ABSTRACT
Background
The association between newer classes of glucose‐lowering drugs (GLDs) and the risk of Parkinson's disease (PD) remains unclear.
Objective
The aim was to examine the effect of newer GLDs on the risk of PD through a meta‐analysis of randomized outcome trials.
Methods
The methods included randomized placebo‐controlled outcome trials that reported PD events associated with three newer classes of GLDs (ie, dipeptidyl peptidase‐4 inhibitors, glucagon‐like peptide‐1 receptor agonists, and sodium‐glucose co‐transporter‐2 inhibitors) in participants with or without type 2 diabetes. The pooled odds ratio (OR) and 95% confidence interval (CI) were estimated using Peto's method.
Results
The study included 24 trials involving 33 PD cases among 185,305 participants during a median follow‐up of 2.2 years. Newer GLDs were significantly associated with a lower PD risk (OR: 0.50; 95% CI: 0.25–0.98) than placebo.
Conclusion
Newer GLDs may possibly be associated with a decreased risk of PD; however, larger datasets are required to confirm or refute this notion.
Keywords: glucose‐lowering drugs, meta‐analysis, Parkinson's disease, randomized outcome trials, type 2 diabetes
Parkinson's disease (PD), the fastest‐growing neurological disorder, affects over 1 million persons in the United States. 1 , 2 With increases in the aging population, the number of PD cases is expected to increase to 1.2 million by 2030. 1 PD contributes to a large US economic burden, with an estimated cost of $51.9 billion in 2017. 3 There is currently no cure for PD, and the existing treatments primarily focus on alleviating symptoms and enhancing or preserving the quality of life of patients. 4
Newer classes of glucose‐lowering drugs (GLDs) (Table S1), including dipeptidyl peptidase‐4 (DPP4) inhibitors, glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs), and sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors, have been increasingly used for treating type 2 diabetes (T2D) due to their cardiovascular and renal benefits. T2D and PD both share some common parts of signaling pathways such as insulin resistance. 5 Newer GLDs have been shown to improve insulin resistance and mediate other pathways (eg, mitochondrial dysfunction); thus, they may be potential therapeutic strategies for preventing or treating PD. 5 , 6 Cardiovascular outcome trials of the newer GLDs, as in T2D, 7 recommended by the U.S. Food and Food Administration to evaluate the cardiovascular outcomes of all newer GLDs, enable us to evaluate the impact of newer GLDs on the risk of PD. However, PD was not the prespecified outcome, which may lead to insufficient statistical power from individual trials. To address this, we conducted a meta‐analysis of randomized outcome trials to examine the association between newer GLDs (eg, DPP4 inhibitors, GLP‐1RAs, and SGLT2 inhibitors) and the risk of PD among individuals with or without T2D.
Methods
We used the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement to conduct this analysis.
Search Strategy and Study Selection
Following the previously reported search strategy, 8 we updated the search of PubMed, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) until December 2022. Additionally, we performed a manual search to identify any other eligible publications by examining the references of relevant reviews.
Two reviewers (H.T. and Y.L.) independently selected the studies based on the following inclusion criteria: (1) randomized placebo‐controlled cardiovascular and renal outcome trials; (2) trials enrolled adults (≥18 years) with or without T2D; (3) trials compared newer GLDs, including DPP‐4 inhibitors, GLP‐1RAs, and SGLT2 inhibitors, with placebo; and (4) trials reported the events of PD. PD was identified using one preferred term—“Parkinson's disease”—using the Medical Dictionary for Regulatory Activities (MedDRA). Because PD was not the prespecified outcome, it was more likely to be reported as an adverse event by patients and confirmed by physicians.
Data Extraction and Quality Assessment
We extracted the following data from each study: first author, publication year, baseline participant characteristics, inclusion criteria, study drug and control treatments, follow‐up duration, and the number of PD cases (extracted from trial results published on www.clinicaltrials.gov). We also assessed the quality of each included study using the Cochrane risk of bias assessment tool. 9
Statistical Analysis
We calculated a pooled odds ratio (OR) and 95% confidence interval (CI) for risk of PD using Peto's method, the least biased and most powerful method for assessing rare events (<1%). 10 We conducted the following two subgroup analyses: (1) based on the class of newer GLDs (DPP4 inhibitors, GLP‐1RAs, and SGLT2 inhibitors vs. placebo) and (2) based on the type of participants included (among participants with T2D only and those with or without T2D). We assessed heterogeneity between studies and the interaction between subgroups using χ2 test. Sensitivity analyses using the Mantel–Haenszel method and a 0.5 continuity correction for zero events in both arms were conducted to test the robustness of the results. Potential publication bias was assessed using a funnel plot, Begg's test, or Egger's test. A two‐sided P‐value <0.05 was considered statistically significant. The statistical analyses were performed using Stata (version 16; Stata Corp., College Station, TX).
Results
Twenty‐four trials met our inclusion criteria and were included in this meta‐analysis (Figure S1). 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 The baseline characteristics of studies are presented in Table 1. A total of 185,305 participants with a mean age of 65.1 years were randomly allocated to either a newer GLD or placebo group. Twenty trials included T2D participants only, 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 33 , 34 whereas four trials included participants with or without T2D (enrolling patients with heart failure or chronic kidney disease). 29 , 30 , 31 , 32 There were five trials for DPP‐4 inhibitors, 11 , 12 , 13 , 14 , 15 eight trials for GLP‐1RAs, 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 and 11 trials for SGLT2 inhibitors. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 Across the trials, there were 33 PD cases during a median follow‐up of 2.2 years (range: 0.8–5.4 years). The risk of bias for the selective reporting domain was determined as unclear because PD was not the prespecified outcome, whereas the other five domains were determined as low risk of bias (Table S2).
TABLE 1.
Basic characteristics of 24 randomized placebo‐controlled cardiovascular and renal outcome trials
| Study | Trial Name | N | Population | Age (y) | Men (%) | White (%) | Treatment | Follow‐up (y) | |
|---|---|---|---|---|---|---|---|---|---|
| Scirica et al. (2013) 11 | SAVOR‐TIMI 53 | 16,492 | T2D patients with a history of, or at risk for, CVD | 65 | 67 | 75 | Saxagliptin | 2.1 | |
| White et al. (2013) 12 | EXAMINE | 5380 | T2D patients with CVD | 61 | 68 | 73 | Alogliptin | 1.5 | |
| Green et al. (2015) 13 | TECOS | 14,671 | T2D patients with CVD | 66 | 71 | 68 | Sitagliptin | 3 | |
| Gantz et al. (2017) 14 | OMNEON | 4192 | T2D patients with CVD | 64 | 70 | 81 | Omarigliptin | 1.8 | |
| Rosenstock et al. (2019) 15 | CARMELINA | 6979 | T2D patients with high cardiovascular risk | 66 | 63 | 80 | Linagliptin | 2.2 | |
| Pfeffer et al. (2015) 16 | ELIXA | 6068 | T2D patients with CVD | 60 | 70 | 75 | Lixisenatide | 2.1 | |
| Marso et al. (2016) 17 | SUSTAIN‐6 | 3297 | T2D patients with established CVD or high cardiovascular risk | 65 | 61 | 83 | Semaglutide | 2.1 | |
| Marso et al. (2016) 18 | LEADER | 9340 | T2D patients with high cardiovascular risk | 64 | 64 | 78 | Liraglutide | 3.8 | |
| Holman et al. (2017) 19 | EXSCEL | 14,752 | T2D patients with or without previous CVD | 62 | 62 | 76 | Exenatide | 3.2 | |
| Hernandez et al. (2018) 20 | HARMONY | 9432 | T2D patients with CVD | 64 | 70 | 70 | Albiglutid | 1.6 | |
| Gerstein et al. (2019) 21 | REWIND | 9901 | T2D patients with previous CVD or at high cardiovascular risk | 66 | 54 | 76 | Dulaglutide | 5.4 | |
| Husain et al. (2019) 22 | PIONEER‐6 | 3183 | T2D patients at high cardiovascular risk | 66 | 68 | 72 | Semaglutide | 1.3 | |
| Gerstein et al. (2021) 23 | AMPLITUDE‐O | 4076 | T2D patients with CVD or CKD at high cardiovascular risk | 72 | 55 | 76 | Efpeglenatide | 2.2 | |
| Zinman et al. (2015) 24 | EMPA‐REG OUTCOME | 7020 | T2D patients with CVD | 63 | 72 | 72 | Empagliflozin | 3.1 | |
| Neal et al. (2017) 25 | CANVAS Program | 10,142 | T2D patients with high CV risk | 63 | 64 | 78 | Canagliflozin | 2.4 | |
| Wiviott et al. (2019) 26 | DECLARE–TIMI 58 | 17,161 | T2D patients had or were at risk for atherosclerotic CVD | 64 | 63 | 80 | Dapagliflozin | 4.2 | |
| Perkovic et al. (2019) 27 | CREDENCE | 4401 | T2D patients with albuminuric CKD | 63 | 66 | 67 | Canagliflozin | 2.6 | |
| Cannon et al. (2020) 28 | VERTIS‐CV | 8246 | T2D patients with atherosclerotic CVD | 64 | 70 | 88 | Ertugliflozin | 3.5 | |
| McMurray et al. (2019) 29 | DAPA‐HF | 4744 | Patients with HFrEF, regardless of the presence or absence of T2D | 66 | 77 | 70 | Dapagliflozin | 1.5 | |
| Heerspink et al. (2020) 30 | DAPA‐CKD | 4304 | Patients with CKD | 62 | 67 | 53 | Dapagliflozin | 2.4 | |
| Packer et al. (2020) 31 | EMPEROR‐Reduced | 3730 | Patients with HFrEF | 67 | 76 | 70 | Empagliflozin | 1.3 | |
| Anker et al. (2021) 32 | EMPEROR‐Preserved | 5988 | Patients with HFpEF | 72 | 55 | 76 | Empagliflozin | 2.2 | |
| Bhatt et al. (2021) 33 | SCORED | 10,584 | T2D patients with CKD and risks for CVD | 69 | 55 | 83 | Sotagliflozin | 1.3 | |
| Bhatt et al. (2021) 34 | SOLOIST‐WHF | 1222 | T2D patients hospitalized for worsening heart failure | 69 | 66 | 93 | Sotagliflozin | 0.75 | |
Abbreviations: CKD, chronic kidney disease; CVD, cardiovascular disease; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; N, number of population; T2D, type 2 diabetes.
Our meta‐analysis of 24 trials showed that there was an association between newer GLDs and a lower risk of PD compared to placebo (OR: 0.50; 95% CI: 0.25–0.98) (Fig. 1). Meta‐analysis of 11 trials showed that there was weak evidence of an association between SGLT2 inhibitors and a decreased risk of PD (OR: 0.37; 95% CI: 0.13–1.01). However, there was a lack of evidence regarding the association between GLP‐1RAs and a decrease in PD risk (OR: 0.51; 95% CI: 0.10–2.55) and between DPP‐4 inhibitors and a decrease in PD risk (OR: 0.71; 95% CI: 0.23–2.22). Further subgroup analyses by type of participants found similar effects between trials including participants with T2D only (OR: 0.60; 95% CI: 0.29–1.23) and trials including participants with and without T2D (OR: 0.14; 95% CI: 0.02–0.96), with a P for interaction of 0.16 (Figure S2).
FIG. 1.
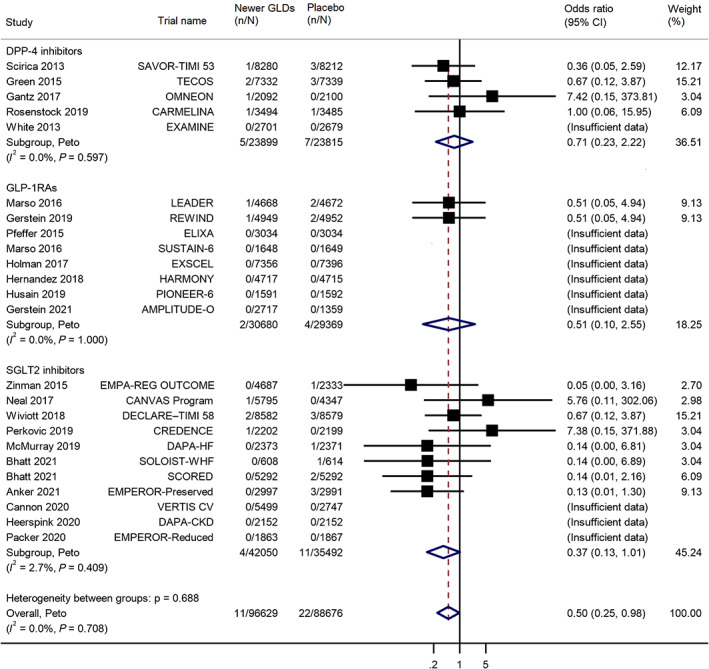
Meta‐analysis of the effects of newer glucose‐lowering drugs on the risk of Parkinson's disease in participants with or without type 2 diabetes, subgroup by type of newer GLDs (glucose‐lowering drug). CI, confidence interval; DPP‐4 inhibitors, dipeptidyl peptidase‐4 inhibitors; GLP‐1RAs, glucagon‐like peptide‐1 receptor agonists; SGLT2 inhibitors, sodium‐glucose co‐transporter‐2 inhibitors.
Our sensitivity analyses using the Mantel–Haenszel method (OR: 0.55; 95% CI: 0.29–1.04) (Figure S3) and a 0.5 continuity correction for zero events in both arms (OR: 0.57; 95% CI: 0.31–1.03) (Figure S4) further confirm our primary results. No evidence of statistical heterogeneity was observed in meta‐analyses (all P > 0.05). Also, there was no evidence of publication bias based on Begg's test (P = 0.91), Egger's test (P = 0.71), or funnel plot (Figure S5).
Discussion
The results of this meta‐analysis showed an association between newer classes of GLDs and a reduced risk of PD. In the subgroup analyses by drug class, there was weak evidence regarding the association between SGLT2 inhibitors and a reduced risk of PD. However, lack of evidence supported the association between GLP‐1RAs and DPP‐4 inhibitors and a decrease in PD risk. It should be noted that the findings should be interpreted with caution due to the low number of events and short duration of follow‐up of trials included.
This study found a decreased risk of PD associated with newer GLDs; however, the underlying mechanism of this effect is not completely clear. 5 Previous studies have reported T2D to be an independent risk factor for PD development, 5 , 35 and both conditions share similar pathophysiological pathways, such as impaired insulin signaling, mitochondrial dysfunction, oxidative damage, and inflammation. 5 Newer GLDs have generated significant interest in their neuroprotective effects by improving insulin resistance and reducing oxidative damage and inflammation, which make them promising therapeutic options for the management of PD. 6
The results of this study indicated a potential benefit of SGLT2 inhibitors in reducing the risk of PD, which aligns with previous research findings. 36 , 37 A recently published population‐based cohort study involving people with T2D found a significant association between SGLT2 inhibitors and a lower risk of PD compared to DPP‐4 inhibitors (hazard ratio [HR]: 0.28; 95% CI: 0.09–0.91). 36 Cumulative studies have also shown the neuroprotective effects of GLP‐1RAs. 5 One clinical trial showed that exenatide, a specific GLP‐1RA, exhibited the ability to alleviate cognitive, motor, and nonmotor symptoms in patients with PD. 38 One meta‐analysis of two observational studies showed that GLP‐1RAs were significantly associated with a 59% decrease in the risk of PD compared to no use (HR: 0.41; 95% CI: 0.19–0.87). 39 However, our study found a nonsignificant decrease in PD risk (OR: 0.51; 95% CI: 0.10–2.55), which could potentially be attributed to the limited number of PD cases (6 of 60,049 participants), resulting in insufficient statistical power to detect a significant difference. In our study, DPP‐4 inhibitors were not significantly associated with a decreased risk of PD, which was consistent with results from a meta‐analysis of three observational studies (HR: 0.69; 95% CI: 0.35–1.38). 39 Recent population studies have provided encouraging and inspiring insights into the potential benefits of newer GLDs in reducing PD risk. However, current evidence on the matter is still limited, and it needs to be further explored.
Our study findings must be interpreted with caution considering the following limitations. First, PD was not the prespecified outcome in these trials and primarily relied on patient‐report/physician confirmation; thus, PD events may not be completely reported during follow‐up, resulting in missed cases. The underreporting of PD cases might explain the neutral effects observed in each class of newer GLDs and the results of sensitivity analyses. It is important to note that we cannot completely rule out a significant reduction in PD risk associated with each class of newer GLD. Second, due to the unavailability of individual participant data from the trials, we were unable to determine the PD status of participants at baseline. Furthermore, a clinical diagnosis of PD typically takes 3 to 10 years to confirm. 40 Thus, PD recorded in these trials might be prevalent cases rather than incident events. Third, further analyses (eg, the association between change in glucose levels and PD risk) were not possible because individual participant data from these trials were unavailable.
In conclusion, our meta‐analysis of randomized outcome trials suggests that there may be a potential association between newer GLDs and a reduced risk of developing PD. Our findings also highlight the possibility of repurposing newer GLDs for the treatment of PD. However, further studies using real‐world data are required to confirm or refute this notion.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
H.T.: 1A, 1B, 1C, 2A, 2B, 3A
Y.L.: 1B, 1C, 3B
M.S.O.: 2C, 3B
W.T.D: 2C, 3B
A.R‐Z.: 2C, 3B
F.W.: 2C, 3B
Y.H.: 2C, 3B
W‐H.C.: 2C, 3B
B.A.V.: 2C, 3B
J.B.: 1A, 1B, 2C, 3B
J.G.: 1A, 1B, 2C, 3B
Disclosures
Ethical Compliance Statement: Institutional review board approval and informed patient consent were not necessary for this work. We confirm we have read the journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: This project was funded by the American Foundation for Pharmaceutical Education (AFPE) Predoctoral Fellowship in Pharmaceutical Sciences, the National Institute of Diabetes and Digestive and Kidney (NIDDK) of the National Institutes of Health (R01DK133465), and the National Institute on Aging (NIA) of the National Institutes of Health (R01AG080991, R01AG076234, and R56AG069880). The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months: J.G. received consulting fee from Pfizer which was outside of the current work. The other authors declare that there are no additional disclosures to report.
Supporting information
Table S1. Classes of glucose‐lowering drugs (excluding insulin) available for treating type 2 diabetes.
Table S2. Risk of bias of each domain for each study.
Figure S1. Flowchart of the study selection. CENTRAL, Cochrane Central Register of Controlled Trials; DPP‐4 inhibitors, dipeptidyl peptidase‐4 inhibitors; GLP‐1RAs, glucagon‐like peptide‐1 receptor agonists; SGLT2 inhibitors, sodium‐glucose co‐transporter‐2 inhibitors.
Figure S2. Meta‐analysis of the effects of newer glucose‐lowering drugs (GLDs) on the risk of Parkinson's disease in participants with or without type 2 diabetes (T2D), subgroup by type of participants included. CI, confidence interval; OR, odds ratio.
Figure S3. Meta‐analysis of the effects of newer glucose‐lowering drugs (GLDs) on the risk of Parkinson's disease in participants with or without type 2 diabetes using Mantel–Haenszel method. CI, confidence interval; DPP‐4 inhibitors, dipeptidyl peptidase‐4 inhibitors; GLP‐1RAs, glucagon‐like peptide‐1 receptor agonists; OR, odds ratio; SGLT2 inhibitors, sodium‐glucose co‐transporter‐2 inhibitors.
Figure S4. Meta‐analysis of the effects of newer glucose‐lowering drugs (GLDs) on the risk of Parkinson's disease in participants with or without type 2 diabetes using a 0.5 continuity correction for zero events in both arms. CI, confidence interval; DPP‐4 inhibitors, dipeptidyl peptidase‐4 inhibitors; GLP‐1RAs, glucagon‐like peptide‐1 receptor agonists; OR, odds ratio; SGLT2 inhibitors, sodium‐glucose co‐transporter‐2 inhibitors.
Figure S5. Funnel plot of the effects of newer glucose‐lowering drugs on the risk of Parkinson's disease in participants with or without type 2 diabetes. OR, odds ratio.
Acknowledgments
This work was supported by American Foundation for Pharmaceutical Education (AFPE) Predoctoral Fellowship in Pharmaceutical Sciences, National Institute of Diabetes and Digestive and Kidney (NIDDK) of the National Institutes of Health (R01DK133465), National Institute on Aging (NIA) of the National Institutes of Health (R01AG080991, R01AG076234, and R56AG069880).
References
- 1. Marras C, Beck JC, Bower JH, et al. Prevalence of Parkinson's disease across North America. NPJ Parkinsons Dis 2018;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Willis AW, Roberts E, Beck JC, et al. Incidence of Parkinson disease in North America. NPJ Parkinsons Dis 2022;8:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang W, Hamilton JL, Kopil C, et al. Current and projected future economic burden of Parkinson's disease in the U.S. NPJ Parkinsons Dis 2020;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA 2020;323:548–560. [DOI] [PubMed] [Google Scholar]
- 5. Labandeira CM, Fraga‐Bau A, Arias Ron D, Alvarez‐Rodriguez E, Vicente‐Alba P, Lago‐Garma J, Rodriguez‐Perez AI. Parkinson's disease and diabetes mellitus: common mechanisms and treatment repurposing. Neural Regen Res 2022;17:1652–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Q, Cao T, Li N, et al. Repurposing of anti‐diabetic agents as a new opportunity to alleviate cognitive impairment in neurodegenerative and neuropsychiatric disorders. Front Pharmacol 2021;12:667874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. US Food and Drug Administration . Type 2 Diabetes Mellitus: Evaluating the Safety of New Drugs for Improving Glycemic Control Guidance for Industry; 2020.
- 8. Tang H, Kimmel SE, Smith SM, et al. Comparable cardiorenal benefits of SGLT2 inhibitors and GLP‐1RAs in Asian and white populations: an updated meta‐analysis of results from randomized outcome trials. Diabetes Care 2022;45:1007–1012. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Stat Med 2004;23:1351–1375. [DOI] [PubMed] [Google Scholar]
- 11. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326. [DOI] [PubMed] [Google Scholar]
- 12. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–1335. [DOI] [PubMed] [Google Scholar]
- 13. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–242. [DOI] [PubMed] [Google Scholar]
- 14. Gantz I, Chen M, Suryawanshi S, et al. A randomized, placebo‐controlled study of the cardiovascular safety of the once‐weekly DPP‐4 inhibitor omarigliptin in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2017;16:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA 2019;321:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–2257. [DOI] [PubMed] [Google Scholar]
- 17. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 18. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet 2018;392:1519–1529. [DOI] [PubMed] [Google Scholar]
- 21. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet 2019;394:121–130. [DOI] [PubMed] [Google Scholar]
- 22. Husain M, Birkenfeld AL, Donsmark M, et al. Oral Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019;381:841–851. [DOI] [PubMed] [Google Scholar]
- 23. Gerstein HC, Sattar N, Rosenstock J, et al. Cardiovascular and renal outcomes with Efpeglenatide in type 2 diabetes. N Engl J Med 2021;385:896–907. [DOI] [PubMed] [Google Scholar]
- 24. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 25. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 26. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 27. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 28. Cannon CP, Pratley R, Dagogo‐Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020;383:1425–1435. [DOI] [PubMed] [Google Scholar]
- 29. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 30. Heerspink HJL, Stefansson BV, Correa‐Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446. [DOI] [PubMed] [Google Scholar]
- 31. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 32. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 33. Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med 2021;384:129–139. [DOI] [PubMed] [Google Scholar]
- 34. Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–128. [DOI] [PubMed] [Google Scholar]
- 35. Chohan H, Senkevich K, Patel RK, et al. Type 2 diabetes as a determinant of Parkinson's disease risk and progression. Mov Disord 2021;36:1420–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mui JV, Zhou J, Lee S, et al. Sodium‐glucose cotransporter 2 (SGLT2) inhibitors vs. dipeptidyl peptidase‐4 (DPP4) inhibitors for new‐onset dementia: a propensity score‐matched population‐based study with competing risk analysis. Front Cardiovasc Med 2021;8:747620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin KJ, Wang TJ, Chen SD, et al. Two birds one Stone: the neuroprotective effect of antidiabetic agents on Parkinson disease‐focus on sodium‐glucose cotransporter 2 (SGLT2) inhibitors. Antioxidants (Basel) 2021;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double‐blind, placebo‐controlled trial. Lancet 2017;390:1664–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qin X, Zhang X, Li P, Wang M, Yan L, Bao Z, Liu Q. Association between diabetes medications and the risk of Parkinson's disease: a systematic review and meta‐analysis. Front Neurol 2021;12:678649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30:1591–1601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Classes of glucose‐lowering drugs (excluding insulin) available for treating type 2 diabetes.
Table S2. Risk of bias of each domain for each study.
Figure S1. Flowchart of the study selection. CENTRAL, Cochrane Central Register of Controlled Trials; DPP‐4 inhibitors, dipeptidyl peptidase‐4 inhibitors; GLP‐1RAs, glucagon‐like peptide‐1 receptor agonists; SGLT2 inhibitors, sodium‐glucose co‐transporter‐2 inhibitors.
Figure S2. Meta‐analysis of the effects of newer glucose‐lowering drugs (GLDs) on the risk of Parkinson's disease in participants with or without type 2 diabetes (T2D), subgroup by type of participants included. CI, confidence interval; OR, odds ratio.
Figure S3. Meta‐analysis of the effects of newer glucose‐lowering drugs (GLDs) on the risk of Parkinson's disease in participants with or without type 2 diabetes using Mantel–Haenszel method. CI, confidence interval; DPP‐4 inhibitors, dipeptidyl peptidase‐4 inhibitors; GLP‐1RAs, glucagon‐like peptide‐1 receptor agonists; OR, odds ratio; SGLT2 inhibitors, sodium‐glucose co‐transporter‐2 inhibitors.
Figure S4. Meta‐analysis of the effects of newer glucose‐lowering drugs (GLDs) on the risk of Parkinson's disease in participants with or without type 2 diabetes using a 0.5 continuity correction for zero events in both arms. CI, confidence interval; DPP‐4 inhibitors, dipeptidyl peptidase‐4 inhibitors; GLP‐1RAs, glucagon‐like peptide‐1 receptor agonists; OR, odds ratio; SGLT2 inhibitors, sodium‐glucose co‐transporter‐2 inhibitors.
Figure S5. Funnel plot of the effects of newer glucose‐lowering drugs on the risk of Parkinson's disease in participants with or without type 2 diabetes. OR, odds ratio.


