Abstract
Background
Mycoplasma genitalium (MG) is on the CDC Watch List of Antimicrobial Resistance Threats, yet there is no systematic surveillance to monitor change.
Methods
We initiated surveillance in sexual health clinics in 6 cities, selecting a quota sample of urogenital specimens tested for gonorrhea and/or chlamydia. We abstracted patient data from medical records and detected MG and macrolide-resistance mutations (MRMs) by nucleic acid amplification testing. We used Poisson regression to estimate adjusted prevalence ratios (aPRs) and 95% CIs, adjusting for sampling criteria (site, birth sex, symptom status).
Results
From October–December 2020 we tested 1743 urogenital specimens: 57.0% from males, 46.1% from non-Hispanic Black persons, and 43.8% from symptomatic patients. MG prevalence was 16.6% (95% CI: 14.9–18.5%; site-specific range: 9.9–23.5%) and higher in St Louis (aPR: 1.9; 1.27–2.85), Greensboro (aPR: 1.8; 1.18–2.79), and Denver (aPR: 1.7; 1.12–2.44) than Seattle. Prevalence was highest in persons <18 years (30.4%) and declined 3% per each additional year of age (aPR: .97; .955–.982). MG was detected in 26.8%, 21.1%, 11.8%, and 15.4% of urethritis, vaginitis, cervicitis, and pelvic inflammatory disease (PID), respectively. It was present in 9% of asymptomatic males and 15.4% of asymptomatic females, and associated with male urethritis (aPR: 1.7; 1.22–2.50) and chlamydia (aPR: 1.7; 1.13–2.53). MRM prevalence was 59.1% (95% CI: 53.1–64.8%; site-specific range: 51.3–70.6%). MRMs were associated with vaginitis (aPR: 1.8; 1.14–2.85), cervicitis (aPR: 3.5; 1.69–7.30), and PID cervicitis (aPR: 1.8; 1.09–3.08).
Conclusions
MG infection is common in persons at high risk of sexually transmitted infections; testing symptomatic patients would facilitate appropriate therapy. Macrolide resistance is high and azithromycin should not be used without resistance testing.
Keywords: Mycoplasma genitalium, surveillance, antimicrobial resistance, epidemiology
In initial surveillance of sexual health clinic patients from 6 cities, Mycoplasma genitalium prevalence was 16%; macrolide-resistance prevalence was 59%. Testing patients with urogenital symptoms for M. genitalium will facilitate appropriate therapy. Without resistance testing, azithromycin should not be used.
Nations around the world have implemented public health and clinical programs to mitigate the morbidity associated with sexually transmitted infections (STIs). In the United States, chlamydia, gonorrhea, and syphilis are reportable infections; screening guidelines exist for specific populations; and state and national surveillance systems monitor trends in infections including antimicrobial resistance (AMR) in Neisseria gonorrhoeae (GC). Like Chlamydia trachomatis (CT) and GC, Mycoplasma genitalium (MG) causes male urethritis [1] and is associated with cervicitis, pelvic inflammatory disease (PID), infertility, preterm delivery, and human immunodeficiency virus (HIV) [2–4]. Unlike CT and GC, MG is not reportable and there is no systematic surveillance, hampering our ability to determine whether population rates of infection are changing. This is complicated by rapidly expanding AMR [5] and the emergence of untreatable infections. Mycoplasma genitalium is on the Centers for Disease Control and Prevention (CDC) Watch List for Antimicrobial Resistance Threats [6], highlighting organisms that could become a greater threat.
Despite documented associations with STI syndromes and concerns about AMR, there are surprisingly limited data on MG prevalence in US populations at risk of the infection. Although MG was measured in the National Health and Nutrition Examination Survey (NHANES) for the first time in 2017–2018 [7], NHANES surveys a population at substantially lower STI risk than patients attending sexual health clinics (SHCs). The contribution of MG to STI syndromes remains ill defined; current CDC guidelines limit diagnostic testing for MG to recurrent urethritis or cervicitis (with consideration for PID) [8], and MG infections that clear after empiric therapy are not captured, potentially underestimating the contribution of MG. Finally, most data on AMR in MG are derived from research studies whose participants usually do not represent all clinic attendees.
To address these gaps, we initiated systematic surveillance in SHCs in 2020 under the Mycoplasma genitalium Infection in the US project (MyGeniUS). We estimated MG prevalence, correlates, and its contribution to urethritis, vaginitis, cervicitis, and PID in patients attending urban SHCs in 4 geographic regions. We also estimated the prevalence and correlates of macrolide-resistant MG.
METHODS
Eight SHCs in the Western (Denver, CO; Seattle, WA), Southern (Greensboro, NC), Central (Indianapolis, IN; St Louis, MO), and Northeastern (3 clinics in New York City, NY) regions participated in 2020. Although two 3-month data-collection cycles were planned, it was not possible to collect specimens during the first half of 2020 due to the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A single data-collection cycle occurred from October through December 2020.
Urine and swab specimens (urethral, vaginal, cervical) from persons tested for CT and GC using nucleic acid amplification testing (NAAT; Aptima Combo 2; Hologic, Inc, San Diego, CA, USA) were selected for MG testing. Each clinic identified specimens based on symptoms and birth sex. We categorized sex as male or female based on what was recorded, or inferred sex from the anatomic site of specimen collection when birth sex and gender identity were not differentiated. Each site aimed to identify 100 specimens per cycle from each of 4 groups (symptomatic males, asymptomatic males, symptomatic females, asymptomatic females). The target sample size provides precision of ±2.6% for MG prevalence estimates and 80% or greater power to detect an absolute change of 7% or greater in the prevalence of macrolide resistance each year. Specimens were de-identified and frozen at −80°C after collection in all but 1 site where they were held at 4°C prior to shipping (New York). Specimens were shipped on dry ice (cold packs in New York) to the Global Health STI Laboratory at the University of Washington (UW) and stored at −80°C prior to testing. Mycoplasma genitalium was detected by transcription-mediated amplification using the Food and Drug Administration (FDA)–cleared Aptima Mycoplasma genitalium assay. Macrolide-resistance mutations (MRMs) in MG 23S rRNA (A2058C, A2058G, A2058T, A2059C, A2059G) were detected using a research-use-only reverse transcription–polymerase chain reaction (RT-PCR) assay consisting of general-purpose RT-PCR reagents and analyte-specific reagent primers and probes on the Panther Fusion instrument (Hologic, Inc).
We abstracted data on age, birth sex, gender identity, race, ethnicity, sex/gender of sexual partners, symptoms, and diagnoses from electronic medical records (EMRs) where EMRs were available (Denver, Seattle, New York) or entered abstracted data into a REDCap [9] questionnaire where EMRs were not available (Indianapolis, St Louis, Greensboro). Only 3 sites could provide data on transgender, nonbinary, and other gender identities so we categorized gender identity as cisgender male, cisgender female, or another identity. We collected data on race (Black, White, Asian, American Indian/Alaska Native, Native Hawaiian/other Pacific Islander, multiracial, other, unknown) and ethnicity (Hispanic/Latinx, non-Hispanic [NH]/Latinx, unknown) and simplified this to 5 mutually exclusive groups (NH Black, NH White, NH other race, Hispanic, unknown).
The sex/gender of sexual partners was derived from the patient’s birth sex and sex/gender of sexual partners. We defined men who have sex with men (MSM) as males who reported any male sexual partners, men who have sex with women (MSW) as males who reported only female partners, women who have sex with women (WSW) as females who reported any female partner, and women who have sex with men (WSM) as females who reported only male partners. Results were similar when we included categories for MSM-only and WSW-only. Four sites were able to abstract current GC and CT results (Denver, New York, Seattle, St Louis); data on history of GC/CT infection were not available.
After linking clinic records data, we deleted identifying information and forwarded anonymized data to the UW coordinating center. To assess the representativeness of the data, we compared each site's surveillance population with that clinic's population during the collection period (Supplementary Table 1). We calculated MG prevalence and binomial exact 95% confidence intervals (CIs), overall and by sociodemographic and clinical characteristics, using Pearson's chi-square and Cochrane-Armitage tests of trend to determine statistical significance. We used Poisson regression with robust standard errors to estimate prevalence ratios (PRs), adjusting for site, sex, and symptom status to account for quota sampling (Supplementary Table 2), with 3 exceptions. As race/ethnicity was highly correlated with site (Supplementary Table 3), PRs for sites were adjusted for sex, symptom status, and race/ethnicity and PRs for race/ethnicity were only adjusted for sex and symptom status. Prevalence ratios for symptom status by sex were only adjusted for site. Further adjustment for age or sex/gender of sexual partners did not appreciably change PRs and neither was included. Analyses used R studio (version 4.0.5; R Foundation for Statistical Computing, Vienna, Austria) and Stata/BE (version 17.0; StataCorp, College Station, TX, USA).
This was considered a public health surveillance activity in most sites and informed consent was not required. Only Indianapolis required review of surveillance procedures by the local institutional review board and obtained written consent from persons contributing specimens.
RESULTS
We collected 1745 specimens between 1 October and 31 December 2020. Birth sex was not recorded for 1 specimen and MG results were not available for 1 specimen, leaving 400 specimens from Denver, 236 from Greensboro, 9 from Indianapolis, 319 from New York, 384 from Seattle, and 395 from St Louis. Males contributed 993 specimens (514 symptomatic, 479 asymptomatic). Females contributed 750 specimens (465 symptomatic, 285 symptomatic). Most were from NH Black persons (46.1%), with 24.0% from NH White and 17.2% from Hispanic persons. Included persons were similar to the underlying clinic population with 3 exceptions: sex and symptom status (due to quota sampling), age in St Louis and Indianapolis, and race/ethnicity in Indianapolis and Greensboro (Supplementary Table 1).
Prevalence and Geographic Regions
Mycoplasma genitalium was detected in 290 of 1743 specimens (prevalence: 16.6%; 95% CI: 14.9–18.5%) (Table 1). Site-specific prevalence ranged from 9.9% (Seattle) to 23.5% (St Louis). Relative to Seattle, MG prevalence was significantly higher in St Louis (adjusted PR [aPR]: 1.9; 95% CI: 1.27–2.85), Greensboro (aPR: 1.8; 1.18–2.79), and Denver (aPR: 1.7; 1.12–2.44).
Table 1.
Prevalence and Association of Mycoplasma genitalium With Sociodemographic and Clinical Characteristics Among 1743 Patients Attending Urban Sexual Health Clinics, September–December 2020
| Characteristic | Prevalence | aPRb | 95% CI | P | |
|---|---|---|---|---|---|
| (MG+/n) | % (95% CI)a | ||||
| Overall | 290/1743 | 16.6 (14.9–18.5) | … | … | |
| Sitec | |||||
| Denver, CO | 65/400 | 16.3 (12.8–20.2) | 1.7 | 1.12–2.44 | .01 |
| Greensboro, NC | 52/236 | 22.0 (16.9–27.9) | 1.8 | 1.18–2.79 | .007 |
| Indianapolis, IN | 2/9 | 22.2 (2.8–60.0) | 1.7 | .47–6.06 | .42 |
| New York, NY | 40/319 | 12.5 (9.1–16.7) | 1.3 | .83–2.02 | .26 |
| Seattle, WA | 38/384 | 9.9 (7.1–13.3) | 1.0 | ref | |
| St Louis, MO | 93/395 | 23.5 (19.4–28.0) | 1.9 | 1.27–2.85 | .002 |
| Sociodemographic characteristics | |||||
| Sexd | |||||
| Male | 158/993 | 15.9 (13.7–18.3) | 1.0 | ref | |
| Female | 132/750 | 17.6 (14.9–20.5) | 1.8 | 1.22–2.59 | .003 |
| Age | |||||
| Age continuous (per year) | 1743 | … | .97 | .955–.982 | <.001 |
| Age categories | |||||
| <18 y | 7/23 | 30.4 (13.2–52.9) | 1.0 | ref | |
| 18–24 y | 104/437 | 23.8 (19.9–28.1) | .8 | .38–1.53 | .44 |
| 25–29 y | 77/460 | 16.7 (13.4–20.5) | .5 | .27–1.11 | .10 |
| 30–39 y | 79/505 | 15.6 (12.6–19.1) | .5 | .27–1.09 | .08 |
| ≥40 y | 23/317 | 7.3 (4.7–10.7) | .2 | .11–.53 | <.001 |
| Race/ethnicitye | |||||
| NH Black | 168/804 | 20.9 (18.1–23.9) | 1.0 | ref | |
| NH White | 48/419 | 11.5 (8.6–14.9) | .6 | .46–.85 | .003 |
| NH Other | 17/115 | 14.8 (8.9–22.6) | .8 | .50–1.27 | .34 |
| Hispanic or Latinx | 36/299 | 12.0 (8.6–16.3) | .7 | .47–.93 | .02 |
| Unknown/missing | 21/106 | 19.8 (12.7–28.7) | 1.1 | .76–1.70 | .52 |
| Gender identityf | |||||
| Cisgender male | 157/979 | 16.0 (13.8–18.5) | 1.0 | ref | |
| Cisgender female | 131/737 | 17.8 (15.1–20.7) | .7 | .28–1.70 | .42 |
| Another | 2/27 | 7.4 (0.9–24.3) | … | … | |
| Sex/gender of sexual partnersg | |||||
| Males | |||||
| MSM any | 41/365 | 11.2 (8.2–14.9) | 1.0 | ref | |
| MSW only | 110/582 | 18.9 (15.8–22.3) | 1.2 | .80–1.66 | .45 |
| Females | |||||
| WSW any | 10/78 | 12.8 (6.3–22.3) | 1.0 | ref | |
| WSM only | 118/638 | 18.5 (15.6–21.7) | 1.2 | .67–2.30 | .49 |
| No sex in past year | 1/11 | 9.1 (0.2–41.3) | … | … | |
| Unknown/another | 10/69 | 14.5 (7.2–25.0) | … | … | |
| Clinical characteristics | |||||
| Symptom statush | |||||
| Males | |||||
| Asymptomatic | 43/479 | 9.0 (6.6–11.9) | 1.0 | ref | |
| Symptomatic | 115/514 | 22.4 (18.8–26.2) | 2.2 | 1.55–3.19 | <.001 |
| Females | |||||
| Asymptomatic | 44/285 | 15.4 (11.4–20.2) | 1.0 | ref | |
| Symptomatic | 88/465 | 18.9 (15.5–22.8) | 1.2 | .83–1.62 | .40 |
| Diagnosis (excluding people with diagnoses other than those listed)i | |||||
| Males | |||||
| No diagnosis | 64/631 | 10.1 (7.9–12.8) | 1.0 | ref | |
| Male urethritis | 63/235 | 26.8 (21.3–33.0) | 1.7 | 1.22–2.50 | .002 |
| Females | |||||
| No diagnosis | 65/427 | 15.2 (11.9–19.0) | 1.0 | ref | |
| Vaginitis | 55/261 | 21.1 (16.3–26.5) | 1.1 | .72–1.63 | .69 |
| Cervicitis | 2/17 | 11.8 (1.5–36.4)b | .7 | .18–2.44 | .53 |
| PID | 2/13 | 15.4 (1.9–45.4)b | .9 | .24–3.24 | .86 |
| Chlamydia (n = 1152)j | |||||
| Chlamydia negative | 119/1003 | 11.9 (9.9–14.0) | 1.0 | ref | |
| Chlamydia positive | 42/149 | 28.2 (21.1–36.1) | 1.7 | 1.13–2.53 | .01 |
| Gonorrhea (n = 1149) j | |||||
| Gonorrhea negative | 122/1019 | 12.0 (10.0–14.1) | 1.0 | ref | |
| Gonorrhea positive | 31/130 | 23.8 (16.8–32.1) | 1.6 | .99–2.43 | .053 |
Bolded values are statistically significant at P < .05.
Abbreviations: aPR, adjusted prevalence ratio; CI, confidence interval; MG, Mycoplasma genitalium; MSM, men who have sex with men; MSW, men who have sex with women; NH, non-Hispanic; PID, pelvic inflammatory disease; ref, reference; WSM, women who have sex with men; WSW, women who have sex with women.
aBinomial exact 95% CIs.
bAll prevalence ratio (PRs) adjusted for site, sex, and symptom status unless otherwise specified.
cPRs for site adjusted for race-ethnicity, sex, and symptom status.
dSpecimens were identified for surveillance based on recorded sex and the anatomic site from which the specimen was collected; sex does not account for gender identity.
ePRs for race-ethnicity adjusted for sex and symptom status but not site due to collinearity.
fDue to concerns about potential deductive disclosure, transgender, gender-diverse, and gender nonconforming persons in Denver were not included. The category of “Another” gender identity included those who indicated gender-diverse (n = 12), transgender male (n = 2), transgender female (n = 4), another (n = 1), and unknown (n = 8).
gMSM include male sex (index) who report sex with any male sex/gender partner either alone or in combination with other gender partners (eg, cis-female, trans male/female, nonbinary, or other gender identity); n = 43 MSM who identified as men who have sex with women and men (MSWM). MSW include male sex (index) who only report sex with female partners. WSM include female sex (index) who only had sex with male partners. WSW include female sex (index) who report sex with any female sex/gender either alone or in combination with other gender partners (eg, cis-female, trans male/female, nonbinary, other gender identity); n = 69 identified as women who have sex with women and men (WSWM). The unknown/another category includes 62 persons with missing information on sex/gender of sex partner.
hPRs for symptom status by sex adjusted for site only.
iDiagnoses are not mutually exclusive. There were 127 males with diagnoses other than urethritis who were excluded from the no-other-diagnoses denominator. There were 43 females with diagnoses other than vaginitis, cervicitis, or PID who were excluded from the no-other-diagnoses denominator, as well as 7 females with vaginitis and cervicitis and 4 females with vaginitis and PID. Although clinic records indicated “other diagnosis,” the specific diagnoses were rarely recorded. In models for diagnosis, MG was modeled as the exposure and the diagnosis was modeled as the outcome. In all other models, MG was modeled as the outcome.
jGonorrhea and chlamydia status documented in only 4 sites (Denver, New York City, Seattle, St Louis).
Sociodemographic Characteristics
Mycoplasma genitalium prevalence was higher in females than in males (17.6% vs 15.9%; aPR: 1.8; 1.22–2.59) (Table 1). Prevalence was somewhat higher in people with opposite-sex than with same-sex sexual partners, but there were no significant differences in adjusted analyses comparing MSW to MSM (aPR: 1.2; .80–1.66) or WSM to WSW (aPR: 1.2; .67–2.30). Mycoplasma genitalium prevalence was highest in NH Black and persons with unknown race/ethnicity (20.9% and 19.8%, respectively) and significantly lower in NH White (aPR: .6; .46–.85) and Hispanic (aPR: .7; .47–.93) persons. Relationships were similar when we evaluated expanded race/ethnicity groups (Supplementary Table 4).
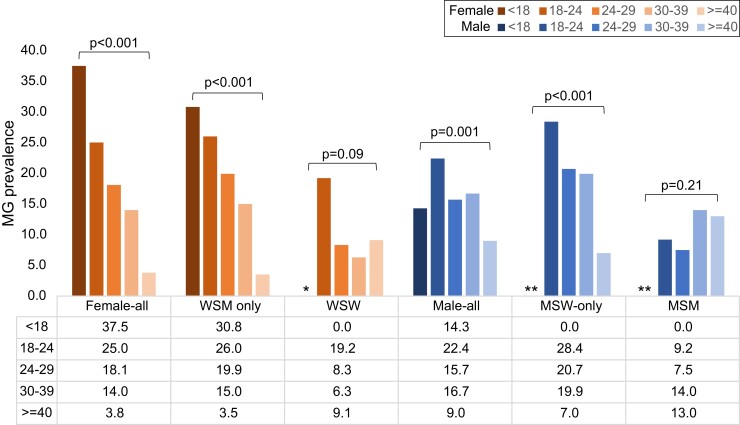
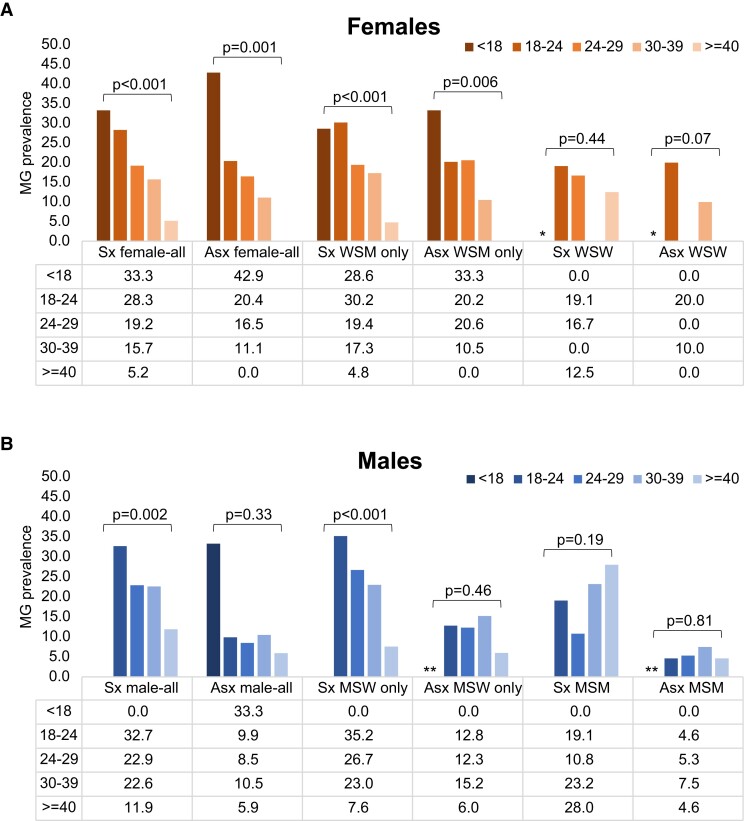
Persons with MG were younger than those without MG (median age: 27 [interquartile range (IQR): 22–32] vs 29 [25–37] y; P < .001). The highest prevalence was in those aged younger than 18 years (30.4%) and prevalence declined by 3% per each additional year of age (aPR: .97; .955–.982) (Table 1). This relationship varied by sex and sex/gender of sexual partner (Figure 1). Prevalence was highest among the youngest females (37.5% in those <18 y), with a linear decline as age increased (P-trend < .001), primarily among WSM and symptomatic MSW (P-trend < .001 for both). This did not occur among people with same-sex sexual partners (P > .05 for all; Figures 1 and 2).
Figure 1.
Age-specific prevalence of Mycoplasma genitalium among persons attending urban sexual health clinics during September–December 2020, stratified by sex and sex/gender of sex partner. Abbreviations: Asx, asymptomatic; MG, Mycoplasma genitalium; MSM, men who have sex with men; MSW, men who have sex with women; Sx, symptomatic; WSM, women who have sex with men; WSW, women who have sex with women. *WSW: Any <18 years suppressed as there was only 1 person in that age category; **n = 4 males who reported another/unknown gender of sex partner and were excluded from calculations among MSW and MSM.
Figure 2.
(A, B) Age-specific prevalence of Mycoplasma genitalium among persons attending urban sexual health clinics during September–December 2020 stratified by symptom status, sex, and sex of sex partner. Abbreviations: Asx, asymptomatic; MG, Mycoplasma genitalium; MSM, men who have sex with men; MSW, men who have sex with women; Sx, symptomatic; WSM, women who have sex with men; WSW, women who have sex with women. *WSW: Any <18 years suppressed as there was only 1 person in that age category; **n = 4 males who reported another/unknown gender of sex partner and were excluded from calculations among MSW and MSM.
Contribution to Urogenital Syndromes
Mycoplasma genitalium prevalence was 2-fold higher in males with than in those without urogenital symptoms (22.4% vs 9.0%; aPR: 2.2; 95% CI: 1.55–3.19) (Table 1). Mycoplasma genitalium was detected in over one-quarter (26.8%) of males with urethritis, and significantly associated with urethritis (aPR: 1.7; 1.22–2.50). No other clinical characteristics were associated with male MG infection (Table 1, Supplementary Table 5).
Mycoplasma genitalium prevalence was similar in females with and without symptoms (18.9% vs 15.4%; aPR: 1.2; .83–1.62) (Table 1). Mycoplasma genitalium was detected in 21.1% with vaginitis, 11.8% with cervicitis, 15.4% with PID, and 15.2% with no diagnosed syndrome. No clinical characteristics were associated with female MG infection in adjusted analyses (Table 1, Supplementary Table 5).
Coinfection With Chlamydia trachomatis/Neisseria gonorrhoeae
A subset of people had CT (n = 1152) and GC (n = 1149) data. The prevalence of CT was 12.9%; GC prevalence was 11.3%. Mycoplasma genitalium was detected in 28.2% of CT and 23.9% of GC infections. After adjusting for sampling criteria (site, birth sex, symptoms), MG was associated with CT (aPR: 1.7; 1.13–2.53) but not GC (Table 1). Mycoplasma genitalium was detected in 26.5% of CT/GC-negative urethritis, 10.8% of CT/GC-negative vaginitis, and 11.1% of CT/GC-negative PID, but not in CT/GC-negative cervicitis. Because many diagnoses lacked data on CT/GC (38% urethritis, 65% vaginitis, 47% cervicitis) or numbers were small (PID), we do not report associations adjusted for CT/GC.
Macrolide-Resistance Mutations
Of 290 MG-positive specimens, 286 (98.6%) had valid MRM results (2 were invalid, 2 had insufficient volume). Overall MRM prevalence was 59.1% (95% CI: 53.1–64.8%), with site-specific prevalences of 51.3–70.6% (MRM prevalence in Indianapolis was based on <5 infections and suppressed) (Table 2). Relative to Seattle, MRM prevalence was higher in Greensboro (aPR: 1.6; 1.06–2.31), but not significantly different in other sites.
Table 2.
Prevalence and Association of Macrolide Resistance With Sociodemographic and Clinical Characteristics Among 286 Mycoplasma genitalium–Positive Patients Attending Urban Sexual Health Clinics, September–December 2020
| Characteristic | Macrolide Resistance | ||||
|---|---|---|---|---|---|
| MRM+/Total Tested | Prevalence, % (95% CI)a | aPRb | 95% CI | P | |
| Overall | 169/286 | 59.1 (53.1–64.8) | … | … | |
| Sitec | |||||
| Denver, CO | 34/65 | 52.3 (39.5–64.9) | 1.0 | .69–1.52 | .91 |
| Greensboro, NC | 36/51 | 70.6 (56.2–82.5) | 1.6 | 1.06–2.31 | .02 |
| Indianapolis, IN | … | … | … | … | |
| New York NY | 20/39 | 51.3 (34.8–67.6) | .9 | .55–1.36 | .53 |
| Seattle, WA | 20/38 | 52.6 (35.8–69.0) | 1.0 | ref | |
| St Louis, MO | 57/91 | 62.6 (51.9–72.6) | 1.3 | .91–1.99 | .14 |
| Sociodemographic characteristics | |||||
| Sexd | |||||
| Male | 94/155 | 60.6 (52.5–68.4) | 1.0 | ref | |
| Female | 75/131 | 57.3 (48.3–65.9) | 1.0 | .74–1.40 | .93 |
| Age | |||||
| Age continuous (per year) | 169/286 | … | 1.0 | .976–1.001 | .08 |
| Age categories | |||||
| <18 y | 5/7 | 71.4 (29.0–96.3) | 1.0 | ref | |
| 18–24 y | 66/103 | 64.1 (54.0–73.3) | .8 | .54–1.33 | .47 |
| 25–29 y | 46/76 | 60.5 (48.6–71.6) | .8 | .51–1.29 | .38 |
| 30–39 y | 39/77 | 50.6 (39.0–62.2) | .7 | .42–1.12 | .13 |
| ≥40y | 13/23 | 56.5 (34.5–76.8) | .7 | .41–1.34 | .33 |
| Race/ethnicitye | |||||
| NH Black | 97/165 | 58.8 (50.9–66.4) | 1.0 | ref | |
| NH White | 26/48 | 54.2 (39.2–68.6) | .9 | .70–1.25 | .64 |
| NH Other | 11/16 | 68.8 (41.3–89.0) | 1.2 | .83–1.78 | .32 |
| Hispanic/Latinx | 20/36 | 55.6 (38.1–72.1) | .9 | .69–1.29 | .70 |
| Unknown/missing | 15/21 | 71.4 (47.8–88.7) | 1.2 | .89–1.66 | .23 |
| Gender identityf | |||||
| Cisgender male | 94/154 | 61.0 (52.9–68.8) | 1.0 | ref | |
| Cisgender female | 75/130 | 57.7 (48.7–66.3) | .8 | .05–14.03 | .90 |
| Another | 0/2 | 0 (0) | … | … | |
| Sex/gender of sex partnersg | |||||
| Males | |||||
| MSM any | 21/41 | 51.2 (35.1–67.1) | 1.0 | ref | |
| MSW only | 68/107 | 63.6 (53.7–72.6) | 1.1 | .78–1.58 | .56 |
| Females | |||||
| WSW any | 6/10 | 60.0 (26.2–87.8) | 1.0 | ref | |
| WSM only | 66/117 | 56.4 (46.9–65.6) | 1.0 | .56–1.64 | .88 |
| Unknown | 7/10 | 70.0 (34.8–93.3) | … | … | |
| No sex in past year | 1/1 | 100.0 (25.0–100.0) | … | … | |
| Clinical characteristics | |||||
| Symptom statush | |||||
| Males | |||||
| Asymptomatic | 23/42 | 54.8 (38.7–70.2) | 1.0 | ref | |
| Symptomatic | 71/113 | 62.8 (53.2–71.7) | 1.1 | .81–1.43 | .63 |
| Females | |||||
| Asymptomatic | 22/44 | 50.0 (34.6–65.4) | 1.0 | ref | |
| Symptomatic | 53/87 | 60.9 (49.9–71.2) | 1.2 | .87–1.73 | .24 |
| Diagnosisi | |||||
| Males | |||||
| No diagnosis | 38/64 | 59.4 (46.4–71.5) | 1.0 | ref | |
| Male urethritis | 39/62 | 62.9 (49.7–74.8) | .9 | .63–1.16 | .33 |
| Females | |||||
| No diagnosis | 32/64 | 50.0 (37.2–62.8) | 1.0 | ref | |
| Vaginitis | 36/55 | 65.5 (51.4–77.8) | 1.8 | 1.14–2.85 | .01 |
| Cervicitis | 2/2 | 100.0 (15.8–100.0) | 3.5 | 1.69–7.30 | .001 |
| PID | 2/2 | 100.0 (15.8–100.0) | 1.8 | 1.09–3.08 | .02 |
| Chlamydiaj | |||||
| Chlamydia negative | 62/118 | 52.5 (43.1–61.8) | 1.0 | ref | |
| Chlamydia positive | 25/41 | 61.0 (44.5–75.8) | .9 | .56–1.41 | .62 |
| Gonorrheaj | |||||
| Gonorrhea negative | 66/121 | 54.5 (45.2–63.6) | 1.0 | ref | |
| Gonorrhea positive | 13/29 | 44.8 (26.4–64.3) | .6 | .34–1.23 | .19 |
Bolded values are statistically significant at P < .05.
Abbreviations: aPR, adjusted prevalence ratio; CI, confidence interval; MRM, macrolide-resistance mutation; MSM, men who have sex with men; MSW, men who have sex with women; NH, non-Hispanic; PID, pelvic inflammatory disease; ref, reference; WSM, women who have sex with men; WSW, women who have sex with women.
aBinomial exact 95% CIs.
bAll prevalence ratios (PRs) adjusted for site, sex, and symptom status unless otherwise specified.
cPRs for site were adjusted for race-ethnicity, sex, and symptom status. Prevalence of MRM in Indianapolis was based on fewer than 5 specimens; the estimate and PR is suppressed, but data are included in the global estimate of MRM prevalence across sites.
dSpecimens were identified for surveillance based on recorded sex and the anatomic site from which the specimen was collected; sex does not account for gender identity.
ePRs for race-ethnicity adjusted for sex and symptom status but not site due to collinearity.
fDue to concerns about potential deductive disclosure, transgender, gender-diverse, and gender nonconforming persons in Denver were not included.
gMSM include male sex (index) who report sex with any male sex/gender partner either alone or in combination with other gender partners (eg, cis-female, trans male/female, nonbinary, or other gender identity); n = 43 MSM who identified as men who have sex with women and men (MSWM). MSW include male sex (index) who only report sex with female partners. WSM include female sex (index) who only had sex with male partners. WSW include female sex (index) who report sex with any female sex/gender either alone or in combination with other gender partners (eg, cis-female, trans male/female, nonbinary, other gender identity); n = 69 identified as women who have sex with women and men (WSWM). The unknown/another category includes 62 persons with missing information on sex/gender of sex partner.
hPRs for symptom status by sex adjusted for site only.
iDiagnoses are not mutually exclusive; therefore, summed counts may exceed the total population.
jGonorrhea and chlamydia status documented in only 4 sites (Denver, New York City, Seattle, St Louis).
Macrolide-resistance mutations were associated with vaginitis (aPR: 1.8; 1.14–2.85), cervicitis (aPR: 3.5; 1.69–7.30), and PID (aPR: 1.8; 1.09–3.08), but not with other characteristics (Table 2, Supplementary Tables 5 and 6). There was no association between MRMs and CT/GC in persons with those data.
DISCUSSION
Mycoplasma genitalium prevalence among symptomatic and asymptomatic SHC attendees in 6 US cities was 16.6%; site-specific prevalence ranged from 9.9% in Seattle to 23.5% in St Louis. Only Seattle had implemented MG testing prior to the start of surveillance (October 2018) and Seattle's lower prevalence may reflect previous detection and treatment of long-duration prevalent infections. The overall prevalence was nearly 10 times higher than the prevalence in reproductive-age persons participating in NHANES 2017–2018 (1.7%: 95% CI 1.1–2.7%) [7], which is not surprising. Most NHANES participants had a low likelihood of STIs (nearly 50% had <5 lifetime partners), whereas SHC attendees report more sexual risk behaviors. The 16.6% prevalence we observed was remarkably similar to a multicenter study in SHCs from 2013–2014 [10], but somewhat higher than diverse US clinic types (10.3%) [11] or Midwestern primary care clinics (6.8% in males, 11.4% in females) [12, 13]. This difference is likely attributable to higher overall STI prevalence in SHCs.
As with most bacterial STIs, MG was more common among females than males. The higher prevalence in females may reflect a longer duration of genital infection (possibly due to the absence of symptoms in many) and/or more efficient transmission through penile-vaginal sex than other sexual behaviors. The higher prevalence in females may also be related to bacterial vaginosis (BV); BV in women is common and may enhance susceptibility to MG [14–16]. The lower prevalence in males may also reflect anatomic site of infection. We tested only urogenital specimens and MG prevalence was higher in rectal specimens from MSM with paired samples [17–20]. Finally, the lower MG prevalence in males may reflect higher levels of STI screening and treatment in MSM, notably those on HIV pre-exposure prophylaxis for whom STI screening is recommended every 3 months [21]. Although antibiotics used to treat CT/GC have relatively low efficacy against MG [22], some MG coinfections are likely eradicated when CT/GC is treated.
Although few people were younger than 18 years, our observation that MG was most common in younger people and prevalence declined with increasing age was consistent with population-level data from the United Kingdom [23]. This age trend suggests that partial immunity may develop, a hypothesis supported by the detection of local and systemic anti-MG antibodies in other studies [24, 25]. Notably, the decline in infection with increasing age was most evident in symptomatic people, suggesting that any partial immunity may protect against symptomatic infection. Although the age trend was clearest in females, it was also present in symptomatic MSW shifted by approximately 5 years, consistent with heterosexual age-mixing patterns [26].
Our findings confirm the association of MG with male urethritis [1]. The proportion of male urethritis cases with MG we observed in 2020 (26.8%) was similar to US SHC patients in 2017–2018 (28.7%) [27], suggesting little change over time. In contrast, we observed no significant relationship between MG and female STI syndromes, reflecting either a true absence of association or the small number of cervicitis and PID diagnoses. Cervical infections are not always associated with prominent symptoms, and speculum examinations—required to make a clinical diagnosis of cervicitis—are generally not performed in asymptomatic women in our clinics. Cervicitis was probably incompletely ascertained, hindering our ability to evaluate associations with MG. Larger, carefully designed prospective studies are needed to define the contribution of MG to female syndromes and determine the implications of asymptomatic infection. The association of MG with CT suggests that both pathogens circulate in similar sexual networks, consistent with observed associations between CT and GC.
The high prevalence of macrolide resistance (59.1%) is consistent with other recent reports. In systematic surveillance in 2019, Public Health England detected MRMs in 69% of symptomatic MG infections identified in public clinics [20], only slightly higher than the prevalence in our symptomatic and asymptomatic patients. Previous estimates of macrolide resistance range widely, including 0–11% in non–US settings with infrequent azithromycin use [28–30], 30–41% in US pregnant women [31, 32], and 60–90% in US clinic populations [18, 27, 33–35]. The global increase in macrolide resistance in MG [5] motivated the development of resistance-guided therapy approaches [36] and contributed to the replacement of azithromycin with doxycycline as first-line therapy for urethritis and cervicitis in the 2021 CDC STI Treatment Guidelines [8]. Ideally, MG treatment decisions would not be made without resistance testing. However, resistance testing is not widely available in the United States. Given this, the high prevalence of macrolide resistance that we observed supports CDC guidelines to use moxifloxacin instead of azithromycin to treat MG until resistance testing is possible.
The association of vaginitis, cervicitis, and PID diagnoses with MRMs may reflect previous azithromycin treatment, subsequent symptom resolution, and eventual recrudescence of a macrolide-resistant infection. More data on MRMs in women are needed. The lack of association between MRMs and current CT/GC coinfection emphasizes that prior rather than current azithromycin treatment selects for resistance. Unlike other reports, we observed no association between MRMs and MSM. In the United Kingdom, MRMs were over twice as common in MSM as MSW (adjusted odds ratio: 2.64; 1.09–6.38) [20]. In Australia, MRM prevalence in MSM (89.7%) was substantially higher than in MSW (50.0%) [37]. Our non-inclusion of rectal specimens and different underlying transmission networks may explain these differences.
Our analysis has several strengths. First, our large sample size yielded high precision of prevalence estimates. Second, including public SHC attendees from 4 US regions provided broad geographic representation. Specimens from symptomatic and asymptomatic males and females captured the full spectrum of patients in clinical care and adjusting for site, sex, and symptoms largely accounted for any differential distribution of these groups across sites. Third, we utilized a highly sensitive NAAT to detect MG and a novel assay to detect MRMs. Fourth, the use of primarily remnant specimens was efficient and minimized selection bias that occurs in research studies when persons decline to enroll.
There were also limitations. First, we leveraged routine data collection and there was variability between clinics in how some characteristics were defined. Second, there were few persons from Indianapolis; those prevalence estimates should be interpreted with caution. Third, we did not collect data systematically on CT/GC, Trichomonas vaginalis, BV, vulvovaginal candidiasis, or HIV. We are systematically collecting information on CT/GC and BV in subsequent years. Fourth, although we collected data from 6 cities, this does not represent all US geographic areas. Fifth, the MRM assay does not detect wild-type MG. Some specimens classified as MRM-negative may have been nontypeable, introducing some uncertainty to the MRM estimates. This was minimized, in part, by an internal control, validating successful PCR amplification in MG-negative specimens. Sixth, we do not have information on quinolone resistance–associated mutations; we are validating an assay to do this and will report on this in the future. Seventh, we launched data collection during the last quarter of 2020 as SHCs were re-opening after the initial wave of the SARS-CoV-2 pandemic. Patient characteristics here may differ from those of current or pre-pandemic attendees. Notably, few asymptomatic females attended SHCs during this time. Eighth, we only evaluated urogenital specimens; MG and AMR prevalence may differ at extragenital sites.
This initial effort demonstrated that MG surveillance using remnant specimens from sentinel clinics, previously implemented for human papillomavirus [38], is feasible and provides an important complement to MG testing in NHANES. High MG prevalence and widespread macrolide resistance across the United States underscore the need to detect MG in symptomatic patients to guide therapy. Reductions in persistent urethritis after implementing routine MG testing highlight the value of this approach [39]. The high prevalence in asymptomatic females emphasizes the need to determine how often reproductive sequelae occur. The removal of azithromycin as first-line therapy for STI syndromes may slow the expansion of macrolide-resistant MG and preserve azithromycin for some patients. However, ongoing surveillance will be critical to determine whether this occurs or whether we will need other strategies to curb the spread of resistance.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Lisa E Manhart, Department of Epidemiology and Center for AIDS and STD, University of Washington, Seattle, Washington, USA.
Gina Leipertz, Department of Epidemiology, University of Washington, Seattle, Washington, USA; Elson S. Floyd College of Medicine, Washington State University, Spokane, Washington, USA.
Olusegun O Soge, Department of Global Health, and Division of Infectious Diseases, University of Washington, Seattle, Washington, USA.
Stephen J Jordan, Division of Infectious Diseases, Department of Medicine, Indiana University, Indianapolis, Indiana, USA.
Candice McNeil, Department of Medicine, Section on Infectious Diseases, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Preeti Pathela, New York City Department of Health and Mental Hygiene, Queens, New York, USA.
Hilary Reno, Division of Infectious Diseases, Washington University, St Louis, Missouri, USA.
Karen Wendel, Public Health Institute at Denver Health, Denver, Colorado, USA.
Anika Parker, Department of Epidemiology and Center for AIDS and STD, University of Washington, Seattle, Washington, USA.
William M Geisler, Division of Infectious Diseases, Department of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Damon Getman, Hologic, Inc, San Diego, California, USA.
Matthew R Golden, Center for AIDS and STD, University of Washington, Seattle, Washington, USA.
for the MyGeniUS Study Team:
Anna Berzkalns, Alfred Iqbal, Rushlenne Pascual, Erika Wakatake, Paul Swenson, Lora Fortenberry, Lisa Coss, Kevin Kamis, Masayo Nishiyama, Lucy Alderton, Lawrence Weingarten, Laura Blair, Dana Strope, Andrea Lewis, and Kelly Jamison
Notes
Author Contributions. L. E. M., M. R. G., and D. G. conceptualized the project. G. L. developed data-collection instruments and managed all study activities. O. O. S. oversaw all laboratory testing. S. J. J., C. M., P. P., H. R., K. W., W. M. G., and M. R. G. oversaw data collection in their clinics. A. P. and G. L. performed data management. L. E. M., G. L., and A. P. conducted data analyses. L. E. M. wrote the first draft of the manuscript. All authors provided substantive input into the final manuscript.
Acknowledgments. The authors thank Sean Proll for assistance in creating the figures. They also thank the clinic patients who contributed specimens and the clinic staff in each site for accommodating this surveillance activity.
Financial support. This work was supported by Hologic, Inc, the manuacturer of the Aptima M. genitalium diagnostic test used in this study.
MyGeniUS Study Team. Members of the MyGeniUS Study Team are as follows: Anna Berzkalns, Alfred Iqbal, Rushlenne Pascual, Erika Wakatake, and Paul Swenson from the University of Washington; Lora Fortenberry and Lisa Coss from Indiana University; Kevin Kamis, Masayo Nishiyama, and Lucy Alderton from Denver Health; Lawrence Weingarten from St Louis County Sexual Health Clinic; Laura Blair from the Washington University Clinical Research Unit; Dana Strope from Missouri State Public Health Laboratory; Andrea Lewis from Wake Forest University School of Medicine and Guilford County Department of Public Health; and Kelly Jamison from the NYC Health Department.
Research electronic data capture. Study data that were not extracted from SHC electronic medical record systems were collected and managed using REDCap electronic data-capture tools hosted at the University of Washington/Institute of Translation Health Sciences. REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for data integration and interoperability with external sources.
References
- 1. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24:498–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 2015; 61:418–26. [DOI] [PubMed] [Google Scholar]
- 3. Frenzer C, Egli-Gany D, Vallely LM, Vallely AJ, Low N. Adverse pregnancy and perinatal outcomes associated with Mycoplasma genitalium: systematic review and meta-analysis. Sex Transm Infect 2022; 98:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Napierala Mavedzenge S, Weiss HA. Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS 2009; 23:611–20. [DOI] [PubMed] [Google Scholar]
- 5. Machalek DA, Tao Y, Shilling H, et al. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect Dis 2020; 20:1302–14. [DOI] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention . Antibiotic resistance threats in the United States, 2019. Atlanta, GA: US Department of Health and Human Services; CDC, 2019. [Google Scholar]
- 7. Torrone EA, Kruszon-Moran D, Philips C, et al. Prevalence of urogenital Mycoplasma genitalium infection, United States, 2017 to 2018. Sex Transm Dis 2021; 48:e160–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 2021; 70:1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris PA, Taylor R, Thielke R, Payne J, Gonzales N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Getman D, Jiang A, O'Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 2016; 54:2278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaydos CA, Manhart LE, Taylor SN, et al. Molecular testing for Mycoplasma genitalium in the United States: results from the AMES prospective multicenter clinical study. J Clin Microbiol 2019; 57:e01125-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Napierala M, Munson E, Wenten D, et al. Detection of Mycoplasma genitalium from male primary urine specimens: an epidemiologic dichotomy with Trichomonas vaginalis. Diagn Microbiol Infect Dis 2015; 82:194–8. [DOI] [PubMed] [Google Scholar]
- 13. Munson E, Bykowski H, Munson KL, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-mediated amplification using primary female urogenital specimens. J Clin Microbiol 2016; 54:432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lokken EM, Balkus JE, Kiarie J, et al. Recent bacterial vaginosis is associated with acquisition of Mycoplasma genitalium. Am J Epidemiol 2017; 186:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balkus JE, Manhart LE, Lee J, et al. Periodic presumptive treatment for vaginal infections may reduce the incidence of sexually transmitted bacterial infections. J Infect Dis 2016; 213:1932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asare K, Osman F, Ngcapu S, et al. Burden of sexually transmitted infections from acute HIV infection among women in South Africa: evidence from a prospective cohort study. Ann Epidemiol 2022; 74:132–9. [DOI] [PubMed] [Google Scholar]
- 17. Latimer RL, Shilling HS, Vodstrcil LA, et al. Prevalence of Mycoplasma genitalium by anatomical site in men who have sex with men: a systematic review and meta-analysis. Sex Transm Infect 2020; 96:563–70. [DOI] [PubMed] [Google Scholar]
- 18. Allan-Blitz LT, Mokany E, Campeau S, Wee R, Shannon C, Klausner JD. Prevalence of Mycoplasma genitalium and azithromycin-resistant infections among remnant clinical specimens, Los Angeles. Sex Transm Dis 2018; 45:632–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Munson E, Morgan E, Sienkiewicz L, et al. Molecular screening in a longitudinal cohort of young men who have sex with men and young transgender women: associations with focus on the emerging sexually transmitted pathogen Mycoplasma genitalium. Sex Transm Infect 2021; 97:434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fifer H, Merrick R, Pitt R, et al. Frequency and correlates of Mycoplasma genitalium antimicrobial resistance mutations and their association with treatment outcomes: findings from a national sentinel surveillance pilot in England. Sex Transm Dis 2021; 48:951–4. [DOI] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention . Pre-exposure prophylaxis for the prevention of HIV infection in the United States—2021 update. A clinical practice guideline. Atlanta, GA: Centers for Disease Control and Prevention, 2021. [Google Scholar]
- 22. Jensen JS, Bradshaw C. Management of Mycoplasma genitalium infections—can we hit a moving target? BMC Infect Dis 2015; 15:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sonnenberg P, Ison CA, Clifton S, et al. Epidemiology of Mycoplasma genitalium in British men and women aged 16–44 years: evidence from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Int J Epidemiol 2015; 44:1982–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wood GE, Iverson-Cabral SL, Gillespie CW, Lowens MS, Manhart LE, Totten PA. Sequence variation and immunogenicity of the Mycoplasma genitalium MgpB and MgpC adherence proteins during persistent infection of men with non-gonococcal urethritis. PLoS One 2020; 15:e0240626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Svenstrup HF, Fedder J, Kristoffersen SE, Trolle B, Birkelund S, Christiansen G. Mycoplasma genitalium, Chlamydia trachomatis, and tubal factor infertility—a prospective study. Fertil Steril 2008; 90:513–20. [DOI] [PubMed] [Google Scholar]
- 26. White JL, Patel EU, Grabowski MK, et al. Trends and correlates of age-disparate sexual partnerships in the United States: the National Health and Nutrition Examination Surveys. Sex Transm Dis 2022; 49:e17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bachmann LH, Kirkcaldy RD, Geisler WM, et al. Prevalence of Mycoplasma genitalium infection, antimicrobial resistance mutations and symptom resolution following treatment of urethritis. Clin Infect Dis 2020; 71:e624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jonduo ME, Vallely AJ, Whiley DM, et al. Mycoplasma genitalium macrolide and fluoroquinolone resistance in pregnant women in Papua New Guinea. Sex Transm Infect 2022; 99:71–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mahlangu MP, Muller EE, Da Costa Dias B, Venter JME, Kularatne RS. Molecular characterization and detection of macrolide and fluoroquinolone resistance determinants in Mycoplasma genitalium in South Africa, 2015 to 2018. Sex Transm Dis 2022; 49:511–6. [DOI] [PubMed] [Google Scholar]
- 30. Melendez JH, Hardick J, Onzia A, et al. Retrospective analysis of Ugandan men with urethritis reveals Mycoplasma genitalium and associated macrolide resistance. Microbiol Spectr 2022; 10:e0230421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stafford IA, Hummel K, Dunn JJ, et al. Retrospective analysis of infection and antimicrobial resistance patterns of Mycoplasma genitalium among pregnant women in the southwestern USA. BMJ Open 2021; 11:e050475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu M, Souder JP, Subramaniam A, et al. Prevalence of Mycoplasma genitalium infection and macrolide resistance in pregnant women receiving prenatal care. Int J Gynaecol Obstet 2022; 160:341–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao L, Waites KB, Van Der Pol B, Aaron KJ, Hook EW 3rd, Geisler WM. Mycoplasma genitalium infections with macrolide and fluoroquinolone resistance-associated mutations in heterosexual African American couples in Alabama. Sex Transm Dis 2019; 46:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dionne-Odom J, Geisler WM, Aaron KJ, et al. High prevalence of multidrug-resistant Mycoplasma genitalium in human immunodeficiency virus-infected men who have sex with men in Alabama. Clin Infect Dis 2018; 66:796–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chambers LC, Jensen JS, Morgan JL, et al. Lack of association between the S83I ParC mutation in Mycoplasma genitalium and treatment outcomes among men who have sex with men with nongonococcal urethritis. Sex Transm Dis 2019; 46:805–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Durukan D, Read TRH, Murray G, et al. Resistance-guided antimicrobial therapy using doxycycline-moxifloxacin and doxycycline-2.5 g azithromycin for the treatment of Mycoplasma genitalium infection: efficacy and tolerability. Clin Infect Dis 2020; 71:1461–8. [DOI] [PubMed] [Google Scholar]
- 37. McIver R, Jalocon D, McNulty A, et al. Men who have sex with men with Mycoplasma genitalium-positive nongonococcal urethritis are more likely to have macrolide-resistant strains than men with only female partners: a prospective study. Sex Transm Dis 2019; 46:513–7. [DOI] [PubMed] [Google Scholar]
- 38. Meites E, Winer RL, Newcomb ME, et al. Vaccine effectiveness against prevalent anal and oral human papillomavirus infection among men who have sex with men—United States, 2016–2018. J Infect Dis 2020; 222:2052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johnson KA, Sankaran M, Kohn RP, Bacon O, Cohen SE. Testing for Mycoplasma genitalium and using doxycycline as first-line therapy at initial presentations for non-gonococcal urethritis (NGU) correlate with reductions in persistent NGU. Clin Infect Dis 2022; 76:1674–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.