Abstract
BACKGROUND:
The impact of gender-affirming testosterone on fertility is poorly understood, with ovarian histopathologic studies showing variable results, some with a detrimental effect on reproductive capacity and uncertain reversibility. Assisted reproductive outcome data are restricted to small case series that lack the ability to inform clinical practice guidelines and limit fertility preservation counseling for transgender and nonbinary individuals.
OBJECTIVE:
This study aimed to determine the impact of current testosterone and testosterone washout on in vitro fertilization outcomes in a mouse model for gender-affirming hormone treatment. We hypothesized that current or previous testosterone treatment would not affect in vitro fertilization outcomes.
STUDY DESIGN:
C57BL/6N female mice (n=120) were assigned to 4 treatment groups: (1) current control, (2) current testosterone, (3) control washout, and (4) testosterone washout. Testosterone implants remained in situ for 6 or 12 weeks, representing the short- and long-term treatment arms, respectively. Current treatment groups underwent ovarian stimulation with implants in place, and washout treatment groups were explanted and had ovarian stimulation after 2 weeks. Oocytes were collected, fertilized, and cultured in vitro, with one arm continuing to the blastocyst stage and the other having transfer of cleavage-stage embryos. Statistical analysis was performed using GraphPad Prism, version 9.0 and R statistical software, version 4.1.2, with statistical significance defined by P<.05.
RESULTS:
Current long-term testosterone treatment impaired in vitro fertilization outcomes, with fewer mature oocytes retrieved (13.7±5.1 [standard deviation] vs 28.6±7.8 [standard deviation]; P<.0001) leading to fewer cleavage-stage embryos (12.1±5.1 vs 26.5±8.2; P<.0001) and blastocysts (10.0±3.2 vs 25.0±6.5; P<.0001). There was recovery of in vitro fertilization outcomes following washout in the short-term treatment cohort, with incomplete reversibility in the long-term cohort. Testosterone did not negatively affect maturity, fertilization, or blastulation rates.
CONCLUSION:
In a mouse model of gender-affirming hormone treatment, testosterone negatively affected oocyte yield without affecting oocyte quality. Our findings suggest that testosterone reversibility is duration-dependent. These results demonstrate the feasibility of in vitro fertilization without testosterone discontinuation while supporting a washout period for optimization of mature oocyte yield.
Keywords: assisted reproductive technology, fertility, fertility preservation, transgender medicine
Introduction
Transgender and nonbinary individuals represent approximately 1.6 million people in the United States and 25 million adults worldwide.1,2 Transgender adolescents and young adults have demonstrated high interest in parenting; however, <20% of transgender and nonbinary individuals are parents in the United States.3,4 This discrepancy likely reflects multifactorial challenges faced by transgender and gender-diverse (TGD) populations, including discrimination in the health care setting, potential for gender dysphoria associated with fertility treatment, and potential impact of gender-affirming treatments on reproductive capacity.
Masculinizing hormone treatment for TGD individuals consists of testosterone administration, typically by intramuscular or subcutaneous injection or via a transdermal route. There is consensus among major medical societies for universal fertility preservation counseling before initiation of gender-affirming treatments; however, such counseling is often inconsistent and incomplete.5-7 Limited studies of ovaries removed from transgender men with previous testosterone treatment have shown variable histopathologic findings,8,9 and ovarian reserve testing is similarly inconsistent.10-12 In an effort to better characterize the impact of testosterone on reproduction, Kinnear et al13,14 developed a mouse model for testosterone as gender-affirming hormone treatment (GAHT) in which subcutaneous testosterone implants led to cessation of estrous cycles and absence of corpus lutea with recovery following washout.
Current evidence on the impact of testosterone on in vitro fertilization (IVF) demonstrates variable results with potential for a detrimental effect on reproductive capacity and uncertain reversibility. IVF outcome data are limited to small case series of transgender men with previous testosterone treatment, with 2 studies demonstrating no difference in oocytes retrieved and a third finding a negative impact on oocyte yield.15-17 Clinically, it remains uncertain whether testosterone should be discontinued before ovarian stimulation, with further evidence needed to guide patient-centered decision-making. Our objective was to determine the impact of current testosterone and cessation on IVF outcomes, after both short and long testosterone exposure. Our primary outcome was 2-cell embryo development. We hypothesized that current or previous testosterone treatment would not significantly affect IVF outcomes.
Materials and Methods
Ethical approval
This study was completed in compliance with the protocol approved at the University of Michigan (PRO00009635) by the Institutional Animal Care and Use Committee guidance.
Experimental design
C57BL/6N female mice (n=120) were used for this study. All mice were housed in ventilated cages in groups of 5 in a nonbarrier facility at the University of Michigan, Ann Arbor. Mice had access to food and water ad libitum and were exposed to regular 12-hour light and dark cycles. Within each study arm, mice were assigned to 4 treatment groups: (1) current control, (2) current testosterone, (3) control cessation, and (4) testosterone cessation. Subcutaneous implants remained in place for the 6- or 12-week treatment duration for the short- and long-term treatment arms, respectively. Ovarian stimulation was performed with implants in place for the current treatment groups and 2 weeks after explantation in the washout cohorts (Figure 1, A and B). Oocytes were fertilized in vitro with sperm from B6D2F1/J male mice and embryos cultured in Global media (LifeGlobal, Guilford, CT).
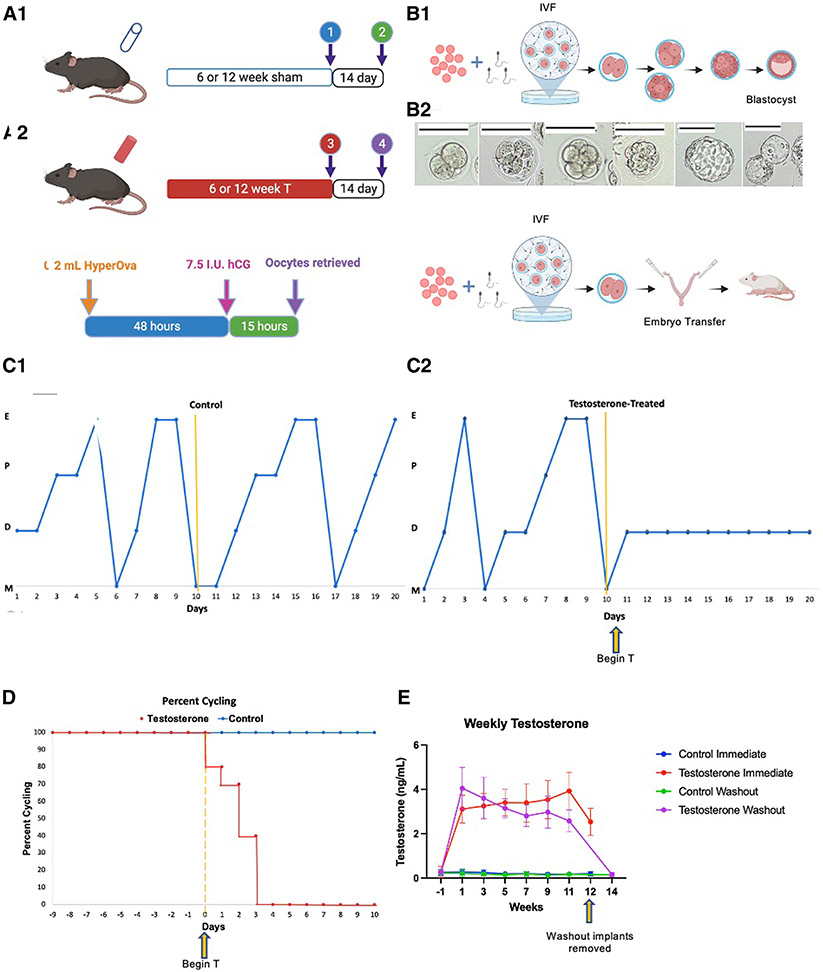
FIGURE 1. Experimental design and model profile.
(A1) Control or T silastic tubing implants placed subcutaneously for 6 or 12 weeks representing short and long-term treatment cohorts. For immediate groups, implants remained in place at time of IVF while in cessation groups implants were removed with a 14 day washout period before IVF. (A2) Ovarian stimulation protocol utilized for all mice. (B) Embryo culture design with (B1) arm cultured to blastocyst stage and (B2) cultured to 2-cell embryo stage with subsequent transfer into pseudopregnant mice. (C1) Cyclicity of one representative control and (C2) one representative T-treated mouse. (D) Percent cyclicity for mice after implant placement in control and T cohorts. (E) Weekly T levels obtained prior to implant placement then biweekly throughout study duration for all treatment cohorts.
hCG, human chorionic gonadotropin; IVF, in vitro fertilization; T, testosterone.
Subcutaneous implants
Silastic tubing implants of 16 mm were filled with 200-proof ethanol (control) or 10 mg of testosterone enanthate dissolved in ethanol. Subcutaneous implant placement was performed using sterile technique at 10 weeks under isoflurane anesthesia, with incisions closed using 4-0 suture in an interrupted fashion.
Vaginal cytology and serum hormone analysis
Daily vaginal cytology was performed before implant placement to establish cyclicity and continued after implant placement until all testosterone-treated mice ceased cycling. Serum testosterone levels were measured before implant placement and biweekly thereafter with serum collected from the lateral tail vein. At the time of sacrifice, terminal blood was collected via decapitation. Blood samples were kept overnight at 4°C, centrifuged for 10 minutes (9200 g), and then stored at −20°C. Hormone analysis was performed in singlets at the Ligand Assay and Analysis Core Facility, University of Virginia Center for Research in Reproduction. The following reportable ranges were able to be detected with a coefficient of variation of <20%: 5.0 to 3200 pg/mL for estradiol enzyme-linked immunosorbent assay (ELISA), 0.15 to 40.0 ng/mL (or 0.75–200.00 ng/mL with a 5× dilution) for progesterone ELISA, 10.0 to 1600 ng/dL for testosterone ELISA, and 4.8 to 260 ng/mL for Ansh anti-Müllerian hormone (AMH) ELISA (Ansh Labs, Webster, TX).
Ovarian stimulation and oocyte collection
Intraperitoneal injection of 0.2-mL HyperOva (Cosmo Bio Ltd, Tokyo, Japan) was used for ovarian stimulation, followed by intraperitoneal injection of 7.5 international units of human chorionic gonadotropin (hCG) 48 hours later (Figure 1, B) per the Center for Animal Resources and Development (CARD) protocol.18 Washing dishes were prepared with Global media covered with OVOIL (Vitrolife, Gothenburg, Sweden) and placed in the incubator to equilibrate. Female mice were euthanized at 15 hours after hCG injection. Abdominal dissection was performed with removal of the ampulla portion of the bilateral oviducts. Using a dissection needle, the ampulla was opened with release of the cumulus-oocyte complex, and the complex was transferred into the media.
Sperm collection and in vitro fertilization
The 12-week male B6D2F1/J mice were paired with a single C57BL/6N mouse 1 week before IVF, and then unpaired and singly housed. Sperm collection and fertilization dishes were prepared, and fresh sperm retrieved according to protocol.18 Fresh sperm from the sperm dishes of 2 male mice was transferred into the fertilization media containing the cumulus-oocyte complexes from a single female mouse.
Embryo culture
Four hours after insemination, oocytes were washed and transferred to a new drop of Global media. For the embryo transfer experiment, embryos were cultured to the 2-cell stage. For the extended embryo culture experiment, embryos were cultured to the blastocyst stage (Figure 1, C-E).
Embryo transfer
B6D2F1/J female pseudopregnant mice were obtained by mating 2- to 6-month females with vasectomized B6D2F1 male mice. Embryo transfers were performed on anesthetized pseudopregnant females with embryos introduced into the infundibulum of the oviduct. A maximum of 20 embryos were transferred into a single pseudopregnant female in an effort to optimize live birth rate (LBR). Female mice were individually housed following transfer, with the number of pups delivered used to calculate LBR.
Statistical analysis
Statistical analysis was performed using GraphPad Prism, version 9.0 (Dot-matics, Boston, MA) and R statistical software, version 4.1.2 (R Core Team, Vienna, Austria). Quantitative variables were analyzed using unpaired t tests, and chi-square tests were used for comparing differences in rates. Shapiro–Wilk tests for normality were performed for each of the quantitative variables before conducting t-tests. Two-way analysis of variance was used in weekly testosterone analysis. For hormone analysis, levels below the limit of detection of the ELISA assay for estradiol (14/110 samples; 12.7%) and testosterone (87/642 samples; 13.5%) utilized the lower limit for quantification. Statistical significance was defined by P<.05.
Results
Testosterone induced acyclicity and elevation of serum testosterone levels with return to baseline following washout
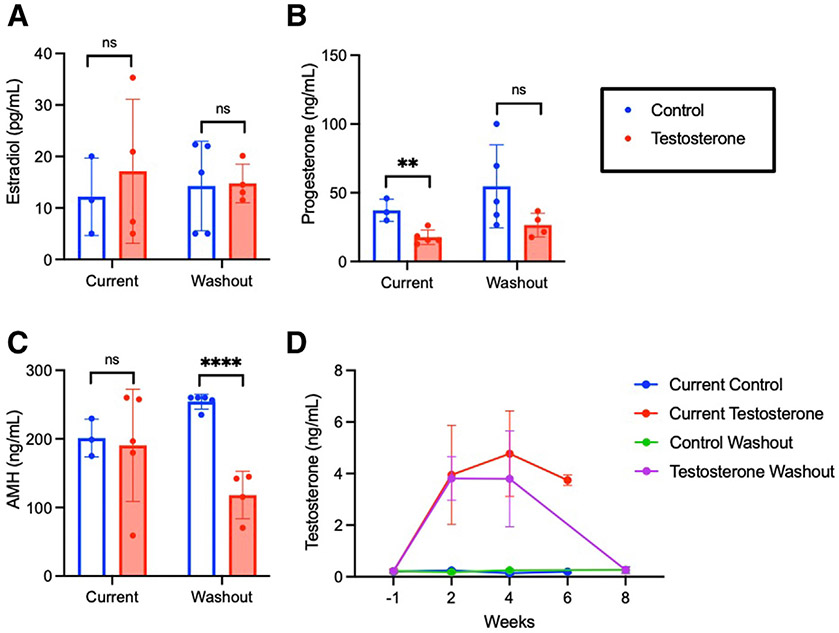
Testosterone levels remained elevated during the treatment period for all testosterone cohorts (Figure 1, I). Terminal testosterone levels in the current treatment group remained elevated compared with controls (2.53±0.61 vs 0.20±0.09 ng/mL; P<.0001), with return to control levels for the washout cohort (0.16±0.09 vs 0.15±0.05 ng/mL; P=.63). Testosterone led to cessation of estrous cycles by postimplantation day 4 (Figure 1, F-H), which is consistent with previous observations in our model.14 One mouse in the current testosterone cohort developed severe vaginal prolapse after 9 weeks of testosterone and was euthanized. Two mice intended for the control cessation cohort died of uncertain etiology before implantation.
Long-term testosterone decreased progesterone and Ansh anti-Müllerian hormone levels, with Ansh anti-Müllerian hormone levels remaining decreased following washout
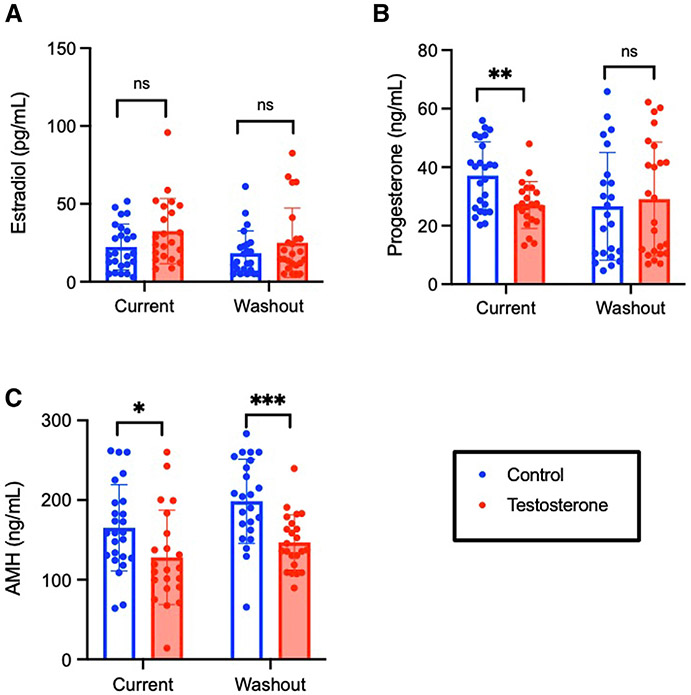
Testosterone did not significantly affect estradiol levels for either current treatment (P=.18) or washout cohorts (P=.23) (Figure 2, A). Current testosterone led to a decrease in terminal progesterone levels compared with controls (27.1±8.0 vs 37.1±11.5 ng/mL; P=.0014), with no difference following washout (29.1±19.5 vs 26.6±18.4 ng/mL; P=.65) (Figure 2, B). Both current testosterone (128.1±59.2 vs 165.1±54.2 ng/mL; P=.031) and testosterone washout (146.8±34.7 vs 198.6±52.9 ng/mL; P=.0003) significantly decreased terminal AMH levels compared with controls (Figure 2, C).
FIGURE 2. Hormones for long-term treatment cohorts.
A, Terminal estradiol, B, progesterone, and C, AMH for immediate and washout cohorts. Bars are mean±SD. Significance was determined by P<.05.
AMH, anti-Müllerian hormone; SD, standard deviation.
Long-term testosterone negatively affected in-vitro fertilization outcomes, with evidence of partial recovery following washout
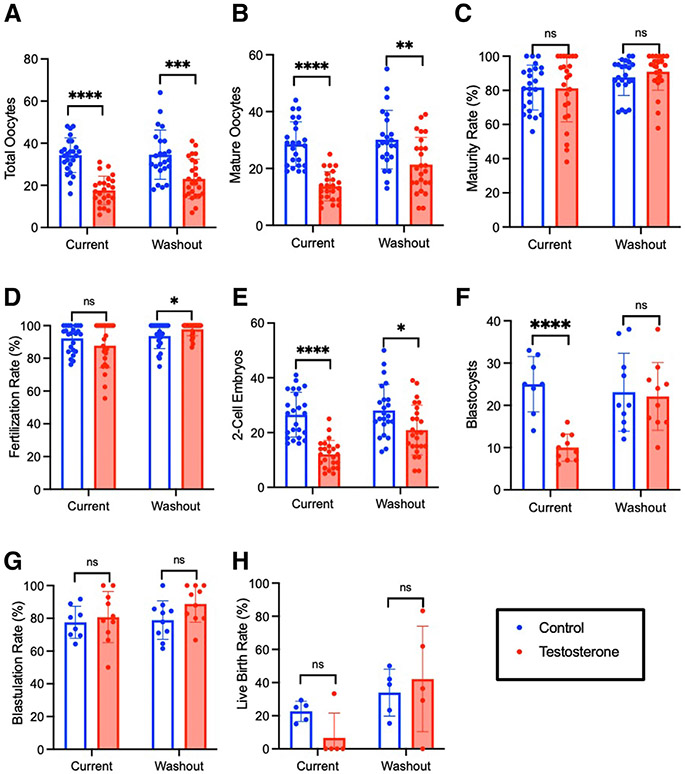
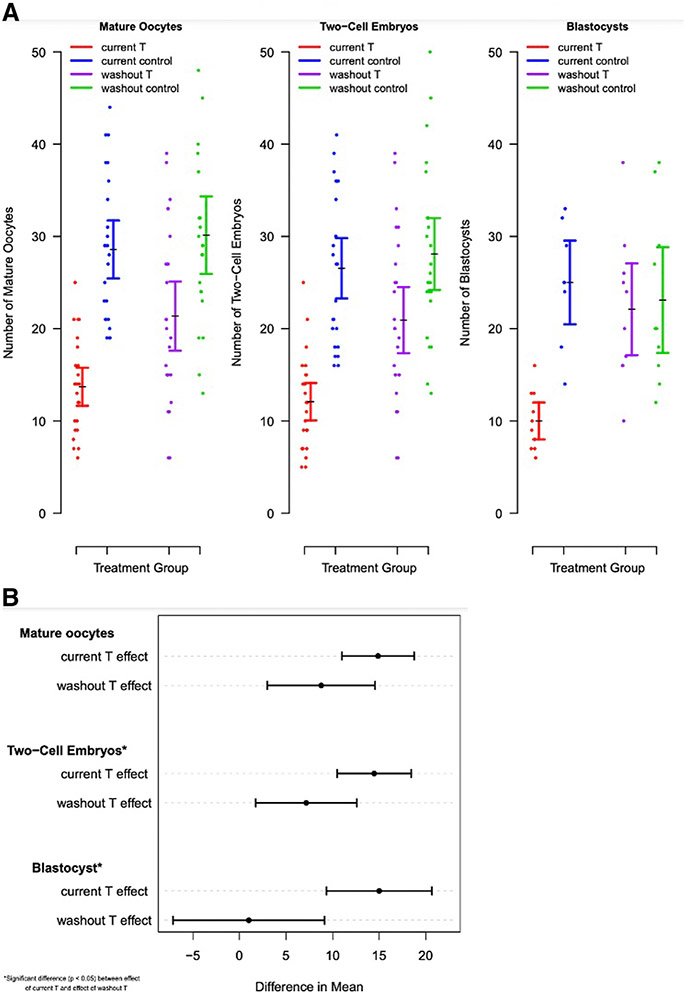
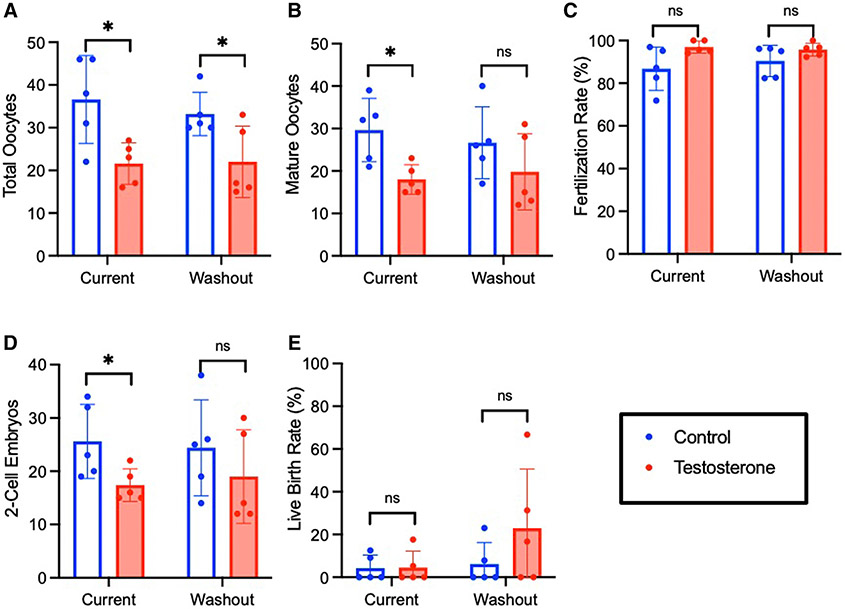
Mice receiving 12 weeks of current testosterone demonstrated lower oocyte yield (17.6±6.7 vs 34.2±8.2; P<.0001) and fewer mature oocytes (13.7±5.1 vs 28.6±5.1; P<.0001), 2-cell embryos (12.5±3.4 vs 30.4±7.6; P=.0276), and blastocysts (10.0±3.2 vs 25.0±6.5; P<.0001) compared with controls (Figure 3). Testosterone did not impair rates of oocyte maturity (81.2%±19.6% vs 81.7%±13.1%; P=.93), fertilization (87.8%±13.4% vs 92.2%±8.0%; P=.17), or blastulation (80.7%±15.6% vs 77.6%±9.8%; P=.62). After testosterone washout, there was improvement in IVF outcomes relative to controls, with no significant difference in number of blastocysts (22.1±8.0 vs 23.1±9.2; P=.80). Although mature oocytes (21.4±9.5 vs 30.1±10.3; P=.0036) and 2-cell embryos (20.9±9.2 vs 28.1±9.5; P=.011) remained lower in the testosterone washout group compared with controls, there was partial recovery of IVF outcomes compared with current testosterone (Figure 4, A). This partial recovery is evidenced by the difference in the effect of active testosterone vs washout testosterone on mature oocytes (P=.08), 2-cell embryos (P=.03), and blastocysts (P=.005) (Figure 4, B). The LBR was lower after 12 weeks of current testosterone (6.7%±14.9% vs 22.6%±6.1%; P=.057), but the decrease was not statistically significant (Figure 3, H).
FIGURE 3. In vitro fertilization and embryo culture outcomes.
Ovarian stimulation outcomes for long-term treatment cohort including (A) total oocytes and (B) mature oocytes for both immediate treatment and washout cohorts. (C) Maturity and (D) fertilization rates for immediate and washout cohorts. Culture outcomes including (E) 2-cell embryos, (F) blastocysts, (G) blastulation rates, and (H) live birth rates for long-term treatment and washout cohorts.
ns, not significant.
FIGURE 4. Assessment of reversibility following testosterone washout in long-term cohort.
A, Dot-plot of IVF outcomes in long-term cohort with mean and confidence interval represented. B, Difference in means for IVF outcomes between T and T washout cohorts. Significance was determined by P<.05.
IVF, in vitro fertilization; T, testosterone.
Short-term testosterone impaired in-vitro fertilization outcomes, with recovery following washout
Among the short-term treatment cohorts, terminal testosterone was elevated with current treatment (3.75±0.20 vs 0.20±0.06 ng/mL; P<.0001) and returned to control levels with testosterone washout (0.26±0.13 vs 0.27±0.10; P=.91) (Figure 5). Short-term testosterone decreased serum progesterone levels in a pattern similar to that observed with long-term treatment (17.7±5.2 vs 37.2±8.1 ng/mL; P=.006). Short-term testosterone did not decrease terminal AMH (190.6±81.2 vs 201.2±27.4 ng/mL; P=.84), but a decrease in AMH (118.2±34.6 vs 254.3±10.8 ng/mL; P<.0001) was observed among the testosterone washout cohort compared with controls. Six weeks of testosterone treatment led to a decrease in mature oocyte yield (18.9±1.3 vs 28.1±2.1; P=.014), contributing to fewer 2-cell embryos (Figure 6). After testosterone washout, there was recovery in IVF outcomes, with no difference in 2-cell embryos between cohorts (19.0±8.8 vs 24.4±9.0; P=.36). Overall, LBRs were low, with no significant difference among treatment and control cohorts (4.6%±7.7% vs 4.3%±6.0%; P=.95).
FIGURE 5. Hormones for short-term treatment cohorts.
Terminal (A) estradiol, (B) progesterone, and (C) AMH for immediate and washout cohorts. (D) Testosterone levels obtained before implant placement and then biweekly for study duration of short-term treatment cohorts. Bars are mean±SD. Significance was determined by P<.05.
AMH, anti-Müllerian hormone; ns, not significant; SD, standard deviation.
FIGURE 6. In vitro fertilization and embryo transfer outcomes following short-term treatment.
Ovarian stimulation results for immediate and washout cohorts including (A) total oocytes, (B) mature oocytes, (C) fertilization rate, (D) 2-cell embryos, and (E) live birth rate.
Comment
Principal findings
In our mouse model of GAHT, current testosterone impaired IVF outcomes, with evidence of reversibility following washout after short-term treatment, and partial reversibility following washout in the long-term treatment cohort. The lower oocyte yield contributed to fewer cleavage-stage embryos and blastocysts. Importantly, there was no decline in rates of maturity, fertilization, or blastulation, indicating that oocyte quality was not significantly affected by testosterone. The reversibility of IVF outcomes demonstrated in the short-term cohort as opposed to partial reversibility in the long-term cohort supports a potential duration-dependent impact of testosterone on reproductive capacity.
Results in the context of what is known
This model of GAHT investigated the impact of both current treatment (short and long) and washout on fertility beyond the cleavage-stage embryo. Bartels et al19 previously investigated the impact of testosterone on murine fertility, finding no difference in oocyte yield, fertilization, or initial cleavage. In contrast, we found impaired IVF outcomes with current treatment and evidence toward recovery with washout. However, findings of our mouse model using subcutaneous testosterone implants and a 2-week washout differ markedly from findings with testosterone injections that required a 6- to 7-week washout. Our study also had substantially higher power to detect a difference in the number of embryos and evaluated additional fertility outcomes.
Clinical implications
Our results are consistent with a recent study of assisted reproductive technology outcomes comparing embryos between transgender men with previous testosterone treatment and cisgender women, which found no difference in fertilization or early embryonic development.20 Similarly, studies of in vitro maturation of oocytes collected from ovaries of transgender men receiving testosterone treatment found a high percentage of normal-appearing spindles and aligned chromosomes representing preserved oocyte quality.21,22
A key finding of our study is that ovarian stimulation and IVF were feasible, with formation of blastocysts in both current testosterone and washout cohorts. In the absence of standard practice guidelines, many reproductive endocrinologists recommend testosterone discontinuation before oocyte cryopreservation. However, this is a challenging decision for TGD individuals because testosterone discontinuation may cause significant gender dysphoria, the fear of which prohibits some TGD individuals from pursuing fertility preservation altogether.23-26 Our findings are consistent with recent case reports of transgender men continuing gender-affirming testosterone treatment during gonadotropin stimulation with successful retrieval of mature oocytes.27-29 Our results support consideration of testosterone continuation in a patient-centered decision-making model using ovarian reserve assessment, duration of testosterone treatment, and concerns for gender dysphoria to optimize counseling.
Research implications
Our research provides important insight into the impact of current testosterone vs discontinuation on IVF outcomes. Following long-term treatment, AMH levels were lower in both testosterone cohorts, indicating an impairment of ovarian reserve, which may require a longer washout period to improve. Our results raise the question of whether a longer washout period would allow for complete reversibility after long-term testosterone treatment. Future studies are needed to determine the reversibility of long-term testosterone treatment, quantify the benefit and ideal duration of a washout period, and investigate the impact on offspring conceived from testosterone-exposed oocytes.
Strengths and limitations
A strength of this research was the study design with designated cohorts for embryo culture to the blastocyst stage and for embryo transfer to allow for a comprehensive evaluation of the impact of testosterone on fertility outcomes. Expanding fertility measures beyond the 2-cell embryo stage provides important insight into oocyte quality with the ability to form blastocysts and to successfully implant. These results demonstrate feasibility of IVF during testosterone therapy despite a decreased oocyte yield. Another strength of this study is the use of both short-and long-term treatment cohorts to assess potential duration-dependent effects of testosterone on ovarian function. Although both regimens impaired IVF outcomes, complete reversibility following washout was observed for short-term treatment, with only partial reversibility following long-term testosterone, supporting duration-dependent testosterone reversibility. The reversibility of testosterone following short-term treatment lends support for considering ovarian stimulation earlier in one’s GAHT course.
A limitation of our study is the inability to power for LBR as the primary outcome. Because of seasonal variations in murine litter size, it was imperative to perform the embryo transfers at the same time of year. Thus, we were limited by the number of pseudopregnant mice available for embryo transfer and cohort size that could be reasonably completed. Although there was a lower LBR in the current long-term testosterone cohort compared with controls (P=.0573), this did not reach statistical significance. Assessment of implantation sites or gestational ultrasound may be considered in future studies to provide further insight into the impact of testosterone on reproductive capacity. Our method of oocyte collection was not compatible with survival, which prohibited autologous embryo transfer and our ability to evaluate the uterine impact of testosterone. In addition, we were unable to assess for any long-term consequences of testosterone-exposed oocytes in offspring due to potential epigenetic changes.
Importantly, our findings are limited by the use of a translational mouse model to examine the impact of testosterone on mouse fertility outcomes, which may not perfectly mirror its effect on human reproductive physiology. Advantages of the mouse model in the study of reproductive function include the well-characterized reproductive cycles, short gestational period, and close resemblance of the follicles in the mouse ovary to those in the human ovary, with high conservation of genes and proteins expressed.30,31 Our key findings provide insight into the impact of testosterone on reproductive potential, with further exploration needed to guide fertility preservation counseling for gender-diverse individuals. We investigated a single 2-week washout time period, which was adequate for recovery from short-term but not long-term testosterone exposure. A recent survey of reproductive endocrinologists providing care to transgender individuals found that most (75.8%) would recommend testosterone discontinuation for 1 to 3 months before ovarian stimulation, citing concerns for oocyte quality and stimulation success.32 This 2-week washout period was selected for our study to assess for reversibility of the impact of testosterone after serum levels returned to baseline and in a time frame that translated to current clinical practice.
Conclusions
Although there was a decrease in oocyte yield, gonadotropin stimulation and creation of blastocysts were achievable with current testosterone in a mouse model of GAHT. Our findings provide insight into the feasibility of ovarian stimulation during testosterone therapy and the potential benefit of a washout period to increase mature oocyte yield. Further investigation into the reproductive consequences of testosterone for oocytes and offspring should be a research priority to provide individualized counseling and to inform clinical practice guidelines for TGD individuals seeking fertility preservation.
AJOG at a Glance.
Why was this study conducted?
The impact of gender-affirming testosterone on fertility is poorly understood. This study aimed to determine the impact of current testosterone and testosterone washout on in vitro fertilization (IVF) outcomes.
Key findings
Testosterone led to a lower oocyte yield with subsequently fewer embryos at both the cleavage and blastocyst stage. Testosterone did not impair rates of oocyte maturity, fertilization, or blastulation in either current treatment or washout arms. These findings suggest that testosterone does not affect oocyte quality and thus support the feasibility of ovarian stimulation and IVF in the continued presence of testosterone. A 2-week washout period led to improvement in IVF outcomes, which supports the potential reversibility of testosterone effects and suggests that a testosterone washout period may optimize results for transgender and gender-diverse individuals seeking fertility preservation who are open to testosterone discontinuation.
What does this add to what is known?
While there was a decrease in oocyte yield, ovarian stimulation and creation of blastocysts was achievable with current testosterone in a mouse model of gender-affirming hormone treatment. These findings are consistent with recent case reports of transgender men continuing gender-affirming testosterone treatment during gonadotropin stimulation with successful retrieval of mature oocytes. These findings support consideration of testosterone continuation in a patient-centered decision making model. Further research is needed to assess the possible benefit of a washout period to increase mature oocyte yield and to investigate the impact on offspring conceived from testosterone-exposed oocytes.
Acknowledgments
This research was funded by the National Institutes of Health (Grant 1R01HD098233-01).
Footnotes
The authors report no conflict of interest.
The findings of this study were presented at the 78th annual meeting of the American Society for Reproductive Medicine, Anaheim, CA, October 22–26, 2022.
Contributor Information
Amanda R. Schwartz, Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI.
Min Xu, Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI.
Nicholas C. Henderson, Department of Biostatistics, University of Michigan School of Public Health, Ann Arbor, MI.
Cynthia Dela Cruz, Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI.
Daniel Pfau, Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI.
Vasantha Padmanabhan, Department of Pediatrics, University of Michigan, Ann Arbor, MI.
Ariella Shikanov, Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI.
Molly B. Moravek, Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI.
References
- 1.Herman JL, Flores AR, O’Neill KK. How many adults and youth identify as transgender in the United States. The Williams Institute, UCLA School of Law; 2022. [Google Scholar]
- 2.Winter S, Diamond M, Green J, et al. Transgender people: health at the margins of society. Lancet 2016;388:390–400. [DOI] [PubMed] [Google Scholar]
- 3.Auer MK, Fuss J, Nieder TO, et al. Desire to have children among transgender people in Germany: a cross-sectional multi-center study. J Sex Med 2018;15:757–67. [DOI] [PubMed] [Google Scholar]
- 4.Carone N, Rothblum ED, Bos HMW, Gartrell NK, Herman JL. Demographics and health outcomes in a U.S. probability sample of transgender parents. J Fam Psychol 2021;35:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ethics Committee of the American Society for Reproductive Medicine. asrm@asrm.org. Access to fertility services by transgender and nonbinary persons: an Ethics Committee opinion. Fertil Steril 2021;115:874–8. [DOI] [PubMed] [Google Scholar]
- 6.Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2017;102:3869–903. [DOI] [PubMed] [Google Scholar]
- 7.Standards of care for the health of transsexual, transgender, and gender-conforming people. World Professional Association for Transgender Health. 2012. Available at: http://www.wpath.org/publications/soc. Accessed December 20, 2022. [Google Scholar]
- 8.Grimstad FW, Fowler KG, New EP, et al. Ovarian histopathology in transmasculine persons on testosterone: a multicenter case series. J Sex Med 2020;17:1807–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loverro G, Resta L, Dellino M, et al. Uterine and ovarian changes during testosterone administration in young female-to-male transsexuals. Taiwan J Obstet Gynecol 2016;55:686–91. [DOI] [PubMed] [Google Scholar]
- 10.Caanen MR, Soleman RS, Kuijper EAM, et al. AntiMüllerian hormone levels decrease in female-to-male transsexuals using testosterone as cross-sex therapy. Fertil Steril 2015;103:1340–5. [DOI] [PubMed] [Google Scholar]
- 11.Tack LJW, Craen M, Dhondt K, Vanden Bossche H, Laridaen J, Cools M. Consecutive lynestrenol and cross-sex hormone treatment in biological female adolescents with gender dysphoria: a retrospective analysis. Biol Sex Differ 2016;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaish I, Tordjman K, Amir H, et al. Functional ovarian reserve in transgender men receiving testosterone therapy: evidence for preserved anti-Müllerian hormone and antral follicle count under prolonged treatment. Hum Reprod 2021;36:2753–60. [DOI] [PubMed] [Google Scholar]
- 13.Kinnear HM, Constance ES, David A, et al. A mouse model to investigate the impact of testosterone therapy on reproduction in transgender men. Hum Reprod 2019;34:2009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinnear HM, Hashim PH, Dela Cruz C, et al. Reversibility of testosterone-induced acyclicity after testosterone cessation in a transgender mouse model. F S Sci 2021;2:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amir H, Yaish I, Samara N, Hasson J, Groutz A, Azem F. Ovarian stimulation outcomes among transgender men compared with fertile cisgender women. J Assist Reprod Genet 2020;37:2463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung A, Sakkas D, Pang S, Thornton K, Resetkova N. Assisted reproductive technology outcomes in female-to-male transgender patients compared with cisgender patients: a new frontier in reproductive medicine. Fertil Steril 2019;112:858–65. [DOI] [PubMed] [Google Scholar]
- 17.Adeleye AJ, Cedars MI, Smith J, Mok-Lin E. Ovarian stimulation for fertility preservation or family building in a cohort of transgender men. J Assist Reprod Genet 2019;36:2155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reproductive engineering technique in mice. Kumamoto University. Available at: http://card.medic.kumamoto-u.ac.jp/card/english/sigen/manual/onlinemanual.html. Accessed December 10, 2022. [Google Scholar]
- 19.Bartels CB, Uliasz TF, Lestz L, Mehlmann LM. Short-term testosterone use in female mice does not impair fertilizability of eggs: implications for the fertility care of transgender males. Hum Reprod 2021;36:189–98. [DOI] [PubMed] [Google Scholar]
- 20.Israeli T, Preisler L, Kalma Y, et al. Similar fertilization rates and preimplantation embryo development among testosterone-treated transgender men and cisgender women. Reprod Biomed Online 2022;45:448–56. [DOI] [PubMed] [Google Scholar]
- 21.De Roo C, Lierman S, Tilleman K, et al. Ovarian tissue cryopreservation in female-to-male transgender people: insights into ovarian histology and physiology after prolonged androgen treatment. Reprod Biomed Online 2017;34:557–66. [DOI] [PubMed] [Google Scholar]
- 22.Lierman S, Tilleman K, Braeckmans K, et al. Fertility preservation for trans men: frozenthawed in vitro matured oocytes collected at the time of ovarian tissue processing exhibit normal meiotic spindles. J Assist Reprod Genet 2017;34:1449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James-Abra S, Tarasoff LA, Green D, et al. Trans people’s experiences with assisted reproduction services: a qualitative study. Hum Reprod 2015;30:1365–74. [DOI] [PubMed] [Google Scholar]
- 24.Armuand G, Dhejne C, Olofsson JI, Rodriguez-Wallberg KA. Transgender men’s experiences of fertility preservation: a qualitative study. Hum Reprod 2017;32:383–90. [DOI] [PubMed] [Google Scholar]
- 25.Lai TC, McDougall R, Feldman D, Elder CV, Pang KC. Fertility counseling for transgender adolescents: a review. J Adolesc Health 2020;66:658–65. [DOI] [PubMed] [Google Scholar]
- 26.Persky RW, Gruschow SM, Sinaii N, Carlson C, Ginsberg JP, Dowshen NL. Attitudes toward fertility preservation among transgender youth and their parents. J Adolesc Health 2020;67:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stark BA, Mok-Lin E. Fertility preservation in transgender men without discontinuation of testosterone. F S Rep 2022;3:153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gale J, Magee B, Forsyth-Greig A, Visram H, Jackson A. Oocyte cryopreservation in a transgender man on long-term testosterone therapy: a case report. F&S Reports 2021;2:249–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moravek MB, Dixon M, Pena SM, Obedin-Maliver J. Management of testosterone around ovarian stimulation in transmasculine patients: challenging common practices to meet patient needs-2 case reports. Hum Reprod 2023;38:482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walters KA, Allan CM, Handelsman DJ. Rodent models for human polycystic ovary syndrome. Biol Reprod 2012;86:1–12. [DOI] [PubMed] [Google Scholar]
- 31.Smith P, Wilhelm D, Rodgers RJ. Development of mammalian ovary. J Endocrinol 2014;221:R145–61. [DOI] [PubMed] [Google Scholar]
- 32.Yost SK, Kobernik EK, Moravek MB. Current practices and knowledge surrounding ovarian stimulation in transgender men. SRI 2021: Scientific Abstracts. Reprod Sci (Suppl 1) 2021;28(Suppl 1):291–2. [Google Scholar]