Note, despite a low risk of LVOT obstruction, this patient was denied therapy (TMVI using a SAPIEN 3 THV) due to the dimensions of the annulus. CT: computed tomography; LVOT: left ventricular outflow tract; MV: mitral valve; THV: transcatheter heart valve; TMVI: transcatheter mitral valve implantation; ViMAC: valve-in-MAC
LVOT ASSESSMENT
The spatial proximity of the MV to the LVOT, especially of the larger anterior MV leaflet, carries the danger of postinterventional-relevant LVOT obstruction. According to the Mitral Valve Academic Research Consortium (MVARC) criteria, significant LVOT obstruction is defined as (1) acute, intraprocedural haemodynamic deterioration after TMVI with evidence of displacement of the prosthetic valve or the native anterior MV leaflet obstructing the LVOT and (2) an increase in the mean LVOT gradient ≥10 mmHg from baseline37.
Several anatomical factors might influence the risk of LVOT obstruction and need to be considered in preprocedural CT. An acute aortomitral angle is associated with a higher chance of LVOT obstruction (Figure 3E)38. Modern post-processing software offers the opportunity to estimate the size of the neo-LVOT, enabling a virtual simulation of the adequate prosthetic valve (type and size)39. Of note, the dimensions of the neo-LVOT are highly dependent on the size and the shape of the LV cavity, which therefore needs a preprocedural evaluation. Albeit an end-systolic neo-LVOT area ≤1.7 cm² was shown to be high risk for relevant LVOT obstruction33, a novel multiphase assessment throughout the cardiac cycle indicates that a single cut-off might be too conservative40.
As the neo-LVOT is circumscribed by the interventricular septum anteriorly and by the displaced anterior MV leaflet posteriorly, the extent of septal hypertrophy and the magnitude of the anterior MV leaflet need to be assessed.
For the assessment of the risk of LVOT obstruction when using the SAPIEN 3 (Edwards Lifesciences) THV, the concept of “skirt neo-LVOT” has been developed. It is defined as the neo-LVOT obtained at the level of the THV skirt, since the skirt-covered part of the prosthesis may contribute to the risk of LVOT obstruction in patients in whom the anterior leaflet is removed or split. Figure 4C, Figure 4D and Figure 4F show the preprocedural assessment of LVOT obstruction in patients with MAC.
ACCESS PLANNING
Many different technologies for TMVI are currently under investigation10. Transseptal and transapical access are the two most frequent approaches. In both cases, precise preprocedural CT-based planning would enable and optimise a safe and successful procedure. Even though transseptal access is guided by periprocedural transoesophageal echocardiography, preprocedural planning of the location of the transseptal puncture on anatomical landmarks could optimise angles, achieving a perpendicular coaxial trajectory for optimal axial TMVI device deployment30,41. Furthermore, positional relationships to important surrounding structures can be evaluated. Hereby, serious complications like obstruction or even violation of the coronary sinus and left circumflex artery could effectively be avoided42,43. Additionally, a CT body scan allows for the evaluation of the patency of transfemoral access.
For transapical access, the above-mentioned principal considerations regarding optimal coaxial trajectory can be adopted. Modern post-processing software allows for the generation of a virtual model of the heart and ribcage to evaluate the best placed intercostal space for transapical access, ensuring a straight trajectory, coaxial deposition and anchoring in the mitral annulus centroid (Figure 3F). In the absence of apical left ventricular abnormalities, the ideal access point is commonly anterior or anterolateral of the true left ventricular apex incorporating an offset from the mitral annulus coaxial trajectory. Most often, this access point is located in the left-sided fifth intercostal space41. Further evaluation of epicardial coronaries and the subvalvular MV apparatus with its papillary muscles and chordae would help to anticipate and prevent possible complications44.
Indications for TMVI
Indications for TMVI depend on the type of MR and the presence of MAC.
1. DMR
Since long-term outcomes after surgical MV repair of DMR are excellent45,46, the 2020 American and 2021 European Guidelines recommend heart surgery as the reference standard in patients at low to intermediate risk1,2. Transcatheter approaches are reserved for high-risk patients with reasonable life expectancy, favouring repair techniques1,2. In the context of DMR, TMVI can be considered for high-risk patients with an unfavourable anatomy for transcatheter MV repair.
2. FMR
In this predominantly high-risk population, surgical approaches often lead to suboptimal results and increased mortality47. Therefore, current guidelines recommend MV surgery for FMR only in cases with concomitant indication for coronary artery bypass grafting1,2, and MV repair is favoured over MV replacement. Since no device is currently approved, no specific recommendations are available regarding TMVI in this clinical scenario. Nonetheless, it can be considered for patients with an unfavourable anatomy for transcatheter MV repair in clinical trials.
3. MAC
No specific recommendations are given in the latest guidelines regarding patients with MAC1,2. In clinical practice, both patients with MR and those with symptomatic mitral stenosis may be candidates for TMVI if they are anatomically eligible and are considered at high surgical risk.
Given the simpler screening process, the availability of commercial devices, the potentially lower costs but also the more straightforward postprocedural management, TEER is often considered to be the default approach for transcatheter mitral valve therapies, whereas TMVI seems to be reserved for those patients who are unsuitable for TEER. Needless to say, TMVI has the potential to offer greater and more reproducible MR reduction and to also potentially treat stenosis. As such, a pre-existing gradient across the mitral valve, small mitral valve area, previous mitral valve annuloplasty and/or calcifications are features that are more suitable for TMVI48. Other features that favour TMVI include those indicating inadequate MR reduction with TEER, such as short posterior leaflet, complex Barlow’s disease, multisegmental pathologies, significant leaflet tethering, leaflet clefts or indentations, leaflet perforation, and large coaptation defects48. The Heart Valve Collaboratory has published definitions of anatomical features that are considered not suitable for TEER49.
The main anatomical features causing screening failure for TMVI can be divided into two categories12:
1. Those causing left ventricular outflow tract obstruction (LVOTO) (anticipated neo-LVOT area under 1.7 cm2), septal hypertrophy (>15 mm thickness), long (>25 mm) anterior MV leaflet with redundant chordae, small LV (end-diastolic diameter <48 mm), narrow aorto-mitral annular angle, and preserved ejection fraction;
2. Those causing poor device sealing, leading to paravalvular leakage (PVL), device migration or embolisation (moderate and non-circumferential MAC).
In addition to morphological considerations, left ventricular function (potentially higher risk for TMVI than TEER in patients with a severely reduced LV function), the need for oral anticoagulation post-TMVI and the durability of bioprostheses in the mitral position should also be taken into account when deciding on TMVI versus TEER. However, these aspects are still debated, and evidence or robust recommendations remain scarce.
Lastly, surgical mitral valve repair is preferred to replacement when results are expected to be durable, as the first is associated with better survival1,2,50. Nonetheless, it has not been demonstrated for percutaneous therapies. In the CHOICE-MI registry, TMVI was associated with superior reduction of MR and more pronounced symptomatic improvement compared to TEER. However, periprocedural mortality was higher51. Ongoing studies comparing TEER versus TMVI will shed light on this question. Figure 5 suggests a decision algorithm for TMVI versus TEER.
Figure 5. Proposed algorithm for the decision of TMVI versus TEER.

ASA: alcohol septal ablation; gen: generation; LAMPOON: Laceration of the Anterior Mitral leaflet to Prevent Outflow ObtructioN; LVEDD: left ventricular end-diastolic diameter; LVOT: left ventricular outflow tract; MAC: mitral annular calcification; MV: mitral valve; TEER: transcatheter edge-to-edge repair; TMVI: transcatheter mitral valve implantation
Devices and procedures
DEDICATED DEVICES
TMVI is most commonly performed with either transseptal or transapical access. In broad terms, these procedures can be grouped into either single- or multistep approaches. In the latter method, first, an anchoring member is placed, followed by insertion of the valve prosthesis. Examples of single-step devices are the Intrepid (Medtronic), Tendyne (Abbott Structural), EVOQUE Eos (Edwards Lifesciences), AltaValve (4C Medical), CardioValve (Venus MedTech), and Cephea (Abbott Structural). For multistep devices, examples include the HighLife (HighLife SAS), SAPIEN M3 (Edwards Lifesciences) and Saturn (InnovHeart). Table 2 summarises the device-specific characteristics. While direct comparisons of these methods have not been performed, the multistep approach generally allows for the use of lower profile systems.
Table 2. Device-specific characteristics.
| Device | Access | Sheath | Design | Shape | Size | Anchoring | Active studies |
|---|---|---|---|---|---|---|---|
| Single-step devices | |||||||
| Tendyne (Abbott Structural) | Transapical | 38 Fr | Self-expanding nitinol double frame Trileaflet porcine | D-shape | External frame: 35-40 mm in the SL dimension and 34-50 mm in the IC dimension | Apical tether | NCT03433274 NCT04898335 NCT04818502 NCT02321514 |
| Intrepid (Medtronic) | Transapical Transseptal | 35 Fr | Self-expanding nitinol double frame Trileaflet bovine | Circular | 27 mm Outer frame sizes: 43, 46, 50 mm | Annular anchoring by cleats, radial force | NCT05496998 NCT03242642 |
| AltaValve (4C Medical) | Transapical Transseptal | 32 Fr | Self-expanding nitinol spherical frame Trileaflet bovine | Circular | 27 mm Annular ring sizes: 40, 46, 54 | Left atrium anchoring (oversized nitinol frame) | NCT03997305 |
| CardioValve (Venus MedTech) | Transseptal | 28 Fr | Dual self-expanding nitinol frame Trileaflet bovine | Circular | 36-53 mm | Atrial flanges, annular anchoring | NCT03813524 NCT03339115 NCT05486832 NCT03958773 NCT04100720 |
| Cephea (Abbott Structural) | Transseptal | 36-38 Fr | Self-expanding nitinol, double-disc frame Trileaflet bovine | Circular | 36 mm | Annular anchoring (axial compression forces) | NCT05061004 |
| EVOQUE Eos (Edwards Lifesciences) | Transseptal | 28 Fr | Self-expanding nitinol frame Trileaflet bovine | Circular | 44-48 mm | Annulus, leaflet and chord anchoring by multiple anchors | NCT02718001 NCT03230747 |
| Multistep devices | |||||||
| HighLife (HighLife SAS) | Transapical Transseptal Transfemoral | 18 Fr and 39 Fr | Self-expanding nitinol stent frame Subannular ring Trileaflet bovine | Circular | 28 mm, 31 mm | Atrial and ventricular flanges, subannular ring | NCT02974881 NCT04029337 NCT04029363 NCT04888247 NCT05610566 |
| SAPIEN M3 (Edwards Lifesciences) | Transseptal | 20 Fr | Nitinol dock balloon-expandable cobalt-chromium alloy Trileaflet bovine | Circular | 29 mm | Docking, radial force | NCT04153292 |
| Saturn (InnovHeart) | Transapical Transseptal | 10 Fr | Self-expanding nitinol stent frame Trileaflet bovine Annular structure | Circular | 28 mm Broad range of annulus sizes | Subannular ring | NCT04464876 |
| IC: intercommissural SL: septo-lateral | |||||||
TRANSAPICAL
Currently, the most common approach for TMVI procedures is transapical (or transventricular), as this mode permits the placement of large delivery systems (e.g., ~35 Fr or larger). While an analogy to transapical TAVR can be easily assumed, transapical TMVI is wholly different with respect to tissue quality and puncture location. For patients undergoing TMVI, the myocardium is relatively thinner owing to the lack of pressure hypertrophy and the frequent presence of dilated cardiomyopathy. As stated before, particular care is needed for determining the access location from both preoperative contrast-enhanced cardiac CT imaging and intraprocedural TOE. Using the proper access site helps to avoid entanglement with cords and papillary muscle interactions, and, in some technologies (e.g., Tendyne), allows for the optimal fixation of the final orientation of TMVI device. The fixation of the final orientation is particularly important to help ensure prosthetic sealing within the native anatomy and minimise the risk of paravalvular regurgitation. Tendyne (Figure 6A) and Intrepid (Figure 6B) are the two most common TMVI platforms that currently use transapical access. A transfemoral system has been developed for Intrepid and has been used with success, although the early-generation device required a transapical delivery system52,53,54.
Figure 6. Mitral transcatheter heart valves.
A) Tendyne. B) Intrepid TMVR system. C) SAPIEN M3 transseptal TMVR system. D) Cephea. E) CardioValve. F) EVOQUE Eos. G) AltaValve. H) HighLife. I) Saturn THV system. THV: transcatheter heart valve; TMVR: transmitral valve replacement
Using the preprocedural CT, a left anterolateral thoracotomy in the appropriate rib space is performed, followed by biplane TOE imaging to determine an access site that is orthogonal to the commissural and anteroposterior planes. Following placement of pledgeted sutures, an 18 gauge access needle is used to place a 6-8 Fr sheath, followed by retrograde wiring of the MV with a 0.035” guidewire. A balloon catheter is used to floss the valve to confirm no chordal crossing. For Tendyne cases, the delivery system is inserted over the wire, de-aired, passed to the left atrium, and rotated while the atrial portion is being extruded into the correct anatomical orientation. The valve is then seated intra-annularly without the need for rapid pacing. The prosthesis is secured in a stable position using a braided, high-molecular-weight polyethylene tether, which is attached to an epicardial pad and adjusted to optimise seating of the prosthesis for MR reduction and to minimise the risk of device displacement. For Intrepid cases, the Intrepid delivery catheter is advanced into the left atrium, with exposure of a circular atrial brim using a hydraulic delivery mechanism. Under TOE imaging, the brim is aligned and retracted to the native valve, using a short run of rapid ventricular pacing during deployment. A number of other TMVI platforms (e.g., AltaValve) have also been deployed with transapical delivery, with similar approaches used with regard to orthogonal access.
TRANSSEPTAL
For transseptal TMVI, there is no defined cut-off for use of percutaneous access, though surgical cut-downs are still commonly performed, as most platforms are currently >30 Fr. For transseptal puncture access, the mitral height requirements and location of the puncture is device-specific, with posterior access of >3.5-4.0 cm being most common. The following are some of the most common examples with human experience and are representative of the field of transseptal TMVI.
Intrepid
The transseptal Intrepid system utilises the same valve as is employed for transapical approaches, mounted on a delivery system that is currently ~35 Fr. Either a straight or curved sheath is used to position the Intrepid valve in the left atrium, followed by steering to the MV with control knobs, without the need for a guidewire. Similar to the transapical approach, the atrial brim is exposed, followed by use of a hydraulic system to deploy the prosthesis under rapid ventricular pacing. Due to its circular configuration, there is no need to rotate the prosthesis for anatomical alignment. The prosthesis anchors with multiple small cleats and a cork-like effect in the MV apparatus.
SAPIEN M3
The SAPIEN M3 comprises a nitinol dock to provide anchoring for a balloon-expandable THV, similar to the SAPIEN 3, with some modifications including a skirt that covers the entire frame to decrease paravalvular leakage (Figure 6C). The SAPIEN M3 platform is a 2-step approach, in which a subvalvular “dock” is first placed in the left ventricle by encircling the MV chordae and leaflets. The anchoring member is placed through the same transseptal access for prosthesis delivery, which is, notably, a relatively low-profile 20 Fr system. The left ventricle is entered at the site of the medial commissure, using the back flexion and the curve of the delivery catheter, followed by passage of the anchor member counterclockwise several times in the surgeon’s view of the MV to place the dock. TOE confirmation of passage around the mitral leaflets in multiple views is essential. A 29 mm prosthesis that shares similarities to the TAVI prosthesis is then passed antegrade and seated within the dock in the MV apparatus using rapid ventricular pacing for deployment, similar to a transseptal mitral valve-in-valve procedure.
Cephea
The Cephea prosthesis is placed into the left atrium using a relatively superior and short transseptal height, steered towards the MV, and passed over a wire into the left ventricle, followed by extrusion of the ventricular portion of the prosthesis (Figure 6D). Posterior torque is then applied to the catheter to gain height on the delivery system and move the prosthesis to the mitral annulus, followed by extrusion of the atrial portion.
CardioValve
The CardioValve system (Figure 6E) is delivered by the transseptal approach using a 28 Fr multisteerable catheter in a 3-step procedure. First, the mitral inflow portion is unsheathed, and the mitral leaflets and subvalvular apparatus are grasped. Then, the atrial flange is exposed, and the device is released.
EVOQUE Eos
The EVOQUE Eos MV is delivered via a 28 Fr system following placement of a Safari wire in the left ventricle via transseptal access (Figure 6F). Control knobs are used to steer the valve to the left ventricle, followed by deployment of a control capsule that holds the ventricular outflow portion. Depth control allows movement of ventricular anchors without loss of coaxiality, leading to engagement of the leaflets and subvalvular apparatus, with confirmation on TOE. The atrial inflow portion with a sealing skirt is then released.
AltaValve
The AltaValve (4C Medical) prosthesis is a low-profile system, where the mitral inflow portion protrudes minimally (<15 mm) into the left ventricle and orients parallel to the outflow tract (Figure 6G). Anchoring is achieved by a nitinol frame that is oversized relative to the left atrium. Placement can be performed via transapical and transseptal approaches. For the latter, the valve is steered towards the native MV and the mitral inflow portion is unsheathed, followed by the use of a positioner catheter to centre the prosthesis in the native MV as the atrial supporting frame is exposed. The entire system is fully retrievable after full deployment.
HighLife
The HighLife (HighLife SAS) system is also a 2-step approach, in which the anchoring member is placed using an 18 Fr guide catheter placed retroaortic (Figure 6H). The encircling of the MV is first performed with a low-profile guidewire that is passed clockwise around the MV, once in the surgeon’s view, followed by insertion of the subannular implant (SAI) ring. During deployment of the SAI, retraction is performed (i.e., cinching) to confirm capture of the mitral leaflets and chords with TOE imaging. Transseptal puncture is then performed. A 28 mm prothesis is placed antegrade, with extrusion of the left ventricular portion distal to the SAI, followed by retraction and exposure of the atrial portion.
Saturn
The Saturn system is a multistep device including a subannular ring and a trileaflet bovine valve (Figure 6I).
AORTIC THV
Aortic devices are used for TMVI for the treatment of patients with severe MAC. The SAPIEN 3 THV is the most used device. Three approaches are mainly used: transapical, transseptal and transatrial. Albeit the transapical access used to be the most frequently used, the transseptal route is gaining popularity and has become the preferred access route30. While the procedure is essentially similar to valve-in-valve and valve-in-ring TMVI, TOE plays a more important role in the positioning of the prosthesis55. Transatrial access may be proposed in operable patients. By allowing the resection of the anterior leaflet and an active fixation of the THV by sutures, this route may be particularly attractive for patients at risk for LVOT obstruction and those with incomplete calcification of the mitral annulus56. Of note, when crimping the THV, the orientation of the THV varies according to the selected access.
CONCOMITANT PROCEDURES
One of the most common exclusion criteria for TMVI candidacy is the risk of LVOT obstruction. Methods to reduce this risk include septal reduction strategies such as pre-emptive alcohol septal ablation (ASA), pre-emptive radiofrequency ablation, and SESAME (Septal Scoring Along the Midline Endocardium), or leaflet laceration strategies such as LAMPOON (Laceration of the Anterior Mitral leaflet to Prevent Outflow ObstructioN). In choosing the method to address LVOT obstruction or its potential obstruction, it is important to note the differences in TMVI designs, as leaflet modification techniques will be relatively less effective for fully covered frames.
For ASA, the procedure is as conventionally performed for the treatment of patients with obstructive hypertrophic cardiomyopathy, but the dose of alcohol is considerably lower owing to the absence of myocardial hypertrophy. Typical doses are 0.5 to 1.0 ml. Very slow injections (e.g., >3 to 5 min) are especially advised with a normal thickness of the myocardium in order to reduce risk of spillage through septal collaterals into the left ventricular cavity. Significant time, approximately 1 month, is required to fully assess the effect on LVOT anatomy, and the remodelling response can be variable. Of note, if performed after TMVI placement, LVOT obstruction can acutely worsen with ASA because of myocardial oedema. Wang and colleagues investigated the use of alcohol septal ablation to prevent LVOT obstruction in patients undergoing valve-in-MAC (ViMAC) procedures. The procedure resulted in a significant increase in the predicted neo-LVOT area but was associated with 10% mortality at 30-day follow-up57. This study collected early experience, and outcomes have improved. A contemporary single-centre study that analysed the outcomes of patients undergoing pre-emptive alcohol septal ablation to reduce the risk of TMVI-induced LVOTO compared with patients who received alcohol ablation to treat hypertrophic obstructive cardiomyopathy found zero mortality at 30 days in the TMVI group58.
For the leaflet modification technique, LAMPOON with either an antegrade or retrograde approach can be performed pre-emptively or, in some cases, after TMVI implantation. The approaches are similar to what have been used for mitral valve-in-valve and valve-in-ring therapies. For TMVI in native valves, preservation of leaflet tissue carries a relatively higher importance for the sealing of the prosthesis to minimise the risk of paravalvular regurgitation.
In a case series including 21 patients with LAMPOON during valve-in-valve or valve-in-ring TMVI, all patients survived to 30 days, and none of them had significant LVOT obstruction at discharge59.
SESAME is a newly described technique in which a coronary catheter and guidewire are used to mechanically enter the basal interventricular septum and traverse the myocardium to a mid-ventricular exit point. The wire is snared and exchanged for a wire that is energised and used to perform a myotomy. Preclinical data have shown promising results60.
Results
DEDICATED DEVICES
For the purposes of this review, we have summarised the published results on dedicated devices that are already being evaluated in pivotal trials. These are the Tendyne MV system, the Intrepid TMVI system and the SAPIEN M3 system. Regarding aortic devices, we report the results of the main registries.
Tendyne Mitral Valve system
The Tendyne Mitral Valve was the first transcatheter MV implanted in humans61, and is the device with most data available. It obtained the CE mark on 30 January 2020.
The Global Feasibility Trial (Expanded Clinical Study of the Tendyne Mitral Valve System; ClinicalTrials.gov: NCT02321514) enrolled symptomatic patients with primary or secondary mitral regurgitation who were at high or prohibitive surgical risk. The primary performance endpoint was reduction of MR to ≤2+ at 1 month post-procedure. The primary safety endpoint was evaluated at 30 days and was a composite of device success and freedom from cardiovascular death, reintervention for valve-related dysfunction, disabling stroke, myocardial infarction, life-threatening bleeding, major vascular complications, renal failure requiring dialysis, and other device- or procedure-related serious adverse events. The results observe in the first 100 patients treated were favourable, with an observed 30-day all-cause mortality of 6%, which was lower than that predicted by the Society of Thoracic Surgeons (STS) score of 7.8±5.7%. The mean age was 75.4±8.1 years, and 31% were women. Technical success was achieved in 96% of the patients, who all met the primary performance endpoint of ≤2+ MR at 1 month (98.8% none or trivial MR and 1.2% 1+ MR)62. The improvement in MR reduction remained stable, with 98.5% having none or trivial MR at 1 year and 93.2% at 2 years63. The Kaplan-Meier estimate of all-cause mortality at 1 year was 27% and 41.6% at 2 years. There was significant improvement of symptoms which was sustained at 2 years, 66% of patients were in New York Heart Assocation (NYHA) Class III or IV at baseline, and 81.6% were in NYHA Functional Class I or II at 2 years (p<0.0001). Similarly, there was a significant increase in quality-of-life scores, the overall Kansas City Cardiomyopathy Questionnaire (KCCQ) score increased from 49.0±22.8 at baseline to 67.2±26.2 at 2 years (p<0.0001). In addition, the annualised heart failure hospitalisation rate decreased from 1.3 to 0.51 (p<0.0001)63.
The early experience with Tendyne in mitral annular calcification has also been favourable. In the initial report of the first 9 patients treated (8 with compassionate use) − the mean age was 77±6 years (44% women), and the mean STS score was 7.4±3.6% − all-cause mortality at 30 days was zero. Technical success was achieved in 8 patients (89%). One patient developed LVOT obstruction due to the inadvertent rotation of a standard profile prosthesis, which was only recognised after surgical closure of the transapical access. This event was successfully treated with alcohol septal ablation. There was no residual MR in any patient64. Given these encouraging results, an MAC feasibility trial (n=11; Feasibility Study of the Tendyne Mitral Valve System in Mitral Annular Calcification; ClinicalTrials.gov: NCT03539458) was initiated. A subsequent publication summarising the 1-year outcomes of 20 patients treated either as compassionate use (n=9) or within the MAC feasibility trial, reports elimination of MR in all patients. The mean age was 78±6 years, and 45% were women. The STS score in patients treated in the MAC feasibility trial was 9.0±8.1%, whereas it was 7.0±3.5% in the compassionate-use group. The 1-year mortality was 40% (20% cardiac). This is higher than the 27% mortality observed in non-calcified valves but similar to the 39.9% 1-year mortality reported in the Mitral Implantation of TRAnscatheter vaLves (MITRAL) Early Feasibility Trial evaluating valve-in-MAC using aortic THVs (ClinicalTrials.gov: NCT02370511)65. Among the 12 survivors at 1 year, 92% were in NYHA Class I or II, and 80% were in NYHA III or IV prior to the procedure. Paired analysis of KCCQ scores was available for 7 survivors. Among these, the mean improvement in KCCQ score was 29.9±26.3 with an increase ≥10 points in 5 (71.4%) patients.
Considering the favourable outcomes in selected MAC patients and the need to develop a treatment option for these high-risk patients, an arm with 103 MAC patients was added to the prospective SUMMIT pivotal trial (Clinical Trial to Evaluate the Safety and Effectiveness of Using the Tendyne Mitral Valve System for the Treatment of Symptomatic MITral Regurgitation; ClinicalTrials.gov: NCT03433274). The SUMMIT trial is the only pivotal trial evaluating mitral transcatheter valves that has a randomised arm against transcatheter edge-to-edge repair with MitraClip for patients who have favourable anatomy for either TEER or TMVI with Tendyne (n=382). In addition, it has a TMVI with Tendyne arm for patients who do not have favourable anatomy for TEER (n=313) or the MAC arm (n=103) mentioned above. Enrolment was completed in 2023.
Intrepid
In the initial report of the first 50 consecutive patients treated, the device was successfully implanted in 48 (96%). The mean age was 73±9 years, and 32% were women. They had multiple comorbidities, the STS score was 6.4±5.5%, and most (86%) were in NYHA Class III or IV at baseline. Among patients who received the implant, MR was mild or absent at 30 days in all patients. At 30 days, 79% were in NYHA Class I or II, and significant improvements in Minnesota Living With Heart Failure (MLWHF) Questionnaire scores (56.2±26.8 vs 31.7±22.1; p=0.011) were observed. Thirty-day mortality was 14%, and Kaplan-Meier analysis estimated survival at 1 year was 76.5%53.
The transfemoral Intrepid EFS trial (TMVR Pilot Study & EFS of the TMVR Transseptal System; ClinicalTrials.gov: NCT02322840) collects outcomes of patients treated with the transfemoral device. In the initial report describing outcomes of the first 15 patients treated with the transfemoral/transseptal Intrepid system (mean age 80 years, STS score 4.7% and 23% female), 14 underwent successful implantation. One patient had conversion to sternotomy due to valve migration during the index procedure. All implanted patients had trace or no MR, and the mean gradient was 4.7±1.8 mmHg at 30 days. Patients experienced an improvement of symptoms, 67% were in NYHA Class III or IV at baseline, and 86% were in NYHA Class I or II at 30 days. There was improvement in KCCQ scores of at least 10 points in 46%, and 39% of patients demonstrated an improvement in the MLWHF questionnaire (defined as a decrease >5 points). The 30-day 6-minute walk test data demonstrated a 50-metre improvement in the median metres walked. There were no deaths at 30 days54.
The APOLLO trial (Transcatheter Mitral Valve Replacement With the Medtronic Intrepid TMVI System in Patients With Severe Symptomatic Mitral Regurgitation; ClinicalTrials.gov: NCT03242642) is a pivotal trial evaluating the transapical Intrepid TMVI system in patients with symptomatic primary or secondary MR who are not candidates for approved transcatheter or surgical MV intervention. It used to have a randomised arm comparing TMVI with Intrepid against standard surgery or TEER. This arm was closed because of poor enrolment and now has a single-arm primary cohort of patients who do not have favourable anatomy for TEER (250-500 patients); an arm of MAC patients (up to 300) was also added. Recruitment is currently ongoing at the time of writing this manuscript.
SAPIEN M3
The initial report of the first-in-human experience included 10 patients (mean age 76.1±5.0 years, STS score 3.8%±2.5% and 50% women). The primary endpoint was technical success, as defined by MVARC criteria, at completion of the index procedure. Technical success was achieved in 90%. At 30 days, MR was ≤1+ in 89% of patients and severe in 1 due to severe PVL; this was successfully reduced to moderate with percutaneous PVL closure. The median transmitral gradient at 30 days was 6 mmHg (Q1, Q3: 5, 6 mmHg), and there was no LVOT obstruction. Functional class improved, all were in NYHA Class III or IV at baseline, and 90% were in Class I or II at follow-up. There were no statistically significant improvements in KCCQ scores (baseline 288 vs 350; p=0.812) or 6-min walk distance (50.00 vs 73.45 metres; p=0.195). There were no deaths at 30 days66. The experience in MAC is limited but growing at the time of writing this manuscript. Clinical outcomes in MAC patients have not been published.
The ENCIRCLE trial (SapiEN M3 system transCatheter mItral valve ReplaCement via transseptaL accEss; ClinicalTrials.gov: NCT04153292) is a prospective multicentre pivotal trial evaluating the SAPIEN M3 valve in symptomatic patients with primary or secondary MR who are not candidates or considered unsuitable for commercially available options. The main cohort of this study aims to enrol 300 patients who are not good candidates for standard surgery or TEER; prior surgical repair with an annuloplasty ring is not an exclusion criterion. It also has a registry arm of failed TEER (n=100), and an arm of MAC patients (n=100) was recently added.
AORTIC TRANSCATHETER VALVES
The largest case series is an international registry including 116 patients at high surgical risk (STS score 15.3±12.0%). In this registry, the rate of technical success was 77%. However, the 30-day mortality was as high as 25%, and at 1 year, 54% had died. LVOT obstruction with haemodynamic compromise was observed in 11.2% of patients and was associated with a 50% mortality rate. The main case series analysing the results of TMVI in patients with MAC are shown in Table 365,67,68,69,70,71. Despite differences regarding 30-day mortality (11-40%), the 1-year mortality rate was near to 50% in all retrospective studies (50% mortality at 1 year in the TMVI in MAC registry and 62.8% in the TMVI Registry). Nonetheless, outcomes improved with experience and better patient selection. In the first prospective clinical trial evaluating ViMAC (Mitral Implantation of TRAnscatheter vaLves [MITRAL] Trial), the role of pre-emptive alcohol septal ablation was implemented and evaluated to decrease the risk for TMVI-induced LVOT obstruction65. A total of 31 high surgical risk patients (mean STS score 8.6±8.2%) underwent TMVI (transseptal=15, transatrial=15, transapical=1) using the SAPIEN family of THVs (XT=2, SAPIEN 3=29). The 30-day mortality in patients treated with transseptal access was 6.7% and in patients treated with surgical transatrial access was 21.4%. At 1 year, all-cause mortality was 34.5% (combined transatrial and transseptal access) in the entire cohort and 26.7% in patients treated with transseptal access. At 2 years, all-cause mortality was 39.9%, and at 5 years was 67.9% (combined transatrial and transseptal access)72.
Table 3. Main results of TMVI in MAC with aortic devices.
| n | Age, years | STS score/ EuroSCORE II | Technical success | 30-day mortality | 30-day LVOT obstruction | 1-year mortality | |
|---|---|---|---|---|---|---|---|
| Guerrero, 201870 | 116 | 78 | 5.3±11.6% | 77.6% | 25.0% | 11.2% | 53.7% |
| Praz, 201868 | 26 | 100 | 9.4±4.8% | 100% | 26.9% | 0% | - |
| Urena, 201869 | 27 | 78 | 7.3 (3.0-14.0) | 77.7% | 11.2% | 7.4% | 41.7% |
| Yoon, 201867 | 58 | 62 | 10.1±6.9% | 62.1% | 34.5% | 39.7% | 62.8% |
| Guerrero, 202071 | 100 | 74 | 10.3 (6.8-17.3) | 74.0% | 21.8% | 10.0% | - |
| Guerrero, 202165 | 31 | 74.5 | 8.6±8.2% | 74.2% | 16.7% | 9.7% | 39.9% |
| EuroSCORE: European System for Cardiac Operative Risk Evaluation; LVOT: left ventricular outflow tract; MAC: mitral annular calcification; STS: Society of Thoracic Surgeons; TMVI: transcatheter mitral valve implantation | |||||||
Unknowns and future directions
The Central illustration provides an overview of the current use of TMVI in patients with native mitral valve disease and summarises the main findings from pivotal trials. TMVI demonstrates promising results in terms of safety and efficacy, and it has the potential to become the preferred therapy for patients with FMR if the durability of dedicated devices is confirmed to be similar to surgical bioprostheses. However, in patients with DMR, when durable repair is feasible, surgery is likely to remain the first-choice therapy even with technological advancements and improved technique. In this scenario, TMVI will be probably restricted to patients considered to be at high surgical risk.
Central illustration. Summarising the main results of TMVI nowadays.

CE: European conformity; CT: computed tomography; MAC: mitral annular calcification; THV: transcatheter heart valve; TMVI: transcatheter mitral valve implantation; TOE: transoesophageal echocardiography; TTE: transthoracic echocardiography
However, there are remaining challenges that need be addressed. One of the main shortcomings is the narrow eligibility criteria, with screening failure rates as high as 89%11,12,13. As patients left untreated have poor outcomes12,13(Figure 7), all efforts should be done to optimise candidacy. The reasons for denying therapy vary across different studies, anatomical criteria are the most frequently reported ones, followed by a concomitant tricuspid regurgitation and the risk of medical futility. Among the anatomical factors, the risk of LVOT obstruction, the size of the mitral annulus, and the presence of MAC are the main reasons. Thus, developing therapies to reduce the risk of LVOT obstruction is crucial for expanding eligibility for TMVI. While most devices are contraindicated in patients with MAC, at least one dedicated device has shown efficacy in these patients, and another is being evaluated. Concomitant severe TR is also frequently reported as a reason to refuse therapy11,12,13. MR is frequently associated with TR, and, therefore, a percutaneous option for treating TR is mandatory for the development of TMVI. Several percutaneous tricuspid repair and replacement therapies are currently in evaluation with promising preliminary results73. Finally, medical futility is another common reason to contraindicate the procedure11,12,13. The use of the transapical approach may increase the risk of futile procedures, particularly in frail patients. Although it has not been determined to what extent the use of the transseptal approach might favour eligibility for TMVI, the transseptal route will probably become the preferred access in the future. Referring patients earlier may also help to prevent futility. The main reasons for non-eligibility and potential strategies to broadening eligibility are displayed in Figure 6.
Figure 7. Non-eligibility for TMVI.
The main reported reasons for non-eligibility for TMVI are the risk of futility, the presence of severe tricuspid regurgitation and anatomical constraints. Potential solutions to improve eligibility for TMVI are proposed. LVOT: left ventricular outflow tract; MAC: mitral annular calcification; THV: transcatheter heart valve; TMVI: transcatheter mitral valve implantation; TR: tricuspid regurgitation
The development of the technology and the enhancement of the technique will probably result in better candidacy. A wider annulus size range − with increased availability of sizes and/or the use of mechanisms to reduce the dimensions of the mitral annulus to fit to available device sizes − mechanisms of trapping the anterior leaflet to reduce the risk of LVOT obstruction, reducing the size of the sheaths with better navigability leading to increased use of the transseptal approach, may address, at least in part, these challenges.
In addition, several unknowns still remain and should be evaluated in future trials. Firstly, the risk of valve thrombosis and the need for long-term anticoagulation as well as the most appropriate antithrombotic therapy (anti-vitamin K vs new anticoagulants) remain unclear. Data from TMVI using aortic THV have revealed a higher risk of valve thrombosis compared to surgical bioprostheses, particularly in the first year after the procedure74. Furthermore, the risk of thrombosis may vary depending on the type of THV, with larger stent frames in the left atrium potentially increasing the risk. Although most cases of thrombosis are subclinical and resolved with optimisation of antithrombotic therapy74, it has been suggested that this might be an early phase of valve degeneration75. Secondly, the decision of whether to systematically close residual atrial septal defects (ASD; sheath diameters ranging between 20-39 Fr) remains unknown. While closure may be necessary for patients with significant tricuspid regurgitation, the decision should be based on weighing the long-term consequences of large ASDs against the potential risks of percutaneous closure (e.g., compromising reintervention). Finally, the durability of dedicated THVs should be demonstrated to be comparable to surgery, and the need and possibility for reintervention are unknown. Furthermore, it remains to be elucidated if the need for reintervention is similar to that of TEER. So far, no head-to-head comparisons have been done between both therapies. Although we might speculate that reintervention will probably be feasible using aortic devices, at least for circular-shaped devices, it has yet to be demonstrated.
The pivotal trials evaluating TMVI devices, which have included a randomised arm that compares the new TMVI device to standard surgery (SUMMIT and APOLLO trials), and ongoing randomised trials comparing TEER and TMVI will shed more light on the roles of each therapy for the treatment of patients with MR. However, since the results of the COAPT trial were published and clinical practice guidelines changed their recommendations to provide TEER with a higher indication than surgery in patients with secondary MR, enrolling in clinical trials has become more challenging. Physicians and patients may find it easier to proceed with TEER, which is considered the new standard of care for FMR, even for patients who do not have an anatomy favourable for TEER. This might result in suboptimal procedural outcomes and poor overall clinical outcomes, as well as setbacks in the development of TMVI. Further efforts must be made to include patients in these trials.
Patients with MAC, in addition to their anatomical factors, pose a clinical challenge regarding the risk of futility. Even when 30-day mortality was low (0-10%), 1-year mortality has been as high as 40%64,65,69. The causes of early death were both cardiovascular and non-cardiovascular, (50% cardiovascular and 50% non-cardiovascular), heart failure being the main cause of cardiovascular death in one study76. Thus, much effort should be made in order to identify patients who will ultimately benefit from this therapy. Of note, in the MITRAL Trial, for patients treated by transseptal access, all-cause mortality was 26.7%65, which is similar to the mortality observed in patients treated with TEER using MitraClip in the US77. Whether the use of the transseptal approach might improve survival in these patients should be confirmed in future studies. Likewise, all-cause mortality at 2 years was 39.9% (combined transatrial and transseptal access)78, which is comparable to the 43.3% mortality rate at 2 years in extremely high surgical risk patients treated with TAVI in the PARTNER 1 trial79. However, the prognosis of patients with MAC is not fully understood, and it is likely that other factors impacting the survival of these patients should be identified. In the meantime, before considering TMVI valve-in-MAC, patients should be assessed for the risk of clinical futility, and anatomical factors increasing the risk of periprocedural complications should be ruled out.
Conclusions
The initial results of TMVI for the treatment of native MV disease using both dedicated and aortic THVs are encouraging. However, eligibility criteria for TMVI are narrow, and only a few patients ultimately qualify for this therapy. The development of concomitant therapies aimed at reducing the risk of LVOT obstruction, the advancement of percutaneous therapies for treating TR and the wider use of the transseptal approach are likely to broaden the eligibility criteria for TMVI and to facilitate its incorporation into clinical practice. Future research will determine the role of this therapy in the management of native MV disease.
Acknowledgments
Conflict of interest statement
M. Urena has received speaker honoraria from Edwards Lifesciences. P. Lurz has received institutional research grant support from Edwards Lifesciences and ReCor. P. Sorajja is on the advisory boards for Anteris, Abbott Structural, Boston Scientific, Medtronic, and VDyne; is a consultant for Anteris, Abbott Structural, Boston Scientific, Evolution Medical, Half Moon Medical, Medtronic, Neovasc, TeleFlex, Shifamed, TriFlo, and W.L. Gore & Associates; is on the executive committees of Abbott Structural, Medtronic, Neovasc, and W.L. Gore & Associates; and is a National or Global Principal Investigator for Abbott Structural, Medtronic, HighLife, and VDyne. D. Himbert is a proctor for Abbott and Edwards Lifesciences. M. Guerrero has received institutional research grant support from Edwards Lifesciences.
Abbreviations
- CT
computed tomography
- DMR
degenerative mitral regurgitation
- FMR
functional mitral regurgitation
- LAMPOON
Laceration of the Anterior Mitral leaflet to Prevent Outflow ObtructioN
- LVOT
left ventricular outflow tract
- MAC
mitral annular calcification
- MR
mitral regurgitation
- MV
mitral valve
- SAI
subannular implant
- SESAME
Septal Scoring Along the Midline Endocardium
- TAVI
transcatheter aortic valve implantation
- TOE
transoesophageal echography
- TEER
transcatheter edge-to-edge repair
- THV
transcatheter heart valve
- TMVI
transcatheter mitral valve implantation
- TR
tricuspid regurgitation
- ASA
alcohol septal ablation
Contributor Information
Marina Urena, Department of Cardiology, Hôpital Bichat Claude-Bernard, Assistance Publique Hôpitaux de Paris, Université Paris Cité, Paris, France.
Philipp Lurz, Department of Cardiology, Zentrum für Kardiologie, Universitätsmedizin Mainz, Mainz, Germany.
Paul Sorajja, Department of Cardiology, Allina Health Minneapolis Heart Institute, Abbott Northwestern Hospital, Minneapolis, MN, USA.
Dominique Himbert, Department of Cardiology, Hôpital Bichat Claude-Bernard, Assistance Publique Hôpitaux de Paris, Université Paris Cité, Paris, France.
Mayra Guerrero, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA.
References
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis, De Paulis, Delgado V, Freemantle N, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. EuroIntervention. 2022;17:e1126–96. doi: 10.4244/EIJ-E-21-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing Committee Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:e25–197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- Iung B, Delgado V, Rosenhek R, Price S, Prendergast B, Wendler O, Bonis MD, Tribouilloy C, Evangelista A, Bogachev-Prokophiev A, Apor A, Ince H, Laroche C, Popescu BA, Piérard L, Haude M, Hindricks G, Ruschitzka F, Windecker S, Bax JJ, Maggioni A, Vahanian A EORP VHD II Investigators. Contemporary Presentation and Management of Valvular Heart Disease: The EURObservational Research Programme Valvular Heart Disease II Survey. Circulation. 2019;140:1156–69. doi: 10.1161/CIRCULATIONAHA.119.041080. [DOI] [PubMed] [Google Scholar]
- Iung B, Delgado V, Lazure P, Murray S, Sirnes PA, Rosenhek R, Price S, Metra M, Carrera C, De Bonis, Haude M, Hindricks G, Bax J, Vahanian A. Educational needs and application of guidelines in the management of patients with mitral regurgitation. A European mixed-methods study. Eur Heart J. 2018;39:1295–303. doi: 10.1093/eurheartj/ehx763. [DOI] [PubMed] [Google Scholar]
- Mirabel M, Iung B, Baron G, Messika-Zeitoun D, Détaint D, Vanoverschelde JL, Butchart EG, Ravaud P, Vahanian A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28:1358–65. doi: 10.1093/eurheartj/ehm001. [DOI] [PubMed] [Google Scholar]
- Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ COAPT Investigators. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med. 2018;379:2307–18. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- Feldman T, Kar S, Elmariah S, Smart SC, Trento A, Siegel RJ, Apruzzese P, Fail P, Rinaldi MJ, Smalling RW, Hermiller JB, Heimansohn D, Gray WA, Grayburn PA, Mack MJ, Lim DS, Ailawadi G, Herrmann HC, Acker MA, Silvestry FE, Foster E, Wang A, Glower DD, Mauri L EVEREST II Investigators. Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation: 5-Year Results of EVEREST II. J Am Coll Cardiol. 2015;66:2844–54. doi: 10.1016/j.jacc.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L EVEREST II Investigators. Percutaneous Repair or Surgery for Mitral Regurgitation. N Engl J Med. 2011;364:1395–406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- Obadia JF, Armoiry X, Iung B, Lefèvre T, Mewton N, Messika-Zeitoun D, Cormier B, Berthiller J, Maucort-Boulch D, Boutitie F, Vaz B, Trochu JN, Vahanian A. The MITRA-FR study: design and rationale of a randomised study of percutaneous mitral valve repair compared with optimal medical management alone for severe secondary mitral regurgitation. EuroIntervention. 2015;10:1354–60. doi: 10.4244/EIJV10I11A232. [DOI] [PubMed] [Google Scholar]
- Hensey M, Brown RA, Lal S, Sathananthan J, Ye J, Cheung A, Blanke P, Leipsic J, Moss R, Boone R, Webb JG. Transcatheter Mitral Valve Replacement: An Update on Current Techniques, Technologies, and Future Directions. JACC Cardiovasc Interv. 2021;14:489–500. doi: 10.1016/j.jcin.2020.12.038. [DOI] [PubMed] [Google Scholar]
- Urena M, Vahanian A, Søndergaard L. Patient selection for transcatheter mitral valve implantation: why is it so hard to find patients? EuroIntervention. 2018;14:AB83–90. doi: 10.4244/EIJ-D-18-00510. [DOI] [PubMed] [Google Scholar]
- Ben Ali, Ludwig S, Duncan A, Weimann J, Nickenig G, Tanaka T, Coisne A, Vincentelli A, Makkar R, Webb JG, Akodad M, Muller DWM, Praz F, Wild MG, Hausleiter J, Goel SS, von Ballmoos, Denti P, Chehab O, Redwood S, Dahle G, Baldus S, Adam M, Ruge H, Lange R, Kaneko T, Leroux L, Dumonteil N, Tchetche D, Treede H, Flagiello M, Obadia JF, Walther T, Taramasso M, Søndergaard L, Bleiziffer S, Rudolph TK, Fam N, Kempfert J, Granada JF, Tang GHL, von Bardeleben, Conradi L, Modine T CHOICE-MI Investigators. Characteristics and outcomes of patients screened for transcatheter mitral valve implantation: 1-year results from the CHOICE-MI registry. Eur J Heart Fail. 2022;24:887–98. doi: 10.1002/ejhf.2492. [DOI] [PubMed] [Google Scholar]
- Niikura H, Gössl M, Kshettry V, Olson S, Sun B, Askew J, Stanberry L, Garberich R, Tang L, Lesser J, Bae R, Harris KM, Bradley SM, Sorajja P. Causes and Clinical Outcomes of Patients Who Are Ineligible for Transcatheter Mitral Valve Replacement. JACC Cardiovasc Interv. 2019;12:196–204. doi: 10.1016/j.jcin.2018.10.042. [DOI] [PubMed] [Google Scholar]
- Wyler von, Kalra A, Reardon MJ. Complexities of transcatheter mitral valve replacement (TMVR) and why it is not transcatheter aortic valve replacement (TAVR). Ann Cardiothorac Surg. 2018;7:724–30. doi: 10.21037/acs.2018.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–43. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- Mack M, Carroll JD, Thourani V, Vemulapalli S, Squiers J, Manandhar P, Deeb GM, Batchelor W, Herrmann HC, Cohen DJ, Hanzel G, Gleason T, Kirtane A, Desai N, Guibone K, Hardy K, Michaels J, DiMaio JM, Christensen B, Fitzgerald S, Krohn C, Brindis RG, Masoudi F, Bavaria J. Transcatheter Mitral Valve Therapy in the United States: A Report From the STS-ACC TVT Registry. J Am Coll Cardiol. 2021;78:2326–53. doi: 10.1016/j.jacc.2021.07.058. [DOI] [PubMed] [Google Scholar]
- Van Mieghem, Piazza N, Anderson RH, Tzikas A, Nieman K, De Laat, McGhie JS, Geleijnse ML, Feldman T, Serruys PW, de Jaegere. Anatomy of the mitral valvular complex and its implications for transcatheter interventions for mitral regurgitation. J Am Coll Cardiol. 2010;56:617–26. doi: 10.1016/j.jacc.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Guerrero M, Dvir D, Himbert D, Urena M, Eleid M, Wang DD, Greenbaum A, Mahadevan VS, Holzhey D, O’Hair D, Dumonteil N, Rodes-Cabau J, Piazza N, Palma JH, DeLago A, Ferrari E, Witkowski A, Wendler O, Kornowski R, Martinez-Clark P, Ciaburri D, Shemin R, Alnasser S, McAllister D, Bena M, Kerendi F, Pavlides G, Sobrinho JJ, Attizzani GF, George I, Nickenig G, Fassa AA, Cribier A, Bapat V, Feldman T, Rihal C, Vahanian A, Webb J, O’Neill W. Transcatheter Mitral Valve Replacement in Native Mitral Valve Disease With Severe Mitral Annular Calcification: Results From the First Multicenter Global Registry. JACC Cardiovasc Interv. 2016;9:1361–71. doi: 10.1016/j.jcin.2016.04.022. [DOI] [PubMed] [Google Scholar]
- Naoum C, Leipsic J, Cheung A, Ye J, Bilbey N, Mak G, Berger A, Dvir D, Arepalli C, Grewal J, Muller D, Murphy D, Hague C, Piazza N, Webb J, Blanke P. Mitral Annular Dimensions and Geometry in Patients With Functional Mitral Regurgitation and Mitral Valve Prolapse: Implications for Transcatheter Mitral Valve Implantation. JACC Cardiovasc Imaging. 2016;9:269–80. doi: 10.1016/j.jcmg.2015.08.022. [DOI] [PubMed] [Google Scholar]
- Elmariah S, Fearon WF, Inglessis I, Vlahakes GJ, Lindman BR, Alu MC, Crowley A, Kodali S, Leon MB, Svensson L, Pibarot P, Hahn RT, Thourani VH, Palacios IF, Miller DC, Douglas PS, Passeri JJ PARTNER Trial Investigators and PARTNER Publications Office. Transapical Transcatheter Aortic Valve Replacement Is Associated With Increased Cardiac Mortality in Patients With Left Ventricular Dysfunction: Insights From the PARTNER I Trial. JACC Cardiovasc Interv. 2017;10:2414–22. doi: 10.1016/j.jcin.2017.09.023. [DOI] [PubMed] [Google Scholar]
- Whisenant B, Kapadia SR, Eleid MF, Kodali SK, McCabe JM, Krishnaswamy A, Morse M, Smalling RW, Reisman M, Mack M, O’Neill WW, Bapat VN, Leon MB, Rihal CS, Makkar RR, Guerrero M. One-Year Outcomes of Mitral Valve-in-Valve Using the SAPIEN 3 Transcatheter Heart Valve. JAMA Cardiol. 2020;5:1245–152. doi: 10.1001/jamacardio.2020.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regueiro A, Granada JF, Dagenais F, Rodés-Cabau J. Transcatheter Mitral Valve Replacement: Insights From Early Clinical Experience and Future Challenges. J Am Coll Cardiol. 2017;69:2175–92. doi: 10.1016/j.jacc.2017.02.045. [DOI] [PubMed] [Google Scholar]
- Guerrero M, Urena M, Pursnani A, Wang DD, Vahanian A, O’Neill W, Feldman T, Himbert D. Balloon expandable transcatheter heart valves for native mitral valve disease with severe mitral annular calcification. J Cardiovasc Surg (Torino) 2016;57:401–9. [PubMed] [Google Scholar]
- Silbiger JJ. Anatomy, mechanics, and pathophysiology of the mitral annulus. Am Heart J. 2012;164:163–76. doi: 10.1016/j.ahj.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- Fox E, Harkins D, Taylor H, McMullan M, Han H, Samdarshi T, Garrison R, Skelton T. Epidemiology of mitral annular calcification and its predictive value for coronary events in African Americans: the Jackson Cohort of the Atherosclerotic Risk in Communities Study. Am Heart J. 2004;148:979–84. doi: 10.1016/j.ahj.2004.05.048. [DOI] [PubMed] [Google Scholar]
- Carpentier AF, Pellerin M, Fuzellier JF, Relland JY. Extensive calcification of the mitral valve anulus: pathology and surgical management. J Thorac Cardiovasc Surg. 1996;111:718–29. doi: 10.1016/s0022-5223(96)70332-x. [DOI] [PubMed] [Google Scholar]
- Reddy YNV, Murgo JP, Nishimura RA. Complexity of Defining Severe “Stenosis” From Mitral Annular Calcification. Circulation. 2019;140:523–5. doi: 10.1161/CIRCULATIONAHA.119.040095. [DOI] [PubMed] [Google Scholar]
- Massera D, Kizer JR, Dweck MR. Mechanisms of mitral annular calcification. Trends Cardiovasc Med. 2020;30:289–95. doi: 10.1016/j.tcm.2019.07.011. [DOI] [PubMed] [Google Scholar]
- Urena M, Himbert H, Brochet E, Carrasco JL, Iung B, Nataf P, Vahanian A. Transseptal Transcatheter Mitral Valve Replacement Using Balloon-Expandable Transcatheter Heart Valves. A Step-by-Step Approach. JACC Cardiovasc Interv. 2017;10:1905–19. doi: 10.1016/j.jcin.2017.06.069. [DOI] [PubMed] [Google Scholar]
- Douglas PS, Carabello BA, Lang RM, Lopez L, Pellikka PA, Picard MH, Thomas JD, Varghese P, Wang TY, Weissman NJ, Wilgus R. 2019 ACC/AHA/ASE Key Data Elements and Definitions for Transthoracic Echocardiography: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards) and the American Society of Echocardiography. J Am Coll Cardiol. 2019;7:403–69. doi: 10.1016/j.jacc.2019.02.027. [DOI] [PubMed] [Google Scholar]
- Natarajan N, Patel P, Bartel T, Kapadia S, Navia J, Stewart W, Tuzcu EM, Schoenhagen P. Peri-procedural imaging for transcatheter mitral valve replacement. Cardiovasc Diagn Ther. 2016;6:144–59. doi: 10.21037/cdt.2016.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SH, Bleiziffer S, Latib A, Eschenbach L, Ancona M, Vincent F, Kim WK, Unbehaum A, Asami M, Dhoble A, Silaschi M, Frangieh AH, Veulemans V, Tang GHL, Kuwata S, Rampat R, Schmidt T, Patel AJ, Nicz PFG, Nombela-Franco L, Kini A, Kitamura M, Sharma R, Chakravarty T, Hildick-Smith D, Arnold M, de Brito, Jensen C, Jung C, Jilaihawi H, Smalling RW, Maisano F, Kasel AM, Treede H, Kempfert J, Pilgrim T, Kar S, Bapat V, Whisenant BK, Van Belle, Delgado V, Modine T, Bax JJ, Makkar RR. Predictors of Left Ventricular Outflow Tract Obstruction After Transcatheter Mitral Valve Replacement. JACC Cardiovasc Interv. 2019;12:182–93. doi: 10.1016/j.jcin.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Blanke P, Dvir D, Cheung A, Ye J, Levine RA, Precious B, Berger A, Stub D, Hague C, Murphy D, Thompson C, Munt B, Moss R, Boone R, Wood D, Pache G, Webb J, Leipsic J. A simplified D-shaped model of the mitral annulus to facilitate CT-based sizing before transcatheter mitral valve implantation. J Cardiovasc Comput Tomogr. 2014;8:459–67. doi: 10.1016/j.jcct.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnken AH, Mühlenbruch G, Das M, Wildberger JE, Kühl HP, Günther RW, Kelm M, Koos R. MDCT detection of mitral valve calcification: prevalence and clinical relevance compared with echocardiography. AJR Am J Roentgenol. 2007;188:1264–9. doi: 10.2214/AJR.06.1002. [DOI] [PubMed] [Google Scholar]
- Guerrero M, Wang DD, Pursnani A, Eleid M, Khalique O, Urena M, Salinger M, Kodali S, Kaptzan T, Lewis B, Kato N, Cajigas HM, Wendler O, Holzhey D, Pershad A, Witzke C, Alnasser S, Tang GHL, Grubb K, Reisman M, Blanke P, Leipsic J, Williamson E, Pellikka PA, Pislaru S, Crestanello J, Himbert D, Vahanian A, Webb J, Hahn RT, Leon M, George I, Bapat V, O’Neill W, Rihal C. A Cardiac Computed Tomography-Based Score to Categorize Mitral Annular Calcification Severity and Predict Valve Embolization. JACC Cardiovasc Imaging. 2020;13:1945–57. doi: 10.1016/j.jcmg.2020.03.013. [DOI] [PubMed] [Google Scholar]
- Stone GW, Adams DH, Abraham WT, Kappetein AP, Généreux P, Vranckx P, Mehran R, Kuck KH, Leon MB, Piazza N, Head SJ, Filippatos G, Vahanian AS Mitral Valve Academic Research Consortium (MVARC) Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 2: endpoint definitions: A consensus document from the Mitral Valve Academic Research Consortium. Eur Heart J. 2015;36:1878–91. doi: 10.1093/eurheartj/ehv333. [DOI] [PubMed] [Google Scholar]
- Murphy DJ, Ge Y, Don CW, Keraliya A, Aghayev A, Morgan R, Galper B, Bhatt DL, Kaneko T, Carli MD, Shah P, Steigner M, Blankstein R. Use of Cardiac Computerized Tomography to Predict Neo Left Ventricular Outflow Tract Obstruction Before Transcatheter Mitral Valve Replacement. J Am Heart Assoc. 2017;6:e007353. doi: 10.1161/JAHA.117.007353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke P, Naoum C, Dvir D, Bapat V, Ong K, Muller D, Cheung A, Ye J, Min JK, Piazza N, Theriault-Lauzier P, Webb J, Leipsic J. Predicting LVOT Obstruction in Transcatheter Mitral Valve Implantation: Concept of the Neo-LVOT. JACC Cardiovasc Imaging. 2017;10:482–5. doi: 10.1016/j.jcmg.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Meduri CU, Reardon MJ, Lim DS, Howard E, Dunnington G, Lee DP, Liang D, Gooley R, O’Hair D, Ng MK, Walton A, Spargias K, Blackman D, Coisne A, Hildick-Smith D, De Gouy, Chenoweth S, Kar S, McCarthy PM, Piazza N, Qasam A, Martin RP, Leon MB, Mack MJ, Adams DH, Bapat V. Novel Multiphase Assessment for Predicting Left Ventricular Outflow Tract Obstruction Before Transcatheter Mitral Valve Replacement. JACC Cardiovasc Interv. 2019;12:2402–12. doi: 10.1016/j.jcin.2019.06.015. [DOI] [PubMed] [Google Scholar]
- Blanke P, Park JK, Grayburn P, Naoum C, Ong K, Kohli K, Norgaard BL, Webb JG, Popma J, Boshell D, Sorajja P, Muller D, Leipsic J. Left ventricular access point determination for a coaxial approach to the mitral annular landing zone in transcatheter mitral valve replacement. J Cardiovasc Comput Tomogr. 2017;11:281–7. doi: 10.1016/j.jcct.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Blanke P, Dvir D, Naoum C, Cheung A, Ye J, Theriault-Lauzier P, Spaziano M, Boone RH, Wood DA, Piazza N, Webb JG, Leipsic J. Prediction of fluoroscopic angulation and coronary sinus location by CT in the context of transcatheter mitral valve implantation. J Cardiovasc Comput Tomogr. 2015;9:183–92. doi: 10.1016/j.jcct.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Weir-McCall JR, Blanke P, Naoum C, Delgado V, Bax JJ, Leipsic J. Mitral Valve Imaging with CT: Relationship with Transcatheter Mitral Valve Interventions. Radiology. 2018;288:638–55. doi: 10.1148/radiol.2018172758. [DOI] [PubMed] [Google Scholar]
- Ranganath P, Moore A, Guerrero M, Collins J, Foley T, Williamson E, Rajiah P. CT for Pre- and Postprocedural Evaluation of Transcatheter Mitral Valve Replacement. Radiographics. 2020;40:1528–53. doi: 10.1148/rg.2020200027. [DOI] [PubMed] [Google Scholar]
- David TE, Armstrong S, McCrindle BW, Manlhiot C. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation. 2013;127:1485–92. doi: 10.1161/CIRCULATIONAHA.112.000699. [DOI] [PubMed] [Google Scholar]
- David TE, David CM, Tsang W, Lafreniere-Roula M, Manlhiot C. Long-Term Results of Mitral Valve Repair for Regurgitation Due to Leaflet Prolapse. J Am Coll Cardiol. 2019;74:1044–53. doi: 10.1016/j.jacc.2019.06.052. [DOI] [PubMed] [Google Scholar]
- Conradi L, Treede H, Rudolph V, Graumüller P, Lubos E, Baldus S, Blankenberg S, Reichenspurner H. Surgical or percutaneous mitral valve repair for secondary mitral regurgitation: comparison of patient characteristics and clinical outcomes. Eur J Cardiothorac Surg. 2013;44:490–6. doi: 10.1093/ejcts/ezt036. [DOI] [PubMed] [Google Scholar]
- Hausleiter J, Stocker TJ, Adamo M, Karam N, Swaans MJ, Praz F. Mitral valve transcatheter edge-to-edge repair. EuroIntervention. 2023;18:957–76. doi: 10.4244/EIJ-D-22-00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DS, Herrmann HC, Grayburn P, Koulogiannis K, Ailawadi G, Williams M, Ng VG, Chau KH, Sorajja P, Smith RL, Guerrero M, Daniels D, Granada JF, Mack MJ, Leon MB, McCarthy P. Consensus Document on Non-Suitability for Transcatheter Mitral Valve Repair by Edge-to-Edge Therapy. Structural Heart. 2021;5:227–33. [Google Scholar]
- Lazam S, Vanoverschelde JL, Tribouilloy C, Grigioni F, Suri RM, Avierinos JF, de Meester, Barbieri A, Rusinaru D, Russo A, Pasquet A, Michelena HI, Huebner M, Maalouf J, Clavel MA, Szymanski C, Enriquez-Sarano M MIDA (Mitral Regurgitation International Database) Investigators. Twenty-Year Outcome After Mitral Repair Versus Replacement for Severe Degenerative Mitral Regurgitation: Analysis of a Large, Prospective, Multicenter, International Registry. Circulation. 2017;135:410–22. doi: 10.1161/CIRCULATIONAHA.116.023340. [DOI] [PubMed] [Google Scholar]
- Ludwig S, Kalbacher D, Ali WB, Weimann J, Adam M, Duncan A, Webb JG, Windecker S, Orban M, Giannini C, Coisne A, Karam N, Scotti A, Sondergaard L, Adamo M, Muller DWM, Butter C, Denti P, Melica B, Regazzoli D, Garatti A, Schmidt T, Andreas M, Dahle G, Taramasso M, Nickenig G, Dumonteil N, Walther T, Flagiello M, Kempfert J, Fam N, Ruge H, Rudolph TK, Wyler von, Metra M, Redwood S, Granada JF, Tang GHL, Latib A, Lurz P, von Bardeleben, Modine T, Hausleiter J, Conradi L the CHOICE-MI and the EuroSMR Investigators (see online Appendix S1) Transcatheter mitral valve replacement or repair for secondary mitral regurgitation: a propensity score-matched analysis. Eur J Heart Fail. 2023;25:399–410. doi: 10.1002/ejhf.2797. [DOI] [PubMed] [Google Scholar]
- Muller DWM, Farivar RS, Jansz P, Bae R, Walters D, Clarke A, Grayburn PA, Stoler RC, Dahle G, Rein KA, Shaw M, Scalia GM, Guerrero M, Pearson P, Kapadia S, Gillinov M, Pichard A, Corso P, Popma J, Chuang M, Blanke P, Leipsic J, Sorajja P Tendyne Global Feasibility Trial Investigators. Transcatheter Mitral Valve Replacement for Patients With Symptomatic Mitral Regurgitation: A Global Feasibility Trial. J Am Coll Cardiol. 2017;69:381–91. doi: 10.1016/j.jacc.2016.10.068. [DOI] [PubMed] [Google Scholar]
- Bapat V, Rajagopal V, Meduri C, Farivar RS, Walton A, Duffy SJ, Gooley R, Almeida A, Reardon MJ, Kleiman NS, Spargias K, Pattakos S, Ng MK, Wilson M, Adams DH, Leon M, Mack MJ, Chenoweth S, Sorajja P Intrepid Global Pilot Study Investigators. Early Experience With New Transcatheter Mitral Valve Replacement. J Am Coll Cardiol. 2018;71:12–21. doi: 10.1016/j.jacc.2017.10.061. [DOI] [PubMed] [Google Scholar]
- Zahr F, Song HK, Chadderdon SM, Gada H, Mumtaz M, Byrne T, Kirshner M, Bajwa T, Weiss E, Kodali S, George I, Heiser J, Merhi WM, Thaden JJ, Zhang A, Lim DS, Reardon MJ, Adams DH, Mack MJ, Leon MB. 30-Day Outcomes Following Transfemoral Transseptal Transcatheter Mitral Valve Replacement: Intrepid TMVR Early Feasibility Study Results. JACC Cardiovasc Interv. 2022;15:80–9. doi: 10.1016/j.jcin.2021.10.018. [DOI] [PubMed] [Google Scholar]
- Urena M, Vahanian A, Brochet E, Ducrocq G, Iung B, Himbert D. Current Indications for Transcatheter Mitral Valve Replacement Using Transcatheter Aortic Valves: Valve-in-Valve, Valve-in-Ring, and Valve-in-Mitral Annulus Calcification. Circulation. 2021;143:178–96. doi: 10.1161/CIRCULATIONAHA.120.048147. [DOI] [PubMed] [Google Scholar]
- Russell HM, Guerrero ME, Salinger MH, Manzuk MA, Pursnani AK, Wang D, Nemeh H, Sakhuja R, Melnitchouk S, Pershad A, Fang HK, Said SM, Kauten J, Tang GHL, Aldea G, Feldman TE, Bapat VN, George IM. Open Atrial Transcatheter Mitral Valve Replacement in Patients With Mitral Annular Calcification. J Am Coll Cardiol. 2018;72:1437–48. doi: 10.1016/j.jacc.2018.07.033. [DOI] [PubMed] [Google Scholar]
- Wang DD, Eng MH, Greenbaum AB, Myers E, Forbes M, Karabon P, Pantelic M, Song T, Nadig J, Guerrero M, O’Neill WW. Validating a prediction modeling tool for left ventricular outflow tract (LVOT) obstruction after transcatheter mitral valve replacement (TMVR). Catheter Cardiovasc Interv. 2017;92:379–87. doi: 10.1002/ccd.27447. [DOI] [PubMed] [Google Scholar]
- Elhadi M, Guerrero M, Collins JD, Rihal CS, Eleid MF. Safety and Outcomes of Alcohol Septal Ablation Prior to Transcatheter Mitral Valve Replacement. JSCAI. 2022;1:100396. doi: 10.1016/j.jscai.2022.100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisko JC, Babaliaros VC, Khan JM, Kamioka N, Gleason PT, Paone G, Byku I, Tiwana J, McCabe JM, Cherukuri K, Khalil R, Lasorda D, Goel SS, Kleiman NS, Reardon MJ, Daniels DV, Spies C, Mahoney P, Case BC, Whisenant BK, Yadav PK, Condado JF, Koch R, Grubb KJ, Bruce CG, Rogers T, Lederman RJ, Greenbaum AB. Tip-to-Base LAMPOON for Transcatheter Mitral Valve Replacement With a Protected Mitral Annulus. JACC Cardiovasc Interv. 2021;14:541–50. doi: 10.1016/j.jcin.2020.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan JM, Bruce CG, Greenbaum AB, Babaliaros VC, Jaimes AE, Schenke WH, Ramasawmy R, Seemann F, Herzka DA, Rogers T, Eckhaus MA, Campbell-Washburn A, Guyton RA, Lederman RJ. Transcatheter Myotomy to Relieve Left Ventricular Outflow Tract Obstruction: The Septal Scoring Along the Midline Endocardium Procedure in Animals. Circ Cardiovasc Interv. 2022;15:e011686. doi: 10.1161/CIRCINTERVENTIONS.121.011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter G, Lozonschi L, Ebner A, Gallo S, Marin y, Missov E, de Marchena. First-in-human off-pump transcatheter mitral valve replacement. JACC Cardiovasc Interv. 2014;7:1077–8. doi: 10.1016/j.jcin.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Sorajja P, Moat N, Badhwar V, Walters D, Paone G, Bethea B, Bae R, Dahle G, Mumtaz M, Grayburn P, Kapadia S, Babaliaros V, Guerrero M, Satler L, Thourani V, Bedogni F, Rizik D, Denti P, Dumonteil N, Modine T, Sinhal A, Chuang ML, Popma JJ, Blanke P, Leipsic J, Muller D. Initial Feasibility Study of a New Transcatheter Mitral Prosthesis: The First 100 Patients. J Am Coll Cardiol. 2019;73:1250–60. doi: 10.1016/j.jacc.2018.12.066. [DOI] [PubMed] [Google Scholar]
- Muller DWM, Sorajja P, Duncan A, Bethea B, Dahle G, Grayburn P, Babaliaros V, Guerrero M, Thourani VH, Bedogni F, Denti P, Dumonteil N, Modine T, Jansz P, Chuang ML, Blanke P, Leipsic J, Badhwar V. 2-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Severe Symptomatic Mitral Regurgitation. J Am Coll Cardiol. 2021;78:1847–59. doi: 10.1016/j.jacc.2021.08.060. [DOI] [PubMed] [Google Scholar]
- Sorajja P, Gossl M, Babaliaros V, Rizik D, Conradi L, Bae R, Burke RF, Schafer U, Lisko JC, Riley RD, Guyton R, Dumonteil N, Berthoumieu P, Tchetche D, Blanke P, Cavalcante JL, Sun B. Novel Transcatheter Mitral Valve Prosthesis for Patients With Severe Mitral Annular Calcification. J Am Coll Cardiol. 2019;74:1431–40. doi: 10.1016/j.jacc.2019.07.069. [DOI] [PubMed] [Google Scholar]
- Guerrero M, Wang DD, Eleid MF, Pursnani A, Salinger M, Russell HM, Kodali SK, George I, Bapat VN, Dangas GD, Tang GHL, Inglesis I, Meduri CU, Palacios I, Reisman M, Whisenant BK, Jermihov A, Kaptzan T, Lewis BR, Tommaso C, Krause P, Thaden J, Oh JK, Douglas PS, Hahn RT, Leon MB, Rihal CS, Feldman T, O’Neill WW. Prospective Study of TMVR Using Balloon-Expandable Aortic Transcatheter Valves in MAC: MITRAL Trial 1-Year Outcomes. JACC Cardiovasc Interv. 2021;14:830–45. doi: 10.1016/j.jcin.2021.01.052. [DOI] [PubMed] [Google Scholar]
- Webb JG, Murdoch DJ, Boone RH, Moss R, Attinger-Toller A, Blanke P, Cheung A, Hensey M, Leipsic J, Ong K, Sathananthan J, Wood DA, Ye J, Tartara P. Percutaneous Transcatheter Mitral Valve Replacement: First-in-Human Experience With a New Transseptal System. J Am Coll Cardiol. 2019;73:1239–46. doi: 10.1016/j.jacc.2018.12.065. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Whisenant BK, Bleiziffer S, Delgado V, Dhoble A, Schofer N, Eschenbach L, Bansal E, Murdoch DJ, Ancona M, Schmidt T, Yzeiraj E, Vincent F, Niikura H, Kim WK, Asami M, Unbehaun A, Hirji S, Fujita B, Silaschi M, Tang GHL, Kuwata S, Wong SC, Frangieh AH, Barker CM, Davies JE, Lauten A, Deuschl F, Nombela-Franco L, Rampat R, Nicz PFG, Masson JB, Wijeysundera HC, Sievert H, Blackman DJ, Gutierrez-Ibanes E, Sugiyama D, Chakravarty T, Hildick-Smith D, de Brito, Jensen C, Jung C, Smalling RW, Arnold M, Redwood S, Kasel AM, Maisano F, Treede H, Ensminger SM, Kar S, Kaneko T, Pilgrim T, Sorajja P, Van Belle, Prendergast BD, Bapat V, Modine T, Schofer J, Frerker C, Kempfert J, Attizzani GF, Latib A, Schaefer U, Webb JG, Bax JJ, Makkar RR. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J. 2019;40:441–51. doi: 10.1093/eurheartj/ehy590. [DOI] [PubMed] [Google Scholar]
- Praz F, Khalique OK, Lee R, Veeragandham R, Russell H, Guerrero M, Islam AM, Deaton DW, Kaneko T, Kodali SK, Leon MB, Bapat V, Takayama H, Borger MA, George I. Transatrial implantation of a transcatheter heart valve for severe mitral annular calcification. J Thorac Cardiovasc Surg. 2018;156:132–42. doi: 10.1016/j.jtcvs.2018.03.016. [DOI] [PubMed] [Google Scholar]
- Urena M, Brochet E, Lecomte M, Kerneis C, Carrasco JL, Ghodbane W, Abtan J, Alkhoder S, Raffoul R, Iung B, Nataf P, Vahanian A, Himbert D. Clinical and haemodynamic outcomes of balloon-expandable transcatheter mitral valve implantation: a 7-year experience. Eur Heart J. 2018;39:2679–89. doi: 10.1093/eurheartj/ehy271. [DOI] [PubMed] [Google Scholar]
- Guerrero M, Urena M, Himbert D, Wang DD, Eleid M, Kodali S, George I, Chakravarty T, Mathur M, Holzhey D, Pershad A, Fang HK, O’Hair D, Jones N, Mahadevan VS, Dumonteil N, Rodés-Cabau J, Piazza N, Ferrari E, Ciaburri D, Nejjari M, DeLago A, Sorajja P, Zahr F, Rajagopal V, Whisenant B, Shah PB, Sinning JM, Witkowski A, Eltchaninoff H, Dvir D, Martin B, Attizzani GF, Gaia D, Nunes NSV, Fassa AA, Kerendi F, Pavlides G, Iyer V, Kaddissi G, Witzke C, Wudel J, Mishkel G, Raybuck B, Wang C, Waksman R, Palacios I, Cribier A, Webb J, Bapat V, Reisman M, Makkar R, Leon M, Rihal C, Vahanian A, O’Neill W, Feldman T. 1-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Severe Mitral Annular Calcification. J Am Coll Cardiol. 2018;71:1841–53. doi: 10.1016/j.jacc.2018.02.054. [DOI] [PubMed] [Google Scholar]
- Guerrero M, Vemulapalli S, Xiang Q, Wang DD, Eleid M, Cabalka AK, Sandhu G, Salinger M, Russell H, Greenbaum A, Kodali S, George I, Dvir D, Whisenant B, Russo MJ, Pershad A, Fang K, Coylewright M, Shah P, Babaliaros V, Khan JM, Tommaso C, Saucedo J, Kar S, Makkar R, Mack M, Holmes D, Leon M, Bapat V, Thourani VH, Rihal C, O’Neill W, Feldman T. Thirty-Day Outcomes of Transcatheter Mitral Valve Replacement for Degenerated Mitral Bioprostheses (Valve-in-Valve), Failed Surgical Rings (Valve-in-Ring), and Native Valve With Severe Mitral Annular Calcification (Valve-in-Mitral Annular Calcification) in the United States: Data From the Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv. 2020;13:e008425. doi: 10.1161/CIRCINTERVENTIONS.119.008425. [DOI] [PubMed] [Google Scholar]
- Guerrero ME, Eleid MF, Wang DD, Pursnani A, Kodali SK, George I, Palacios I, Russell H, Makkar RR, Kar S, Satler LF, Rajagopal V, Dangas G, Tang GHL, McCabe JM, Whisenant BK, Fang K, Balan P, Smalling R, Kaptzan T, Lewis B, Douglas PS, Hahn RT, Thaden J, Oh JK, Leon M, O'Neill W, Rihal C. 5-Year Prospective Evaluation of Mitral Valve-in-Valve, Valve-in-Ring, and Valve-in-MAC Outcomes: MITRAL Trial Final Results. JACC Cardiovasc Interv. 2023;16:2211–2227. doi: 10.1016/j.jcin.2023.06.041. [DOI] [PubMed] [Google Scholar]
- Russo G, Taramasso M, Pedicino D, Gennari M, Gavazzoni M, Pozzoli A, Muraru D, Badano LP, Metra M, Maisano F. Challenges and future perspectives of transcatheter tricuspid valve interventions: adopt old strategies or adapt to new opportunities? Eur J Heart Fail. 2022;24:442–54. doi: 10.1002/ejhf.2398. [DOI] [PubMed] [Google Scholar]
- Kikoïne J, Urena M, Nguyen CC, Fischer Q, Carrasco JL, Brochet E, Ducrocq G, Vahanian A, Iung B, Himbert D. Predictors and clinical impact of thrombosis after transcatheter mitral valve implantation using balloon-expandable bioprostheses. EuroIntervention. 2021;16:1455–62. doi: 10.4244/EIJ-D-20-00991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urena M, Lemann T, Chong-Nguyen C, Brochet E, Ducrocq G, Carrasco JL, Iung B, Vahanian A, Himbert D. Causes and predictors of mortality after transcatheter mitral valve implantation in patients with severe mitral annulus calcification. Catheter Cardiovasc Interv. 2021;98:981–9. doi: 10.1002/ccd.29874. [DOI] [PubMed] [Google Scholar]
- Mirsadraee S, Sellers S, Duncan A, Hamadanchi A, Gorog DA. Bioprosthetic valve thrombosis and degeneration following transcatheter aortic valve implantation (TAVI). Clin Radiol. 2021 doi: 10.1016/j.crad.2020.08.015. [DOI] [PubMed] [Google Scholar]
- Sorajja P, Mack M, Vemulapalli S, Holmes DR, Stebbins A, Kar S, Lim DS, Thourani V, McCarthy P, Kapadia S, Grayburn P, Pedersen WA, Ailawadi G. Initial Experience With Commercial Transcatheter Mitral Valve Repair in the United States. J Am Coll Cardiol. 2016;67:1129–40. doi: 10.1016/j.jacc.2015.12.054. [DOI] [PubMed] [Google Scholar]
- Eleid MF, Wang DD, Pursnani A, Kodali SK, George I, Palacios I, Russell H, Makkar RR, Kar S, Satler LF, Rajagopal V, Dangas G, Tang GHL, McCabe JM, Whisenant BK, Fang K, Kaptzan T, Lewis B, Douglas P, Hahn R, Thaden J, Oh JK, Leon M, O’Neill W, Rihal CS, Guerrero ME. 2-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Annular Calcification, Rings, and Bioprostheses. J Am Coll Cardiol. 2022;80:2171–83. doi: 10.1016/j.jacc.2022.09.037. [DOI] [PubMed] [Google Scholar]
- Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, Bavaria JE, Kodali S, Brown DL, Bowers B, Dewey TM, Svensson LG, Tuzcu M, Moses JW, Williams MR, Siegel RJ, Akin JJ, Anderson WN, Pocock S, Smith CR, Leon MB PARTNER Trial Investigators. Transcatheter Aortic-Valve Replacement for Inoperable Severe Aortic Stenosis. N Engl J Med. 2012;366:1696–704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]