Key Points
Question
Is liquid biopsy testing a cost-effective method for colorectal cancer screening, particularly in patients who refuse traditional screening methods?
Findings
In this economic evaluation using a simulated cohort of patients aged 45 years with average risk of colorectal cancer, colonoscopy was the preferred (most cost-effective) screening method, with an incremental cost-effectiveness ratio of $28 071 per life-year gained. Offering liquid biopsy testing to patients who refused colonoscopy resulted in the greatest gain of life-years but was not cost-effective.
Meaning
These findings suggest that in patients who refuse traditional screening for colorectal cancer, liquid biopsy is not cost-effective as an alternative screening method.
This economic evaluation estimates the cost-effectiveness of liquid biopsy for colorectal screening in the US.
Abstract
Importance
Despite recommendations for universal screening, adherence to colorectal cancer screening in the US is approximately 60%. Liquid biopsy tests are in development for cancer early detection, but it is unclear whether they are cost-effective for colorectal cancer screening.
Objective
To estimate the cost-effectiveness of liquid biopsy for colorectal cancer screening in the US.
Design, Setting, and Participants
In this economic evaluation, a Markov model was developed to compare no screening and 5 colorectal cancer screening strategies: colonoscopy, liquid biopsy, liquid biopsy following nonadherence to colonoscopy, stool DNA, and fecal immunochemical test. Adherence to first-line screening with colonoscopy, stool DNA, or fecal immunochemical test was assumed to be 60.6%, and adherence for liquid biopsy was assumed to be 100%. For colonoscopy, stool DNA, and fecal immunochemical test, patients who did not adhere to testing were not offered other screening. In colonoscopy–liquid biopsy hybrid, liquid biopsy was second-line screening for those who deferred colonoscopy. Scenario analyses were performed to include the possibility of polyp detection for liquid biopsy.
Exposures
No screening, colonoscopy, fecal immunochemical test, stool DNA, liquid biopsy, and colonoscopy–liquid biopsy hybrid screening.
Main Outcomes and Measures
Model outcomes included life expectancy, total cost, and incremental cost-effectiveness ratios. A strategy was considered cost-effective if it had an incremental cost-effectiveness ratio less than the US willingness-to-pay threshold of $100 000 per life-year gained.
Results
This study used a simulated cohort of patients aged 45 years with average risk of colorectal cancer. In the base case, colonoscopy was the preferred, or cost-effective, strategy with an incremental cost-effectiveness ratio of $28 071 per life-year gained. Colonoscopy–liquid biopsy hybrid had the greatest gain in life-years gained but had an incremental cost-effectiveness ratio of $377 538. Colonoscopy–liquid biopsy hybrid had a greater gain in life-years if liquid biopsy could detect polyps but remained too costly.
Conclusions and Relevance
In this economic evaluation of liquid biopsy for colorectal cancer screening, colonoscopy was a cost-effective strategy for colorectal cancer screening in the general population, and the inclusion of liquid biopsy as a first- or second-line screening strategy was not cost-effective at its current cost and screening performance. Liquid biopsy tests for colorectal cancer screening may become cost-effective if their cost is substantially lowered.
Introduction
Colorectal cancer (CRC) is the third most common cancer1 and second most common cause of cancer deaths in the US.2 However, there are robust screening methods for identifying both cancerous and precancerous colorectal lesions.3 Despite decades of evidence-based recommendations for CRC screening and precancerous polyp surveillance,4 screening uptake remains poor. Currently, recommended CRC screening modalities include noninvasive tests, such as fecal immunochemical test (FIT) and stool DNA, and invasive tests, such as colonoscopy.3,5,6 Of these, colonoscopy has the best performance for both cancer and adenoma detection, and is the most used CRC screening modality in the US.7 As of 2020, according to the American Cancer Society (ACS) and US Centers for Disease Control and Prevention (CDC), the average adherence to colonoscopy in US populations aged 50 to 75 years is only 60.6%.8 When stool tests are offered after colonoscopy is refused, the incremental improvement in screening uptake is modest, with a cumulative adherence of only 67%.8 This rate remains below the goal of 80% national adherence to CRC screening set by the National Colorectal Cancer Roundtable and ACS.9 Notably, CRCs that develop in unscreened patients are estimated to account for 28% to 44% of CRC deaths.10
Currently there are no blood tests recommended for CRC screening. New blood tests or liquid biopsies (LBs) using circulating tumor DNA–based markers are in development for single cancer and multicancer early detection (MCED), including CRC.11,12,13,14 These tests have better performance than previous blood-based cancer detection tests,15 and LBs may present a more appealing CRC screening option especially among individuals who are unscreened.16 While there has been increasing investment in LB for its potential to detect early cancer, it remains unclear whether it is a cost-effective CRC screening strategy in the US.
The purpose of this study was to estimate the cost-effectiveness of LB as a first- or second-line CRC screening strategy in the US compared with no screening and 3 approved screening strategies colonoscopy, FIT, and stool DNA. Our hypothesis was that LB would improve CRC detection and decrease the number of CRC deaths.
Methods
This study is not considered human participants research at Columbia University; therefore, institutional review board approval was not sought, and informed consent was not needed. Reporting of this study is consistent with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline.
Model Overview
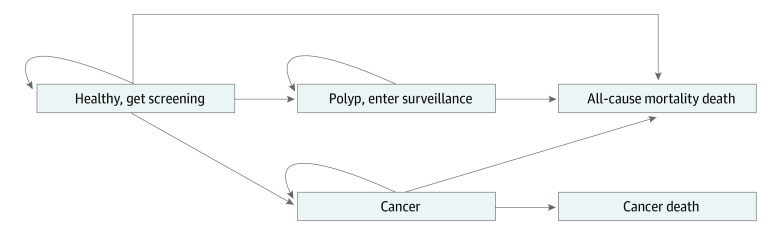
We created a state-transition, cohort level Markov model to assess the cost-effectiveness of LB for CRC screening compared with no screening and currently accepted screening strategies (Figure 1). The model simulated a hypothetical cohort of patients with average CRC risk in the US with a starting age of 45 years, aligning with the newest US Preventive Services Task Force (USPSTF) recommendation for CRC screening.17 Patients with average risk of developing CRC were not screened after age 75 years. We assumed that patients with colonic polyps were above average risk and continued polyp surveillance until age 85 years.18 The model had an annual cycle length, and patients were followed up until death or age 100 years. Cancer incidence and mortality data were obtained from Surveillance, Epidemiology, and End Results (SEER) registries.19,20 Baseline CRC incidence was derived using SEER data prior to 2000 to reflect a CRC incidence rate prior to screening guideline implementation. CRC mortality was estimated from post-2000 SEER data to reflect the most recent advances in CRC treatment. All-cause mortality rates were derived using CDC life tables.21 Base-case first-line screening adherence for our hypothetical cohort was 60.6% based on data from the CDC.8
Figure 1. Markov Model Schematic.
All patients began in the Markov model healthy at age 45 years, with average risk of developing colorectal cancer. If patients were not screened, as in the natural history, there was no polyp detection or polyp surveillance, and the cancer state was entered only after diagnostic colonoscopy due to symptomatic presentation. For all other strategies, screening could detect either polyps or cancer. Death was determined by age-specific all-cause mortality, or age and stage-specific cancer death.
Strategies
The strategies analyzed were screening with colonoscopy, FIT, stool DNA (S-DNA), LB, colonoscopy-LB screening hybrid (C-LB), and no screening or natural history (NH) (eFigure 1 in Supplement 1). In the NH strategy, patients did not undergo any CRC screening. The only CRCs accounted for were those found via diagnostic colonoscopy due to symptomatic presentation. In colonoscopy, FIT, and S-DNA strategies, 60.6% of the population underwent screening while 39.4% were modeled to not adhere to screening, akin to NH.8 For LB, we assumed patients would agree to receive a blood test for cancer screening and adherence was set to 100%; this assumption was varied broadly in sensitivity analysis.16 In the C-LB group, 60.6% of patients received colonoscopy, and all unscreened patients underwent LB. Patients at average risk received colonoscopy every 10 years, S-DNA and LB every 3 years, and FIT annually. We included the possibility of complications due to colonoscopy,22 but did not include complications from stool tests or blood tests.
Test Performance Characteristics
Base case estimates for sensitivity and specificity of CRC and polyp detection by colonoscopy, FIT, and stool DNA were obtained from previously published literature23,24,25,26,27 (Table 1). Several investigators are in the process of developing LB tests for cancer early detection, but few have detailed, published results available for analysis. For base case estimates for LB, we used data published by GRAIL for their MCED test Galleri,11 and extracted sensitivity and specificity for CRC detection only. This study had no data on precancerous lesions; thus, in our base case scenario, LB had no ability to detect polyps. In sensitivity and scenario analyses, we varied the performance of LB to account for the many tests in development.28
Table 1. Markov Model Inputs.
| Test performance characteristics | Base case | Range | Distribution | Source |
|---|---|---|---|---|
| Colonoscopya | ||||
| Cancer detection rate | 92.8 | 95% CI | Beta | Cooper et al,26 2012 |
| Interval cancer Rate | 7.2 | 95% CI | Beta | Cooper et al,26 2012 |
| High-risk polyp sensitivity | Age specific | 95% CI | Beta | Lieberman et al,30 2008 |
| Low-risk polyp sensitivity | Age specific | 95% CI | Beta | Lieberman et al,30 2008; Lowenfels et al,31 2011 |
| Complications | Age specific | 95% CI | Beta | Knudsen et al,23 2012 |
| FITa | ||||
| Cancer sensitivity | 73.8 | 95% CI | Beta | Knudsen et al,28 2016 |
| High-risk polyp sensitivity | 23.8 | 95% CI | Beta | Knudsen et al,28 2016 |
| Low-risk polyp sensitivity | 7.6 | 95% CI | Beta | Knudsen et al,28 2016 |
| Specificity | 96.4 | 95% CI | Beta | Knudsen et al,28 2016 |
| Stool DNAa | ||||
| Cancer sensitivity | 92.3 | 95% CI | Beta | Knudsen et al,28 2016 |
| High-risk polyp sensitivity | 42.4 | 95% CI | Beta | Knudsen et al,28 2016 |
| Low-risk polyp sensitivity | 17.2 | 95% CI | Beta | Knudsen et al,28 2016 |
| Specificity | 89.8 | 95% CI | Beta | Knudsen et al,28 2016 |
| Liquid biopsy | ||||
| Cancer sensitivity | 82.0 | 70-90 | Beta | Klein et al,11 2021 |
| Specificity | 99.5 | Not varied | Beta | Klein et al,11 2021 |
| Costs, 2022 US $ | ||||
| Colonoscopy with polypectomy | 1366 | 1024 to 1707 | Gamma | Lansdorp-Vogelaar et al,27 2010 |
| Colonoscopy without polypectomy | 1119 | 839 to 1399 | Gamma | Lansdorp-Vogelaar et al,27 2010 |
| Fecal immunochemical test | 52 | 39 to 65 | Gamma | Lansdorp-Vogelaar et al,27 2010 |
| Stool DNA test | 492 | 369 to 615 | Gamma | Lansdorp-Vogelaar et al,27 2010 |
| Liquid biopsy test | 949 | 0 to 949 | Gamma | GRAIL website,59 2023 |
| First year costs of local cancer | 42 347 | 31 760 to 52 934 | Gamma | Lang et al,35 2009 |
| Continuing costs of local cancer | 3941 | 2956 to 4926 | Gamma | Lang et al,35 2009 |
| Final year costs of local cancer | 14 158 | 10 618 to 17 697 | Gamma | Lang et al,35 2009 |
| First year costs of regional cancer | 56 019 | 42 014 to 70 024 | Gamma | Lang et al,35 2009 |
| Continuing costs of regional cancer | 8188 | 6141 to 10 235 | Gamma | Lang et al,35 2009 |
| Final year costs of regional cancer | 25 270 | 18 952 to 31 587 | Gamma | Lang et al,35 2009 |
| First year costs of distant cancer | 55 705 | 41 779 to 69 632 | Gamma | Lang et al,35 2009 |
| Continuing costs of distant cancer | 27 586 | 20 689 to 34 482 | Gamma | Lang et al,35 2009 |
| Final year costs of distant cancer | 34 464 | 25 848 to 43 080 | Gamma | Lang et al,35 2009 |
| Cost of colonoscopy complication | Age specific | 95% CI | Gamma | Knudsen et al,23 2012 |
Abbreviation: FIT, fecal immunochemical test.
Model inputs for colonoscopy, FIT, and stool DNA were applied to natural history incidence data and therefore vary by age. Sensitivity analysis was done around the 95% CI of the table mean.
Polyps
Data about national polyp prevalence by age group were obtained from the Clinical Outcomes Research Initiative (CORI) database.29,30 Polyps were defined as high risk (HR) or low risk (LR). Patients with polyps 10 mm or greater in size or with villous or high-grade dysplasia on histology were considered to have a HR polyp; patients with polyps less than 10 mm without villous or high-grade dysplasia on histology were considered to have a LR polyp.31 Once a polyp was diagnosed, patients underwent surveillance colonoscopy every 3 years for HR polyps and every 5 years for LR polyps until age 85 years.32,33
Effectiveness and Costs
Effectiveness for each screening strategy was assessed through life-years gained (LYG) after age 45 years, indicating the average number of years a patient would live under each screening group. This modeling analysis was performed from a societal cost perspective and therefore included direct health care use costs and indirect costs, such as time lost from work. Medicare inflation-adjusted costs for colonoscopy, colonoscopy with polypectomy, FIT, and stool DNA were estimated from a previously published analysis (Table 1).26 Once a patient was diagnosed with CRC, costs associated with CRC treatment and living with cancer were applied. These costs were obtained from SEER-Medicare estimates and divided into first year of living with cancer, continuing cancer costs, and last year of living before death due to cancer.34 All costs were adjusted to 2022 US dollars using the Consumer Price Index for health care costs.35 Costs and LYG were discounted at an annual rate of 3%. Base-case cost of LB was approximated as the current market price of the MCED test Galleri.36
The primary end points were LYG, total costs in 2022 US dollars, and incremental cost-effectiveness ratios (ICERs) (eMethods in Supplement 1). We used the commonly accepted US willingness-to-pay (WTP) threshold of $100 000/LYG to determine cost-effectiveness. Secondary end points included total CRCs diagnosed and overall death due to CRC.
Statistical Analyses
All analyses were performed using TreeAge Pro Healthcare Version 2022 R2.1 (TreeAge software). To determine the impact of model input uncertainty on cost-effectiveness results, we performed 1-way and probabilistic sensitivity analyses. In the scenario analyses, we considered cases where LB had sensitivity for HR polyps and LR polyps, adjusted LR polyp surveillance intervals to 7 and 10 years, and extended screening end age to 85 years. HR polyp sensitivity was varied between 10% and 100%, and LR polyp detection sensitivity was varied between 5% and 50%.28
In 1-way sensitivity analyses, model parameters are varied 1 at a time around the mean value, holding all other parameters constant. Upper and lower bounds for parameters were determined from 95% CIs around the parameter mean. For parameters where the lower bound yielded a negative value, the lower bound was set to 0. For most costs, we set the upper and lower bounds to 25% above and 25% below the mean cost, respectively. To determine at what cost LB became cost-effective compared with other strategies, we set the upper bound to its current estimated cost ($949), and the lower bound to $0.
In a probabilistic sensitivity analysis, each model input is sampled simultaneously from a probability distribution. The mean values for these distributions were base case values, and SDs were determined from literature or the corresponding database. Gamma distributions were used for costs and β distributions for all other variables.37,38,39 Probabilistic sensitivity analyses were performed over 5000 iterations for each strategy and the scenario where LB included polyp sensitivity.
Results
Base Case Results
In the NH, or no intervention strategy, 5.2% of the population developed CRC and 1.6% died from CRC, which is consistent with SEER CRC incidence from earlier decades20 (Table 2; eFigure 2 in Supplement 1). The population in the NH had 35.57 LYG after age 45 years with a mean cost per person of $6283. Colonoscopy was the most cost-effective screening strategy with an ICER of $28 071. It was associated with a mean cost of $9037 per person and 35.67 LYG. FIT and S-DNA strategies were both dominated by colonoscopy, meaning fewer life-years were gained compared with colonoscopy (FIT, 35.62 LYG; S-DNA, 35.64 LYG), though FIT was less expensive at $8223 and S-DNA more expensive at $11 583. C-LB had the greatest gain in life-years (35.68 LYG) and decreased total cancers and cancer deaths compared with colonoscopy. However, it was not cost-effective due to its cost of $12 006, yielding an ICER of $377 538 per LYG, above the WTP threshold of $100 000 per LYG. LB was dominated by other strategies and had a price of $15 562, though a higher proportion of cancers were diagnosed at an early stage compared with NH (eTable 1 in Supplement 1).
Table 2. Base Case Results.
| Strategy | Cost, $ | LYG | Incremental cost, $ | Incremental LYG | ICER, $/LYGa | Total cancer, %b | Deaths due to cancer, %b |
|---|---|---|---|---|---|---|---|
| Natural history | 6284 | 35.574 | [Referenc] | [Reference] | [Reference] | 5.2 | 1.6 |
| FIT | 8223 | 35.624 | 1939 | 0.050 | Extendedly dominatedc | 4.2 | 1.2 |
| Colonoscopy | 9037 | 35.672 | 2753 | 0.098 | 28 071 | 3.2 | 1.0 |
| Stool DNA | 11 583 | 35.641 | 2546 | −0.031 | Strictly dominatedd | 3.7 | 1.1 |
| Colonoscopy–LB | 12 006 | 35.680 | 2969 | 0.008 | 377 538 | 3.0 | 0.9 |
| LB | 15 562 | 35.581 | 3555 | −0.099 | Strictly dominatedd | 5.1 | 1.5 |
Abbreviations: ICER, incremental cost-effectiveness ratio; FIT, fecal immunochemical test; LB, liquid biopsy; LYG, life-years gained.
ICER is calculated relative to the next least costly, nondominated strategy.
Total cancer and deaths due to cancer represent cancer incidence and mortality of the entire population.
Extendedly dominated indicates that the strategy resulted in fewer LYG at a higher cost per life-year gained compared with another strategy.
Strictly dominated indicates that the strategy resulted in higher costs and fewer LYG compared with another strategy.
Scenario Analyses
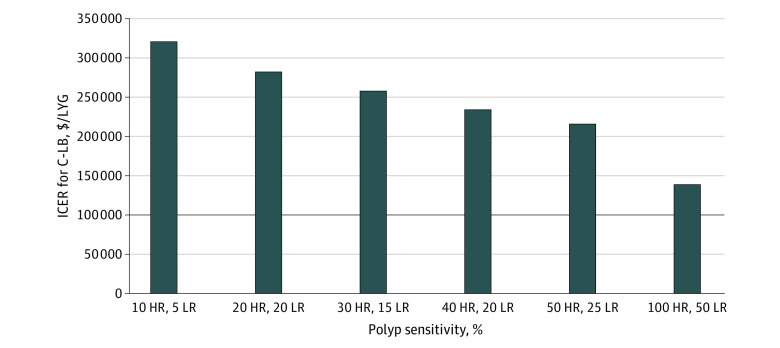
We varied the ability of LB to detect polyps over a range of sensitivities (Figure 2; eTable 2 in Supplement 1). At 10% HR polyp sensitivity and 5% LR polyp sensitivity, C-LB screening had more LYG than the base case (0.009 LYG). The ICER decreased to $319 834 per LYG but was still above the WTP threshold and was therefore not cost-effective. LB remained dominated, though LYG increased to 35.60 from 35.58 base case. C-LB and LB remained not cost-effective even with polyp sensitivity as high as 50% to 100%. Scenario analyses adjusting number of years between LR polyp screening (eTable 3 in Supplement 1), and end age of screening (eTable 4 in Supplement 1) were also done. Neither analysis differed from base-case model conclusions.
Figure 2. Scenario Analysis for LB Polyp Detection in C-LB.
Incremental cost-effectiveness ratios (ICERs) associated with varying liquid biopsy (LB) polyp sensitivity in the colonoscopy-LB (C-LB) screening group. The black line indicates an ICER of $100 000 per life-year gained (LYG), or the US willingness-to-pay threshold. At its current price, even with polyp sensitivity as high as 100% sensitivity for high-risk polyps and 50% sensitivity for low risk polyps, C-LB ICER compared with traditional screening strategies does not reach the US willingness-to-pay threshold. HR indicates high-risk polyp; LR, low-risk polyp.
One-Way Sensitivity Analyses
Results from 1-way sensitivity analyses were compared between 2 strategies. We compared NH with LB; colonoscopy with LB with polyp sensitivity (20% HR polyp sensitivity; 10% LR polyp sensitivity); and colonoscopy with C-LB with polyp sensitivity. We also directly compared the cost of LB with all other strategies (Figure 3).
Figure 3. Sensitivity Analyses for Liquid Biopsy (LB) Cancer Sensitivity and Cost.
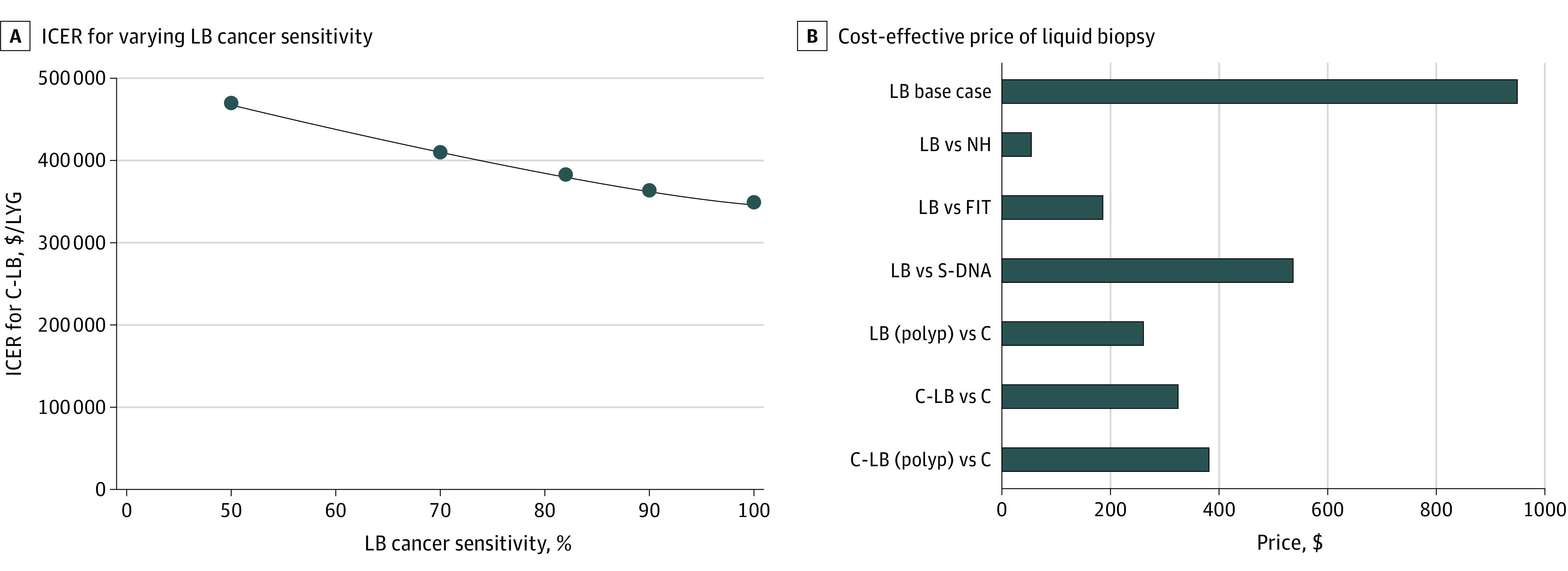
A, Incremental cost-effectiveness ratios (ICERs) associated with varying liquid biopsy (LB) cancer sensitivity in the colonscopy-LB (C-LB) screening arm. The base case sensitivity for LB cancer detection was 82%. When cancer sensitivity for LB was varied over a range of 50% to 100%, the ICER for LB remained dominated (not pictured) and the ICER for C-LB remained above the willingness-to-pay threshold of $100 000 per life-year gained (LYG), represented by the black line. ICERs are calculated relative to traditional screening strategies included in base case results. B, Analysis for cost-effective price of LB. The base case price of LB was set at $949, as shown in the top bar. Cost-effective price of LB compared with other strategies was determined via 1-way sensitivity analysis. LB (polyp) and C-LB (polyp) represent scenarios where LB included polyp sensitivity, with high-risk polyp sensitivity at 20% and low risk polyp sensitivity at 10%. FIT indicates fecal immunochemical test; NH, natural history; S-DNA, stool DNA.
Comparing NH with LB, the model was most sensitive to the cost of LB testing, followed by the performance characteristics of LB. The cost of LB had to be decreased to $56.16 for it to be cost-effective with an ICER below the $100 000 per LYG WTP threshold. When comparing colonoscopy with LB with polyp sensitivity, the parameters the model was most sensitive to were the cost of LB, followed by cost of colonoscopy and sensitivity of LB for polyps. Cost of LB had to be decreased to $260.95 for it to become cost-effective. Lastly, when comparing colonoscopy with C-LB with polyp sensitivity, the model was most sensitive to the performance of LB detection for cancer, LB cost, and the ability for LB to detect polyps. The cost of LB had to be reduced to $382.10 for C-LB to become cost-effective. We performed further sensitivity analyses by adjusting performance estimates and adherence rates for LB. The base case test sensitivity for cancer was 82% (range, 50%-100%), based on data from literature. We varied this sensitivity between 50% and 100%. Across the range of values for this parameter, total cancers and cancer deaths for LB and for C-LB remained stable. Overall cost of the screening strategies changed minimally and ICERs remained over a $100 000 per LYG WTP threshold in the case of C-LB, or dominated in the case of LB. Reductions in LB adherence led to decreased costs but increased cancer burden and cancer deaths for both LB and C-LB. Summary of 1-way sensitivity analyses is included in Figure 3 and eTables 5 and 6 in Supplement 1.
Probabilistic Sensitivity Analyses
Using a WTP threshold of $100 000 per LYG, colonoscopy was the cost-effective strategy in 86.8% of the 5000 iterations, C-LB hybrid was cost-effective in 12.2% of iterations, FIT was cost-effective in 1% of iterations, and stool DNA and LB were the cost-effective strategy in less than 1% of iterations (eFigure 3 in Supplement 1). In the scenario where LB testing included polyp sensitivity, with a mean sensitivity of 20% for HR polyp detection and 10% for LR polyp detection, colonoscopy remained the most cost-effective screening strategy 96.9% of the time at a WTP of $100 000 per LYG (eFigure 4 in Supplement 1).
Discussion
In this study, we used a Markov simulation to analyze the cost-effectiveness of LB, used both as a novel first or second-line screening modality. We present the first analysis that integrates novel LB into paradigms for CRC screening and systematically explores scenarios to determine the cost-effectiveness of LB.
The most cost-effective screening strategy in our base-case model was colonoscopy, with an ICER of $28 071 per LYG. While C-LB had the highest number of LYG and prevented the most cancers, the cost of LB would have to reduce by 66% (from $949 to $324) for the C-LB strategy to become cost-effective in our model. Compared with NH, the cost of LB would have to be reduced by 94% for its ICER to drop below the WTP threshold of $100 000 per LYG. When compared with stool-based tests, the cost of LB would have to decrease by 43% to 80% to be cost-effective. LB and C-LB had more LYG when polyp detection was introduced, but they did not achieve cost-effectiveness at LB’s current price even with perfect performance.
Adherence to CRC screening recommendations continues to be a significant public health concern. Patients cite many reasons for not getting CRC screened, including distress with bowel preparations, discomfort handling stool samples, and difficulty taking time off work.40,41,42,43 Given the numerous barriers, there is tremendous opportunity to engage unscreened patients. LB is far easier to complete than stool tests or colonoscopy, because it can be done alongside routine bloodwork. Our LB strategy modeled whether offering LB to an unscreened population would be cost-effective compared with no screening.
Prior analyses have studied the cost-effectiveness of CRC screening strategies,44,45 including less sensitive blood-based biomarkers.15 Our analysis focuses on a new category of blood-based screening tests that could have better performance with the advent of efficient next-generation sequencing and application of artificial intelligence for cancer prediction. Other studies have analyzed the use of LB for risk assessment of patients living with advanced cancers.46,47,48 To our knowledge, this is the first study that also considers novel LB in a cost-effectiveness analysis for CRC screening.
While LB is not currently widely available for routine clinical use, it will likely become easy to request by physician prescription.49 Many LB tests are in preparation for FDA approval, and more than one test has been awarded breakthrough device designation as recently as January of 2023, including one for MCED (pancreatic, ovarian, esophageal, liver, and lung cancer).50 With multiple LB tests soon to enter the market, and a potential reduction in price pending FDA approval, conducting a cost-effectiveness analysis is timely. Our analysis provides threshold targets for LB performance and cost to guide future test development and clinical and policy decision making.
Limitations
This study has limitations. In the absence of observed data to compare our results against, we calibrated our model to historical SEER data and cross-validated our results against previous CRC screening models. We made the assumption that patients would be 100% adherent to any follow-up testing. This included follow-up colonoscopy after a positive stool or LB test, or long-term polyp surveillance. Incorporating a patient’s prior behavior and allowing it to affect future choices would have greatly increased the complexity of our model and results. To address this, we performed sensitivity analysis on overall adherence rates.
Base case sensitivity and cost for LB testing was limited to the results of a single peer-reviewed study. Other LB companies have shared data in press releases and abstracts.28,51,52 Differences in their test characteristics are accounted for in sensitivity analyses for LB cancer detection and scenario analyses for polyp detection. We similarly varied cost in sensitivity analysis as cost of LB will likely decrease with advancing technology. We did not adjust specificity, because this was already set at 99.5%, and adjusting any lower would worsen the performance of LB. While many LB tests are CRC specific, others are marketed as MCED tests.53 Our analysis only took into consideration the cost-effectiveness for CRC screening; future analyses could take into account the cumulative result of screening for multiple cancers at once. We did not include the possible harms from LB testing, such as patient anxiety due to false-positive results.54,55,56
Our model approximates CRC incidence, mortality, and screening adherence for the total US population. The model does not account for racial and ethnic variation, thereby mitigating the known increased cancer mortality among people who belong to minoritized racial and ethnic groups.57,58 We do not believe the outcomes of this study would significantly change if the analyses were done for separate racial and ethnic groups because LB was only marginally more effective than no screening.
Conclusions
In this economic evaluation of LB for CRC screening in the US, screening with LB was not cost-effective compared with current screening strategies. Furthermore, for individuals who refused traditional screening, it was not cost-effective to offer LB over no screening. LB tests for CRC screening may become cost-effective in the future if they are significantly less expensive or if polyp detection is introduced coupled with a decrease in cost.
eFigure 1. CRC Screening Strategies Considered
eMethods. Evaluating Cost-Effectiveness
eTable 1. Cancer Stage Distribution by Screening Strategy
eFigure 2. Cost-Effectiveness Plane
eTable 2. Liquid Biopsy Polyp Sensitivity Scenario Analyses Clinical End Points
eTable 3. Scenario Analyses for Low Risk Polyp Surveillance Interval
eTable 4. Scenario Analyses For Screening End Age
eTable 5. Sensitivity Analysis for Liquid Biopsy Adherence
eTable 6. Sensitivity Analysis for Liquid Biopsy Cancer Sensitivity and Cost
eFigure 3. Probabilistic Sensitivity Analysis for Base-Case Screening Strategies
eFigure 4. Probabilistic Sensitivity Analysis for Liquid Biopsy with Polyp Detection
Data Sharing Statement
References
- 1.Common Cancer Types. National Cancer Institute. Published April 21, 2015. Accessed February 15, 2023. https://www.cancer.gov/types/common-cancers
- 2.Key statistics for colorectal cancer. American Cancer Society. Accessed February 15, 2023. https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html
- 3.Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(19):1978-1998. doi: 10.1001/jama.2021.4417 [DOI] [PubMed] [Google Scholar]
- 4.Winawer SJ. The history of colorectal cancer screening: a personal perspective. Dig Dis Sci. 2015;60(3):596-608. doi: 10.1007/s10620-014-3466-y [DOI] [PubMed] [Google Scholar]
- 5.Patel SG, May FP, Anderson JC, et al. Updates on age to start and stop colorectal cancer screening: recommendations from the US Multisociety Task Force on Colorectal Cancer. Gastrointest Endosc. 2022;95(1):1-15. doi: 10.1016/j.gie.2021.06.012 [DOI] [PubMed] [Google Scholar]
- 6.Day LW, Bhuket T, Allison J. FIT testing: an overview. Curr Gastroenterol Rep. 2013;15(11):357. doi: 10.1007/s11894-013-0357-x [DOI] [PubMed] [Google Scholar]
- 7.Stracci F, Zorzi M, Grazzini G. Colorectal cancer screening: tests, strategies, and perspectives. Front Public Health. 2014;2:210. doi: 10.3389/fpubh.2014.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Street W. Colorectal cancer facts and figures 2020-2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf
- 9.Miller-Wilson LA, Limburg P, Helmueller L, João Janeiro M, Hartlaub P. The impact of multi-target stool DNA testing in clinical practice in the United States: A real-world evidence retrospective study. Prev Med Rep. 2022;30:102045. doi: 10.1016/j.pmedr.2022.102045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stock C, Knudsen AB, Lansdorp-Vogelaar I, Haug U, Brenner H. Colorectal cancer mortality prevented by use and attributable to nonuse of colonoscopy. Gastrointest Endosc. 2011;73(3):435-443.e5. doi: 10.1016/j.gie.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 11.Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;32(9):1167-1177. doi: 10.1016/j.annonc.2021.05.806 [DOI] [PubMed] [Google Scholar]
- 12.Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV; CCGA Consortium . Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020;31(6):745-759. doi: 10.1016/j.annonc.2020.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hubbell E, Clarke CA, Aravanis AM, Berg CD. Modeled reductions in late-stage cancer with a multi-cancer early detection test. Cancer Epidemiol Biomarkers Prev. 2021;30(3):460-468. doi: 10.1158/1055-9965.EPI-20-1134 [DOI] [PubMed] [Google Scholar]
- 14.Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. Accessed February 23, 2023. https://www.science.org/doi/10.1126/science.aar3247?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed& [DOI] [PMC free article] [PubMed]
- 15.Ladabaum U, Allen J, Wandell M, Ramsey S. Colorectal cancer screening with blood-based biomarkers: cost-effectiveness of methylated septin 9 DNA versus current strategies. Cancer Epidemiol Biomarkers Prev. 2013;22(9):1567-1576. doi: 10.1158/1055-9965.EPI-13-0204 [DOI] [PubMed] [Google Scholar]
- 16.Lee MJ, Hueniken K, Kuehne N, et al. Cancer patient-reported preferences and knowledge for liquid biopsies and blood biomarkers at a comprehensive cancer center. Cancer Manag Res. 2020;12:1163-1173. doi: 10.2147/CMAR.S235777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Screening for Colorectal Cancer . US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(19):1965-1977. doi: 10.1001/jama.2021.6238 [DOI] [PubMed] [Google Scholar]
- 18.Qaseem A, Crandall CJ, Mustafa RA, et al. ; Clinical Guidelines Committee of the American College of Physicians . Screening for colorectal cancer in asymptomatic average-risk adults: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;171(9):643-654. doi: 10.7326/M19-0642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.*STAT Database: Mortality - All COD, Aggregated Total U.S. (1990-2017) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, SUrveillance Research Program, released December 2019. Underlying mortality data provided by NCHS (https://www.cdc.gov/nchs).
- 20.SEER Program. SEER stat databases: SEER November 2020 submission. National Cancer Institute. Accessed October 10, 2023. https://seer.cancer.gov/data-software/documentation/seerstat/nov2020
- 21.Arias E, Xu J. United States life tables, 2019. Natl Vital Stat Rep. 2022;70(19):1-59. doi: 10.15620/cdc:113096 [DOI] [PubMed] [Google Scholar]
- 22.Knudsen AB, Hur C, Gazelle GS, Schrag D, McFarland EG, Kuntz KM. Rescreening of individuals with a negative colonoscopy: a comparative effectiveness analysis. Ann Intern Med. 2012;157(9):611-620. doi: 10.7326/0003-4819-157-9-201211060-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287-1297. doi: 10.1056/NEJMoa1311194 [DOI] [PubMed] [Google Scholar]
- 24.García-Albéniz X, Hsu J, Bretthauer M, Hernán MA. Effectiveness of screening colonoscopy to prevent colorectal cancer among Medicare beneficiaries aged 70-79 years: a prospective observational study. Ann Intern Med. 2017;166(1):18-26. doi: 10.7326/M16-0758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper GS, Xu F, Barnholtz Sloan JS, Schluchter MD, Koroukian SM. Prevalence and predictors of interval colorectal cancers in Medicare beneficiaries. Cancer. 2012;118(12):3044-3052. doi: 10.1002/cncr.26602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, Wilschut JA, Zauber AG, van Ballegooijen M. Stool DNA testing to screen for colorectal cancer in the Medicare population: a cost-effectiveness analysis. Ann Intern Med. 2010;153(6):368-377. doi: 10.7326/0003-4819-153-6-201009210-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315(23):2595-2609. doi: 10.1001/jama.2016.6828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guardant Health announces positive results from pivotal ECLIPSE study evaluating a blood test for the detection of colorectal cancer. Guardant Health. Accessed February 23, 2023. https://investors.guardanthealth.com/press-releases/press-releases/2022/Guardant-Health-announces-positive-results-from-pivotal-ECLIPSE-study-evaluating-a-blood-test-for-the-detection-of-colorectal-cancer/default.aspx
- 29.Lieberman DA, Holub JL, Moravec MD, Eisen GM, Peters D, Morris CD. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic Black and White patients. JAMA. 2008;300(12):1417-1422. doi: 10.1001/jama.300.12.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowenfels AB, Williams JL, Holub JL, Maisonneuve P, Lieberman DA. Determinants of polyp size in patients undergoing screening colonoscopy. BMC Gastroenterol. 2011;11:101. doi: 10.1186/1471-230X-11-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. doi: 10.1053/j.gastro.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 32.Gupta S, Jacobs ET, Baron JA, et al. Risk stratification of individuals with low-risk colorectal adenomas using clinical characteristics: a pooled analysis. Gut. 2017;66(3):446-453. doi: 10.1136/gutjnl-2015-310196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Updated Polypectomy Surveillance Recommendations. Accessed August 31, 2023. https://www.jwatch.org/na50884/2020/02/12/updated-polypectomy-surveillance-recommendations
- 34.Lang K, Lines LM, Lee DW, Korn JR, Earle CC, Menzin J. Lifetime and treatment-phase costs associated with colorectal cancer: evidence from SEER-Medicare data. Clin Gastroenterol Hepatol. 2009;7(2):198-204. doi: 10.1016/j.cgh.2008.08.034 [DOI] [PubMed] [Google Scholar]
- 35.BEA interactive data application. Accessed February 23, 2023. https://apps.bea.gov/iTable/?reqid=19&step=2&isuri=1&categories=survey#eyJhcHBpZCI6MTksInN0ZXBzIjpbMSwyLDNdLCJkYXRhIjpbWyJjYXRlZ29yaWVzIiwiU3VydmV5Il0sWyJOSVBBX1RhYmxlX0xpc3QiLCI2OSJdXX0=
- 36.Cancer screening cost with the Galleri test. Galleri. Accessed February 23, 2023. https://www.galleri.com/patient/the-galleri-test/cost
- 37.Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation: a practical approach. Med Decis Making. 1985;5(2):157-177. doi: 10.1177/0272989X8500500205 [DOI] [PubMed] [Google Scholar]
- 38.Briggs A. Probabilistic analysis of cost-effectiveness models: statistical representation of parameter uncertainty. Value Health. 2005;8(1):1-2. doi: 10.1111/j.1524-4733.2005.08101.x [DOI] [PubMed] [Google Scholar]
- 39.Claxton K, Sculpher M, McCabe C, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ. 2005;14(4):339-347. doi: 10.1002/hec.985 [DOI] [PubMed] [Google Scholar]
- 40.Jones RM, Devers KJ, Kuzel AJ, Woolf SH. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010;38(5):508-516. doi: 10.1016/j.amepre.2010.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones RM, Woolf SH, Cunningham TD, et al. The relative importance of patient-reported barriers to colorectal cancer screening. Am J Prev Med. 2010;38(5):499-507. doi: 10.1016/j.amepre.2010.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bie AKL, Brodersen J. Why do some participants in colorectal cancer screening choose not to undergo colonoscopy following a positive test result: a qualitative study. Scand J Prim Health Care. 2018;36(3):262-271. doi: 10.1080/02813432.2018.1487520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilkins T, Gillies RA, Harbuck S, Garren J, Looney SW, Schade RR. Racial disparities and barriers to colorectal cancer screening in rural areas. J Am Board Fam Med. 2012;25(3):308-317. doi: 10.3122/jabfm.2012.03.100307 [DOI] [PubMed] [Google Scholar]
- 44.Ran T, Cheng CY, Misselwitz B, Brenner H, Ubels J, Schlander M. Cost-effectiveness of colorectal cancer screening strategies: a systematic review. Clin Gastroenterol Hepatol. 2019;17(10):1969-1981.e15. doi: 10.1016/j.cgh.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 45.Zauber AG. Cost-effectiveness of colonoscopy. Gastrointest Endosc Clin N Am. 2010;20(4):751-770. doi: 10.1016/j.giec.2010.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashktorab H, Brim H. Blood-based liquid biopsies: a noninvasive and cost-effective tool for improved risk assessment and identification of lymph node metastasis in patients with submucosal T1 colorectal cancer. Gastroenterol. 2021;161(1):29-31. https://www.gastrojournal.org/article/S0016-5085(21)00662-4/fulltext [DOI] [PubMed] [Google Scholar]
- 47.Sánchez-Calderón D, Pedraza A, Mancera Urrego C, Mejía-Mejía A, Montealegre-Páez AL, Perdomo S. Analysis of the cost-effectiveness of liquid biopsy to determine treatment change in patients with HER2-positive advanced breast cancer in Colombia. Clinicoecon Outcomes Res. 2020;12:115-122. doi: 10.2147/CEOR.S220726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ezeife DA, Spackman E, Juergens RA, et al. The economic value of liquid biopsy for genomic profiling in advanced non-small cell lung cancer. Ther Adv Med Oncol. 2022;14. doi: 10.1177/17588359221112696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frequently asked questions from patients. Galleri. Accessed March 9, 2023. https://www.galleri.com/patient/support/faqs
- 50.Rock B. Burning Rock received FDA breakthrough device designation for its OverCTM multi-cancer detection blood test. Accessed April 14, 2023. https://www.prnewswire.com/news-releases/burning-rock-received-fda-breakthrough-device-designation-for-its-overc-multi-cancer-detection-blood-test-301712390.html
- 51.Validation of a panel of methylated DNA and protein markers for multi-cancer detection in plasma. Abstracts Online. Accessed August 31, 2023. https://www.abstractsonline.com/pp8/#!/10517/presentation/20605
- 52.Putcha G, Xu C, Shaukat Md, Mph A, Levin TR. Prevention of colorectal cancer through multiomics blood testing: The PREEMPT CRC study. J Clin Oncol. 2022;40(4)(suppl):TPS208-TPS208. doi: 10.1200/JCO.2022.40.4_suppl.TPS208 [DOI] [Google Scholar]
- 53.Chen M, Zhao H. Next-generation sequencing in liquid biopsy: cancer screening and early detection. Hum Genomics. 2019;13(1):34. doi: 10.1186/s40246-019-0220-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brodersen J, Siersma VD. Long-term psychosocial consequences of false-positive screening mammography. Ann Fam Med. 2013;11(2):106-115. doi: 10.1370/afm.1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McNaughton-Collins M, Fowler FJ Jr, Caubet JF, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med. 2004;117(10):719-725. doi: 10.1016/j.amjmed.2004.06.036 [DOI] [PubMed] [Google Scholar]
- 56.Harris RP, Sheridan SL, Lewis CL, et al. The harms of screening: a proposed taxonomy and application to lung cancer screening. JAMA Intern Med. 2014;174(2):281-285. doi: 10.1001/jamainternmed.2013.12745 [DOI] [PubMed] [Google Scholar]
- 57.Esnaola NF, Ford ME. Racial differences and disparities in cancer care and outcomes: where’s the rub? Surg Oncol Clin N Am. 2012;21(3):417-437, viii. doi: 10.1016/j.soc.2012.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Augustus GJ, Ellis NA. Colorectal cancer disparity in African Americans: risk factors and carcinogenic mechanisms. Am J Pathol. 2018;188(2):291-303. doi: 10.1016/j.ajpath.2017.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- SEER Program. SEER stat databases: SEER November 2020 submission. National Cancer Institute. Accessed October 10, 2023. https://seer.cancer.gov/data-software/documentation/seerstat/nov2020
Supplementary Materials
eFigure 1. CRC Screening Strategies Considered
eMethods. Evaluating Cost-Effectiveness
eTable 1. Cancer Stage Distribution by Screening Strategy
eFigure 2. Cost-Effectiveness Plane
eTable 2. Liquid Biopsy Polyp Sensitivity Scenario Analyses Clinical End Points
eTable 3. Scenario Analyses for Low Risk Polyp Surveillance Interval
eTable 4. Scenario Analyses For Screening End Age
eTable 5. Sensitivity Analysis for Liquid Biopsy Adherence
eTable 6. Sensitivity Analysis for Liquid Biopsy Cancer Sensitivity and Cost
eFigure 3. Probabilistic Sensitivity Analysis for Base-Case Screening Strategies
eFigure 4. Probabilistic Sensitivity Analysis for Liquid Biopsy with Polyp Detection
Data Sharing Statement